Abstract
Systemic treatment options for patients with locally advanced or metastatic basal cell carcinoma (BCC) are limited, particularly when tumors are refractory to anti-programmed cell death protein-1 (PD-1). A better understanding of immune checkpoint expression within the BCC tumor microenvironment may inform combinatorial treatment strategies to optimize response rates. CD3, PD-1, programmed death ligand-1 (PD-L1), lymphocyte activation gene 3 (LAG-3), and T-cell immunoglobulin domain and mucin domain 3 (TIM-3)+ cell densities within the tumor microenvironment of 34 archival, histologically aggressive BCCs were assessed. Tumor infiltrating lymphocyte (TIL) expression of PD-1, PD-L1, and LAG-3, and to a lesser degree TIM-3, correlated with increasing CD3+ T-cell densities (Pearson’s r=0.89, 0.72, 0.87, and 0.63, respectively). 100% of BCCs (34/34) demonstrated LAG-3 and PD-1 expression in >1% TIL; and the correlation between PD-1 and LAG-3 densities was high (Pearson’s r=0.89). LAG-3 was expressed at ~50% of the level of PD-1. Additionally, we present a patient with locally-advanced BCC who experienced stable disease during and after 45 weeks of first-line anti-PD-1 (nivolumab), followed by a partial response after the addition of anti-LAG-3 (relatlimab). Longitudinal biopsies throughout the treatment course showed a graduated increase in LAG-3 expression after anti-PD-1 therapy, lending support for coordinated immunosuppression and suggesting LAG-3 as a co-target for combination therapy to augment the clinical impact of anti-PD-(L)1.
Keywords: pathology; carcinoma, basal cell; biomarkers, tumor; immunohistochemistry; tumor microenvironment
Introduction
Most basal cell carcinomas (BCCs) are effectively treated with topical therapy or surgical resection. However, for some patients, BCC invades surrounding anatomical structures, destroying soft tissue, cartilage and bone. Because BCCs frequently present on the head and neck, locally destructive tumors can cause substantial disfigurement and functional impairment. In rare cases, BCC metastasizes to distant sites. Hedgehog pathway inhibitors (HHI) are standard-of-care treatment for patients with locally-advanced BCC (laBCC) or metastatic BCC (mBCC). Although objective response rates (ORRs) are ~30–70%, durations of response are often suboptimal, and patients frequently discontinue therapy due to adverse events.1
Immune checkpoint inhibitors (ICI), for example, anti-programmed cell death protein-1 (PD-1), have demonstrated efficacy in treating patients with a broad array of advanced cancers, including melanoma, Merkel cell carcinoma, and cutaneous squamous cell carcinoma. In February 2021, the US Food and Drug Administration approved cemiplimab-rwlc (anti-PD-1) for patients with laBCC or mBCC who were previously treated with an HHI, or for whom an HHI is not appropriate. Clinical trial data informing this approval (NCT03132636) reported ORRs of 31% among 84 patients with laBCC (median duration of response (DOR) not reached (range: 2.1–21.4+ months)), and 21% among 28 patients with mBCC (median DOR not reached (range: 9–23.0+ months)).2
Across various tumor types, antitumor responses to programmed cell death protein-(death-ligand)1 (PD-(L)1) antibodies are frequently associated with a high tumor mutational burden and PD-L1 expression in the pretreatment tumor microenvironment (TME). BCC has both features3 yet the majority of patients do not experience tumor regression. Immunoregulatory molecules such as lymphocyte activation gene 3 (LAG-3) or T-cell immunoglobulin domain and mucin domain 3 (TIM-3) appear to play a role in ICI resistance in other tumor types,4 and may act similarly in BCC. The aim of the present study was to characterize PD-L1, PD-1, LAG-3, and TIM-3 expression in a cohort of BCCs to potentially identify targetable checkpoint molecules that may confer resistance to PD-(L)1 blockade.
Materials and methods
Case selection
Following Institutional Review Board (IRB) approval (JHU IRB-X; NA_0085595), 34 formalin-fixed paraffin-embedded (FFPE) specimens from patients with histologically-aggressive BCC (defined as those with perineural or bony invasion and/or size >1 cm) were identified in the surgical pathology archives. Clinicopathologic parameters were collected, including patient anatomic site, recurrence, metastasis, and whether systemic treatment was received prior to biopsy (online supplemental table S1).
jitc-2023-007463supp001.pdf (6.8MB, pdf)
Immunohistochemistry and in-situ hybridization
Immunohistochemical (IHC) stains for CD3, PD-L1, PD-1, LAG-3, and TIM-3 were performed on FFPE tissue sections. IHC for CD3 was performed per routine automated clinical staining. Staining for PD-L1, PD-1, LAG-3, and TIM-3 were performed as previously described,5 with a revised primary antibody for TIM-3 (clone F38-2E2 at a concentration of 5 ug/mL) and amplification system (TSA plus biotin kit, Perkin Elmer NEL749B001KT, 1:50 dilution).
In-situ hybridization (ISH) for PD-1 was performed on select cases to confirm observed protein expression patterns. Specifically, ISH for PD-1 mRNA expression was performed using an automated stainer (Leica Bond, Leica Biosystems) using the RNAscope Kit (Advanced Cell Diagnostics) according to the manufacturer’s instructions.
Scoring of IHC stains
CD3, PD-L1, PD-1, LAG-3, and TIM-3 immunostains were scanned (Hamamatsu NanozoomerXR). A pathologist annotated the TME using image analysis software (HALO V.3.5, Indica Labs), and tumor infiltrating lymphocyte (TIL) densities for each marker within the TME were determined (online supplemental figure S1). The relative percentage of tumor cells (TCs) expressing each marker were also assessed by a pathologist. TC expression >1% was considered ‘positive’ expression.
Results
PD-1 and PD-L1 expression
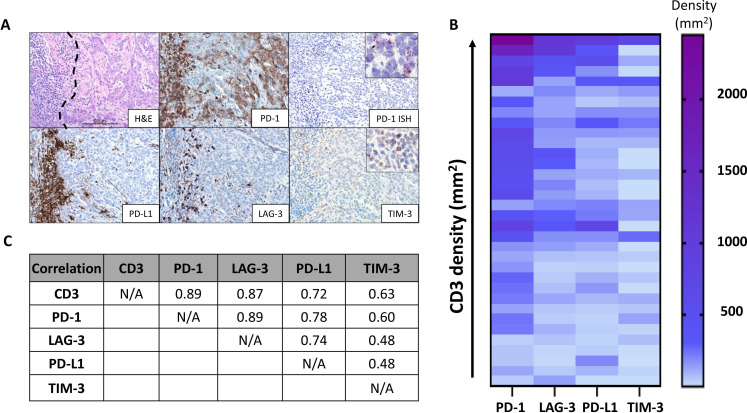
While 100% (34/34) of BCCs exhibited PD-1 and PD-L1 expression on TIL (PD-1 average density: 471/mm2, range 33–2447/mm2; PD-L1 average density: 178/mm2, range 9–985/mm2), only 9% (3/34) of specimens showed PD-L1 expression by TCs. In these few cases, the average proportion of TCs displaying PD-L1 was 2% (range of 1–5%). Unexpectedly, 56% (19/34) of specimens showed TC PD-1 expression. In these cases, the average proportion of TCs displaying PD-1 was 10% (range of 2–90%). TC expression of PD-1 was confirmed by ISH (figure 1A).
Figure 1.
Patterns of immunoactive marker expression in aggressive basal cell carcinomas (BCCs). (A) Photomicrograph (top left) displaying a representative tumor-immune cell interface within BCC (immune cells on left and tumor on right of black dotted line, H&E staining). Programmed cell death protein-1 (PD-1) immunohistochemistry highlights PD-1 expression by tumor infiltrating lymphocyte (TIL) and tumor cells (TCs) (top middle). PD-1 amplified in-situ hybridization (ISH) was used to verify the PD-1 expression by TCs (top right). Inset shows positive signal in the cytoplasm (red, punctate dots). Programmed death ligand-1 (PD-L1), lymphocyte activation gene 3 (LAG-3), and T-cell immunoglobulin domain and mucin domain 3 (TIM-3) expressions were also present on TIL in this same region. Scale bar=50 µm. (B) Heat map relating CD3+ T-cell densities with PD-1, PD-L1 LAG-3, and TIM-3 lymphocyte expression on a per-specimen basis. Specifically, each row in the heat map represents one specimen from an individual patient. In contrast to the other markers, TIM-3 densities were relatively low and did not associate with an increasing T-cell infiltrate. (C) Pearson’s correlation coefficients, r, showing the strong relationship between CD3, PD-1, and LAG-3 expression.
LAG-3 and TIM-3 expression
One hundred percent (34/34) of cases showed LAG-3 and TIM-3 expression by TIL (LAG-3 average density 208/mm2; range 14–1132/mm2; TIM-3 average density 91/mm2; range 0.4–705/mm2), with a LAG-3/CD3+ T-cell ratio of 47% (range 8.5–100%), and a TIM-3/CD3+ T-cell ratio of 19% (range of 1–89%). No LAG-3 or TIM-3 expression by TCs was present.
Correlation of checkpoint expression with T-cell infiltrates and each other
The densities of PD-1+, PD-L1+, LAG-3+, and TIM-3+ TIL from each specimen were correlated against CD3+ densities to assess whether expression of these molecules was associated with T-cell infiltration (figure 1B). PD-1, PD-L1, and LAG-3 strongly correlated with the density of T-cell infiltrates (r=0.89, 0.72, 0.87, respectively) (figure 1C), though there were some cases that showed expression of PD-1 and LAG-3 out of proportion to the T-cell infiltrate (online supplemental figure S2). In contrast, TIM-3 expression by TIL was only moderately correlated with T-cell infiltration (r=0.63). PD-1, PD-L1, and LAG-3 expressions were also highly correlated with each other, but not with TIM-3. Of these molecules, PD-1 was expressed at the highest density. On average, LAG-3 and PD-L1 were expressed at~50% and 35% of PD-1 levels, respectively (p<0.0001 for both, paired t-test).
Case presentation
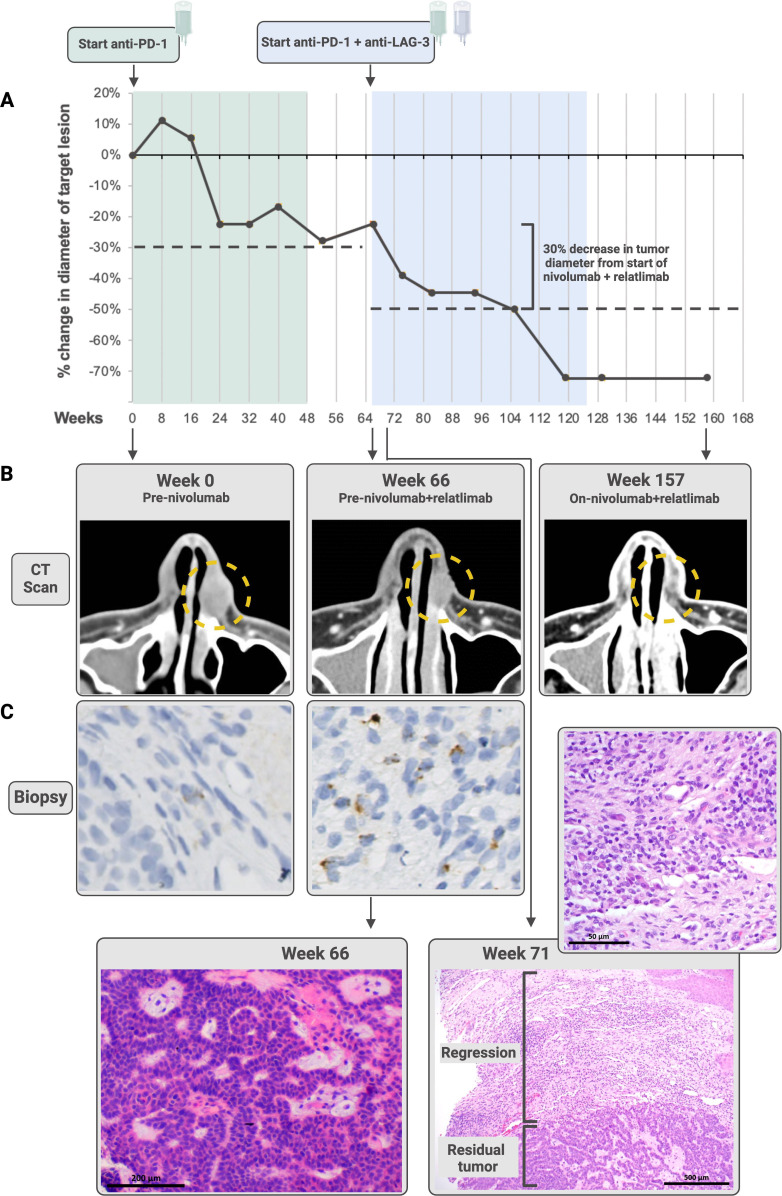
A patient in his early 50s presented with a treatment-naïve 18×14 mm BCC of the left nasal ala. After undergoing evaluation by a multidisciplinary team, the patient opted to forego surgical resection and pursue medical therapy on an IRB-approved clinical trial which included consent for biospecimen analysis (NCT03521830, JHU IRB:00166274). He began first-line nivolumab (anti-PD-1) 480 mg intravenously every 4 weeks. Over the next 48 weeks, the patient experienced stable disease per Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1 with a maximum decrease in tumor diameter of 27.7% (figure 2A,B). Nivolumab was discontinued per protocol, and the patient was monitored for the next 18 weeks, during which the BCC remained stable. He then began nivolumab 480 mg intravenously every 4 weeks plus relatlimab (anti-LAG-3) 960 mg intravenously every 4 weeks, experienced a partial response 39 weeks later, and discontinued therapy per protocol at 59 weeks. At 21.5 months, a partial response was ongoing. Treatment-related adverse effects included a rash and pruritus (grade 1, Common Terminology Criteria for Adverse Events (CTCAE) V.5), and arthritis affecting the hips, elbows and shoulders (grade 2).
Figure 2.
The patient whose advanced basal cell carcinoma stabilized on anti-programmed cell death protein-1 (PD-1), then regressed following the addition of anti-lymphocyte activation gene 3 (LAG-3) therapy. (A) the patient received nivolumab (anti-PD-1) over 48 weeks (green) during which he experienced stable disease per Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1. The tumor remained stable in size for 18 weeks after discontinuation of therapy, at which point the patient received nivolumab plus relatlimab (anti-LAG-3; blue). He experienced a partial response 39 weeks later, ongoing at 21.5 months from nivolumab/relatlimab initiation. Dashed lines show a 30% decrease in tumor diameter from baseline during each treatment regimen. Both CT scans (B) and paired pretreatment and on-treatment biopsies stained with H&E (C) demonstrate tumor regression. The high-powered view of the regression area shown for Week 71 shows fibrosis, plasma cells, foamy macrophages and numerous lymphocytes. The LAG-3 immunohistochemistry (IHC) staining performed on the Week 0 (immediate pretreatment) and Week 66 biopsies shows an increase in LAG-3 expression after administration of anti-PD-1 monotherapy prior to anti-PD-1+LAG-3 treatment. The patient went on to demonstrate an objective response to combinatorial therapy. Additional images for LAG-3 expression over the course of therapy are shown in online supplemental figure S3. Some figure elements created with BioRender.com.
H&E staining and IHC for LAG-3 were performed on serial biopsies from this patient (pretreatment, 4 weeks on-treatment, and 15 months after the first dose of anti-PD-1, figure 2C, online supplemental figure S3). The LAG-3 IHC showed 1%, 5%, and 10% expression in immune cells (ICs), respectively, demonstrating a graduated increase in expression. An on-treatment biopsy was performed at 5 weeks after the first dose of combination nivolumab plus relatlimab. H&E sections revealed partial regression of the tumor (figure 2C). The regression bed (where tumor used to be), was characterized by features of pan-tumor immune-related pathologic response,6 including chronic inflammation (lymphocytes and plasma cells) and histologic findings associated with wound healing (neovascularization and proliferative fibrosis).
Discussion
The current study describes expression of four immunoregulatory proteins—PD-L1, PD-1, LAG-3, and TIM-3—in the TME of BCCs in order to investigate potential mechanisms driving resistance to PD-1 blockade and to provide a rationale for the design of clinical trials testing combinatorial ICI regimens.
LAG-3 is an immunoregulatory molecule that is expressed in some tumors resistant to PD-1-pathway blockade. Blockade of LAG-3, especially in patients whose tumors exhibit LAG-3 expression on tumor-associated ICs, can overcome resistance and reinvigorate antitumor immunity.7 Similarly, antibodies blocking TIM-3 can trigger antitumor immunity after progression on anti-PD-1 therapy.8 We found LAG-3 to be a prominent feature of the BCC TME and demonstrated its coordinate expression with PD-1 and PD-L1, akin to what has been described in melanoma.9 As in melanoma, LAG-3 expression appears to be subdominant to PD-1/PD-L1 expression in the BCC TME. TIM-3 expression on TIL was also observed, though it was less coordinated with CD3+ T-cell infiltration and PD-1 expression than LAG-3. TIM-3 could also be considered as a potential target along with anti-PD-1 to further augment response rates in BCCs refractory to anti-PD-1 monotherapy.
While PD-L1 expression by TCs and ICs and PD-1 expression by lymphocytes have been widely studied across various tumor types, less is known about intrinsic PD-1 expression by TCs. Our study identified PD-1 expression by TCs in 38% of BCCs. In preclinical melanoma models, such expression is associated with tumor-promoting effects, which can be suppressed by inhibiting PD-1.10 Thus, PD-1 expression by TCs—in patients with BCC and other malignancies—should be explored as a candidate biomarker of tumor regression in response to PD-1 blocking drugs.
In the current case report, the patient received the full course of anti-PD-1 allowed on the trial before anti-LAG-3 was ultimately added to their regimen. During this time, the patient’s best overall response was stable disease for over a year. However, as this was not a controlled study, it is not possible to entirely exclude the possibility that continuation of anti-PD-1 alone could have ultimately further facilitated disease clearance. Taken together with the IHC characterization of the BCC TME, our findings provide a rationale supporting translational trials exploring the synergistic blockade of LAG-3 and anti-PD-(L)1 for patients with advanced BCC resistant to PD-1 pathway blockade. Further investigation into expression of immunoregulatory molecules in the BCC TME may identify additional therapeutic targets and candidate biomarkers predicting tumor regression in response to ICI.
Footnotes
Contributors: JSD, JL and JMT had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JSD, AS, EJL, JMT. Acquisition, analysis, and interpretation of data: JSD, AS, JL, EMW, KMS, HX, AO, DW, LLE, PN, BFG, JKC, MDS, EJL, JMT. Drafting of the manuscript: JSD, AS, KMS, EJL, JMT. Critical revision of the manuscript for important intellectual content: JSD, JL, EMW, AS, KMS, EJL, JMT. Statistical analysis: JSD, AS, JMT. Obtained funding: JL, JMT. Administrative, technical, or material support: JSD, AS, EMW, JL, JMT. Study supervision: EJL, JMT.
Funding: This work was supported by The Mark Foundation for Advanced Genomics and Imaging at Johns Hopkins (JMT, EJL); National Cancer Institute R01 CA142779 (JMT), National Institutes of Health Training grant T32CA193145 (AS, JSD), The Bloomberg~Kimmel Institute for Cancer Immunotherapy (JSD, KMS, LLE, EJL, JMT), The Marilyn and Michael Glosserman Fund for Basal Cell Carcinoma and Melanoma Research (KMS, EJL), and Bristol-Myers Squibb (JSD, EJL, JMT).
Competing interests: JSD receives research funding from Bristol-Myers Squibb. DW is an employee of Merck. EJL serves as a consultant/advisory board member for Array BioPharma, Bristol-Myers Squibb, EMD Serono, Genentech, Macrogenics, Merck, Millennium, Novartis, Sanofi/Regeneron, and receives institutional research funding from Bristol-Myers Squibb, Merck and Regeneron. JMT serves as a consultant/advisory board member for Bristol-Myers Squibb, Merck, AstraZeneca, Compugen, and Akoya Biosciences. No additional potential conflicts of interest were disclosed.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by JHU IRB-X; NA_00085595 JHU IRB: 00166274 for the case report patient. Participants gave informed consent to participate in the study before taking part.
References
- 1. Tay EY-X, Teoh Y-L, Yeo MS-W. Hedgehog pathway inhibitors and their utility in basal cell carcinoma: A comprehensive review of current evidence. Dermatol Ther (Heidelb) 2019;9:33–49. 10.1007/s13555-018-0277-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol 2021;22:848–57. 10.1016/S1470-2045(21)00126-1 [DOI] [PubMed] [Google Scholar]
- 3. Lipson EJ, Lilo MT, Ogurtsova A, et al. Basal cell carcinoma: PD-L1/PD-1 Checkpoint expression and tumor regression after PD-1 blockade. J Immunother Cancer 2017;5:23. 10.1186/s40425-017-0228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lei Q, Wang D, Sun K, et al. Resistance mechanisms of anti-Pd1/Pdl1 therapy in solid tumors. Front Cell Dev Biol 2020;8:672. 10.3389/fcell.2020.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duffield AS, Ascierto ML, Anders RA, et al. Th17 immune Microenvironment in Epstein-Barr virus-negative Hodgkin lymphoma: implications for Immunotherapy. Blood Adv 2017;1:1324–34. 10.1182/bloodadvances.2017007260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stein JE, Lipson EJ, Cottrell TR, et al. Pan-tumor pathologic scoring of response to PD-(L)1 blockade. Clin Cancer Res 2020;26:545–51. 10.1158/1078-0432.CCR-19-2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puhr HC, Ilhan-Mutlu A. New emerging targets in cancer Immunotherapy: the role of Lag3. ESMO Open 2019;4:e000482. 10.1136/esmoopen-2018-000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Acharya N, Sabatos-Peyton C, Anderson AC. Tim-3 finds its place in the cancer Immunotherapy landscape. J Immunother Cancer 2020;8:e000911. 10.1136/jitc-2020-000911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taube JM, Young GD, McMiller TL, et al. Differential expression of immune-regulatory genes associated with PD-L1 display in Melanoma: implications for PD-1 pathway blockade. Clin Cancer Res 2015;21:3969–76. 10.1158/1078-0432.CCR-15-0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kleffel S, Posch C, Barthel SR, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell 2015;162:1242–56. 10.1016/j.cell.2015.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-007463supp001.pdf (6.8MB, pdf)