Abstract
Following the commodity risk assessments of bonsai plants from China consisting of Pinus parviflora grafted on P. thunbergii performed by EFSA, the EFSA Plant Health Panel performed a pest categorisation of Pestalotiopsis disseminata, a clearly defined plant pathogenic fungus of the family Pestalotiopsidaceae. The pathogen has been reported on herbaceous, woody and ornamental plants causing symptoms such as leaf blight, shoot blight, seedling blight, pod canker, pre‐ and post‐harvest fruit rot, and gummosis. Moreover, the fungus was reported as an endophyte on a wide range of asymptomatic hosts. The pathogen is present in Africa, North and South America, Asia, Europe and Oceania. It has been reported from the EU, with a restricted distribution (Portugal). There is a key uncertainty on the geographical distribution of P. disseminata in the EU and worldwide, because of the endophytic nature of the fungus, the lack of surveys and since the pathogen might have been misidentified based only on morphology and pathogenicity tests. The pathogen is not included in Commission Implementing Regulation (EU) 2019/2072. This pest categorisation focuses on those hosts that are relevant for the EU and for which there is robust evidence that the pathogen was formally identified by a combination of morphology, pathogenicity and multilocus sequence analysis. Plants for planting, fresh fruits, bark and wood of host plants as well as soil and other plant growing media are the main pathways for the entry of the pathogen into the EU. Host availability and climate suitability factors occurring in parts of the EU are favourable for the establishment of the pathogen. Despite the low aggressiveness observed in most reported hosts, and the fact that P. disseminata may colonise plants as an endophyte, its introduction and spread in the EU may have an economic and environmental impact (with a key uncertainty) where susceptible hosts are grown. Phytosanitary measures are available to prevent the introduction and spread of the pathogen. The Panel cannot conclude on whether P. disseminata satisfies all the criteria that are within the remit of EFSA to assess for this species to be regarded as potential Union quarantine pest, because of the key uncertainties on the restricted distribution in the EU and the magnitude of the impact.
Keywords: endophytes, eucalyptus leaf spot, Euonymus japonicus, pest risk, pine shoot blight, plant pest, Psidium guava
1. INTRODUCTION
1.1. Background and Terms of Reference as provided by the Requestor
1.1.1. Background
The new Plant Health Regulation (EU) 2016/2031, on the protective measures against pests of plants, is applying from 14 December 2019. Conditions are laid down in this legislation in order for pests to qualify for listing as Union quarantine pests, protected zone quarantine pests or Union regulated non‐quarantine pests. The lists of the EU regulated pests together with the associated import or internal movement requirements of commodities are included in Commission Implementing Regulation (EU) 2019/2072. Additionally, as stipulated in the Commission Implementing Regulation 2018/2019, certain commodities are provisionally prohibited to enter in the EU (high risk plants, HRP). EFSA is performing the risk assessment of the dossiers submitted by exporting countries to the EU of the HRP commodities, as stipulated in Commission Implementing Regulation 2018/2018. Furthermore, EFSA has evaluated a number of requests from exporting countries to the EU for derogations from specific EU import requirements.
In line with the principles of the new plant health law, the European Commission with the Member States are discussing monthly the reports of the interceptions and the outbreaks of pests notified by the Member States. Notifications of an imminent danger from pests that may fulfil the conditions for inclusion in the list of the Union quarantine pest are included. Furthermore, EFSA has been performing horizon scanning of media and literature.
As a follow‐up of the above‐mentioned activities (reporting of interceptions and outbreaks, HRP, derogation requests and horizon scanning), a number of pests of concern have been identified. EFSA is requested to provide scientific opinions for these pests, in view of their potential inclusion by the risk manager in the lists of Commission Implementing Regulation (EU) 2019/2072 and the inclusion of specific import requirements for relevant host commodities, when deemed necessary by the risk manager.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinions in the field of plant health.
EFSA is requested to deliver 53 pest categorisations for the pests listed in Annex 1A, 1B, 1D and 1E (for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Additionally, EFSA is requested to perform pest categorisations for the pests so far not regulated in the EU, identified as pests potentially associated with a commodity in the commodity risk assessments of the HRP dossiers (Annex 1C; for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Such pest categorisations are needed in the case where there are not available risk assessments for the EU.
When the pests of Annex 1A are qualifying as potential Union quarantine pests, EFSA should proceed to phase 2 risk assessment. The opinions should address entry pathways, spread, establishment, impact and include a risk reduction options analysis.
Additionally, EFSA is requested to develop further the quantitative methodology currently followed for risk assessment, in order to have the possibility to deliver an express risk assessment methodology. Such methodological development should take into account the EFSA Plant Health Panel Guidance on quantitative pest risk assessment and the experience obtained during its implementation for the Union candidate priority pests and for the likelihood of pest freedom at entry for the commodity risk assessment of High Risk Plants.
1.2. Interpretation of the Terms of Reference
Pestalotiopsis disseminata is one of a number of pests listed in Annex 1C to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a potential Union quarantine pest for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores, and so inform EU decision making as to its appropriateness for potential inclusion in the lists of pests of Commission Implementing Regulation (EU) 2019/ 2072. If a pest fulfils the criteria to be potentially listed as a Union quarantine pest, risk reduction options will be identified.
1.3. Additional information
This pest categorisation was initiated following the commodity risk assessments of bonsai plants from China consisting of Pinus parviflora grafted on Pinus thunbergii performed by EFSA (EFSA PLH Panel, 2022), in which P. disseminata was identified as a relevant non‐regulated EU pest which could potentially enter the EU on bonsai plants.
2. DATA AND METHODOLOGIES
2.1. Data
2.1.1. Information on pest status from NPPOs
In the context of the current mandate, EFSA is preparing pest categorisations for new/emerging pests that are not yet regulated in the EU. When official pest status is not available in the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, online), EFSA consults the NPPOs of the relevant MSs. To obtain information on the official pest status for P. disseminata, EFSA has consulted the NPPO of Portugal.
2.1.2. Literature search
A literature search on P. disseminata was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as search term. Papers relevant for the pest categorisation were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.3. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, online), the CABI databases and scientific literature databases as referred above in Section 2.1.1.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt and TRACES databases were consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission as a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. TRACES is the European Commission's multilingual online platform for sanitary and phytosanitary certification required for the importation of animals, animal products, food and feed of non‐animal origin and plants into the European Union, and the intra‐EU trade and EU exports of animals and certain animal products. Up until May 2020, the Europhyt database managed notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States and the phytosanitary measures taken to eradicate or avoid their spread. The recording of interceptions switched from Europhyt to TRACES in May 2020.
GenBank was searched to determine whether it contained any nucleotide sequences for P. disseminata which could be used as reference material for molecular diagnosis. GenBank® (www.ncbi.nlm.nih.gov/genbank/) is a comprehensive publicly available database that as of August 2019 (release version 227) contained over 6.25 trillion base pairs from over 1.6 billion nucleotide sequences for 450,000 formally described species (Sayers et al., 2020).
2.2. Methodologies
The Panel performed the pest categorisation for P. disseminata, following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018), the EFSA guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee et al., 2017) and the International Standards for Phytosanitary Measures No. 11 (FAO, 2013).
The criteria to be considered when categorising a pest as a potential Union quarantine pest (QP) is given in Regulation (EU) 2016/2031 Article 3 and Annex I, Section 1 of the Regulation. Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. In judging whether a criterion is met the Panel uses its best professional judgement (EFSA Scientific Committee, 2017) by integrating a range of evidence from a variety of sources (as presented above in Section 2.1) to reach an informed conclusion as to whether or not a criterion is satisfied.
TABLE 1.
Pest categorisation criteria under evaluation, as derived from Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column).
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest (article 3) |
|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in and spread within, the EU territory? If yes, briefly list the pathways for entry and spread |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests' introduction have an economic or environmental impact on the EU territory? |
| Available measures (Section 3.6 ) | Are there measures available to prevent pest entry, establishment, spread or impacts? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met |
The Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, deemed to be a risk management decision, the Panel will present a summary of the observed impacts in the areas where the pest occurs, and make a judgement about potential likely impacts in the EU. Whilst the Panel may quote impacts reported from areas where the pest occurs in monetary terms, the Panel will seek to express potential EU impacts in terms of yield and quality losses and not in monetary terms, in agreement with the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Article 3 (d) of Regulation (EU) 2016/2031 refers to unacceptable social impact as a criterion for quarantine pest status. Assessing social impact is outside the remit of the Panel.
3. PEST CATEGORISATION
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and/or to be transmissible?
Yes, the identity of Pestalotiopsis disseminata (Thüm.) Steyaert is clearly defined and the pathogen has been shown to produce consistent symptoms and to be transmissible.
P. disseminata (Thüm.) Steyaert (Steyaert, 1949) has been recognised as a plant pathogenic fungus of the family Pestalotiopsidaceae (Index Fungorum, accessed Nov 2023); nevertheless, P. disseminata has been commonly isolated as endophyte or saprobe on a wide range of plants (Maharachchikumbura et al., 2011).
The classification of the Pestalotiopsis genus at the family level has been controversial given the divergence or heterogeneity of morphological characters. Indeed, some authors have been accommodating this genus into the family Sporocadaceae (Nag Raj, 1993) or Amphisphaeriaceae (Jeewon et al., 2003). More recently, Senanayake et al. (2015) introduced the family Pestalotiopsidaceae (derived from Amphisphaeriaceae) to accommodate Pestalotiopsis spp. together with other genera, based on morphological and molecular data. However, the introduction of this new family was not accepted by some authors (Jaklitsch et al., 2016; Liu et al., 2019) that revived the older family name Sporocadaceae to accommodate the Pestalotiopsis genus.
The EPPO Global Database (EPPO, online) provides the following taxonomic identification for P. disseminata:
Preferred name: Pestalotiopsis disseminata (von Thüm.) Steyaert
Order: Amphisphaeriales
Family: Sporocadaceae
Genus: Pestalotiopsis
Species: Pestalotiopsis disseminata
The global fungal nomenclature database Index Fungorum (https://www.indexfungorum.org/) accommodates the genus Pestalotiopsis in the family Pestalotiopsidaceae (accessed on 31 August 2023). In this pest categorisation, the Panel decided to adopt the nomenclature provided by Index Fungorum.
Synonyms: the EPPO Global Database (EPPO, 2022) also reports the following scientific name:
Pestalotia disseminata von Thümen
Common names: The following common name is provided by the EPPO Global Database (EPPO, online): leaf spot of eucalyptus.
The EPPO code 1 (EPPO, 2019; Griessinger & Roy, 2015) for this species is: PESTDI (EPPO, online).
3.1.2. Biology of the pest
P. disseminata is a plant pathogenic fungus of the family Pestalotiopsidaceae. Like other Pestalotiopsis species, P. disseminata displays different lifestyles. It has been reported as a pathogen causing diseases on monocotyledonous, dicotyledonous and gymnosperm host plants, as a saprophyte, commonly found on dead leaves and woody plant tissues (Sharma et al., 2011), or as mycoparasite (Hwang et al., 2016). Moreover, it may occur as an endophyte in asymptomatic plant tissues and may eventually switch to a pathogenic behaviour when its host is stressed (Maharachchikumbura et al., 2012). P. disseminata is also known to produce a wide range of secondary metabolites with various bioactivities (Hwang et al., 2016) and some isolates were reported as entomopathogenic (Lyu et al., 2014).
In general, species in the genus Pestalotiopsis are not host‐specific, having the ability to infect a wide range of hosts (Hopkins & McQuilken, 2000; Keith et al., 2006), on which they may cause a variety of diseases, including canker lesions, gummosis, shoot dieback, needle blight, tip blight, grey blight, scabby canker, severe chlorosis, fruit rots and leaf spots (Maharachchikumbura et al., 2011). Pestalotiopsis spp. are considered as weak or opportunistic pathogens (Madar et al., 1991), but some species may cause serious damage and the number of known hosts is increasing (Jeewon et al., 2004).
Pestalotiopsis species infect their host through natural openings such as stomata, lenticels and hydathodes or through wounds (Wright et al., 1998). Along with other species of Pestalotiopsis, P. disseminata is frequently isolated as an endophyte from healthy plant tissues (e.g. Lateef et al., 2018; Liu et al., 2012; Tejesvi et al., 2009; Wei et al., 2007). As many other endophytic species, it may remain dormant until the plant is stressed, and then displays a pathogenic behaviour (Maharachchikumbura et al., 2011). Aging and stress inducers such as pruning, insect damage, high temperatures, strong wind and rainfall may act as triggers of infection or shift to pathogenicity (Tuset et al., 1999; McQuilken and Hopkins, 2004; Keith et al., 2006).
P. disseminata has no known sexual stage, therefore the primary inoculum is likely to be conidia. These are released from acervuli (Keith et al., 2006; Maharachchikumbura et al., 2011; Nag Raj, 1993), that are formed on symptomatic plant tissues during wet weather and are washed‐off or splash‐dispersed by water to infect susceptible host tissues. In addition to the typical appendage‐bearing six‐celled alpha‐conidia, P. disseminata may also produce beta‐conidia in culture, but their biological and epidemiological role is unknown (Crous et al., 2006). The sources of the primary inoculum may include infected plant parts (Keith et al., 2006; Pandey, 1990), debris from a previous crop, used growing media and soil (Hopkins and McQuilken, 2000). Secondary inoculum produced on diseased tissues causes secondary infections, thereby increasing the incidence and severity of the disease (Maharachchikumbura et al., 2011).
Optimum conditions for conidial germination and leaf infection were determined at 25°C and 70% RH (Das et al., 2010). Watanabe et al. (2000) provided a detailed description of the adhesion, germination and infection process in the closely related species P. neglecta. In the first stage, the lower median cell germinates by firmly attaching to the substrate. Successive infections are achieved by two upper median cells. Adhesion is favoured by a mucilaginous matrix coating the conidia and by the release of fibrillar adhesive substances along the length of the pedicel to the apex of the basal cell, from the apical appendages and at the point of germ tube emergence (Watanabe et al., 2000).
Seeds of certain host plants may represent a source of primary inoculum of P. disseminata. The pathogen has been detected by sequencing of the ITS2 region from seeds of P. pinea, P elliottii, P. patula, P. radiata, P. taeda and P. pinaster from various origins (Cleary et al., 2019), and has been isolated from stored seed lots of Eucalyptus pellita in Australia (Yuan et al., 1997). Similarly, the closely related species P. pinicola has been isolated from pine seed endosperm (Tibpromma et al., 2019), while P. brassicae and P. oryzae were isolated from Brassica napus and Oryza sativa seeds, respectively (Maharachchikumbura et al., 2014).
Insects are likely to act as carriers of the pathogen: although not specifically reported for P. disseminata, their role has been demonstrated for other Pestalotiopsis species, such as P. funerea on Cupressus sempervirens (Battisti et al., 1999) and Pestalotiopsis sp. (possibly P. palmarum) on Elaeis guineensis (Martínez & Plata‐Rueda, 2013).
3.1.3. Host range/species affected
P. disseminata has been reported on a wide range of monocotyledonous, dicotyledonous and gymnosperms, cultivated and wild plant species worldwide. It was described for the first time from dead leaves of E. globulus in Portugal (von Thümen, 1881). Subsequently, the pathogen has been associated with fruit gummosis on Prunus persica (Singh et al., 2000), fruit scab on Psidium guajava (Bhargava et al., 2003; Keith et al., 2006; El‐Argawy, 2016), fruit rot on Feijoa sellowiana (Naeimi et al., 2015), Malus domestica (Hino, 1966) (also mentioned in a commodity risk assessment; AQIS, 1998), Persea americana (Liu et al., 2019) and Musa sapientium (Al Ameen et al., 2017), pod canker on Vicia faba (Singh & Tombisana Devi, 2001) and E. pellita (Yuan et al., 1997), shoot blight on Pinus spp. (Cleary et al., 2019; Silva et al., 2020; Watanabe et al., 2010), grey leaf blight on Persea bombycina (Das et al., 2010; Paliwal and Paliwal, 2015; Ray et al., 2019), Euonymus japonicus (Wang et al., 2023), Eucalyptus spp. (Crous et al., 2006) and Morus alba (Philip, 1995). More recently, P. disseminata has attracted the interest of many scientists due to its wide array of bioactive secondary metabolites (Deyrup et al., 2006; Hwang et al., 2015; Hwang et al., 2016) and, consequently, this species has been repeatedly isolated from wild species along with other endophytic fungi (Lateef et al., 2018; Liu et al., 2012; Tejesvi et al., 2009; Wei et al., 2007).
A detailed list of the cultivated and wild hosts of P. disseminata reported so far in the literature is included in Appendix A. Most of the reports refer to P. disseminata as an endophyte, rather than a pathogen. Because of the wide host range of the fungus, this pest categorisation will focus on those hosts that are relevant for the EU and for which there is robust evidence in the literature that (a) the pathogen was isolated and identified by both morphological and molecular (multilocus gene sequencing analysis) methods, (b) the Koch's postulates were fulfilled through pathogenicity tests and (c) impacts on affected crops were reported. Using the above criteria, the Panel identified the following hosts (crops and ornamentals) as main hosts of P. disseminata: Euonymus japonicus (Wang et al., 2023) and Psidium guajava (Keith et al., 2006).
Nevertheless, the actual host range of P. disseminata is still largely unknown, because of the different lifestyles of the fungus (saprobe, endophyte, opportunistic pathogen). Moreover, there is uncertainty on the reports where the identification of the pathogen was based merely on morphology, not supported by multigene phylogenetic analysis.
3.1.4. Intraspecific diversity
No information on intraspecific diversity of P. disseminata was found in the available literature. In addition, the sexual stage of the pathogen, which could potentially enhance its genomic plasticity and adaptation to various adverse environmental conditions, including fungicide exposure, is still unknown.
3.1.5. Detection and identification of the pest
Are detection and identification methods available for the pest?
YES, methods are available for the detection and identification of P. disseminata and for its distinction from other closely related taxa in the family Pestalotiopsidaceae.
Symptoms induced by P. disseminata on susceptible hosts include: fruit gummosis (Singh et al., 2000), fruit scab (Bhargava et al., 2003; Keith et al., 2006; El‐Argawy, 2016), fruit rot (Al Ameen et al., 2017; Liu et al., 2019; Naeimi et al., 2015), pod canker (Singh & Tombisana Devi, 2001), seedling blight (Cleary et al., 2019; Yuan et al., 1997), shoot blight (Cleary et al., 2019; Silva et al., 2020; Watanabe et al., 2010) and grey leaf blight (Philip, 1995; Crous et al., 2006; Das et al., 2010; Paliwal and Paliwal, 2015; Ray et al., 2019; Wang et al., 2023). Such symptoms are also produced by other pests. As a consequence, the pathogen cannot be detected based merely on symptoms.
The following description of the morphological features of P. disseminata is provided by Crous et al. (2006): ‘Colonies on OA (oatmeal agar) reaching 52–54 mm diam in 7 days with an even, glabrous, colourless margin; immersed mycelium colourless, aerial mycelium pure white, fluffy, covering most of the colony surface and very dense and high in the centre and in concentric zones after 7 days; reverse in the centre buff. Colonies on CMA (corn meal agar) reaching 52–55 mm diam after 7 days, as on OA, but aerial mycelium less well‐developed and reverse colourless. Colonies on MEA (malt extract agar) reaching 56 mm diam in 7 days, with an even or slightly undulating colourless margin; immersed mycelium colourless, but surface of the colony completely covered by a high, dense mat of pure white, in the centre yellowish, fluffy aerial mycelium, the margin also covered by a diffuse layer of aerial hyphae; reverse with a faint cinnamon tinge. Conidiomata developing from 10 to 14 days (none after 7 days) mainly on the surface of the colony’.
Conidia (alpha‐conidia) of P. disseminata are described by Crous et al. (2006) as: ‘broadly fusoid to fusoid‐clavate, straight or somewhat curved, five‐celled, upper cell conical to cylindrical, hyaline, fairly thin‐walled, apical setulae central, (2–)3(–4), rather stout, up to 1.2 μm wide, 11–20 μm long, with a blunt tip, three intermediate cells concolorous or the upper two intermediate cells slightly darker, dull olivaceous‐brown to vinaceous‐brown, contents guttulate, walls smooth, slightly constricted at the septa when mounted in water and thickened up to 1 μm especially in the upper two intermediate cells and in the septa, basal cell hyaline, thin‐walled, tapering into a filiform pedicel (2–)2.5–4.5(–5) μm long; conidium body (18–)20–24(–25) × 6.5–7(–8) μm (OA)’ (Figure 1).
FIGURE 1.
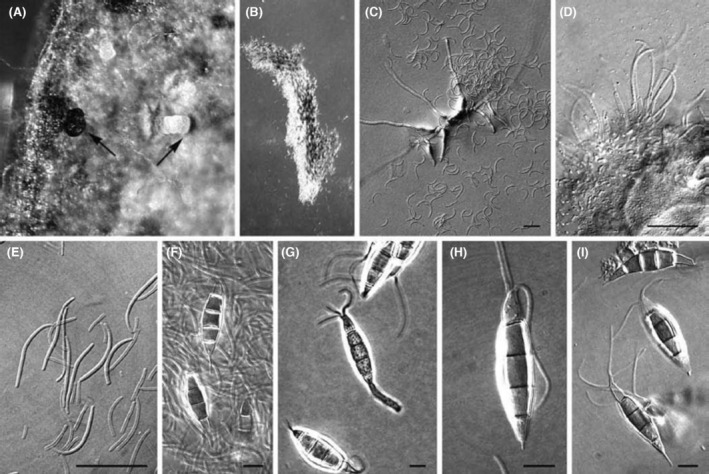
Pestalotiopsis disseminata morphological features: (A) Conidiomata with exuding alpha‐ (black) and beta‐ (cream) conidial masses (arrowed). (B) Conidial cirrus containing back (alpha‐) and hyaline (beta‐) conidia. (C) Germinating alpha‐conidium, among infertile beta‐conidia on MEA plate. (D) Conidiogenous cells giving rise to beta‐conidia. (E) Beta‐conidia. (F–I) Alpha‐conidia. Scale bars: C–E = 10 μm, F–I = 7 μm (from Crous et al., 2006).
In addition to the typical alpha‐conidia, Crous et al. (2006) reported the production of beta‐conidia by two isolates of P. disseminata from Colombia and New Zealand grown in vitro. Beta‐conidia (Figure 1) occurred in the same conidioma with alpha‐conidia, but none could be induced to germinate on malt extract agar, while all alpha‐conidia could germinate within 1–2 days. Moreover, none of the colonies derived from alpha‐conidia could be induced to form beta‐conidia on different substrates (Crous et al., 2006). Hence, the epidemiological and biological role of beta‐conidia still needs to be clarified under natural conditions.
It should be noted that the morphological features in Pestalotiopsis species are subject to various degrees of cultural variation even within the same species, particularly for such characters as growth rate, conidial morphology and fruiting structures (reviewed by Jeewon et al., 2003). Hu et al. (2007) showed that colony morphology (colour, growth rate and texture) is highly variable even within single isolates of Pestalotiopsis during repeated subculturing. Based on the above, it is unlikely that P. disseminata could be detected only by visual inspection of its host plants.
Attempts to distinguish Pestalotiopsis spp. by molecular markers have been reported by Hu et al. (2007), who suggested a combination of the ITS gene and the β‐tubulin gene to differentiate endophytic species of Pestalotiopsis in P. armandii and Ribes spp.. Liu et al. (2010) considered that proper analysis and alignment of the ITS region can be a useful character in grouping Pestalotiopsis spp. isolates presenting different types of pigmentation, which is used as a key character for the phylogeny of the species. Tejesvi et al. (2009) tested an identification approach based on the ITS – restriction fragment length polymorphism (ITS‐RFLP) but failed to achieve clustering of endophytic isolates of Pestalotiopsis spp. that could reflect either their host, colonised plant parts or location. Tsai et al. (2021) examined a collection of 98 isolates (including four strains of P. disseminata) causing tea grey blight disease in Taiwan by using a multilocus sequencing (MLS) approach based on the combination of ITS, β‐tubulin, translation elongation factor 1‐α, together with morphological features, and resolved most of the tested Pestalotiopsis‐like species. As for other fungi, the MLS approach is now considered the most reliable to identify P. disseminata from other species, albeit with some degree of uncertainty due to some sequences that may have been misidentified (Tsai et al., 2021). Therefore, a combination of morphological and molecular methods is recommended for a reliable identification of the fungus.
Nucleotide sequences of P. disseminata are available in GenBank (https://www.ncbi.nlm.nih.gov/nucleotide/; 89 sequences retrieved on 30 August 2023) and could be used as reference material for molecular diagnosis.
No EPPO Standard is available for the detection and identification of P. disseminata and no species‐specific primers for PCR‐based identification are available.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
P. disseminata has been reported to be present in Europe (UK), Africa (Congo, Ghana, Kenya, Malawi, Nigeria, South Africa, Tanzania, Zimbabwe), North America (USA, Hawaii), South America (Brazil, Cuba, Venezuela, West Indies), Asia [Brunei, China, India, Japan, Malaysia, Myanmar, Philippines, Türkiye] and Oceania [Australia, Cook Islands, Fiji, Micronesia, New Zealand (North Island), Papua New Guinea, Samoa, Solomon Islands]. The current geographical distribution of P. disseminata is shown in Figure 2.
FIGURE 2.
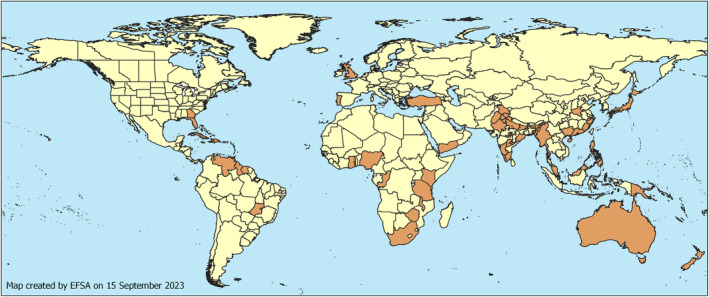
Global distribution of Pestalotiopsis disseminata (Source: EPPO Global Database accessed on 28 April 2023 and literature).
A list of the countries and states/provinces from where P. disseminata has been reported is included in Appendix B. The records are based on CABI (2019), EPPO (online), Farr et al. (2021) and other literature sources (Appendix B).
Nevertheless, the current geographical distribution of P. disseminata outside the EU might be wider than that reported, as in the past, when molecular tools (particularly multigene phylogenetic analysis) were not fully developed, the pathogen might have been misidentified based only on morphology and pathogenicity tests, which cannot reliably differentiate species within the genus Pestalotiopsis or from other closely related species of the genera Pestalotia and Neopestalotiopsis.
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed.
YES, P. disseminata has been reported from Portugal.
P. disseminata has been first described (as Pestalozzia disseminata) on dead leaves of E. globulus in Coimbra, Portugal (von Thümen, 1881). There are two additional collections from Portugal in herbaria listed at mycoportal.org, in 1883 by Rabenhorst and Winter, and in 1879 by Moller). Silva et al. (2020) detected P. disseminata in blighted shoots of P. pinea collected in Cascais (Portugal). While the molecular identification was based on MLS, and the isolates from Portugal were clustered with two Westerdijk Institute isolates from Persea americana and E. botryoides from New Zealand, the authors did not perform any pathogenicity assay on pine. Cleary et al. (2019) detected operational taxonomic units corresponding to the fungus in P. pinaster seed lots from Portugal. However, it is worth mentioning that there is uncertainty about the origin of the seed lots and that this study was based merely on the sequence of ITS2 region, hence the identity of the fungus may be questionable as more loci are needed for a reliable identification; moreover, there is no evidence for its pathogenicity in the positive seed lots.
The current geographical distribution of P. disseminata in the EU might be wider than that reported, as in the past, when molecular tools (particularly multigene phylogenetic analysis) were not fully developed, the pathogen might have been misidentified based only on morphology and pathogenicity tests, which cannot reliably differentiate species within the genus Pestalotiopsis or from other closely related species of the genera Pestalotia and Neopestalotiopsis.
3.3. Regulatory status
3.3.1. Commission Implementing Regulation 2019/2072
P. disseminata is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072, an implementing act of Regulation (EU) 2016/2031, or in any emergency plant health legislation.
3.3.2. Hosts or species affected that are prohibited from entering the Union from third countries
None of the main hosts identified in Section 3.1.3 are included in Commission Implementing Regulation 2019/2072.
A list of commodities included in Annex VI of Commission Implementing Regulation (EU) 2019/2072 is provided in Table 2. Also, hosts of the genera Acer L., Albizia Durazz., Persea Mill. and Prunus L. are included in the Commission Implementing Regulation (EU) 2018/2019 on high‐risk plants.
TABLE 2.
List of plants, plant products and other objects that are Pestalotiopsis disseminata hosts whose introduction into the Union from certain third countries is prohibited (Source: Commission Implementing Regulation (EU) 2019/2072, Annex VI).
| List of plants, plant products and other objects whose introduction into the Union from certain third countries is prohibited | |||
|---|---|---|---|
| Description | CN code | Third country, group of third countries or specific area of third country | |
| 19. | Soil as such consisting in part of solid organic substances | ex 2530 90 00 ex 3824 99 93 | Third countries other than Switzerland |
| 20. | Growing medium as such, other than soil, consisting in whole or in part of solid organic substances, other than that composed entirely of peat or fibre of Cocos nucifera L., previously not used for growing of plants or for any agricultural purposes | ex 2530 10 00 ex 2530 90 00 ex 2703 00 00 ex 3101 00 00 ex 3824 99 93 | Third countries other than Switzerland |
3.4. Entry, establishment and spread in the EU
3.4.1. Entry
Is the pest able to enter into the EU territory? If yes, identify and list the pathways.
Yes, the pathogen can enter into the EU, via host plants for planting, fruits, parts of host plants (e.g. foliage, branches, bark, wood) and soil/plant growing media.
Comment on plants for planting as a pathway.
Plants for planting are a main pathway for the entry of the pathogen into the EU.
The Panel identified the following main pathways for the entry of P. disseminata into the EU:
host plants for planting,
fresh fruits of host plants,
bark and wood of host plants and
soil and other plant growing media, associated with infected host plant debris, all originating in infested third countries.
The pathogen is frequently isolated as an endophyte, hence it may enter into the EU territory on asymptomatic plant parts (e.g. stems, branches, fruits) of its hosts. Moreover, the ability to survive as a saprobe in dead plant tissues (leaves, bark, wood) may favour its entry into the EU through compost and potting substrate imported from infested countries.
P. disseminata and other species of the family Pestalotiopsidaceae have been detected on seeds (Cleary et al., 2019; Hu et al., 2007; Maharachchikumbura et al., 2014; Tibpromma et al., 2019; Yuan et al., 1997). Although there is no evidence so far of P. disseminata being transmitted from the seeds to the emerging seedlings, seeds of host plants are likely to be a pathway of entry of the pathogen into the EU. Pine nuts for consumption are also considered a possible entry pathway, although minor.
The pathogen is unlikely to enter into the EU by natural means (e.g. rain, wind‐driven rain, insects) because of the long distance or natural barriers between the infested third countries and the EU MSs.
Although there are no data available, conidia of the pathogen may also be present as contaminants on other substrates or objects (e.g. non‐host plants, second hand agricultural machinery and equipment, crates, etc.) imported into the EU. Nevertheless, these are considered minor pathways for the entry of the pathogen into the EU territory.
A list of all the potential pathways for the entry of the pathogen into the EU territory is included in Table 3.
TABLE 3.
Potential pathways for the entry of Pestalotiopsis disseminata into the EU.
| Pathways (e.g. host/intended use/source) | Life stage | Relevant mitigations [e.g., prohibitions (Annex VI), special requirements (Annex VII) or phytosanitary certificates (Annex XI) within Implementing Regulation 2019/2072) |
|---|---|---|
| Host plants for planting, other than seeds | Mycelium, acervuli, alpha‐conidia | Plants for planting, other than seeds, that are hosts of P. disseminata and are prohibited from being imported from third countries (Annex VI of Commission Implementing Regulation (EU) 2019/2072) are listed in Table 2. There is a temporary prohibition for high‐risk plants (Regulation 2018/2019) |
| Seeds of host plants for sowing | Mycelium, acervuli, alpha‐conidia | A phytosanitary certificate is required for the introduction into the Union from third countries, other than Switzerland, of seeds of host plants for sowing |
| Pine seeds (with and without teguments) for consumption | Mycelium, acervuli, alpha‐conidia | |
| Fresh fruits of host plants | Mycelium, acervuli, alpha‐conidia | A phytosanitary certificate is required for the introduction into the Union from third countries other than Switzerland, of guava fruits fresh or dried [Annex XI, Part A, point 5 of Commission Implementing Regulation (EU) 2019/2072] |
| Parts of host plants, other than fruits and seeds | Mycelium, acervuli, alpha‐conidia | A phytosanitary certificate is required for the introduction into the Union from third countries other than Switzerland, of parts of host plants other than fruits and seeds [Annex XI, Part B of Commission Implementing Regulation (EU) 2019/2072] |
| Bark of host plants | Mycelium, acervuli, alpha‐conidia | A phytosanitary certificate is required for the introduction into the Union from certain third countries of isolated bark of Conifers (Pinales) [Annex XI, Part A (11) of Commission Implementing Regulation (EU) 2019/2072] |
| Wood of host plants | Mycelium, acervuli, alpha‐conidia | A phytosanitary certificate is required for the introduction into the Union from certain third countries of wood of Conifers (Pinales) and including wood, which has not kept its natural round surface [Annex XI, Part A (12) of Commission Implementing Regulation (EU) 2019/2072] |
| Soil as such not attached or associated with plants for planting | Mycelium, alpha‐conidia | The introduction into the Union from third countries, other than Switzerland, of soil as such consisting in part of solid organic substances is banned [Annex VI (19) of Commission Implementing Regulation (EU) 2019/2072] |
| Growing medium as such, other than soil not attached or associated with plants for planting | Mycelium, alpha‐conidia | A phytosanitary certificate is required for the introduction into the Union from third countries, other than Switzerland, of growing medium attached to or associated with plants, intended to sustain the vitality of the plants [Annex XI, Part A (1) of Commission Implementing Regulation (EU) 2019/2072]. Special requirements also exist for this commodity [Annex VII (1) of Commission Implementing Regulation (EU) 2019/2072] |
| Growing medium, attached to or associated with host and non‐host plants for planting, carrying infected plant debris, with the exception of sterile medium of in‐vitro plants | Mycelium, alpha‐conidia | A phytosanitary certificate is required for the introduction into the Union from third countries, other than Switzerland, of growing medium attached to or associated with plants, intended to sustain the vitality of the plants [Annex XI, Part A (1) of Commission Implementing Regulation (EU) 2019/2072]. Special requirements also exist for this commodity [Annex VII (1) of Commission Implementing Regulation (EU) 2019/2072] |
| Machinery and vehicles with contaminated soil and/or infected debris of host plants | Mycelium, acervuli, alpha‐conidia | A phytosanitary certificate is required for the introduction into the Union from third countries, other than Switzerland, of machinery and vehicles [Annex XI, Part A (1) of Commission Implementing Regulation (EU) 2019/2072]. Special requirements also exist for this commodity [Annex VII (2) of Commission Implementing Regulation (EU) 2019/2072] |
The quantity of fresh produce of main hosts imported into the EU from countries where P. disseminata is present is provided in Table 4 and Appendix C.
TABLE 4.
EU annual imports of fresh produce from countries where Pestalotiopsis disseminata is present, 2017–2021 (in 100 kg) Source: Eurostat (accessed on 1 June 2023).
| Commodity* | HS code | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Fresh or dried guavas, mangoes and mangosteens | 0804 50 00 | 1,195,960 | 1,278,765 | 1,475,892 | 1,625,763 | 1,839,990 |
Hosts are in bold.
Notifications of interceptions of harmful organisms began to be compiled in Europhyt in May 1994 and in TRACES in May 2020. As of June 2023, there were no records of interception of P. disseminata in the Europhyt and TRACES databases.
3.4.2. Establishment
Is the pest able to become established in the EU territory?
YES, P. disseminata has been already reported in Portugal (see Section 3.2.2). Both the biotic (host availability) and abiotic (climate suitability) factors occurring in the EU suggest that the pathogen could establish in other parts of the EU territory where susceptible hosts are grown.
Based on its biology, P. disseminata could potentially be transferred from the pathways of entry to the host plants grown in the EU via splash‐dispersed alpha‐conidia, contaminated soil or other plant growing media associated with plants for planting, as well as by surface (rain or irrigation) water. Other potential means of dispersion include insects, similarly to other Pestalotiopsis species (Martínez & Plata‐Rueda, 2013), as well as birds and small animals. The frequency of this transfer will depend on the volume and frequency of the imported commodities, their destination (e.g. nurseries, retailers, packinghouses) and its proximity to the hosts grown in the EU territory, as well as on the management of plant debris and fruit waste.
3.4.2.1. EU distribution of main host plants
As noted above and shown in Appendix A, P. disseminata has a wide host range, as it is able to colonise several plant species endophytically. Some of its hosts (e.g. Pinus spp) are widely distributed in the EU, both in commercial production (nurseries, open fields, orchards) and in home gardens or forests. Of the two main hosts, Euonymus japonicus is cultivated as an ornamental and Psidium guajava is cultivated in Greece and Spain (Rojas‐Sandoval & Acevedo‐Rodríguez, 2013).
3.4.2.2. Climatic conditions affecting establishment
Based on the data available in the literature on the geographic coordinates of the locations from where P. disseminata has been reported, the pathogen is present in non‐EU areas with BSh, BSk, Cfa, Cfb, Cfc, Csa, Csb, Dfb and Dfc Köppen‐Geiger climate zones. These climate zones also occur in the EU territory, where hosts of P. disseminata are also grown (Figure 3).
FIGURE 3.
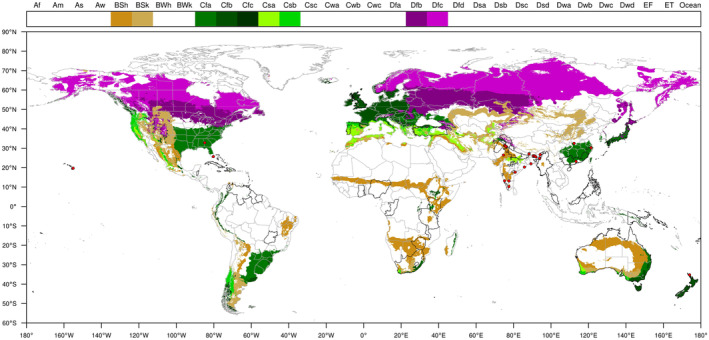
Distribution of seven Köppen–Geiger climate types, i.e. BSh, BSk, Cfa, Cfb, Cfc, Csa, Csb, Dfb and Dfc that occur in the EU and in third countries where Pestalotiopsis disseminata has been reported. The legend shows the list of Köppen–Geiger climates. Red dots indicate point locations where P. disseminata was reported.
3.4.3. Spread
Describe how the pest would be able to spread within the EU territory following establishment?
P. disseminata could potentially spread within the EU by both natural and human‐ assisted means.
Host plants for planting are a main means of spread of the pathogen within the EU territory.
P. disseminata could potentially spread within the EU via natural and human‐assisted means.
Spread by natural means. Alpha‐conidia of Pestalotiopsis spp. are able to spread over relatively short distances by water (rain, overhead irrigation) splash (McQuilken and Hopkins, 2004; Tuset et al., 1999). Wind may increase the dispersal distance of water‐splashed conidia (Xu et al., 1999). Although not specifically reported for P. disseminata, the role of insects as means of spread has been demonstrated for other Pestalotiopsis species (Battisti et al., 1999; Martínez & Plata‐Rueda, 2013).
Spread by human‐assisted means. The pathogen can spread over long distances via the movement of infected host plants for planting (rootstocks, grafted plants, scions, seeds, etc.), including dormant plants, as well as fresh fruits, contaminated soil /plant growing media and agricultural machinery, tools, etc.
P. disseminata can also spread via contaminated/infected seed of host plants, such as Pinus spp. (Cleary et al., 2019) and Eucalyptus spp. (Yuan et al., 1997), with some uncertainty.
3.5. Impacts
Would the pests' introduction have an economic or environmental impact on the EU territory?
Yes, despite the low aggressiveness observed in most reported hosts, and the fact that P. disseminata has often been found as an endophyte, the introduction and/or spread of this fungus into the EU may have an economic impact in the territory where susceptible hosts are grown, with uncertainty on the magnitude of such impact.
P. disseminata has been reported as a weak parasite and an endophyte on a wide range of plant hosts. However, few reports quantified the extent of the disease or the economic impact on cultivated crops.
Recently, the pathogen was reported as causing a ‘serious grey blight disease’ on E. japonicus grown in the Henan Province (China), determining severe defoliation with a disease incidence ranging from 52% to 70% (Wang et al., 2023).
Although peach is not considered here as a main host (Koch postulates were fulfilled, but without molecular identification; see Section 3.1.3), Singh et al. (2000) defined gummosis on peach fruits caused by P. disseminata as ‘a critical disease’, widely distributed in all surveyed orchards in the Manipur district (India); the loss in fruit production in some areas reached 85% and the disease was recurrent during several years.
Albeit not relevant to the EU, P. disseminata has been reported as the main foliar pathogen on som (Persea bombycina Kost.), the primary food plant of muga silkworm (Antheraea assamensis Helfer), grown in the northeastern regions of India (particularly in Assam) to produce the shiny golden silk (Das et al., 2010).
Despite the lack of information on the impact of the pathogen in Portugal, its introduction and/or spread in the EU could have an economic and environmental impact, with a key uncertainty concerning the magnitude of such impact, particularly considering the increased frequency of heavy precipitations and extreme extratropical cyclones forecast in Europe as a consequence of global warming (Priestley & Catto, 2022), that may act as stress factors favouring the shift of the fungus from endophytic to pathogenic.
3.6. Available measures and their limitations
Are there measures available to prevent pest entry, establishment, spread or impacts such that the risk becomes mitigated?
Yes. Although not specifically targeted against P. disseminata, existing phytosanitary measures (see Sections 3.3.2 and 3.4.1) mitigate the likelihood of the pathogen's entry into the EU territory on certain host plants. Potential additional measures are also available to further mitigate the risk of entry, establishment, spread and impacts of the pathogen in the EU (see Section 3.6.1).
3.6.1. Identification of potential additional measures
Phytosanitary measures (prohibitions) are currently applied to some host plants for planting (see Section 3.3.2).
Additional potential risk reduction options and supporting measures are shown in Sections 3.6.1.1 and 3.6.1.2.
3.6.1.1. Additional potential risk reduction options
Potential additional control measures are listed in Table 5.
TABLE 5.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways. Control measures are measures that have a direct effect on pest abundance.
| Control measure/risk reduction option (blue underline = Zenodo doc, blue = WIP) | RRO summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Require pest freedom | Plants, plant products and other objects come from a pest‐free country or a pest‐free area or a pest‐free place of production | Entry/Spread |
| Growing plants in isolation | Growing nursery plants in isolation may represent an effective control measure | Entry/Establishment/Spread |
| Managed growing conditions | Proper field drainage, plant distancing, use of pathogen‐free agricultural tools (e.g. pruning scissors, saws and grafting blades) and removal of infected plants and plant debris in the field could potentially mitigate the likelihood of infection at origin as well as the spread of the pathogen | Entry/Spread/Impact |
| Crop rotation, associations and density, weed/volunteer control | Although P. disseminata has been isolated over a wide range of potential hosts (Appendix A), crop rotation (wherever feasible) may represent an effective means to reduce inoculum sources and potential survival of the pathogen. Although weeds have not been reported as hosts of P. disseminata, their control could potentially make the micro‐climatic conditions less favourable (e.g. by reducing moisture) to pathogen infection and spread | Establishment/Spread/Impact |
| Use of resistant and tolerant plant species/varieties | Although limited information is available only on the differential susceptibility of guava cultivars (Keith et al., 2006), the identification and selection of resistant and tolerant host species/varieties may contribute to the restriction of the growth and development of P. disseminata | Establishment/Spread/Impact |
| Roguing and pruning | P. disseminata overwinters on infected attached plant parts which can act as inoculum sources. Thus, pruning of the symptomatic plant organs may be important in reducing the sources of inoculum and spread capacity | Spread/Impact |
| Biological control and behavioural manipulation | No data are available on the biocontrol of P. disseminata. However, biocontrol agents such as Trichoderma, Gliocladium and Pseudomonas proved effective in field control of the grey blight disease on Camellia sinensis caused by Pestalotiopsis theae (Sanjay et al., 2008). Moreover, Won et al. (2021) achieved effective control of leaf blight disease caused by P. maculans on Quercus acutissima seedlings grown in containers by the application of Bacillus velezensis | Entry/Establishment/Spread/Impact |
| Chemical treatments on crops including reproductive material | The resistance inducer of natural origin chitosan (2.5%) proved effective against scab disease caused by P. disseminata and other Pestalotiopsis species in guava fruits (El‐Argawy, 2016). Fungicide application achieved field control of grey blight disease on Camellia sinensis caused by P. theae (Sanjay et al., 2008). Chemical control has been also reported on Pestalotiopsis spp. affecting ornamental Camellia spp. (reviewed by Hopkins, 1996). | Entry/Establishment/Spread/Impact |
| Chemical treatments on consignments or during processing | The application of fungicides to plants or plant products after harvest, during process or packaging operations and storage may contribute to mitigate the likelihood of entry or spread of P. disseminata | Entry/Spread/Impact |
| Physical treatments on consignments or during processing | Physical treatments (irradiation, mechanical cleaning, sorting, etc.) may reduce or mitigate the risk of entry/spread, but no specific information for P. disseminata is available | Entry/Spread |
| Cleaning and disinfection of facilities, tools and machinery | P. disseminata may also infect its host plants through wounds created by pruning or grafting. Therefore, although no specific information is available on this species, cleaning and surface sterilisation of pruning and grafting tools as well as of equipment and facilities (including premises, storage areas) are good cultural and handling practices employed in the production and marketing of any commodity and may mitigate the likelihood of entry or spread of the pathogen | Entry/Spread |
| Limits on soil | Pestalotiopsis spp. survive in plant debris (e.g. bark, wood, leaf litter) in soil. Therefore, plants, plant products and other objects (e.g. used farm machinery) should be free from soil to ensure freedom from the pathogen | Entry/Establishment/Spread |
| Soil treatment | Given that Pestalotiopsis spp. survive in soil associated with plant debris and despite the lack of specific studies for this pathogen, it is reasonable to assume that soil and substrate disinfestation with chemical, biological or physical (heat, soil solarisation) means could potentially reduce the persistence and availability of inoculum sources | Entry/Establishment/Spread/Impact |
| Use of non‐contaminated water | Considering that P. disseminata may spread via contaminated irrigation water, physical or chemical treatment of irrigation water may be applied in nurseries and greenhouses | Entry/Spread/Impact |
| Waste management | Waste management in authorised facilities and official restriction on the movement of infected plant material prevent the pest from escaping. On‐site proper management of pruning residues is recommended as an efficient measure | Entry/Establishment/Spread |
| Heat and cold treatments | No specific studies are available for P. disseminata. However, protection of guava fruit from decay was achieved by hot water treatment at 50°C for 30 min after artificial inoculation with Pestalotiopsis versicolor (Madhukar & Reddy, 1990) | Entry/Spread |
| Conditions of transport | If plant material, potentially infected or contaminated with P. disseminata (including waste material) must be transported, specific transport conditions (type of packaging/protection, transport means) should be defined to prevent the pathogen from escaping. These may include, albeit not exclusively: physical protection, sorting prior to transport, sealed packaging, etc. | Entry/ Spread |
| Controlled atmosphere | Although no specific reports are available on P. disseminata, controlled atmosphere could be employed to achieve prevention/delay of symptoms in infected commodities, particularly fruit. Storage in the presence of 9%–12% carbon dioxide extended shelf‐life of rambutan fruits (Nephilium lappaceum L.) infected by Pestalotiopsis spp., whereas ozone treatment has been successfully applied against P. mangiferae on mango fruit (Guillen et al., 1999) | Spread |
| Post‐entry quarantine and other restrictions of movement in the importing country | Recommended for plant species known to be hosts of P. disseminata. This measure does not apply to fruits of host plants | Establishment/Spread |
3.6.1.2. Additional supporting measures
Potential additional supporting measures are listed in Table 6.
TABLE 6.
Selected supporting measures (a full list is available in EFSA PLH Panel, 2018) in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance.
| Supporting measure (blue underline = Zenodo doc, blue = WIP) | Summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Inspection and trapping |
Inspection is defined as the official visual examination of plants, plant products or other regulated articles to determine if pests are present or to determine compliance with phytosanitary regulations (ISPM 5) The effectiveness of sampling and subsequent inspection to detect pests may be enhanced by including trapping and luring techniques Pestalotiopsis disseminata may remain quiescent or latent within the asymptomatic host tissues. On symptomatic hosts, symptoms may be confused with those caused by other pathogens or abiotic disorders, making it unlikely that the pathogen could be detected based on visual inspection only |
Entry/Establishment/Spread |
| Laboratory testing |
Examination, other than visual, to determine if pests are present using official diagnostic protocols. Diagnostic protocols describe the minimum requirements for reliable diagnosis of regulated pests Multilocus gene sequencing analysis combined with the observation of cultural and morphological characteristics of fungal colonies, conidiomata with alpha‐ and possibly beta conidia is required for the reliable detection and identification of P. disseminata (see Section 3.1.5) |
Entry/Establishment/Spread |
| Sampling |
According to ISPM 31, it is usually not feasible to inspect entire consignments, so phytosanitary inspection is performed mainly on samples obtained from a consignment. It is noted that the sampling concepts presented in this standard may also apply to other phytosanitary procedures, notably selection of units for testing For inspection, testing and/or surveillance purposes the sample may be taken according to a statistically based or a non‐statistical sampling methodology Necessary as part of other risk reduction options |
Entry/Establishment/Spread |
| Phytosanitary certificate and plant passport |
An official paper document or its official electronic equivalent, consistent with the model certificates of the IPPC, attesting that a consignment meets phytosanitary import requirements (ISPM 5) a) export certificate (import) b) plant passport (EU internal trade) Recommended for plant species known to be hosts of P. disseminata, including plant parts and seeds for sowing |
Entry/Spread |
| Certified and approved premises |
Mandatory/voluntary certification/approval of premises is a process including a set of procedures and of actions implemented by producers, conditioners and traders contributing to ensure the phytosanitary compliance of consignments. It can be a part of a larger system maintained by the NPPO in order to guarantee the fulfilment of plant health requirements of plants and plant products intended for trade. Key property of certified or approved premises is the traceability of activities and tasks (and their components) inherent the pursued phytosanitary objective. Traceability aims to provide access to all trustful pieces of information that may help to prove the compliance of consignments with phytosanitary requirements of importing countries Certified and approved premises may reduce the likelihood of the plants and plant products originating in those premises to be infected by P. disseminata |
Entry/Spread |
| Certification of reproductive material (voluntary/official) |
Plants come from within an approved propagation scheme and are certified pest free (level of infestation) following testing; Used to mitigate against pests that are included in a certification scheme The risk of entry and/or spread of P. disseminata is reduced if host plants for planting, including seeds for sowing, are produced under an approved certification scheme and tested free of the pathogen |
Entry/Spread |
| Delimitation of Buffer zones |
ISPM 5 defines a buffer zone as ‘an area surrounding or adjacent to an area officially delimited for phytosanitary purposes in order to minimise the probability of spread of the target pest into or out of the delimited area, and subject to phytosanitary or other control measures, if appropriate’ (ISPM 5). The objectives for delimiting a buffer zone can be to prevent spread from the outbreak area and to maintain a pest‐free production place (PFPP), site (PFPS) or area (PFA) Delimitation of a buffer zone around an outbreak area can prevent spread of the pathogen and maintain a pest‐free area, site or place of production |
Spread |
| Surveillance |
Surveillance to guarantee that plants and produce originate from a pest‐free area could be an option Pestalotiopsis disseminata has been reported in the EU (Portugal). Therefore, surveillance would be an efficient supporting measure to define pest‐free areas or pest‐free places of production as well as to prevent spread of the pathogen |
Entry/Establishment/Spread |
3.6.1.3. Biological or technical factors limiting the effectiveness of measures
Latently infected (asymptomatic) host plants and plant products are unlikely to be detected by visual inspection.
The similarity of symptoms caused by P. disseminata and of signs (e.g. acervuli with alpha‐ and beta conidia) formed by the pathogen with those of other Pestalotiopsis species poses a serious challenge to the detection and identification of the pathogen based solely on visual inspection.
The lack of rapid diagnostic methods based on molecular approaches (i.e. species‐specific primers) does not allow proper in planta identification of the pathogen at entry. In addition, thorough post‐entry laboratory analyses may not be feasible for certain commodities as isolation in pure culture is needed prior to DNA extraction as well as molecular identification based on multigene sequencing.
The wide host range of the pathogen and its ability to survive endophytically on asymptomatic plants limits the possibility to develop standard diagnostic protocols for all potential hosts.
3.7. Uncertainty
Uncertainty applies over the current geographical distribution of P. disseminata, because of the lack of surveys, and because in the past, when molecular tools (particularly multigene phylogenetic analysis) were not fully developed, the pathogen might have been misidentified based only on morphology and pathogenicity tests, which cannot reliably differentiate species within the genus Pestalotiopsis. Moreover, the pathogen may colonise endophytically a wide range of host plants, therefore its distribution might be wider than reported.
The magnitude of the impact of the pest is also a key uncertainty.
4. CONCLUSIONS
P. disseminata has been reported in the EU (Portugal, where the species was originally described in 1881), with a restricted distribution (with a key uncertainty). There is also a key uncertainty on the magnitude of the impact. Therefore, the Panel cannot conclude on whether the pathogen satisfies all the criteria that are within the remit of EFSA to assess for this species to be regarded as potential Union quarantine pest (Table 7).
TABLE 7.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column).
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Key uncertainties |
|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of P. disseminata is clearly defined. The pathogen has been shown to produce consistent symptoms and to be transmissible | None |
| Absence/presence of the pest in the EU (Section 3.2 ) | Pestalotiopsis disseminata is known to be present in the EU, with a restricted distribution (Portugal) | The geographical distribution of P. disseminata in the EU |
| Pest potential for entry, establishment and spread in the EU (Section 3.4 ) | Pestalotiopsis disseminata has already been reported to be present in the EU and it may spread within the EU. The main pathways for the additional entry of the pathogen into and spread within the EU are: (i) host plants for planting (ii) fresh fruits of host plants, (iii) bark and wood of host plants and (iv) soil and other plant growing media, originating in infested third countries. Both the biotic (host availability) and abiotic (climate suitability) factors occurring in parts of the EU are favourable for the establishment of the pathogen. Pestalotiopsis disseminata could potentially spread within the EU by both natural and human‐assisted means | None |
| Potential for consequences in the EU (Section 3.5 ) | Despite the low aggressiveness observed in most reported hosts, and the fact that P. disseminata has often been found as an endophyte, the introduction and/or spread of this fungus into the EU may have an economic and environmental impact where susceptible hosts are grown | There is uncertainty about the magnitude of the impacts |
| Available measures (Section 3.6 ) | Although not specifically targeted against P. disseminata, existing phytosanitary measures mitigate the likelihood of the pathogen's introduction and spread in the EU. Potential additional measures also exist to mitigate the risk of introduction and spread of the pathogen in the EU | None |
| Conclusion (Section 4 ) | The Panel cannot conclude on whether Pestalotiopsis disseminata satisfies all the criteria that are within the remit of EFSA to assess for this species to be regarded as potential Union quarantine pest, because of the key uncertainties on the restricted distribution in the EU and the magnitude of the impact | The geographical distribution of P. disseminata in the EU and the magnitude of the impact |
| Aspects of assessment to focus on/scenarios to address in future if appropriate: | The main knowledge gap concerns the present geographical distribution of P. disseminata within the EU. To reduce this uncertainty, systematic surveys would need to be carried out and isolates of Pestalotiopsis spp. and of related genera (e.g. Pestalotia, Neopestalotiopsis, etc.) available in culture collections would need to be re‐evaluated using appropriate pest identification methods (e.g. multilocus gene sequencing analysis) to define the current geographical distribution of the pathogen in the EU. Further research is needed on the role of seeds as dispersal pathway and on the magnitude of impacts on hosts of EU relevance | |
ABBREVIATIONS
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PFA
pest‐free production area
- PFPP
pest‐free production place
- PFPS
pest‐free production site
- PZ
protected zone
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
GLOSSARY
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 2022)
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 2022)
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2022)
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2022)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2022)
- Greenhouse
A walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment
- Hitchhiker
An organism sheltering or transported accidentally via inanimate pathways including with machinery, shipping containers and vehicles; such organisms are also known as contaminating pests or stowaways (Toy & Newfield, 2010)
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2022)
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2022)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2022)
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2022)
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2022)
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2023‐00346
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A. Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L. Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen, and Lucia Zappalà.
MAP DISCLAIMER
The designations employed and the presentation of material on any maps included in this scientific output do not imply the expression of any opinion whatsoever on the part of the European Food Safety Authority concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
ACKNOWLEDGEMENTS
EFSA wishes to acknowledge the contribution of Oresteia Sfyra (ISA expert) for the literature review and the climate suitability analysis of this opinion.
APPENDIX A. Pestalotiopsis disseminata host plants/species affected
A.1.
Source: EPPO Global Database (EPPO online), CABI (2019) and literature.
| Host status | Host name | Plant family | Common name | ReferenceA |
|---|---|---|---|---|
| Cultivated hosts | Acer laevigatum | Sapindaceae | – | Lu et al. (2000) |
| Agropyron cristatum | Poaceae | Crested couch grass | Mathur (1979) | |
| Albizia odoratissima | Fabaceae | Black siris | Mathur (1979) | |
| Aleurites montana | Euphorbiaceae | Mu‐oil tree | Peregrine and Siddiqi (1972) | |
| Anacardium occidentale | Anacardiaceae | Cashew apple | Peregrine and Ahmad (1982) | |
| Camellia sinensis | Theaceae | Tea plant | Zhang et al. (2012) | |
| Cocos nucifera | Arecaceae | Common coconut palm | Shaw (1984) | |
| Coffea arabica | Rubiaceae | Arabian coffee | Hughes (1953) | |
| Comocladia dentata | Anacardiaceae | – | Arnold (1986) | |
| Dactylis glomerata | Poaceae | Hardgrass | Roane and Roane (1996) | |
| Desmodium ovalifolium | Fabaceae | – | Lenne (1990) | |
| Desmodium floribundum | Fabaceae | – | Adhikari (1989) | |
| Elaeis guineensis | Arecaceae | African oil palm | Williams and Liu (1976) | |
| Eucalyptus | Myrtaceae | – | EPPO (online) | |
| Eucalyptus alba | Myrtaceae | White gum | Morales‐Rodríguez et al. (2019) | |
| Eucalyptus botryoides | Myrtaceae | Southern mahogany | Crous et al. (2006) | |
| Eucalyptus citriodora | Myrtaceae | Lemon gum | Crous et al. (1989) | |
| Eucalyptus globulus | Myrtaceae | Southern blue gum | Maharachchikumbura et al. (2011) | |
| Eucalyptus lehmannii | Myrtaceae | Lehmann's gum | Crous et al. (2000) | |
| Eucalyptus maidenii | Myrtaceae | Maiden's gum | Nattrass (1961) | |
| Eucalyptus robusta | Myrtaceae | Beakpod eucalyptus | Lu et al. (2000) | |
| Eucalyptus saligna | Myrtaceae | Sydney blue gum | Urtiaga (1986) | |
| Eugenia sp. | Myrtaceae | – | Mathur (1979) | |
| Euonymus japonicus | Cleastraceae | Japanese spindle | Wang et al. (2023) | |
| Euphorbia milii | Euphorbiaceae | Christ thorn | Urtiaga (1986) | |
| Fragaria vesca | Rosaceae | Wild strawberry | Mathur (1979) | |
| Helianthus annuus | Asteraceae | Sunflower | Kumar and Dwivedi (1981) | |
| Hemidesmus indicus | Apocynaceae | – | Mathur (1979) | |
| Hymenaea torreana | Fabaceae | – | Urtiaga (1986) | |
| Ixora sp. | Rubiaceae | – | Johnston (1960) | |
| Ixora coccinea | Rubiaceae | Burning love | Thaung (2008) | |
| Juniperus lucayana | Cupressaceae | – | Urtiaga (1986) | |
| Kandelia candel | Rhizophoraceae | – | Carrieri et al. (2013) | |
| Lagerstroemia indica | Lythraceae | Cannonball | Ge et al. (2009) | |
| Litchi chinensis | Sapindaceae | Litchee | Liu (1977) | |
| Macadamia sp. | Proteaceae | – | Peregrine and Siddiqi (1972) | |
| Machilus bombycina | Lauraceae | – | Sarbhoy et al. (1971) | |
| Manglietia fordiana | Magnoliaceae | – | Ge et al. (2009) | |
| Morus | Moraceae | Mulberry tree | CABI (2019) | |
| Musa x paradisiaca | Musaceae | Banana | Johnston (1960) | |
| Oryza sativa | Poaceae | Rice | CABI (2019) | |
| Persea americana | Lauraceae | Avocado | Silva et al. (2020) | |
| Persea bombycina | Lauraceae | Som | Ray et al. (2019) | |
| Pieris japonica | Ericaceae | Japanese pieris | Watanabe et al. (2012) | |
| Pinus armandi | Pinaceae | Armand's pine | Silva et al. (2020) | |
| Pinus densiflora | Pinaceae | Japanese red pine | Kobayashi (2007) | |
| Pinus elliottii | Pinaceae | American pitch pine | Silva et al. (2020) | |
| Pinus parviflora | Pinaceae | Japanese white pine | Silva et al. (2020) | |
| Pinus patula | Pinaceae | Mexican weeping pine | Silva et al. (2020) | |
| Pinus pentaphylla | Pinaceae | – | Watanabe et al. (2010) | |
| Pinus pinaster | Pinaceae | Bournemouth pine | Silva et al. (2020) | |
| Pinus pinea | Pinaceae | Italian stone pine | EPPO (online) | |
| Pinus radiata | Pinaceae | Monterey pine | Silva et al. (2020) | |
| Pinus taeda | Pinaceae | Loblolly pine | Silva et al. (2020) | |
| Pithecolobium bigeminum | Fabaceae | – | Thaung (2008) | |
| Podocarpus imbricatus | Podocarpaceae | Java dacryberry | Tejesvi et al. (2009) | |
| Podocarpus macrophyllus | Podocarpaceae | Big‐leaf podocarp | Zhuang (2001) | |
| Podocarpus macrophyllus var. maki | Podocarpaceae | – | Lu et al. (2000) | |
| Prunus persica | Rosaceae | Peach | CABI (2019) | |
| Psidium guajava | Myrtaceae | Guava | Keith et al. (2006) | |
| Rhizophora mucronata | Rhizophoraceae | – | Mathur (1979) | |
| Rhodomyrtus tomentosa | Myrtaceae | Hill gooseberry | Ge et al. (2009) | |
| Saraca indica | Fabaceae | Asoka tree | Urtiaga (1986) | |
| Sideroxylon tomentosum | Sapotaceae | Hairy xantolis | Thaung (2008) | |
| Stigmaphyllon sagraeanum | Malpighiaceae | – | Urtiaga (1986) | |
| Strychnos sp. | Loganiaceae | – | ||
| Syzygium cumini | Myrtaceae | Java plum | Mathur (1979) | |
| Terminalia arjuna | Combretaceae | – | Mathur (1979) | |
| Terminalia catappa | Combretaceae | Barbados almond | Arnold (1986) | |
| Terminalia ivorensis | Combretaceae | – | Ebbels and Allen (1979) | |
| Trachycarpus fortunei | Arecaceae | Chinese windmill palm | Taylor and Hyde (2003) | |
| Tripterygium wilfordii | Celastraceae | – | Kumar and Hyde (2004) | |
| Vicia faba | Fabaceae | Broad bean | Singh and Tombisana Devi (2001) | |
| Wild weed hosts | – | – | – | – |
| Artificial/experimental host | Malus domestica | Rosaceae | Apple | Wollenweber and Hochapfel (1936), Hino (1966) |
APPENDIX B. Distribution of Pestalotiopsis disseminata
B.1.
Distribution records based on EPPO Global Database (EPPO, online) and CABI (2019).
| Region | Country | Sub‐national (e.g. state) | Status | References |
|---|---|---|---|---|
| North America | USA | Present, no details | EPPO (online) | |
| Florida | Present, no details | Jenkins (1943) | ||
| Georgia | Present, no details | Hwang et al. (2016) | ||
| Hawaii | Present, no details | EPPO (online) | ||
| Asia | Bangladesh | Present, no details | Al Ameen et al. (2017) | |
| Brunei Darussalam | Present, no details | Peregrine and Ahmad (1982) | ||
| China | Fujian | Present, no details | Liu et al. (2012) | |
| Guangxi | Present, no details | Huang et al. (2002) | ||
| Guangzhou | Present, no details | Kumar and Hyde (2004) | ||
| Hainan | Present, no details | Liu et al. (2012) | ||
| Hangzhou | Present, no details | Lou et al. (2002), Chen et al. (2013) | ||
| Henan | Present, no details | Wang et al. (2023) | ||
| Hong Kong | Present, no details | Lu et al. (2000) | ||
| Japan | Present, no details | EPPO (online) | ||
| Fukuoka | Present, no details | Suto and Kobayashi (1993) | ||
| Kumamoto | Present, no details | Suto and Kobayashi (1993) | ||
| India | Andhra Pradesh | Present, no details | Giri et al. (1996) | |
| Assam | Present, no details | Ray et al. (2019) | ||
| Bihar | Present, no details | Singh et al. (2004) | ||
| Delhi | Present, no details | Singh et al. (2004) | ||
| Himachal Pradesh | Present, no details | Singh et al. (2004) | ||
| Haryana | Present, no details | Singh et al. (2004) | ||
| Kerala | Present, no details | CABI (2019) | ||
| Madhya Pradesh | Present, no details | Rai (1986) | ||
| Maharashtra | Present, no details | Thite and Patil (1975) | ||
| Manipur | Present, no details | CABI (2019) | ||
| Odisha | Present, no details | CABI (2019) | ||
| Punjab | Present, no details | Singh et al. (2004) | ||
| Uttarakhand | Present, no details | Singh et al. (2004) | ||
| Uttar Pradesh | Present, no details | Singh et al. (2004) | ||
| Sundarbans | Present, no details | Purkait and Purkayastha (1999) | ||
| Malaysia | Present, no details | Johnston (1960) | ||
| Myanmar | Present, no details | Thaung (2008) | ||
| Philippines | Present, no details | Kobayashi & de Guzman (1988) | ||
| Türkiye | Present, no details | Cleary et al. (2019) | ||
| South America | Brazil | Present, no details | Mendes et al. (1998) | |
| Venezuela | Present, no details | Turner (1971) | ||
| Caribbean | Cuba | Present, no details | Arnold (1986) | |
| West Indies | Present, no details | Minter et al. (2001) | ||
| Africa | Congo | Present, no details | Turner (1971) | |
| Ghana | Present, no details | Turner (1971) | ||
| Kenya | Present, no details | Nattrass (1961) | ||
| Malawi | Present, no details | Peregrine and Siddiqi (1972) | ||
| Nigeria | Present, no details | Turner (1971) | ||
| South Africa | Present, no details | Crous et al. (1989) | ||
| Tanzania | Present, no details | Ebbels and Allen (1979) | ||
| Zimbabwe | Present, no details | Whiteside (1966) | ||
| Oceania | Australia | Present, no details | Morales‐Rodríguez et al. (2019) | |
| Cook Islands | Present, no details | Dingley et al. (1981) | ||
| Fiji | Present, no details | Dingley et al. (1981) | ||
| Micronesia | Present, no details | EPPO (online) | ||
| New Zealand | North Island | Present, no details | Crous et al. (2006) | |
| Papua New Guinea | Present, no details | Shaw (1984) | ||
| Samoa | Present, no details | Dingley et al. (1981) | ||
| Solomon Islands | Present, no details | EPPO (online) | ||
| EU | Portugal | Cascais | Present, no details | Silva et al. (2020) |
| Coimbra | Present, no details | Maharachchikumbura et al. (2011) | ||
| Other Europe | UK | Present, no details | Taylor and Hyde (2003), Mycoportal.org |
APPENDIX C. EU annual imports of fresh produce of main hosts from countries where Pestalotiopsis disseminata is present, 2017–2021 (in 100 kg)
C.1.
Source: Eurostat accessed on 1 June 2023
| 2017 | 2018 | 2019 | 2020 | 2021 | ||
|---|---|---|---|---|---|---|
| Fresh or dried guavas, mangoes and mangosteens | New Zealand | 0.08 | 0.09 | 0.07 | 0.10 | 0.22 |
| South Africa | 13,015.45 | 9739.99 | 12,116.95 | 8656.28 | 5777.96 | |
| United States | : | : | : | : | 103.68 | |
| Australia | 94.18 | 62.92 | : | : | 0.01 | |
| Kenya | 4.08 | 65.09 | 10.30 | 66.53 | 1497.11 | |
| Malaysia | 197.22 | 170.64 | 72.72 | 44.56 | 19.01 | |
| Philippines | 519.88 | 795.56 | 368.97 | 128.10 | 153.67 | |
| Ghana | 9114.51 | 10,672.35 | 11,138.06 | 30,296.55 | 15,263.44 | |
| Malawi | : | : | : | 648.00 | 110.83 | |
| Nigeria | 0.10 | 1.13 | 1.95 | 0.03 | 28.59 | |
| Venezuela | 2033.75 | 2401.44 | 1939.11 | 282.69 | 522.30 | |
| India | 8148.87 | 9470.36 | 9315.51 | 7347.61 | 16576.61 | |
| Japan | : | : | : | 0.01 | 7.66 | |
| Brazil | 1,158,717.06 | 1,241,860.63 | 1,437,569.20 | 1,577,043.99 | 1,799,012.86 | |
| Myanmar | 0.28 | 1.47 | 1.00 | : | : | |
| Tanzania | 0.50 | 1.14 | : | 0.09 | ||
| China | 51.87 | 180.81 | 78.23 | 104.34 | 248.77 | |
| Cuba | 216.57 | 14.36 | 103.34 | 230.60 | 135.11 | |
| Türkiye | 0.21 | 24.09 | 68.86 | 38.93 | 86.48 | |
| Sum | 1,195,960.47 | 1,278,764.57 | 1,475,892.29 | 1,625,763.33 | 1,839,989.72 |
EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Baptista, P. , Chatzivassiliou, E. , Di Serio, F. , Gonthier, P. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Stefani, E. , Thulke, H.‐H. , Van der Werf, W. , Civera, A. V. , Yuen, J. , … Reignault, P. L. (2023). Pest categorisation of Pestalotiopsis disseminata . EFSA Journal, 21(12), e8494. 10.2903/j.efsa.2023.8494
Adopted: 30 November 2023
Notes
An EPPO code, formerly known as a Bayer code, is a unique identifier linked to the name of a plant or plant pest important in agriculture and plant protection. Codes are based on genus and species names. However, if a scientific name is changed the EPPO code remains the same. This provides a harmonised system to facilitate the management of plant and pest names in computerised databases, as well as data exchange between IT systems (EPPO, 2019; Griessinger & Roy, 2015).
REFERENCES
- Adhikari, R. S. (1989). New host records of some foliicolous fungi from India: II. Indian Phytopathology, 42, 481. [Google Scholar]
- Al Ameen, M. , Shamsi, S. , & Bashar, M. A. (2017). Mycoflora associated with infected fruits of different varieties of Musa sapientum L. and their pathogenic potentiality. Dhaka university. Journal of Biological Sciences, 26(1), 101–110. [Google Scholar]
- AQIS (Australian Quarantine and Inspection Service) . (1998). Pest risk analysis of the importation of Fuji apple from Japan into Australia (p. 30). Australian Quarantine and Inspection Service. [Google Scholar]
- Arnold, G. R. W. (1986). Lista de Hongos Fitopatogenos de Cuba. Ministerio de Cultura Editorial Cientifico‐Tecnica, 207.
- Battisti, A. , Roques, A. , Colombari, F. , Frigimelica, G. , & Guido, M. (1999). Efficient transmission of an introduced pathogen via an ancient insect‐fungus association. Naturwissenschaften, 86, 479–483. [DOI] [PubMed] [Google Scholar]
- Bhargava, A. , Sobti, A. , & Ghasolia, R. (2003). Studies on guava (Psidium guajava L.) drying/wilt disease in orchards of Pushkar Valley. Journal of Phytological Research, 16(1), 81–84. [Google Scholar]
- CABI . (2019). Pestalotia disseminata (leaf spot: Eucalyptus spp.). CABI Compendium. 10.1079/cabicompendium.39798 [DOI] [Google Scholar]
- Carrieri, R. , Carotenuto, G. , & Lahoz, E. (2013). Characterization and pathogenicity of Pestalotiopsis uvicola causing black leaf spot on carob (Ceratonia siliqua L.) in Italy. European Journal of Plant Pathology, 137(4), 655–661. [Google Scholar]
- Chen, W. J. , Gong, Z. , Wu, Y. , Zhong, G. , Wu, X. , Lin, X. , & Zhu, J. (2013). Investigation on the pests and pathogen identification in Pinus parviflora of Hangzhou in China. Proceedings of the Chinese Society of Plant Protection in 2013, Shandong, China, 327–330. [Google Scholar]
- Cleary, M. , Oskay, F. , Doğmuş, H. T. , Lehtijärvi, A. , Woodward, S. , & Vettraino, A. M. (2019). Cryptic risks to forest biosecurity associated with the global movement of commercial seed. Forests, 10(5), 459. [Google Scholar]
- Crous, P. W. , Knox‐Davies, P. S. , & Wingfield, M. J. (1989). A list of eucalyptus leaf fungi and their potential importance to south African forestry. South African Forestry Journal, 149(1), 17–29. [Google Scholar]
- Crous, P. W. , Phillips, A. J. L. , & Baxter, A. P. (2000). Phytopathogenic fungi from South Africa (p. 358). University of Stellenbosch, Department of Plant Pathology Press. [Google Scholar]
- Crous, P. W. , Verkley, G. J. , & Groenewald, J. Z. (2006). Eucalyptus microfungi known from culture. 1. Cladoriella and Fulvoflamma genera nova, with notes on some other poorly known taxa. Studies in Mycology, 55(1), 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, R. , Chutia, M. , Das, K. , & Jha, D. K. (2010). Factors affecting sporulation of Pestalotiopsis disseminata causing grey blight disease of Persea bombycina Kost., the primary food plant of muga silkworm. Crop Protection, 29(9), 963–968. [Google Scholar]
- Deyrup, S. T. , Swenson, D. C. , Gloer, J. B. , & Wicklow, D. T. (2006). Caryophyllene sesquiterpenoids from a fungicolous isolate of Pestalotiopsis disseminata . Journal of Natural Products, 69(4), 608–611. [DOI] [PubMed] [Google Scholar]
- Dingley, J. M. , Fullerton, R. A. , & McKenzie, E. H. C. (1981). Survey of agricultural pests and diseases. Technical Report Volume 2. Records of Fungi, bacteria, algae, and angiosperms pathogenic on plants in Cook Islands, Fiji, Kiribati, Niue, Tonga, Tuvalu, and Western Samoa. F.A.O, 485.
- Ebbels, D. L. , & Allen, D. J. (1979). A supplementary and annotated list of plant diseases, pathogens and associated fungi in Tanzania. Phytopathological Papers, 22, 1–89. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Baptista, P. , Chatzivassiliou, E. , Di Serio, F. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Stefani, E. , Thulke, H.‐H. , Van der Werf, W. , Vicent Civera, A. , Yuen, J. , … Gonthier, P. (2022). Scientific opinion on the commodity risk assessment of bonsai plants from China consisting of Pinus parviflora grafted on Pinus thunbergii . EFSA Journal, 20(2), 7077. 10.2903/j.efsa.2022.7077I [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger, M. , Bragard, C. , Caffier, D. , Candresse, T. , Chatzivassiliou, E. , Dehnen‐Schmutz, K. , Gregoire, J.‐C. , Jaques Miret, J. A. , MacLeod, A. , Navajas Navarro, M. , Niere, B. , Parnell, S. , Potting, R. , Rafoss, T. , Rossi, V. , Urek, G. , Van Bruggen, A. , Van Der Werf, W. , … Gilioli, G. (2018). Guidance on quantitative pest risk assessment. EFSA Journal, 16(8), 5350. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy, A. , Benford, D. , Halldorsson, T. , Jeger, M. J. , Knutsen, H. K. , More, S. , Naegeli, H. , Noteborn, H. , Ockleford, C. , Ricci, A. , Rychen, G. , Schlatter, J. R. , Silano, V. , Solecki, R. , Turck, D. , Benfenati, E. , Chaudhry, Q. M. , Craig, P. , … Younes, M. (2017). Scientific opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal, 15(8), 4971. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Argawy, E. (2016). Characterization and control of Pestalotiopsis spp. the causal fungus of guava scabby canker in el‐beheira governorate, Egypt. International Journal of Phytopathology, 4, 121–136. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) . (2019). EPPO codes. https://www.eppo.int/RESOURCES/eppo_databases/eppo_codes
- EPPO (European and Mediterranean Plant Protection Organization) . (online). EPPO Global Database. https://gd.eppo.int
- FAO (Food and Agriculture Organization of the United Nations) . (2013). ISPM (international standards for Phytosanitary measures) 11—Pest risk analysis for quarantine pests (p. 36). FAO. https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014‐04‐30_201405121523‐494.65%20KB.pdf [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) . (2022). International standards for Phytosanitary measures. ISPM 5 glossary of phytosanitary terms. FAO. https://www.fao.org/3/mc891e/mc891e.pdf [Google Scholar]
- Farr D. F., Rossman A. Y., & Castlebury L. A. (2021). United States National Fungus Collections Fungus‐Host Dataset. Available online [accessed Nov 2023]: https://nt.ars‐grin.gov/fungaldatabases
- Ge, Q. X. , Xu, T. , Cao, R. B. , Sun, X. A. , Zhu, P. L. , Zhang, J. X. , Sun, H. T. , Xu, M. Q. , Chen, G. G. , Liu, A. R. , & Chen, Y. X. (2009). Flora Fungorum Sinicorum, 38.
- Giri, R. Y. , Chaudhary, V. , Bhanja, M. R. , & Reddy, S. M. (1996). Fungal diseases of eucalyptus from Warangal‐II. Indian Forester, 122(9), 817–822. [Google Scholar]
- Griessinger D and Roy A‐S, 2015. EPPO codes: a brief description. https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Guillen, G. E. , Nieto, A. D. , Sepulveda, S. J. , Ponce de Leon, G. L. , & Barbosa, M. C. (1999). Effect of O3, I2 and CL2 in Colletotrichum gloeosporioides Penz, Fusarium oxysporum Schlecht, Lasiodiplodia theobromae pat. And Pestalotiopsis mangiferae P. Heen control. In 6. International Mango Symposium: Working Abstracts and Program, Chon Buri (Thailand), 6–9 April 1999 .
- Hino, T. (1966). Two species of Pestalotia parasitic on fruits of Malus pumila var. dulcissima. Transactions of the Mycological Society of Japan, 7, 2, 193–194. [Google Scholar]
- Hopkins, K. E. (1996). Aspects of the biology and control of Pestalotiopsis on hardy ornamental nursery stock. MSc Thesis. University of Glasgow. https://www.proquest.com/openview/6539b57421f026ff7c572bccee783240/1?pq‐origsite=gscholar&cbl=2026366&diss=y [Google Scholar]
- Hopkins, K. E. , & McQuilken, M. P. (2000). Characteristics of Pestalotiopsis associated with hardy ornamental plants in the UK. European Journal of Plant Pathology, 106, 77–85. [Google Scholar]
- Hu, H. , Jeewon, R. , Zhou, D. , Zhou, T. , & Hyde, K. D. (2007). Phylogenetic diversity of endophytic Pestalotiopsis species in Pinus armandii and Ribes spp.: Evidence from rDNA and β–tubulin gene phylogenies. Fungal Diversity, 24, 1–22. [Google Scholar]
- Huang, S. L. , Yan, W. H. , & Cen, Z. L. (2002). Field trials on the control of banana leaf spot diseases with Diniconazole. Journal of the Yunnan Agricultural University, 17(4), 405–407. [Google Scholar]
- Hughes, S. J. (1953). Fungi from the Gold Coast II. Mycological Paper, 50, 1–104. [Google Scholar]
- Hwang, I. H. , Swenson, D. C. , Gloer, J. B. , & Wicklow, D. T. (2015). New polyketides from a fungicolous isolate of Pestalotiopsis disseminata . Planta Medica, 81(11), PT29. [Google Scholar]
- Hwang, I. H. , Swenson, D. C. , Gloer, J. B. , & Wicklow, D. T. (2016). Disseminins and spiciferone analogues: Polyketide‐derived metabolites from a fungicolous isolate of Pestalotiopsis disseminata . Journal of Natural Products, 79(3), 523–530. [DOI] [PubMed] [Google Scholar]
- Jaklitsch, W. M. , Gardiennet, A. , & Voglmayr, H. (2016). Resolution of morphology‐based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria . Persoonia‐Molecular Phylogeny and Evolution of Fungi, 37(1), 82–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeewon, R. , Liew, E. C. , Simpson, J. A. , Hodgkiss, I. J. , & Hyde, K. D. (2003). Phylogenetic significance of morphological characters in the taxonomy of Pestalotiopsis species. Molecular Phylogenetics and Evolution, 27(3), 372–383. [DOI] [PubMed] [Google Scholar]
- Jeewon, R. E. , Liew, E. C. , & Hyde, K. D. (2004). Phylogenetic evaluation of species nomenclature of Pestalotiopsis in relation to host association. Fungal Diversity, 17, 39–55. [Google Scholar]
- Jenkins, A. E. (1943). Leaf spot on Terminalia arjuna . Phytopathology, 33(5), 404–405. [Google Scholar]
- Johnston, A. (1960). A supplement to a host list of plant diseases in Malaya. Mycological Papers, 77, 1–30. [Google Scholar]
- Keith, L. M. , Velasquez, M. E. , & Zee, F. T. (2006). Identification and characterization of Pestalotiopsis spp. causing scab disease of guava, Psidium guajava, in Hawaii. Plant Disease, 90(1), 16–23. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T. 2007. Index of fungi inhabiting woody plants in Japan. Host, distribution and literature. Zenkoku‐Noson‐Kyoiku Kyokai publishing Co., ltd., 1227.
- Kobayashi, T. , & de Guzman, D. (1988). Notes on tree diseases and associated micro‐organisms observed from 1977 to 1985 in The Philippines. Japan Agricultural Research Quarterly, 22(1), 64–70. [Google Scholar]
- Kumar, D. S. S. , & Hyde, K. D. (2004). Biodiversity and tissue‐recurrence of endophytic fungi in Tripterygium wilfordii . Fungal Diversity, 17, 69–90. [Google Scholar]
- Kumar, V. , & Dwivedi, R. S. (1981). Mycoflora associated with floral parts of sunflower. Indian Phytopathology, 34, 314–317. [Google Scholar]
- Lateef, A. , Sepiah, M. , & Bolhassan, M. H. (2018). Molecular identification and diversity of Pestalotiopsis, Neopestalotiopsis and Pseudopestalotiopsis species from four host plants in Sarawak, Borneo Island (Malaysia). Journal of Science and Technology, 10(1). [Google Scholar]
- Lenne, J. M. (1990). World list of fungal diseases of tropical pasture species. Phytopathological Papers, 31, 1–162. [Google Scholar]
- Liu, A. R. , Chen, S. C. , Jin, W. J. , Zhao, P. Y. , Jeewon, R. , & Xu, T. (2012). Host specificity of endophytic Pestalotiopsis populations in mangrove plant species of South China. African Journal of Microbiology Research, 6(33), 6262–6269. [Google Scholar]
- Liu, A. R. , Chen, S. C. , Wu, S. Y. , Xu, T. , Guo, L. D. , Jeewon, R. , & Wei, J. G. (2010). Cultural studies coupled with DNA based sequence analyses and its implication on pigmentation as a phylogenetic marker in Pestalotiopsis taxonomy. Molecular Phylogenetics and Evolution, 57, 528–535. [DOI] [PubMed] [Google Scholar]
- Liu, F. , Bonthond, G. , Groenewald, J. Z. , Cai, L. , & Crous, P. W. (2019). Sporocadaceae, a family of coelomycetous fungi with appendage‐bearing conidia. Studies in Mycology, 92, 287–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P. S. W. (1977). A supplement to a host list of plant diseases in Sabah, Malaysia. Phytopathological Papers, 21, 1–49. [Google Scholar]
- Lou, B. G. , Xia, X. D. , & Lou, X. M. (2002). Studies on needle blight of Pinus parviflora in Hangzhou. Acta Agriculturae Zhejiangensis, 14(5), 269–272. [Google Scholar]
- Lu, B. , Hyde, K. D. , Ho, W. H. , Tsui, K. M. , Taylor, J. E. , Wong, K. M. , & Zhou, D. (2000). Checklist of Hong Kong fungi (p. 207). Fungal Diversity Press. [Google Scholar]
- Lyu, C. , Huang, B. , Ren, H. , Li, W. , Huang, R. , & Guo, L. (2014). Virulence of Pestalotiopsis disseminata GH10 strain to Hemiberlesia pitysophila . Journal of Southern Agriculture, 45(6), 980–983. [Google Scholar]
- Madar, Z. , Solel, Z. , & Kimchi, M. (1991). Pestalotiopsis canker of cypress in Israel. Phytoparasitica, 19, 79–81. [Google Scholar]
- Madhukar, J. , & Reddy, S. M. (1990). Control of fruit‐rot of guava by hot water treatment. Indian Phytopathology, 43(2), 234–236. [Google Scholar]
- Maharachchikumbura, S. S. , Guo, L. D. , Cai, L. , Chukeatirote, E. , Wu, W. P. , Sun, X. , Crous, P. W. , Bhat, D. J. , McKenzie, E. H. , Bahkali, A. H. , & Hyde, K. D. (2012). A multi‐locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Diversity, 56, 95–129. [Google Scholar]
- Maharachchikumbura, S. S. , Guo, L. D. , Chukeatirote, E. , Bahkali, A. H. , & Hyde, K. D. (2011). Pestalotiopsis—Morphology, phylogeny, biochemistry and diversity. Fungal Diversity, 50, 167–187. [Google Scholar]
- Maharachchikumbura, S. S. N. , Hyde, K. D. , Groenewald, J. Z. , Xu, J. , & Crous, P. W. (2014). Pestalotiopsis revisited. Studies in Mycology, 79, 121–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez, L. C. , & Plata‐Rueda, A. (2013). Lepidoptera vectors of Pestalotiopsis fungal disease: First record in oil palm plantations from Colombia. International Journal of Tropical Insect Science, 33, 239–246. [Google Scholar]
- Mathur, R. S. (1979). The Coelomycetes of India. In Bishen Singh Mahendra pal Singh (p. 460). [Google Scholar]
- McQuilken, M. P. , & Hopkins, K. E. (2004). Biology and integrated control of Pestalotiopsis on container‐grown ericaceous crops. Pest Management Science, 60(2), 135–142. [DOI] [PubMed] [Google Scholar]
- Mendes, M. A. S. , da Silva, V. L. , Dianese, J. C. , et al. (1998). Fungos em Plants no Brasil. Embrapa‐SPI/Embrapa‐Cenargen, Brasilia, 555.
- Minter, D. W. , Rodriguez Hernandez, M. , & Mena Portales, J. (2001). Fungi of the Caribbean: An annotated checklist (p. 946). PDMS Publishing. [Google Scholar]
- Morales‐Rodríguez, C. , Dalla Valle, M. , Aleandri, M. , & Vannini, A. (2019). Pestalotiopsis biciliata, a new leaf pathogen of eucalyptus spp. recorded in Italy. Forest Pathology, 49(2), e12492. [Google Scholar]
- Naeimi, S. , Javadi, L. , & Javadi, A. R. (2015). First report of Pestalotia disseminata, the causal agent of feijoa fruit rot in Iran. Mycologia Iranica, 2(1), 75–76. [Google Scholar]
- Nag Raj, T. R. (1993). Coelomycetous anamorphs with appendage bearing conidia. Mycologue. [Google Scholar]
- Nattrass, R. M. (1961). Host lists of Kenya fungi and bacteria. Mycological Papers, 81, 1–46. [Google Scholar]
- Paliwal, A. K. , & Paliwal, D. P. (2015). Occurrence of diseases on muga silkworm host plant Persea bombycina Kost. In district Bageshwar, Uttarakhand – A survey report. Journal of Functional and Environmental Botany, 5(2), 132–136. [Google Scholar]
- Pandey, R. R. (1990). Mycoflora associated with floral parts of guava (Psidium guajava L.). Acta Botanica Indica, 18(1), 59–63. [Google Scholar]
- Peregrine, W. T. H. , & Ahmad, K. B. (1982). Brunei: A first annotated list of plant diseases and associated organisms. Phytopathological Papers, 27, 1–87. [Google Scholar]
- Peregrine, W. T. H. , & Siddiqi, M. A. (1972). A revised and annotated list of plant diseases in Malawi. Phytopathological Papers, 16, 1–51. [Google Scholar]
- Philip, T. (1995). Pestalotiopsis disseminata (Thum.) Steyaert‐a new pathogen on mulberry. Indian. Journal of Sericulture, 34(2), 159–160. [Google Scholar]
- Priestley, M. D. , & Catto, J. L. (2022). Future changes in the extratropical storm tracks and cyclone intensity, wind speed, and structure. Weather and Climate Dynamics, 3(1), 337–360. [Google Scholar]
- Purkait, R. , & Purkayastha, R. P. (1999). Evaluation of CMC‐induced cellulase activities of a wide variety of foliar fungi isolated from mangrove plants of Sundarbans. Journal of Mycopathological Research, 37(2), 69–72. [Google Scholar]
- Rai, M. K. (1986). New host records of Pestalotiopsis from India. Indian Botanical Reporter, 5(1), 1–98. [Google Scholar]
- Ray, M. K. , Baruah, P. K. , Mishra, P. K. , & Das, S. (2019). A comprehensive mycofloral diversity of pedosphere, phyllosphere and aerosphere of Som.(Persea bombycina Kost.) in lower Brahmaputra valley of Assam. Aerobiologia, 35(3), 553–566. [Google Scholar]
- Roane, C. W. , & Roane, M. K. (1996). Graminicolous fungi of Virginia: Fungi associated with genera Aegilops to Digitaria . Virginia Journal of Science, 47, 197–224. [Google Scholar]
- Rojas‐Sandoval, J. , & Acevedo‐Rodríguez, P. (2013). Psidium guajava (guava). CABI. Compendium. 10.1079/cabicompendium.45141 [DOI] [Google Scholar]
- Sanjay, R. , Ponmurugan, P. , & Baby, U. I. (2008). Evaluation of fungicides and biocontrol agents against grey blight disease of tea in the field. Crop Protection, 27(3–5), 689–694. [Google Scholar]
- Sarbhoy, A. K. , Lal, G. , & Varshney, J. L. (1971). Fungi of India (1967–71) (p. 148). Navyug Traders. [Google Scholar]
- Sayers, E. W. , Cavanaugh, M. , Clark, K. , Ostell, J. , Pruitt, K. D. , & Karsch‐Mizrachi, I. (2020). Genbank. Nucleic Acids Research, (Database issue), 48. 10.1093/nar/gkz956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senanayake, I. C. , Maharachchikumbura, S. S. , Hyde, K. D. , Bhat, J. D. , Jones, E. G. , McKenzie, E. H. , Dai, D. Q. , Daranagama, D. A. , Dayarathne, M. C. , Goonasekara, I. D. , Konta, S. et al., (2015). Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Diversity, 73, 73–144. [Google Scholar]
- Sharma, G. , Pandey, R. R. , & Singh, M. S. (2011). Microfungi associated with surface soil and decaying leaf litter of Quercus serrata in a subtropical natural oak forest and managed plantation in northeastern India. African Journal of Microbiology Research, 5(7), 777–787. [Google Scholar]
- Shaw, D. E. (1984). Microorganisms in Papua New Guinea. Dept. primary Ind. Research Bulletin, 33, 1–344. [Google Scholar]
- Silva, A. C. , Diogo, E. , Henriques, J. , Ramos, A. P. , Sandoval‐Denis, M. , Crous, P. W. , & Bragança, H. (2020). Pestalotiopsis pini sp. nov., an emerging pathogen on stone pine (Pinus pinea L.). Forests, 11(8), 805. [Google Scholar]
- Singh, N. I. , & Tombisana Devi, R. (2001). First report of broad bean canker caused by Pestalotiopsis disseminata in India. Plant Disease, 85(2), 229. [DOI] [PubMed] [Google Scholar]
- Singh, N. I. , Tombisana Devi, R. K. , & Imotomba, P. K. (2000). Peach gummosis: A new disease of peach fruit caused by Pestalotiopsis disseminata . Indian Phytopathology, 53(3), 335. [Google Scholar]
- Singh, S. K. , Srivastava, K. D. , & Singh, D. V. (2004). Pathogenic behaviour of leaf blight organisms on wheat. Indian Phytopathology, 57(3), 319–322. [Google Scholar]
- Steyaert, R. L. (1949). Contribution à l'étude monographique de Pestalotia de Not. et Monochaetia Sacc.(Truncatella gen. nov. et Pestalotiopsis gen. nov.). Bulletin du Jardin Botanique de l'Etat, Bruxelles, 19(3), 285–354. [Google Scholar]
- Suto, Y. , & Kobayashi, T. (1993). Taxonomic studies on the species of Pestalotiopsis, parasitic on conifers in Japan. Trans. Mycol. Soc. Japan, 34, 323–344. [Google Scholar]
- Taylor, J. E. , & Hyde, K. D. (2003). Microfungi of tropical and temperate palms. Fungal Diversity Press, Hong Kong, 459. [Google Scholar]
- Tejesvi, M. V. , Tamhankar, S. A. , Kini, K. R. , Rao, V. S. , & Prakash, H. S. (2009). Phylogenetic analysis of endophytic Pestalotiopsis species from ethnopharmaceutically important medicinal trees. Fungal Diversity, 38, 167. [Google Scholar]
- Thaung, M. M. (2008). Biodiversity survey of coelomycetes in Burma. Australas. Mycol., 27, 74–110. [Google Scholar]
- Thite, A. N. , & Patil, M. S. (1975). Some parasitic fungi from Kolhapur (Maharashtra). Botanique, 6(2–3), 109–116. [Google Scholar]
- Tibpromma, S. , Mortimer, P. E. , Karunarathna, S. C. , Zhan, F. , Xu, J. , Promputtha, I. , & Yan, K. (2019). Morphology and multi‐gene phylogeny reveal Pestalotiopsis pinicola sp. nov. and a new host record of Cladosporium anthropophilum from edible pine (Pinus armandii) seeds in Yunnan province, China. Pathogens, 8(4), 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy, S. J. , & Newfield, M. J. (2010). The accidental introduction of invasive animals as hitchhikers through inanimate pathways: A New Zealand perspective. Revue Scientifique et Technique (International Office of Epizootics)., 29(1), 123–133. [DOI] [PubMed] [Google Scholar]
- Tsai, I. , Chung, C. L. , Lin, S. R. , Hung, T. H. , Shen, T. L. , Hu, C. Y. , … Ariyawansa, H. A. (2021). Cryptic diversity, molecular systematics, and pathogenicity of genus Pestalotiopsis and allied genera causing gray blight disease of tea in Taiwan, with a description of a new Pseudopestalotiopsis species. Plant Disease, 105(2), 425–443. [DOI] [PubMed] [Google Scholar]
- Turner, P. D. (1971). Micro‐organisms associated with oil palm (Elaeis guineensis Jacq.). Phytopathological Papers, 14, 1–58. [Google Scholar]
- Tuset, J. J. , Hinarejos, C. , & Mira, J. L. (1999). First report of leaf blight on sweet persimmon tree by Pestalotiopsis theae in Spain. Plant Disease, 83(11), 1070. [DOI] [PubMed] [Google Scholar]
- Urtiaga, R. (1986). Indice de enfermedades en plantas de Venezuela y Cuba. Impresos en Impresos Nuevo Siglo. S.R.L., Barquisimeto, Venezuela: 202.
- Von Thümen, F. Contributiones ad Floram Mycologicam Lusitanicam. Cont. No. 9. Inst. Coimbra 1881, 28, 501–505. https://digitalis‐dsp.sib.uc.pt/institutocoimbra/UCBG‐A‐24‐37a41_v028/UCBGA‐24‐37a41_v028_item1/P348.html
- Wang, T. , Xu, G. , Zhou, S. , Niu, Q. , Zang, J. , Yang, T. , Pang, F. , & Tian, F. (2023). Gray blight disease on Euonymus japonicus caused by Pestalotiopsis disseminata in China. Plant Disease, 107(4), 1229. [DOI] [PubMed] [Google Scholar]
- Watanabe, K. , Motohashi, K. , & Ono, Y. (2010). Description of Pestalotiopsis pallidotheae: A new species from Japan. Mycoscience, 51(3), 182–188. [Google Scholar]
- Watanabe, K. , Nakazono, T. , & Ono, Y. (2012). Morphology evolution and molecular phylogeny of Pestalotiopsis (Coelomycetes) based on ITS2 secondary structure. Mycoscience, 53(3), 227–237. [Google Scholar]
- Watanabe, K. , Parbery, D. G. , Kobayashi, T. , & Doi, Y. (2000). Conidial adhesion and germination of Pestalotiopsis neglecta . Mycological Research, 104(8), 962–968. [Google Scholar]
- Wei, J. G. , Xu, T. , Guo, L. D. , Liu, A. R. , Zhang, Y. , & Pan, X. H. (2007). Endophytic Pestalotiopsis species associated with plants of Podocarpaceae, Theaceae and Taxaceae in southern China. Fungal Diversity, 24, 55–74. [Google Scholar]
- Whiteside, J. O. (1966). A revised list of plant diseases in Rhodesia. Kirkia, 5, 87–196. [Google Scholar]
- Williams, T. H. , & Liu, P. S. W. (1976). A host list of plant diseases in Sabah, Malaysia. Phytopathology Paper, 19, 1–67. [Google Scholar]
- Wollenweber, H. W. , & Hochapfel, H. (1936). Contributions to the knowledge of parasitic and saprophytic fungi. II. Monochaetia and Pestalotia, and their relationship to fruit‐rotting. Zeitschrift Fur Pflanzenkrankheiten, Pflanzenpathologie Und Pflanzenschutz, 46, 401–411. [Google Scholar]
- Won, S. J. , Moon, J. H. , Ajuna, H. B. , Choi, S. I. , Maung, C. E. H. , Lee, S. , & Ahn, Y. S. (2021). Biological control of leaf blight disease caused by Pestalotiopsis maculans and growth promotion of Quercus acutissima Carruth container seedlings using bacillus velezensis CE 100. International Journal of Molecular Sciences, 22(20), 11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, E. R. , Rivera, M. C. , & Flynn, M. J. (1998). First report of Pestalotiopsis guepini and Glomerella cingulata on blueberry in Buenos Aires (Argentina). EPPO Bulletin, 28(1‐2), 219–220. [Google Scholar]
- Xu, L. , Kusakari, S. , Hosomi, A. , Toyoda, H. , & Ouchi, A. (1999). Postharvest disease of grape caused by Pestalotiopsis species. Annals of the Phytopathological Society of Japan, 65, 305–311. [Google Scholar]
- Yuan, Z. Q. , Old, K. M. , Midgley, S. J. , & Solomon, D. (1997). Mycoflora and pathogenicity of fungi present on stored seeds from provenances of Eucalyptus pellita . Australasian Plant Pathology, 26(3), 195–202. [Google Scholar]
- Zhang, Y. M. , Maharachchikumbura, S. S. N. , Wei, J. G. , McKenzie, E. H. C. , & Hyde, K. D. (2012). Pestalotiopsis camelliae, a new species associated with grey blight of Camellia japonica in China. Sydowia, 64(2), 335–344. [Google Scholar]
- Zhuang, W.‐Y. (Ed.). (2001). Higher fungi of tropical China (p. 485). Mycotaxon, Ltd. [Google Scholar]


