Abstract
Coronafacic acid (CFA) is the polyketide component of coronatine (COR), a phytotoxin produced by the plant-pathogenic bacterium Pseudomonas syringae. The genes involved in CFA biosynthesis are encoded by a single transcript which encompasses 19 kb of the COR gene cluster. In the present study, the nucleotide sequence was determined for a 4-kb region located at the 3′ end of the CFA biosynthetic gene cluster. Three open reading frames were identified and designated cfa8, cfa9, and tnp1; the predicted translation products of these genes showed relatedness to oxidoreductases, thioesterases, and transposases, respectively. The translational products of cfa8 and cfa9 were overproduced in Escherichia coli BL21; however, tnp1 was not translated in these experiments. Mutagenesis and complementation analysis indicated that cfa8 is required for the production of CFA and COR. Analysis of a cfa9 mutant indicated that this gene is dispensable for CFA and COR production but may increase the release of enzyme-bound products from the COR pathway; tnp1, however, had no obvious function in CFA or COR biosynthesis. A genetic strategy was used to produce CFA in a P. syringae strain which lacks the COR gene cluster; this approach will be useful in future studies designed to investigate biosynthetic products of the CFA gene cluster.
Coronatine (COR) is a chlorosis-inducing, non-host-specific phytotoxin which functions as an important virulence factor in diseases incited by several pathovars of Pseudomonas syringae (9, 36, 37). COR consists of two distinct moieties, a polyketide component, coronafacic acid (CFA), and a cyclized amino acid, coronamic acid (CMA). Both CFA and CMA are derived from separate biosynthetic pathways and coupled via amide bond formation (8).
The genes for COR biosynthesis in P. syringae pv. glycinea PG4180 are encoded within a 32-kb region of a 90-kb plasmid designated p4180A. The entire COR cluster has been physically mapped with the restriction enzymes BamHI and SstI (5). The biosynthetic genes for CMA and CFA are located on opposing ends of the COR gene cluster and are separated by a 3.4-kb region consisting of three regulatory genes, corP, corR, and corS (Fig. 1A and B) (65). These three genes encode a modified two-component regulatory system in which CorS is the putative histidine protein kinase and CorR and CorP are response regulators (65). The nucleotide sequence of the 6.9-kb region containing the CMA biosynthetic gene cluster revealed the presence of four genes, designated cmaA, cmaB, cmaT, and cmaU (10, 45, 62) (Fig. 1A). The deduced amino acid sequence of cmaA indicates that the enzyme contains an amino-acid-activating domain and a putative iron-binding region; the latter is significant because of its conservation in the active site of certain enzymes which catalyze oxidative cyclizations. cmaB showed extensive homology with syrB, a gene encoding a peptide synthetase required for the synthesis of the phytotoxin syringomycin (70). Furthermore, the deduced amino acid sequence of cmaT suggests that it functions as a thioesterase, providing further support for the role of a thiotemplate mechanism for CMA biosynthesis (62). The function of cmaU remains unclear, since this gene was not related to sequences deposited in various databases (62).
FIG. 1.
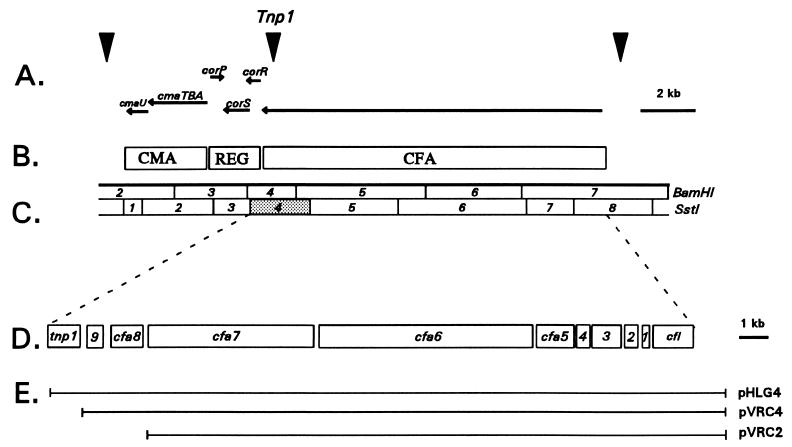
Functional and physical map of the COR biosynthetic gene cluster. (A) Inverted triangles show the location of three ORFs encoding putative transposases. Horizontal lines with arrowheads indicate the transcriptional organization of the COR gene cluster. (B) Functional regions of the COR biosynthetic cluster: CMA, CMA biosynthetic gene cluster; REG, regulatory region gene cluster; and CFA, CFA biosynthetic gene cluster. (C) Physical map of the COR gene cluster; enzymes used for restriction mapping were SstI and BamHI. SstI no. 4, the fragment sequenced in the present study, is shaded. (D) Expanded view of SstI fragments no. 4 to 8, which contain the CFA biosynthetic gene cluster. Abbreviations: 1, cfa1; 2, cfa2; 3, cfa3; 4, cfa4; and 9, cfa9. (E) Cosmid clones containing portions of the CFA gene cluster. pHLG4 contains SstI fragments no. 4 to 8 and cfl, cfa1–9, and tnp1; pVRC4 contains cfl and cfa1–9; and pVRC2 contains cfl and cfa1–7.
Precursor feeding studies with 13C-labeled substrates demonstrated that CFA is a novel polyketide synthesized from 1 U of pyruvate, 1 U of butyrate, and three acetate residues (44). Recent studies have suggested that the pyruvate used for CFA biosynthesis is actually converted into α-ketoglutarate before incorporation into CFA and that α-ketoglutarate may serve as the starter unit for CFA assembly (43). Little information is available regarding potential intermediates in the biosynthetic route to CFA, probably because such intermediates remain enzyme bound and are not released into the cytoplasm. However, Mitchell et al. (38) identified a cyclopentenone com- pound, 2-[1-oxo-2-cyclopenten-2-ylmethyl]-butanoic acid (CPE), which may function as an intermediate or shunt product of the CFA biosynthetic pathway.
Polyketide synthetases (PKS) are generally classified as type I or II enzyme systems and consist of protein complexes that act on covalently bound substrates which are attached as thioesters to an acyl carrier protein (ACP) (24). Type I PKS synthesize polyketides with a reduced structure and consist of large multifunctional proteins which can be divided into domains that catalyze discrete reactions in a nonreiterative fashion (20). Conversely, type II PKS are generally associated with the synthesis of aromatic polyketides, and biosynthesis occurs on monofunctional proteins which associate in a complex. Unlike the type I system, the type II PKS may utilize one or more enzymes in a reiterative fashion. Most of the research on polyketide synthesis in bacteria has focused on compounds synthesized by Streptomyces or other actinomycetes. However, in addition to coronatine, it is important to note that Pseudomonas produces a variety of antimicrobial compounds from the polyketide pathway, including mupirocin (pseudomonic acid) (17), pyoluteorin (15), and 2,4-diacetylphloroglucinol (56). Recently, Nowak-Thompson et al. (40) showed that the biosynthesis of pyoluteorin requires a type I PKS, and Thomashow and coworkers (3, 61) have demonstrated that production of 2,4-diacetylphloroglucinol requires a PKS similar to chalcone synthase (55).
The CFA biosynthetic gene cluster is encoded by a single transcript spanning 19 kb of the COR gene cluster (31) (Fig. 1A). Previous studies have focused on the 5′ end of the transcript, where six open reading frames (ORFs) were detected and named cfl, cfa1, cfa2, cfa3, cfa4, and cfa5 (32, 47) (Fig. 1D). The gene encoding coronafacate ligase (cfl) functions in the ligation of CFA and CMA via amide bond formation and may also have a role in CFA biosynthesis (5, 32, 50). Sequence analysis of cfa1, cfa2, and cfa3 revealed relatedness to monofunctional proteins in the type II class of PKS (20). The translation products of cfa1, cfa2, and cfa3 showed relatedness to ACP, fatty acid dehydratase, and β-ketoacyl synthetase, respectively (47). ACP and β-ketoacyl synthetase have structural roles in the assembly of precursors into the nascent polyketide, whereas fatty acid dehydratase is involved in the modification (dehydration) of the polyketide. The function of cfa4 could not be predicted from database searches, whereas the translation product of cfa5 showed relatedness to acyl-coenzyme A ligases (47). Both cfa1 and cfa3 were overexpressed in Escherichia coli, and protein products close to the predicted size were visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (47). Recently, two additional genes, cfa6 and cfa7, mapped to the CFA gene cluster (Fig. 1D) and were predicted to encode very large proteins (>200 kDa) (49).
The present study focuses on the 3′ end of the CFA biosynthetic gene cluster, which is encoded within SstI fragment no. 4 (Fig. 1C). A previous study indicated that this fragment was required for CFA biosynthesis (31); however, only two mutations mapped to this fragment, and their phenotype was not well characterized (5). In the present study, mutagenesis and complementation analysis were utilized to evaluate the role of this region in COR biosynthesis. Nucleotide sequence analysis of this region revealed two genes related to those associated with polyketide biosynthesis. The protein product of cfa8 showed similarity to crotonyl-coenzyme A reductases and was absolutely required for biosynthesis of CFA and COR. The translational product of cfa9 was related to thioesterases and dispensable for CFA and COR production. Furthermore, a method was developed to produce compounds from the CFA biosynthetic pathway in a COR nonproducer, P. syringae pv. glycinea 18a/90.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Pseudomonas strains were routinely cultured on King’s medium B (27) or mannitol-glutamate medium (25) at 28°C. E. coli cultures were grown on Luria-Bertani medium at 37°C (52).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Source or reference |
|---|---|---|
| Escherichia coli | ||
| DH5α | Δ(lacZYA-argF)U169 | 52 |
| BL21 | F−ompT rB− mB− | Novagen, Madison, Wis. |
| Pseudomonas syringae pv. glycinea | ||
| PG4180 | CFA+ COR+; contains p4180A | 5 |
| PG4180.D5 | CFA− COR− Kmr; cfa8::Tn5 | 64 |
| PG4180.D1 | CFA+ COR+ Kmr; cfa8::Tn5 | 69 |
| 18a/90 | CFA− COR−; lacks COR gene cluster | 63 |
| PG4180.V2 | CFA+ COR+ Kmr Cmr; cfa9::Cmr | This study |
| PG4180.N9 | CFA+ COR+ Kmr | 64 |
| PG4180.P1 | CFA− COR− Gmr; corS::Gmr | 65 |
| Plasmids | ||
| pLAFR3 | Tcr; RK2-derived cosmid vector | 57 |
| pHLG4 | Tcr; contains SstI fragments no. 4 to 8 in pLAFR3 (cfl through tnp1; Fig. 1E) | 31 |
| pVRC4 | Tcr; contains SstI fragments no. 5 to 8 in pLAFR3 (cfl through cfa9; Fig. 1E) | This study |
| pVRC2 | Tcr; contains SstI fragments no. 5 to 8 in pLAFR3 (cfl through cfa7) | This study |
| pBluescript SK+ | Apr; ColEI origin | Stratagene |
| pBluescript KS− | Apr; ColEI origin | Stratagene |
| pSL1 | Apr Cmr; contains Cmr cassette in pBluescript | 33 |
| pBTE | Apr; contains cfa9 on a 0.76-kb BamHI/HindIII fragment in pBluescript | This study |
| pBTE.Cm | Apr Cmr; derived from pBTE; contains cfa9::Cmr | This study |
| pRK415 | Tcr; IncP; RK2-derived vector | 26 |
| pMUH34 | Tcr; contains corRPS on a 3.4-kb HindIII/EcoRI fragment in pRK415 | 65 |
| pBBR1MCS | Cmr; broad-host-range vector compatible with IncP plasmids | 28 |
| pBBR.Reg | Cmr; contains corRPS from pMUH34 in pBBR1MCS | This study |
| pRKS4A | Tcr; 4-kb SstI fragment in pRK415 in the transcriptionally active orientation (Plac-cfa8-cfa9-tnp1) | This study |
| pRKS4I | Tcr; 4-kb SstI fragment in pRK415 in the transcriptionally inactive orientation (Plac-tnp1-cfa9-cfa8) | This study |
| pBS4A | Apr; 4-kb SstI fragment in pBluescript SK+ in the transcriptionally active orientation with respect to the T7 promoter (T7-cfa8-cfa9-tnp1) | This study |
| pBS4I | Apr; 4-kb SstI fragment in pBluescript SK+ in the transcriptionally inactive orientation with respect to the T7 promoter (T7-tnp1-cfa9-cfa8) | This study |
| pBES18A | Apr; 1.8-kb SstI-EcoRI from pBS4L was cloned in pBluescript KS− in the transcriptionally active orientation with respect to the T7 promtoer (T7-cfa8ΔC) | This study |
| pBES18I | Apr; 1.8-kb SstI-EcoRI from pBS4L was cloned in pBluescript KS− in the transcriptionally inactive orientation with respect to the T7 promoter | This study |
| pSE08 | Apr; 0.8-kb SstI-EcoRI fragment in pBluescript SK+ | This study |
| pE09 | Apr; 0.9-kb EcoRI fragment in pBluescript SK+ | This study |
| pE18 | Apr; 1.8-kb EcoRI fragment in pBluescript SK+ | This study |
| pBE078 | Apr; 0.78-kb BamHI/EcoRI fragment in pBluescript SK+ | This study |
Reagents and buffers.
All chemicals were purchased from Sigma Chemical Co. (St. Louis, Mo.); restriction enzymes and T4 DNA ligase were from BRL Life Technologies (Rockville, Md.); [α-32P]dCTP was from New England Nuclear (Boston, Mass.); Pro-mix, consisting of [35S]methionine and [35S]cysteine, was from Amersham (Arlington Heights, Ill.); and the digoxigenin DNA labeling kit was from Boehringer Mannheim (Indianapolis, Ind.). Protein concentrations were determined with the Bio-Rad (Richmond, Calif.) protein assay kit as recommended by the manufacturer. Antibiotics were added to media in the following concentrations (micrograms/milliliter): ampicillin, 100; chloramphenicol, 25; kanamycin, 25; tetracycline, 25.
DNA manipulations.
Agarose gel electrophoresis, restriction digests, purification of DNA fragments from agarose gels, electroporations, and minipreparations of plasmid DNA were performed by standard procedures (52). The procedure utilized for Southern hybridizations has been described previously (59). Large-scale preparations of plasmid DNA were isolated from E. coli with the Qiagen plasmid Midi kit (Qiagen, Chatsworth, Calif.). Plasmid DNA was isolated from Pseudomonas strains as described by Kado and Liu (21). Triparental matings with pRK2013 as the mobilizing plasmid were performed by established techniques (6). A cosmid library of the COR plasmid p4180A was constructed in pLAFR3 as described previously (31). Cosmids pVRC4 and pVRC2 were selected for further analysis based on their hybridization to SstI fragments no. 5 and 8.
Cloning of cfa8 into pBluescript KS−.
A truncated version of cfa8 (cfa8ΔC) was constructed by subcloning the 1.8-kb SstI-EcoRI fragment in pBS4I into pBluescript KS−; the construct which resulted was designated pBES18A and contained 1,536 nucleotides of cfa8 in the transcriptionally active orientation with respect to the T7 promoter (Fig. 2B). This construct was transformed into E. coli BL21, and proteins were overproduced and detected as described below. E. coli BL21 was also transformed with a second construct, pBES18I, which contained cfa8ΔC in the transcriptionally inactive orientation.
FIG. 2.
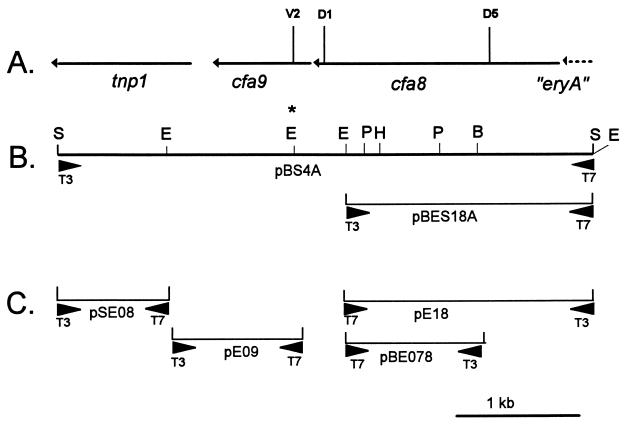
(A) Physical location of cfa8, cfa9, and tnp1 on SstI fragment no. 4. Vertical lines with letter-and-number designations indicate the locations of insertion mutations in cfa8 and cfa9. The region designated eryA extends into SstI fragment no. 5 and shows relatedness to the multifunctional type I PKS involved in erythromycin synthesis. (B) Physical map of two constructs used for protein overproduction in the current study. pBS4A contains SstI fragment no. 4 in pBluescript SK+ with cfa8, cfa9, and tnp1 in the transcriptionally active orientation with respect to the T7 promoter. The EcoRI site marked with an asterisk was used to insert a Cmr cassette into cfa9. pBES18A contains a 1.8-kb SstI-EcoRI fragment containing a truncated cfa8 (cfa8ΔC) in pBluescript KS−; cfa8ΔC is oriented in the transcriptionally active position with respect to the T7 promoter. (C) pSE08, pE09, pE18, and pBE078 are subclones used for sequence analysis. Internal primers were used to fill in sequencing gaps.
Construction of a mutation in cfa9.
cfa9 was cloned into pBluescript SK+ as a 0.76-kb PCR product. Plasmid pBS4I was used as template, and the following oligonucleotides were used as primers in the PCR: forward primer, 5′GCGGATCCTGAGACCTAGTCATG (the BamHI recognition site is shown in bold; the following sequence corresponds to nucleotides 1969–1983); and reverse primer, 5′GCAAGCTTTGTGTAGTGGCTGGCT (the HindIII site is shown in bold; the following sequence corresponds to nucleotides 2741–2730). Following amplification of the 0.76-kb PCR product, ligation in pBluescript SK+, and transformation into E. coli DH5α, plasmid pBTE was recovered. To assess the function of cfa9, we constructed a nonpolar mutation in this gene. The chloramphenicol resistance (Cmr) gene from pSL1 (33) was cloned into the EcoRI site of cfa9. pBTE-Cm, the clone containing the mutant allele (cfa9::Cmr), was electroporated into PG4180.N9, followed by the selection of Cmr derivatives.
DNA sequencing and analysis.
Manual nucleotide sequencing reactions were performed by the dideoxynucleotide method (53) and Sequenase version 2.0 (U.S. Biochemicals, Cleveland, Ohio) and [α-32P]dCTP. Automated nucleotide sequencing was performed with AmpliTaq DNA polymerase, an ABI 373A apparatus, and the ABI PRISM dye primer cycle sequencing kit (Perkin-Elmer, Foster City, Calif.). Automated sequencing was provided by the Oklahoma State University Recombinant DNA/Protein Resource Facility. A series of subclones (0.7 to 4.0 kb) was generated in pBluescript SK+ (Stratagene, La Jolla, Calif.) (Fig. 2C) and sequenced with the T7 and T3 primers. Gaps were filled by generating sequence from internal primers. The Tn5 insertions in selected mutants were localized by sequencing the DNA flanking the transposon with the oligonucleotide 5′GGTTCCGTTCAGGACGCTAC, which is derived from the border region of IS50 (51). Sequence data were aligned, and homology searches were executed with the Wisconsin sequence analysis package (version 9.0; Genetics Computer Group).
Detection and quantitation of coronafacoyl compounds.
Pseudomonas strains were incubated in 10-ml volumes of Hoitink-Sinden medium optimized for COR production at 18°C as described previously (42). Organic acids were extracted from culture supernatants and analyzed for COR and CFA by high-pressure liquid chromatography (HPLC) as described by Palmer and Bender (42). A calibration curve for quantifying CPE was constructed with an authentic standard of CPE (38) and Gold Nouveau chromatography software (Beckman Instruments, Fullerton, Calif.). The HPLC was calibrated by injecting a dilution series of known CPE concentration and measuring A208. The programmed calibration curve made it possible to obtain quantitative information on CPE yields from the peak areas produced during each chromatographic separation.
35S labeling of proteins.
Proteins were labeled with [35S]methionine and [35S]cysteine by the method described by Ohnishi et al. (41). E. coli BL21(DE3)-containing constructs cloned into pBluescript were overexpressed with the T7 promoter. Transformants were inoculated into 10 ml of Luria-Bertani broth containing ampicillin and incubated at 37°C to an optical density at 600 nm of 0.5. Cells from this culture (0.5 ml) were pelleted and washed with 10 volumes of M9 medium (52). Cells were collected by centrifugation and resuspended in 1 ml of M9 medium supplemented with 0.5% glycerol, 10 μg of thiamine per ml, and all essential amino acids (excluding methionine and cysteine) at 10 μg/ml. The expression of genes controlled by T7 polymerase was induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG) to 1 mM (final concentration) and incubating at 37°C for 1 h. Rifampin was added at 0.2 mg/ml to inactivate the host RNA polymerase, and cells were incubated for an additional 30 min. Proteins were then labeled by adding 15 μCi of Pro-mix and incubating an additional 5 min. After labeling, the cells were pelleted and resuspended in protein sample buffer containing 1% SDS (52). Proteins were separated by SDS-PAGE (10% acrylamide), blotted to Immobilon-P membranes (Millipore Corp., Bedford, Mass.), and exposed to X-ray film.
Nucleotide sequence accession number.
The nucleotide sequence of SstI fragment no. 4 was deposited in GenBank under accession no. AF061506.
RESULTS
Nucleotide sequence analysis.
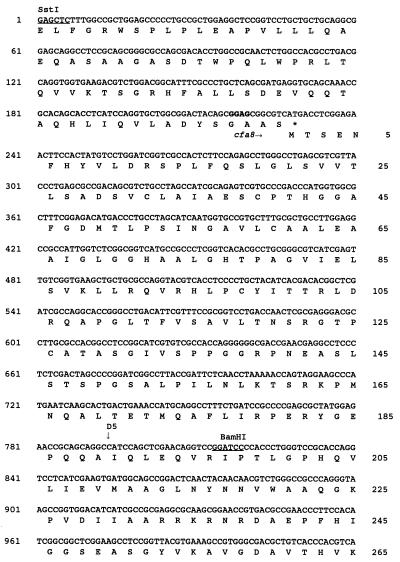
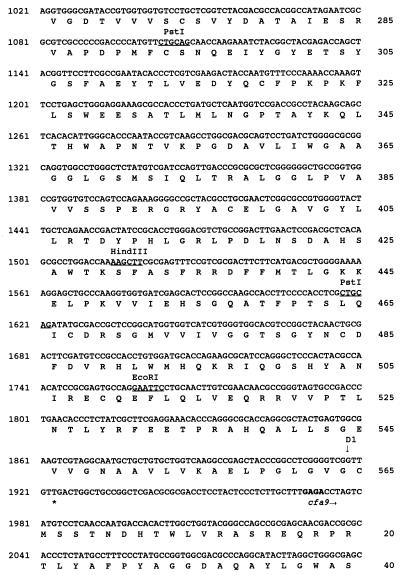
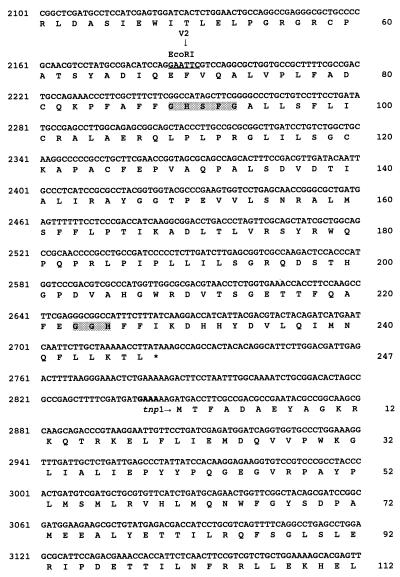
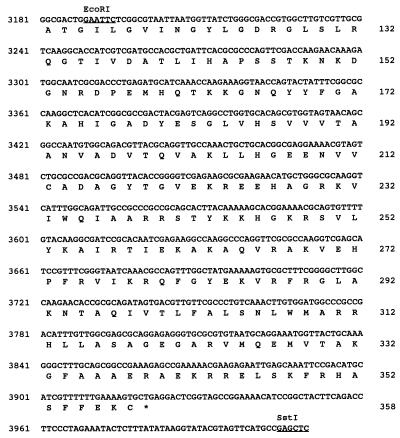
The nucleotide sequence of SstI fragment no. 4 was determined by a combination of manual and automated sequencing of both DNA strands (Fig. 3). Three ORFs having a common orientation for translation were detected and designated cfa8 (1,696 bp), cfa9 (743 bp), and tnp1 (1,075 bp) (Fig. 3). Each ORF had a potential ribosome binding site 7 to 11 bp upstream of the translational start. The authenticity of the three ORFs was validated by codon preference analysis with the University of Wisconsin Genetics Computer Group program CODONPREFERENCE in default mode and a Pseudomonas codon usage table (11).
FIG. 3.
Nucleotide sequence of a 4.0-kb DNA region required for COR biosynthesis, containing genes cfa8, cfa9, and tnp1. The translational product of the first 230 nucleotides of SstI fragment no. 4 shows similarity to the type I PKS enzyme system (16). Numbers for nucleotides and amino acid residues are shown on the left and right, respectively. Restriction sites are underlined and indicated above the nucleotides. Putative Shine-Dalgarno sequences are indicated in bold, and translational stop codons are indicated by asterisks. Vertical arrows are used to mark the Tn5 insertion sites in cfa8 and the insertion of the chloramphenicol resistance cassette in cfa9. The active site motifs in the thioesterase-like gene encoded by cfa9 are indicated by shading of the appropriate amino acid residues.
To identify enzymes related to the protein products of cfa8, cfa9, and tnp1, nucleotide sequence data were compared with GenBank, EMBL, SWISSPROT, and PIR database entries with the FASTEMBL and BLASTX programs. Cfa8 had a predicted mass of 61 kDa, and the N-terminal 177 amino acids of Cfa8 did not show significant relatedness to any sequences deposited in the databases. However, the C-terminal 388 amino acids of Cfa8 showed similarity to various members of the quinone oxidoreductase superfamily, also known as lens crystallins in certain mammals (66). Examples of this class of sequences and their relatedness to the C-terminal portion of Cfa8 include alcohol dehydrogenase from Methylobacterium extorquens (40% identity and 55% similarity) (12), crotonyl-coenzyme A reductase from Streptomyces collinus (32% identity and 45% similarity) (66), and crotonyl-CoA reductase from Streptomyces fradiae (30% identity and 42% similarity) (19).
Cfa9, which had a predicted mass of 27.5 kDa, showed relatedness (25 to 37% identity; 35 to 40% similarity) to several thioesterases, including those involved in the synthesis of gramicidin, tyrocidine, and tylosin (29, 35, 39). Cfa9 contained the GXSXG and GXH motifs characteristic of diisopropyl fluorophosphate-sensitive animal and avian thioesterases (Fig. 3) (13). Several studies have indicated that the serine and histidine residues in the GXSXG and GXH motifs are active-site residues in thioesterases and essential for enzymatic activity (46, 60, 67).
The deduced amino acid sequence of tnp1 indicated that the gene encoded a protein with a predicted molecular mass of 41 kDa. Database searches indicated that Tnp1 was related (47% identity, 71% similarity) to a protein encoded by IS5 from E. coli and various bacteriophages (30, 54).
Characterization of mutations in cfa8.
Two mutants, PG4180.D5 and PG4180.D1, were shown to contain Tn5 insertions in cfa8 (Fig. 2A and Fig. 3). Although PG4180.D5 was completely defective in the synthesis of both CFA and COR, PG4180.D1 synthesized 7.6 μg of COR per ml, a level approximately 75% of that obtained for the wild-type PG4180 (Table 2). This result indicated that the mutation in PG4180.D1 had little effect on COR production and was probably nonpolar with respect to downstream genes. Furthermore, PG4180.D1 synthesized 0.7 μg of CFA/ml, a level fivefold higher than that of CFA production in PG4180 (Table 2). The precise location of the Tn5 insertions in PG4180.D5 and PG4180.D1 was determined by the subcloning strategy described by Ullrich and Bender (62) and a primer complementary to the IS50 portion of Tn5. The Tn5 insertions in PG4180.D5 and PG4180.D1 mapped downstream of the presumed translational start site for cfa8 at nucleotides 567 and 1690, respectively (Fig. 2A and Fig. 3).
TABLE 2.
Production of COR, CFA, and CPE by P. syringae pv. glycinea strains used in the present study
| Straina | Productionb (in μg/ml) of:
|
||
|---|---|---|---|
| COR | CFA | CPE | |
| PG4180 | 9.97 ± 1.8 | 0.13 ± 0.03 | 0.35 ± 0.05 |
| PG4180.D5 | ND | ND | ND |
| PG4180.D1 | 7.6 ± 0.7 | 0.7 ± 0.15 | 0.08 ± 0.03 |
| PG4180.D5(pHLG4) | 1.27 ± 0.2 | 0.07 ± 0.03 | ND |
| PG4180.D5(pVRC4) | 2.15 ± 1.3 | 0.2 ± 0.05 | 0.12 ± 0.1 |
| PG4180.D5(pVRC2) | ND | ND | ND |
| PG4180.D5(pRKS4A) | 1.8 ± 0.72 | 0.02 ± 0.03 | ND |
| PG4180.D5(pRKS4I) | ND | ND | ND |
| PG4180.N9 | 16.5 ± 2.5 | 9.7 ± 0.26 | 0.92 ± 0.15 |
| PG4180.V2 | 2.2 ± 0.4 | 2.3 ± 0.3 | 0.06 ± 0.03 |
| 18a/90 | ND | ND | ND |
| 18a/90(pHLG4) | ND | ND | ND |
| 18a/90(pVRC4) | ND | ND | ND |
| 18a/90(pHLG4, pBBR.Reg) | ND | 0.13 ± 0.08 | 5.2 ± 1.8 |
| 18a/90(pVRC4, pBBR.Reg) | ND | 0.1 ± 0.05 | 6.1 ± 0.5 |
Plasmid phenotypes are as follows: pHLG4, cfl-cfa1–9-tnp1; pVRC4, cfl-cfa1–9; pVRC2, cfl-cfa1–7; pRKS4A, Plac-cfa8-cfa9-tnp1; pRKS4I, Plac-tnp1-cfa9-cfa8; and pBBR.Reg, corRPS. PG4180 is the wild-type strain; PG4180.N9 is a Tn5 mutant derived from the wild type, which overproduces COR (64).
Detection limits for COR, CFA, and CPE are 0.1, 0.02, and 0.015 μg, respectively. ND, not detected.
Since PG4180.D5 was totally defective in COR and CFA production, attempts were made to complement this mutant. pHLG4, a cosmid clone containing the entire CFA biosynthetic gene cluster (SstI fragments no. 4 to 8, Fig. 1E) (31), was introduced into PG4180.D5. The transconjugant PG4180.D5(pHLG4) produced 1.27 μg of COR/ml (Table 2), indicating that pHLG4 could partially complement the mutation in PG4180.D5. Another cosmid clone, pVRC4, contained SstI fragments no. 5 to 8 and a portion of SstI fragment no. 4; PG4180.D5(pVRC4) produced 2.15 μg of COR/ml, indicating that part of SstI fragment no. 4 was dispensable for COR production. Sequence analysis of the 3′ region of the CFA gene cluster in pVRC4 indicated that the cosmid contained cfa8 and cfa9 but lacked tnp1. This result indicated that tnp1 is not required for COR biosynthesis. Cosmid pVRC2 contained SstI fragments no. 5 to 8 but lacked cfa8, cfa9, and tnp1; not surprisingly, transconjugants of PG4180.D5(pVRC2) failed to produce COR or CFA at detectable levels. The partial, rather than full, complementation of PG4180.D5 by pHLG4 and pVRC4 was unexpected but could be attributed to differences which occur when the CFA gene cluster is present in trans relative to p4180A.
Construction and characterization of a cfa9 insertional mutant.
To further investigate the role of cfa9 in COR biosynthesis, we constructed a nonpolar mutation in this gene. pBTE.Cm, the clone containing the mutant allele (cfa9::Cmr), was electroporated into PG4180.N9, a COR-producing derivative of PG4180, and Cmr derivatives were selected. Recombination of the Cmr cassette into cfa9 and loss of the vector were verified by plasmid isolation and Southern blot analysis (data not shown). The cfa9 mutant resulting from this experiment, PG4180.V2, produced 2.2, 2.3, and 0.06 μg of COR, CFA, and CPE, respectively, per ml (Table 2). The levels of these compounds were significantly lower (P = 0.05) than those produced by the parent strain, PG4180.N9, which biosynthesized per ml 16.5, 9.7, and 0.9 μg of COR, CFA, and CPE, respectively (Table 2). These results suggest that cfa9 is not absolutely required for the secretion of COR or CFA but that it may increase the release of these compounds from enzyme-bound complexes inside the cell.
CFA biosynthesis by P. syringae pv. glycinea 18a/90.
P. syringae pv. glycinea 18a/90 was previously reported to be a nonproducer of COR and was found to lack DNA homologous to the COR gene cluster by Southern hybridization experiments (63). In a later study, CMA production by 18a/90 was observed when both the CMA biosynthetic gene cluster and regulatory region were coexpressed in this strain (64). In the present study, we investigated whether P. syringae pv. glycinea 18a/90 derivatives containing the CFA biosynthetic gene cluster and the regulatory region could produce CFA. Cosmids pHLG4 (31) and pVRC4 (this study) contain the entire CFA biosynthetic gene cluster and were introduced into 18a/90. Since both pHLG4 and pVRC4 are IncP derivatives, it was necessary to introduce the regulatory genes on a second replicon compatible with IncP. The broad-host-range vector pBBR1MCS was chosen for this purpose, since it was previously shown to be compatible with IncP, IncW, and IncQ replicons (28). The 3.4-kb HindIII/EcoRI fragment containing corRSP was excised from pMUH34 and cloned into pBBR1MCS; the plasmid which resulted, pBBR.Reg, was checked for functionality by introducing it into PG4180.P1, a corS mutant. PG4180.P1(pBBR.Reg) produced COR at levels equivalent to PG4180, indicating that the regulatory genes cloned into pBBR.Reg complemented the corS mutation in PG4180.P1 (data not shown). In a subsequent experiment, pBBR.Reg was introduced into 18a/90(pHLG4) and 18a/90(pVRC4); the transconjugants which resulted produced 0.13 and 0.1 μg of CFA/ml, respectively (Table 2). Although the amount was low, the presence of CFA in the two transconjugants was confirmed by gas chromatography-mass spectrometry analysis of the derivatized organic acid extracts (7). Interestingly, both 18a/90(pHLG4, pBBR.Reg) and 18a/90(pVRC4, pBBR.Reg) produced substantial levels (approximately 5 to 6 μg/ml) of CPE. This amount of CPE synthesis far exceeds that observed in COR producers, which secrete relatively small amounts of this compound (<1.0 μg; Table 2). 18a/90 and 18a/90 constructs lacking the regulatory region produced no detectable levels of CPE, CFA, or COR.
Identification of products encoded by SstI fragment no. 4.
In the present study, nucleotide sequence analysis indicated that SstI fragment no. 4 contained three discrete genes; therefore, we hypothesized that these genes could be expressed in trans if transcribed from a promoter which functioned in P. syringae. Previous experiments indicated that the Plac promoter functioned in P. syringae pv. glycinea (65), so two constructs were tested for their ability to restore COR production to PG4180.D5. pRKS4A contains SstI fragment no. 4, with Plac upstream of cfa8 in the transcriptionally active orientation (Plac-cfa8-cfa9-tnp1); whereas pRKS4I contained the same fragment, but with Plac in the transcriptionally inactive orientation (Plac-tnp1-cfa9-cfa8). PG4180.D5(pRKS4A) produced 1.8 μg of COR per ml, whereas PG4180.D5(pRKS4I) failed to produce CFA or COR (Table 2). These results indicated that the genes encoded on SstI fragment no. 4 could function in trans to partially complement the Tn5 insertion in PG4180.D5.
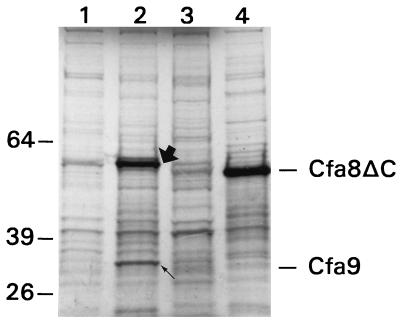
The 4-kb SstI fragment was then subcloned into pBluescript SK+ in transcriptionally active (pBS4A) and inactive (pBS4I) orientations with respect to the T7 promoter (Table 1). Both constructs (pBS4A and pBS4I) were transformed into E. coli BL21 cells which were lysogenic for DE3; these cells carried a chromosomal copy of T7 RNA polymerase under control of the lacUV5 promoter (58). Expression of the ORFs contained in pBS4A and pBS4I was induced with IPTG, and radiolabeled cells were harvested and analyzed by SDS-PAGE and autoradiography (Fig. 4). A control lane contained proteins induced from BL21(pBluescript SK+) (Fig. 4, lane 1). Two bands corresponding to 60 and 32 kDa were induced in the lane containing total cell extracts from BL21(pBS4A) (Fig. 4, lane 2). These values are close to the predicted sizes for cfa8 (∼61 kDa) and cfa9 (∼27.5 kDa). To confirm whether the 60-kDa protein was the translational product of cfa8, a truncated clone was constructed (cfa8ΔC) in pBluescript KS−. This clone was designated pBES18A and lacked approximately 170 bp from the 3′ end of cfa8 (Fig. 2B). BL21(pBES18A) produced a 55-kDa protein product when induced with IPTG (Fig. 4, lane 4), correlating perfectly with the predicted mass for the translational product of cfa8ΔC (∼55 kDa). BL21 cells containing pBES18I, a construct carrying cfa8ΔC in the transcriptionally inactive orientation, did not produce the 55-kDa protein (data not shown). It is important to note that BL21 cells containing pBES18A (Fig. 4, lane 4) and pBES18I (data not shown), did not produce Cfa9.
FIG. 4.
Overexpression of cfa8 and cfa9. Proteins were labeled with [35S]methionine and [35S]cysteine as described in the Materials and Methods. Constructs were transformed into E. coli BL21(DE3) and overexpressed from the T7 promoter. Lanes: 1, BL21(pBluescript SK+); 2, BL21(pBS4A); 3, BL21(pBS4I); and 4, BL21(pBES18A). Molecular size markers (in kilodaltons) are shown on the left; the migrations of Cfa8ΔC and Cfa9 are indicated on the right. The large and small arrows in lane 2 indicate the locations of Cfa8 and Cfa9, respectively.
DISCUSSION
Although sequence analysis of SstI fragment no. 4 revealed the presence of three genes, cfa8, cfa9, and tnp1, only cfa8 is essential for CFA and COR biosynthesis. The translation product of cfa8 showed relatedness to crotonyl-coenzyme A reductases from Streptomyces spp. CCR catalyzes the conversion of acetoacetyl-coenzyme A to butyryl-coenzyme A, and the latter product is used as an extender in polyketide synthesis (18). Consequently, the recruitment of a ccr-like gene into the CFA gene cluster may reflect the requirement for butyryl-coenzyme A as a precursor for CFA synthesis.
Thioesterases are sometimes found at the carboxyl terminus of PKS gene clusters and catalyze the release of the full-length polyketide chain by hydrolyzing the thioester bonds. cfa9, a putative thioesterase, was located at the 3′ end of the CFA transcript and was initially hypothesized to mediate the release of free CFA. The cfa9 mutant PG4180.V2 produced both CFA and COR, although the amounts relative to the parent strain PG4180.N9 were reduced 4- and 7.5-fold, respectively. The reduction in COR synthesis by PG4180.V2 could be explained by the decrease in available CFA; however, it is also possible that the thioesterase encoded by cfa9 functions in the enzymatic release of both compounds. These results indicate that some release of CFA and COR occurs in the absence of a functional cfa9. This result is similar to that obtained by Kao et al. (22), who showed that a thioesterase domain is not essential for release of the triketide lactone produced by DEBS1, one of three multifunctional PKS involved in the synthesis of 6-deoxyerythronolide B (6-dEB), an intermediate in the biosynthetic route to erythromycin. Furthermore, several polyketide gene clusters do not contain thioesterase domains (2, 4, 68). For example, PKS1, a gene encoding a type I fungal polyketide (T toxin), lacks a thioesterase domain, suggesting that release occurs by an unknown mechanism or by the action of an enzyme encoded elsewhere in the fungal genome (68). Also, type II bacterial PKS generally lack an obvious thioesterase component, implying that release does not always require a dedicated thioesterase (20).
The third gene described in the present study, tnp1, is related to the transposase encoded by IS5. tnp1 had no obvious role in CFA or COR production in the present study and was not translated by E. coli BL21. However, tnp1 could be involved in the regulation of COR biosynthesis, possibly by providing a promoter for corR, a downstream regulatory gene which is transcribed independently (Fig. 1A). The transcriptional start site and promoter region for corR have not been defined, and such an analysis could indicate whether tnp1 influences the expression of corR.
It is interesting to speculate on the potential role of tnp1 in the evolution of the COR biosynthetic gene cluster. tnp1 is strategically situated between the CFA and regulatory (REG) gene clusters (Fig. 1A). We have also identified two additional ORFs flanking the COR gene cluster, which are related to transposases; one ORF is located immediately downstream of the CMA gene cluster (62), and a third transposase-like ORF is positioned upstream of the CFA gene cluster (1) (Fig. 1A, inverted triangles). Perhaps the COR regulatory genes were first associated with the CMA gene cluster and a second transposition event positioned the CFA gene cluster adjacent to the regulatory region. Such an event would explain how the CFA and CMA gene clusters were merged under the coordinated control of the corRPS regulatory genes. Also relevant to this discussion is the low level of relatedness between cfa9 and another thioesterase in the COR gene cluster, cmaT. CmaT is thought to mediate the release of enzyme-bound CMA (62); however, cmaT shows very little relatedness to cfa9 (9% identity, 13% similarity). The regions of identity between the two genes are concentrated at the active site motifs (GXSXG and GXH); however, the nucleotide sequence flanking these motifs is highly diverged in the two genes, supporting the theory that the CMA and CFA gene clusters evolved independently.
In the present study, CFA was produced by P. syringae pv. glycinea 18a/90 derivatives containing the CFA and REG gene clusters. These results indicate that production of CFA in a heterologous background is possible. This approach will be extremely useful in future experiments designed to produce potential intermediates in the CFA biosynthetic pathway. Furthermore, the current study indicates that cfa9 may increase the release of intermediates in the CFA pathway; consequently, it may be possible to use cfa9 to facilitate the release of intermediates which generally remain enzyme bound. This approach is analogous to that utilized by several groups to study the synthesis of intermediates in the dEB pathway (14, 23, 34, 48).
Both the current study and previous studies have indicated that CFA biosynthesis requires monofunctional proteins typical of a type II PKS (32, 47). However, type II PKS are more commonly associated with the production of aromatic polyketides, whereas CFA has a highly reduced structure that is more typical of polyketides synthesized by type I systems. This inconsistency was somewhat explained by sequencing 230 additional bp upstream of cfa8. This region showed relatedness (28% identity, 42% similarity) to the eryA genes involved in the synthesis of 6-dEB, a polyketide synthesized by a type I PKS enzyme system (16) (Fig. 2A). Our results now suggest that CFA may be biosynthesized by a unique combination of mono- and multifunctional enzymes, indicating a cooperative activity which is unprecedented in polyketide synthesis.
ACKNOWLEDGMENTS
This work was supported by the Oklahoma Agricultural Experiment Station and by NSF grant MCB-9603618.
We thank F. Alarcón-Chaidez, A. Peñaloza-Vázquez, and L. Keith for reviewing the manuscript. We also thank R. Parry, S. Jiralerspong, and J. Patel for helpful and stimulating discussions.
REFERENCES
- 1.Alarcón-Chaidez, F., A. Peñaloza-Vázquez, and C. L. Bender. Unpublished data.
- 2.Aparicio J F, Molnar I, Schwecke T, Konig A, Haydock S F, Khaw L E, Staunton J, Leadlay P F. Organisation of the biosynthetic gene cluster for rapamycin in Streptomyces hygroscopicus: analysis of the enzymatic domains in the modular polyketide synthase. Gene. 1996;169:9–16. doi: 10.1016/0378-1119(95)00800-4. [DOI] [PubMed] [Google Scholar]
- 3.Bangera M G, Thomashow L S. Characterization of a genomic locus required for synthesis of the antibiotic 2,4-diacetylphloroglucinol by the biological control agent Pseudomonas fluorescens Q2-87. Mol Plant-Microbe Interact. 1996;9:83–90. doi: 10.1094/mpmi-9-0083. [DOI] [PubMed] [Google Scholar]
- 4.Beck J, Ripka S, Siegner A, Schlitz E, Schweizer E. The multifunctional 6-methylsalicylic acid synthase gene of Penicillium patulum: its gene structure relative to that of other polyketide synthases. Eur J Biochem. 1990;192:487–498. doi: 10.1111/j.1432-1033.1990.tb19252.x. [DOI] [PubMed] [Google Scholar]
- 5.Bender C, Liyanage H, Palmer D, Ullrich M, Young S, Mitchell R. Characterization of the genes controlling biosynthesis of the polyketide phytotoxin coronatine including conjugation between coronafacic acid and coronamic acid. Gene. 1993;133:31–38. doi: 10.1016/0378-1119(93)90221-n. [DOI] [PubMed] [Google Scholar]
- 6.Bender C L, Cooksey D A. Molecular cloning of copper resistance genes from Pseudomonas syringae pv. tomato. J Bacteriol. 1987;169:470–474. doi: 10.1128/jb.169.2.470-474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender C L, Malvick D K, Mitchell R E. Plasmid-mediated production of the phytotoxin coronatine in Pseudomonas syringae pv. tomato. J Bacteriol. 1989;171:807–812. doi: 10.1128/jb.171.2.807-812.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender C, Palmer D, Peñaloza-Vázquez A, Rangaswamy V, Ullrich M. Biosynthesis of coronatine, a thermoregulated phytotoxin produced by the phytopathogen Pseudomonas syringae. Arch Microbiol. 1996;166:71–75. [Google Scholar]
- 9.Bender C L, Stone H E, Sims J J, Cooksey D A. Reduced pathogen fitness of Pseudomonas syringae pv. tomato Tn5 mutants defective in coronatine production. Physiol Mol Plant Pathol. 1987;30:272–283. [Google Scholar]
- 10.Budde I P, Rohde B H, Bender C L, Ullrich M S. Growth phase and temperature influence promoter activity, transcript abundance and protein stability during biosynthesis of the Pseudomonas syringae phytotoxin coronatine. J Bacteriol. 1998;180:1360–1367. doi: 10.1128/jb.180.6.1360-1367.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherry J M. (Stanford University). 1994. Personal communication. [Google Scholar]
- 12.Chistoserdova L V, Lidstrom M E. Molecular characterization of a chromosomal region involved in the oxidation of acetyl-CoA to glyoxylate in the isocitrate-lyase-negative methylotroph Methylobacterium extorquens AM1. Microbiology. 1996;142:1459–1468. doi: 10.1099/13500872-142-6-1459. [DOI] [PubMed] [Google Scholar]
- 13.Cho H, Cronan J E., Jr Escherichia coli thioesterase I, molecular cloning and sequencing of the structural gene and identification as a periplasmic enzyme. J Biol Chem. 1993;268:9238–9245. [PubMed] [Google Scholar]
- 14.Cortes J, Wiesmann K E H, Roberts G A, Brown M J B, Staunton J, Leadlay P F. Repositioning of a domain in a modular polyketide synthase to promote specific chain cleavage. Science. 1995;268:1487–1489. doi: 10.1126/science.7770773. [DOI] [PubMed] [Google Scholar]
- 15.Cuppels D A, Howell C R, Stipanovic R D, Stoessl A, Stothers J B. Biosynthesis of pyoluteorin: a mixed polyketide-tricarboxylic acid cycle origin demonstrated by [1,2-13C2]acetate incorporation. Z Naturforsch. 1986;41:532–536. [Google Scholar]
- 16.Donadio S, Staver M J, McAlpine J B, Swanson S J, Katz L. Modular organization of genes required for complex polyketide biosynthesis. Science. 1991;252:675–679. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- 17.Feline T C, Jones R B, Mellows G, Phillips L. Pseudomonic acid. Part 2. Biosynthesis of pseudomonic acid A. J Chem Soc Dalton Trans. 1977;I:309–318. [PubMed] [Google Scholar]
- 18.Gandecha A R, Large S L, Cundliffe E. Analysis of four tylosin biosynthetic genes from tylM region of the Streptomyces fradiae genome. Gene. 1997;184:197–203. doi: 10.1016/s0378-1119(96)00595-1. [DOI] [PubMed] [Google Scholar]
- 19.Gandecha, A. R. GenBank accession no. X81885.
- 20.Hutchinson C R, Fujii I. Polyketide synthase gene manipulation: a structure-function approach in engineering novel antibiotics. Annu Rev Microbiol. 1995;49:201–238. doi: 10.1146/annurev.mi.49.100195.001221. [DOI] [PubMed] [Google Scholar]
- 21.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao C M, Luo G, Katz L, Cane D E, Khosla C. Engineered biosynthesis of a triketide lactone from an incomplete modular polyketide synthase. J Am Chem Soc. 1994;116:11612–11613. [Google Scholar]
- 23.Kao C M, Luo G, Katz L, Cane D E, Khosla C. Manipulation of macrolide ring size by directed mutagenesis of a modular polyketide synthase. J Am Chem Soc. 1995;117:9105–9106. [Google Scholar]
- 24.Katz L, Donadio S. Polyketide synthesis: prospects for hybrid antibiotics. Annu Rev Microbiol. 1993;47:875–912. doi: 10.1146/annurev.mi.47.100193.004303. [DOI] [PubMed] [Google Scholar]
- 25.Keane P J, Kerr A, New P B. Crown gall of stone fruit. II. Identification and nomenclature of Agrobacterium isolates. Aust J Biol Sci. 1970;23:585–595. [Google Scholar]
- 26.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 27.King E O, Ward M K, Raney D E. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 28.Kovach M E, Phillips R W, Elzer P H, Roop III R M, Peterson K M. pBBR1MCS: a broad-host-range cloning vector. BioTechniques. 1994;16:800–802. [PubMed] [Google Scholar]
- 29.Krätschmar J, Krause M, Marahiel M A. Gramicidin S biosynthesis operon containing the structural genes grsA and grsB has an open reading frame encoding a protein homologous to fatty acid thioesterases. J Bacteriol. 1989;171:5422–5429. doi: 10.1128/jb.171.10.5422-5429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kröger M, Hobom G. Structural analysis of insertion sequence IS5. Nature. 1982;297:159–162. doi: 10.1038/297159a0. [DOI] [PubMed] [Google Scholar]
- 31.Liyanage H, Palmer D A, Ullrich M, Bender C L. Characterization and transcriptional analysis of the gene cluster for coronafacic acid, the polyketide component of the phytotoxin coronatine. Appl Environ Microbiol. 1995;61:3843–3848. doi: 10.1128/aem.61.11.3843-3848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liyanage H, Penfold C, Turner J, Bender C L. Sequence, expression and transcriptional analysis of the coronafacate ligase-encoding gene required for coronatine biosynthesis by Pseudomonas syringae. Gene. 1995;153:17–23. doi: 10.1016/0378-1119(94)00661-b. [DOI] [PubMed] [Google Scholar]
- 33.Lukomski S, Hull R A, Hull S I. Identification of the O antigen polymerase (rfc) gene in Escherichia coli O4 by insertional mutagenesis using a nonpolar chloramphenicol cassette. J Bacteriol. 1996;178:240–247. doi: 10.1128/jb.178.1.240-247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDaniel R, Kao C M, Hwang S J, Khosla C. Engineered intermodular and intramodular polyketide synthase fusions. Chem Biol. 1997;4:667–674. doi: 10.1016/s1074-5521(97)90222-2. [DOI] [PubMed] [Google Scholar]
- 35.Merson-Davies L A, Cundliffe E. Analysis of five tylosin biosynthetic genes from the tyl1BA region of the Streptomyces fradiae genome. Mol Microbiol. 1994;13:349–355. doi: 10.1111/j.1365-2958.1994.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell R E. Coronatine production by some phytopathogenic pseudomonads. Physiol Plant Pathol. 1982;13:37–49. [Google Scholar]
- 37.Mitchell R E, Hale C N, Shanks J C. Production of different pathogenic symptoms and different toxins by strains of Pseudomonas syringae pv. tomato not distinguishable by gel-immunodiffusion assay. Physiol Plant Pathol. 1983;23:315–322. [Google Scholar]
- 38.Mitchell R E, Young H, Liddell M J. Isolation and structural characterization of 2-[1-oxo-2-cyclopenten-2-ylmethyl]-butanoic acid, a polyketide product of coronatine-producing Pseudomonas spp. Tetrahedron Lett. 1995;36:3237–3240. [Google Scholar]
- 39.Mootz H D, Marahiel M A. The tyrocidine biosynthesis operon of Bacillus brevis: complete nucleotide sequence and biochemical characterization of functional internal adenylation domains. J Bacteriol. 1997;179:6843–6850. doi: 10.1128/jb.179.21.6843-6850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowak-Thompson B, Gould S J, Loper J E. Identification and sequence analysis of the genes encoding a polyketide synthase required for pyoluteorin biosynthesis in Pseudomonas fluorescens Pf-5. Gene. 1997;204:17–24. doi: 10.1016/s0378-1119(97)00501-5. [DOI] [PubMed] [Google Scholar]
- 41.Ohnishi K, Fan F, Schoenhals G J, Kihara M, Macnab R M. The FliO, FliP, FliQ, and FliR proteins of Salmonella typhimurium: putative components for flagellar assembly. J Bacteriol. 1997;179:6092–6099. doi: 10.1128/jb.179.19.6092-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer D A, Bender C L. Effects of environmental and nutritional factors on production of the polyketide phytotoxin coronatine by Pseudomonas syringae pv. glycinea. Appl Environ Microbiol. 1993;59:1619–1623. doi: 10.1128/aem.59.5.1619-1626.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parry R J, Jiralerspong S, Mhaskar S, Alemany L, Wilcott R. Investigations of coronatine biosynthesis. Elucidation of the mode of incorporation of pyruvate into coronafacic acid. J Am Chem Soc. 1996;118:703–704. [Google Scholar]
- 44.Parry R J, Mhaskar S V, Lin M T, Walker A E, Mafoti R. Investigations of the biosynthesis of the phytotoxin coronatine. Can J Chem. 1994;72:86–99. [Google Scholar]
- 45.Patel, J., C. Bender, and R. Parry. Unpublished data.
- 46.Pazirandeh M, Chirala S S, Wakil S J. Site-directed mutagenesis studies on the recombinant thioesterase domain of chicken fatty acid synthase expressed in Escherichia coli. J Biol Chem. 1991;266:20946–20952. [PubMed] [Google Scholar]
- 47.Penfold C N, Bender C L, Turner J G. Characterisation of genes involved in biosynthesis of coronafacic acid, the polyketide component of the phytotoxin coronatine. Gene. 1996;183:167–173. doi: 10.1016/s0378-1119(96)00550-1. [DOI] [PubMed] [Google Scholar]
- 48.Pieper R, Luo G, Cane D, Khosla C. Cell-free synthesis of polyketides by recombinant erythromycin polyketide syntheases. Nature. 1995;378:263–266. doi: 10.1038/378263a0. [DOI] [PubMed] [Google Scholar]
- 49.Rangaswamy, V., S. Jiralerspong, R. Parry, and C. L. Bender. Unpublished data.
- 50.Rangaswamy V, Ullrich M, Jones W, Mitchell R, Parry R, Reynolds P, Bender C L. Expression and analysis of coronafacate ligase, a thermoregulated gene required for production of the phytotoxin coronatine in Pseudomonas syringae. FEMS Microbiol Lett. 1997;154:65–72. doi: 10.1111/j.1574-6968.1997.tb12625.x. [DOI] [PubMed] [Google Scholar]
- 51.Rich J J, Willis D K. A single oligonucleotide can be used to rapidly isolate DNA sequences flanking a transposon Tn5 insertion by the polymerase chain reaction. Nucleic Acids Res. 1990;18:6673–6676. doi: 10.1093/nar/18.22.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 53.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schoner B, Kahn M. The nucleotide sequence of IS5 from Escherichia coli. Gene. 1981;14:165–174. doi: 10.1016/0378-1119(81)90112-8. [DOI] [PubMed] [Google Scholar]
- 55.Schröder J. A family of plant-specific polyketide synthases: facts and predictions. Trends Plant Sci. 1997;2:373–378. [Google Scholar]
- 56.Shanahan P, Glennon J D, Crowley J J, Donnelly D F, O’Gara F. Liquid chromotographic assay of microbially derived phloroglucinol antibiotics for establishing the biosynthetic route to production, and the factors affecting their regulation. Anal Chim Acta. 1993;272:271–277. [Google Scholar]
- 57.Staskawicz B, Dahlbeck D, Keen N, Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987;169:5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Studier F W, Moffatt B A. Use of bacteriophage T7 polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 59.Sundin G W, Bender C L. Ecological and genetic analysis of copper and streptomycin resistance in Pseudomonas syringae pv. syringae. Appl Environ Microbiol. 1993;59:1018–1024. doi: 10.1128/aem.59.4.1018-1024.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tai M-H, Chirala S S, Wakil S J. Roles of Ser101, Asp236, and His237 in catalysis of thioesterase II and the C-terminal region of the enzyme in its interaction with fatty acid synthase. Proc Natl Acad Sci USA. 1993;90:1852–1856. doi: 10.1073/pnas.90.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomashow L S, Bangera M G, Bonsall R F, Kim D-S, Raaijmakers J, Weller D M. 2,4-Diacetylphloroglucinol, a key antibiotic in soilborne pathogen suppression by fluorescent Pseudomonas spp. In: Stacey G, Mullin B, Gresshoff P M, editors. Biology of plant-microbe interactions. St. Paul, Minn: International Society for Plant-Microbe Interactions; 1996. pp. 469–474. [Google Scholar]
- 62.Ullrich M, Bender C L. The biosynthetic gene cluster for coronamic acid, an ethylcyclopropyl amino acid, contains genes homologous to amino acid activating enzymes and thioesterases. J Bacteriol. 1994;176:7574–7586. doi: 10.1128/jb.176.24.7574-7586.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ullrich M, Bereswill S, Voelksch B, Fritsche W, Geider K. Molecular characterization of field isolates of Pseudomonas syringae pv. glycinea differing in coronatine production. J Gen Microbiol. 1993;139:1927–1937. doi: 10.1099/00221287-139-8-1927. [DOI] [PubMed] [Google Scholar]
- 64.Ullrich M, Guenzi A C, Mitchell R E, Bender C L. Cloning and expression of genes required for coronamic acid (2-ethyl-1-aminocyclopropane 1-carboxylic acid), an intermediate in the biosynthesis of the phytotoxin coronatine. Appl Environ Microbiol. 1994;60:2890–2897. doi: 10.1128/aem.60.8.2890-2897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ullrich M, Peñaloza-Vázquez A, Bailey A M, Bender C L. A modified two-component regulatory system is involved in temperature-dependent biosynthesis of the Pseudomonas syringae phytotoxin coronatine. J Bacteriol. 1995;177:6160–6169. doi: 10.1128/jb.177.21.6160-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wallace K K, Bao Z Y, Dai H, Digate R, Schuler G, Speedie M K, Reynolds K A. Purification of crotonyl-CoA reductase from Streptomyces collinus and cloning, sequencing and expression of the corresponding gene in Escherichia coli. Eur J Biochem. 1995;233:954–962. doi: 10.1111/j.1432-1033.1995.954_3.x. [DOI] [PubMed] [Google Scholar]
- 67.Witkowski A, Naggert J, Wessa B, Smith S. A catalytic role for histidine 237 in rat mammary gland thioesterase II. J Biol Chem. 1991;266:18514–18519. [PubMed] [Google Scholar]
- 68.Yang G, Rose M S, Turgeon B G, Yoder O C. A polyketide synthase is required for fungal virulence and production of the polyketide T-toxin. Plant Cell. 1996;8:2139–2150. doi: 10.1105/tpc.8.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Young S A, Park S K, Rodgers C, Mitchell R E, Bender C L. Physical and functional characterization of the gene cluster encoding the polyketide phytotoxin coronatine in Pseudomonas syringae pv. glycinea. J Bacteriol. 1992;174:1837–1843. doi: 10.1128/jb.174.6.1837-1843.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J-H, Quigley N B, Gross D C. Analysis of the syrB and syrC genes of Pseudomonas syringae pv. syringae indicates that syringomycin is synthesized by a thiotemplate mechanism. J Bacteriol. 1995;177:4009–4020. doi: 10.1128/jb.177.14.4009-4020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]