Abstract
Each year, SARS-CoV-2 is infecting an increasingly unprecedented number of species. In the present article, we combine mammalian phylogeny with the genetic characteristics of isolates found in mammals to elaborate on the host-range potential of SARS-CoV-2. Infections in nonhuman mammals mirror those of contemporary viral strains circulating in humans, although, in certain species, extensive viral circulation has led to unique genetic signatures. As in other recent studies, we found that the conservation of the ACE2 receptor cannot be considered the sole major determinant of susceptibility. However, we are able to identify major clades and families as candidates for increased surveillance. On the basis of our findings, we argue that the use of the term panzootic could be a more appropriate term than pandemic to describe the ongoing scenario. This term better captures the magnitude of the SARS-CoV-2 host range and would hopefully inspire inclusive policy actions, including systematic screenings, that could better support the management of this worldwide event.
Keywords: SARS-CoV-2, evolution, mammals, panzootic, mutations
Even before the COVID-19 pandemic, humans had already been challenged with several emerging viral respiratory infections with pandemic potential, including two coronaviruses, SARS-CoV-1 and MERS-CoV. SARS-CoV-2 (species severe acute respiratory syndrome-related coronavirus, subgenus Sarbecovirus, genus Betacoronavirus, family Coronaviridae; Coronaviridae Study Group of the International Committee on Taxonomy of Viruses 2020) is a virus of zoonotic origin. All known human coronaviruses (Decaro and Lorusso 2020, Tagliamonte et al. 2020), including recent porcine deltacoronaviruses (Lednicky et al. 2021) and canine alphacoronaviruses (Lednicky et al. 2022, Vlasova et al. 2022), are of zoonotic origin. However, SARS-CoV-2 has been unique, in that it has been able to ignite a pandemic with catastrophic consequences around the globe (CRS 2021). To date, it appears that the first viral strains of SARS-CoV-2 were detected in Wuhan City (Hubei Province, China, December 2019) at the Huanan seafood wholesale market (Liu et al. 2020). The Huanan market, which is the largest of its kind in central China, sells many different species of farm and wild animals and is visited by thousands of people daily. Globally, as of 16 February 2023, there have been 756,411,740 confirmed cases of COVID-19, including 6,842,468 deaths reported to the World Health Organization (WHO; https://covid19.who.int).
As the COVID-19 pandemic continues, the number of mammal species that are susceptible to infection with SARS-CoV-2 increases. We are at the beginning of a macrocycle that may become the first documented case of a viral infection with exceptional characteristics in terms of host-range susceptibility. Coronavirus infections in humans find their origins in viruses circulating in wildlife—in particular, bats—through cross-species transmission that often includes intermediate hosts, such as alpacas, palm civets, rodents, cattle, and dromedary camels (Cui et al. 2019). Overall, the transmission of pathogens is not unidirectional; ecosystem disruptions and alterations to the human–wildlife interface also create opportunities for circulating infectious agents to undergo reverse zoonotic transmission, or spillback, into domestic and wild animal species. The transmission of pathogens between different species—that is, crossing species barriers—is an ecological phenomenon known as host jump, cross-species transmission, zoonotic transfer, pathogen spillover, and zoonotic spillover. Specifically, spillover can be defined as the “cross-species transmission of a parasite into a host population not previously infected” (Plowright et al. 2017). Usually, spillover refers to the cross-species transmission of pathogens from wildlife (usually vertebrates) to humans (Plowright et al. 2017, Wells and Clark 2019), whereas spillback is defined as the transmission of a pathogen from humans to wildlife (reverse zoonosis) by direct contact between species or mediated by vectors (Weaver 2013, Olival et al. 2020, Hendy et al. 2020). Because of the massive spread of SARS-CoV-2 in humans, the direction of spillover events has been mostly from humans to animals in a zooanthroponotic fashion, although over time these interspecies transmissions have become anthropozoonotic (animals to humans). Along these lines, the SARS-CoV-2 pandemic has already set off a series of potential spillback risk scenarios and venues for transmission, because many domesticated pets, mammals in zoos, and livestock are susceptible to SARS-CoV-2 infection (Hobbs and Reid 2021, Qiu et al. 2023). For example, tigers in the Bronx Zoo (New York, New York, in the United States) acquired SARS-CoV-2 infection possibly from zookeepers (McAloose et al. 2020), and mink farms in the United States, as well as in Europe, have reported outbreaks of SARS-CoV-2 linked to human spillback. In addition, there is a documented event of spillback from human to mink with further spillover back to humans (Chaintoutis et al. 2021, Eckstrand et al. 2021, Koopmans 2021, Oude Munnink et al. 2021). A similar scenario is currently described in North American white-tail deer (Kuchipudi et al. 2022, Martins et al. 2022, Pickering et al. 2022) and in pet hamsters (Yen et al. 2022). The possibility of spillback is a critical concern, because it could result in an enzootic establishment (Manes et al. 2020) and future spillover of SARS-CoV-2 to humans (Fischhoff et al. 2021), both of which have implications for human and nonhuman mammal health, including wildlife (Gryseels et al. 2020, Olival et al. 2020, Audino et al. 2021). Therefore, understanding the trajectory and dynamics of SARS-CoV-2 in mammals is of utmost importance. In this study, we investigate the potential of SARS-CoV-2 to infect additional species to those already reported by combining virological data and inferences of mammalian species susceptibility obtained by mapping infection and angiotensin-converting enzyme 2 (ACE2) receptor conservation onto a phylogenetic tree of mammal species. We hope that the results in the present article can guide policy to support systematic screening of wildlife to monitor SARS-CoV-2 circulation and evolution in mammals, preserve endangered species, and further prevent virus spillover to humans.
Tracking coronaviruses
Coronaviruses possess the largest genomes of any RNA viruses, 27.6–31 kilobases (kb) in size (other RNA viruses are typically 10 kb in size; Belshaw et al. 2007). The 5′-most (i.e., from the initiation point) two-thirds of the genome includes the replicase gene, which consists of two overlapping open reading frames (ORF), 1a and 1b. Located downstream of ORF1b are four ORFs that code for a set common to all coronaviruses of structural proteins: spike, envelope, membrane, and nucleocapsid proteins. Coronavirus entry into host cells is mediated by its transmembrane spike glycoprotein that forms homotrimers protruding from the viral surface (Tortorici and Veesler 2019). The spike glycoprotein includes two functional subunits responsible either for binding to the host cell receptor (S1 subunit including the receptor-binding domain; RBD) or for fusion of the viral and cellular membranes (S2 subunit). The ACE2, previously identified as the cellular receptor for SARS-CoV-1 (Hamming et al. 2004), also acts as a receptor for SARS-CoV-2 (Yan et al. 2020). In particular, the SARS-CoV-2 spike protein is activated by TMPRSS2 protease expressed at the apical surface of the airway epithelium to mediate fusion (Hoffmann et al. 2020). The ACE2 receptor was first reported in 2000 (Imai et al. 2008), with a sequence of approximately 800 amino acids. It is found in various species and in multiple tissues, including the small and large intestines, kidneys, testes, heart (Sun et al. 2021a), and brain (Xu and Lazartigues 2020).
Since the start of the COVID-19 pandemic, virus genomic sequences have been generated and shared at an unprecedented rate, with more than 15 million SARS-CoV-2 sequences available via the Global Initiative on Sharing All Influenza Data (GISAID; www.gisaid.org), permitting near real-time surveillance of the pandemic (Meredith et al. 2020). The use of pathogen genomes on this scale to track the spread of the virus internationally (Di Giallonardo et al. 2020), study local outbreaks (Amato et al. 2022), and inform public health policy signifies a new age in virus genomic investigations. To further understand the epidemiology, sequencing enables the identification of emerging SARS-CoV-2 variants and mutations potentially linked to changes in viral properties, tissue and host-species tropism, and adaptation. Since late 2020, SARS-CoV-2 variants circulating globally that pose an increased risk to global public health have been classified as variants of interest and variants of concern to prioritize global monitoring and research efforts (WHO 2021). These variants are characterized by specific multiple genomic mutations with respect to the Wuhan-Hu strain, and this characterization may cause diagnostic detection failures and reduced efficacy of treatments (CDC 2023).
Documented susceptibility of mammals to SARS-CoV-2 infection and conservation concerns
To review the status of known SARS-CoV-2 infection in mammals, we surveyed the cases noted by the National Veterinary Services around the world. The National Veterinary Services are responsible for collecting, managing, and reporting SARS-CoV-2 infections in mammals to the World Organization for Animal Health (WOAH; founded as the Office International des Épizooties). The tripartite of WOAH, WHO, and the Food and Agriculture Organization of the United Nations have emphasized the importance of monitoring wildlife populations for SARS-CoV-2 infection as a means of early detection of potential reservoir species. A case in point was the detection of SARS-CoV-2 in white-tail deer (Chandler et al. 2021, Kuchipudi et al. 2022) and mule deer (WOAH 2022a) in the United States and Canada based on the monitoring efforts of those countries. Conversely, routine or systematic testing for SARS-CoV-2 in domestic animals has only been recommended under very specific conditions (WOAH 2022b). It is important to stress that the active monitoring for SARS-CoV-2 in wildlife varies depending on the country. Some countries, such as the United States, have established a systematic surveillance plan for targeted species (USDA 2023), whereas other countries lack a proper surveillance strategy, which makes it difficult to quantify the surveillance efforts. Limited disease surveillance (not only SARS-CoV-2) in wildlife is a common problem in several countries, because of the lack of funding and to prioritization of activities in domestic animals (Delgado et al. 2023). Considering the above, data on SARS-CoV-2 infection officially reported to the WOAH were coupled with gray literature (e.g., newspaper articles) and references in scientific journal articles in our compilation reported in the present article. In particular, the study of Meekins and colleagues (2021) was used as an initial reference to outline SARS-CoV-2 susceptibility and disease course in different mammal species on the basis of both experimental and natural infections.
Overall, 51 nonhuman mammal species from 22 taxonomic families were identified as having SARS-CoV-2 infection (table 1). We found 24 species with natural infection, 20 with experimental infection and found to be susceptible, and 8 with experimental infection and not found to be susceptible. Remarkably, differences were evident within a family. For example, in Procyonidae, the South American coati (Nasua nasua) has shown natural infection, whereas raccoons (Procyon lotor) have been experimentally infected and found to be not susceptible. In addition, in the Canidae, wolves (Canis lupus) have been shown to be naturally infected by SARS-CoV-2, whereas the common raccoon dog and the red fox (Nyctereutes procyonoides and Vulpes vulpes, respectively) have only shown susceptibility through experimental infection, and coyotes (Canis latrans) are not susceptible through experimental infection.
Table 1.
SARS-CoV-2 susceptibility, clinical signs, and International Union for Conservation of Nature (IUCN) status for nonhuman mammal species.
| Species | Family | IUCN status | Clinical signs | Source |
|---|---|---|---|---|
| Domestic cattle (Bos taurus) | Bovidae | NA | Most subclinical, some increased temperature and coughing in calves | Falkenberg et al. 2021 |
| Common marmoset (Callithrix jacchus) | Callitrichidae | LC | Most subclinical, increased body temperature | Lu et al. 2020, Singh et al. 2021 |
| Coyote (Canis latrans) | Canidae | LC | Not susceptible | Porter et al. 2022 |
| Domestic dog (Canis lupus) | Canidae | NA | Subclinical | Shi et al. 2020, Bosco-Lauth et al. 2021 |
| Raccoon dog (Nyctereutes procyonoides) | Canidae | LC | Most subclinical, isolated lethargy observed | Freuling et al. 2020 |
| Red fox (Vulpes vulpes) | Canidae | LC | Lethargy and sneezing | Porter et al. 2022 |
| Mongolian Beaver (Castor fiber) | Castoridae | LC | Cough, runny nose, sticky eyes, and mortality | Xinhua 2021 |
| African green monkey (Chlorocebus aethiops) | Cercopithecidae | LC | Decreased appetite, anorexia, elevated body temperature, changes in respiratory rate | Cross et al. 2020, Hartman et al. 2020, Blair et al. 2021, Woolsey et al. 2021 |
| Baboon (Papio hamadryas) | Cercopithecidae | LC | Not reported | Singh et al. 2021 |
| Cynomolgus macaque (Macaca fascicularis) | Cercopithecidae | VU | Subclinical, elevated body temperature, decreased appetite, weight loss | Koo et al. 2020, Lu et al. 2020, Ishigaki et al. 2021, Salguero et al. 2021 |
| Northern pig-tailed macaque (Macaca leonina) | Cercopithecidae | VU | Increase in body temperature | Song 2021 |
| Rhesus macaque (Macaca mulatta) | Cercopithecidae | LC | Subclinical, elevated body temperature, decreased activity, decreased appetite, weight loss, changes in respiratory pattern | Chandrashekar et al. 2020, Deng et al. 2020, Lu et al. 2020, Munster et al. 2020, Shan et al. 2020, Yu et al. 2020, Zheng et al. 2020, Song et al. 2020a, Blair et al. 2021, Salguero et al. 2021, Singh et al. 2021 |
| White-tailed deer (Odocoileus virginianus) | Cervidae | LC | Most subclinical; elevated body temperature, some ocular or nasal discharge | Chandler et al. 2021, Cool et al. 2021, Palmer et al. 2021 |
| Bank vole (Myodes glareolus) | Cricetidae | LC | Subclinical | Ulrich et al. 2020 |
| Bushy-tailed woodrat (Neotoma cinerea) | Cricetidae | LC | Subclinical | Bosco-Lauth et al. 2021 |
| Campbell's dwarf hamster (Phodopus campbelli) | Cricetidae | LC | Subclinical | Trimpert et al. 2020 |
| Chinese hamster (Cricetulus barabensis) | Cricetidae | LC | Weight loss | Bertzbach et al. 2021 |
| Deer mouse (Peromyscus maniculatus) | Cricetidae | LC | Most subclinical; isolated ruffled fur, isolated weight loss | Bosco-Lauth et al. 2021, Fagre et al. 2021, Griffin et al. 2021 |
| Djungarian dwarf hamster (Phodopus sungorus) | Cricetidae | LC | Subclinical | Trimpert et al. 2020 |
| Roboroversuski dwarf hamster (Phodopus roboroversuskii) | Cricetidae | LC | Decreased body temperature, severe weight loss, dyspnoea, ruffled fur, depressed behavior, required euthanasia | Trimpert et al. 2020 |
| Syrian golden hamster (Mesocricetus auratus) | Cricetidae | VU | Weight loss, lethargy, ruffled fur, hunched posture, respiratory signs, fatal disease reported in older hamsters | Chan et al. 2020, Imai et al. 2020, Osterrieder et al. 2020, Rosenke et al. 2020, Sia et al. 2020, Song et al. 2020b, Mohandas et al. 2021, Monchatre-Leroy et al. 2021, Selvaraj et al. 2021, Yen et al. 2022 |
| Canada lynx (Lynx canadensis) | Felidae | LC | Coughing and lethargy | USDA 2021a |
| Domestic cat (Felis catus) | Felidae | NA | Most subclinical (adults or subadults), behavior changes, diarrhea, weight loss, potential severe clinical signs in juveniles | Barrs et al. 2020, Bosco-Lauth et.al 2020 et al. 2020, Gaudreault et al. 2020, Halfmann et al. 2020, Newman et al. 2020, Sailleau et al. 2020, Segales et al. 2020, Shi et al. 2020, Bao et al. 2021, Gaudreault et al. 2021, Hamer et al. 2021, Hosie et al. 2021, Pagani et al. 2021, Ruiz-Arrondo et al. 2021, Klaus et al. 2021a, Klaus et al. 2021b |
| Eurasian lynx (Lynx lynx) | Felidae | LC | Respiratory clinical signs | WOAH 2022c |
| Fishing cat (Prionailurus viverrinus) | Felidae | VU | Not reported | USDA 2021b |
| Leopard (Panthera pardus) | Felidae | VU | Not reported | Mahajan et al. 2022 |
| Lion (Panthera leo) | Felidae | VU | Loss of appetite, nasal discharge, sore throat, coughing, fever | McAloose et al. 2020, Fernandez-Bellon et al. 2021, Mishra et al. 2021 |
| Puma (Puma concolor) | Felidae | LC | Subclinical, coughing and wheezing | Giraldo-Ramirez et al. 2021 |
| Snow Leopard (Panthera uncia) | Felidae | VU | Respiratory clinical signs | Giraldo-Ramirez et al. 2021 |
| Tiger (Panthera tigris) | Felidae | EN | Intermittent cough and audible wheezing | McAloose et al. 2020 |
| Hippo (Hippopotamus amphibius) | Hippopotamidae | VU | Runny nose | BBC News 2021 |
| Chimpanzee (Pan troglodytes) | Hominidae | EN | Subclinical | Farzan 2021 |
| Orangutan (Pongo abelii) | Hominidae | CR | Subclinical | Farzan 2021 |
| Western lowland gorilla (Gorilla gorilla) | Hominidae | CR | Respiratory clinical signs | USDA 2021c |
| Spotted Hyenas (Crocuta crocuta) | Hyaenidae | LC | Not reported | USDA 2021d |
| New Zealand white rabbit (Oryctolagus cuniculus) | Leporidae | EN | Not susceptible | Mykytyn et al. 2021, Fritz et al. 2022 |
| Striped skunk (Mephitis mephitis) | Mephitidae | LC | Subclinical | Bosco-Lauth et al. 2021 |
| Wild House mouse (Mus musculus) | Muridae | LC | Not susceptible | Bosco-Lauth et al. 2021 |
| American mink (Neovison vison) | Mustelidae | LC | Many asymptomatic, weight loss, nasal discharge, respiratory distress, reduced activity/feed intake, mortality | Hammer et al. 2021, Molenaar et al. 2020, Oreshkova et al. 2020, Boklund et al. 2021, Larsen et al. 2021, Oude Munnink et al. 2021, Shuai et al. 2021 |
| Asian small-clawed otter (Aonyx cinerea) | Mustelidae | VU | Respiratory signs, lethargy | USDA 2021e |
| Domestic ferret (Mustela putorius) | Mustelidae | LC | Most subclinical; isolated increased body temperature, reduced activity, respiratory signs, reduced appetite, ruffled fur | Kim et al. 2020, Richard et al. 2020, Schlottau et al. 2020, Shi et al. 2020, Everett et al. 2021, Giner et al. 2021, Gortazar et al. 2021, Kim et al. 2021, Kutter et al. 2021, Monchatre-Leroy et al. 2021, Ryan et al. 2021 |
| Raccoon (Procyon lotor) | Procyonidae | LC | Not susceptible | Bosco-Lauth et al. 2021 |
| South American Coati (Nasua nasua) | Procyonidae | LC | Not reported | USDA 2021f |
| Egyptian fruit bat (Rousettus aegyptiacus) | Pteropodidae | LC | Subclinical | Schlottau et al. 2020 |
| Black-tailed prairie dog (Cynomys ludovicianus) | Sciuridae | LC | Not susceptible | Bosco-Lauth et al. 2021 |
| Fox squirrel (Sciurus niger) | Sciuridae | LC | Not susceptible | Bosco-Lauth et al. 2021 |
| Wyoming ground squirrel (Urocitellus elegans) | Sciuridae | LC | Not susceptible | Bosco-Lauth et al. 2021 |
| Domestic pig (Sus scrofa) | Suidae | LC | Most subclinical; isolated ocular nasal discharge, mild depression, cough | Meekins et al. 2020, Schlottau et al. 2020, Shi et al. 2020, Buckley et al. 2021, Pickering et al. 2021, Vergara-Alert et al. 2021 |
| Northern Tree shrew (Tupaia belangeri) | Tupaiidae | LC | Most subclinical, increased body temperature | Xu et al. 2020, Zhao et al. 2020 |
| Big brown bat (Eptesicus fuscus) | Vespertilionidae | LC | Not susceptible | Hall et al. 2020 |
| Binturong (Arctictis binturong) | Viverridae | VU | Not reported | USDA 2021b |
Abbreviations: CR, critically endangered; EN, endangered; LC, least concern; NA, not listed; VU, vulnerable.
Of the mammalian species infected and susceptible to SARS-CoV-2, almost 30% (15 species) belong to the Threatened categories specified by the International Union for Conservation of Nature (IUCN). These categories identify species threatened with global extinction and are Vulnerable, Endangered, and Critically Endangered. This observation is not surprising given that 27% of all assessed mammal species are endangered (IUCN Red List, www.iucnredlist.org); however, it is worth noting how alarming disease spread can be if it occurrs in populations whose existence is already threatened. The repeated occurrence of natural infection in species known to be susceptible to SARS-CoV-2 is concerning from a conservation standpoint. Western lowland gorillas (Gorilla gorilla), for example, were found to be susceptible to COVID-19 infection, with some individuals developing severe respiratory symptoms such as pneumonia (Gibbons 2021). Gorillas are Critically Endangered, and an outbreak in wild populations could be catastrophic for the conservation of the species. Moreover, four species of big cats, ranging in categories from Vulnerable to Endangered, are susceptible to infection and exhibit severe clinical signs (McAloose et al. 2020, Fernández-Bellon et al. 2021, Giraldo-Ramirez et al. 2021, Mishra et al. 2021, Mahajan et al. 2022). Tigers (Panthera tigris) in captivity have shown symptoms ranging from intermittent cough to audible wheezing (McAloose et al. 2020). Such a clinical condition can be treated and managed in captive mammals but could be fatal in the wild. It is therefore essential that we gain knowledge on the susceptibility to SARS-CoV-2 of endangered species that are at risk of infection.
We mapped the geographic distribution of SARS-CoV-2 natural infection in domestic mammals and wildlife on the basis of the definition of the WOAH (2020;supplemental figure S1, supplemental table S1). The geographic distribution was plotted using the countries’ centroids and the point displacement tool in QGIS version 3.16 (QGIS 2022). Global administrative boundaries were retrieved from the Database of Global Administrative Areas (https://gadm.org/index.html). The maps showed an uneven distribution of domestic and wild mammal species reported with a SARS-CoV-2 infection, with the majority of the species reported in the United States and Europe. Most likely, this observation reflects an increased surveillance effort for SARS-CoV-2 in some countries rather than a deterioration of the epidemiological situation of the disease. In this sense, it is not surprising that the virus has been reported in 15 different mammal species in the United States, because the USDA Animal and Plant Health Inspection Service began implementing active surveillance (proactive testing) of SARS-CoV-2 in animals soon after the start of the pandemic. Conversely, other countries (such as Brazil) have reported SARS-CoV-2 natural infection in zero species.
Phylogenetic analysis of SARS-CoV-2 highlights spillback potential and the need for viral surveillance
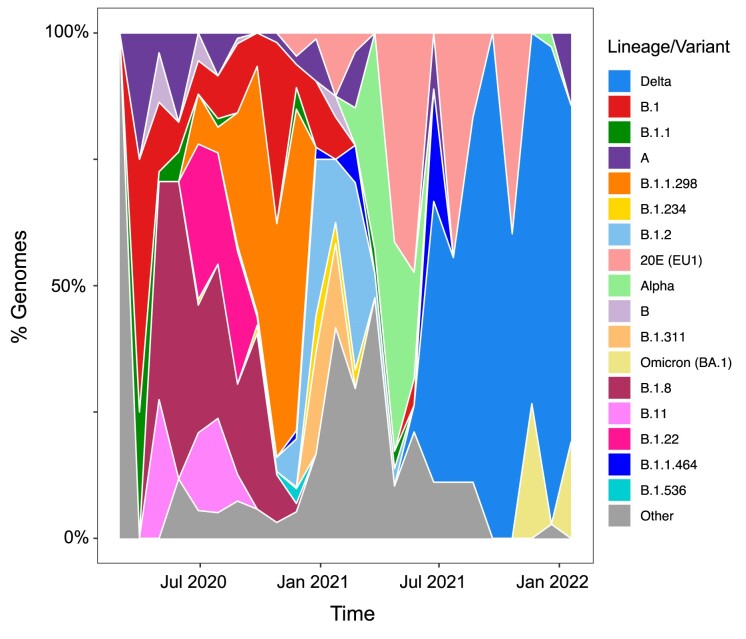
The global data set of mammal-related SARS-CoV-2 genome sequences obtained from GISAID consists of data from 41 countries, with 18 PANGO lineages (Phylogenetic Assignment of Named Global Outbreak Lineages; O'Toole et al. 2022) that have a frequency greater than 0.5% (supplemental table S2). The dynamics of the lineages over time in nonhuman mammals mirror the waves of variants that have been seen in the human population (figure 1). In the first year of the pandemic, both human and nonhuman mammal infections were caused by a mix of low-frequency lineages, none of which was from variants of concern. Early variants of concern, such as Alpha and Delta, were found in nonhuman mammals well after infection was reported in humans (January 2021 and May 2021, respectively). In fact, humans were predominantly infected with the Alpha variant (PANGO lineage B.1.1.7), which was first reported in September 2020 in the United Kingdom and then was exported worldwide. Subsequently, humans were infected by the Delta variant (PANGO lineage B.1.617.2), which was first detected in India in October 2020 and rapidly dominated human infections globally. While Delta was still spreading across the globe (in November 2021), a new variant of concern, Omicron (PANGO lineage B.1.1.529), quickly emerged and replaced Delta. The Omicron variant was found in nonhuman mammals soon after the onset of infection in humans. In addition, it has been suggested that Omicron emerged either as the result of a spillback into humans after evolving in a nonhuman mammal host (e.g., mouse) or as a result of evolution in immunocompromised human host (Wei et al. 2021, Du et al. 2022). Specifically, the 45 point mutations unique to the Omicron lineage have evolutionary markers similar to mouse-adapted lineages. Indeed, most of these mutations cluster within the spike gene sequence, where many mutations overlap with mutations arising from chronic SARS-CoV-2 infection in mice (Wei et al. 2021) that increase the binding affinity to mouse ACE2 (mACE2; Cameroni et al. 2022). In the United Kingdom, the dominant strain detected in domestic cats also appears to have trailed the emergence of each variant of concern into the human population (Tyson et al. 2023). Although the theory of spillover from mice to humans has never been refuted, the origin of this variant remains mysterious.
Figure 1.
Diversity of SARS-CoV-2 in animals. The plot shows the distribution of SARS-CoV-2 variants of concern or interest and lineages assessed by full genome sequences obtained from infected animals worldwide across time. The lineages with frequency less than 0.005 have been collapsed into the “Other” category.
A set of 1527 complete genomes of SARS-CoV-2 strains collected from mammals (other than humans) and available on GISAID (www.gisaid.org) was aligned as previously described (Magalis et al. 2022a). Briefly, we used viralMSA (Moshiri 2021) and the SARS-CoV-2 genome (NIH 2020) as a reference to align the sequences. Mutations that were potentially associated with contamination, recurrent sequencing errors, or hypermutability were masked using a VCF filter provided by De Maio and colleagues (2020). A maximum likelihood phylogenetic tree was built using IQ-TREE, with the best-fitting evolutionary model chosen according to Bayesian information criteria and ultrafast bootstrap approximation used to provide support (Minh et al. 2013, Nguyen et al. 2015). The presence of sufficient phylogenetic signal was evaluated by performing a likelihood mapping analysis in IQ-TREE. Migration analysis (i.e., mapping of ancestral traits on nodes and branches through phylodynamic maximum-likelihood reconstruction) was carried out using TreeTime (Sagulenko et al. 2018) and the maximum-likelihood tree obtained with IQ-TREE with rooting on the MN908947.3 reference isolate. As TreeTime requires a rooting method, the MN908947.3 isolate was chosen for rooting of the timed tree corresponding to the oldest sample within the SARS-CoV-2 genomes and to the theoretical last common ancestor of all taxonomic units included in the tree, therefore allowing assessment of valid evolution of clades within each tree. MN908947.3 has been largely accepted as the reference in the context of SARS-CoV-2 when modeling evolution without dating the tree in time (Tagliamonte et al. 2022, Magalis et al. 2022b).
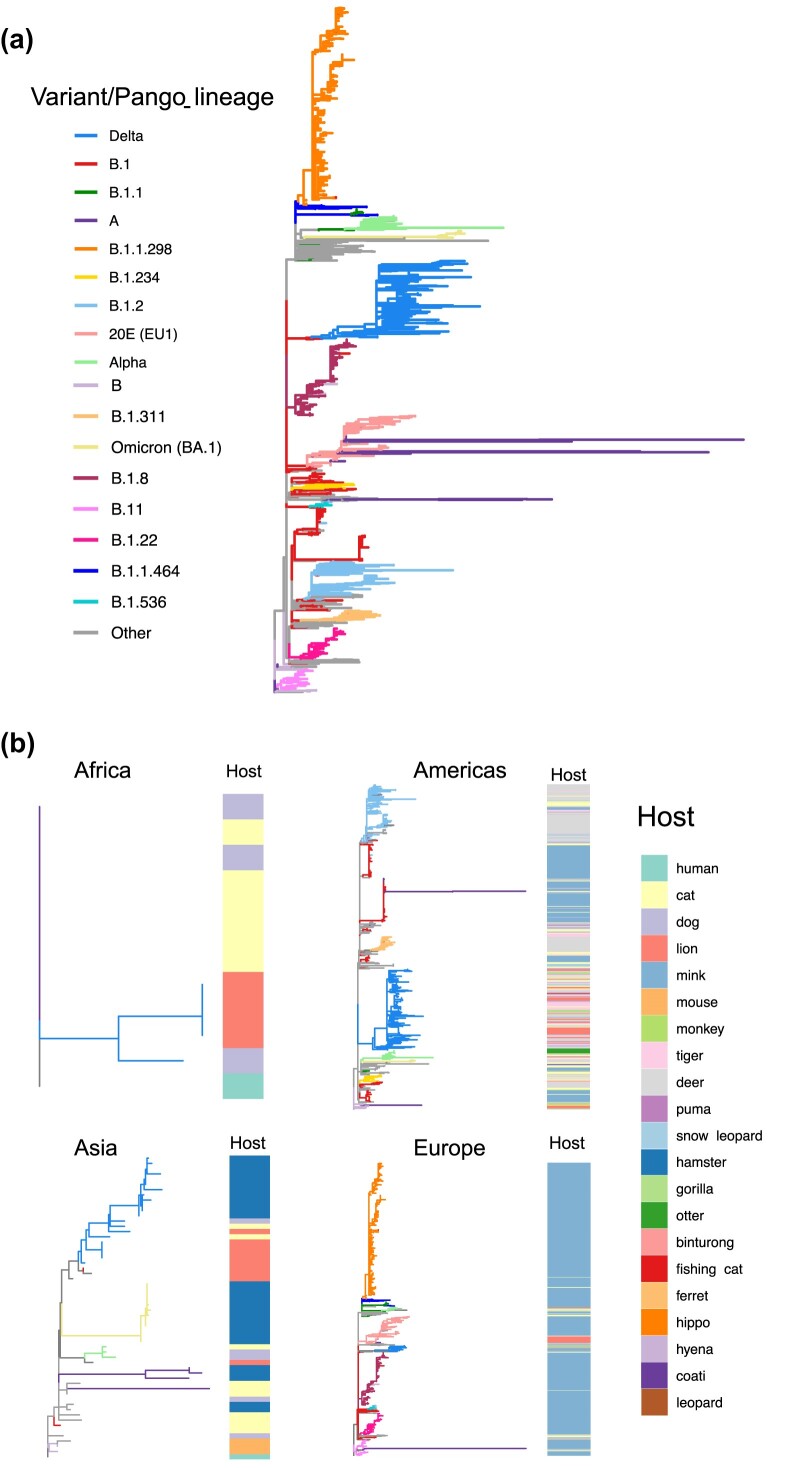
When we examined the phylogenetic relationships across isolates from several parts of the world, we found that isolates cluster by variants, forming monophyletic groups (figure 2a). When comparing the location and host of origin, no clear patterns were observed, especially in the case of the Delta and Omicron clades. The host of origin is also intermixed as per the early B.1.2 lineage that was circulating in North America (figure 2b). By comparing the dynamics across continents, it is striking that the multiple outbreaks in mink appear to be monophyletic by variant rather than host. The phylogeny also suggests multiple spillback events to mink or deer populations in the Americas and in Europe (figure 2b) on the basis of the presence of multiple independent SARS-CoV-2 lineages in mink and deer. Moreover, their rather large clades also show a high degree of diversity within each clade, likely evidencing viral evolution within the nonhuman mammal populations.
Figure 2.
(a) Global phylogeny of SARS-CoV-2 sequences obtained from infected animals. The maximum likelihood tree is rooted in the first genome of SARS-CoV-2 obtained from a human case in Wuhan, China. The branches are colored on the basis of the ancestral state reconstruction using PANGO lineages or variants as traits. (b) Continent specific phylogenetic reconstruction of SARS-CoV-2 sequences obtained from infected animals. The phylogenetic trees with branches are colored on the basis of variant of SARS-CoV-2 sequences and heatmap depicting host of origin (the infected animal from which the genome was obtained).
When looking at the number of genome sequences by continent, we noticed that surveillance of nonhuman mammals in Asia and Africa was limited compared with the Americas (with most sequences from North America) and Europe (figure 2, supplemental tables S2 and S3). This is likely because of differences in surveillance infrastructure between the Global North and Global South. As we expected, our study reveals that nonhuman mammals have become infected with strains that were circulating in humans. In the case of early variants, spillback from humans into new animal hosts was detected following extensive circulation in humans. This was not the case for Omicron, which, instead, was identified in multiple species shortly after its emergence. Whether this is linked to a sampling bias or to a greater spillback potential remains unclear.
Variants continue to evolve and adapt in nonhuman mammal hosts
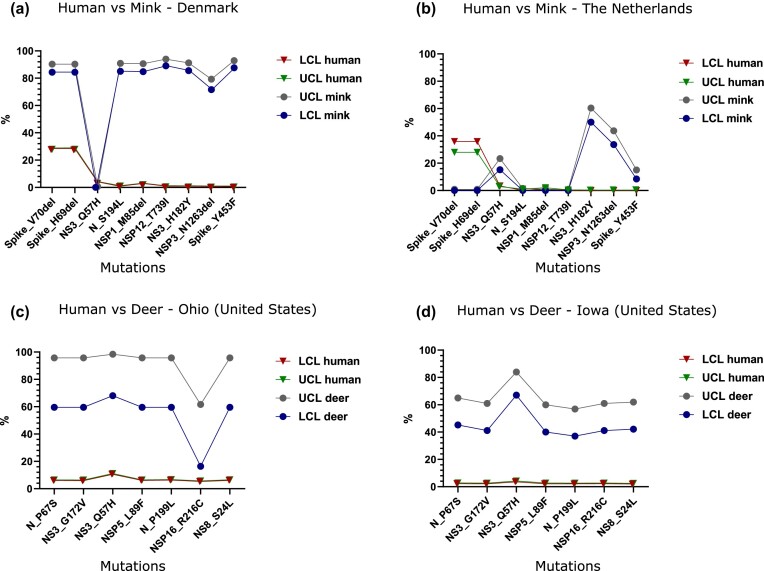
The repeated interspecies transmission of a virus presents the potential for the acceleration of viral evolution and a possible source of novel strain emergence. This potential was demonstrated by reverse zoonosis of SARS-CoV-2 from humans to mink (zooanthroponosis), followed by selection in mink and spillback into humans (anthropozoonosis; Oreshkova et al. 2020, Eckstrand et al. 2021, Lu et al. 2021, Oude Munnink et al. 2021, Li et al. 2022a). Using the same set of complete genomes described above of SARS-CoV-2 collected from nonhuman host species (GISAID), we extracted sequences from mink (n = 1067) and deer (n = 138) and used them to identify occurrences of mutations with respect to the reference genome circulating in humans. Mink and deer were selected because they have the highest numbers of related SARS-CoV-2 genomes present in GISAID. For each identified mutation, we calculated the difference between the percentage obtained from human samples and from the two nonhuman mammal species under investigation. Because an analysis at a global scale may be biased by sampling and availability of data on GISAID, comparisons were made by country. Accordingly, for human sequences, we selected from GISAID sequences belonging to the same lineage detected in mink and deer for each country with evidence of infection in nonhuman mammals, including PANGO lineages B.1, B.1.160, B.1, B.1.2, B.1.50, B.1.594 for mink and B.1, B.1.119, B.1.2, B.1.311, B.1.58, and B.1.596 for deer (Pangolin COVID-19 Lineage Assigner; https://pangolin.cog-uk.io). As for the lineage origin, we used the country or US state most represented by the sample numbers, including The Netherlands, Denmark, Oregon, Wisconsin, Michigan, and Utah for mink and Ohio and Iowa for deer. The percentages of samples with a mutation of interest in mink and deer in different countries were compared using a Bayesian approach with a beta distribution β(n + 1, n – s + 1), where n is the total number of tested sequences in each species or country and s is the number of samples with the mutation.
While taking into consideration the limits of this analysis due to the relatively low number of sequences from mink when compared with human sequences, we identified mink-specific mutations (Spike_Y453F, NS3_H182Y, and NSP3_N1263del) with statistical support, apparently only in the outbreaks in Denmark and The Netherlands (figure 3a, 3b). Mutations Spike_V70del, Spike_H69del, N_S194L, NSP1_M85del, and NSP12_T739I are unique to Denmark, whereas NS3_Q57H was only found in the outbreak in The Netherlands. Sequences from deer in Ohio and Iowa also share a common set of deer-specific mutations, including N_P67S, NS3_G172V, NS3_Q57H, NSP5_L89F, N_P199L, NSP16_R216C, and NS8_S24L (figure 3c, 3d).
Figure 3.
SARS-CoV-2 mink (a and b) and deer (c and d) specific mutations. The percentage equals the number of samples that contain each mutation. The percentages of samples with mutation in the two species in different countries were compared with those of humans using a Bayesian approach with beta distribution β(n + 1, n – s + 1), where n is the total number of tested sequences in each species or country and s is the number of samples with mutation. Abbreviations: LCL, lower control limit; UCL, upper control limit.
The findings in mink and deer are of great interest given that they are the only two species for which we have documented evidence of sustained intraspecies transmission, although in two different biological landscapes, farm (mink) and free range (deer). In both cases, our analysis identified genetic signatures, scattered in structural and nonstructural viral proteins, that have been acquired by deer- and mink-adapted strains regardless of the infecting lineage or the country (or US state) where they were identified.
SARS-CoV-2 evades the human immune system in several ways, such as accumulating mutations on the spike protein that reduce binding to host antibodies. Significantly, many sites of mutations in variants of concern are located on the RBD that engages ACE2. Mutations that endow the ability for the spike protein to evade antibodies in humans may also play an additional role by influencing ACE2 binding specificity and host range. Mutation Y453F, located in the RBD of the spike protein, occurs in the mink outbreaks in both Denmark and The Netherlands; however, in sequences from The Netherlands, it is only present in a limited number of sequences that are divergent from the rest of the group. In the Danish group, the same mutation occurs in all sequences except one, which was one of the earliest collected samples.
A recent study by Porter and colleagues (2023), through an elegant evolutionary approach, confirmed the importance of the Y453F mutation as a signature of adaptation to the mink host and further analyzed additional mutations in the spike protein of mink-related sequences, including S1147L, F486L, and Q314K not evidenced in our analysis but apparently showing an increased evolutionary rate. Although the biological role is known for some of these mutations, such as Y453F, which enhances interaction with the mink ACE2 receptor and resistance to convalescent serum (Bayarri-Olmos et al. 2021, Ren et al. 2021, Zhou et al. 2022), further experiments are warranted for the others. Systematic screening of viral sequences obtained from mammals and analyses inferred on a global scale are therefore essential to confirm host-specific mutations and to immediately identify any clusters in humans related to any nonhuman mammal species. From a practical perspective, understanding the prompt identification of novel sequences may allow us to study viral properties in vivo using animal models, correlate with pathology or transmission in humans or animals, and develop (or update) diagnostic assays.
Taking into account the global spread of the Omicron lineage and its offspring, a recent study also highlighted residues on the spike protein that increase the number of interactions with the murine ACE2, including N501Y, Q493R, G496S, and Q498R (Ni et al. 2023). This structural observation is consistent with literature reports that spike mutations N501Y, Q493H, and K417N increase binding to ACE2 from mice (mACE2) and other species (Dinnon III et al. 2020, Sun et al. 2021b, Li et al. 2022b). Structural data and binding analysis of mACE2 to SARS-CoV-2 variant of concern highlight how mutations that lead to evasion of the immune response in humans may have an impact on the binding to ACE2 receptors of other species, possibly increasing host susceptibility. Binding assays and structural analysis also identified four of the mutations found in SARS-CoV-2 spikes and known to be associated with immune escape, including N501Y, E484A, Q493R, and Q498R, because critical mutations involved in high-affinity binding of variant of concern spikes to the mACE2 receptor, and as such potentially allowing for expansion of SARS-CoV-2 host range.
Phylogenetic mapping of ACE2 receptor does not correlate with infection
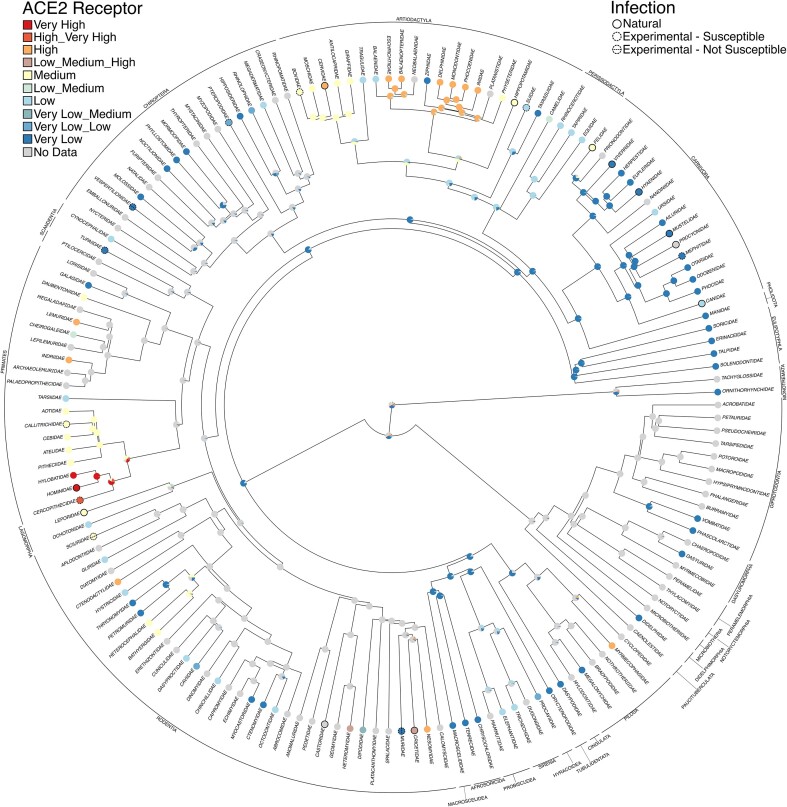
Previous attempts to predict species susceptible to SARS-CoV-2 have compared sequences of ACE2 orthologs across species (Damas et al. 2020), modeled the structure of the spike protein bound to ACE2 orthologs (Lam et al. 2020, Rodrigues et al. 2020), or combined structural modeling with machine learning of species ecology and biology (Fischhoff et al. 2021). In the present article, to use phylogenetic relatedness, we mapped two traits associated with SARS-CoV-2 onto a recently published phylogenetic tree of mammals to predict potential susceptibility for infection in species for which infection has not yet been reported. The “DNA-only,” maximum clade credibility tree with a nearly complete sampling of 4098 mammal species from Upham and colleagues (2019) was downloaded and used for character mapping analyses, as recommended. The phylogeny was time calibrated using 17 fossils placed at nodes and one root constraint. Two traits of interest were mapped to the phylogeny: infection and conservation of the ACE2 receptor. Infection was determined using the literature search described above, distinguishing between natural and experimental infection. The ACE2 receptor data were compiled from Damas and colleagues (2020), specifically figures 1 and 2 in which the authors group species into very high, high, medium, low, and very low conservation relative to humans. For our analyses, 248 species (out of 252 described in Damas et al. 2020) were also found in the phylogeny, belonging to 101 families across the mammal tree of life. In particular, 19 have very high conservation, 27 have high conservation, 56 have medium conservation, 47 have low conservation, and 99 have very low conservation of ACE2. Most families have the same ACE2 receptor conservation designation (i.e., all Hominidae have very high ACE2 receptor conservation), although some families such as Heteromyidae, represented by Ord's kangaroo rat (Dipodomys ordii), Stephens's kangaroo rat (Dipodomys stephensi), and the little pocket mouse (Perognathus longimembris), are heteromorphic in having low, high, and medium ACE2 receptor conservation, respectively. Remarkably, only 28 species had both infection and ACE2 information, again highlighting the concerning lack of effort that has gone into systematic screening of nonhuman mammals. Species without data were scored as N for “no data.” These two traits are summarized in supplemental table S4. Using the ace function in Ape v. 5.6.2 (Paradis et al. 2004), likelihood scores for a one parameter equal rates model, a symmetric model with forward and reverse transitions between states constrained to be equal, and an all-rates different matrix were compared for best fit. Model selection was determined using the Akaike information criterion (supplemental table S5). Following model choice, ancestral character estimation was also performed using Phytools v. 1.0.3 (Revell 2012) to compare stochastic character mapping methods (SIMMAP; Bollback 2006; make.simmap with 10,000 simulations).
Both ancestral state reconstruction methods (simmap and ace) recovered congruent patterns of SARS-CoV-2 infection and the ACE2 receptor. At the species level—especially for infection—the limited data points (52 of 4098 species: 24 with natural infection, 20 susceptible with experimental infection, and 8 not susceptible with experimental infection) prevented us from having the statistical power to identify large-scale patterns. Therefore, we focused on mapping the conservation of the ACE2 receptor from Damas and colleagues (2020) and secondarily added if a species had a documented infection (both natural and experimental; supplemental figures S2 and S3). Even with the additional data (248 of 4098 species), patterns were still difficult to identify, so we also conducted additional analyses at the family level.
To generate a family-level tree, the species tree was collapsed to monophyletic families using custom scripts (05_Species2FamilyTree.R) to produce a tree that had 159 families. For analyses performed at the family level, states were combined if species within the family were classified as both low and medium (i.e., a new state named Low_Medium was made), and analyses were run as described above. Mapping of infection was still underpowered for identification of patterns (22 of 159 families). However, analyses of the ACE2 receptor with infection mapped at the tips were insightful (101 data points of 159 families). In general, we found that infection is not associated with receptor conservation. For example, a family could have low ACE2 receptor conservation and still have SARS-CoV-2 infection. This is true of the Carnivora clade, which has six natural infections and one experimental infection but is recovered as having very low ACE2 receptor conservation (figure 4, supplemental figure S4). Conversely, a large clade within the Artiodactyla composed of Iniidae (river dolphins), Delphinidae (oceanic dolphins), Phocoenidae (porpoises), Monodontidae (narwhals and belugas), Ziphiidae (beaked whales), Physeteridae (sperm whales, pygmy sperm whales, and dwarf sperm whales), Balaenopteridae (baleen whales), Eschrichtiidae (gray whales), Neobalaenidae (pygmy right whales), and Balaenidae (right whales and bowhead whales) is recovered as having high conservation of the ACE2 receptor but no documented SARS-CoV-2 infection. The latter may be linked to very low exposure risk for these mammals in the wild, although some of these species are reared in captivity and could have been exposed by infected caretakers or could be infected through wastewater (Audino et al. 2021). Perhaps unsurprisingly, a clade within the primates composed of Cercopithecidae (Old World monkeys), Hominidae (great apes, including humans), and Hylobatidae (gibbons) is recovered as having very high ACE2 receptor conservation with both natural and experimental infection. The sister clade (also in primates) composed of Aotidae (night monkeys) and four families of New World monkeys (Atelidae, Cebidae, Callitrichidae, and Pitheciidae) is modeled as medium conservation and has just one experimental infection and no natural infections known. However, experimental infections should be further investigated as species in the same family may have been inoculated with different strains or different doses than species with natural infections. In general, like the results of Fischhoff and colleagues (2021), our results suggest that within mammalian orders there is a lot of variation in susceptibility.
Figure 4.
Stochastic character mapping of ACE2 receptor conservation with SARS-CoV-2 infection secondarily mapped at the tips. The orders are indicated around the outside. Our phylogeny was limited to just mammal families with DNA, and the data were time scaled using node dating (TopoFree_ND; Upham et al. 2019).
To demonstrate the potential of using phylogenetic character mapping, additional analyses were performed to investigate infection using both stochastic mapping (make.simmap) and ancestral character estimation (ace) for the Felidae (cat) family alone, which had densely sampled trait data. We found that two clades, Panthera and Lynx, have a high probability for infection (supplemental figures S5 and S6). Currently, five of six species of Panthera have known SARS-CoV-2 infection, whereas two of four species of Lynx have documented natural infection.
Because developing predictive models for SARS-CoV-2 has proved to be difficult, we had hoped that by including phylogenetic relatedness, we could identify patterns that could provide insights for leading infection surveillance initiatives. Phylogenetic character mapping methods that aim to reconstruct trait states at nodes can suffer from issues of sampling bias, missing lineages, and model inadequacy. This issue, combined with the limited data for SARS-CoV-2 infection across mammals, suggests that these methods are not yet well suited for making these predictions. Sampling bias of captive animals influences the representation of species in such analyses, because captive populations are more likely to be surveyed for infection and often experience different environmental conditions, diets, and social structures compared with their wild counterparts. If more surveillance data were available, especially for species outside of captivity, these types of analyses could be powerful tools in the identification of SARS-CoV-2-susceptible mammals and intermediate host species for SARS-CoV-2, guide the selection of mammal models of COVID-19, and assist the conservation of nonhuman mammals in both native habitats and human care. However, even with our limited data set, we are able to highlight species in the Felidae that should be monitored and further investigated. Additional nonhuman mammal surveillance efforts are needed to enable this type of predictive research, particularly for conservation efforts.
In addition, ACE2 receptor conservation should not be considered as the sole major determinant of species susceptibility, but rather as one of the factors that may contribute to susceptibility. In our study, ACE2 receptor conservation is not always associated with infection. However, we also question how characterizing ACE2 conservatism related to humans rather than bats, in particular Rhinolophus bats because those are the presumable natural hosts of the SARS2 ancestor (e.g., in northern Laos; Temmam et al. 2022), affects results. Furthermore, recent work has found that ACE2 does not play a mandatory role in the conformational activation of the spike protein necessary for infection (Cervantes et al. 2023).
Our findings suggest that evolutionary proximity to species that have been infected could be useful for developing informed surveillance programs in the future. As we attempt to establish the extent of SARS-CoV-2 circulation in mammals, it would seem reasonable to concentrate and intensify surveillance efforts in species that are closely related to existing clusters of positivity. In addition, because ACE2 receptor conservation was determined on the basis of sequence similarity to human ACE2 binding residues, the imperfect correspondence with infection perhaps highlights the danger of using a human-first approach when humans are just one lineage in the mammal tree of life.
Conclusions
In this study, we highlighted several priorities that should be included in future efforts aimed at understanding the host range and ramifications of multispecies SARS-CoV-2 infection. First, increased surveillance efforts in nonhuman mammal populations are necessary in Africa, South America, the Indian subcontinent, and Southeast Asia, all areas that appear to be greatly underrepresented from a surveillance perspective. We want to emphasize, however, that this data collection should follow both FAIR (Wilkinson et al. 2016) and CARE (Carroll et al. 2020) principles for data management and stewardship to support data provenance and equity. In addition, surveillance in nonhuman mammals should be expanded both qualitatively and quantitatively. Work using host ecology machine learning has been fundamental in providing susceptibility predictions at the global scale for emerging pathogens and potential host–pathogen systems to help direct surveillance programs toward specific geographic regions and targeted for specific pathogens and hosts (Becker et al. 2022, Robles-Fernández et al. 2022). However, we should also develop surveillance programs that aim to understand the real extent of infection in wild and domestic mammals, with particular focus on certain endangered species that are closely related to species shown to be susceptible to infection. This surveillance effort should also be able to identify early spillover events caused by novel variants as well as specific genetic signatures that can be associated with heavily infected populations. Although we were not able to infer the likelihood of novel infection of additional mammal species, other populations will undoubtedly become infected in the years to come, and spillover events with mammal-adapted viruses could become a serious public health problem. Surveillance for SARS-CoV-2 has been predominantly human-centric, with enormous financial and organizational efforts being performed in humans. Our findings suggest that a less human-centric approach could be developed for this type of emergency. Therefore, surveillance in nonhuman mammal populations would be an essential component of preparedness and response. For this reason, we believe that the exceptional circumstances that could be determined by the expanding host range of SARS-CoV-2 should be reflected in the terminology we use to describe this event.
Human diseases caused by animal pathogens are known as zoonoses. Zoonotic pathogens may cause a variety of outcomes, from sporadic outbreaks to pandemic events. SARS-CoV-2 has caused what has been defined as a pandemic. The term pandemic comes from the two Greek words pan and demos, which mean, respectively, “all” and “people” (Agnelli and Capua 2022). For the sake of clarity, the term pandemic does not encompass the infection of all mammals. There is another term that would perhaps be more suitable to define the magnitude of what we are experiencing, as was suggested by Gollakner and Capua (2020). The term panzootic, which literally means “affecting all animals,” has been used only very rarely to describe extensive multispecies infections by a single pathogen (Agnelli and Capua 2022). However, on the basis of its use to date, it remains unclear how many species, across how many clades, are required for an event to be considered a panzootic. In addition, Agnelli and Capua (2022) found no reference to how many species (symptomatic or not) should be included in a disease defined as a panzootic or whether humans (Homo sapiens) are included or not in the zootic part of the word. Therefore, we believe that the most relevant occurrence to ignite a panzootic is infection, whether clinically overt or asymptomatic. Asymptomatic infections may develop into a clinical illness after adaptation in a given species. This discourse is relevant not only to Sars-CoV-2 infection, but also to many other zoonotic pathogens, such as, for example, avian influenza. The latter, because of extensive circulation in wildfowl and domestic poultry, has caused infection of wild mammals such as foxes and bears, and given its interspecies transmission potential, it may also be a leading candidate to be defined as a panzootic.
In the present article, we have generated and analyzed data that support the fact that infection of mammals in the COVID-19 setting is a relevant component of the global problem. We believe that, for the sake of clearer communication with the public and with decision-makers about the complexity of this infection, the term panzootic could be introduced in the general discourse, and if animal infections continue to increase, possibly replace the term pandemic.
Supplementary Material
Acknowledgments
We acknowledge Adriano Di Pasquale, Anna Conte, and Marialuigia Caporale (IZSAM) for their technical assistance in genome analysis. AF is supported by a fellowship provided by the One Health Center of Excellence at the University of Florida. AL is supported by funding from the European Union's Horizon 2020 Research and Innovation Programme One Health European Joint Programme under grant agreement no. 773,830. MEM and PSS are supported by iDigBio through National Science Foundation grants no. DBI-1,547,229, no. DBI-2,027,654, and by the NSF directly through grant no. DBI-2,037,937.
Author Biography
Makenzie E. Mabry (mmabry44@gmail.com) and Pamela S. Soltis (psoltis@flmnh.ufl.edu) are affiliated with the Florida Museum of Natural History, at the University of Florida, in Gainesville, Florida, in the United States. Angela Fanelli is affiliated with the Department of Veterinary Medicine at the University of Bari, in Valenzano, Bari, Italy. Carla Mavian is affiliated with the Emerging Pathogens Institute and with the Department of Pathology at the University of Florida, in Gainesville, Florida, in the United States. Alessio Lorusso (a.lorusso@izs.it) is affiliated with the Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise G. Caporale, in Teramo, Italy. Costanza Manes is affiliated with the Department of Wildlife Ecology and Conservation and with the One Health Center of Excellence, at the University of Florida, in Gainesville, Florida, in the United States. Ilaria Capua is affiliated with the One Health Center of Excellence, at the University of Florida, in Gainesville, Florida, in the United States, and with the School of International Advanced Studies, at Johns Hopkins University, in Bologna, Italy. MEM, AF, and CM are co-first authors.
Contributor Information
Makenzie E Mabry, Florida Museum of Natural History, University of Florida, Gainesville, Florida, United States.
Angela Fanelli, Department of Veterinary Medicine, University of Bari, Valenzano, Bari, Italy.
Carla Mavian, Emerging Pathogens Institute and with the Department of Pathology, University of Florida, Gainesville, Florida, United States.
Alessio Lorusso, Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise G. Caporale, Teramo, Italy.
Costanza Manes, Department of Wildlife Ecology and Conservation and with the One Health Center of Excellence, University of Florida, Gainesville, Florida, United States.
Pamela S Soltis, Florida Museum of Natural History, University of Florida, Gainesville, Florida, United States.
Ilaria Capua, One Health Center of Excellence, University of Florida, Gainesville, Florida, United States; School of International Advanced Studies, Johns Hopkins University, Bologna, Italy.
Supplemental material
Supplemental data are available at BIOSCI online.
The scripts used for performing character state reconstruction for the mammal phylogeny can be found at https://github.com/mmabry/PanzooticProject. The data sets, including tree files and character matrices used for analyses and for figures, can be found at https://doi.org/10.5281/zenodo.8326720.
Author contributions
Makenzie E. Mabry (Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing), Angela Fanelli (Data curation, Investigation, Validation, Writing – original draft, Writing – review & editing), Carla Mavian (Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing), Alessio Lorusso (Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing), Costanza Manes (Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing), Pamela S. Soltis (Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing), and Ilaria Capua (Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing).
References cited
- Agnelli S, Capua I. 2022. Pandemic or panzootic: A reflection on terminology for SARS-CoV-2 infection. Emerging Infectious Diseases 28: 2552–2555. [Google Scholar]
- Amato L, et al.. 2022. Epidemiological and genomic findings of the first documented Italian outbreak of SARS-CoV-2 alpha variant of concern. Epidemics 39: 100578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audino T, et al.. 2021. SARS-CoV-2, a threat to marine mammals? A study from Italian seawaters. Animals (Basel) 11: 1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, et al.. 2021. Susceptibility and attenuated transmissibility of SARS-CoV-2 in domestic cats. Journal of Infectious Diseases 223: 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrs VR, Peiris M, Tam KWS, Law PYT, Brackman CJ, To EMW, Yu VYT, Chu DKW, Perera R, Sit THC. 2020. SARS-CoV-2 in quarantined domestic cats from COVID-19 households or close contacts, Hong Kong, China. Emerging Infectious Diseases 26: 3071–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayarri-Olmos R, Rosbjerg A, Johnsen LB, Helgstrand C, Bak-Thomsen T, Garred P, Skjoedt M-O. 2021. The SARS-CoV-2 Y453F mink variant displays a pronounced increase in ACE-2 affinity but does not challenge antibody neutralization. Journal of Biological Chemistry 296: 100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BBC News . 2021. Belgian zoo hippos test positive for Covid. BBC (4 December 2021). www.bbc.com/news/world-europe-59516896.
- Becker DJ, et al.. 2022. Optimising predictive models to prioritise viral discovery in zoonotic reservoirs. Lancet Microbe 3e625–e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshaw R, Pybus OG, Rambaut A. 2007. The evolution of genome compression and genomic novelty in RNA viruses. Genome Research 171496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertzbach LD, Vladimirova D, Dietert K, Abdelgawad A, Gruber AD, Osterrieder N, Trimpert J. 2021. SARS-CoV-2 infection of Chinese hamsters (Cricetulus griseus) reproduces COVID-19 pneumonia in a well-established small animal model. Transboundary and Emerging Diseases 68: 1075–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RV, et al.. 2021. Acute respiratory distress in aged, SARS-CoV-2-infected African green monkeys but not Rhesus macaques. American Journal of Pathology 191: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boklund A, et al.. 2021. SARS-CoV-2 in Danish mink farms: Course of the epidemic and a descriptive analysis of the outbreaks in 2020. Animals 11: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollback JP. 2006. SIMMAP: Stochastic character mapping of discrete traits on phylogenies. BMC Bioinformatics 7: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco-Lauth AM, Hartwig AE, Porter SM, Gordy PW, Nehring M, Byas AD, VandeWoude S, Ragan IK, Maison RM, Bowen RA. 2020. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proceedings of the National Academy of Sciences 117: 26382–26388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco-Lauth AM, et al.. 2021. Peridomestic mammal susceptibility to Severe Acute Respiratory Syndrome coronavirus 2 infection. Emerging Infectious Diseases 27: 2073–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley A, Falkenberg S, Martins M, Laverack M, Palmer MV, Lager K, Diel DG. 2021. Intravenous, intratracheal, and intranasal inoculation of swine with SARS-CoV-2. Viruses 13: 1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameroni E, et al.. 2022. Broadly neutralizing antibodies overcome SARS-CoV-2 omicron antigenic shift. Nature 602: 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S, et al.. 2020. The CARE Principles for indigenous data governance. Data Science Journal 19: 43. 10.5334/dsj-2020-043. [DOI] [Google Scholar]
- [CDC] Center for Disease Control and Prevention . 2023. SARS-CoV-2 variant classifications and definitions. CDC (1 September 2023). www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html. [Google Scholar]
- Cervantes M, Hess T, Morbioli GG, Sengar A, Kasson PM. (2023). The ACE2 receptor accelerates but is not biochemically required for SARS-CoV-2 membrane fusion. Chemical Science 146997–7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaintoutis SC, et al.. 2021. Outbreaks of SARS-CoV-2 in naturally infected mink farms: Impact, transmission dynamics, genetic patterns, and environmental contamination. PLOS Pathogens 17: e1009883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JF, et al.. 2020. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a Golden Syrian Hamster model: Implications for disease pathogenesis and transmissibility. Clinical Infectious Diseases 71: 2428–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler JC, et al.. 2021. SARS-CoV-2 exposure in wild white-tailed deer (Odocoileus virginianus). Proceedings of the National Academy of Sciences 118e2114828118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar A, et al.. 2020. SARS-CoV-2 infection protects against rechallenge in Rhesus macaques. Science 369: 812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool K, et al.. 2021. Infection and transmission of ancestral SARS-CoV-2 and its alpha variant in pregnant white-tailed deer. Emerging Microbes and Infections 1195–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . 2020. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology 5: 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross RW, Agans KN, Prasad AN, Borisevich V, Woolsey C, Deer DJ, Dobias NS, Geisbert JB, Fenton KA, Geisbert TW. 2020. Intranasal exposure of African green monkeys to SARS-CoV-2 results in acute phase pneumonia with shedding and lung injury still present in the early convalescence phase. Virology Journal 17: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [CRS] Congressional Research Service . 2021. Global economic effects of COVID-19. CRS. Report no. R46270. [Google Scholar]
- Cui J, Li F, Shi Z-L. 2019. Origin and evolution of pathogenic coronaviruses. Nature Reviews Microbiology 17: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas J, et al.. 2020. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proceedings of the National Academy of Sciences 117: 22311–22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N, Lorusso A. 2020. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Veterinary Microbiology 244: 108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Ferrari N, Fanelli A, Muset S, Thompson L, Sleeman JM, White CL, Walsh D, Wanous C, Tizzani P. 2023. Wildlife health surveillance: Gaps, needs and opportunities. Scientific and Technical Review 41: 1–27. [DOI] [PubMed] [Google Scholar]
- De Maio N, Walker C, Borges R, Weilguny L, Slodkowicz G, Goldman N. 2020. Masking strategies for SARS-CoV-2 alignments. Virological.org (May 2020). https://virological.org/t/masking-strategies-for-sars-cov-2-alignments/480. [Google Scholar]
- Deng W, et al.. 2020. Primary exposure to SARS-CoV-2 protects against reinfection in Rhesus macaques. Science 369: 818–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giallonardo F, Duchene S, Puglia I, Curini V, Profeta F, Cammà C, Marcacci M, Calistri P, Holmes EC, Lorusso A. 2020. Genomic epidemiology of the first wave of SARS-CoV-2 in Italy. Viruses 12: 1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnon KH III, et al. 2020. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 586: 560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du P, Gao GF, Wang Q. 2022. The mysterious origins of the Omicron variant of SARS-CoV-2. Innovation 3: 100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstrand CD, Baldwin TJ, Rood KA, Clayton MJ, Lott JK, Wolking RM, Bradway DS, Baszler T. 2021. An outbreak of SARS-CoV-2 with high mortality in mink (Neovison vison) on multiple Utah farms. PLOS Pathogens 17: e1009952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett HE, et al.. 2021. Intranasal infection of Ferrets with SARS-CoV-2 as a model for asymptomatic human infection. Viruses 13: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagre A, et al.. 2021. SARS-CoV-2 infection, neuropathogenesis and transmission among deer mice: Implications for spillback to New World rodents. PLOS Pathogens 17: e1009585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg S, Buckley A, Laverack M, Martins M, Palmer MV, Lager K, Diel DG. 2021. Experimental inoculation of young calves with SARS-CoV-2. Viruses 13: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan Z. 2021. Chimpanzees and Orangutans at Dehiwala Zoo test COVID positive. Newsfirst.lk (18 July 2021). www.newsfirst.lk/2021/07/18/chimpanzees-orangutans-at-dehiwala-zoo-test-covid-positive. [Google Scholar]
- Fernández-Bellon H, et al.. 2021. Monitoring natural SARS-CoV-2 infection in lions (Panthera leo) at the Barcelona Zoo: Viral dynamics and host responses. Viruses 13: 1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff IR, Castellanos AA, Rodrigues JPGLM, Varsani A, Han BA. 2021. Predicting the zoonotic capacity of mammals to transmit SARS-CoV-2. Proceedings of the Royal Society B 288: 20211651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freuling CM, et al.. 2020. Susceptibility of raccoon dogs for experimental SARS-CoV-2 infection. Emerging Infectious Diseases 26: 2982–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M, de Riols de Fonclare D, Garcia D, Beurlet S, Becquart P, Rosolen SG, Briend-Marchal A, Leroy EM. 2022. First evidence of natural SARS-CoV-2 infection in domestic rabbits. Veterinary Sciences 9: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreault NN, et al.. 2020. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerging Microbes and Infections 9: 2322–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreault NN, et al.. 2021. Experimental re-infected cats do not transmit SARS-CoV-2. Emerging Microbes and Infections 10: 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons A. 2021. Captive gorillas test positive for coronavirus. Science (12 January 2021). www.science.org/content/article/captive-gorillas-test-positive-coronavirus. [Google Scholar]
- Giner J, et al.. 2021. SARS-CoV-2 seroprevalence in household domestic Ferrets (Mustela putorius furo). Animals 11: 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Ramirez S, Rendon-Marin S, Jaimes JA, Martinez-Gutierrez M, Ruiz-Saenz J. 2021. SARS-CoV-2 clinical outcome in domestic and wild cats: A systematic review. Animals 11: 2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollakner R, Capua I. 2020. Is COVID-19 the first pandemic that evolves into a panzootic? Veterinaria Italiana 56: 7–8. [DOI] [PubMed] [Google Scholar]
- Gortazar C, Barroso-Arevalo S, Ferreras-Colino E, Isla J, de la Fuente G, Rivera B, Dominguez L, de la Fuente J, Sanchez-Vizcaino JM. 2021. Natural SARS-CoV-2 infection in kept Ferrets, Spain. Emerging Infectious Diseases 27: 1994–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin BD, et al.. 2021. SARS-CoV-2 infection and transmission in the North American deer mouse. Nature Communications 12: 3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryseels S, De Bruyn L, Gyselings R, Calvignac-Spencer S, Leendertz FH, Leirs H. 2020. Risk of human-to-wildlife transmission of SARS-CoV-2. Mammal Review 51: 272–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann PJ, et al.. 2020. Transmission of SARS-CoV-2 in domestic cats. New England Journal of Medicine 383: 592–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JS, Knowles S, Nashold SW, Ip HS, Leon AE, Rocke T, Keller S, Carossino M, Balasuriya U, Hofmeister E. 2020. Experimental challenge of a North American bat species, big brown bat (Eptesicus fuscus), with SARS-CoV-2. Transboundary and Emerging Diseases 68: 3443–3452. [DOI] [PubMed] [Google Scholar]
- Hamer SA, et al.. 2021. SARS-CoV-2 infections and viral isolations among serially tested cats and dogs in households with infected owners in Texas, USA. Viruses. 13: 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer AS, et al.. 2021. SARS-CoV-2 transmission between mink (Neovison vison) and humans, Denmark. Emerging Infectious Diseases 27: 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. 2004. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal of Pathology 203: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, et al.. 2020. SARS-CoV-2 infection of African green monkeys results in mild respiratory disease discernible by PET/CT imaging and shedding of infectious virus from both respiratory and gastrointestinal tracts. PLOS Pathogens 16: e1008903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendy A, et al.. 2020. The vertical stratification of potential bridge vectors of mosquito-borne viruses in a central Amazonian forest bordering Manaus, Brazil. Scientific Reports 10: 18254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs EC, Reid TJ. 2021. Animals and SARS-CoV-2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transboundary and Emerging Diseases 68: 1850–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, et al.. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie MJ, et al.. 2021. Detection of SARS-CoV-2 in respiratory samples from cats in the UK associated with human-to-cat transmission. Veterinary Record 188: e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Kuba K, Penninger JM. 2008. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Experimental Physiology 93: 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M, et al.. 2020. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proceedings of the National Academy of Sciences 117: 16587–16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigaki H, Nakayama M, Kitagawa Y, Nguyen CT, Hayashi K, Shiohara M, Gotoh B, Itoh Y. 2021. Neutralizing antibodydependent and -independent immune responses against SARS-CoV-2 in cynomolgus macaques. Virology 554: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, et al.. 2020. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host and Microbe 27: 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, et al.. 2021. Critical role of neutralizing antibody for SARS-CoV-2 reinfection and transmission. Emerging Microbes and Infections 10: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus J, et al.. 2021a. Detection and genome sequencing of SARS-CoV-2 in a domestic cat with respiratory signs in Switzerland. Viruses 13: 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus J, Palizzotto C, Zini E, Meli ML, Leo C, Egberink H, Zhao S, Hofmann-Lehmann R. 2021b. SARS-CoV-2 infection and antibody response in a symptomatic cat from Italy with intestinal B-cell lymphoma. Viruses 13: 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BS, Oh H, Kim G, Hwang EH, Jung H, Lee Y, Kang P, Park JH, Ryu CM, Hong JJ. 2020. Transient lymphopenia and interstitial pneumonia with endotheliitis in SARS-CoV-2-infected macaques. Journal of Infectious Diseases 222: 1596–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans M. 2021. SARS-CoV-2 and the human-animal interface: Outbreaks on mink farms. Lancet Infectious Diseases 21: 18–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchipudi SV, et al.. 2022. Multiple spillovers from humans and onward transmission of SARS-CoV-2 in white-tailed deer. Proceedings of the National Academy of Sciences 119: e2121644119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter JS, de Meulder D, Bestebroer TM, Lexmond P, Mulders A, Richard M, Fouchier RAM, Herfst S. 2021. SARS-CoV and SARS-CoV-2 are transmitted through the air between ferrets over more than one meter distance. Nature Communications 12: 1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SD, et al.. 2020. SARS-CoV-2 spike protein predicted to form complexes with host receptor protein orthologues from a broad range of mammals. Scientific Reports 10: 16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen HD, et al.. 2021. Preliminary report of an outbreak of SARS-CoV-2 in mink and mink farmers associated with community spread, Denmark, June to November 2020. Eurosurveillance 26: 2100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky JA, et al.. 2021. Independent infections of porcine deltacoronavirus among Haitian children. Nature 600: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky JA, Tagliamonte MS, White SK, Blohm GM, Alam MM, Iovine NM, Salemi M, Mavian C, Morris GJ. 2022. Isolation of a novel recombinant canine coronavirus from a visitor to Haiti: Further evidence of transmission of coronaviruses of zoonotic origin to humans. Clinical Infectious Diseases 75: e1184–e1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al.. 2022b. Broader-species receptor binding and structural bases of Omicron SARS-CoV-2 to both mouse and palm-civet ACE2s. Cell Discovery 8: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Cheng X, Qin C. 2022a. Reverse spillover of SARS-CoV-2 from human to wild animals. Science China Life Sciences 65: 1902–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Kuo RL, Shih SR. 2020. COVID-19: The first documented coronavirus pandemic in history. Biomedical Journal 43: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, et al.. 2020. Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduction and Targeted Therapy 5: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, et al.. 2021. Adaptation, spread and transmission of SARS-CoV-2 in farmed minks and associated humans in the Netherlands. Nature Communications 12: 6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalis BR, et al.. 2022a. Severe Acute Respiratory Syndrome coronavirus 2 Delta vaccine breakthrough transmissibility in Alachua County, Florida. Clinical Infectious Diseases 75: 1618–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalis BR, et al.. 2022b. Low-frequency variants in mildly symptomatic vaccine breakthrough infections presents a doubled-edged sword. Journal of Medical Virology 94: 3192–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S, et al.. 2022. Detection of SARS-CoV-2 in a free ranging leopard (Panthera pardus fusca) in India. Eur J Wildl Res 68: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes C, Gollakner R, Capua I. 2020. Could Mustelids spur COVID-19 into a panzootic? Veterinaria Italiana 56: 65–66. [DOI] [PubMed] [Google Scholar]
- Martins M, et al.. 2022. From Deer-to-deer: SARS-CoV-2 is efficiently transmitted and presents broad tissue tropism and replication sites in white-tailed deer. PLOS Pathogens 18: e1010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAloose D, et al.. 2020. From people to Panthera: Natural SARS-CoV-2 infection in tigers and lions at the Bronx Zoo. Mbio 11: e02220–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meekins DA, et al.. 2020. Susceptibility of swine cells and domestic pigs to SARS-CoV-2. Emerging Microbes and Infections 9: 2278–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meekins DA, Gaudreault NN, Richt JA. 2021. Natural and experimental SARS-CoV-2 infection in domestic and wild animals. Viruses 13:1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith LW, et al.. 2020. Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: A prospective genomic surveillance study. Lancet Infectious Diseases 20: 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh BQ, Nguyen MAT, von Haeseler A. 2013. Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution 30: 1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, et al.. 2021. SARS-CoV-2 Delta variant among Asiatic lions, India. Emerging Infectious Diseases 27: 2723–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas S, Yadav PD, Shete A, Nyayanit D, Sapkal G, Lola K, Gupta N. 2021. SARS-CoV-2 Delta variant pathogenesis and host response in Syrian Hamsters. Viruses 13: 1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar RJ, et al.. 2020. Clinical and pathological findings in SARS-CoV-2 disease outbreaks in farmed mink (Neovison vison). Veterinary Pathology 57: 653–657. [DOI] [PubMed] [Google Scholar]
- Monchatre-Leroy E, et al.. 2021. Hamster and ferret experimental infection with intranasal low dose of a single strain of SARS-CoV-2. Journal of General Virology 102: 001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshiri N. 2021. ViralMSA: Massively scalable reference-guided multiple sequence alignment of viral genomes. Bioinformatics 37: 714–716. [DOI] [PubMed] [Google Scholar]
- Munster VJ, et al.. 2020. Respiratory disease in Rhesus macaques inoculated with SARS-CoV-2. Nature 585: 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytyn AZ, et al.. 2021. Susceptibility of rabbits to SARS-CoV-2. Emerging Microbes and Infections 10: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A, et al.. 2020. First reported cases of SARS-CoV-2 infection in companion animals: New York, March–April 2020. Morbidity and Mortality Weekly Report 69: 710–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni D, Turelli P, Beckert B, Nazarov S, Uchikawa E, Myasnikov A, Pojer F, Trono D, Stahlberg H, Lau K. 2023. Cryo-EM structures and binding of mouse and human ACE2 to SARS-CoV-2 variants of concern indicate that mutations enabling immune escape could expand host range. PLOS Pathogens 19: e1011206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [NIH] National Institutes of Health . 2020. Severe acute respiratory syndrome coronavirus 2 isolate Wuhan-Hu-1, complete genome. GenBank: MN908947.3. National Library of Medicine. www.ncbi.nlm.nih.gov/nuccore/MN908947. [Google Scholar]
- Olival KJ, et al.. 2020. Possibility for reverse zoonotic transmission of SARS-CoV-2 to free-ranging wildlife: A case study of bats. PLOS Pathogens 16: e1008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova N, et al.. 2020. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance 25: 2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieder N, Bertzbach LD, Dietert K, Abdelgawad A, Vladimirova D, Kunec D, Hoffmann D, Beer M, Gruber AD, Trimpert J. 2020. Age-dependent progression of SARS-CoV-2 infection in Syrian hamsters. Viruses 12: 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole Á, Pybus OG, Abram ME, Kelly EJ, Rambaut A. 2022. Pango lineage designation and assignment using SARS-CoV-2 spike gene nucleotide sequences. BMC Genomics 23: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink BB, et al.. 2021. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 371: 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani G, et al.. 2021. Human-to-cat SARS-CoV-2 transmission: Case report and full-genome sequencing from an infected pet and its owner in Northern Italy. Pathogens 10: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MV, et al.. 2021. Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2. Journal of Virology 95: e00083–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K. 2004. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Pickering BS, Smith G, Pinette MM, Embury-Hyatt C, Moffat E, Marszal P, Lewis CE. 2021. Susceptibility of domestic swine to experimental infection with Severe Acute Respiratory Syndrome coronavirus 2. Emerging Infectious Diseases 27: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering B, et al.. 2022. Divergent SARS-CoV-2 variant emerges in white-tailed deer with deer-to-human transmission. Nature Microbiology 7: 2011–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, Lloyd-Smith JO. 2017. Pathways to zoonotic spillover. Nature Reviews Microbiology 15: 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SM, Hartwig AE, Bielefeldt-Ohmann H, Bosco-Lauth AM, Root JJ. 2022. Susceptibility of wild canids to SARS-CoV-2. Emerging Infectious Diseases 28: 1852–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AF, Purcell DFJ, Howden BP, Duchene S. 2023. Evolutionary rate of SARS-CoV-2 increases during zoonotic infection of farmed mink. Virus Evolution 9: vead002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QGIS . 2022. QGIS Geographic Information System. QGIS Association. www.qgis.org. [Google Scholar]
- Qiu X, Liu Y, Sha A. 2023. SARS-CoV-2 and natural infection in animals. Journal of Medical Virology 95: e28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, et al.. 2021. Mutation Y453F in the spike protein of SARS-CoV-2 enhances interaction with the mink ACE2 receptor for host adaption. PLOS Pathogens 17: e1010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell LJ. 2012. phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution /British Ecological Society 3: 217–223. [Google Scholar]
- Richard M, et al. 2020. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nature Communications 11: 3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Fernández ÁL, Santiago-Alarcon D, Lira-Noriega A. 2022. Wildlife susceptibility to infectious diseases at global scales. Proceedings of the National Academy of Sciences 119: e2122851119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues JPGLM, Barrera-Vilarmau S, Teixeira JMC, Sorokina M, Seckel E, Kastritis PL, Levitt M. 2020. Insights on cross-species transmission of SARS-CoV-2 from structural modeling. PLOS Computational Biology 16: e1008449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenke K, et al. 2020. Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerging Microbes and Infections 9: 2673–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Arrondo I, Portillo A, Palomar AM, Santibáñez S, Santibáñez P, Cervera C, Oteo JA. 2021. Detection of SARS-CoV-2 in pets living with COVID-19 owners diagnosed during the COVID-19 lockdown in Spain: A case of an asymptomatic cat with SARS-CoV-2 in Europe. Transboundary and Emerging Diseases 68: 973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KA, et al.. 2021. Dose-dependent response to infection with SARS-CoV-2 in the ferret model and evidence of protective immunity. Nature Communications 12: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagulenko P, Puller V, Neher RA. 2018. TreeTime: Maximum-likelihood phylodynamic analysis. Virus Evolution 4: vex042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailleau C, et al.. 2020. First detection and genome sequencing of SARS-CoV-2 in an infected cat in France. Transboundary and Emerging Diseases 67: 2324–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salguero FJ.et al.. 2021. Comparison of Rhesus and cynomolgus macaques as an infection model for COVID-19. Nature Communications 12: 1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlottau K, et al.. 2020. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: An experimental transmission study. Lancet Microbe 1: e218–e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segales J, et al.. 2020. Detection of SARS-CoV-2 in a cat owned by a COVID-19-affected patient in Spain. Proceedings of the National Academy of Sciences 117: 24790–24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj P, et al.. 2021. SARS-CoV-2 infection induces protective immunity and limits transmission in Syrian hamsters. Life Science Alliance 4: e202000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C, et al.. 2020. Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques. Cell Research 30: 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, et al.. 2020. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368: 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai L, et al.. 2021. Replication, pathogenicity, and transmission of SARS-CoV-2 in minks. National Science Review 8: nwaa291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia SF, et al.. 2020. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 583: 834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK, et al.. 2021. Responses to acute infection with SARS-CoV-2 in the lungs of Rhesus macaques, baboons and marmosets. Nature Microbiology 6: 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song TZ. 2021. Northern pig-tailed macaques (Macaca leonina) infected with SARS-CoV-2 show rapid viral clearance and persistent immune response. Zoological Research 42: 350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song TZ, et al.. 2020a. Delayed severe cytokine storm and immune cell infiltration in SARS-CoV-2-infected aged Chinese Rhesus macaques. Zoological Research 41: 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, et al.. 2020b. SARS-CoV-2 causes a systemically multiple organs damages and dissemination in Hamsters. Frontiers in Microbiology 11: 618891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Gu L, Ma L, Duan Y 2021a. Atlas of ACE2 gene expression reveals novel insights into transmission of SARS-CoV-2. Heliyon 7: e05850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, et al.. 2021b. Characterization and structural basis of a lethal mouse-adapted SARS-CoV-2. Nature Communications 12: 5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliamonte MS, Abid N, Borocci S, Sangiovanni E, Ostrov DA, Kosakovsky Pond SL, Salemi M, Chillemi G, Mavian C. 2020. Multiple recombination events and strong purifying selection at the origin of SARS-CoV-2 spike glycoprotein increased correlated dynamic movements. International Journal of Molecular Science 105: 93–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliamonte MS, et al.. 2022. Rapid emergence and spread of severe acute respiratory syndrome coronavirus 2 gamma (p. 1) variant in Haiti. Clinical Infectious Diseases 74: 2057–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmam S, et al.. 2022. Bat coronaviruses related to SARS-CoV-2 and infectious for human cells. Nature 604: 330–336. [DOI] [PubMed] [Google Scholar]
- Tortorici MA, Veesler D. 2019. Structural insights into coronavirus entry. Advances in Virus Research 105: 93–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimpert J, Vladimirova D, Dietert K, Abdelgawad A, Kunec D, Dokel S, Voss A, Gruber AD, Bertzbach LD, Osterrieder N. 2020. The Roborovski Dwarf Hamster is a highly susceptible model for a rapid and fatal course of SARS-CoV-2 infection. Cell Reports 33: 108488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson GBet al.. 2023. Increase in SARS-CoV-2 seroprevalence in UK domestic felids despite weak immunogenicity of post-omicron variants. Viruses 15: 1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich L, Michelitsch A, Halwe N, Wernike K, Hoffmann D, Beer M. 2020. Experimental SARS-CoV-2 infection of bank voles-general susceptibility but lack of direct transmission. Emerging and Infectious Diseases 27: 1193–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upham NS, Esselstyn JA, Jetz W. 2019. Inferring the mammal tree: Species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLOS Biology 17: e3000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [USDA] US Department of Agriculture . 2021a. Confirmation of COVID-19 in a Canada Lynx at a Pennsylvania Zoo. Animal and Plant Health Inspection Service (21 December 2021). www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2021/sa-12/covid-lynx-pa [Google Scholar]
- [USDA] US Department of Agriculture . 2021b. Confirmation of COVID-19 in a Binturong and a Fishing Cat at an Illinois Zoo. Animal and Plant Health Inspection Service (6 October 2021). www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2021/sa-10/covid-binturong-fishing-cat [Google Scholar]
- [USDA] US Department of Agriculture . 2021c. Confirmation of COVID-19 in Gorillas at a California Zoo. Animal and Plant Health Inspection Service (11 January 2021). www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2021/sa-01/ca-gorillas-sars-cov-2. [Google Scholar]
- [USDA] US Department of Agriculture . 2021d. Confirmation of COVID-19 in Hyenas at a Colorado Zoo. Animal and Plant Health Inspection Service (5 November 2021). www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2021/sa-11/covid-hyenas. [Google Scholar]
- [USDA] US Department of Agriculture . 2021e. Confirmation of COVID-19 in Otters at an Aquarium in Georgia. Animal and Plant Health Inspection Service (22 April 2021). https://www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2021/sa-04/covid-georgia-otters. [Google Scholar]
- [USDA] US Department of Agriculture . 2021f. Confirmation of COVID-19 in a Coatimundi at an Illinois Zoo. Animal and Plant Health Inspection Service (14 October 2021). www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2021/sa-10/covid-coatimundi. [Google Scholar]
- [USDA] US Department of Agriculture . 2023. One health: SARS-CoV-2 - Projects. Animal and Plant Health Inspection Service (15 November 2023) https://www.aphis.usda.gov/aphis/ourfocus/onehealth/one-health-projects.
- Vergara-Alert J, et al.. 2021. Pigs are not susceptible to SARS-CoV-2 infection but are a model for viral immunogenicity studies. Transboundary and Emerging Diseases 68: 1721–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova AN, Diaz A, Damtie D, Xiu L, Toh T-H, Lee JS-Y, Saif LJ, Gray GC. 2022. Novel canine coronavirus isolated from a hospitalized patient with pneumonia in East Malaysia. Clinical Infectious Diseases 74: 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC. 2013. Urbanization and geographic expansion of zoonotic arboviral diseases: Mechanisms and potential strategies for prevention. Trends in Microbiology 21: 360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Shan K, Wang W, Zhang S, Huan Q, Qian W. 2021. Evidence for a mouse origin of the SARS-CoV-2 Omicron variant. Journal of Genetics and Genomics 12: 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells K, Clark NJ. 2019. Host specificity in variable environments. Trends in Parasitology 35: 452–465. [DOI] [PubMed] [Google Scholar]
- [WHO] Word Health Organization . 2021. Guidance for surveillance of SARS-CoV-2 variants. WHO (9 August 2021). www.who.int/publications/i/item/WHO_2019-nCoV_surveillance_variants.
- Wilkinson MD, et al.. 2016. The FAIR Guiding Principles for scientific data management and stewardship. Scientific Data 3: 160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [WOAH] World Organization for Animal Health . 2020. Considerations for Sampling, Testing, and Reporting of SARS-CoV-2 in Animals, vers. 1.1. WOAH. www.woah.org/fileadmin/Home/MM/A_Sampling_Testing_and_Reporting_of_SARS-CoV-2_in_animals_3_July_2020.pdf.
- [WOAH] World Organization for Animal Health . 2022a. SARS-CoV-2 in Animals. WOAH. Situation report no. 11. www.woah.org/app/uploads/2022/04/sars-cov-2-situation-report-11.pdf.
- [WOAH] World Organization for Animal Health . 2022b. Considerations on Monitoring SARS-CoV-2 in Animals. WOAH. www.woah.org/app/uploads/2022/08/en-sars-cov-2-surveillance.pdf.
- [WOAH] World Organization for Animal Health . 2022c. SARS-CoV-2 in Animals. WOAH. Situation report no. 8. www.oie.int/app/uploads/2022/01/sars-cov-2-situation-report-8.pdf.
- Woolsey C, et al.. 2021. Establishment of an African green monkey model for COVID-19 and protection against re-infection. Nature Immunology 22: 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xinhua . 2021. Mongolia confirms first animal deaths due to COVID-19. Xinhuanet.com (15 September 2021). www.xinhuanet.com/english/asiapacific/2021-09/15/c_1310188921.htm.
- Xu J, Lazartigues E. 2020. Expression of ACE2 in human neurons supports the neuro-invasive potential of COVID-19 virus. Cellular and Molecular Neurobiology 42: 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, et al.. 2020. COVID-19-like symptoms observed in Chinese tree shrews infected with SARS-CoV-2. Zoological Research 41: 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. 2020. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367: 1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HL, et al.. 2022. Transmission of SARS-CoV-2 delta variant (AY. 127) from pet hamsters to humans, leading to onward human-to-human transmission: A case study. Lancet 399: 1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, et al.. 2020. DNA vaccine protection against SARS-CoV-2 in Rhesus macaques. Science 369: 806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, et al.. 2020. Susceptibility of tree shrew to SARS-CoV-2 infection. Scientific Reports 10: 16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, et al.. 2020. Virulence and pathogenesis of SARS-CoV-2 infection in Rhesus macaques: A nonhuman primate model of COVID-19 progression. PLOS Pathogens 16: e1008949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, et al.. 2022. Mutations that adapt SARS-CoV-2 to mink or ferret do not increase fitness in the human airway. Cell Reports 38: 110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.