Highlights
-
•
Little evidence of causal associations of inflammatory markers (CRP, GlycA, IL-6, IL-6R, sIL-6R) and cognition in Mendelian randomization analyses in ALSPAC.
-
•
Little evidence of causal effect of same inflammatory markers on general cognitive ability in Mendelian randomization analyses.
-
•
General cognitive ability may be causally associated with lower inflammation.
-
•
Larger genome-wide association studies on individual cognitive domains are needed.
Keywords: Cognition, Inflammation, Mendelian randomization, Observational, ALSPAC, CRP, IL-6, GlycA, Emotion recognition, Working memory, Response inhibition
Abstract
Background
Inflammation is associated with cognitive functioning and dementia in older adults, but whether inflammation is related to cognitive functioning in youth and whether these associations are causal remains unclear.
Methods
In a population-based cohort (Avon Longitudinal Study of Parents and Children; ALSPAC), we investigated cross-sectional associations of inflammatory markers (C-reactive protein [CRP], Interleukin-6 [IL-6] and Glycoprotein acetyls [GlycA]) with measures of cold (working memory, response inhibition) and hot (emotion recognition) cognition at age 24 (N = 3,305 in multiple imputation models). Furthermore, we conducted one-sample and two-sample bidirectional Mendelian randomization (MR) analyses to examine potential causal effects of genetically-proxied inflammatory markers (CRP, GlycA, IL-6, IL-6 receptor, soluble IL-6 receptor) on cognitive measures (above) and on general cognitive ability.
Results
In the ALSPAC cohort, there was limited evidence of an association between standardised inflammatory markers and standardised cognitive measures at age 24 after adjusting for potential confounders (N = 3,305; beta range, −0.02 [95 % confidence interval (CI) −0.06 to 0.02, p = 0.27] to 0.02 [95 % CI −0.02 to 0.05, p = 0.33]). Similarly, we found limited evidence of potential effects of 1-unit increase in genetically-proxied inflammatory markers on standardised working memory, emotion recognition or response inhibition in one-sample MR using ALSPAC data (beta range, −0.73 [95 % CI −2.47 to 1.01, p = 0.41] to 0.21 [95 % CI −1.42 to 1.84, p = 0.80]; or on standardised general cognitive ability in two-sample MR using the latest Genome-Wide Association Study (GWAS) datasets (inverse-variance weighted beta range, −0.02 [95 % CI −0.05 to 0.01, p = 0.12] to 0.03 [95 % CI −0.01 to 0.07, p = 0.19]).
Conclusions
Our MR findings do not provide strong evidence of a potential causal effect of inflammatory markers (CRP, IL-6, IL-6 receptor, GlycA) on the cognitive functions examined here. Given the large confidence intervals in the one-sample MR, larger GWAS of specific cognitive measures are needed to enable well-powered MR analyses to investigate whether inflammation causally influences specific cognitive domains.
1. Introduction
Cognitive function predicts many important life outcomes including educational attainment (Deary et al., 2007, Strenze, 2007), occupation status (Schmidt and Hunter, 2004, Strenze, 2007) health-related mortality (Calvin et al., 2017), and quality of life (Cumming et al., 2014). Cognitive dysfunction is a core feature of many mental health disorders including depression, schizophrenia and Alzheimer’s disease (Bolt et al., 2019, Dalili et al., 2015, Fusar-Poli et al., 2012, Nikolin et al., 2021, Rock et al., 2014), and is highly prevalent in physical illnesses including cancer and long COVID (Janelsins et al., 2014, Van Dyk and Ganz, 2021). Despite this, there are few treatments which effectively address cognitive dysfunction (see (Pan et al., 2017) for a review), making it an unmet clinical need. Instead, some treatments may result in worse cognition (Van Dyk & Ganz, 2021). Therefore, there is a need to identify modifiable risk factors that may be targets for prevention and treatment of cognitive dysfunction.
One promising intervention target could be inflammation (Khandaker et al., 2018). There is some evidence that systemic inflammatory markers such as C-reactive protein (CRP) are associated with cognitive dysfunction in people with physical or mental health conditions (Mac Giollabhui et al., 2020b, Misiak et al., 2018, Morrens et al., 2022). Observational studies in the general population have also reported associations between inflammatory markers, such as CRP, Interleukin-6 (IL-6) and Glycoprotein acetyls (GlycA), and poorer general cognitive ability and specific cognitive domains (Conole et al., 2021, Cullen et al., 2017, Fard et al., 2022, Kokosi et al., 2021, Mac Giollabhui et al., 2021b, Sartori et al., 2012, Shields et al., 2021, Van der Lee et al., 2018) although improved cognition has also been reported (Milton et al., 2021). Regarding the human experimental literature, the effect of acute inflammatory challenges (endotoxin and vaccines) on hot (processing of emotionally valanced stimuli e.g., emotion recognition (Roiser & Sahakian, 2013)) and cold (processing of emotionally neutral stimuli e.g., memory of neutral words (Roiser & Sahakian, 2013)) cognitive domains have yielded inconsistent findings (Balter et al., 2018, Bollen et al., 2017, Brydon et al., 2008, Handke et al., 2020, Harrison et al., 2014). For example, a systematic review reported conflicting results within cold cognitive domains (attention, executive function, memory), with some studies reporting decreased or enhanced performance after an inflammatory challenge, whilst others did not (Bollen et al., 2017). In contrast, there was more consistent evidence in hot cognitive domains of social and emotion processing, where inflammation reduced performance (Bollen et al., 2017). However, most experimental studies are restricted to males and small sample sizes (N < 50).
Whilst there is some evidence of an association between inflammation and cognition, there are gaps in the literature. First, current studies are often restricted to small samples (Bollen et al., 2017). Larger studies in the general population are needed to provide more reliable evidence. Second, few studies have examined the association between inflammation and cognition in youth (although see (Cullen et al., 2017, Mac Giollabhui et al., 2021a, Shields et al., 2021)), instead most studies have examined older adults (see (Fard et al., 2022) for a review). Third, the direction and causality of association are unknown. Specifically, it is unclear whether inflammation affects cognition or if observed associations are due to residual confounding or reverse causation. Fourth, most studies examined commonly measured markers such as IL-6 and CRP (Conole et al., 2021, Kokosi et al., 2021, Mac Giollabhui et al., 2021b, Shields et al., 2021). Whilst these are useful markers of systemic inflammation, their levels vary over time within individuals (Bogaty et al., 2013). Investigating novel inflammatory markers such as GlycA, which are thought to be more stable and better reflect chronic inflammation (Connelly et al., 2017, Otvos et al., 2015, Ritchie et al., 2015), could be more fruitful. Fifth, few studies have examined the effect of inflammation on hot and cold cognitive domains. As there is evidence of cognitive sub-groups within clinical populations (people experiencing deficits in hot cognition, cold cognition, or in both domains) (Dam et al., 2021), investigating both domains may propose novel treatment targets for sub-groups of individuals experiencing specific cognitive impairments.
In this study, we investigated the role of inflammation in cognition, both a broad measure of general cognitive ability and specific cognitive domains. We first examined associations between inflammatory markers (CRP, GlycA and IL-6) and cold (working memory and response inhibition) and hot (emotion recognition) cognitive measures within a large population-based cohort (Avon Longitudinal Study of Parents and Children; ALSPAC). To better assess causality, we then used Mendelian randomization (MR). MR is a genetic epidemiological method that helps to overcome the limitations of observational studies (particularly, residual confounding and reverse causation) by using genetic variants (Single Nucleotide Polymorphisms, SNPs) strongly associated with an exposure (identified in genome-wide association studies, GWAS) as proxies for the exposure (Davey Smith and Ebrahim, 2003, Sanderson et al., 2022). This approach is less susceptible to these limitations because genetic variants are randomly assigned during gamete formation and conception (making them less likely to be associated with confounders) and fixed at conception (precluding reverse causation) (Davey Smith & Ebrahim, 2003). MR can be conducted using SNP-exposure and SNP-outcome effect sizes from the same sample (one-sample MR) or from two samples (two-sample MR) (Lawlor, 2016, Sanderson et al., 2022). Whilst each approach has its own strengths and limitations, they are conceptually similar. See (Sanderson et al., 2022) for a recent review. Here, we conducted one-sample MR to examine associations between inflammatory markers (CRP, IL-6, IL-6 receptor [IL-6R], soluble IL-6R [sIL-6R], GlycA) and the same cognitive domains within ALSPAC. Inflammatory exposures were selected based on their well-studied associations with mental health conditions (CRP, IL-6). We also included GlycA as it is thought to provide a more stable marker of chronic inflammation (Connelly et al., 2017, Otvos et al., 2015, Ritchie et al., 2015). To increase statistical power, we used two-sample MR to examine potential causal relationships between the same inflammatory markers and general cognitive ability (GCA). As there were no large GWAS on specific cognitive domains, we focused on the broader construct of GCA in the two-sample MR. Given the possibility that the relationship between inflammation and cognition could be in either direction, we also conducted bidirectional analyses which tests both possibilities. Triangulating across multiple methods (non-genetic observational analysis and Mendelian randomization) which have different sources of bias will enable more robust conclusions to be drawn (Hammerton and Munafò, 2021, Lawlor et al., 2016). Based on previous research, we hypothesised that higher levels of inflammation would be associated with poorer cognition.
2. Materials and methods
This study was pre-registered (Open Science Framework: https://osf.io/892wr/), with deviations justified (Supplementary Table S26). Ethics approval was obtained in original studies.
2.1. Cross-sectional analysis in the ALSPAC cohort
2.1.1. Cohort description
ALSPAC is a longitudinal population-based birth cohort which initially recruited 14,541 pregnant women in the Avon area (UK) with expected delivery dates between 1st April 1991 and 31st December 1992 (Boyd et al., 2013, Fraser et al., 2013, Northstone et al., 2019). There were 14,062 live births and 13,988 children who were alive at age one. An attempt was made to increase the original sample size when the eldest children were approximately aged seven. This resulted in a total sample size of 15,454 pregnancies (14,901 alive at 1 year of age) when using data after age seven. A vast range of variables are available including data on genetics (Supplementary methods 1.1), mental and physical health, and cognition. The study website contains details of the available data through a fully searchable data dictionary and variable search tool: https://www.bristol.ac.uk/alspac/researchers/our-data/. Although the ALSPAC cohort is broadly representative of the general population in Britain in 1991, it is important to note that the cohort is less representative of less affluent families (e.g., living in rented accommodation, not having a car) and ethnic minority mothers. For further details, see: https://www.bristol.ac.uk/alspac/researchers/cohort-profile/.
2.1.2. Inflammatory exposures at age 24
High sensitivity CRP, GlycA (mainly a1-acid glycoprotein) and IL-6 were assessed in blood samples collected from participants after fasting for at least 6 h. CRP (mg/l), GlycA (mmol/l) and IL-6 (normalised protein expression [NPX] values on log2 scale) were quantified using an immunoturbidimetric assay (Roche Diagnostics), 1D proton (1H) Nuclear Magnetic Resonance spectroscopy-based platform (Nightingale Health, Helsinki, Finland), and Olink Proteomics assay, respectively. For intra-assay coefficient of variance for CRP and IL-6, see Supplementary methods 1.2.
2.1.3. Cognitive outcomes at age 24
Working memory, the ability to temporarily store and manipulate information, was assessed using the N-back task (two-back design) (Kirchner, 1958). On each trial, a number is briefly presented (500 ms) and participants are asked to report whether this number is the same or different from the number presented two trials earlier. This task consists of 48 trials with no feedback (8 trials are matches). Prior to this, there are 12 practice trials with feedback. The primary outcome is discriminability index (d’) which provides an overall performance estimate. A higher d’ indicates better working memory. Individuals who either did not respond on > 50 % trials or had a negative d’ were removed from analyses (N = 78).
Emotion recognition, the ability to identify emotion expressions, was assessed using the Emotion Recognition Task (Penton-Voak et al., 2012). On each trial, a face displaying one of six basic emotions (happy, sad, anger, fear, disgust, or surprise) is briefly presented (200 ms) and then immediately covered up. Following this, participants report which emotion was displayed using the six labels. For each emotion, there are eight levels of intensity. This task consists of 96 trials (each emotion presented 16 times). The primary outcome is hits (i.e., number of emotion expressions correctly identified), with a higher score indicating better emotion recognition.
Response inhibition, the ability to suppress a prepotent response, was assessed using the Stop-Signal Task (Logan et al., 1984). On each trial, a letter (X or O; 1,000 ms) is presented and participants are asked to report which letter was displayed, as quickly as possible. However, on 25 % trials a tone is presented after the letter (“stop signal”). Participants are asked to inhibit responding on these trials. The task consists of 256 trials (four blocks of 64 trials). The primary outcome is stop-signal reaction time (SSRT). A lower SSRT indicates better response inhibition.
All exposure and outcomes are continuous measures. For distributions of variables, see Supplementary Table S2. Of the 15,645 participants in ALSPAC, 3,305 individuals had data on all three cognitive measures (working memory, emotion recognition, response inhibition) at age 24.
2.1.4. Potential confounders
Potential confounders were chosen based on evidence that these variables may be risk factors for inflammation (O’Connor et al., 2009) and cognition and thus may confound the inflammation-cognition association. Potential confounders included sex, ethnicity, BMI (age 24), maternal education (Degree, A level, O level, Vocational or CSE), maternal socioeconomic status (SES), smoking status (age 24), alcohol use (age 24) and IQ (age 8). For more details, see Supplementary methods 1.3.
2.1.5. Statistical analysis
Prior to analysis, exposure and outcome variables were standardised. Data were analysed using Stata 16 (StataCorp, 2019) using mvreg command. Linear regression models examined the cross-sectional association between inflammation (CRP, GlycA and IL-6) and cognitive measures at age 24. An unadjusted model was first examined (model 1); followed by models adjusted for sex, ethnicity, and BMI at age 24 (model 2); additionally adjusted for maternal education and SES (model 3); additionally adjusted for smoking and alcohol use at age 24 (model 4); additionally adjusted for IQ at age 8 (model 5). We conducted a sensitivity analysis excluding ALSPAC participants who had CRP ≥ 10 mg/l, an indicator of current infection, at age 24 (N = 114); see (Mac Giollabhui et al., 2020a) for a review on using this cut-off as an indicator of current infection. Working memory was not normally distributed and natural log transforming this variable did not correct for this. As such, the untransformed raw variable was used in analyses. For complete case and sensitivity analyses, see Supplementary Table S4–S9.
2.1.5.1. Dealing with missing data
Given the possibility that data are not missing completely at random (MCAR) in ALSPAC (Taylor et al., 2018), we conducted multiple imputation (MI). The rationale for using MI is (1) to improve power in the complete case analyses by imputing covariates and exposures (N range in fully adjusted complete case models: 1,686–1,902), (2) we assume the outcome is missing at random (MAR) given the variables in the analysis model, and (3) we assume every exposure and covariate is MAR given the variables in the imputation model. Participants who had data on all three cognitive outcomes at age 24 were included in the analysis (N = 3,305). Exposures (inflammatory markers) and potential confounders were imputed. For each set of imputations, 100 datasets were imputed using chained equations. We included the standardised exposures, standardised outcomes, potential confounders, and auxiliary variables in all models. To increase the plausibility that data are MAR, auxiliary variables were included in the MI (Madley-Dowd et al., 2019, White et al., 2011). These were chosen based on their association with incomplete variables (Supplementary methods 1.4). For each exposure, we report linear regression models with imputed data as our primary analysis. For more detail on the MI and variables included in the imputation models, see Supplementary methods 1.4 and Table S1.
2.2. Genetic Mendelian randomization (MR) analysis
MR is a method used to assess causality (Davey Smith and Ebrahim, 2003, Sanderson et al., 2022). Genetic variants strongly associated with an exposure are used as proxies for the exposure; which makes this method less susceptible to reverse causation and confounding (Davey Smith & Ebrahim, 2003). The validity of causal inferences drawn from MR relies on three key assumptions: (1) genetic variants are robustly associated with the exposure, (2) genetic variants are not associated with potential confounders, (3) genetic variants are associated with the outcome only via the exposure. Here, we conducted one-sample MR within the ALSPAC cohort and two-sample MR using publicly available GWAS. Given the possibility that the relationship between inflammation and cognition could be in either direction, we conducted bidirectional analyses to investigate both possibilities. For more details, see Supplementary methods 1.5.
2.2.1. One-sample bidirectional Mendelian randomization in the ALSPAC cohort
One-sample MR assessed whether there is a potential causal relationship between the same inflammatory markers and cognitive measures reported in the cross-sectional analysis (N range: 1,677–2,193).
2.2.1.1. Data sources
The GWAS listed in Table 1 were used to identify genetic variants (Single Nucleotide Polymorphisms; SNPs) associated with inflammation (CRP, IL-6, IL-6R, sIL-6R and GlycA) and cognition (working memory, emotion recognition, response inhibition) (Ahluwalia et al., 2021, Han et al., 2020, Kettunen et al., 2016, Ligthart et al., 2018, Mahedy et al., 2021, Rosa et al., 2019, Sarwar et al., 2012, Swerdlow et al., 2012). For details on GWAS (including any overlap with ALSPAC) and accessing data, see Supplementary methods 1.6, Tables S11 and S13. For number of genetic variants included in each SNP set, see Table 1.
Table 1.
GWAS and instruments used to extract SNPs for inflammation (CRP, IL-6, IL-6R, sIL-6R, GlycA) and cognition (working memory, emotion recognition, response inhibition).
| Phenotype | GWAS/Instrument | N | SNP location | N SNP | 1SMR | 2SMR |
|---|---|---|---|---|---|---|
| Inflammation | ||||||
| Primary analysis | ||||||
| CRP | Ligthart et al. (2018) | 204,402 |
CRP gene cis SNPs |
6 | 6 | 6 |
| Han et al. (2020) | 418,642 |
CRP gene cis SNPs |
20 | 18 | 13 | |
| IL-6R | Ahluwalia et al. (2021) | 52,654 |
IL6R gene cis SNPs |
2 | 2 | 2 |
| sIL-6R | Rosa et al. (2019) | 3,301 | IL6R gene cis SNPs | 34 | 34 | 22 |
| GlycA | Borges et al. (2020) | 115,078 | Genome-wide SNPs |
87 | 82 | 82 |
| Secondary analysis | ||||||
| CRP | Ligthart et al. (2018) | 204,402 | Genome-wide SNPs |
78 | 76 | 77 |
| Han et al. (2020) | 418,642 | Genome-wide SNPs |
552 | 520 | 494 | |
| IL-6 |
Ahluwalia et al. (2021) | 52,654 | Genome-wide SNPs |
3 | 3 | 3 |
| IL-6R | Sarwar et al. (2012) | 27,185 | IL6R gene cis SNPs | 1 | 1 | 1 |
| Swerdlow et al. (2012) | ≤ 4,479/ SNP |
IL6R gene cis SNPs | 3 | 3 | 3 | |
| GlycA | Kettunen et al. (2016) | 19,270 | Genome-wide SNPs |
10 | 10 | 10 |
| Cognition | ||||||
| Working memory | Mahedy et al. (2021) | 2,471 | Genome-wide SNPs |
3 | 3 | N/A |
| Emotion recognition | Mahedy et al. (2021) | 2,560 | Genome-wide SNPs |
6 | 6 | N/A |
| Response inhibition | Mahedy et al. (2021) | 2,446 | Genome-wide SNPs |
6 | 6 | N/A |
| General cognitive ability | Lam et al. (2021) | 373,617 | Genome-wide SNPs | 250 | N/A | Range: 219–250 |
N, number of participants; SNP location (Genome-wide SNPs [independent significant genome-wide SNPs] or cisSNPs [SNPs within +/- 1mB from protein coding gene]), N, number of SNPs identified from each GWAS/instrument (see section 2.2.1.2 for criteria applied); 1SMR, Final N SNPs included in genetic risk scores (including proxies) for one-sample MR; 2SMR, Final N SNPs included in instruments (including proxies) for two-sample MR; CRP, c-reactive protein; IL-6, interleukin-6; IL-6R, interleukin-6 receptor, sIL-6R, soluble interleukin-6 receptor; GlycA, glycoprotein acetyls.Note:genetic variants for some instruments were pre-selected:Rosa et al. (2019)selected independent SNPs (r2 < 0.1) located within 250kb fromIL6R;Sarwar et al. (2012)selected one SNP (rs2228145) located withinIL6R;Swerdlow et al. (2012)selected independent SNPs located within 55kb fromIL6R. For CRP, we pre-specified theLigthart et al. (2018)GWAS in our pre-registration; however, as a larger GWAS was available (Han et al., 2020), we included this GWAS to increase statistical power. For location of protein coding gene, see Supplementary Table S14 footnote.
2.2.1.2. Extracting genetic variants for inflammation and cognition
SNPs were extracted from GWAS full summary statistics based on the following criteria: (1) p-value threshold for inflammatory markers (p < 5 × 10-8) and for cognitive measures (p < 5 × 10-6; due to no SNPs meeting criteria at 5 × 10-8), (2) linkage disequilibrium (LD) clumping (r2 = 0.01, kb = 1000) using ld_clump() in the ieugwasr package and (3) minor allele frequency > 0.01. LD clumping ensures that SNPs are not highly correlated with each other and are therefore independent. For ease of interpretation, all effect alleles refer to the exposure-increasing allele. Where possible, SNPs were divided into cis variants (SNPs +/- 1mB from protein coding gene based on Genome Reference Consortium Human Build 37 or 38, see Supplementary Table S14 footnote for location of protein coding genes) and genome-wide variants (SNPs that met statistical criteria based on p-value and LD thresholds). Cis variants, due to their proximity to the protein coding gene, are less likely to be pleiotropic (i.e., less likely to influence the outcome via pathways other than the exposure) and therefore may provide more valid instruments. Genome-wide variants may increase statistical power in the analyses due to the larger number available. The primary analysis was performed using cis SNPs extracted from the largest available GWAS, except for GlycA which does not have a protein coding gene. Instead, we used the largest GlycA GWAS to date in the primary analysis. Secondary analyses used smaller GWAS/instruments. For number of SNPs available after applying each criterion, see Supplementary Table S14.
2.2.1.3. Creating genetic risk scores for inflammation and cognition
Genetic and phenotypic data were available for 8,130 ALSPAC participants. For one-sample MR, identified SNP sets (Table 1) were combined into a weighted genetic risk score for inflammatory (CRP, IL-6, IL-6R, sIL-6R, GlycA) and cognitive (working memory, emotion recognition, response inhibition) phenotypes for each ALSPAC participant (Purcell et al., 2007). Specifically, risk alleles were weighted by the effect size (beta) reported in the GWAS and then summed to provide a risk score. Unrelated individuals were kept, withdrawals of consent were removed, missing genotypes were not imputed. For SNPs not available in ALSPAC, proxies were identified that had: r2 > 0.8 (using LDproxy_batch function in EUR population in R), rsID available, SNP available in full summary statistics and ALSPAC. For number of SNPs included in each genetic risk score and quality checks, see Table 1, Table S15 and Supplementary methods 1.7.
2.2.1.4. Data analysis
Outcome variables (not exposures) are standardised for direct comparison. Analyses were carried out in R 4.1.1 (R Core Team, 2020). Genetic risk scores were created in Plink v1.90 and two-stage least squares regressions were conducted using the AER package (Kleiber & Zeileis, 2008). Exposures were CRP (mg/l, age 24), GlycA (mmol/l, age 24), IL-6 (pg/ml, age 9). Models included top 10 genetic principal components to adjust for genetic ancestry. As CRP and IL-6 were highly skewed, they were log transformed.
2.2.1.5. Two-sample bidirectional Mendelian randomization
Two-sample MR assessed whether there is a potential causal relationship between the same inflammatory markers and general cognitive ability. For sample sizes, see Table 1.
2.2.1.6. Genetic instruments
For inflammatory markers, we extracted SNPs from the same GWAS/instruments used in the one-sample MR (Table 1). For GCA, we used the largest combined GWAS on GCA to date (N = 373,617) (Lam et al., 2021), see Supplementary methods 1.6 for more detail. SNPs for GCA were identified using the same criteria outlined in 2.2.1.2, identifying 250 SNPs. Proxies were identified for SNPs not available in the outcome GWAS using the following criteria: R2 > 0.8, SNP with highest R2 available in both the exposure and outcome summary statistics. For final N SNPs used in each instrument, see Table 1, Supplementary Tables S19 and S22.
2.2.1.7. Additional MR assumption
The same assumptions for one-sample MR apply, with the additional assumption that samples are non-overlapping but come from the same population (Lawlor, 2016). Whilst it is difficult to determine the exact percentage of overlap, there is overlap between most exposure and outcome GWAS due to the use of large data sources (cohorts in CHARGE consortium and UK Biobank). Consequently, some advantages (e.g., weak instruments biasing results towards the null) of two-sample MR may be reduced (Burgess et al., 2016, Lawlor, 2016), although the main advantage of increased statistical power remains (Lawlor, 2016). Nevertheless, recent work suggests that sample overlap may not bias MR results as much as previously thought (Burgess et al., 2016, Sanderson et al., 2022).
2.2.1.8. Data analysis
Two-sample MR was conducted using the TwoSampleMR Package 0.5.6 (Hemani et al., 2018) in R 4.1.1 (R Core Team, 2020). For each instrument, this package harmonises the SNP-exposure and SNP-outcome data. Palindromic SNPs were excluded if the strand could not be inferred from the minor allele frequency (>0.42), if available. The primary analysis used either the inverse-variance weighted (IVW) method (>1 SNP available) or Wald ratio (1 SNP available). Sensitivity analyses included MR-Egger (Bowden et al., 2015), weighted median (Bowden et al., 2016), weighted mode (Hartwig et al., 2017), and MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) (Verbanck et al., 2018). As these MR methods have different assumptions regarding the validity of the genetic instruments, we can be more confident in our causal inferences if the relationship between inflammation and cognition is observed across methods. Some MR methods require a minimum number of SNPs; therefore, sensitivity analyses are reported when enough SNPs are available. For details on each method, see Supplementary methods 1.9. Additional sensitivity analyses included Steiger filtering to check that SNPs have a stronger association with the exposure than the outcome (Hemani et al., 2017), checking for heterogeneity (Cochran’s Q-statistic), checking for pleiotropy (Egger intercept).
3. Results
Fig. 1 presents an overview of the analyses conducted in this study. For descriptive statistics on cognitive measures, inflammatory markers, and confounders in ALSPAC, see Supplementary Table S2. For correlations between variables of interest, see Supplementary Table S3.
Fig. 1.
Overview of analyses performed in this study.Note: MR was run bidirectionally. Manhattan plot image taken from Ligthart et al. (2018).
3.1. Association between standardised inflammatory markers and standardised cognitive function at age 24 in the ALSPAC cohort estimated using multivariate regression
There was limited evidence of an association between CRP and cognitive measures (ps ≥ 0.32; Table 2). GlycA was associated with poorer working memory (β = −0.08, 95 % CI = −0.11 to −0.04, p <.001), emotion recognition (β = −0.05, 95 % CI = −0.09 to −0.01, p =.007) and response inhibition at age 24 (β = 0.05, 95 % CI = 0.01 to 0.08, p =.011) (Table 2), but these associations did not persist after adjusting for potential confounders. IL-6 was also associated with poorer working memory (β = −0.05, 95 % CI = −0.08 to −0.01, p =.017), emotion recognition (β = −0.05, 95 % CI = −0.09 to −0.02, p =.003) and response inhibition at age 24 (β = 0.03, 95 % CI = −0.004 to 0.07, p =.084) (Table 2), but associations did not persist after adjusting for confounders. For MI sensitivity analyses, see Supplementary results 1.1. This is broadly consistent with complete case analyses and sensitivity analyses (removing individuals with possible infection) (Supplementary Table S7–S9).
Table 2.
Linear regression models of cross-sectional associations between inflammatory markers (CRP, GlycA and IL-6) and cognitive measures at age 24 in ALSPAC (N = 3,305; multiply imputed models).
| Models | b | 95 % CI | p |
|---|---|---|---|
| C-reactive Protein (CRP, mg/l) | |||
| Outcome: Working memory | |||
| Model 1 | -0.02 | -0.05 to 0.02 | 0.32 |
| Model 2 | -0.0002 | -0.04 to 0.04 | 0.99 |
| Model 3 | 0.001 | -0.04 to 0.04 | 0.95 |
| Model 4 | 0.006 | -0.03 to 0.04 | 0.74 |
| Model 5 | 0.01 | -0.02 to 0.05 | 0.48 |
| Outcome: Emotion recognition | |||
| Model 1 | -0.003 | -0.04 to 0.04 | 0.88 |
| Model 2 | 0.007 | -0.03 to 0.05 | 0.73 |
| Model 3 | 0.008 | -0.03 to 0.05 | 0.68 |
| Model 4 | 0.01 | -0.03 to 0.05 | 0.53 |
| Model 5 | 0.02 | -0.02 to 0.05 | 0.33 |
| Outcome: Response inhibition | |||
| Model 1 | 0.02 | -0.02 to 0.05 | 0.36 |
| Model 2 | -0.004 | -0.04 to 0.03 | 0.85 |
| Model 3 | -0.003 | -0.04 to 0.03 | 0.87 |
| Model 4 | -0.009 | -0.05 to 0.03 | 0.63 |
| Model 5 | -0.01 | -0.05 to 0.02 | 0.45 |
| Glycoprotein Acetyls (GlycA, mmol/l) | |||
| Outcome: Working memory | |||
| Model 1 | -0.08 | -0.11 to -0.04 | <0.001 |
| Model 2 | -0.05 | -0.09 to -0.01 | 0.019 |
| Model 3 | -0.04 | -0.08 to 0.002 | 0.062 |
| Model 4 | -0.03 | -0.07 to 0.01 | 0.15 |
| Model 5 | -0.02 | -0.06 to 0.02 | 0.28 |
| Outcome: Emotion recognition | |||
| Model 1 | -0.05 | -0.09 to -0.01 | 0.007 |
| Model 2 | -0.01 | -0.05 to 0.03 | 0.57 |
| Model 3 | -0.001 | -0.04 to 0.04 | 0.97 |
| Model 4 | 0.006 | -0.04 to 0.05 | 0.79 |
| Model 5 | 0.01 | -0.03 to 0.05 | 0.53 |
| Outcome: Response inhibition | |||
| Model 1 | 0.05 | 0.01 to 0.08 | 0.011 |
| Model 2 | 0.007 | -0.03 to 0.05 | 0.73 |
| Model 3 | 0.002 | -0.04 to 0.04 | 0.94 |
| Model 4 | -0.01 | -0.05 to 0.03 | 0.62 |
| Model 5 | -0.02 | -0.06 to 0.02 | 0.43 |
| Interleukin-6 (IL-6, normalized protein expression on log2 scale) | |||
| Outcome: Working memory | |||
| Model 1 | -0.05 | -0.08 to -0.01 | 0.017 |
| Model 2 | -0.02 | -0.06 to 0.03 | 0.43 |
| Model 3 | -0.01 | -0.06 to 0.03 | 0.52 |
| Model 4 | -0.001 | -0.04 to 0.04 | 0.95 |
| Model 5 | 0.001 | -0.04 to 0.04 | 0.97 |
| Outcome: Emotion recognition | |||
| Model 1 | -0.05 | -0.09 to -0.02 | 0.003 |
| Model 2 | -0.02 | -0.06 to 0.02 | 0.38 |
| Model 3 | -0.02 | -0.06 to 0.02 | 0.45 |
| Model 4 | -0.005 | -0.05 to 0.04 | 0.82 |
| Model 5 | -0.003 | -0.04 to 0.04 | 0.90 |
| Outcome: Response inhibition | |||
| Model 1 | 0.03 | -0.004 to 0.07 | 0.084 |
| Model 2 | -0.004 | -0.04 to 0.04 | 0.83 |
| Model 3 | -0.006 | -0.05 to 0.03 | 0.79 |
| Model 4 | -0.02 | -0.06 to 0.02 | 0.31 |
| Model 5 | -0.02 | -0.06 to 0.02 | 0.27 |
Multiply imputed models (100 imputations). N = 3,305 individuals who had data on all three cognitive measures at age 24 in ALSPAC. 95 % CI = 95 % Confidence Interval. Model 1: unadjusted; Model 2: adjusted for sex, ethnicity, and BMI at age 24; Model 3: additionally adjusted for maternal education and SES; Model 4: additionally adjusted for smoking and alcohol use at age 24; Model 5: additionally adjusted for IQ at age 8. Exposure and outcome are standardised.
3.2. One-sample bidirectional Mendelian randomization in ALSPAC
3.2.1. MR assumptions
Regression models examined whether genetic risk scores, based on the SNP instrument set, predicted the relevant exposures (circulating levels of inflammatory markers and cognitive performance) in ALSPAC. All instruments had F-statistics > 10 (range:15.8 to 112.1), indicating adequate instrument strength (Table 3) (Burgess and Thompson, 2011, Staiger and Stock, 1997). re-running the analysis removing individuals with high CRP levels (>10 mg/l) did not substantially change the results, suggesting that these individuals are not driving associations.
Table 3.
Association between genetic risk scores and exposures within ALSPAC.
| Outcome | Instrument | F | R2 | N |
|---|---|---|---|---|
| Inflammation | ||||
| Primary analysis | ||||
| Log CRP (mg/l, age 24) | Ligthart et al. (cis) | 26.7 | 1.2 % | 2,222 |
| Han et al. (cis) | 15.8 | 0.7 % | 2,222 | |
| Log IL-6 (pg/ml, age 9) | Ahluwalia et al. (cis) | 87.6 | 2.1 % | 4,184 |
| Rosa et al. (cis) | 76.1 | 1.8 % | 4,184 | |
| GlycA (mmol/l, age 24) | Borges et al. (genome-wide) | 65.7 | 2.7 % | 2,392 |
| Secondary analysis | ||||
| Log CRP (mg/l, age 24) | Ligthart et al. (genome-wide) | 99.9 | 4.3 % | 2,222 |
| Han et al. (genome-wide) | 79.9 | 3.5 % | 2,222 | |
| Log IL-6 (pg/ml, age 9) | Ahluwalia et al. (genome-wide) | 77.3 | 1.8 % | 4,184 |
| Sarwar et al. (cis) | 87.9 | 2.1 % | 4,184 | |
| Swerdlow et al. (cis) | 95.4 | 2.2 % | 4,184 | |
| GlycA (mmol/l, age 24) | Kettunen et al. (genome-wide) | 51.8 | 2.1 % | 2,392 |
| Cognition | ||||
| Working memory (age 24) | Mahedy et al. | 59.7 | 2.3 % | 2,534 |
| Emotion recognition (age 24) | Mahedy et al. | 112.1 | 4.1 % | 2,626 |
| Response inhibition (age 24) | Mahedy et al. | 112.1 | 4.3 % | 2,508 |
Separate regression models examined whether genetic risk scores were associated with potential confounders (Davies et al., 2018, Yang et al., 2022) (Supplementary Table S16). Top 10 genetic principal components were added to all models to adjust for genetic ancestry. There was evidence that one CRP instrument (Han-genome-wide) was associated with maternal education (p=.00006) and alcohol use (p =.015); and one cognition instrument (Mahedy-emotion recognition) was associated with maternal SES (p =.013). This violates one of the MR assumptions and thus caution should be taken when interpreting findings using these two instruments. Evidence for other instruments was weak (Supplementary Table S16).
3.2.2. Potential causal relationships between inflammatory markers and cognitive measures
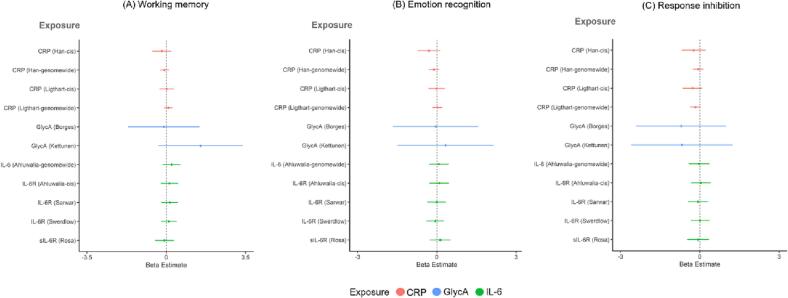
We did not find strong evidence of a causal effect of genetically-proxied CRP, IL-6, IL-6R, sIL-6R or GlycA on standard deviation change in cognitive measures in ALSPAC at age 24 in our primary analysis using cis variants and larger GlycA GWAS (beta range: −0.73 [95 % CI −2.47 to 1.01, p =.41] to 0.21 [95 % CI = −1.42 to 1.84, p =.80]) or secondary analysis using genome-wide significant variants and smaller GWAS (beta range: −0.73 [95 % CI = −2.67 to 1.21, p =.46] to 1.54 [95 % CI = −0.34 to 3.42, p =.11]; N range: 1,677 to 2,193; Fig. 2 and Supplementary Table S17;). There was also not strong evidence of a causal effect of genetically-proxied cognition on standard deviation change in inflammatory markers (ps ≥ 0.19; Supplementary Table S18).
Fig. 2.
One-sample MR: effect of genetically-proxied inflammatory markers on (A) working memory, (B) emotion recognition, and (C) response inhibition at age 24 years. Outcomes are standardised, exposures are not standardised. Points represent beta estimates and 95 % CI. For values, see Supplementary Table S17.
3.3. Two-sample bidirectional Mendelian randomization
3.3.1. Effect of inflammatory markers on general cognitive ability
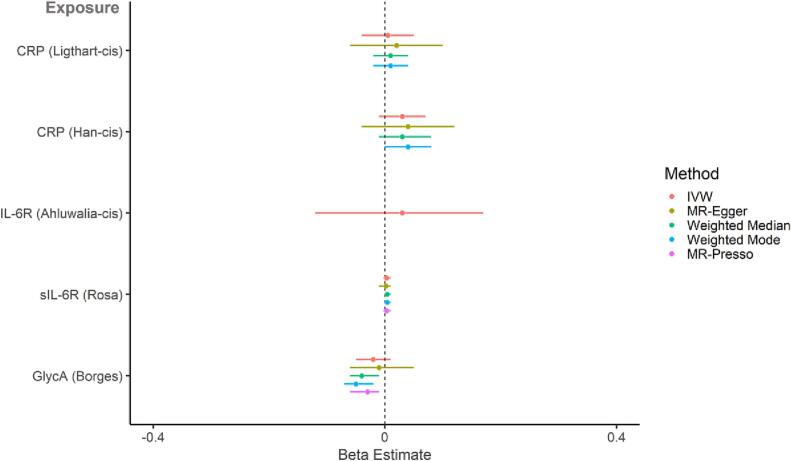
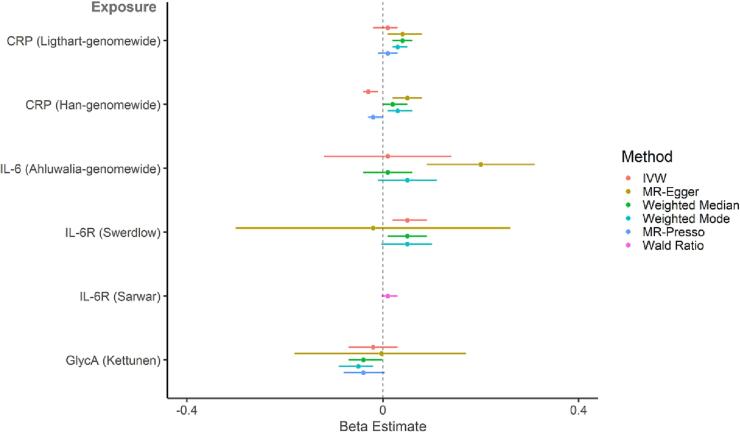
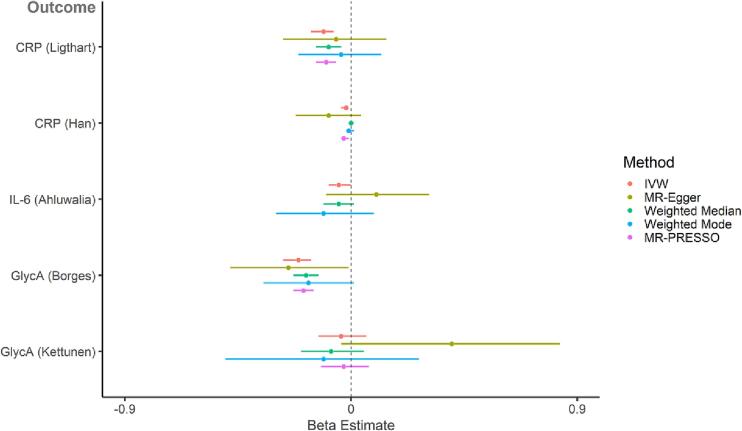
In the primary analysis, there was not strong evidence for a causal effect of genetically-proxied inflammatory markers (CRP, IL-6R, sIL-6R and GlycA) on standard deviation change in GCA (IVW estimates range: −0.02 [95 % CI = −0.05 to 0.01, p =.12] to 0.03 [95 % CI = −0.01 to 0.07, p =.19]; Fig. 3 and Supplementary Table S20). However, there was a pattern towards CRP increasing GCA and GlycA decreasing GCA, which was consistent amongst most sensitivity analyses. In the secondary analyses, there was some evidence for a causal effect of genetically-proxied IL-6R (Swerdlow 3 SNP instrument) on higher cognition (IVW estimate: 0.05 [95 % CI = 0.02 to 0.09, p =.006]) and CRP on poorer cognition (IVW estimate: −0.03 [95 % CI = −0.04 to −0.01, p =.01), although the direction of effects did not replicate across different MR methods (Fig. 4; Supplementary Table S20). Steiger filtering showed that one CRP instrument (Han – 494 genome-wide SNPs) had four invalid variants (i.e., variants had stronger associations with the outcome than the exposure). re-running the analysis with these variants removed did not substantially change the results.
Fig. 3.
Two-sample MR (primary analysis - cis variants for CRP, IL-6R and sIL-6R; larger GlycA GWAS): causal effect of genetically-proxied inflammatory markers on standardised general cognitive ability. Points represent beta estimates and 95 % CI.
Fig. 4.
Two-sample MR (secondary analysis – genome-wide significant variants and smaller GWAS): causal effect of genetically-proxied inflammatory markers on standardised general cognitive ability. Points represent beta estimates and 95 % CI.
3.3.2. Effect of general cognitive ability on inflammatory markers
There was some evidence of a causal effect of higher GCA on lower CRP (Ligthart IVW estimate: −0.11, 95 % CI = −0.16 to −0.07, p <.0001]; Han IVW estimate: −0.02, 95 % CI = −0.04 to −0.01, p =.005), IL-6 (Ahluwalia IVW estimate: −0.05, 95 % CI = −0.09 to −0.002, p =.039) and GlycA (Borges IVW estimate: −0.21, 95 % CI = −0.27 to −0.16, p <.0001) (Fig. 5; Supplementary Table S23). Effect estimates were broadly consistent across sensitivity analyses. These findings are consistent with Steiger filtered results (Supplementary Table S25).
Fig. 5.
Two-sample MR: causal effect of standardised genetically-proxied general cognitive ability on inflammatory markers. Points represents beta estimates and 95 % CI.
3.3.3. Assessment of heterogeneity and horizontal pleiotropy
For most instruments, there was evidence of heterogeneity based on Cochran’s Q-statistic (range: 0.4 to 1634). There was limited evidence of horizontal pleiotropy for inflammation (ps ≥ 0.42) and cognition (ps ≥ 0.048) instruments based on the Egger intercept, although this was not always consistent with MR-PRESSO Global Test results (Supplementary Table S21, S24). Instruments used in secondary analyses revealed evidence of horizontal pleiotropy using the Egger intercept, highlighting the importance of using cis variants which may be less likely to be pleiotropic and therefore may provide more valid instruments. For MR sensitivity plots, see Supplementary Figures S1–S15.
4. Discussion
We examined associations between inflammatory markers and cognition using a large population-based cohort and complementary MR analyses. Our cross-sectional analyses show that GlycA and IL-6, but not CRP, are associated with poorer working memory, emotion recognition, and response inhibition at 24 years. However, this association was fully explained by potential confounders, namely sex, ethnicity, BMI, maternal education, maternal SES, smoking and alcohol use, childhood IQ. In one-sample MR, there was limited evidence of a causal relationship between inflammatory markers (CRP, IL-6, IL-6R, sIL-6R, GlycA) and the same cognitive measures in ALSPAC, although confidence intervals were large. In two-sample MR, we did not find strong evidence of a causal effect of the same inflammatory markers on GCA. However, there was a pattern towards CRP being associated with higher GCA and GlycA being associated with poorer GCA. There was evidence that higher GCA may be causally related to lower inflammation, with the strongest evidence for GlycA.
4.1. Comparison with previous studies
4.1.1. Inflammatory markers and specific cognitive domains
Our MR analyses found limited evidence of a causal relationship between inflammatory markers and cold cognitive domains (working memory and response inhibition) in early adulthood. Recent observational studies report that CRP and IL-6 (age 9) predict working memory performance one year later in ALSPAC (Kokosi et al., 2021, Shields et al., 2021). Unlike the current study, these studies focused on associations in childhood; although there are other differences which may also account for the discrepant findings (e.g., confounders included, a larger sample size). Consistent with our findings, Proitsi and colleagues (2018) did not find associations between GlycA and a related cognitive domain (short-term memory) in late midlife after adjusting for potential confounders (Proitsi et al., 2018). Moreover, experimental studies did not find consistent evidence that acute inflammatory challenges influence working memory in early adulthood (Bollen et al., 2017). As for response inhibition, to our knowledge, few observational studies have examined its association with inflammation. Experimentally, there is limited evidence that acute inflammation influences response inhibition in early adulthood, as assessed by the Stroop and Go/No-Go tests (Bollen et al., 2017, Handke et al., 2020). In relation to the overarching domain of executive functioning, Mac Giollabhui and colleagues (2021) reported that CRP was associated with reduced executive functioning in 43,896 individuals aged 18–93 after adjusting for confounders (Mac Giollabhui et al., 2021b). Importantly, effect sizes were small (as noted by the authors) and performance on the task used may also reflect processing speed (Kuiper et al., 2017).
In relation to hot cognition, our MR analyses found limited evidence in support of a causal relationship between inflammatory markers and emotion recognition. To our knowledge, there are few observational studies that have examined the association between inflammation and emotion processing. Experimentally, there is some evidence that inflammatory challenges are associated with poorer social and emotional processing (Balter et al., 2018, Bollen et al., 2017). In a double-blind placebo controlled cross-over design, Balter and colleagues (2018) reported that acute inflammation induced via a Typhoid vaccination reduced accuracy on the Reading the Mind in the Eyes Test ∼ 6 h post-injection (Balter et al., 2018). However, it is important to note that these studies typically involve higher acute doses of inflammation, which differ from low-grade inflammation examined here.
Collectively, whilst there is evidence of an association between inflammatory markers (CRP, IL-6, GlycA) and poorer cognition (working memory, response inhibition, emotion recognition), our MR analysis did not find strong evidence of causality in young adults. However, given the large confidence intervals in our MR analyses and the small effect sizes observed (consistent with previous studies) (Mac Giollabhui et al., 2021b), our study may be underpowered to detect these effects (see Supplementary methods 1.8 for power calculation). There is therefore a need to further interrogate causality. This requires large GWAS on specific cognitive domains (for well-powered MR studies) and experimental studies with larger sample sizes. Further studies are also required that test causality using different cognitive tasks, inflammatory markers, and at different ages in the lifespan.
4.1.2. Inflammatory markers and general cognitive ability
Our MR analysis did not find strong evidence that inflammatory markers examined here have a causal effect on GCA across a broad age range. Nevertheless, the pattern of associations consistently showed genetically proxied CRP to be associated with higher GCA and genetically proxied GlycA to be associated with poorer GCA, warranting further research. A previous study including 13,000 individuals reported that GlycA was associated with poorer GCA after adjusting for cardiometabolic correlates of cognition (Van der Lee et al., 2018). In addition, Conole and colleagues (2021) report that a DNA methylation signature of CRP was associated with poorer GCA in elderly individuals (aged 72) (Conole et al., 2021). Whilst these studies provide evidence that CRP and GlycA are associated with poorer GCA, they do not establish causality, as noted by the authors. Consequently, it is possible that potential confounders or reverse causality may account for these findings. A recent MR study that explored a broad range of inflammatory markers on cognitive functioning reported three cytokines (Eotaxin, Interleukin-8 and Monocyte chemotactic protein 1) were associated with higher fluid intelligence and Interleukin-4 associated with lower fluid intelligence (Pagoni et al., 2022). Consistent with our MR analyses, there was limited evidence to suggest a causal effect of IL-6 on fluid intelligence; other inflammatory measures (CRP and GlycA) were not examined (Pagoni et al., 2022). Therefore, although MR analyses report limited evidence of a causal effect of the inflammatory markers examined here, there is some evidence for other inflammatory markers. Taken together, few studies have examined potential causal effects of inflammatory markers on GCA across a broad age range. Given the pattern of findings in our study, further studies investigating the role of these inflammatory markers on cognition are needed.
There was evidence that GCA may be causally related to lower inflammation, with the strongest evidence for GlycA. Arguably, this is not surprising as measures closely related to GCA (higher educational attainment and SES) are associated with lower levels of CRP and IL-6 (Loucks et al., 2010, Muscatell et al., 2018, Nazmi and Victora, 2007, O’Connor et al., 2009, Pollitt et al., 2007). One potential mechanism through which GCA may impact inflammation is via health-related behaviour (e.g., physical activity, smoking): individuals with higher GCA may be more likely to engage in healthier lifestyle choices (e.g., less likely to smoke) which may result in lower levels of inflammation (Davies et al., 2019). Importantly, as GCA is associated with higher SES/education, this may provide people with the means to engage in healthier lifestyle choices (e.g., via higher income) (Friedman & Herd, 2010). An alternative mechanism could involve shared biological pathways linking inflammation and cognition (Zuber et al., 2022). However, this is less likely as we only observe the effect in one direction (GCA on inflammation, not vice versa). A third possible explanation is that the results are due to chance (Type I error) and do not reflect a true casual effect. Further studies are needed to explore possible mechanisms of this relationship.
5. Strengths and limitations
A key strength of this study is the triangulation of findings using complementary analyses (cohort and MR) which provides increased confidence in our inferences drawn. We also used large population-based data, increasing statistical power and generalisability of our findings. Additionally, we examined several inflammatory markers, including a novel marker (GlycA) which may better reflect chronic inflammation. Moreover, in the cohort analyses we considered many potential confounders and in the MR analysis, we used strong instrumental variables making weak instrument bias unlikely and checked key assumptions with no primary instruments being associated with potential confounders.
A limitation is that similar to other cohorts, ALSPAC is less representative of some populations (e.g., less affluent families, ethnic minority individuals). Future studies should examine whether findings generalise in cohorts which have a higher proportion of under-represented populations. Also, there is non-random attrition within ALSPAC (Wolke et al., 2009), which may lead to bias. For example, if inflammation and cognition are related to attrition, results may be biased towards the null. Whilst we find some evidence that IQ scores are related to attrition, evidence for inflammatory markers is less consistent (see Supplementary Table S10). Second, many cognitive tasks (including those used here) have poor-to-moderate test–retest reliability therefore measurement error may mask potential associations by increasing confidence intervals (Hedge et al., 2018). Third, we relied on single measures of inflammatory markers which exhibit high intra-individual variability (Conole et al., 2021). However, we did include a novel inflammatory marker (GlycA) which is thought to provide a more stable measure of inflammation (Connelly et al., 2017, Ritchie et al., 2015). Fourth, MR focuses on lifetime effects of inflammation on cognition. This is informative for understanding lifetime risk of an exposure, but cannot discern effects at specific ages (i.e., potential sensitive periods). Experimental research and MR studies with genetic variants associated with inflammatory markers at specific ages (e.g., early childhood, adulthood) are required to address this temporal aspect. Fifth, we focus on a subset of cognitive domains and inflammatory markers. Future studies should examine other domains (e.g., executive functioning, reward processing) and inflammatory markers (Boyle et al., 2019, Mac Giollabhui et al., 2021a). Additionally, some instruments used in the MR analyses had few SNPs, displayed evidence of heterogeneity, and/or were associated with potential confounders. Nevertheless, there was limited evidence of horizontal pleiotropy (MR-Egger intercept) or associations with potential confounders in primary analyses using cis variants.
5.1. Implications
Our study highlights the need for large-scale GWAS on specific cognitive domains. This will enable well-powered MR studies to examine causal relationships between inflammation and specific cognitive domains. Additionally, our MR analyses found limited evidence that the inflammatory markers CRP, IL-6, IL-6R, sIL-6R and GlycA influence cognition (working memory, response inhibition, emotion recognition, GCA); suggesting that they may not be good intervention targets for poorer cognition (although further evidence is needed to determine this). We also found a potential causal effect of GCA on inflammation and highlight the need for mechanistic studies investigating this relationship. Future studies should also examine potential moderators of the relationship between inflammation and cognition. For example, conditions which impact the immune system, brain, and/or pathways linking the peripheral immune system to the brain (e.g., disruptions to the neural pathway via afferent nerves, humoral pathway, blood–brain barrier) (Dantzer et al., 2008).
6. Conclusions
In summary, we used cross-sectional association and MR analyses to examine the association and potential causal relationship between inflammatory markers and cognition (general and domain-specific) using data from a large population-based cohort (ALSPAC) and publicly available GWAS. The MR analyses did not find strong evidence for a causal effect of inflammatory markers (CRP, IL-6, GlycA) on specific cognitive domains in young adults in the ALSPAC cohort (working memory, response inhibition, emotion recognition) or GCA. There was some evidence that GCA may be causally related to lower inflammation. There is a need for larger GWAS on specific cognitive domains and experimental studies with larger sample sizes to further interrogate causality.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The CHARGE Inflammation Working Group conducted GWAS of inflammation (CRP and IL-6) used in this study. MM is co-director of Jericoe Ltd, which produces software for the assessment and modification of emotion perception. The authors report no other biomedical financial interests or potential conflicts of interest. GDS reports Scientific Advisory Board Membership for Relation Therapeutics and Insitro.
Acknowledgments
Acknowledgements
This work is supported by the Elizabeth Blackwell Institute for Health Research, University of Bristol, and the Wellcome Trust Institutional Strategic Support Fund (204813/Z/16/Z). This research was funded in whole, or in part, by the Wellcome Trust [Grant number 204813/Z/16/Z]. For the purpose of open access, the authors have applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
This work was also supported by the Medical Research Council (MRC) Integrative Epidemiology Unit at the University of Bristol (MC_UU_00011/1, MC_UU_00011/3, MC_UU_00011/7). GMK acknowledges funding support from the Wellcome Trust (Grant No. 201486/Z/16/Z), and the UK Medical Research Council (Grant No. MC_PC_17213, Grant No. MR/S037675/1, and Grant No. MR/W014416/1). HJ is supported by the NIHR Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. GDS and KT work in the MRC Integrative Epidemiology Unit at the University of Bristol which is supported by the Medical Research Council (MC_UU_00011/1 and MC_UU_00011/3). CD is supported by an MRC Integrative Epidemiology Unit Fellowship (MC_UU_00011/1).
The UK Medical Research Council and Wellcome (Grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and CS will serve as a guarantor for the contents of this paper. A comprehensive list of grants funding is available on the ALSPC website (grant-acknowledgements.pdf (bristol.ac.uk); this research was specifically funded by Wellcome Trust and MRC (Grant Ref: 076467/Z/05/Z; MR/L022206/1). GWAS data was generated by Sample Logistics and Genotyping Facilities at Wellcome Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe.
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Members of the CHARGE Inflammation Working Group are Emelia Benjamin, Daniel I. Chasman, Abbas Dehghan, Tarunveer Singh Ahluwalia, James Meigs, Russell Tracy, Behrooz Z. Alizadeh, Symen Ligthart, Josh Bis, Gudny Eiriksdottir, Nathan Pankratz, Myron Gross, Alex Rainer, Harold Snieder, James G. Wilson, Bruce M. Psaty, Josee Dupuis, Bram Prins, Urmo Vaso, Maria Stathopoulou, Lude Franke, Terho Lehtimaki, Wolfgang Koenig, Yalda Jamshidi, Sophie Siest, Ali Abbasi, Andre G. Uitterlinden, Mohammadreza Abdollahi, Renate Schnabel, Ursula M. Schick, Ilja M. Nolte, Aldi Kraja, Yi-Hsiang Hsu, Daniel S. Tylee, Alyson Zwicker, Rudolf Uher, George Davey Smith, Alanna C. Morrison, Andrew Hicks, Cornelia M. van Duijn, Cavin Ward-Caviness, Eric Boerwinkle, J. Rotter, Ken Rice, Leslie Lange, Markus Perola, Eco de Geus, Andrew P. Morris, Kari Matti Makela, David Stacey, Johan Eriksson, Tim M. Frayling, and Eline P. Slagboom.
Ethics statement
Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee (http://www.bristol.ac.uk/alspac/researchers/research-ethics/) and the Local Research Ethics Committees. Consent for biological samples has been collected in accordance with the Human Tissue Act (2004). Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time. ALSPAC study data were collected and managed using REDCap electronic data capture tools hosted at the University of Bristol (Harris et al., 2009). REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2023.02.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- Ahluwalia T.S., Prins B.P., Abdollahi M., Armstrong N.J., Aslibekyan S., Bain L., Jefferis B., Baumert J., Beekman M., Ben-Shlomo Y., Bis J.C., Mitchell B.D., de Geus E., Delgado G.E., Marek D., Eriksson J., Kajantie E., Kanoni S., Kemp J.P., Alizadeh B.Z. Genome-wide association study of circulating interleukin 6 levels identifies novel loci. Hum. Mol. Genet. 2021;30(5):393–409. doi: 10.1093/hmg/ddab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balter L.J.T., Hulsken S., Aldred S., Drayson M.T., Higgs S., Veldhuijzen van Zanten J.J.C.S., Raymond J.E., Bosch J.A. Low-grade inflammation decreases emotion recognition – Evidence from the vaccination model of inflammation. Brain Behav. Immun. 2018;73:216–221. doi: 10.1016/J.BBI.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Bogaty P., Dagenais G.R., Joseph L., Boyer L., Leblanc A., Bélisle P., Brophy J.M. Time Variability of C-Reactive Protein: Implications for Clinical Risk Stratification. PLoS One. 2013;8(4):e60759. doi: 10.1371/journal.pone.0060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen J., Trick L., Llewellyn D., Dickens C. The effects of acute inflammation on cognitive functioning and emotional processing in humans: A systematic review of experimental studies. J. Psychosom. Res. 2017;94:47–55. doi: 10.1016/J.JPSYCHORES.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Bolt L.K., Amminger G.P., Farhall J., McGorry P.D., Nelson B., Markulev C., Yuen H.P., Schäfer M.R., Mossaheb N., Schlögelhofer M., Smesny S., Hickie I.B., Berger G.E., Chen E.Y.H., de Haan L., Nieman D.H., Nordentoft M., Riecher-Rössler A., Verma S., Allott K.A. Neurocognition as a predictor of transition to psychotic disorder and functional outcomes in ultra-high risk participants: Findings from the NEURAPRO randomized clinical trial. Schizophr. Res. 2019;206:67–74. doi: 10.1016/j.schres.2018.12.013. [DOI] [PubMed] [Google Scholar]
- Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden J., Davey Smith G., Haycock P.C., Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Golding J., Macleod J., Lawlor D.A., Fraser A., Henderson J., Molloy L., Ness A., Ring S., Davey Smith G. Cohort Profile: The ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2013;42(1):111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle C.C., Kuhlman K.R., Dooley L.N., Haydon M.D., Robles T.F., Ang Y.S., Pizzagalli D.A., Bower J.E. Inflammation and dimensions of reward processing following exposure to the influenza vaccine. Psychoneuroendocrinology. 2019;102:16–23. doi: 10.1016/J.PSYNEUEN.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L., Harrison N.A., Walker C., Steptoe A., Critchley H.D. Peripheral Inflammation is Associated with Altered Substantia Nigra Activity and Psychomotor Slowing in Humans. Biol. Psychiatry. 2008;63(11):1022–1029. doi: 10.1016/J.BIOPSYCH.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S., Thompson S.G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011;40(3):755–764. doi: 10.1093/IJE/DYR036. [DOI] [PubMed] [Google Scholar]
- Burgess S., Davies N.M., Thompson S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016;40(7):597. doi: 10.1002/GEPI.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin C.M., Batty G.D., Der G., Brett C.E., Taylor A., Pattie A., Čukić I., Deary I.J. Childhood intelligence in relation to major causes of death in 68 year follow-up: prospective population study. BMJ. 2017;357 doi: 10.1136/BMJ.J2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly M.A., Otvos J.D., Shalaurova I., Playford M.P., Mehta N.N. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J. Transl. Med. 2017;15(1) doi: 10.1186/S12967-017-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conole, E. L. S., Stevenson, A. J., Maniega, S. M., Harris, S. E., Green, C., Valdés Hernández, M. D. C., Harris, M. A., Bastin, M. E., Wardlaw, J. M., Deary, I. J., Miron, V. E., Whalley, H. C., Marioni, R. E., & Cox, S. R. (2021). DNA Methylation and Protein Markers of Chronic Inflammation and Their Associations With Brain and Cognitive Aging. Neurology, 97(23), e2340–e2352. 10.1212/WNL.0000000000012997. [DOI] [PMC free article] [PubMed]
- Cullen A.E., Tappin B.M., Zunszain P.A., Dickson H., Roberts R.E., Nikkheslat N., Khondoker M., Pariante C.M., Fisher H.L., Laurens K.R. The relationship between salivary C-reactive protein and cognitive function in children aged 11–14 years: Does psychopathology have a moderating effect? Brain Behav. Immun. 2017;66:221–229. doi: 10.1016/J.BBI.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming T.B., Brodtmann A., Darby D., Bernhardt J. The importance of cognition to quality of life after stroke. J. Psychosom. Res. 2014;77(5):374–379. doi: 10.1016/J.JPSYCHORES.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Dalili M.N., Penton-Voak I.S., Harmer C.J., Munafò M.R. Meta-analysis of emotion recognition deficits in major depressive disorder. Psychol. Med. 2015;45(6):1135–1144. doi: 10.1017/S0033291714002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam V.H., Stenbæk D.S., Köhler-Forsberg K., Ip C., Ozenne B., Sahakian B.J., Knudsen G.M., Jørgensen M.B., Frokjaer V.G. Hot and cold cognitive disturbances in antidepressant-free patients with major depressive disorder: a NeuroPharm study. Psychol. Med. 2021;51(14):2347–2356. doi: 10.1017/S0033291720000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46. doi: 10.1038/NRN2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith G., Ebrahim S. “Mendelian randomization”: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003;32(1):1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- Davies N.M., Holmes M.V., Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (Clinical Research Ed.) 2018;362 doi: 10.1136/BMJ.K601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.M., Hill W.D., Anderson E.L., Sanderson E., Deary I.J., Smith G.D. Multivariable two-sample Mendelian randomization estimates of the effects of intelligence and education on health. Elife. 2019;8 doi: 10.7554/ELIFE.43990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary I.J., Strand S., Smith P., Fernandes C. Intelligence and educational achievement. Intelligence. 2007;35(1):13–21. doi: 10.1016/J.INTELL.2006.02.001. [DOI] [Google Scholar]
- Fard M.T., Savage K.M., Stough C.K. Peripheral inflammation marker relationships to cognition in healthy older adults – A systematic review. Psychoneuroendocrinology. 2022;144 doi: 10.1016/J.PSYNEUEN.2022.105870. [DOI] [PubMed] [Google Scholar]
- Fraser, A., Macdonald-wallis, C., Tilling, K., Boyd, A., Golding, J., Davey smith, G., Henderson, J., Macleod, J., Molloy, L., Ness, A., Ring, S., Nelson, S. M., & Lawlor, D. A. (2013). Cohort profile: The avon longitudinal study of parents and children: ALSPAC mothers cohort. International Journal of Epidemiology, 42(1), 97–110. 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed]
- Friedman E.M., Herd P. Income, Education, and Inflammation: Differential Associations in a National Probability Sample (The MIDUS Study) Psychosom. Med. 2010;72(3):290. doi: 10.1097/PSY.0B013E3181CFE4C2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Deste G., Smieskova R., Barlati S., Yung A.R., Howes O., Stieglitz R.D., Vita A., McGuire P., Borgwardt S. Cognitive functioning in prodromal psychosis: A meta-analysis. Arch. Gen. Psychiatry. 2012;69(6):562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Hammerton G., Munafò M.R. Causal inference with observational data: the need for triangulation of evidence. Psychol. Med. 2021;51(4):563–578. doi: 10.1017/S0033291720005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Ong J.S., An J., Hewitt A.W., Gharahkhani P., MacGregor S. Using Mendelian randomization to evaluate the causal relationship between serum C-reactive protein levels and age-related macular degeneration. Eur. J. Epidemiol. 2020;35(2):139–146. doi: 10.1007/S10654-019-00598-Z. [DOI] [PubMed] [Google Scholar]
- Handke A., Axelsson J., Benson S., Boy K., Weskamp V., Hasenberg T., Remy M., Hebebrand J., Föcker M., Brinkhoff A., Unteroberdörster M., Engler H., Schedlowski M., Lasselin J. Acute inflammation and psychomotor slowing: Experimental assessment using lipopolysaccharide administration in healthy humans. Brain, Behavior, & Immunity - Health. 2020;8 doi: 10.1016/J.BBIH.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N.A., Doeller C.F., Voon V., Burgess N., Critchley H.D. Peripheral Inflammation Acutely Impairs Human Spatial Memory via Actions on Medial Temporal Lobe Glucose Metabolism. Biol. Psychiatry. 2014;76(7):585–593. doi: 10.1016/J.BIOPSYCH.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig F.P., Davey Smith G., Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017;46(6):1985–1998. doi: 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedge C., Powell G., Sumner P. The reliability paradox: Why robust cognitive tasks do not produce reliable individual differences. Behav. Res. Methods. 2018;50(3):1166–1186. doi: 10.3758/s13428-017-0935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemani G., Tilling K., Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13(11):e1007081. doi: 10.1371/journal.pgen.1007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemani G., Zheng J., Elsworth B., Wade K.H., Haberland V., Baird D., Laurin C., Burgess S., Bowden J., Langdon R., Tan V.Y., Yarmolinsky J., Shihab H.A., Timpson N.J., Evans D.M., Relton C., Martin R.M., Davey Smith G., Gaunt T.R., Haycock P.C. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018;7 doi: 10.7554/ELIFE.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins M.C., Kesler S.R., Ahles T.A., Morrow G.R. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry. 2014;26(1):102–113. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen J., Demirkan A., Würtz P., Draisma H.H.M., Haller T., Rawal R., Vaarhorst A., Kangas A.J., Lyytikäinen L.P., Pirinen M., Pool R., Sarin A.P., Soininen P., Tukiainen T., Wang Q., Tiainen M., Tynkkynen T., Amin N., Zeller T., Ala-Korpela M. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat. Commun. 2016;7(1):1–9. doi: 10.1038/ncomms11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Zammit S., Burgess S., Lewis G., Jones P.B. Association between a functional interleukin 6 receptor genetic variant and risk of depression and psychosis in a population-based birth cohort. Brain Behav. Immun. 2018;69:264–272. doi: 10.1016/j.bbi.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner W.K. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 1958;55(4):352–358. doi: 10.1037/h0043688. [DOI] [PubMed] [Google Scholar]
- Kleiber C., Zeileis A. Springer-Verlag; 2008. Applied Econometrics with R. [Google Scholar]
- Kokosi T., Flouri E., Midouhas E. The role of inflammation in the association between poverty and working memory in childhood. Psychoneuroendocrinology. 2021;123 doi: 10.1016/J.PSYNEUEN.2020.105040. [DOI] [PubMed] [Google Scholar]
- Kuiper J.S., Oude Voshaar R.C., Verhoeven F.E.A., Zuidema S.U., Smidt N. Comparison of cognitive functioning as measured by the Ruff Figural Fluency Test and the CogState computerized battery within the LifeLines Cohort Study. BMC Psychology. 2017;5(1) doi: 10.1186/S40359-017-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M., Chen C.-Y., Ge T., Xia Y., Hill D.W., Trampush J.W., Yu J., Knowles E., Davies G., Stahl E.A., Huckins L., Liewald D.C., Djurovic S., Melle I., Christoforou A., Reinvang I., DeRosse P., Lundervold A.J., Steen V.M., Lencz T. Identifying nootropic drug targets via large-scale cognitive GWAS and transcriptomics. Neuropsychopharmacology. 2021;17:47. doi: 10.1038/s41386-021-01023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D.A. Commentary: Two-sample Mendelian randomization: opportunities and challenges. Int. J. Epidemiol. 2016;45(3):908. doi: 10.1093/IJE/DYW127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D.A., Tilling K., Smith G.D. Triangulation in aetiological epidemiology. Int. J. Epidemiol. 2016;45(6):1866–1886. doi: 10.1093/IJE/DYW314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligthart S., Vaez A., Võsa U., Stathopoulou M.G., de Vries P.S., Prins B.P., Van der Most P.J., Tanaka T., Naderi E., Rose L.M., Wu Y., Karlsson R., Barbalic M., Lin H., Pool R., Zhu G., Macé A., Sidore C., Trompet S., Alizadeh B.Z. Genome Analyses of >200,000 Individuals Identify 58 Loci for Chronic Inflammation and Highlight Pathways that Link Inflammation and Complex Disorders. Am. J. Hum. Genet. 2018;103(5):691–706. doi: 10.1016/j.ajhg.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan G.D., Cowan W.B., Davis K.A. On the ability to inhibit simple and choice reaction time responses: A model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984;10(2):276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Loucks E.B., Pilote L., Lynch J.W., Richard H., Almeida N.D., Benjamin E.J., Murabito J.M. Life course socioeconomic position is associated with inflammatory markers: The Framingham Offspring Study. Soc Sci Med. 2010;71(1):187–195. doi: 10.1016/J.SOCSCIMED.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Giollabhui N., Ellman L.M., Coe C.L., Byrne M.L., Abramson L.Y., Alloy L.B. To exclude or not to exclude: considerations and recommendations for C-Reactive Protein values higher than 10 mg/L. Brain Behav. Immun. 2020;87:898. doi: 10.1016/J.BBI.2020.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Giollabhui N., Swistun D., Murray S., Moriarity D.P., Kautz M.M., Ellman L.M., Olino T.M., Coe C.L., Abramson L.Y., Alloy L.B. Executive dysfunction in depression in adolescence: the role of inflammation and higher body mass. Psychol. Med. 2020;50(4):683–691. doi: 10.1017/S0033291719000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Giollabhui N., Alloy L.B., Hartman C.A. Investigating whether depressed youth exhibiting elevated C reactive protein perform worse on measures of executive functioning, verbal fluency and episodic memory in a large, population based sample of Dutch adolescents. Brain Behav. Immun. 2021;94:369–380. doi: 10.1016/J.BBI.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Giollabhui N., Alloy L.B., Schweren L.J.S., Hartman C.A. Investigating whether a combination of higher CRP and depression is differentially associated with worse executive functioning in a cohort of 43,896 adults. Brain Behav. Immun. 2021;96:127–134. doi: 10.1016/J.BBI.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madley-Dowd P., Hughes R., Tilling K., Heron J. The proportion of missing data should not be used to guide decisions on multiple imputation. J. Clin. Epidemiol. 2019;110:63–73. doi: 10.1016/j.jclinepi.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahedy L., Suddell S., Skirrow C., Fernandes G.S., Field M., Heron J., Hickman M., Wootton R., Munafò M.R. Alcohol use and cognitive functioning in young adults: improving causal inference. Addiction. 2021;116(2):292–302. doi: 10.1111/add.15100. [DOI] [PubMed] [Google Scholar]
- Milton D.C., Ward J., Ward E., Lyall D.M., Strawbridge R.J., Smith D.J., Cullen B. The association between C-reactive protein, mood disorder, and cognitive function in UK Biobank. Eur. Psychiatry. 2021;64(1) doi: 10.1192/J.EURPSY.2021.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak B., Stańczykiewicz B., Kotowicz K., Rybakowski J.K., Samochowiec J., Frydecka D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: A systematic review. Schizophr. Res. 2018;192:16–29. doi: 10.1016/j.schres.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Morrens M., Overloop C., Coppens V., Loots E., Van Den Noortgate M., Vandenameele S., Leboyer M., De Picker L. The relationship between immune and cognitive dysfunction in mood and psychotic disorder: a systematic review and a meta-analysis. Mol. Psychiatry. 2022:1–10. doi: 10.1038/s41380-022-01582-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Brosso S.N., Humphreys K.L. Socioeconomic status and inflammation: a meta-analysis. Mol. Psychiatry. 2018;25(9):2189–2199. doi: 10.1038/s41380-018-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazmi A., Victora C.G. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7 doi: 10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolin S., Tan Y.Y., Schwaab A., Moffa A., Loo C.K., Martin D. An investigation of working memory deficits in depression using the n-back task: A systematic review and meta-analysis. J. Affect. Disord. 2021;284:1–8. doi: 10.1016/j.jad.2021.01.084. [DOI] [PubMed] [Google Scholar]
- Northstone K., Lewcock M., Groom A., Boyd A., Macleod J., Timpson N., Wells N. The Avon Longitudinal Study of Parents and Children (ALSPAC): an update on the enrolled sample of index children in 2019. Wellcome Open Research. 2019;4 doi: 10.12688/wellcomeopenres.15132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M.F., Bower J.E., Cho H.J., Creswell J.D., Dimitrov S., Hamby M.E., Hoyt M.A., Martin J.L., Robles T.F., Sloan E.K., Thomas K.M.S., Irwin M.R. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun. 2009;23(7):887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos J.D., Shalaurova I., Wolak-Dinsmore J., Connelly M.A., Mackey R.H., Stein J.H., Tracy R.P. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin. Chem. 2015;61(5):714–723. doi: 10.1373/CLINCHEM.2014.232918. [DOI] [PubMed] [Google Scholar]
- Pagoni P., Korologou-Linden R.S., Howe L.D., Davey Smith G., Ben-Shlomo Y., Stergiakouli E., Anderson E.L. Causal effects of circulating cytokine concentrations on risk of Alzheimer’s disease and cognitive function. Brain Behav. Immun. 2022;104:54–64. doi: 10.1016/J.BBI.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z., Grovu R.C., Cha D.S., Carmona N.E., Subramaniapillai M., Shekotikhina M., Rong C., Lee Y., McIntyre R.S. Pharmacological Treatment of Cognitive Symptoms in Major Depressive Disorder. CNS & Neurological Disorders Drug Targets. 2017;16(8) doi: 10.2174/1871527316666170919115100. [DOI] [PubMed] [Google Scholar]
- Penton-Voak I.S., Bate H., Lewis G., Munafò M.R. Effects of emotion perception training on mood in undergraduate students: Randomised controlled trial. Br. J. Psychiatry. 2012;201(1):71–72. doi: 10.1192/bjp.bp.111.107086. [DOI] [PubMed] [Google Scholar]
- Pollitt R.A., Kaufman J.S., Rose K.M., Diez-Roux A.V., Zeng D., Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. Eur. J. Epidemiol. 2007;22(1):55–66. doi: 10.1007/S10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Proitsi P., Kuh D., Wong A., Maddock J., Bendayan R., Wulaningsih W., Hardy R., Richards M. Lifetime cognition and late midlife blood metabolites: findings from a British birth cohort. Transl. Psychiatry. 2018;8(1):1–11. doi: 10.1038/s41398-018-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., Maller J., Sklar P., De Bakker P.I.W., Daly M.J., Sham P.C. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007;81(3):559. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . In R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A language and environment for statistical computing. [Google Scholar]
- Ritchie S.C., Würtz P., Nath A.P., Abraham G., Havulinna A.S., Fearnley L.G., Sarin A.P., Kangas A.J., Soininen P., Aalto K., Seppälä I., Raitoharju E., Salmi M., Maksimow M., Männistö S., Kähönen M., Juonala M., Ripatti S., Lehtimäki T., Inouye M. The Biomarker GlycA Is Associated with Chronic Inflammation and Predicts Long-Term Risk of Severe Infection. Cell Syst. 2015;1(4):293–301. doi: 10.1016/J.CELS.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Rock P.L., Roiser J.P., Riedel W.J., Blackwell A.D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol. Med. 2014;44(10):2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- Roiser J.P., Sahakian B.J. Hot and cold cognition in depression. CNS Spectr. 2013;18(3):139–149. doi: 10.1017/S1092852913000072. [DOI] [PubMed] [Google Scholar]
- Rosa M., Chignon A., Li Z., Boulanger M.C., Arsenault B.J., Bossé Y., Thériault S., Mathieu P. A Mendelian randomization study of IL6 signaling in cardiovascular diseases, immune-related disorders and longevity. NPJ Genom. Med. 2019;4(1):1–10. doi: 10.1038/s41525-019-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson E., Glymour M.M., Holmes M.V., Kang H., Morrison J., Munafò M.R., Palmer T., Schooling C.M., Wallace C., Zhao Q., Davey Smith G. Mendelian randomization. Nature Reviews Methods Primers. 2022;2(1):1–21. doi: 10.1038/s43586-021-00092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori A.C., Vance D.E., Slater L.Z., Crowe M. The impact of inflammation on cognitive function in older adults: Implications for healthcare practice and research. J. Neurosci. Nurs. 2012;44(4):206–217. doi: 10.1097/JNN.0b013e3182527690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar N., Butterworth A.S., Freitag D.F., Gregson J., Willeit P., Gorman D.N., Gao P., Saleheen D., Rendon A., Nelson C.P., Braund P.S., Hall A.S., Chasman D.I., Tybjærg-Hansen A., Chambers J.C., Benjamin E.J., Franks P.W., Clarke R., Wilde A.A.M., Buring J. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet. 2012;379(9822):1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F.L., Hunter J. General Mental Ability in the World of Work: Occupational Attainment and Job Performance. J. Pers. Soc. Psychol. 2004;86(1):162–173. doi: 10.1037/0022-3514.86.1.162. [DOI] [PubMed] [Google Scholar]
- Shields G.S., Deer L.K., Hastings P.D., Hostinar C.E. Adiposity, inflammation, and working memory: Evidence for a vicious cycle. Brain, Behavior, & Immunity - Health. 2021;13 doi: 10.1016/J.BBIH.2021.100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger D., Stock J.H. Instrumental Variables Regression with Weak Instruments. Econometrica. 1997;65(3):557. doi: 10.2307/2171753. [DOI] [Google Scholar]
- StataCorp. (2019). Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.
- Strenze T. Intelligence and socioeconomic success: A meta-analytic review of longitudinal research. Intelligence. 2007;35(5):401–426. doi: 10.1016/J.INTELL.2006.09.004. [DOI] [Google Scholar]
- Swerdlow D.I., Holmes M.V., Kuchenbaecker K.B., Engmann J.E.L., Shah T., Sofat R., Guo Y., Chung C., Peasey A., Pfister R., Mooijaart S.P., Ireland H.A., Leusink M., Langenberg C., Li K., Palmen J., Howard P., Cooper J.A., Drenos F., Casas J.P. The interleukin-6 receptor as a target for prevention of coronary heart disease: A mendelian randomisation analysis. Lancet. 2012;379(9822):1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A.E., Jones H.J., Sallis H., Euesden J., Stergiakouli E., Davies N.M., Zammit S., Lawlor D.A., Munafò M.R., Davey Smith G., Tilling K. Exploring the association of genetic factors with participation in the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2018;47(4):1207–1216. doi: 10.1093/ije/dyy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Lee S.J., Teunissen C.E., Pool R., Shipley M.J., Teumer A., Chouraki V., Melo van Lent D., Tynkkynen J., Fischer K., Hernesniemi J., Haller T., Singh-Manoux A., Verhoeven A., Willemsen G., de Leeuw F.A., Wagner H., van Dongen J., Hertel J., Budde K., van Duijn C.M. Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimer’s and Dementia. 2018;14(6):707–722. doi: 10.1016/j.jalz.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Van Dyk K., Ganz P.A. Cancer-related cognitive impairment in patients with a history of breast cancer. JAMA Insights. 2021;326(17):1736–1737. doi: 10.1001/jama.2021.13309. [DOI] [PubMed] [Google Scholar]
- Verbanck M., Chen C.-Y., Neale B., Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011;30(4):377–399. doi: 10.1002/SIM.4067. [DOI] [PubMed] [Google Scholar]
- Wolke D., Waylen A., Samara M., Steer C., Goodman R., Ford T., Lamberts K. Selective drop-out in longitudinal studies and non-biased prediction of behaviour disorders. Br. J. Psychiatry. 2009;195(3):249–256. doi: 10.1192/BJP.BP.108.053751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Sanderson E., Tilling K., Borges M.C., Lawlor D.A. Exploring and mitigating potential bias when genetic instrumental variables are associated with multiple non-exposure traits in Mendelian randomization. Eur. J. Epidemiol. 2022 doi: 10.1007/s10654-022-00874-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber V., Grinberg N., Gill D., Manipur I., Slob E.A., Patel A., Wallace C., Burgess S. Combining evidence from Mendelian randomization and colocalization: Review and comparison of approaches. Am. J. Hum. Genet. 2022;109(5):767–782. doi: 10.1016/j.ajhg.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.