Abstract
Introduction
Attention deficit hyperactivity disorder (ADHD) affects 5%–10% of paediatric population and is reportedly more common in children with type 1 diabetes (T1D), exacerbating its clinical course. Proper treatment of ADHD in such patients may thus provide neurological and metabolic benefits. To test this, we designed a non-commercial second phase clinical trial comparing the impact of different pharmacological interventions for ADHD in children with T1D.
Methods and analysis
This is a multicentre, randomised, open-label, cross-over clinical trial in children and adolescents with ADHD and T1D. The trial will be conducted in four reference paediatric diabetes centres in Poland. Over 36 months, eligible patients with both T1D and ADHD (aged 8–16.5 years, T1D duration >1 year) will be offered participation. Patients’ guardians will undergo online once-weekly training sessions behaviour management for 10 weeks. Afterward, children will be randomised to methylphenidate (long-release capsule, doses 18-36-54 mg) versus lisdexamphetamine (LDX, 30-50-70 mg). Pharmacotherapy will continue for 6 months before switching to alternative medication. Throughout the trial, the participants will be evaluated every 3 months by their diabetologist and online psychological assessments. The primary endpoint (ADHD symptom severity, Conners 3.0 questionnaire) will be assessed by a blinded investigator. Secondary endpoints will include HbA1c, continuous glucose monitoring indices and quality-of-life (PedsQL).
Ethics and dissemination
The trial is approved by Bioethical Committee at Medical University of Lodz and Polish regulatory agency (RNN/142/22/KE, UR/DBL/D/263/2022). The results will be communicated to the research and clinical community, and Polish agencies responsible for healthcare policy. Patient organisations focused on paediatric T1D will be notified by a consortium member. We hope to use the trial’s results to promote collaboration between mental health professionals and diabetes teams, evaluate the economic feasibility of using LDX in patients with both diseases and the long run improve ADHD treatment in children with T1D.
Trial registration numbers
EU Clinical Trials Register (EU-CTR, 2022-001906-24) and NCT05957055.
Keywords: Behavior, Protocols & guidelines, Paediatric endocrinology, Child & adolescent psychiatry, Randomized Controlled Trial, Impulse control disorders
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Blinded assessment of primary endpoint, use of structured and validated questionnaires for diagnosis and assessment.
Direct comparison of drugs via cross-over design with planned dose-optimisation protocol.
Patients and physicians not blinded to the drug, possible expectation bias.
A moderate risk of selection bias—exclusion of patients with more complex psychiatric phenotypes. Caregivers and patients reluctant to stimulant medication drugs may be unwilling to participate.
Small differences in the efficacy of active compound release between the tested drugs (possible active dose-related bias in clinical effectiveness), dose optimisation for lisdexamphetamine not including all market-available intermediate doses.
Introduction
Attention deficit hyperactivity disorder effect on type 1 diabetes treatment
Type 1 diabetes (T1D) is a common disease affecting over 1 million children worldwide, with an age-standardised incidence of 31/100 000 in Europe, and 16/100 000 in Poland (0–19 years).1 2
Recent advantages in medical technology improved glycaemic control and significantly reduced rates of diabetes complications.3 4 However, state-of-art therapy of T1D requires frequent blood glucose monitoring, counting of carbohydrates intake and adjustments of administered insulin doses. These and other activities put considerable burden on the patient and their guardians. To benefit from such intensive treatment, a child needs efficient executive functioning and high level of self-control—and those who lack those abilities might face the gap between expected and achieved outcomes.
In particular, comorbid psychological and neurodevelopmental disorders were shown to impair diabetes management.5–8 A prime example is attention deficit hyperactivity disorder (ADHD), which affects 5%–10% of children9 and is reported to be up to 35% more frequent (OR 1.35; 95% CI 1.08 to 1.73) in patients with T1D compared with healthy peers.10 A Swedish active screening of children with T1D showed that among children with newly diagnosed ADHD, 77.8% had inadequate metabolic control (mean HbA1c >8.6%) compared with 42.9% in the group of children with treated ADHD.11 Association between ADHD and poor T1D control was also reported by German and Israeli studies.12 13 In addition, those patients experience an elevated risk of life-threatening episodes of severe hypoglycaemia or ketoacidosis, resulting in prolonged hospitalisations10 14 15 and long-term complications, such as diabetic nephropathy.5 From that perspective, the need for evidence regarding the effectiveness and safety of ADHD treatment in paediatric T1D emerges as a pertinent clinical challenge.
Current ADHD treatments and their effects on T1D
No randomised clinical trials (RCTs) have been conducted regarding the effectiveness and safety of ADHD treatment in those with coexisting T1D. Therefore, despite the tremendous impact that both conditions have on patients’ everyday life, current clinical guidelines on the psychological management of T1D do not address the problem of ADHD.16
Many European therapeutic guidelines recommend environmental modifications or psychosocial intervention as first-line treatment for children with ADHD.17–19 Parent training in behaviour management (PT) is a psychosocial intervention aimed at improving caregiver’s understanding of ADHD symptoms and helping them acquire skills to deal with everyday challenging behaviour and support child development. Although PT improves parenting and reduces conduct problems, meta-analyses found no effect of PT on core ADHD symptoms when raters were blinded to the treatment allocations.20 If, despite PT, symptoms of ADHD persist and cause significant impairment of everyday functioning, pharmacotherapy is recommended.
Preferred medications include stimulants, which showed better efficacy (higher effect sizes) on ADHD core symptom reduction and easier dose optimisation protocols than non-psychostimulating medications.21 22 Two psychostimulants with the best evidence for effectiveness and tolerability are methylphenidate (MPH) and lisdexamphetamine (LDX). LDX, contrary to MPH, is an inactive prodrug that requires enzymatic conversion, resulting in an extended and more stable acting time (~13 hours). In most international guidelines, LDX is advised as first-line treatment comparable to long-acting MPH, or as a secondary drug after treatment failure with previous MPH medication attempts.22 In Poland, LDX is neither reimbursed by the National Health Fund (NHF) nor commercially available. As a result, long-acting MPH formulations are considered the first-line pharmacotherapy for ADHD.23
Limited retrospective data demonstrates that patients with ADHD and T1D treated with stimulants show lower HbA1c (8.1±1.0%) compared with children that were diagnosed but not treated pharmacologically (8.5±1.1%).8 At the same time, others reported higher blood pressure and no difference in metabolic control.12 13 However, generalisation of those results remains limited due to the low sample size, lack of evidence from RCTs, and no direct comparisons between LDX and MPH. In conclusion, there is a lack of data on the safety and effectiveness of ADHD medication in children with T1D regarding the effect on ADHD symptoms, quality of life and metabolic control.
Aim of the study
The trial aims to compare the safety and efficacy of ADHD treatment with LDX or MPH in children and adolescents with ADHD and T1D.
Methods and analysis
Study design and population
LAMAinDiab is a second phase randomised cross-over open-label clinical trial with blinded endpoint assessment. Cross-over design was chosen based on the sample size analysis, as well as for ethical reasons—to provide each participant with an active drug with proven efficacy in ADHD. The project is funded by the Medical Research Agency (pol. ‘Agencja Badań Medycznych’), which supports non-commercial clinical trials in Poland through open calls. On grant application, the project consortium was established, and four recruiting sites—that collectively provide care for ~25% of national paediatric population with T1D24 25—were declared.
Patient and public involvement statement
The project was consulted with and was supported by a national patient organisation (Polish Federation for Support of Children and Adolescents with Diabetes, ‘Diabetycy.eu’), which entered the project’s consortium. Clinical trial’s design and protocol were thoroughly consulted with the organisation, and its representative (MZ) was included among the authors to acknowledge her input. Subsequently, the organisation’s qualified representatives agreed to play the roles of independent investigators blinded to the treatment allocation and perform ADHD symptom assessments for participating children.
Inclusion and exclusion criteria
Principal inclusion criteria:
Age 8–16.5 years at trial entry.
T1D diagnosed according to National and International Guidelines26 27 at least 12 months before recruitment, treated with functional intensive insulin therapy.
ADHD diagnosis according to 5th Edition Diagnostic and Statistical Manual of Mental Disorders (DSM-5)28 or International Statistical Classification of Diseases (ICD-10)29 and confirmed as consistent with DSM-5 by a psychiatrist.
Polish citizenship and health insurance.
Principal exclusion criteria:
Clinical partial remission of T1D (daily insulin dose <0.3 units per kilogram and concomitant HbA1c measurement ≤6.5% from the last 3 months) or severely unsatisfactory glycaemic control (mean HbA1c over the past year ≥12%, excluding HbA1c measurement at T1D diagnosis).
Clinically apparent or previously diagnosed cardiovascular disease: haemodynamically significant heart defect, advanced vascular atherosclerosis or documented hypertension (at least stage 2).
Diagnosed intellectual disability or other disability that prevents patient adherence to the therapeutic regimen; history of other mental illness or disorder preventing participation in the trial, for example, bipolar disorder, schizophrenia, other psychotic disorders, history of suicide attempts or present suicide intentions, psychoactive substances abuse.
Contraindications (in line with product characteristics, described in detail at NCT05957055), allergy or hypersensitivity to either studied drug.
Language barrier making it impossible to conduct a full psychological consultation in Polish, lack of permanent residence and national insurance in Poland.
Declared inability or unwillingness of the parents/legal guardians to come to the centre at the time specified by the protocol, in particular—to pick up the study drugs at the dose adjustment stage (the need to pick up 4–5 times over 6–8 weeks, each time within 2–3 days of receiving the recommendations).
Other reasons that, in the opinion of the attending physician, are more likely to result in difficulties in maintaining the participant’s participation in the trial or harm to the participant’s health in case of participation in the trial.
Setting
The trial will be conducted in four paediatric diabetology centres, and apply telemedical tools to facilitate recruitment, improve compliance and reduce the burden on participants and their families. The participating centres provide coordinated paediatric diabetes care for their respective voivodeships (regions of Poland):
Lodzkie voivodeship: Department of Pediatrics, Diabetology, Endocrinology and Nephrology, Pediatric Centre of the Central Clinical Hospital of the Medical University of Lodz.
Silesian voivodeship: Department of Children’s Diabetology, Medical University of Silesia, Upper Silesian John Paul II Child Health Centre.
Pomeranian voivodeship: Department of Pediatrics, Diabetology, and Endocrinology of the Medical University of Gdansk, University Clinical Centre in Gdansk.
Opole voivodeship: Department of Pediatrics of the Institute of Medical Sciences of the University of Opole (Department of Pediatrics and the Diabetology Clinic for Children of the University Clinical Hospital in Opole).
Information about the centres was published on the project’s website (https://lamaindiab.umed.pl/) Paediatric diabetology centres included in the trial are public care providers—the trial visits and procedures will be carried out as add-ons to routine visits related to the management of diabetes. Paediatric healthcare in Poland is tax-financed by the NHF and provides universal, free-of-charge care for all children registered in Poland and their caregivers.
Summary of trial procedures
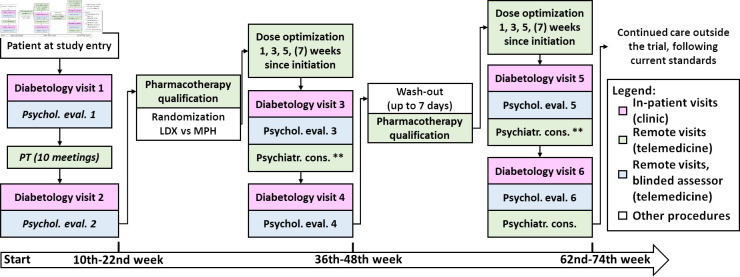
Patients with ADHD and T1D, meeting the clinical trial’s inclusion criteria may enrol into the trial in the designated reference centres. First, their guardians will receive information about the study from the centre’s representative via phone, followed by complete information and consent forms sent to the agreed mail address. During the next routine outpatient consultation, the guardians and children will thoroughly discuss the information provided with an investigator. After answering any questions related to the study and its protocol, the investigator will verify inclusion and exclusion criteria and obtain signed informed consent form in line with current regulations (from both parents and children ≥13 years old). After successful recruitment, study procedures will be initiated. Simplified patient’s course in the clinical trial is demonstrated in figure 1.
Figure 1.
Simplified study design flowchart. LDX, lisdexamphetamine; MPH, methylphenidate; PT, parental training. **If needed on-demand consultation.
Each patient will begin the trial starting with the enrolment appointment, followed by a baseline assessment by a diabetologist and a psychological evaluation. At this stage, all trial participants will be provided with the following devices: pre-configured tablets with appropriate telecommunication software and prepaid internet access, wrist accelerometers, and blood pressure monitors. All devices will be provided by the Medical University of Lodz to assist participants and their parents in following the study procedures and data collection. Additionally, willing patients who did not qualify for reimbursement will be provided with continuous glucose monitoring (CGM) sensors and receivers and instructed in their use.
Next, the patient’s parents/legal guardians will participate in a PT programme of 10 themed online workshops (once-weekly sessions, 90 min long) led by psychotherapists and supplemented with homework and educational materials. PT aims to provide immediate educational and behavioural support for the child’s caretakers by educating them about ADHD and providing tools and skills to understand and modify the child’s behaviours and was demonstrated to strengthen family bonds and improve adherence to future pharmacotherapy. To complete this part of the trial successfully, participant’s guardians must participate in at least eight meetings—with a possible revisit of the missed ones in another cycle.
After completion of PT, each participant will repeat the psychological evaluation to assess the effects of PT intervention alone on ADHD symptoms severity. Those with sustained clinically significant ADHD symptoms will be qualified for pharmacological intervention. Possible contraindications for pharmacotherapy will be assessed during the next diabetological visit. The add-on procedures will include urine tests (pregnancy and panel test for substance use), ECG with QT segment assessment (to exclude long QT syndrome) and ophthalmological consultation (to exclude glaucoma). Subsequent and final assessment and qualification will be performed by a psychiatrist during an online consultation. Afterward, each participant starting pharmacotherapy will be randomised using a digital randomisation system to receive either MPH or LDX as the first drug.
The dose titration protocol was based on Newcorn et al30 flexible-dose design (NCT01552915), with modifications. The dose will be optimised during up to four biweekly psychiatric consultations in a flexible manner (for 5–7 weeks, allowing both one-step increase and decrease between set minimum and maximum dose for each studied drug). After the maximum tolerated dose is established, patients will continue pharmacotherapy for 6 months. During that time, treatment safety and efficacy will be evaluated two times—after first 3 months by psychological and diabetes care team’s evaluation (with small dose adjustments allowed) and after full course (6 months) of therapy. On-demand psychiatric consultations will be allowed. In addition, during both diabetological visits each participant will donate a dry blood sample for evaluation of the concentration of an allocated drug, and another sample will be self-collected on the day of the final psychological assessment for that arm to ensure that endpoint measurements are not biased by incidental non-adherence. After the last evaluation, participants will return the unused drug to their diabetes care centre and will begin a wash-out period.
Qualification for the second arm of pharmacotherapy will be based on the same procedures and consultations which will be performed in parallel with the last diabetological assessment in the first arm. Final switch and start of the second drug (LDX or MPH) will be based on psychiatrist decision. Dose adjustment, safety and efficacy monitoring will follow the same procedures over the next 6 months. Schedule of the trial’s procedures is presented in online supplemental table 1.
bmjopen-2023-078112supp001.pdf (67.8KB, pdf)
At the end of patient’s participation in the trial, all the devices will be returned to the university, and the last safety and efficacy interview will be performed. All the patients will receive further treatment recommendations at NHF facilities.
Randomisation
The starting drug will be determined using block randomisation stratified by the trial centre in a 1:1 ratio between MPH and LDX. The risk of randomisation error will be minimised by using a user-friendly ‘Randomizer’ IT tool provided by the Sponsor, integrated with the electronic case report forms (eCRF). In the event of unexpected randomisation difficulties (eg, lack of internet access or other technical problems), centre’s trial coordinator may request randomisation via backup randomisation list available only for Sponsor’s representative, re-randomisation or patient’s withdrawal.
Blinding
The participant allocation is open both to the participant and their guardians as well as their attending physician (diabetologist and psychiatrist alike). Blinding at participant level was considered but decided against due to practical reasons (ie, costs and difficulties in producing effective over-encapsulation, high risk for spontaneous unblinding due to differences in pharmacokinetics of the studied drugs). However, the people assessing the primary outcome (ie, ADHD symptom severity) will be blinded to the allocation and operating independently from the care centres. No exceptions or unblinding options are planned for those researchers as their assessment serves mostly research purpose, and data collected by them are reviewed by unblinded clinicians.
Endpoints and analysis
The primary endpoints of the trial are:
Efficacy, defined as change in the severity of ADHD symptoms (‘inattention’ and ‘hyperactivity/impulsivity’ measured by Conners 3 questionnaire) compared between LDX versus MPH.
Safety, defined as frequency of Medical Dictionary for Regulatory Activities (MedDRA)-defined adverse events for both drugs.
For each participant, we will calculate the difference in questionnaire scores (‘inattention’ and ‘hyperactivity/impulsivity’) at the end of each arm versus the initiation of drug therapy (before first drug). Separate comparisons will be made for each subscale and informant (guardian/child).
Safety analysis will report the number of recorded events by type and severity and the incidence rate (the number of events divided by the number of patient-months of observation).
The secondary endpoints of the trial are (all compared between LDX and MPH arms):
Diabetes control, as measured by HbA1c and CGM-derived time in target range, mean sensor glucose and coefficient of variation, before and after treatment with each of investigated drugs.
General and diabetes-related quality of life (measured with PedsQL questionnaires), before and after treatment with each of investigated drugs.
Number and percentage of trial participants that achieved improvement of ADHD symptoms (Conners 3 ‘inattention’ and ‘hyperactivity/impulsivity’) defined as 33% reduction in scale values compared with baseline.
The exploratory endpoints of the trial are:
Difference between LDX versus MPH in school attendance during the month preceding the 6-month drug evaluation.
Difference between LDX versus MPH in physical activity (daily steps, % of time in moderate-to-vigorous physical activity) and sleep (duration, latency, efficiency) indices.
Differences between baseline assessment and after PT completion concerning: in ADHD symptoms severity (Conners 3 ‘inattention’ and ‘hyperactivity/impulsivity’), HbA1c and CGM-derived glycaemic control (time in range, mean sensor glucose, coefficient of variation).
Difference between LDX versus MPH in frequency (number of events per patient-months) of acute diabetes complications (severe hypoglycaemia, ketoacidosis) and hospitalisations, and number of days spent in inpatient care (number of days per patient-months).
Tools and parameters used during the trial
During inpatient visits in the trial, standard procedures will be performed, including anthropometric, heart rate and blood pressure measurements. Applicable values will be referenced with Polish percentile charts.
ADHD symptoms will be measured using The Conners’ Rating Scales (The Conners 3.0 scales, Polish version) completed by parents (Conners 3.0-P) and children (Conners 3.0-SR). The Conners’ Rating Scales are validated and most commonly used tools to assess difficulties in children and adolescents with ADHD in research and clinical settings worldwide. The Polish version of Conners 3.0 has proven high psychometric reliability and validity. In the trial, we will focus on changes in content scales of ‘Inattention’ and ‘Hyperactivity/Impulsivity’, two core domains of ADHD symptoms by DSM-5.29
Diabetes control will be assessed using HbA1c measured in local laboratories using methods concordant with the NGSP programme. Moreover, patients will be instructed to use CGM according to their attending physicians’ recommendations and generated data will be collected during diabetes check-ups. In Poland, CGM is reimbursed in the form of intermittently scanned CGM, with possible extension into real-time CGM for those with impaired awareness of hypoglycaemia. If available, CGMs will be linked with appropriate devices: insulin pumps, CGM readers, mobile phones. CGM data will be backed up and processed using GlyCulator 3.0 platform.31 For wrist accelerometer data, manufacturer-provided software will be used to collect the data for further analysis.
Patient’s quality-of-life (QoL) will be measured using the Pediatric Quality of Life Inventory 3.2 Core (PedsQL QoL) and Diabetes Module (PedsQL diab).32 The PedsQL is the most common tool to measure QoL in the paediatric population. The PedsQL diab allows focusing on T1D-specific issues, especially relevant for the ADHD interaction with T1D self-control. PedsQL QoL and diab have been translated into Polish and validated for academic and commercial use.
During the drug dose-optimisation period, patients will be tasked with performing self-assessment, including self-monitoring of blood pressure using automated monitors, diabetes monitoring with CGM and activity monitoring using a wrist accelerometer. Blood pressure monitors data will be periodically uploaded into a central data repository integrated with eCRF, while wrist accelerometry will be stored and analysed at the trial completion. CGM data will be backed up on inpatient clinic visits using appropriate software in each centre.
Statistical analysis
The primary outcome will be compared between the LDX and MPH group using paired t-tests and multivariable regression models to account for clinical covariates. Sensitivity analysis will be performed for primary and secondary endpoints for the subgroup of patients with no imputed data.
For each intervention, the percentage of reported adverse events will be reported with the relevant statistics for paired comparisons (χ2, p), and for the entire table (TMB, p). The incidence rates for individual adverse events will be compared with appropriate statistical tests (Poisson test or equivalent). In addition, each type of event will be compared using the McNemar test, and the frequencies of different events using the McNemar-Bowker global symmetry test.
Treatment safety will be assessed and reported following standard procedures and coded using MedDRA V.24.1 and WHO severity scale, and recorded in eCRF.
Safety and efficacy endpoints will be analysed using data from patients who completed both planned treatments, independent of potential protocol deviations (ie, population ‘as treated’). Data of patients lost to follow-up will be filled in on the basis of last observation carried forward, provided that they have at least one complete timepoint of outcome measures on the current treatment. Patients with deviations from protocol leading to no outcome data will not be included in efficacy analyses.
The secondary and exploratory endpoints will be evaluated using the appropriate statistical methods for either continuous (paired t-test, linear regression) or nominal variables (McNemar test). Interim analyses were not planned within this study.
Sample size estimation
To our knowledge, at the time of planning this trial, no trial with equivalent design and outcome measures in this population was published. Thus, we calculated the sample size to allow for detection of a moderate difference (0.33 SD) difference in score changes between LDX versus MPH for the key Conners 3 measures.33 Such difference was deemed clinically impactful by the clinical team designing the trial. To estimate the sample size, we assumed significance threshold of alpha=0.05, the statistical power of 80%. The risk of applying multiple tests (each scale in each type of responder—four in total) was assessed as minimal due to high intercorrelations among those measures. As such, no alpha adjustment was planned. Such assumptions yielded the minimum number of participants of 89 (rounded to 90). The risk of drop-out during pharmacotherapy was assessed as considerable (~33%) due to the challenging population of interest (children with ADHD and T1D, with ADHD possibly present in parents) and known side effects of tested medications. Thus, the target number of pharmacologically treated children was planned at 135, and 150 recruited given that up to 10% might be disqualified from pharmacotherapy due to drug contraindications or considerable improvement after PT. Assuming recruitment success at 80% (considerable benefit for patients, access to a drug unavailable in Poland, coordinated diabetes and psychiatric care), we estimated that 190 children that should be approached. Based on the general prevalence of ADHD in the paediatric population, the number of patients with T1D to screen for ADHD would be at least 4000—which was a number of patients supervised by the four trial centres.
Data entry and storage
Patient data collected during the clinical trial will be stored within the electronic trial documentation database, following appropriate regulations, with data access provided to appropriate trial personnel. Reported, presented and published data will be anonymised. The clinical trial records will be stored for 25 years after trial completion.
Trial monitoring
Trial monitoring will follow adequate international and national clinical trial regulations. Sponsor’s representatives will visit each site, discuss the clinical trial course, review and validate relevant records, and verify all reference centres’ that partake in the clinical trial. National regulatory authorities may request access to research documentation, source documents, research personnel and facilities. The Sponsor will be notified of any centre’s audits by regulatory agencies, and copies of audit reports will be transferred accordingly.
Trial timeline
Planned date for starting the study is 1 January 2024, but may be prone to change following logistic reasons. The trial is planned to last 47 months, setting the tentative end date of the study at 1 December 2027.
Ethics and dissemination
Ethical considerations
The clinical trial follows principles of the Declaration of Helsinki, current Good Clinical Practice guidelines and other applicable regulations. The clinical trial has been registered in European Union Drug Regulating Authorities Clinical Trials Database and in ClinicalTrials.gov. The clinical trial was approved by the Bioethical Committee at Medical University of Lodz (agreement no. RNN/142/22/KE), and the Polish Office for Registration of Medicinal Products, Medical Devices and Biocidal Products (UR/DBL/D/263/2022). All participants of the clinical trial are insured within the appropriate insurance agreements (policy no. COR233280) and signed informed consent forms from them and their parents will be collected before trial procedures. All changes in the trial are subject to ethical and regulatory review before their incorporation into the clinical trial.
Safety during and postintervention
Before the intervention, the treatment of coexisting diseases will be recorded in the eCRF and continued as is or appropriately modified by the respective specialist. All adverse events will be documented in the electronic trial documentation using MedDRA V.24.1 and graded using applicable WHO standards, with Severe Adverse Events (SAE) reported within 24 hours since the occurrence and evaluated by Safety Monitoring Team (SMT). In case of a SUSAR, the SMT follows the Council for International Organizations of Medical Sciences forms and reports it to appropriate authorities within 15 days (or seven in case of threat to the life or death of the patient) from receiving the report, following the data transfer procedure with the applicable law. The SMT will provide an annual patient safety report throughout the clinical trial, including appropriate information on treatment safety. The Sponsor holds the right to pause or discontinue part of the trial, the entire trial or the participation of an individual patient.
After trial completion, all the patients will receive further treatment instructions, prescription for ADHD treatment and referral to appropriate health provider facilities. Reference diabetology centres will provide continued diabetes care under NHF.
Dissemination plan
Results will be submitted for publication in leading international scientific journals in diabetes care, endocrinology and psychiatry. Results will also be shared during relevant national and international congresses and conferences. The cooperation between the Sponsor and patient organisation (Polish Federation for Support for Children and Adolescents with Diabetes) will be continued after the trial’s completion to increase awareness of the impact of psychiatric diseases in patients with T1D. The study’s results will also be communicated to the funding agency and national healthcare policy makers.
Discussion
Despite the favourable opinion of the Polish Agency for Health Technology Assessment and Tariff System, LDX is still unavailable for Polish patients outside this clinical trial. We will provide data collected during this trial for consideration of the agency, to promote discussion about the availability of LDX and advocate for it being widely accessible (if not reimbursed). We will also perform a specific cost-effectiveness analysis for the particular population of children with ADHD and T1D, and investigate possible costs and benefits of considering LDX as a first-line treatment in this group of patients, justified by the system savings provided by possibly improved diabetes control.
Supplementary Material
Footnotes
Twitter: @FendlerLab
AM and JędC contributed equally.
Contributors: AM, JC, HK-K, WF, AB, KB: written the article, prepared the clinical trial protocol. WF, AB, AM, KB: supervised the clinical trial registration. EK: translated the clinical trial protocol and written the supplementary materials. BM, AS, APC, PJ-C, MM, IM, AK, TW, WF, AB: consulted the manuscript and the clinical trial protocol. MZ: supervised patients consultation of the clinical trial. All authors reviewed the results and approved the final version of the manuscript. We comply to the ICMJE guidelines.
Funding: This work was supported by Medical Research Agency (Agencja Badań Medycznych) grant number 2021/ABM/02/00006/P/03.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a Multicentre prospective registration study. Diabetologia 2019;62:408–17. 10.1007/s00125-018-4763-3 [DOI] [PubMed] [Google Scholar]
- 2.Ogle GD, James S, Dabelea D, et al. Global estimates of incidence of type 1 diabetes in children and adolescents: results from the International diabetes Federation Atlas, 10th edition. Diabetes Res Clin Pract 2022;183:109083. 10.1016/j.diabres.2021.109083 [DOI] [PubMed] [Google Scholar]
- 3.Fang M, Selvin E. Thirty-year trends in complications in U.S. adults with newly diagnosed type 2 diabetes. Diabetes Care 2021;44:699–706. 10.2337/dc20-2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali MK, Pearson-Stuttard J, Selvin E, et al. Interpreting global trends in type 2 diabetes complications and mortality. Diabetologia 2022;65:3–13. 10.1007/s00125-021-05585-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Kuja-Halkola R, Larsson H, et al. Neurodevelopmental disorders, Glycemic control, and diabetic complications in type 1 diabetes: a nationwide cohort study. J Clin Endocrinol Metab 2021;106:e4459–70. 10.1210/clinem/dgab467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butwicka A, Fendler W, Zalepa A, et al. Efficacy of metabolic and psychological screening for mood disorders among children with type 1 diabetes. Diabetes Care 2012;35:2133–9. 10.2337/dc12-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffus SH, Cooper KL, Agans RP, et al. Mental health and behavioral screening in pediatric type 1 diabetes. Diabetes Spectr 2019;32:171–5. 10.2337/ds18-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazor-Aronovitch K, Pinhas-Hamiel O, Pivko-Levy D, et al. Dual diagnosis of type 1 diabetes mellitus and attention deficit hyperactivity disorder. Pediatr Diabetes 2021;22:649–55. 10.1111/pedi.13195 [DOI] [PubMed] [Google Scholar]
- 9.Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol 2018;47:199–212. 10.1080/15374416.2017.1417860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ai Y, Zhao J, Liu H, et al. The relationship between diabetes mellitus and attention deficit hyperactivity disorder: A systematic review and meta-analysis. Front Pediatr 2022;10:936813. 10.3389/fped.2022.936813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Kuja-Halkola R, Larsson H, et al. Poor Glycaemic control is associated with increased risk of neurodevelopmental disorders in childhood-onset type 1 diabetes: a population-based cohort study. Diabetologia 2021;64:767–77. 10.1007/s00125-020-05372-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilgard D, Konrad K, Meusers M, et al. Comorbidity of attention deficit hyperactivity disorder and type 1 diabetes in children and adolescents: analysis based on the Multicentre DPV Registry. Pediatr Diabetes 2017;18:706–13. 10.1111/pedi.12431 [DOI] [PubMed] [Google Scholar]
- 13.Kapellen TM, Reimann R, Kiess W, et al. Prevalence of medically treated children with ADHD and type 1 diabetes in Germany - analysis of two representative databases. J Pediatr Endocrinol Metab 2016;29:1293–7. 10.1515/jpem-2016-0171 [DOI] [PubMed] [Google Scholar]
- 14.Nicolucci A, Buseghin G, De Portu S. Short-term cost analysis of complications related to Glycated hemoglobin in patients with type 1 diabetes in the Italian setting. Acta Diabetol 2016;53:199–204. 10.1007/s00592-015-0755-7 [DOI] [PubMed] [Google Scholar]
- 15.Bansal M, Shah M, Reilly B, et al. Impact of reducing Glycated hemoglobin on Healthcare costs among a population with uncontrolled diabetes. Appl Health Econ Health Policy 2018;16:675–84. 10.1007/s40258-018-0398-2 [DOI] [PubMed] [Google Scholar]
- 16.de Wit M, Gajewska KA, Goethals ER, et al. ISPAD clinical practice consensus guidelines 2022: psychological care of children, adolescents and young adults with diabetes. Pediatr Diabetes 2022;23:1373–89. 10.1111/pedi.13428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nationella vård- och insatsprogram, Available: https://www.vardochinsats.se/adhd/ [Accessed 23 Apr 2023].
- 18.NICE . Recommendations | attention deficit hyperactivity disorder: diagnosis and management | guidance |.
- 19.Millenet . Long version of the Interdisciplinary evidence-and consensus-based (S3) guideline “attention-deficit/hyperactivity disorder (ADHD) in children, adolescents and adults;
- 20.Daley D, van der Oord S, Ferrin M, et al. Behavioral interventions in attention-deficit/hyperactivity disorder: a meta-analysis of randomized controlled trials across multiple outcome domains. J Am Acad Child Adolesc Psychiatry 2014;53:835–47. 10.1016/j.jaac.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 21.Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry 2018;5:727–38. 10.1016/S2215-0366(18)30269-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coghill D, Banaschewski T, Cortese S, et al. The management of ADHD in children and adolescents: bringing evidence to the clinic: perspective from the European ADHD guidelines group (EAGG). Eur Child Adolesc Psychiatry 2023;32:1337–61. 10.1007/s00787-021-01871-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coghill D, Banaschewski T, Zuddas A, et al. Long-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies. BMC Psychiatry 2013;13:237. 10.1186/1471-244X-13-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cukrzyca. Available: https://analizy.mz.gov.pl/app/cukrzyca_pl [Accessed 23 Apr 2023].
- 25.Główny Urząd Statystyczny / Infografiki, widżety / Infografiki / Infografika - Światowy Dzień Walki z Cukrzycą (14 listopada), Available: https://stat.gov.pl/infografiki-widzety/infografiki/infografika-swiatowy-dzien-walki-z-cukrzyca-14-listopada,46,3.html [Accessed 23 Apr 2023].
- 26.Araszkiewicz A, Bandurska-Stankiewicz E, Borys S, et al. Guidelines on the management of patients with diabetes. A position of diabetes Poland. Current Topics in Diabetes 2022;2:1–130. 10.5114/ctd/146259 [DOI] [Google Scholar]
- 27.Libman I, Haynes A, Lyons S, et al. ISPAD clinical practice consensus guidelines 2022: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes 2022;23:1160–74. 10.1111/pedi.13454 [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Diagnostic Stat Man Ment Disord. 22 May 2013. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- 29.ICD-10: international statistical classification of diseases and related health problems: tenth revision, Available: https://apps.who.int/iris/handle/10665/42980 [Accessed 23 Apr 2023].
- 30.Newcorn JH, Nagy P, Childress AC, et al. Randomized, double-blind, placebo-controlled acute comparator trials of Lisdexamfetamine and extended-release methylphenidate in adolescents with attention-deficit/hyperactivity disorder. CNS Drugs 2017;31:999–1014. 10.1007/s40263-017-0468-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chrzanowski J, Grabia S, Michalak A, et al. Glyculator 3.0: A fast, easy-to-use Analytical tool for CGM data analysis, aggregation, center Benchmarking, and data sharing. Diabetes Care 2023;46:e3–5. 10.2337/dc22-0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varni JW, Delamater AM, Hood KK, et al. Pedsql 3.2 diabetes Module for children, adolescents, and young adults: Reliability and validity in type 1 diabetes. Diabetes Care 2018;41:2064–71. 10.2337/dc17-2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soutullo C, Banaschewski T, Lecendreux M, et al. A post hoc comparison of the effects of Lisdexamfetamine Dimesylate and osmotic-release oral system methylphenidate on symptoms of attention-deficit hyperactivity disorder in children and adolescents. CNS Drugs 2013;27:743–51. 10.1007/s40263-013-0086-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-078112supp001.pdf (67.8KB, pdf)