Abstract
Nitrosomonas europaea has two copies of the operon encoding ammonia monooxygenase (AMO). The nucleotide sequences of the two copies of amoA were obtained, and they were found to differ by one nucleotide. To determine if both copies of amoA were functional, insertional mutagenesis was performed to inactivate either copy of amoA alone. A DNA cassette containing the lacZ and kan genes inserted into amoA was constructed. Mutagenesis was done by using transformation and homologous recombination to mobilize the cassette into the chromosomal copies of amoA. Mutations were obtained in both copies of amoA. Either copy of amoA was sufficient to support growth when the other copy was disrupted. However, inactivation of one copy of amoA, but not the other, resulted in slower growth. Measurements of ammonia-dependent O2 consumption, which depends on AMO, confirmed that the slower-growing mutant had lower activity while the faster-growing mutant had near wild-type levels of activity. Similarly, as measured by [14C]acetylene label incorporation, there was less active AMO present in the slower-growing mutant than in the faster-growing mutant or in the wild type. Northern blot analysis of transcription likewise showed that the slower-growing mutant had less full-sized AMO mRNA.
Nitrosomonas europaea is a chemolithoautotrophic soil bacterium that derives its carbon for growth from CO2 and its energy for metabolism by the oxidation of ammonia (NH3) to nitrite (NO2−) in the process of nitrification (40). Nitrification is a bacterial process that affects the availability of NH4+-based fertilizers applied to agricultural soils (18) and plays a role in the reclamation of NH4+-rich wastewaters (30). The oxidation of NH3 to NO2− by N. europaea is carried out in two steps: first, NH3 is oxidized to hydroxylamine (NH2OH) by ammonia monooxygenase (AMO), and second, NH2OH is oxidized to NO2− by hydroxylamine oxidoreductase (HAO).
One unusual genetic feature of nitrifiers is that most of the genes involved in nitrification identified to date are present in more than one copy in the genome. In N. europaea, the genes encoding AMO (amoC, amoA, and amoB) are adjacent to each other and are present in two copies (24, 35). The putative catalytic polypeptide of AMO is encoded in amoA (15). The transcript for AMO includes amoA and amoB (36). In N. europaea, the genes encoding HAO and the genes encoding cytochrome c-554 (hcy or cyc), which are in proximity to HAO, are found in three copies (3, 12, 25, 34). A gene which apparently codes for another c-type cytochrome is present as two copies immediately downstream of two of the three copies of the gene coding for cytochrome c-554 (3). The reason why N. europaea has multiple copies of some genes remains unclear. Each of the three copies of hao could be disrupted by insertional mutagenesis with a marker gene, indicating that none of the three copies of hao were essential (11). Apparently, the remaining copies compensated for the loss of the mutagenized copy. It is not known if all copies of the duplicated genes are expressed concomitantly or if they are differentially regulated.
Multiple copies of amoA, amoB, and amoC have been identified in other nitrifiers such as Nitrosospira sp. strain NpA V, Nitrosospira briensis, Nitrosolobus multiformis, Nitrosomonas eutrophus, and Nitrosovibrio tenuis (19, 27, 28). In Nitrosospira sp. strain NpA V, amoA was present in three copies, with 99.6% DNA similarity among the copies (28). The transcriptional regulation of the multiple gene copies in these nitrifiers has not yet been examined.
Gene duplication in other bacteria appears to be relatively uncommon outside the rRNA and tRNA genes. Nonetheless, a number of cases have been investigated. In some cases, the duplicate genes are silent copies, e.g., the pilin genes in Neisseria gonorrhoeae MS11 (10). In other cases, genes may be duplicated and expressed in a similar manner, e.g., the tuf genes encoding the elongation factor EF-Tu in Escherichia coli and other gram-negative bacteria (37, 39) and the mer genes in Thiobacillus ferrooxidans encoding mercury resistance (16). Genes may be duplicated but expressed differently, e.g., the genes encoding lysyl-tRNA synthetases, lysS and lysU, in E. coli (17, 32) and the psb genes in Synechocystis sp. (4, 21, 26). There are cases of duplicated operons, e.g., the cbb operon in Alcaligenes eutrophus (22) and an operon encoding two multidrug efflux transporters in Bacillus subtilis (1).
Gene function and expression studies in other bacteria showing multiple gene copies have often made use of insertional mutagenesis to characterize the function and expression of each gene copy. The insertion of an exogenous DNA fragment containing a genetic marker serves to disrupt the target gene, preventing the translation of a functional enzyme from that locus. This paper describes the insertional inactivation of the two copies of amoA in N. europaea with cassettes conferring antibiotic resistance.
MATERIALS AND METHODS
Strains and cell cultures.
Strains of N. europaea and E. coli used are described in Table 1. E. coli cells were grown in Luria-Bertani medium as described previously (33). N. europaea cells were grown in liquid medium (6) and on solid medium (11) containing 50 mM NH4+. The solid medium for N. europaea was liquid medium containing 1% Bacto Agar (Difco Laboratories, Detroit, Mich.). The growth plates were prepared by placing an autoclaved Nytran membrane (6 by 6 cm) (Schleicher & Schuell, Keene, N.H.) on the solid medium. The N. europaea cells were then spread on the membrane and incubated at 30°C. The membrane was transferred to fresh plates weekly. Individual colonies were transferred to liquid culture after about 14 days.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| E. coli | ||
| DH5α | F′ φ80dlacZΔM15 endA1 recA1 gyrA96 thi-1 hsdR17(rK− mK+) supE44 relA1 deoR Δ(lacZYA-argF)U169 | 9 |
| InvαF′ | F′ endA1 recA1 hsdR17(rK− mK+) supE44 thi-1 gyrA96 relA1 φ80lacZΔM15 Δ(lacZYA-argF)U169 | Invitrogen |
| N. europaea | ||
| ATCC 19178 | American Type Culture Collection | |
| A141 | Insertion of lacZ/kan into the BamHI site of amoA1 | This study |
| A142 | Insertion of lacZ/kan into the BamHI site of amoA2 | This study |
| A1 | Insertion of kan into the BamHI site of amoA1 | This study |
| A2 | Insertion of kan into the BamHI site of amoA2 | This study |
| Plasmids | ||
| pNHA10 | 842-bp PCR fragment containing most of amoA cloned into pCRII vector | This study |
| pNHA11 | pNHA10 with a mutation in amoA at position 715 (24), creating a BamHI site | This study |
| pNHA12 | amoA insert cut out of pNHA11 with EcoRI and cloned into EcoRI site of pRL139 | This study |
| pNHA14 | lacZ/kan fragment from pKOK6.1 inserted into BamHI site of pNHA12 | This study |
| pNHA15 | kan from pUC4 pKSAC inserted into BamHI site of pNHA12 | This study |
| pUC4 KSAC | Plasmid containing the kan cassette from Tn903 | Pharmacia |
| pKOK6.1 | Plasmid containing a promoterless lacZ and kan with its own promoter | 20 |
| pCRII | TA PCR cloning vector (Kanr AmprlacZa f1ori ColE1 ori) | Invitrogen |
| pRL139 | pUC19 with a symmetrical multiple cloning site | 5 |
The NO2− formation in cultures, which can be correlated to growth, was measured colorimetrically (8). NO2− formation, rather than NH3 consumption, was used as a measure of metabolic activity since it is a simpler assay. In N. europaea, the vast majority of NH3 consumed is oxidized to NO2− rather than incorporated into cell mass such that the rate of NH3 consumption is indistinguishable from that of NO2− formation. Growth was measured as cell density by light scattering at 600 nm in a spectrophotometer (model DU7; Beckman, Palo Alto, Calif.) as well as by protein determinations. The protein content of the cell suspensions was estimated by the biuret assay (7), after the cells were solubilized in 3 N NaOH for 30 min at 65°C. Bovine serum albumin was used as a standard. Protein content was proportional to optical density throughout the course of cell growth. NH3-dependent O2 uptake, which depends on AMO activity, was measured with a Clark-type oxygen electrode (Yellow Springs Instruments Co., Yellow Springs, Ohio) as described previously (13). [U-14C]acetylene labeling of AMO was done as described previously (14).
DNA manipulation.
Genomic and plasmid DNA preparations, DNA restriction digestions, Southern hybridizations, and other standard DNA manipulations were done as described previously (2, 33). DNA probes were labeled by random priming using the Prime-a-Gene kit from Promega Corporation (Madison, Wis.) and with [α-32P]dCTP (either 3,000 or 6,000 Ci/mmol; DuPont NEN Products, Wilmington, Del.). The hybridization signals were visualized on a PhosphorImager (Molecular Dynamics, Sunnyvale Calif.) and analyzed by densitometry by using the ImageQuant (Molecular Dynamics) software. DNA sequencing and oligonucleotide synthesis were done by the Center for Gene Research Central Laboratory, Oregon State University. The PCRs were performed on an Easycycler (Ericomp, San Diego, Calif.) instrument by using either Taq DNA polymerase (Perkin-Elmer, Branchburg, N.J.) or Pfu DNA polymerase (Stratagene, La Jolla, Calif.) in a 25-μl reaction volume with the following program: 1 repetition of cycle A (2 min at 94°C) and then 40 repetitions of cycle B (1 min at 94°C, 1 min at 50°C, and 1.5 min at 72°C).
Total RNA was isolated as described previously (31) with the following modifications. RNA was prepared by inhibiting RNase activity with vanadyl ribonucleoside (Life Technologies, Rockville, Md.) before lysis followed by acid-phenol chloroform extraction. RNA samples were resuspended in diethyl pyrocarbonate-treated H2O and resolved in a denaturing 1.2% agarose gel. Prior to electrophoresis, the RNA was prestained with ≤5 μg of ethidium bromide per ml of loading buffer. The RNA was blotted into Nytran membranes by using a vacuum Hoefer TE70 blotter (Pharmacia Biotech, Piscataway, N.J.), leaving high-molecular-weight DNA in the gel. This technique allowed RNA to be harvested from relatively small volumes (≤200 ml) of low-density cultures (optical density at 600 nm [OD600] of ≤0.03) without the need for CsCl step-gradient centrifugation.
amoA gene nomenclature.
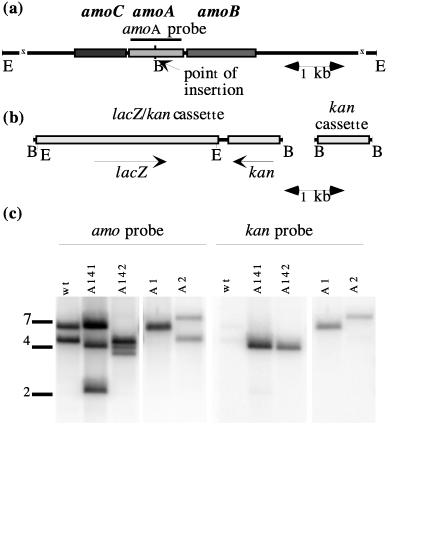
N. europaea contains two copies of amoA which are distinguishable by Southern hybridization (25). An EcoRI digest of N. europaea genomic DNA probed with an amoA probe detects two DNA fragments (6.5 and 5 kb [Fig. 1c]). In this paper, the amo copies contained on the lower and upper EcoRI fragments are referred to as amoA1 and amoA2, respectively. This nomenclature parallels that used for the multiple copies of amoA and amoB in Nitrosospira sp. strain NpA V (28).
FIG. 1.
Physical map of the genes coding for AMO, the DNA cassettes used, and Southern hybridization to a probe for amoA and for the kan cassette. (a) The three genes coding for known and putative AMO peptides and the probe used to detect amoA are shown. The BamHI (B) restriction site created for the insertion of the cassettes is shown. The sizes of the EcoRI (E) fragments for the two gene copies are approximately 5 and 6.5 kb. (b) The lacZ/kan and kan cassettes that were inserted into the BamHI site. (c) Southern hybridization to the amo probe and the kan probe. wt, N. europaea wild-type strain.
DNA amplifications and constructs.
The DNA constructs are described in Table 1. The lacZ/kan cassette used in these experiments was isolated from the plasmid pKOK6.1 (20), which was a gift from W. Lotz and T. Bauer (Friedrich-Alexander-Universität). The lacZ/kan cassette contains a promoterless lacZ gene, and in the opposite orientation it contains a kan gene with its own promoter (Fig. 1b). There is a transcriptional terminator between the two genes. Although the insertion of the cassette into amoA allowed the amo promoter to drive the expression of lacZ, in this study the cassette was used only for purposes of insertional mutagenesis. A second cassette containing the kan gene with its promoter and without lacZ (from pUC4 KSAC; Pharmacia Biotech) was also used (Fig. 1b).
The following manipulations were made to produce the DNA fragments containing amoA and the cassettes that were to be used in the transformation experiments. First, a 0.8-kb DNA fragment containing amoA was amplified by using the oligonucleotide primers ANT and ACT (Table 2). This fragment was cloned into the pCRII (Stratagene) vector to form pNHA10. In order to use the BamHI sites flanking the lacZ/kan cassette, a BamHI insertion site had to be created in amoA. The BamHI site was created by site-directed mutagenesis in which a thymine nucleotide at position 338 of the amoA coding region was changed to cytosine, creating a BamHI site at that location. The DpnI-PCR mutagenesis method (ExSitePCR; Stratagene) uses two overlapping oligonucleotide primers containing the mutant sequence which span the mutation site in opposite directions. PCR was done on a circular plasmid containing wild-type amoA as a template (pNHA10), with the mutagenic primers, AM5 and AM4 (Table 2), and the high-fidelity Pfu DNA polymerase. The PCR parameters were as follows: 12 cycles, with 1 cycle consisting of 30 s at 94°C, 30 s at 50°C, and 15 min at 72°C. The template DNA was removed by digestion with DpnI, which requires methylated DNA for activity. The newly synthesized PCR product, containing the mutated site (pNHA11), was transformed into the E. coli DH5α. To ensure that the newly created BamHI site in amoA would be unique, the EcoRI fragment containing amoA was subcloned out of pNHA11 and inserted between the EcoRI sites of the vector pRL139 (5) (pNHA12). The lacZ/kan cassette, excised from pKOK6.1 with BamHI, was inserted into the BamHI site in amoA on plasmid pNHA12 to form pNHA14. Transformants were selected for kanamycin resistance. Colonies were screened by restriction digestion for clones containing the lacZ/kan cassette inserted into amoA in the correct orientation. In a similar manner, a cassette containing kan alone was excised from pUC4 KSAC with BamHI and cloned into the BamHI site of amoA to form pNHA15.
TABLE 2.
Oligonucleotide primer sequences
| Oligo- nucleotide primer | Sequencea | Refer- ence |
|---|---|---|
| ANT | 5′-GCGGCCAAGATGCCGCCG | 24 |
| ACT | 5′-GATCCCCTCTGGAAAGCC | 24 |
| AM5 | 5′-CTCTGGTTGGAGGTGGATCCTTCGGTC | 24 |
| AM4 | 5′-ACCGAAGGATCCACCTCCAACCAGAGCCG | 24 |
| NPT1 | 5′-ATGAGCCATATTCAACGGGAAACG | 29 |
| NPT2 | 5′-CTGCAGGGGGGGGGGGGCGCTGAG | 29 |
| S2301 | 5′-TTCTGCAGTACCGTGAGGGAAAGG | 37 |
| S23R01 | 5′-GGCTGCTTCTAAGCCAAC | 37 |
The changed nucleotide is shown in bold type.
The amo probe used in the hybridizations was a 0.8-kb PCR fragment containing amoA (Fig. 1a). The amoA probe was amplified by using primers ANT and ACT from genomic DNA or from pNHA10. The kan probe was amplified from the plasmid pUC4 KSAC by using primers NPT1 and NPT2 (Table 2). The probe for the 23S rRNA was produced by DNA amplification of N. europaea genomic DNA by using primers S2301 and S23R01 (Table 2) (38).
The DNA sequence of the two copies of amoA was obtained from at least two PCR clones for each copy. Genomic DNA obtained from mutagenized strains of N. europaea with an insertion of the mutagenic cassette into one of the copies of amoA was used as a template for the PCR reactions. Copy-specific amplification of amoA fragments was obtained by using one primer site within the lacZ/kan cassette and a second primer site outside the coding region for amoA.
Cell transformation by electroporation.
N. europaea cells (0.5 liter) from a liquid culture in early stationary growth phase (OD600 = 0.1) were harvested by centrifugation and washed three times with sterile H2O. The sedimented cells were resuspended in 1.5 ml of H2O and kept on ice until use. Cell transformation with plasmids pNHA14 and pNHA15 was done by electroporation in an ElectroPorator (Invitrogen, Carlsbad, Calif.) in 1-mm-gap cuvettes (Invitrogen). Electroporation was done at 1,200 V, 25 μF, and ∞ resistance. In a prechilled cuvette, 120 μl of cells were mixed with 1 μg (1 μl) of pNHA14 or pNHA15 DNA and pulsed. The cells were transferred to 0.5 liter of fresh medium and allowed to grow for 15 h under nonselective conditions at 30°C while shaking. Cells were then plated on solid nutrient medium as described above containing kanamycin sulfate (10 μg/ml).
Nucleotide sequence accession numbers.
The nucleotide sequences for amoA1 and amoA2 from N. europaea have been submitted to the GenBank database under accession numbers AF058691 and AF058692, respectively.
RESULTS
DNA sequencing, mutagenesis, and corroboration of the recombination events.
The DNA sequences obtained for the coding regions of amoA1 and amoA2 revealed that they differed by only a single nucleotide (position 65 of the amoA coding region, C for amoA1 and T for amoA2), which resulted in an amino acid change (Thr for amoA1; Met for amoA2). The nucleotide sequence of amoA2 was identical to the amoA sequence determined by McTavish et al. (24). In addition, the 165-bp intergenic region between the end of amoC and the beginning of amoA was identical in amoA1 and amoA2.
DNA fragments containing either lacZ/kan or kan were inserted into copies of amoA in N. europaea by electroporation and recombination by using plasmids pNHA14 and pNHA15 (Table 1). Although the fragments were not targeted to a particular copy of amoA, we found roughly equal numbers of recombination events in the copies. The transformation efficiency was calculated to be 2 × 10−6 mutant colonies per cell. Kanamycin-resistant colonies grew sufficiently in 14 days to transfer into liquid medium.
Southern hybridizations were used to locate the point of insertion of the mutagenic cassette in the N. europaea genome. Genomic DNA from kanamycin-resistant strains was digested with the endonuclease EcoRI and blotted onto Nytran Plus nylon membranes. The DNA blots were hybridized to either the amoA or kan probe. In wild-type cells, two fragments (6.5 and 5 kb) were detected with the amo probe after EcoRI digestion of genomic DNA (Fig. 1c). In the mutant N. europaea A141, one wild-type fragment (6.5 kb) was detected, but the second wild-type fragment was replaced by two new fragments (2.4 and 4 kb). An insertion of the lacZ/kan cassette into a copy of amoA would increase the size of the highlighted fragment. However, because two internal EcoRI sites flanking the lacZ gene in the lacZ/kan cassette results in the excision of lacZ by EcoRI, the expected result would be the conversion of the amoA hybridizing fragment into two new smaller hybridizing fragments. Similarly, in strain A142, the 5-kb wild-type fragment is retained, while the 6.5-kb fragment was replaced by 3.8- and 4-kb fragments.
In mutagenesis experiments done with the kan cassette, a different result was expected than that obtained with the lacZ/kan cassette. The kan cassette has no internal EcoRI sites, and therefore an insertion of the cassette into amoA should simply increase the size of the amoA hybridizing fragment. Indeed, when genomic DNA from mutant strain A1 was probed with amoA, the 5-kb fragment had been shifted to about 6.2 kb, which appeared as a doublet with the wild-type 6.5-kb fragment. In strain A2, the wild-type 5-kb fragment was highlighted by the amoA probe, as was a 7.7-kb fragment resulting from an insertion of the kan cassette into the larger amoA fragment. In all cases, the hybridization patterns detected with the amo probe showed that the kanamycin-resistant strains had only one of the two amoA gene copies disrupted, and the pattern was consistent with the insertion of a cassette into one of the copies of amoA.
Hybridizations with the kan probe confirmed that the lacZ/kan and kan cassettes were inserted into the DNA fragments containing amoA. Strains A141 and A142 both had single fragments of about 4 kb highlighted by the kan probe. The kan probe highlighted 6.2- and 7.7-kb fragments in strains A1 and A2, respectively. These results were consistent with insertions of either the lacZ/kan or kan cassette into the two copies of amoA. In all clones examined, no insertions of the cassettes into other locations in the genome were detected. No hybridization to the kan cassette in the wild type was observed.
Thus, clonal cell lines were obtained with insertions of either lacZ/kan (N. europaea A141 and A142) or kan alone (N. europaea A1 and A2) into both copies of amoA alone. The amoA::lacZ/kan mutations in N. europaea have been stable in culture for over a year. The amoA::kan mutations appeared to be as stable as the insertion of the lacZ/kan cassette. The ability to get insertions into both copies of amoA singly indicated that neither copy was essential and both were sufficient to support growth under the conditions used.
Phenotypical differences in the N. europaea mutant strains.
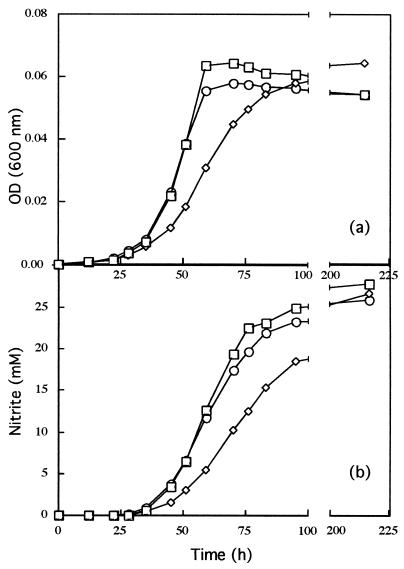
Since N. europaea requires NH3 for rapid growth, AMO plays a critical role in the energetics and metabolism of this organism. We therefore considered whether the mutations in amoA influenced growth rates of the mutant strains. To test this possibility, cells from early-stationary-phase cultures of mutant and wild-type strains of similar optical densities were inoculated into fresh medium to identical optical densities (OD600 = 0.005) and their growth was monitored by their OD600 and by NO2− formation. N. europaea A141, with a lacZ/kan insertion in amoA1 (5-kb DNA fragment [Fig. 1c]) grew slower than wild-type cells, although it eventually reached the same cell density as wild-type cultures did (Fig. 2a). N. europaea A142, with a lacZ/kan insertion in amoA2 (6.5-kb DNA fragment [Fig. 1c]) had a growth curve more similar to that of wild-type cells. N. europaea A1 and A2 exhibited growth patterns similar to N. europaea A141 and A142, respectively, confirming the observed copy-specific phenotypes (data not shown). The growth rates of wild-type and mutant strains were calculated from OD600 measurements of actively growing cultures during exponential growth from several growth curves. Although special attention was paid to consistency while performing replicate growth experiments, variations in the growth rates of the wild-type and mutant strains were observed. In 6 replicate experiments, the mean growth rate for the wild-type strain was 0.093 ± 0.015 h−1. In 8 replicate experiments, the mean growth rate of amoA1 mutants (A141 and A1) was 0.067 ± 0.009 h−1. In 10 replicate experiments, the mean growth rate of amoA2 mutants (A142 and A2) was 0.084 ± 0.017 h−1. The differences in growth rates between strains was more apparent when the growth rates of the mutant strains were expressed as a percentage of the wild-type growth rate in each experiment. When the growth rates were compared this way, the averaged results from all the replicate experiments showed that strains with amoA1 inactivated generally had slower growth rates than did wild-type cells (about 75% of the wild type rate, while strains with amoA2 inactivated had growth rates similar to that of wild-type cells (about 97% of the wild-type growth rate [Table 3]). When metabolic activity was monitored by NO2− formation, curves similar to those based on optical density measurements were obtained (Fig. 2b). All mutants eventually reached NO2− levels similar to that of the wild-type strain. In these experiments, the cultures consumed about half the available NH3.
FIG. 2.
Growth of the wild-type, A141, and A142 strains of N. europaea. (a) OD600 during a 215-h growth comparison experiment (see text). Symbols: □, N. europaea (wild type) showing a growth rate of 0.089 h−1; ◊, N. europaea A141 showing a growth rate of 0.062 h−1; ○, N. europaea A142 showing a growth rate of 0.087 h−1. (b) NO2− accumulation of the growth experiment depicted in panel a.
TABLE 3.
Growth rates, NH3-dependent O2 consumption, [U-14C]acetylene incorporation, and AMO mRNA
| Straina | Growth rateb (n)c | O2 uptaked (n) | [14C] incorp.e (n) | AMO mRNAf (n) |
|---|---|---|---|---|
| Wild type | 100 (6) | 100 (5) | 100 (4) | 100 (12) |
| amoA1 mutants | 75.2 ± 10.0 (8) | 85.2 ± 5.76 (6) | 80.7 ± 10.7 (4) | 63 ± 14 (12) |
| amoA2 mutants | 96.9 ± 6.7 (10) | 104.0 ± 6.9 (14) | 92.4 ± 6.6 (4) | 97 ± 23 (12) |
amoA1 mutants include strains A141 and A1, and amoA2 mutants include strains A142 and A2.
Mean growth rate (hour−1) as a percentage of the wild-type activity ± 1 standard deviation. The rate was calculated from an exponential curve fit from growth curves monitored by OD600.
n is the number of replicates used to calculate the mean and variance.
Mean activity as a percentage of the wild-type activity ± 1 standard deviation.
Mean amount of [14C] incorporated into the 27-kDa peptide of AMO as a percentage of the wild-type amount ± 1 standard deviation.
Mean amount of amo mRNA as a percentage of the wild-type amo mRNA amount ± 1 standard deviation.
NH3-dependent O2 consumption, which requires AMO, was determined for wild-type and mutant strains. To compare cells in a similar physiological state, cells were harvested at the same optical density rather than at the same culture age. These cultures generally had accumulated similar amounts of NO2−. However, the NH3-dependent O2 consumption differed in the wild-type and mutant cells. For example, by using data from a typical experiment where the cells were harvested at about 0.05 OD600, the NH3-dependent O2 uptake values (in nanomoles of O2 consumed minute−1 OD600−1) were 2.62 for the wild-type cells, 2.39 for strain A141, 2.45 for strain A1, 3.09 for strain A142, and 2.97 for strain A2. The activity determinations we observed in a given experiment varied, depending on the optical density at the time of harvest. To allow a comparison of experiments performed as described above but at optical densities other than 0.05, the activities were expressed as a percentage of wild-type activity in each particular experiment. We compared NH3-dependent O2 consumption of the wild type and mutants from cultures harvested at optical densities from 0.028 to 0.050. N. europaea strains with inactivated amoA2 had a mean NH3-dependent O2 consumption of about 104% of the wild-type level (Table 3). N. europaea strains with inactivated amoA1 had a mean NH3-dependent O2 consumption of about 85% of the wild-type level. The same trends were observed when rates were normalized to protein content. Thus, as with the growth rates, the NH3-dependent O2 consumption data showed differences between the mutant strains and wild-type cells. The amoA1 mutant strains had lower rates than wild-type cells, while amoA2 mutants had activities closer to that of wild-type cells.
Given the observed differences between the strains regarding growth rates and NH3-dependent O2 consumption, we were interested to know if these differences were also reflected in the amount of active AMO enzyme. Cells were harvested at the same optical density (OD600 = 0.03), washed, resuspended in fresh medium without NH4+ but containing 10 mM hydrazine sulfate as a reductant source, and incubated with [14C]acetylene (5 × 106 cpm [14]) for 45 min (which is sufficient to reach completion) at 30°C. [14C]acetylene is a suicide substrate, which can be used to label the 27-kDa polypeptide of AMO (14). When the reaction is allowed to go to completion, the amount of label in the 27-kDa polypeptide is proportional to the amount of active AMO present when the acetylene was introduced. Protein extracts were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the labeled peptides analyzed by densitometry on the PhosphorImage of the gel. The mutant strain A141 had less label incorporated (80.7% of the wild-type level) than either wild-type N. europaea (taken as 100%) or strain A142 (92.4% of the wild-type level) (Table 3). These values were based on four replicate samples.
Transcriptional levels in the N. europaea mutant strains.
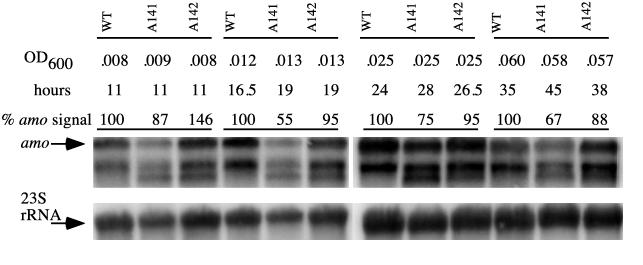
Because one of the copies of amo was affected in each mutant strain, there was the possibility that the strains would show different transcription levels of the amo operon. To test if there were any differences in the mRNA levels transcribed from the two copies of amoA, total RNA was isolated from the mutant (A141 and A142) and wild-type strains and blotted for analysis by Northern hybridization. Cells were harvested at different points on their growth curve starting from early growth (<0.03 OD600) to early stationary growth (≤0.08 OD600). As described above, the different strains were harvested at similar optical densities, albeit at different times, for purposes of comparison (Fig. 3). Total RNA was extracted, blotted, and probed with a probe for amoA or for the 23S rRNA. The amount of RNA loaded onto the gel was approximately the same based on the intensity observed by ethidium bromide staining. The Northern blots with RNA from wild-type N. europaea cells and an amoA probe (Fig. 3) revealed both the full-sized amo transcript (3.5 kb) as well as a second fragment (about 2.4 kb). Both fragments are also highlighted by an amoB probe, but neither fragment has yet been fully characterized (35). The 2.4-kb fragment may be derived from the 3.5-kb transcript or may represent a separate transcript. Northern blots of N. europaea A141 and A142 probed with amoA highlighted an additional smaller transcript (approximately 2.1 kb) not present in wild-type cells (Fig. 3). Since the lacZ/kan cassette had been inserted into amoA as a transcriptional fusion, we expected to find a 4.5-kb fragment highlighted in the Northern blots when probed with amoA or lacZ. Instead, the amoA probe highlighted the 2.1-kb fragment, and no fragment hybridized to the lacZ probe. The amoB probe did not hybridize to the 2.1-kb fragment. However, the Southern hybridization data clearly show that the lacZ/kan cassette was inserted into amoA (Fig. 1). Thus, the transcript containing lacZ is apparently rapidly degraded. The 2.1-kb band would be consistent with a truncated transcript encoding part of amoA and possibly part of another gene. No other labeled fragments were detected.
FIG. 3.
AMO mRNA levels during growth of the wild-type, A141, and A142 strains. The optical densities and the time elapsed at the sampling time are shown. The position for the full-sized AMO mRNA (about 3.5 kb) is indicated by an arrow. The hybridization to the rRNA after stripping the same blot is shown below the amo panel. The amo levels are given as a percent of the wild-type level for each optical density group. These levels were calculated by dividing the intensity observed in the amo signal by the intensity observed in the rRNA signal. The relative intensity for each time point was divided by the wild-type intensity and this value was multiplied by 100. The truncated amo transcript can be observed as an extra band in the amo hybridization and corresponds to approximately 2.1 kb. WT, wild type.
The intensities of the signals detected after hybridizing the amoA probe and, after stripping the blot, hybridizing to the 23S rRNA probe, were quantified by densitometry. The hybridization to the 23S rRNA probe was used to normalize the differences in the amounts of RNA in the blot. These intensities were used to calculate the ratio of AMO mRNA/rRNA (Fig. 3). At early and exponential stages of growth, N. europaea A141 had 20 to 40% less AMO mRNA than the wild-type strain (Fig. 3). N. europaea A142 had a greater amount than the wild type during early growth but 5 to 12% less AMO mRNA during exponential growth. When the results of 12 replicate AMO mRNA level determinations at several time points were averaged, they showed the same trend (Table 3). The N. europaea strains with inactive amoA2 averaged 97% of the AMO mRNA of the wild type. N. europaea strains with inactivated amoA1 averaged 63% of the AMO mRNA level of the wild-type cells during growth (Table 3).
DISCUSSION
N. europaea has two copies of the genes coding for AMO, an enzyme essential for nitrification. The degree of similarity in the DNA sequences of the copies of amoA is striking, but the three copies of amoA in Nitrosospira sp. strain NpA V also have a similar degree of similarity, differing by only one or two nucleotides (28). The sequence of amoA in N. europaea is about 75% similar at the DNA level to that of amoA in Nitrosospira sp. strain NpA V and about 88% similar at the amino acid level.
The existence of almost identical functional copies of genes in the genomes coding for peptide components of a key enzyme in these lithoautothrophic bacteria is intriguing. Basic questions about these genes, whether they are both functional and if either is essential, were addressed by insertional inactivation of the two copies of amoA. This study shows that a DNA cassette can be inserted into either of the two copies of amoA. Presumably (although we do not show this directly), the insertion of the markers precluded the synthesis of a functional AMO enzyme from the affected locus. Because the complete inactivation of AMO will be lethal to a nitrifier and because the inactivation of either copy could be obtained, we conclude that neither copy of amoA is essential to the cell and that both copies are functional.
The growth curves, NO2− production, and activity measurements were different for the two mutants of amo. The mutations in amoA2 resulted in a near wild-type phenotype with regard to growth and NH3-dependent O2 uptake rates. The mutations in amoA1 resulted in slower growth and reduced NH3-dependent O2 consumption (Table 3). Differences in the mutant strains were also revealed at the transcriptional level. An analysis of AMO mRNA levels by Northern hybridizations showed that AMO mRNA levels in the amoA2 mutant were nearly the same as in wild-type cells, while the amoA1 mutant strain consistently showed reduced levels of AMO mRNA (with a mean decrease of 37%). The pattern of mRNA expression in the wild-type strain and strains with either an amoA1 or amoA2 mutation paralleled the results seen for NH3-dependent O2 consumption (Table 3). These results suggested that N. europaea was able to compensate almost entirely for the disruption in the amoA2 gene copy but not the disruption of the amoA1 gene copy under the conditions tested. This observation leads to the conclusion that amoA1 and amoA2 are regulated differently.
A survey of the literature on duplicated genes (see above) shows that these duplications may serve a variety of purposes in the bacterium. Perhaps the two copies of amo provide a mechanism to amplify the rate of transcription. In natural environments, N. europaea seems unlikely to find itself in a situation where NH3 is in an abundant and constant supply. Nonetheless, flushes of ammonia are possible within the local environment of the bacteria. The presence of two copies of the amo operon might allow more-rapid generation of AMO mRNA during a flush of ammonia. In this model, both genes are regulated similarly and both are used to meet the demand for increased transcription. A precedent for an additive gene expression response for optimal growth exists in Salmonella typhimurium where the EF-Tu is encoded by two highly similar genes (37). When either copy is inactivated by insertional mutagenesis, levels of EF-Tu in the cell were reduced by about 65% (39). However, unlike EF-Tu in S. typhimurium, when amoA1 is inactivated in N. europaea, the cell can partially compensate for the loss of that gene copy, and in the case of an inactivated amoA2, near wild-type AMO activity is observed during optimal growth conditions.
Alternatively, differential expression of two highly similar genes is expected when each copy has a primary role associated with particular conditions where the bacterium must survive. In N. europaea, a likely system would be to have a gene copy specialized to suboptimal substrate levels and another specialized to optimal substrate levels where each copy is differentially regulated rather than expressed in an additive form. This system would also allow N. europaea to synthesize a large amount of AMO in a short time when the substrate is abundant and to survive during low substrate levels. Maintaining a basal expression by using one gene copy during suboptimal conditions would be a more efficient use of the vital endogenous energy of the cell for this bacterium with a generation time of 8 h. Differential regulation of expression of either copy of amo in N. europaea in response to environmental conditions would be similar to the case of the lysyl-tRNA synthetase genes of E. coli and other members of the family Enterobacteriaceae. The lysyl-tRNA synthetase genes of E. coli are encoded by two genes, lysS and lysU, which are differentially expressed, depending on the growth conditions of the cell. The nucleotide and deduced amino acid sequences of these genes are highly similar (23, 32). Both genes are expressed, and lysU can functionally replace an inactivated lysS (17). The lysS gene is expressed constitutively, while lysU gene is expressed at low levels but is induced by several factors, including heat shock, low pH, anaerobiosis, or the addition of small hydrophobic leucine peptides or l-alanine or l-leucine to minimal medium.
The differences in growth of the amoA mutants are apparently in contrast to the situation with hao in N. europaea. Insertional mutagenesis of hao showed that none of the three copies was essential, and there was no observed growth phenotype associated with the mutations (11). However, since there are three copies of hao, double mutations might be required to observe differences in their regulation.
Whether the above similarities to other bacteria are relevant to the amo genes of N. europaea is unknown, but they provide models which can be explored. The molecular mechanism by which the expression of the two copies of amo are regulated remains to be elucidated. Also, we have yet to determine if the two transcripts differ in their stability and turnover rate. The purpose for this differential transcription rate between the copies of amo also needs to be determined. Further investigation into the transcription patterns under different growth conditions is needed to characterize the regulation of amo expression.
ACKNOWLEDGMENTS
This work was supported by DOE grant DE-FG03-97ER20266 to D. J. Arp and L. A. Sayavedra-Soto and EPA grant R821405-01 to D. J. Arp and P. J. Bottomley.
REFERENCES
- 1.Ahmed M, Lyass L, Markham P N, Taylor S S, Vázquez-Laslop N, Neyfakh A A. Two highly similar multidrug transporters of Bacillus subtilis whose expression is differentially regulated. J Bacteriol. 1995;177:3904–3910. doi: 10.1128/jb.177.14.3904-3910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, et al. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1993. [Google Scholar]
- 3.Bergmann D J, Arciero D M, Hooper A B. Organization of the hao gene cluster of Nitrosomonas europaea: genes for two tetraheme c cytochromes. J Bacteriol. 1994;176:3148–3153. doi: 10.1128/jb.176.11.3148-3153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bustos S A, Schaefer M R, Golden S S. Different and rapid responses of four cyanobacterial psbA transcripts to changes in light intensity. J Bacteriol. 1990;172:1998–2004. doi: 10.1128/jb.172.4.1998-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elhai J, Wolk C P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988;68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 6.Ensign S A, Hyman M R, Arp D J. In vitro activation of ammonia monooxygenase from Nitrosomonas europaea by copper. J Bacteriol. 1993;175:1971–1980. doi: 10.1128/jb.175.7.1971-1980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gornall A G, Bardawill C J, David M M. Determination of serum proteins by means of the Biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 8.Hageman R H, Hucklesby D P. Nitrate reductase in higher plants. Methods Enzymol. 1971;23:491–503. [Google Scholar]
- 9.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 10.Hass R, Veit S, Meyer T F. Silent pilin genes of Neisseria gonorrhoeae MS11 and the occurrence of related hypervariant sequences among other gonococcal isolates. Mol Microbiol. 1992;6:197–208. doi: 10.1111/j.1365-2958.1992.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 11.Hommes N G, Sayavedra-Soto L A, Arp D J. Mutagenesis of hydroxylamine oxidoreductase in Nitrosomonas europaea by transformation and recombination. J Bacteriol. 1996;178:3710–3714. doi: 10.1128/jb.178.13.3710-3714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hommes N G, Sayavedra-Soto L A, Arp D J. Sequence of hcy, a gene encoding cytochrome c-554 in Nitrosomonas europaea. Gene. 1994;146:87–89. doi: 10.1016/0378-1119(94)90838-9. [DOI] [PubMed] [Google Scholar]
- 13.Hyman M R, Arp D J. 14C2H2- and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J Biol Chem. 1992;267:1534–1545. [PubMed] [Google Scholar]
- 14.Hyman M R, Arp D J. The small-scale production of [U-14C]acetylene from Ba14CO3: application to labeling of ammonia monooxygenase in autotrophic nitrifying bacteria. Anal Biochem. 1990;190:348–353. doi: 10.1016/0003-2697(90)90206-o. [DOI] [PubMed] [Google Scholar]
- 15.Hyman M R, Wood P M. Suicidal inactivation and labeling of ammonia monooxygenase by acetylene. Biochem J. 1985;227:719–725. doi: 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue C, Sugawara K, Kusano T. The merR regulatory gene in Thiobacillus ferrooxidans is spaced apart from the mer structural genes. Mol Microbiol. 1991;5:2707–2718. doi: 10.1111/j.1365-2958.1991.tb01979.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami K, Nakamura Y. Differential regulation of two genes encoding lysyl-tRNA synthetases in Escherichia coli: lysU-constitutive mutations compensate for a lysS null mutation. Mol Microbiol. 1992;65:1739–1745. doi: 10.1111/j.1365-2958.1992.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 18.Keeney D E. Inhibition of nitrification in the soil. In: Prosser J I, editor. Nitrification. Vol. 20. Oxford, England: IRL Press; 1986. pp. 99–115. [Google Scholar]
- 19.Klotz M G, Norton J M. Sequence of an ammonia monooxygenase subunit A-encoding gene from Nitrosospira sp. NpA V. Gene. 1995;163:159–160. doi: 10.1016/0378-1119(95)00392-j. [DOI] [PubMed] [Google Scholar]
- 20.Kokotek W, Lotz W. Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene. 1989;84:467–471. doi: 10.1016/0378-1119(89)90522-2. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni R D, Golden S S. Adaptation to high light intensity in Synechococcus sp. strain PCC 7942: regulation of three psbA genes and two forms of D1 protein. J Bacteriol. 1994;176:959–965. doi: 10.1128/jb.176.4.959-965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusian B, Bednarski R, Husemann M, Bowien B. Characterization of the duplicate ribulose-1,5-bisphosphate carboxylase genes and cbb promoters of Alcaligenes eutrophus. J Bacteriol. 1995;177:4442–4450. doi: 10.1128/jb.177.15.4442-4450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leveque F, Plateau P, Dessen P, Blanquet S. Homology of lysS and lysU, the two Escherichia coli genes encoding distinct lysyl-tRNA synthetase species. Nucleic Acids Res. 1990;18:305–312. doi: 10.1093/nar/18.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McTavish H, Fuchs J A, Hooper A B. Sequence of the gene coding for ammonia monooxygenase in Nitrosomonas europaea. J Bacteriol. 1993;175:2436–2444. doi: 10.1128/jb.175.8.2436-2444.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McTavish H, LaQuier F, Arciero D, Logan M, Mundfrom G, Fuchs J A, Hooper A B. Multiple copies of genes coding for electron transport proteins in the bacterium Nitrosomonas europaea. J Bacteriol. 1993;175:2445–2447. doi: 10.1128/jb.175.8.2445-2447.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohamed A, Eriksson J, Osiewacz H D, Jansson C. Differential expression of the psbA genes in the cyanobacterium Synechocystis 6803. Mol Gen Genet. 1993;238:161–168. doi: 10.1007/BF00279543. [DOI] [PubMed] [Google Scholar]
- 27.Norton J M, Klotz M G. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Homology among ammonia monooxygenase genes from the ammonia oxidizing bacteria, abstr. K-164; p. 564. [Google Scholar]
- 28.Norton J M, Low J M, Klotz M G. The gene encoding ammonia monooxygenase subunit A exists in three nearly identical copies in Nitrosospira sp. NpA V. FEMS Microbiol Lett. 1996;139:181–188. doi: 10.1111/j.1574-6968.1996.tb08200.x. [DOI] [PubMed] [Google Scholar]
- 29.Oka A, Sugisaki H, Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981;147:217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- 30.Painter H A. Nitrification in the treatment of sewage and waste-waters. In: Prosser J I, editor. Nitrification. Vol. 20. Washington, D.C: IRL Press; 1986. pp. 211–213. [Google Scholar]
- 31.Reddy K J, Gilman M. Preparation of bacterial RNA. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley and Sons; 1993. pp. 4.4.1–4.4.7. [Google Scholar]
- 32.Saluta M V, Hirshfield I N. The occurrence of duplicate lysyl-tRNA synthetase gene homologs in Escherichia coli and other procaryotes. J Bacteriol. 1995;177:1872–1878. doi: 10.1128/jb.177.7.1872-1878.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Sayavedra-Soto L A, Hommes N G, Arp D J. Characterization of the gene encoding hydroxylamine oxidoreductase in Nitrosomonas europaea. J Bacteriol. 1994;176:504–510. doi: 10.1128/jb.176.2.504-510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayavedra-Soto, L. A., N. G. Hommes, and D. J. Arp. Unpublished hybridization data.
- 36.Sayavedra-Soto L A, Hommes N G, Russell S A, Arp D J. Induction of ammonia monooxygenase and hydroxylamine oxidoreductase mRNAs by ammonium in Nitrosomonas europaea. Mol Microbiol. 1996;20:541–548. doi: 10.1046/j.1365-2958.1996.5391062.x. [DOI] [PubMed] [Google Scholar]
- 37.Sela S, Yogev D, Razin S, Bercovier H. Duplication of the tuf gene: a new insight into the phylogeny of eubacteria. J Bacteriol. 1989;177:581–584. doi: 10.1128/jb.171.1.581-584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiem S M, Krumme M L, Smith R L, Tiedje J M. Use of molecular techniques to evaluate the survival of a microorganism injected into an aquifer. Appl Environ Microbiol. 1994;60:1059–1067. doi: 10.1128/aem.60.4.1059-1067.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tubulekas I, Hughes D. Growth and translation elongation rate are sensitive to the concentration of EF-Tu. Mol Microbiol. 1993;8:761–770. doi: 10.1111/j.1365-2958.1993.tb01619.x. [DOI] [PubMed] [Google Scholar]
- 40.Wood P M. Nitrification as a bacterial energy source. In: Prosser J I, editor. Nitrification. Oxford, England: Society for General Microbiology, IRL Press; 1986. pp. 39–62. [Google Scholar]