Abstract
The replication terminator protein (RTP) of Bacillus subtilis interacts with its cognate DNA terminators to cause replication fork arrest, thereby ensuring that the forks approaching one another at the conclusion of a round of replication meet within a restricted terminus region. A similar situation exists in Escherichia coli, but it appears that the fork-arrest systems in these two organisms have evolved independently of one another. In the present work, RTP homologs in four species closely related to B. subtilis (B. atrophaeus, B. amyloliquefaciens, B. mojavensis, and B. vallismortis) have been identified and characterized. An RTP homolog could not be identified in another closely related species, B. licheniformis. The nucleotide and amino acid changes from B. subtilis among the four homologs are consistent with the recently established phylogenetic tree for these species. The GC contents of the rtp genes raise the possibility that these organisms arose within this branch of the tree by horizontal transfer into a common ancestor after their divergence from B. licheniformis. Only 5 amino acid residue positions were changed among the four homologs, despite an up to 17.2% change in the nucleotide sequence, a finding that highlights the importance of the precise folded structure to the functioning of RTP. The absence of any significant change in the proposed DNA-binding region of RTP emphasizes the importance of its high affinity for the DNA terminator in its functioning. By coincidence, the single change (E30K) found in the B. mojavensis RTP corresponds exactly to that purposefully introduced by others into B. subtilis RTP to implicate a crucial role for E30 in the fork-arrest mechanism. The natural occurrence of this variant is difficult to reconcile with such an implication, and it was shown directly that RTP.E30K functions normally in fork arrest in B. subtilis in vivo. Additional DNA terminators were identified in the new RTP homolog-containing strains, allowing the definition of a Bacillus terminator consensus and identification of two more terminators in the B. subtilis 168 genome sequence to bring the total to nine.
It is increasingly apparent that the presence of a system to arrest DNA replication is not solely confined to prokaryotic organisms such as Bacillus subtilis and Escherichia coli. The detection of a DNA replication arrest system in the Epstein-Barr virus (12) and more recently in a murine ribosomal DNA (rDNA) system (13) strongly suggests an evolutionary advantage in possessing one. In the murine rDNA system, the advantage appears to be the avoidance of a head-on collision between the replicative and transcription machineries, which may also be the case in the other systems. In bacteria with circular chromosomes, another possible advantage of such an arrest system could be to facilitate some postreplicative event, leading to more efficient coupling of chromosome replication, chromosome partitioning, and cell division. Of the systems identified, a number of similarities are apparent. All involve the binding of a specific protein to a short DNA sequence. In B. subtilis the replication terminator protein (RTP) interacts as a dimer with an ∼30-bp DNA terminator (see reference 39); in E. coli the termination utilization substance (Tus) interacts as a monomer with a 23-bp DNA terminator (see reference 7). The virally encoded Epstein-Barr nuclear antigen type 1 (EBNA-1) interacts as a dimer with a 30-bp sequence located within the origin (oriP) region (12). In the murine system, rDNA replication is arrested by the transcription termination factor 1 (TTF-1) interacting with an 18-bp sequence known as a Sal box, which is adjacent to a short stretch of downstream guanine and cytosine residues (13). None of these terminator proteins demonstrate any sequence or structural similarity to each other and neither do their cognate DNA sequences. For E. coli and B. subtilis, this is somewhat surprising, since many of the enzymes and proteins involved in the initiation and elongation of DNA replication are similar. It would appear that the DNA replication arrest systems of these bacteria have evolved independently. Another feature common to these prokaryotic and eukaryotic organisms is the presence of multiple copies of the target DNA sequences to which the proteins bind. With the exception of Epstein-Barr virus, the orientation of these DNA sequences determines the polarity of fork arrest at these sites. Moreover, these protein-DNA complexes also appear to inhibit RNA polymerase in a polar manner (13, 27).
Resolution of the replication fork-arrest mechanism awaits further study and may indeed be specific to each organism, but it is hoped that progress made with the prokaryotic models may assist in our understanding of the possibly more elaborate eukaryotic systems. The key activity common to the arrest systems of B. subtilis and E. coli appears to be the inhibition of helicase-mediated unwinding of the DNA, a feature also of the EBNA-1 system (9). At present the precise mechanism of helicase blockage remains unresolved. Is the tight binding (clamping) of a terminator protein to its cognate DNA sequence solely responsible for the prevention of further helicase movement? Or does inhibition of helicase activity involve a specific interaction with the terminator protein on the DNA? The most convincing evidence in support of the helicase-terminator protein interaction are the mutagenesis experiments performed by Manna et al. (26). They identified two mutants of RTP that were apparently unaltered in their interaction with the DNA but could not block the E. coli replicative helicase. The results of Manna et al. suggested that a specific interaction of residues E30 and Y33 of RTP with a region of the replicative helicase is required for efficient arrest of the replication fork.
The work described here has focused on whether the RTP-based fork-arrest system of B. subtilis is conserved in other Bacillus species. New RTP sequences have been detected in four closely related Bacillus species. Of the 122 residues of RTP, only five positions differ from the amino acid sequence found in B. subtilis. However, their positioning within the folded structure of RTP is significant in view of the proposed functions of certain regions of this structure. Surprisingly, one of the changes was identical to one of those used to suggest that the residue in question specifically interacts with the replicative helicase (26). The present study has also identified new DNA terminators in the other Bacillus species, leading to the definition of a more comprehensive Bacillus terminator consensus sequence. This has enabled the identification in the recently sequenced B. subtilis chromosome (19) of two additional replication terminators in the terminus region to yield what must be close to the complete suite of such terminators in B. subtilis 168.
MATERIALS AND METHODS
Bacterial strains.
B. subtilis 168 (trpC2) was originally from the Stanford collection, and B. subtilis W23 (NCTC3610) and B. licheniformis (FD01) were obtained from R. Rudner. Other strains of B. licheniformis were obtained from the Bacillus Genetic Stock Centre (5A2) and A. Warth (DSM13). B. atrophaeus (NRS-213), B. amyloliquefaciens (ATCC23350), B. mojavensis (RO-H-1), and B. vallismortis (DV1-F-3) were kindly provided by F. M. Cohan. SU187 was available in this laboratory (6).
Cloning of rtp from B. atrophaeus and B. mojavensis.
The chromosomal EcoRI fragments identified in Fig. 2 that contained potential rtp sequences from B. atrophaeus (1.4 kb) and B. mojavensis (2.3 kb) were purified from low-melting-point agarose by using a Jetsorb DNA extraction kit (GenoMed, Inc., Beverly Hills, Calif.). These fragments were chosen due to their smaller size, reducing the number of cloning steps required. The fragments were ligated into the multiple cloning site of pGEM3Zf(+) (Promega, Madison, Wis.) and transformed into E. coli DH5α. The clones which contained the sequences of interest were identified via colony blot hybridization with the rtp probe (0.53 kb). Once identified, the clones containing rtp from B. atrophaeus and B. mojavensis were given the names pAG15 and pAG21, respectively.
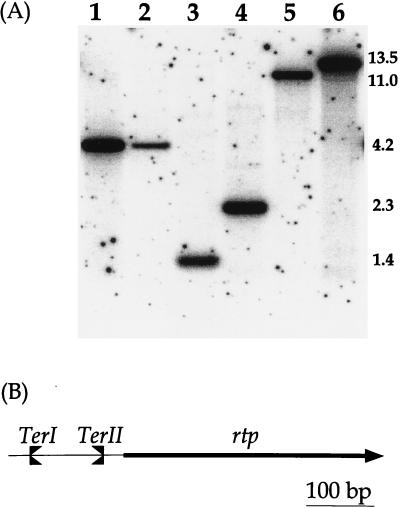
FIG. 2.
(A) Southern transfer and hybridization of EcoRI chromosomal DNA digests from different Bacillus strains with a 32P-labeled 0.53-kb DNA fragment containing rtp as the probe. Lanes: 1, DNA from B. subtilis W23; 2, B. amyloliquefaciens; 3, B. atrophaeus; 4, B. mojavensis; 5, B. vallismortis; 6, B. subtilis 168. Sizes of the hybridizing fragments are in kilobases. (B) Schematic representation of the relative positions of TerI and TerII to rtp in the chromosome of B. subtilis. A clockwise fork would pass through rtp and TerII and be arrested by TerI, while TerII has the potential to arrest a fork approaching from the counterclockwise direction.
DNA probes, Southern transfer, and hybridization.
After agarose gel electrophoresis of restricted chromosomal DNA, the fragments were transferred to an Amersham Hybond-N membrane with a Bio-Rad vacuum blotter. Two of the DNA probes used to detect rtp in other Bacillus strains were available in the laboratory. These were a 1.0-kb HaeIII fragment from pWS43 containing TerI/TerII+rtp, and a 0.53-kb BamHI fragment from pWS53 containing a promoterless rtp. Two short degenerate oligonucleotides were also used as probes. A 17-mer degenerate oligonucleotide [B site; (AT)ATg(AT)AC(TC)AAAT(gA)TTCA] was derived from TerI to TerVI of B. subtilis 168 (14) and was used to detect the presence of multiple DNA terminators in the other Bacillus strains. To determine which of these bands also contained rtp, an 18-mer degenerate oligonucleotide, TTTAA(gA)CC(gA)AA(TC)CATAC, was based on an alignment of the six rtp sequences. These short oligonucleotide probes were labeled at their 5′ ends by using polynucleotide kinase under standard conditions and were hybridized to the DNA as described by Williams and Wake (42). The posthybridization washes were as described by Wood et al. (43); 3 M tetramethylammonium chloride was used to give sequence-independent hybridization. To detect nonperfect matches to the oligonucleotide probes, the stringency was lowered by reducing the temperature of the tetramethylammonium chloride washes to 41°C from 52°C so that only those oligonucleotides bound by more than 11 bp should remain hybridized to the membrane. The larger rtp probes were labeled with 32P by using the Amersham Megaprime DNA-labeling system and were hybridized under standard conditions, that is, at 65°C with two 45-min posthybridization washes in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) followed by another three washes in 1× SSC–0.1% sodium dodecyl sulfate (SDS). To lower this stringency, the temperature was reduced to 58°C, with four posthybridization washes performed in 6× SSC for 20 min, the last two with 0.1% SDS added. A Molecular Dynamics PhosphorImager was used to detect the bound probes.
PCR.
DNA amplifications were performed with an automated thermal cycler (FTS-320 Thermal Sequencer; Corbett Research, Sydney, Australia). Each 50-μl PCR mixture contained 125 ng of both primers, 100 ng of chromosomal template DNA, 1.5 to 4.5 mM MgCl2, 0.8 mM deoxynucleoside triphosphate, the buffer supplied for Tth DNA polymerase (Pharmacia Biotech, Uppsala, Sweden), and a Wax-Aid pellet (Advanced Biotechnologies, Surrey, United Kingdom). The template was denatured at 94°C for 4 min, followed by an incubation at 72°C for 1 min, and then 2.25 U of the DNA polymerase was added. Annealing temperatures ranged from 5 to 20°C below the lowest annealing temperature of the primer in the reaction. For the amplification and sequencing of rtp from other Bacillus strains, primers were derived from the consensus B site of B. subtilis 168 terminators, from within the new rtp sequences, and from a region approximately 50 to 80 bp downstream of rtp found to be almost identical in B. subtilis, B. atrophaeus, and B. mojavensis. For the amplification and sequencing of dnaC from B. mojavensis, three forward and three reverse degenerate primers were derived from an alignment of the helicases dnaC (32) and phage SPP1 G40 (35) from B. subtilis. Fragments obtained from the PCR amplifications were purified with the Jetsorb DNA extraction kit.
DNA replication fork-arrest assays.
The plasmid in vivo fork-arrest assay was as described by Smith and Wake (40). The chromosomal in vivo fork-arrest assay was essentially as described by Franks and Wake (11). Chromosomal DNA was extracted from an exponentially growing culture and digested with SalI. The replication fork intermediates were detected with a 32P-labeled 5.2-kb fragment derived from pALI (11) that contained TerIII.
DNA sequencing and analysis.
Dye terminator cycle sequencing was carried out at the Children’s Medical Research Institute, Westmead, Australia, with the same primers used for PCR. Alignment of DNA and protein sequences were done by using the Australian National Genomic Information Service (ANGIS). Additional DNA terminators in the B. subtilis genome (19) were searched for using the SubtiList database (28).
SDS-polyacrylamide gel electrophoresis, Western blot transfer, and immunodetection of RTP.
Whole-cell extracts from mid-exponential-phase Bacillus cultures were prepared according to the method of Healy et al. (16), except that the 10-ml pellets were resuspended in 200 μl of lysis buffer. Purified RTP was available in the laboratory. The proteins for transfer were separated by SDS-polyacrylamide gel electrophoresis in 1.5-mm 18% (wt/vol) gels as described previously (20). Proteins were then transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, Mass.) by a procedure outlined by Otter et al. (33) with a Mini Trans-Blot cell (Bio-Rad, Hercules, Calif.) at 28 V (100 mA) for 2 h at 4°C. Immunodetection of proteins was performed with the ECL-Plus detection system (Amersham Corp.) according to the manufacturer’s instructions; Tris-buffered saline (pH 7.6) was used, but 5% nonfat dried milk was added to the secondary antibody solution and postprimary antibody washes. The anti-RTP antibodies (1) were purified from rabbit serum with Affigel-10 (Bio-Rad) columns as described by Harry and Wake (15). These purified primary antibodies were then used at a 1/50 dilution, and the horseradish peroxidase-labeled secondary antibodies (Promega) were used at a 1/1,000 dilution.
Nucleotide sequence accession numbers.
The four new rtp sequences have been deposited in GenBank (B. atrophaeus, AF045056; B. amyloliquefaciens, AF045057; B. mojavensis, AF045055; and B. vallismortis, AF045054), as has the partial sequence of dnaC from B. mojavensis (AF045058).
RESULTS
The presence of identical amino acid sequences for RTP in B. subtilis 168 and W23 (25) has raised the question of whether the sequence of RTP is also conserved in other Bacillus strains. This question was partially answered previously by using a Southern hybridization and PCR approach (21). It was not possible to detect RTP in B. licheniformis, B. pumilis, or B. thuringiensis. We focused here on closer relatives of B. subtilis, two of which have become available only recently, B. mojavensis (see reference 38) and B. vallismortis (37). Their evolutionary positions relative to B. subtilis, together with those of B. amyloliquefaciens and B. atrophaeus (30), is shown in the phylogenetic tree (Fig. 1). To test for the presence of RTP in these four species, a Southern hybridization approach that used B. subtilis 168 rtp as a probe was chosen. Cloning and sequencing of one or more of the hybridizing bands would establish whether they corresponded to sequences that resembled the rtp of B. subtilis. If they did, their sequences would assist in the design of degenerate primers for PCR directly off the chromosome in further strains of Bacillus.
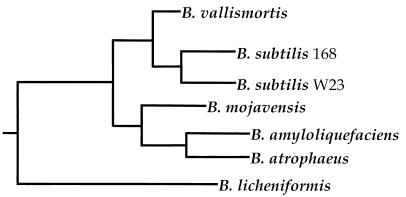
FIG. 1.
Phylogenetic tree showing the position of B. subtilis 168 relative to the Bacillus strains used in this study (37). The horizontal distances correspond to the magnitude of divergence.
Identification of rtp homologs in four other Bacillus species.
An EcoRI chromosomal digest of each species was transferred to a nylon membrane and hybridized to a probe containing TerI/TerII+rtp (1.0 kb) from B. subtilis 168 (Fig. 2B). Under standard stringency conditions (see Materials and Methods), a band was detected only in B. vallismortis and B. mojavensis, suggesting that rtp may be present. At a lower stringency and with a smaller B. subtilis probe that contained only rtp (0.53 kb), a band was detected in all four species (Fig. 2A). The weaker intensities of the bands in B. amyloliquefaciens (lane 2) and B. atrophaeus (lane 3) and their detection only at the lower stringency agrees with their being the most divergent from B. subtilis (see Fig. 1).
Cloning and sequencing the homologs of rtp.
To establish that the hybridizing bands corresponded to homologs of rtp, the chromosomal EcoRI fragments from B. atrophaeus and B. mojavensis were cloned into pGEM3Zf(+) (see Materials and Methods). Sequencing of ∼700 bp of each cloned plasmid established that rtp was indeed present in both of these strains. The sequences of rtp from B. amyloliquefaciens and B. vallismortis were obtained through low-stringency PCR directly off the chromosome (see Materials and Methods). This was possible through the use of degenerate primers derived from the nucleotide sequences of rtp, TerI, and TerII and from a region immediately downstream of rtp in B. subtilis, B. atrophaeus, and B. mojavensis (Fig. 2). The nucleotide sequences were confirmed by three independent PCR amplifications. The validity of this low-stringency PCR was established by showing that the cloned sequences of rtp from B. atrophaeus and B. mojavensis could also be amplified through PCR directly off the chromosome under the same conditions. These latter sequences were found to agree with those obtained with the cloned DNA. In all cases, the new rtp genes were sequenced in both directions.
Features of the nucleotide sequences.
The rtp sequences from five Bacillus species were compared with the 366-nucleotide sequence of the rtp-coding region from B. subtilis 168. The percentages of nucleotide substitutions (Table 1) are consistent with these Bacillus strains being in the same relative phylogenetic positions shown in Fig. 1. The larger number of nucleotide changes in B. amyloliquefaciens and B. atrophaeus agrees with their being the most divergent from B. subtilis 168 of the five organisms listed. Indeed, phylogenetic trees constructed with the very limited rtp nucleotide sequence data gave variable but similar results to that shown in Fig. 1. The nucleotide substitutions in each of the new rtp sequences are equally distributed among those encoding the four α helices, three β strands, and regions between that make up RTP (5) (see Fig. 3). The extreme 3′ and 5′ ends of rtp seem to be the most conserved segments in terms of nucleotide sequence. Of the 102 nucleotide substitutions present overall, the majority do not change the amino acid sequence of RTP; 85 are at the third position, and 10 are at the first position of the codon. However, four substitutions at the first, one substitution at the third, and one substitution where the first and second positions are substituted together within a codon do change the amino acid sequence of RTP. In accordance with the likely importance of this protein, the nucleotide sequences required for its expression are also conserved, at least in B. atrophaeus and B. mojavensis. A potential promoter region consisting of a −10 and −35 sequence together with a good ribosome binding site are located at the same upstream positions as in B. subtilis. The −10 sequence is immediately adjacent to a sequence that strongly resembles TerI of B. subtilis 168, a finding consistent with conservation of autoregulation of rtp expression (2). Also, a potential transcription terminator is present at the equivalent position downstream of these two new rtp sequences (not shown).
TABLE 1.
Nucleotide and amino acid changes in rtp of various Bacillus strains
| Bacillus strain | % Nucleotides differenta | % GC content of:
|
Nature and position of amino acid change in RTP | |
|---|---|---|---|---|
| Genome | rtp | |||
| B. subtilis 168 | 43.5 | 36.3 | Nil | |
| B. subtilis W23 | 6.0 | 43 | 36.9 | Nil |
| B. vallismortis | 9.8 | 43 | 35.5 | Nil |
| B. mojavensis | 10.9 | 43 | 36.6 | E30K |
| B. amyloliquefaciens | 16.9 | 43 | 40.7 | E47P |
| I73V | ||||
| B. atrophaeus | 17.2 | 42 | 39.0 | E47Q |
| V105I | ||||
| K112N | ||||
Differences from B. subtilis 168.
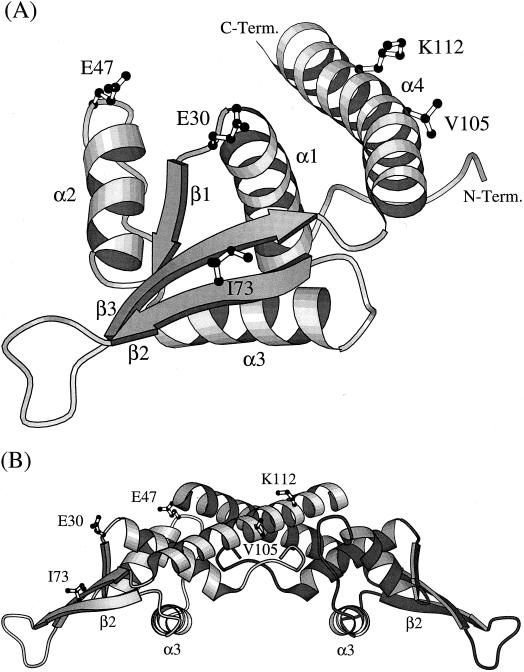
FIG. 3.
(A) A ribbon diagram showing the folded structure of an RTP monomer comprising four α helices, three β strands, and an extended loop connecting the β2 and β3 strands (5). The unstructured amino-terminal region (residues 1 to 6) is not shown. The location of the five amino acids (E30, E47, I73, V105, and K112) that are changed within four closely related Bacillus species are indicated. C-Term., C terminus; N-Term., N. terminus. (B) A ribbon diagram of a dimer of RTP showing the amino acid changes identified. The changes are indicated in only the left monomer, which is less heavily shaded. In binding to the DNA, the dimer is positioned on top of the double helix, such that its lower surface, as well as the unstructured amino-terminal region, make contact with it. The α3 helices dock into adjacent major grooves of the DNA, and the β2 strands dock into flanking minor grooves (34).
A noticeable feature of the rtp sequences is that the GC content is lower (35.5 to 40.7%) than that found in the rest of the chromosome (42 to 43.5% [Table 1]). Generally, the base composition of most genes within a genome is more similar due to exposure to the same selective and mutational pressures (23). As a consequence, sequences with atypical characteristics would tend to stand out, suggesting that they may have been introduced via horizontal transfer and as yet have not fully ameliorated to the recipient genome. The average GC content of the B. subtilis 168 chromosome has recently been established as 43.5% (19). While the content was found to vary throughout the chromosome, only ∼9% of the protein coding sequences were found to have a GC content equal to or less than that of rtp. It is tempting to hypothesize that a common ancestor may have possessed an rtp gene of low GC content and that insufficient time has elapsed for this gene to have taken on fully the sequence characteristics of the recipient genome. Because the gap between the GC content of rtp and the rest of the chromosome in B. amyloliquefaciens and B. atrophaeus is smaller than the other species examined here, this raises the possibility that they have evolved at a faster rate.
Features of the amino acid sequences.
The structural features of RTP from B. subtilis as determined by X-ray crystallography (5) are shown in Fig. 3. RTP shares the motif of the winged-helix family of DNA-binding proteins, such that when a single dimer of RTP binds to the DNA, the α3 helices dock into adjacent major grooves and the two outer β2 strands dock into the outer flanking minor grooves of the DNA (34). Together with the amino-terminal regions of the RTP dimer, the α3 helices and the β2 strands indicated in Fig. 3B would contact the DNA that would be positioned below the structure as it is shown. Only 5 of the 122 amino acids of RTP were found to differ from those of B. subtilis (Table 1). The number of changes in each species, which was greatest in B. amyloliquefaciens and B. atrophaeus, also agrees with the phylogenetic tree (Fig. 1). All of the side chains of those changed are located on the surface of the protein where they would be exposed to solvent (Fig. 3). Three of the changes, E47Q, I73V, and V105I, are conservative and unlikely to alter the folded structure or activity of RTP. These replacements are commonly found within proteins (8). It is also likely that the K112N change, one surface polar residue for another in the α4 helix of B. atrophaeus RTP does not significantly cause it to differ from the RTP of B. subtilis. The E47P change in B. amyloliquefaciens, which is immediately outside the α2 helix, is an infrequent residue change based on Creighton’s analysis (8). Prolines are generally confined to regions away from α helices and β strands due to their restricted phi angle and subsequent disruptive influence on protein structure, as well as their not possessing an available NH group to participate in H bonding. However, in places where proline can be tolerated, it can actually increase the stability of a protein due to its having a smaller loss of entropy on folding than any other residue (36). Indeed, proline is easily modeled into RTP at position 47 without straining the local structure or resulting in torsional angles beyond that permitted within the Ramanchandran plot (22). It is quite likely, therefore, that the E47P change does not influence the folded structure of RTP in a significant way.
The E30K replacement found adjacent to the α1 helix of RTP in B. mojavensis is most interesting. This replacement is infrequently observed in proteins (8). By coincidence, it was this exact amino acid substitution (E30K) that was constructed previously and led to the proposition that E30 specifically interacted with the replicative helicase to arrest replication in vitro (26). With this mutant RTP, a 50% loss of activity against helicase unwinding in a strand displacement assay was found, together with a complete loss of fork-arrest activity in an in vitro replication assay. The presence of E30K in B. mojavensis is difficult to reconcile with these findings. Why would this amino acid change be found naturally if it were essential to fork arrest? This is the only residue change in B. mojavensis RTP, which excludes the possibility of a compensatory residue change taking place elsewhere in the protein. To examine the possibility of a compensatory change within its helicase, we determined the sequence of most of its gene (i.e., the dnaC homolog) by PCR. The DNA sequence was obtained directly off the chromosome with degenerate primers derived from the genes for two replicative helicases of B. subtilis, DnaC (32) and phage SPP1 G40 (35). The DNA sequence yielded the amino acid sequence of 373 residues of B. mojavensis DnaC (corresponding to residues 24 to 396 of B. subtilis DnaC). There were only two differences from B. subtilis DnaC, Y to H at position 177 and T to S at position 191. The hinge region of DnaC helicase, which has been proposed to interact with RTP (26), corresponds to amino acid residues 110 to 153 (on the basis of an alignment with E. coli DnaB helicase and the data presented in reference 29). Thus, the putative hinge region of the B. subtilis and B. mojavensis helicases are identical, and the two proteins overall are almost identical.
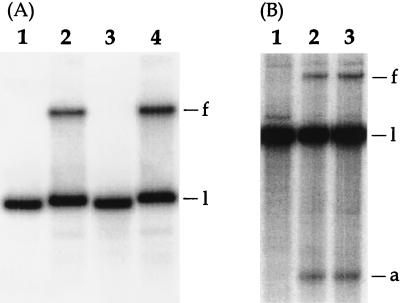
The functioning of RTP.E30K in fork arrest in B. subtilis was tested directly. In another study we had constructed a plasmid that allows insertion of rtp under spac control into the amyE locus of the rtp-deleted SU187 strain of B. subtilis (unpublished data). The rtp-containing plasmid was altered by oligonucleotide insertion to change the E30 codon to that for K and then inserted into the amyE locus of SU187. These RTP- and RTP.E30K-producing strains have been designated SU342 and SU369, respectively. The pWS66-1 fork-arrest assay plasmid containing the TerI terminator (40) was introduced into SU342 and SU369. Figure 4A shows the result of plasmid fork-arrest assays as described previously (40) but in the presence of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Western blot experiments showed that similar levels of RTP and RTP.E30K (slightly less of the latter) were produced in the two strains (data not shown). Lanes 1 and 3 are negative controls in which pWS64-1, lacking TerI, had been introduced into SU342 and SU369, respectively. As expected, only a single plasmid (linearized) band is present; there is no slowly moving band indicative of a forked molecule. On the other hand, lanes 2 and 4, corresponding to the pWS66-1-containing strains producing RTP and RTP.E30K, respectively, show a significant level of retarded fork. Quantitation of the relevant species in each case showed that the E30K-producing strain yielded 140% of the amount of fork (normalized to linear DNA in two independent experiments), as did the wild-type RTP-producing strain. In an extension of this work, the effectiveness of RTP and RTP.E30K in arresting a fork within the bacterial chromosome itself was also assessed. The SU187-derived strains SU342 and SU369 are missing TerI (and TerII) so that TerIII would be the first functionally oriented terminator to encounter the clockwise fork. Figure 4B shows the result of chromosomal fork-arrest assays at TerIII performed as described previously (11). Lane 1 is a negative control in which the parent plasmid lacking rtp had been inserted at the amyE locus of SU187 (yielding SU341) in place of the rtp-containing plasmid (as used to yield SU342). As expected, there is no detectable forked molecule. On the other hand, strains SU342 and SU369 show similar levels of retarded fork (as well as a degraded arm). From two independent experiments and taking into account the degraded-arm species, it was established that the level of chromosomal fork arrest at TerIII in SU369 was essentially the same (98%) as that in SU342. It is concluded that RTP.E30K is as efficient in in vivo fork arrest in B. subtilis as is wild-type RTP.
FIG. 4.
Results of in vivo fork-arrest assays in B. subtilis strains producing wild-type RTP or RTP.E30K. (A) Plasmid fork-arrest assays. Lanes 1 and 3 are negative controls for strains SU342 (RTP) and SU369 (RTP.E30K), respectively, harboring pWS64-1 which lacks TerI. Lanes 2 and 4 show strains SU342 and SU369, respectively, harboring the TerI-containing pWS66-1. The four cultures were grown in the presence of 1 mM IPTG. (B) TerIII chromosomal fork-arrest assays. Lane 1 is a negative control for chromosomal DNA from an isogenic strain, SU341, lacking the rtp gene. Lanes 2 and 3 show SU342 and SU369 chromosomal DNA. The DNA was isolated from cultures grown in Penassay broth–1 mM IPTG. The bands corresponding to the fork (f), linear (l), and arm (a) species are indicated.
Additional DNA terminators.
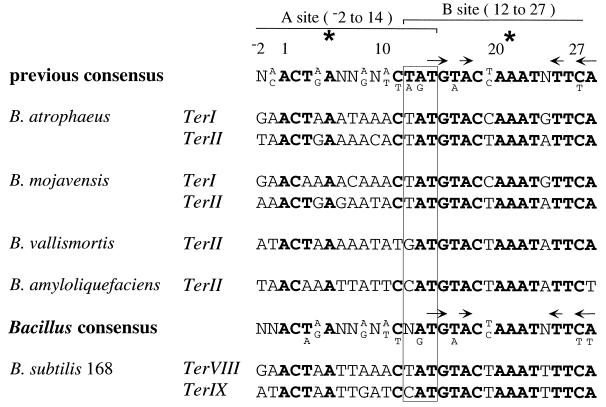
Seven terminus-region chromosomal terminators have been identified in B. subtilis 168 (see reference 14) and two in B. subtilis W23 (25). Each of the terminators is composed of two adjacent and overlapping sites, A and B. When two RTP dimers are bound, it is the B site of the terminator that encounters the DNA replication fork to cause arrest (40). The consensus derived from the previously identified B. subtilis 168 and W23 chromosomal terminators is shown at the top of Fig. 5. The most favored nucleotides in these terminators are shown in boldface. Denoted by an asterisk at nucleotides 5 and 21 are the centers of pseudosymmetry within the A and B sites. The symmetry over the B site (arrows in Fig. 5) is more pronounced than over the A site (see reference 39). It is this feature which presumably gives the B site its higher affinity for the symmetrical RTP dimer compared with the A site (24). In the present study six new chromosomal terminators from other Bacillus strains, which conform to the A- and B-site makeup, were identified. They correspond to TerI and/or TerII, which are located very close to and upstream of rtp in B. subtilis (see Fig. 2). The TerI sequence was not obtained from B. vallismortis and B. amyloliquefaciens due to the TerI region being used as one of the PCR primers for amplifying their homologous rtp genes. These new terminator sequences and a new Bacillus chromosomal consensus are shown in Fig. 5. The importance of the conservation of the B site is highlighted by identical nucleotides being present from nucleotides 13 to 27 in each TerI sequence, except for a guanine at nucleotide 13 in W23. This same region in TerII is identical in all of the strains. The most noticeable feature of the new consensus is the now-variant position at nucleotide 12 in B. amyloliquefaciens and B. vallismortis, within the central trinucleotide (nucleotides 12 to 14 [Fig. 5]). The significance of changing the sequence of the central trinucleotide has been previously examined by Smith et al. (39). Their results suggest that these particular changes would not alter the functioning of these terminators.
FIG. 5.
Alignment of terminator sequences detected in various Bacillus strains. The upper terminator consensus is derived from the previously identified chromosomal terminators of B. subtilis 168 and W23 (14); the lower one takes into account those from the other Bacillus species. The position of overlap between the A and B sites is indicated by the rectangle. The asterisks indicate the centers of pseudosymmetry within the A and B sites, and the arrows above the consensus sequences represent regions of symmetry in the B site. Nucleotides most favored by the 17 terminators now described are shown in boldface; when there are up to only two identical variations this is shown in the consensus sequences in smaller type below the bold.
Searching the entire B. subtilis 168 genome (19) with the new consensus identified two further terminators, TerVIII (163.8°) and TerIX (187.1°), which matched the consensus sequence perfectly (Fig. 5). It is significant that both of these lie within the terminus region. The orientation of TerVIII indicates that it would arrest the counterclockwise fork, whereas TerIX would arrest the clockwise fork. The presence of multiple terminators was also examined in the other Bacillus strains. This was performed by using a 17-mer degenerate probe (B site) derived from the terminators of B. subtilis 168 (see Materials and Methods). The five prominent bands detected in B. subtilis 168 were previously assigned to TerI to TerVI (10), and one of a number of fainter bands was assigned to TerVII (14). Hybridization at low stringency to a membrane containing EcoRI-digested DNA from B. amyloliquefaciens, B. atrophaeus, B. mojavensis, and B. vallismortis showed five to eight bands of variable intensity (data not shown), a result consistent with the presence of multiple terminators.
Tests for a homolog of RTP in B. licheniformis.
In an attempt to identify a more divergent sequence of RTP, B. licheniformis was reexamined due to its likely status as an outgroup to the other strains used in this work (Fig. 1). Using the Southern hybridization and PCR approaches described here did not produce evidence for the presence of RTP or multiple terminators. Under the hybridization conditions described above, no strong hybridizing band could be detected in three strains of B. licheniformis (data not shown). A few bands of weak intensity were detected with the B-site probe, one of which was cloned and sequenced. However, the hybridizing sequence was found to correspond to a poor B-site sequence with no adjacent A site and is thus unlikely to be a terminator. Western transfer of cell proteins to a membrane and immunodetection with antibodies to RTP of B. subtilis 168 was therefore used. Despite the very low concentration of RTP in the cell (1), RTP could be detected in a whole-cell extract from B. subtilis 168 by using horseradish peroxidase in conjunction with the ECL-Plus detection system (Fig. 6, upper band, lane 2). The lower band represents one of a few cross-reacting bands that were also detected with this highly sensitive system despite the affinity purification of the RTP antibodies. Lane 3 is a negative control and contains a cell extract from SU187 from which rtp has been deleted (6). An approximate level of RTP in the B. subtilis cell was estimated by using 5 ng of purified RTP as a standard (lanes 1 and 8). The number of RTP dimers per nucleoid was calculated to be less than 150, which is similar to the approximately 100 monomers of Tus estimated to be present in E. coli (see reference 31). Although a significant number of nucleotide differences exist, RTP could be detected in B. atrophaeus and B. amyloliquefaciens (lanes 4 and 5). However, in several experiments, no protein of the size of RTP could be detected in B. licheniformis 5A2 or FD01 (lanes 6 and 7) under these conditions.
FIG. 6.
Western transfer analysis of total-cell proteins from various Bacillus strains probed with polyclonal B. subtilis 168 RTP antibodies as determined by the ECL-Plus detection system (see Materials and Methods). Only the portion of the membrane relevant to RTP is shown. The position of RTP (5 ng, 14.5 kDa) in lanes 1 and 8 is indicated. Lanes: 2, B. subtilis 168; 3, SU187 (6); 4, B. amyloliquefaciens; 5, B. atrophaeus; 6, B. licheniformis 5A2; 7, B. licheniformis FD01. The lower band in lanes 2 to 7 and the upper band in lanes 6 and 7 represent cross-reacting species. An equal amount of cell protein from each strain as estimated from Coomassie blue staining was loaded into lanes 2 to 7.
DISCUSSION
This work has focused on the level of conservation of the amino acid sequence of RTP in Bacillus species closely related to B. subtilis 168 (B. vallismortis, B. mojavensis, B. amyloliquefaciens, and B. atrophaeus). After determination of the crystal structure of RTP (5), regions of the protein were assigned to potential functions: DNA binding, dimer formation, and contrahelicase (helicase interaction) activity (see reference 3). It was hoped that by determining which residues within RTP were conserved, their importance would be highlighted. Despite a significant number of nucleotide differences in rtp in the four close relatives of B. subtilis (up to 17.2% in B. atrophaeus), only five amino acid residues were changed. The stringent conservation of the primary sequence of RTP emphasizes the importance of the precise folded structure of RTP and its advantage to the cell in spite of the fact that it is not essential for viability (17). All of the residue changes are located on the exterior surface of the protein (Fig. 3), where they would be exposed to solvent and therefore least likely to alter the folded structure of RTP. A significant feature of these changes is that the majority are positioned away from the region of RTP thought to contact the DNA. Interestingly, the only change located close to this region, I73V, is very conservative and unlikely to be significant. The high level of conservation of this region of the RTP dimer almost certainly reflects the need to maintain the high affinity of RTP for its DNA terminators and its primary importance to the fork-arrest mechanism. Two of the five changes, E30K and E47P(Q), are located within a region (residues 17 to 49) previously found to share sequence homology with the DNA replication initiation protein DnaB of B. subtilis (18). The significance of this homology has not been established, but the suggestion of this reflecting a common interaction domain of RTP and DnaB with the replicative helicase (DnaC) has drawn interest to the region and influenced the work of others relating to the possibility of a specific interaction between RTP and the replicative helicase as part of the fork-arrest mechanism (26). Such work has been interpreted to suggest that E30 and Y33 within this region interact specifically with the helicase. This conclusion has depended upon the use of a strand displacement assay with the E. coli helicase, as well as an in vitro replication fork-arrest assay to measure the activity of RTP. However, identification of the E30K change in the B. mojavensis RTP suggests that this particular residue is not as vital to the contrahelicase activity of RTP as previously proposed. The possibility of a compensatory change being present in the B. mojavensis helicase (DnaC) was eliminated. Furthermore, it was shown that RTP.E30K functioned normally in B. subtilis in vivo. Taken together, these findings strongly suggest that E30 is not directly involved in a specific protein-protein interaction as part of the fork-arrest mechanism of B. subtilis.
The orientation and close proximity of TerI and TerII to rtp (Fig. 2) is also conserved in the other four species of Bacillus, as is the distance between TerI and TerII in B. atrophaeus and B. mojavensis (95 to 100 bp between nucleotide 28 of the opposed terminators [data not shown]). It is quite possible that this distance or the sequence context of TerI in the chromosome has some influence on the efficiency of fork arrest. Smith and Wake (40) found that the fork-arrest activity of TerI in a plasmid assay was enhanced when it was adjacent to TerII, as in the chromosome, but the effect of the flanking sequence on terminator efficiency in B. subtilis has not been fully examined. However, it is interesting that the sequence adjacent to the B site in all of the B. subtilis chromosomal terminators, and three of the six new Bacillus chromosomal terminators, is AT-rich (data not shown). As with the new sequences of RTP, the six new terminators are all extremely well conserved, especially over the B site (Fig. 5). It appears that several terminators are present in all rtp-containing Bacillus species which are likely to have similar fork traps based on two groups of opposed terminators.
The detection of two further chromosomal terminators in B. subtilis 168 brings the total to nine (one less than in E. coli [see reference 7]), which must be close to the complete set which conforms to the A-plus-B-site makeup. The two new terminators conform to the arrangement and location of the previously identified seven. They are located within the terminus region, are outside open reading frames, are adjacent to an AT-rich region, and are appropriately oriented within the replication fork trap. TerVIII (163.8°) is oriented to arrest the counterclockwise fork, while TerIX (187.1°) would arrest the clockwise fork. The latter extends the trap to 9.9% of the chromosome (151.5° to 187.1°). The significance of the smaller fork trap in B. subtilis compared to E. coli (42.5% of the chromosome) is not known. However, it is noteworthy that only 55% of the open reading frames in E. coli are transcribed in the same direction as replication (4), whereas in B. subtilis the figure is much higher at 75% (19). Possibly, the smaller fork trap in B. subtilis provides more-stringent coordination between these two processes than that tolerated in E. coli.
In a previous study it was concluded that the B. subtilis chromosomal terminator was made up of 29 bp (positions −2 to 27 of Fig. 5), comprising 16-bp A and B sites overlapping by 3 bp (boxed). The high level of conservation of adenine at nucleotide 28, complementary to thymine at position 14, in the total complement of Bacillus terminators now described indicates that the nucleotide 28 position should be included within the consensus terminator. This increases the B site to 17 bp (nucleotides 12 to 28, the region of pseudosymmetry around nucleotide 21 extending from nucleotide 14 to 28) and the overall terminator to 30 bp (nucleotides −2 to 28).
The number of nucleotide and amino acid changes identified in rtp and its encoded sequence in the four new strains examined here agrees with their established phylogenetic position relative to B. subtilis 168 (Fig. 1). No evidence to suggest that the relatively closely related B. licheniformis possesses a replication fork system based on RTP could be obtained by Southern hybridization, PCR, and Western immunodetection approaches. However, because of the relatively high level of cross-reactivity of RTP antibodies with additional protein species in the immunodetection work described here, we cannot rule out the possibility that an RTP homolog of a different size is present in B. licheniformis. Alternatively, could the acquisition of the RTP-based fork-arrest system be a relatively recent event, one occurring subsequent to the divergence of B. licheniformis and the progenitor of the “B. subtilis-like” group, with the lower GC contents (Table 1) of the rtp sequences reflecting horizontal transfer from another strain or species? To identify what, if any, fork-arrest system is utilized in B. licheniformis would be of great interest and could provide further insight into the relevance and mechanism of replication fork-arrest systems in general. The sporulation approach originally employed to detect fork arrest at the TerI terminator in B. subtilis as well as for the rtp gene (41) could be employed to search for an analogous situation in B. licheniformis.
ACKNOWLEDGMENTS
We thank Jackie Wilce for assistance in the assessment of the structural implications of various amino acid replacements, Bruno Gaeta (ANGIS) for assistance in sequence analysis, and Fred Cohan for comments on the draft manuscript.
This study has been supported by the Australian Research Council.
REFERENCES
- 1.Ahn K S. Expression of the rtp gene of Bacillus subtilis. Ph.D thesis. Sydney, Australia: University of Sydney; 1994. [Google Scholar]
- 2.Ahn K S, Malo M S, Smith M T, Wake R G. Autoregulation of the gene encoding the replication terminator protein of Bacillus subtilis. Gene. 1993;132:7–13. doi: 10.1016/0378-1119(93)90508-z. [DOI] [PubMed] [Google Scholar]
- 3.Bastia D, Mohanty B K. Mechanisms for completing DNA replication. In: DePamphilis M, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 177–214. [Google Scholar]
- 4.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Wayne Davis N, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 5.Bussiere D E, Bastia D, White S W. Crystal structure of the replication terminator protein from B. subtilis at 2.6 Å. Cell. 1995;80:651–660. doi: 10.1016/0092-8674(95)90519-7. [DOI] [PubMed] [Google Scholar]
- 6.Carrigan C M, Pack R A, Smith M T, Wake R G. Normal terC-region of the Bacillus subtilis chromosome acts in a polar manner to arrest the clockwise replication fork. J Mol Biol. 1991;222:197–207. doi: 10.1016/0022-2836(91)90206-l. [DOI] [PubMed] [Google Scholar]
- 7.Coskun-Ari F F, Hill T M. Sequence-specific interactions in the Tus-Ter complex and the effect of base pair substitutions on arrest of DNA replication in Escherichia coli. J Biol Chem. 1997;272:26448–26456. doi: 10.1074/jbc.272.42.26448. [DOI] [PubMed] [Google Scholar]
- 8.Creighton T E. Proteins: structures and molecular properties. 2nd ed. New York, N.Y: W. H. Freeman & Co.; 1993. [Google Scholar]
- 9.Ermakova O V, Frappier L, Schildkraut C L. Role of the EBNA-1 protein in pausing of replication forks in the Epstein-Barr virus genome. J Biol Chem. 1996;271:33009–33017. doi: 10.1074/jbc.271.51.33009. [DOI] [PubMed] [Google Scholar]
- 10.Franks A H, Griffiths A A, Wake R G. Identification and characterisation of new DNA replication terminators in Bacillus subtilis. Mol Microbiol. 1995;17:13–23. doi: 10.1111/j.1365-2958.1995.mmi_17010013.x. [DOI] [PubMed] [Google Scholar]
- 11.Franks A H, Wake R G. Replication fork arrest at relocated replication terminators on the Bacillus subtilis chromosome. J Bacteriol. 1996;178:4258–4265. doi: 10.1128/jb.178.14.4258-4265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gahn T A, Schildkraut C L. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell. 1989;58:527–535. doi: 10.1016/0092-8674(89)90433-9. [DOI] [PubMed] [Google Scholar]
- 13.Gerber J-K, Gögel E, Berger C, Wallisch M, Müller F, Grummt I, Grummt F. Termination of mammalian rDNA replication: polar arrest of replication fork movement by transcription termination factor TTF-I. Cell. 1997;90:559–567. doi: 10.1016/s0092-8674(00)80515-2. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths A A, Wake R G. Search for additional terminators in the Bacillus subtilis 168 chromosome. J Bacteriol. 1997;179:3358–3361. doi: 10.1128/jb.179.10.3358-3361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harry E J, Wake R G. The membrane-bound cell division protein DivIB is localised to the division site in Bacillus subtilis. Mol Microbiol. 1997;25:275–283. doi: 10.1046/j.1365-2958.1997.4581822.x. [DOI] [PubMed] [Google Scholar]
- 16.Healy J, Weir J, Smith I, Losick R. Post-transcriptional control of a sporulation regulatory gene encoding transcription factor ςH in Bacillus subtilis. Mol Microbiol. 1991;5:477–487. doi: 10.1111/j.1365-2958.1991.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 17.Iismaa T P, Wake R G. The normal replication terminus of the Bacillus subtilis chromosome, terC, is dispensable for vegetative growth and sporulation. J Mol Biol. 1987;195:299–310. doi: 10.1016/0022-2836(87)90651-6. [DOI] [PubMed] [Google Scholar]
- 18.Kralicek A V, Day A J, Wake R G, King G F. A sequence similarity between proteins involved in initiation and termination of bacterial chromosome replication. Biochem J. 1990;275:823. doi: 10.1042/bj2750823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunst F, Ogasawara N, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Langley D B. Replication termination in Bacillus subtilis. Ph.D. thesis. Sydney, Australia: University of Sydney; 1996. [Google Scholar]
- 22.Laskowski R A, MacArthur M W, Moss D S, Thorton J M. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst. 1993;26:283–291. [Google Scholar]
- 23.Lawrence J G, Ochman H. Amelioration of bacterial genomes: rates of change and exchange. J Mol Evol. 1997;44:383–397. doi: 10.1007/pl00006158. [DOI] [PubMed] [Google Scholar]
- 24.Lewis P J, Ralston G B, Christopherson R I, Wake R G. Identification of the replication terminator protein binding sites in the terminus region of the Bacillus subtilis chromosome and stoichiometry of the binding. J Mol Biol. 1990;214:73–84. doi: 10.1016/0022-2836(90)90147-E. [DOI] [PubMed] [Google Scholar]
- 25.Lewis P J, Wake R G. DNA and protein sequence conservation at the replication terminus in Bacillus subtilis 168 and W23. J Bacteriol. 1989;171:1402–1408. doi: 10.1128/jb.171.3.1402-1408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manna A C, Pai K S, Bussiere D E, Davies C, White S W, Bastia D. Helicase-contrahelicase interaction and the mechanism of termination of DNA replication. Cell. 1996;87:881–891. doi: 10.1016/s0092-8674(00)81995-9. [DOI] [PubMed] [Google Scholar]
- 27.Mohanty B K, Sahoo T, Bastia D. The relationship between sequence-specific termination of DNA replication and transcription. EMBO J. 1996;15:2530–2539. [PMC free article] [PubMed] [Google Scholar]
- 28.Moszer I, Glaser P, Danchin A. SubtiList: a relational data base for the Bacillus subtilis genome. Microbiology. 1995;141:261–268. doi: 10.1099/13500872-141-2-261. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama N, Arai N, Bond M W, Kaziro Y, Arai K. Nucleotide sequence of dnaB and the primary structure of the dnaB protein from Escherichia coli. J Biol Chem. 1984;259:97–101. [PubMed] [Google Scholar]
- 30.Nakumara L K. Taxonomic relationship of black-pigmented Bacillus subtilis strains and a proposal for Bacillus atrophaeus sp. nov. Int J Syst Bacteriol. 1989;38:295–300. [Google Scholar]
- 31.Natarajan S, Kelley W L, Bastia D. Replication terminator protein of Escherichia coli is a transcriptional repressor of its own synthesis. Proc Natl Acad Sci USA. 1991;88:3867–3871. doi: 10.1073/pnas.88.9.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogasawara N, Nakai S, Yoshikawa H. Systematic sequencing of the 180 kilobase region of the Bacillus subtilis chromosome. DNA Res. 1994;1:1–14. doi: 10.1093/dnares/1.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Otter T, King S M, Witman G M. A two-step procedure for efficient electrotransfer of both high-molecular-weight (>400,000) and low-molecular-weight (<20,000) proteins. Anal Biochem. 1987;162:370–377. doi: 10.1016/0003-2697(87)90406-4. [DOI] [PubMed] [Google Scholar]
- 34.Pai K S, Bussiere D E, Wang F, Hutchison III C A, White S W, Bastia D. The structure and function of the replication terminator protein of Bacillus subtilis: identification of the ‘winged helix’ DNA-binding domain. EMBO J. 1996;15:3164–3173. [PMC free article] [PubMed] [Google Scholar]
- 35.Pedré X, Weise F, Chai S, Lüder G, Alonso J C. Analysis of cis and trans acting elements required for the initiation of DNA replication in the Bacillus subtilis bacteriophage SPP1. J Mol Biol. 1994;236:1324–1340. doi: 10.1016/0022-2836(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 36.Richardson J S, Richardson D C. Principles and patterns of protein conformation. In: Fasman G D, editor. Prediction of protein structure and the principles of protein conformation. New York, N.Y: Plenum Press; 1989. pp. 1–98. [Google Scholar]
- 37.Roberts M S, Nakumara L K, Cohan F M. Bacillus vallismortis sp. nov., a close relative of Bacillus subtilis, isolated from soil in Death Valley, California. Int J Syst Bacteriol. 1996;46:470–475. doi: 10.1099/00207713-46-2-470. [DOI] [PubMed] [Google Scholar]
- 38.Roberts M S, Nakumura L K, Cohan F M. Bacillus mojavensis sp. nov., distinguishable from Bacillus subtilis by sexual isolation, divergence in DNA sequences, and differences in fatty acid composition. Int J Syst Bacteriol. 1994;44:256–264. doi: 10.1099/00207713-44-2-256. [DOI] [PubMed] [Google Scholar]
- 39.Smith M T, de Vries C J, King G F, Wake R G. The Bacillus subtilis DNA replication terminator. J Mol Biol. 1996;260:54–69. doi: 10.1006/jmbi.1996.0381. [DOI] [PubMed] [Google Scholar]
- 40.Smith M T, Wake R G. Definition and polarity of action of DNA replication terminators in Bacillus subtilis. J Mol Biol. 1992;227:648–657. doi: 10.1016/0022-2836(92)90214-5. [DOI] [PubMed] [Google Scholar]
- 41.Weiss A S, Hariharan I K, Wake R G. Analysis of the terminus region of the Bacillus subtilis chromosome. Nature. 1981;293:673–675. doi: 10.1038/293673a0. [DOI] [PubMed] [Google Scholar]
- 42.Williams N K, Wake R G. Sequence limits of DNA strands in the arrested replication fork at the Bacillus subtilis chromosome terminus. Nucleic Acids Res. 1989;17:9947–9956. doi: 10.1093/nar/17.23.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood W I, Gitschier J, Lasky L A, Lawn R M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci USA. 1985;82:1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]