Abstract
Background
Our objective was to explore bone-related outcome and bone turnover markers (BTM) during 2 years of secukinumab treatment in patients with radiographic axial spondyloarthritis (r-axSpA) in daily clinical practice.
Methods
Included were consecutive r-axSpA outpatients from the Groningen Leeuwarden axSpA (GLAS) cohort treated with secukinumab for 2 years. At baseline and 2 years, spinal radiographic damage was assessed using the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS; 0–72), cervical facet joint involvement according the “de Vlam” scoring method (0–15) and radiographic vertebral fractures (VF) using the “Genant” method (grade 0–3). At all visits, BTM reflecting collagen resorption (serum type I collagen C-telopeptide; sCTX), collagen formation (procollagen type 1 N-terminal peptide; PINP) and bone mineralization (bone-specific alkaline phosphatase; BALP) were measured and expressed in Z-scores to correct for the normal influence of age and gender.
Results
17 r-axSpA patients were included; 53% male, mean age was 47±15 years, mean Ankylosing Spondylitis Disease Activity Score (ASDAS) 3.9±1.2, and 53% was biological naïve. The median 2-year progression rates were 1.1 for mSASSS and 0.5 for facet joints, which was less than the smallest detectable change. One traumatic VF (grade 3) occurred. Serum levels of sCTX and PINP remained stable during secukinumab treatment and BALP decreased significantly after 2 years, with median 0–2 year change in Z-scores of +0.1, −0.4, and −1.2, respectively.
Conclusion
This explorative study of r-axSpA patients treated with secukinumab in daily clinical practice showed low radiographic spinal progression during 2 years of follow-up. Collagen resorption and formation markers remained stable, whereas mineralization marker BALP decreased significantly after 2 years. Our results are in line with the results of in vitro studies demonstrating that inhibition of IL17-A resulted in suppression of osteogenic differentiation with significant decrease in mineralization.
Keywords: radiographic axial spondyloarthritis, IL-17 inhibitor, bone metabolism
Introduction
Radiographic axial spondyloarthritis (r-axSpA) is characterized by inflammation, which may result in both excessive bone formation and bone loss. This is reflected in bone-related outcomes including the development of radiographic bone formation with syndesmophytes and ankylosing of the spine, vertebral fractures (VF), and low bone mineral density (BMD). Bone turnover markers (BTM) are biomarkers that are released during bone remodeling by osteoclasts and osteoblasts and, therefore, in vivo mirroring bone metabolism (resorption, formation, and mineralization) and are fundamental to bone-related outcomes in axSpA.
Treatment with TNF-α inhibitors (TNFi) and interleukin-17 inhibitors (IL17i) has been proven to be effective in decreasing disease activity in patients with r-axSpA.1 Regarding bone metabolism, it was observed that BTM balance favors collagen formation (PINP) and mineralization (BALP) during the first 3 years of TNFi, which was accompanied by significant improvement of BMD especially of the lumbar spine.2 Previously, we reported no changes in serum levels of BTM during 1 year of treatment with IL17i secukinumab.3 The objective of this study was to explore bone-related outcomes and BTM more long term, during 2 years of secukinumab treatment in r-axSpA patients in daily clinical practice.
Methods
We included consecutive r-axSpA outpatients, fulfilling the ASAS classification criteria and participating in the Groningen Leeuwarden axSpA (GLAS) cohort, who started treatment with secukinumab because of active disease between April 2016 and June 2020, and continued this treatment for 2 years. Secukinumab was administered as subcutaneous injection (150 mg) every 28 days. In case of inadequate clinical response, the frequency was raised to every 21 or 14 days. Demographic and clinical assessments were obtained from regular GLAS outpatient visits during 2 years of follow-up.4 The GLAS cohort was approved by local ethics committees of the Medical Center Leeuwarden (main protocol assessment, TPO364) and University Medical Center Groningen (local feasibility). All patients provided written informed consent according to the Declaration of Helsinki.
Bone-related outcomes were assessed at baseline and after 2 years in patients with available radiographs. Spinal radiographic damage was assessed using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS; 0–72) and cervical facet joint involvement according the “de Vlam” scoring method (0–15).5,6 Radiographic VFs were assessed using the “Genant” scoring method grade 0–3).7 BMD of the lumbar spine (anterior-posterior projection L1-L4) and hip (total proximal femur) was measured at baseline but not follow-up using dual-energy X-ray absorptiometry (Hologic QDR Discovery, Waltman, MA, USA). The window in which baseline BMD data was obtained was ±1 year from start of biological. Z-scores, the number of SD from the normal mean corrected for age and gender, were calculated using the NHANES reference database.
At all visits, serum bone turnover markers were assessed. sCTX was measured by electro-chemiluminescence (ECLIA; Elecsys 2010 Roche Mannheim, Germany; inter-assay coefficient of variation (IE-CV) 10.8%), PINP by RIA (Orion Diagnostica, Espoo, Finland; IE-CV 9.0%) and BALP by enzyme-linked immunosorbent assay (ELISA; Metra Biosystems, Mountain View, CA, USA; IE-CV 5.5%). Bone turnover markers were measured in a NEN-EN-ISO 9001:2008 certified and NEN-EN-ISO 15189:2012 accredited laboratory. Serum was acquired of non-fasting patients during study visits of the GLAS cohort taking place at fixed hours (same half-day) and were stored within one hour at −20°C until analysis. BTM were measured and expressed in Z-scores, calculated as the number of standard deviations (SD) from the normal mean corrected for age and gender.2
Statistical analysis was performed with SPSS Statistics 23. Bone-related outcomes were explored using descriptive statistics. Generalized estimating equations were used to analyze BTM Z-scores over time within patients. P values <0.05 were considered statistically significant.
Results
Twenty-four patients started secukinumab treatment, of which 17 completed 2 years of treatment and were included in the present analysis; 53% men, mean age 47±15 years, median symptom duration 20 years (interquartile range (IQR) 12–31), 82% HLA-B27+, 53% biological disease-modifying antirheumatic drug (DMARD) naïve and median C-reactive protein (CRP) 5 mg/L (IQR 3–26) (Table 1). Secukinumab was administered as 150 mg per 28 days (n=9), 21 days (n=2), or 14 days (n=6). Mean ASDAS was 3.9±1.2 at baseline and this improved significantly to 2.2±1.1 after 2 year of secukinumab. Mean vitamin D levels were stable during follow-up; 69.9±21.8 at baseline and 72.2±28.7 after 2 years. Regarding bone-related outcome at baseline, median values for BMD Z-score were −0.6 for the lumbar spine and −0.1 for the hip, 3.3 for mSASSS and 1.5 for facet joint score. No radiographic VFs were found at the baseline. Median BTM Z-scores were 0.00, −0.60, and 1.16 for sCTX, PINP, and BALP, respectively.
Table 1.
Baseline Characteristics of r-axSpA Patients Treated with Secukinumab for 2 Years (n=17)
| Characteristics | Patients |
| Age (yrs) | 47 ± 15 |
| Gender (male) (n, %) | 9 (53) |
| Duration of symptoms (yrs) | 20 (12–31) |
| HLA-B27+ (n, %) | 14 (82) |
| History of IBD (n, %) | 1 (6) |
| History of uveitis (n, %) | 3 (18) |
| History of psoriasis (n, %) | 1 (6) |
| History of TNFi use (n, %) | 8 (47) |
| Current NSAID use (n, %) | 13 (76) |
| Current DMARD use (n, %) | 9 (53) |
| BASDAI (range 0–10) | 6.8 ± 1.7 |
| BASDAI ≥4 (n, %) | 16 (94) |
| ASDASCRP | 3.9 ± 1.2 |
| ASDASCRP ≥2.1 (n, %) | 17 (100) |
| CRP (mg/L) | 5 (2.5–26) |
| Increased CRP >5 (n, %) | 8 (47) |
| 25(OH)D (nmol/L) | 69.9 ± 21.8 |
| sCTX (pg/mL) | 287.0 (175.5–370.3) |
| sCTX Z-score | 0.0 (−0.4–1.4) |
| PINP (µg/L) | 49.9 (39.7–73.9) |
| PINP Z-score | 0.6 (0.1–1.6) |
| BALP (U/L) | 21.3 (16.4–29.3) |
| BALP Z-score | 1.2 (−0.2–2.7) |
| LS BMD Z-score | −0.6 (−1.5–−0.4) |
| Hip BMD Z-score | −0.1 (−1.0–0.1) |
| mSASSS (0–72) | 3.3 (1.3–10.2) |
| Facet joint score (0–15) | 1.5 (0.0–2.8) |
Note: Values are mean ± SD or median (range) unless otherwise indicated.
Abbreviations: R-axSpA, radiographic axial spondyloarthritis; HLA-B27+, human leukocyte antigen B27 positive; IBD, inflammatory bowel disease; NSAID, non-steroidal anti-inflammatory drug; DMARD, disease-modifying antirheumatic drug; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; ASDAScrp, ankylosing spondylitis disease activity score with CRP; CRP, C-reactive protein; sCTX, serum C-telopeptide of type I collagen; PINP, procollagen type 1 N-terminal peptide; BALP, bone-specific-alkaline phosphatase; LS BMD, lumbar spine bone mineral density; hip BMD, hip bone mineral density; mSASSS, modified stoke ankylosing spondylitis spine score.
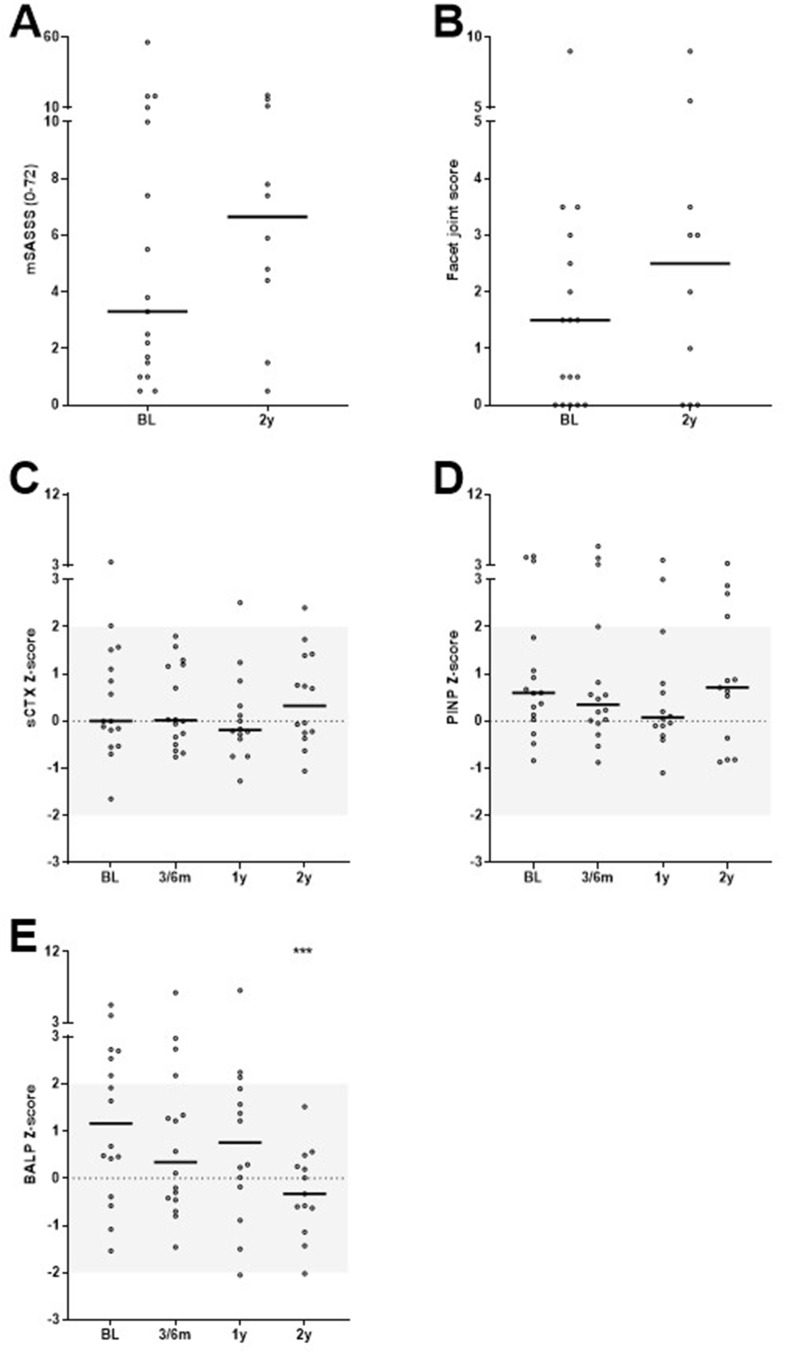
Ten patients had radiographic data available at baseline and 2 years, their median baseline values were 4.7 and 1.0 for mSASSS and facet joint score, respectively, and the median 2-year progression rates were 1.1 and 0.5 units (Figure 1A and B). For both outcomes, this progression rate was less than the smallest detectable change (SDC) of 1.8 and 2.3 units, respectively. One patient developed a traumatic VF (grade 3) after falling from a horse.
Figure 1.
Bone-related outcomes mSASSS (A), facet joint score (B) and bone turnover markers sCTX (C), PINP (D) and BALP (E) in r-axSpA patients treated with secukinumab (IL17i) for 2 years (total group n=17). Bar indicates median value and dots represent individual patient values. ***Indicate p-value of <0.001 compared to baseline.
Serum levels of sCTX and PINP did not significantly change during treatment with a median change in Z-score of +0.1 or −0.4, respectively, after 2 years. Serum levels of BALP decreased significantly with a median change in Z-score of −1.2 after 2 years (Figure 1C–E). Sensitivity analyses in a subgroup of patients who already reached 3 years of follow-up (n=8) indicated that BALP Z-scores remained low after 3 years of secukinumab treatment (data not shown).
Discussion
In this study, we explored the effect of 2 years of secukinumab treatment on the bone metabolism in r-axSpA patients in daily clinical practice. Our results showed low spinal radiographic progression, confirming the previously reported findings from the MEASURE 1 trial and the recent SURPASS trial.8,9 BMD, especially in the lumbar spine, was somewhat lower than in other individuals of the same age and sex, which is comparable to the data of previous studies.10 Due to the short follow-up time, no BMD follow-up data are available. No VF were observed at baseline and only one VF occurred during follow-up, which was related to trauma. Furthermore, we studied biomarkers reflecting the underlying bone metabolism with BTM, using Z-scores to correct for the normal influence of age and gender.
Serum levels of BTM related to collagen resorption (sCTX) and collagen formation (PINP) remained stable, whereas BTM related to mineralization (BALP) decreased significantly after 2 years. In contrast to our findings, absolute values of all BTM remained stable during 2 years of secukinumab in the MEASURE 1 trial. The percentage of females included in our study was larger than in the MEASURE 1 trial. Reduced mineralization, as observed during menopause, is associated with a decrease in BALP.11 However, we observed a decrease in BALP in Z-scores and absolute values in both males and females (data not shown), excluding the difference in gender distribution between our study and the MEASURE 1 study as a possible explanation.
Another difference between our study and the MEASURE 1 trial is the dosage in which secukinumab was administered. In our study, patients started with 150 mg per 28 days, but this was raised to 150 mg per 21 or 14 days in half of the patients. In the MEASURE 1 trial, patients received 75 mg or 150 mg per 28 days. However, it was not mentioned how many and which patients were included in the BTM analyses and whether there was a difference in the course of BALP between both dosing arms. Therefore, the difference in the course of BALP between the two studies could be due to a dose-dependent effect of secukinumab, differences in patient characteristics or both.
The major limitation of our study is the relatively small number of patients in follow-up. In future research, our results should be confirmed in a larger group of patients. However, according to BTM data the decrease in BALP remained in the subgroup of patients who had already reached 3 years of follow-up. Furthermore, our results from patients treated with secukinumab in daily clinical practice are in line with previous in vitro research, demonstrating that IL17 subtypes A and F promote osteogenic differentiation and matrix mineralization. Furthermore, inhibition of IL17 subtypes A and F resulted in suppression of osteogenic differentiation with a significant decrease in mineralization.12 This could be an explanation for the observed course in BALP since secukinumab inhibits IL17-A and BALP is an essential facilitator of bone mineralization.
Conclusion
To conclude, this explorative study of r-axSpA patients receiving secukinumab in daily clinical practice showed low radiographic spinal progression, less than the SDC, during 2 years of treatment. Additionally, serum levels of BTM related to collagen resorption and formation remained stable, whereas BALP, related to mineralization, decreased significantly after 2 years. Longitudinal research in a larger sample of patients is needed to further investigate the effect of IL17i treatment on serum levels of BALP, which may be dose-dependent, and to associate these findings with long-term bone-related outcome, especially with the course of BMD.
Acknowledgments
The authors would like to thank all patients who participated in the GLAS cohort. Furthermore, the authors wish to acknowledge Mrs A Hebels and Mrs B Toonder for their contribution to clinical data collection and L Wagenmakers and K Koerts for their contribution to BTM assessments.
Funding Statement
This study was supported by an investigator initiated research grant from Novartis.
Abbreviations
BALP, bone-specific alkaline phosphatase; BMD, bone mineral density; BTM, bone turnover marker; CRP, C-reactive protein; DMARD, disease-modifying antirheumatic drugs; GLAS, Groningen Leeuwarden axSpA; IL17i, interleukin-17 inhibitor; IQR, interquartile range; MCL, Medical Center Leeuwarden; mSASSS, modified stoke ankylosing spondylitis spine score; PINP, procollagen type 1 N-terminal peptide; sCTX, serum C-telopeptide cross-link of type 1 collagen; R-axSpA; radiographic axial spondyloarthritis; SD, standard deviation; SDC, smallest detectable change; UMCG, University Medical Center Groningen; VF, vertebral fracture.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The GLAS cohort was approved by the local ethics committees of the UMCG and MCL. All patients provided written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Mark Siderius has received consultancy fees from Novartis; Stan Kieskamp reports grants from ASAS, outside the submitted work; Freke Wink has received consultancy fees from AbbVie and Janssen; Frans GM Kroese and Suzanne Arends report no conflicts of interest; Anneke Spoorenberg has received grant/research support from AbbVie, Pfizer, UCB, consultant for AbbVie, Pfizer, MSD, UCB, and Novartis.
References
- 1.Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023;82(1):19–34. doi: 10.1136/ard-2022-223296 [DOI] [PubMed] [Google Scholar]
- 2.Arends S, Spoorenberg A, Houtman PM, et al. The effect of three years of TNF-alpha blocking therapy on markers of bone turnover and their predictive value for treatment discontinuation in patients with ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther. 2012;14(2):R98. doi: 10.1186/ar3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siderius M, Arends S, Wink F, Kroese FGM, Spoorenberg A. Stable bone turnover markers corrected for age and gender during the first year of secukinumab treatment in radiographic axial spondyloarthritis. Clin Exp Rheumatol. 2023. doi: 10.55563/clinexprheumatol/i2fnno [DOI] [PubMed] [Google Scholar]
- 4.Arends S, Brouwer E, van der Veer E, et al. Baseline predictors of response and discontinuation of tumor necrosis factor-alpha blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther. 2011;13(3):R94. doi: 10.1186/ar3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creemers MCW, Franssen MJAM, Van’t Hof MA, Gribnau FWJ, van de Putte LBA, van Riel PLCM. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis. 2005;64(1):127–129. doi: 10.1136/ard.2004.020503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vlam K, Mielants H, Veys EM. Involvement of the zygapophyseal joint in ankylosing spondylitis: relation to the bridging syndesmophyte. J Rheumatol. 1999;26(8):1738–1745. [PubMed] [Google Scholar]
- 7.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–1148. doi: 10.1002/jbmr.5650080915 [DOI] [PubMed] [Google Scholar]
- 8.Braun J, Buehring B, Baraliakos X, et al. Effects of secukinumab on bone mineral density and bone turnover biomarkers in patients with ankylosing spondylitis: 2-year data from a Phase 3 study, MEASURE 1. BMC Musculoskelet Disord. 2021;22(1):1037. doi: 10.1186/s12891-021-04930-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baraliakos X, Van der Heijde D, Machado P, et al. Pos1115 effect of secukinumab versus adalimumab biosimilar on radiographic progression in patients with radiographic axial spondyloarthritis: subgroup analyses by baseline syndesmophytes and C-reactive protein status. In: Scientific Abstracts. BMJ Publishing Group Ltd and European League Against Rheumatism; 2023:882.1–883. doi: 10.1136/annrheumdis-2023-eular.1274 [DOI] [Google Scholar]
- 10.Arends S, Spoorenberg A, Brouwer E, van der Veer E. Clinical studies on bone-related outcome and the effect of TNF-α blocking therapy in ankylosing spondylitis. Curr Opin Rheumatol. 2014;26(3):259–268. doi: 10.1097/BOR.0000000000000053 [DOI] [PubMed] [Google Scholar]
- 11.Rai AD, Sherpa ML, Singh A, Thejaswi SG, Bhutia RD. Bone alkaline phosphatase and urine hydroxyproline assay in pre and postmenopausal women in the state of Sikkim and its correlation with bone mineral density. J Midlife Health. 2021;12(4):304–309. doi: 10.4103/jmh.jmh_73_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah M, Maroof A, Gikas P, et al. Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells. RMD Open. 2020;6(2):e001306. doi: 10.1136/rmdopen-2020-001306 [DOI] [PMC free article] [PubMed] [Google Scholar]