Abstract
Sphingomonas herbicidovorans MH was able to completely degrade both enantiomers of the chiral herbicide dichlorprop [(RS)-2-(2,4-dichlorophenoxy)propanoic acid], with preferential degradation of the (S) enantiomer over the (R) enantiomer. These results are in agreement with the recently reported enantioselective degradation of mecoprop [(RS)-2-(4-chloro-2-methylphenoxy)propanoic acid] by this bacterium (C. Zipper, K. Nickel, W. Angst, and H.-P. E. Kohler, Appl. Environ. Microbiol. 62:4318–4322, 1996). Uptake of (R)-dichlorprop, (S)-dichlorprop, and 2,4-D (2,4-dichlorophenoxyacetic acid) was inducible. Initial uptake rates of cells grown on the respective substrate showed substrate saturation kinetics with apparent affinity constants (Kt) of 108, 93, and 117 μM and maximal velocities (Vmax) of 19, 10, and 21 nmol min−1 mg of protein−1 for (R)-dichlorprop, (S)-dichlorprop, and 2,4-D, respectively. Transport of (R)-dichlorprop, (S)-dichlorprop, and 2,4-D was completely inhibited by various uncouplers and by nigericin but was only marginally inhibited by valinomycin and by the ATPase inhibitor N,N′-dicyclohexylcarbodiimine. Experiments on the substrate specificity of the putative transport systems revealed that (R)-dichlorprop uptake was inhibited by (R)-mecoprop but not by (S)-mecoprop, (S)-dichlorprop, or 2,4-D. On the other hand, the (S)-dichlorprop transport was inhibited by (S)-mecoprop but not by (R)-mecoprop, (R)-dichlorprop, or 2,4-D. These results provide evidence that the first step in the degradation of dichlorprop, mecoprop, and 2,4-D by S. herbicidovorans is active transport and that three inducible, proton gradient-driven uptake systems exist: one for (R)-dichlorprop and (R)-mecoprop, another for (S)-dichlorprop and (S)-mecoprop, and a third for 2,4-D.
Research on the biodegradation of chiral xenobiotics can provide a better understanding of the processes that govern enantioselectivity in the microbial degradation and environmental fate of stereoisomers. We have examined the microbial degradation of the widely used chiral herbicide mecoprop [(RS)-2-(4-chloro-2-methylphenoxy)propanoic acid] by a pure bacterial strain that was isolated from soil. This strain, Sphingomonas herbicidovorans MH, can utilize both enantiomers of mecoprop as sole carbon and energy sources for growth (24). Growth experiments with the pure enantiomers as well as with racemic mecoprop revealed that the (S) enantiomer disappeared much faster from the culture medium than the (R) enantiomer. Based on these results, we concluded that specific catabolic enzymes must be involved in the degradation of each enantiomer of mecoprop (24). This view was confirmed by the finding that two α-ketoglutarate-dependent dioxygenases are involved in the enantioselective degradation of mecoprop in S. herbicidovorans MH, one that is specific for (R)-mecoprop and one that is specific for (S)-mecoprop (17). The (S) enantiomer-specific dioxygenase activity is constitutively expressed, whereas the (R) enantiomer-specific enzyme activity is present only when cells grow on the (R) enantiomer. Both enzymes are present in cells grown on the racemic mixture. Extracts of cells grown on complex medium and extracts of cells grown on (S)-mecoprop contain equally high activities of the (S) enantiomer-specific dioxygenase (17). However, intact cells grown on complex medium show very low oxygen uptake rates with (S)-mecoprop, whereas (S)-mecoprop-grown cells show high oxygen consumption with (S)-mecoprop (24). These findings suggested the existence of specific uptake systems and led us to examine the uptake of phenoxyalkanoic acids by S. herbicidovorans after growth on various substrates.
Many aromatic compounds are taken up by bacteria through energy-dependent transport systems (4, 5, 10, 11, 15, 16). The uptake of 4-chlorobenzoate by the coryneform bacterium NTB-1 is inducible and coupled to the proton motive force (4). The uptake of 4-toluene sulfonate by Comamonas testosteroni T-2 is also inducible and shows substrate saturation kinetics (11). Transport of 4-toluene sulfonate is inhibited completely by uncouplers but only marginally by ATPase inhibitors, and the authors proposed that 4-toluene sulfonate is taken up by an inducible secondary proton symport system (11). The uptake of 4-hydroxybenzoate by Pseudomonas putida is driven by the proton motive force, and the gene that encodes a 4-hydroxybenzoate transporter was identified, cloned, and expressed in Escherichia coli. Furthermore, it was found that 4-hydroxybenzoate transport is an integral feature of the β-ketoadipate pathway in P. putida (5, 15, 16). The uptake of the herbicide 2,4-D (2,4-dichlorophenoxyacetic acid) by Ralstonia eutropha JMP134 is inducible, is sensitive to metabolic inhibitors, and shows substrate saturation kinetics (10). Moreover, the authors identified a gene, designated tfdK, whose translation product shows resemblance to transport proteins of the major facilitator superfamily, and they suggested that tfdK encodes the 2,4-D transporter of R. eutropha JMP134.
Our results indicate that S. herbicidovorans MH has two distinct uptake systems for chiral phenoxypropionic acid herbicides, one for the (R) enantiomer and another one for the (S) enantiomer of dichlorprop and mecoprop. Furthermore, evidence was obtained that 2,4-D was taken up by a third transport system.
MATERIALS AND METHODS
Organism and growth conditions.
S. herbicidovorans MH (DSM 11019) was cultured and maintained as described before (24), unless stated otherwise. Growth experiments were performed in 1-liter Erlenmeyer flasks equipped with magnetic stirring bars. The flasks were equipped with a silicon tube that allowed easy sterile sampling. The experiments were carried out in a water bath at a temperature of 30°C, with stirring held constant at 370 rpm (24).
Uptake assays.
We performed the transport experiments with (R)- and (S)-[14C]dichlorprop synthesized from 14C-labeled 2,4-dichlorophenol (23). Radiolabeled (R)- and (S)-mecoprop were not commercially available. Uptake of 14C-labeled compounds was routinely assayed by a filtration method (18). Cells were harvested at the mid-exponential growth phase by centrifugation (15 min, 7,500 × g, 4°C), washed twice with ice-cold minimal medium (24), and resuspended in a phosphate buffer (20 mM Na2HPO4-KH2PO4 [pH 6.5]) at concentrations of 0.15 ± 0.03 mg of protein per ml. Cell suspensions could be kept on ice and aerated for 2 h without significant loss of transport activity. In some experiments, the cell suspension was preincubated for 5 min with potential uptake inhibitors. The cell suspension was acclimated at 30 ± 2°C for 5 min before the addition of the 14C-labeled compound. At intervals of 15 s, uptake was terminated by filtering a 200-μl sample through a 0.45-μm-pore-size cellulose-nitrate filter (Sartorius AG, Göttingen, Germany) that had been prewashed with 2 ml of phosphate buffer (washing buffer) containing 50 μM of unlabeled 2,4-D or (R)- or (S)-dichlorprop. After filtration, the filters were washed once with 5 ml of the washing buffer to remove extracellular 14C-labeled compounds and then transferred to scintillation vials. After addition of the scintillation cocktail (Filter-Count; Packard Instrument Company, Meriden, Conn.), the radioactivity was determined in a liquid scintillation counter (Kontron Analytical, Zürich, Switzerland). The kinetic uptake parameters Kt (apparent affinity constant) and Vmax (maximum velocity) were calculated with a computer program (IGOR Pro; WaveMetrics Inc., Lake Oswego, Oreg.). The calculations were based on weighted nonlinear regression analysis of the Michaelis-Menten model. Error estimates for the biological variance were obtained from the data of four independent assays with different cell batches at one specific substrate concentration and by making the assumption that the error was heteroscedastically distributed with a constant relative standard deviation.
Estimation of intracellular volume.
The specific intracellular volume of S. herbicidovorans MH was estimated from the dimensions of the cells (average length of the rods, 2.8 μm; average width, 0.7 μm [24]), the protein content (0.15 ± 0.03 mg ml−1 at an optical density at 546 nm of 1), and the cell number ([1.71 ± 0.13] × 108 ml−1 at an optical density at 546 nm of 1). From these data, a specific internal cell volume of 1.2 μl/mg of protein was calculated. This value is in the same range as the volumes of other gram-negative rods (11, 13) and was used for all calculations.
Analytical procedures. (i) HPLC analysis.
High-pressure liquid chromatography (HPLC) analyses were performed on a Gynkotek HPLC system with a M480G pump, a Gina 50T autosampler, and a UVD340S photodiode array detector (Gynkotek GmbH, Germering, Germany). The system was operated isocratically with an eluent consisting of 70% (vol/vol) methanol and 30% (vol/vol) NaH2PO4 (50 mM, pH 3.0) at a flow rate of 0.7 ml/min. Forty microliters of the samples was injected, and the eluting compounds were detected at a wavelength of 230 nm. The analytes were separated on a Nucleodex-α-PM column (200 by 4.0 mm) with permethylated α-cyclodextrin as the chiral stationary phase (Macherey-Nagel, Düren, Germany). Retention times were typically 6.0, 6.7, and 8.6 min for 2,4-D, (R)-dichlorprop, and (S)-dichlorprop, respectively. The detection limits for the dichlorprop enantiomers and 2,4-D were 0.1 and 0.05 μM, and the relative standard deviations (n = 3) were ±0.8% and ±0.7%, respectively.
(ii) Determination of ATP content.
ATP was determined by means of the firefly luciferase assay with an ATP bioluminescence assay kit (HS II; Boehringer, Mannheim, Germany).
(iii) Protein determination.
Protein contents were determined by the method of Lowry et al. (12), with bovine serum albumin as the standard.
(iv) Ion chromatography.
Chloride concentrations were determined with an ion chromatograph equipped with a conductivity detector (Dionex, Olten, Switzerland) after separation on an anion-exchange column (IonPac AS11; Dionex) (24).
Chemicals.
[ring-U-14C]2,4-D (≥98%; 807 GBq/mol) was obtained from Sigma Chemical Co. (St. Louis, Mo.). [ring-U-14C](R)-dichlorprop (≥98.3%; enantiomeric purity, 99.4%; 126 GBq/mol) and [ring-U-14C](S)-dichlorprop (≥98.4%; enantiomeric purity, >99.4%; 166 GBq/mol) were prepared by Amersham, Buckinghamshire, England, as described elsewhere (23). All other chemicals were obtained from Fluka, Buchs, Switzerland, or Merck, Dietikon, Switzerland.
RESULTS
Growth on dichlorprop and 2,4-D.
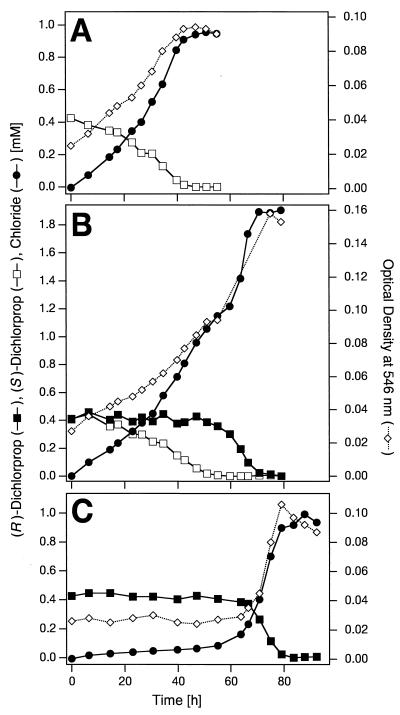
S. herbicidovorans MH utilized both enantiomers of dichlorprop as sole carbon and energy sources for growth (Fig. 1A and C). However, the (S)-dichlorprop was completely removed from the culture medium after 43 h of incubation (Fig. 1A), while in experiments with (R)-dichlorprop as the growth substrate, 82 h passed until the degradation was complete (Fig. 1C). When strain MH was offered racemic dichlorprop as the growth substrate, it could degrade both enantiomers, with the (S)-dichlorprop being preferentially degraded (Fig. 1B). Chloride release was stoichiometric with (R)-, (S)-, and (RS)-dichlorprop as the growth substrate (Fig. 1), which is a strong indication for the mineralization of the aromatic ring. In all growth experiments, strain MH did not excrete any metabolites into the medium that could be detected by HPLC, and the levels of dissolved organic carbon at the beginning and at the end of a growth experiment (data not shown) corresponded to a complete conversion of dichlorprop into biomass and presumably carbon dioxide. 2,4-D was also completely degraded by strain MH, as shown by biomass formation, substrate consumption, and stoichiometric chloride release (data not shown). Typically, growth on 2,4-D started without a lag phase and was complete after 15 h of incubation. The growth rates and the growth yields obtained are listed in Table 1.
FIG. 1.
Growth experiments with S. herbicidovorans MH incubated with (R)-dichlorprop, (S)-dichlorprop, and racemic (RS)-dichlorprop. Precultures were grown in chloride-free complex medium for 12 h at 30°C. (A) Growth on the pure (S) enantiomer; (B) growth on the racemic mixture; (C) growth on the pure (R) enantiomer. An A546 of 0.1 corresponds to 20.8 mg (dry weight) per liter.
TABLE 1.
Growth characteristics of S. herbicidovorans MH grown on (R)-dichlorprop, (S)-dichlorprop, (RS)-dichlorprop, and 2,4-D and of the strain grown on (R)-mecoprop, (S)-mecoprop, and (RS)-mecopropa
| Growth substrate | Mean growth rate (h−1) ± SD | Specific deg- radation rate (mmol h−1 g [dry wt]−1) | Mean yield (g [dry wt] g of substrate−1) ± SD |
|---|---|---|---|
| (R)-Dichlorprop | 0.082 ± 0.006 | 2.3 | 0.37 ± 0.06 |
| (R)-Mecoprop | 0.032 | NDb | 0.30 ± 0.15 |
| (S)-Dichlorprop | 0.036 ± 0.001 | 1.4 | 0.35 ± 0.02 |
| (S)-Mecoprop | 0.039 | ND | 0.35 ± 0.05 |
| Racemic mixture of dichlorprop | 0.29 ± 0.03 | ||
| (R)-Dichlorprop | 0.043 ± 0.003 | 2.4 | |
| (S)-Dichlorprop | 0.029 ± 0.004 | 1.1 | |
| Racemic mixture of mecoprop | 0.016c | ND | 0.30 ± 0.04 |
| 2,4-D | 0.133 ± 0.003 | 4.4 | 0.19 ± 0.05 |
Values for growth on mecoprop were taken from reference 24.
ND, not determined.
In this case, the growth rate was affected by the concentration, which for each enantiomer was only half of what it was in the experiments with the pure mecoprop enantiomers. Additional growth experiments showed increased growth rates in experiments with higher (RS)-mecoprop concentrations (24).
Induction of uptake.
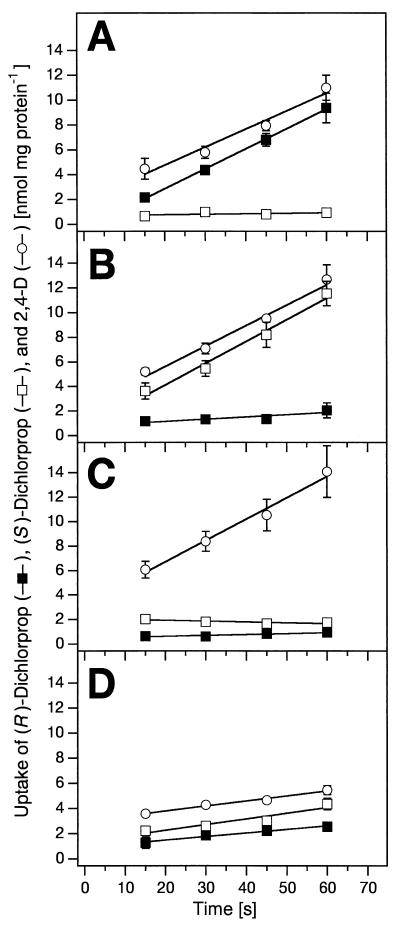
Cells of S. herbicidovorans MH grown on (R)-dichlorprop were induced for the uptake of (R)-dichlorprop and 2,4-D, but such cells did not take up (S)-dichlorprop (Fig. 2A). When S. herbicidovorans MH grew on (S)-dichlorprop, uptake of (S)-dichlorprop and 2,4-D was induced but uptake of (R)-dichlorprop was not (Fig. 2B). Interestingly, cells of S. herbicidovorans MH grown on 2,4-D were able to take up 2,4-D but not the dichlorprop enantiomers (Fig. 2C). Control experiments with succinate-grown cells revealed that these cells took up (R)-dichlorprop, (S)-dichlorprop, and 2,4-D, but with very low rates (Fig. 2D). However, these rates were slightly higher than the rates for uptake by noninduced cells in the experiments described above (Fig. 2A to C).
FIG. 2.
Induction experiments with S. herbicidovorans MH grown on (R)-dichlorprop (A), (S)-dichlorprop (B), 2,4-D (C), and succinate (D). Cells were washed and resuspended in 20 mM phosphate buffer, pH 6.5 (0.15 mg of protein per ml), and uptake was started by the addition of 60 μM 14C-labeled (R)-dichlorprop, (S)-dichlorprop, or 2,4-D. Values represent means of three replicates; standard deviations are indicated by the error bars.
Kinetics of uptake.
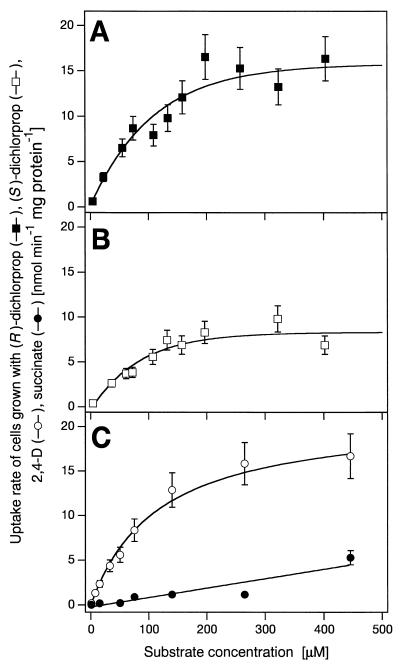
The kinetic parameters of (R)-dichlorprop, (S)-dichlorprop, and 2,4-D uptake were estimated from the initial uptake rates (15 to 60 s) at concentrations from 0.7 to 450 μM. Uptake of (R)-dichlorprop, (S)-dichlorprop, and 2,4-D showed monophasic saturation kinetics (Fig. 3), with Kt values of 108 ± 21, 93 ± 19, and 117 ± 25 μM and Vmax values of 19 ± 2, 10 ± 1, and 21 ± 3 nmol min−1 mg of protein−1, respectively. Succinate-grown cells (Fig. 3C) took up 2,4-D with much lower rates than did 2,4-D-grown cells, and the relationship between 2,4-D concentration and 2,4-D uptake in such cells could be modeled with a linear function.
FIG. 3.
Effect of substrate concentration on uptake of (R)-dichlorprop (A), (S)-dichlorprop (B), and 2,4-D (C) by S. herbicidovorans MH grown on (R)-dichlorprop (A), (S)-dichlorprop (B), and succinate and 2,4-D (C). The initial uptake rates (15 s to 2 min) are plotted versus the external concentration of the substrate in the assay (0.7 to 450 μM). Analysis of the data gave Kt values of 108, 93, and 117 μM and Vmax values of 19, 10, and 21 nmol min−1 mg of protein−1 for (R)- and (S)-dichlorprop and 2,4-D, respectively. Error bars represent relative standard deviations (15%) (see Materials and Methods).
Effect of structurally related compounds on uptake.
To study the substrate specificity of the (R)- and (S)-dichlorprop uptake, we examined how the addition of unlabeled (R)-dichlorprop, (S)-dichlorprop, (R)-mecoprop, (S)-mecoprop, and 2,4-D affected transport. The unlabeled compounds were added at concentrations almost threefold higher than those of the labeled compounds. The presence of (S)-dichlorprop, (S)-mecoprop, and 2,4-D exerted only a small influence on (R)-dichlorprop uptake (Table 2). As expected, (R)-dichlorprop uptake was reduced by unlabeled (R)-mecoprop and (R)-dichlorprop. Similarly, (R)-mecoprop, (R)-dichlorprop, and 2,4-D had only a small effect on (S)-dichlorprop uptake, whereas the (S) enantiomers of mecoprop and dichlorprop reduced the uptake of (S)-dichlorprop (Table 2).
TABLE 2.
Effect of structurally related compounds on uptake of (R)- and (S)-dichlorprop by S. herbicidovorans MHa
| Addition | Uptake of (R)-dichlorpropb ± SD (% of control) | Uptake of (S)-dichlorpropc ± SD (% of control) |
|---|---|---|
| None (control) | 100 ± 3.4 | 100 ± 18.9 |
| 2,4-D | 86 ± 0.9 | 87 ± 10.8 |
| (R)-Dichlorprop | 57 | 95 ± 14.0 |
| (R)-Mecoprop | 50 ± 2.3 | 84 ± 4.5 |
| (S)-Dichlorprop | 89 ± 0.8 | 54 ± 14.9 |
| (S)-Mecoprop | 96 ± 1.8 | 25 ± 10.4 |
Cells grown on (R)-dichlorprop or (S)-dichlorprop were washed and resuspended in 20 mM phosphate buffer (pH 6.5). Initial uptake rates [with 30 μM 14C-labeled (R)- and (S)-dichlorprop] were determined in the presence or absence of an unlabeled, structurally related compound (80 μM). Values represent the means of duplicate determinations.
Uptake by (R)-dichlorprop-grown cells; 100% corresponds to an uptake rate of 2.5 ± 0.2 nmol min−1 mg of protein−1.
Uptake by (S)-dichlorprop-grown cells; 100% corresponds to an uptake rate of 1.5 ± 0.3 nmol min−1 mg of protein−1.
Substrate accumulation.
To measure substrate accumulation, cells were grown on succinate, 2,4-D, (R)-dichlorprop, and (S)-dichlorprop. Cells from each batch were incubated in 60 μM [14C]2,4-D, [14C](R)-dichlorprop, and [14C](S)-dichlorprop solutions for 60 s, and the accumulated radioactivity was determined. Apparent intracellular substrate concentrations were calculated with a cell volume of 1.2 μl/mg of protein. Division of these values by the external substrate concentration yielded the apparent accumulation of the substrates within the cells (Table 3).
TABLE 3.
Accumulation of radioactivity by cells of S. herbicidovorans MH grown on succinate, 2,4-D, (R)-dichlorprop, and (S)-dichlorprop
| Assay substrate | Mean accumulation of radioactivity (c′i/ce)a ± SD of cells grown on:
|
|||
|---|---|---|---|---|
| Succinate | 2,4-D | (R)-Dichlorprop | (S)-Dichlorprop | |
| 2,4-D | 20 ± 1 | 134 ± 27 | 108 ± 14 | 103 ± 15 |
| (R)-Dichlorprop | 15 ± 8 | 3 ± 3 | 114 ± 17 | 5 ± 1 |
| (S)-Dichlorprop | 25 ± 6 | 0 | 0 | 116 ± 13 |
c′i is the apparent intracellular substrate concentration, which was obtained in the presence of catabolic activity and consequently represents the sum of the intracellular concentrations of the substrate and all metabolites. ce is the external substrate concentration in the transport assay (60 μM).
Effect of metabolic inhibitors on uptake.
The uncouplers carbonylcyanide m-chlorophenylhydrazone (CCCP), carbonylcyanide-4-trifluoromethoxyphenylhydrazone (CCFP), and 2,4-dinitrophenol (DNP), which can dissipate the proton motive force Δp (i.e., both the electrical potential Δψ and the pH gradient ΔpH) (9), inhibited the uptake of (R)-dichlorprop, (S)-dichlorprop, and 2,4-D almost completely (Table 4). Almost complete inhibition of (R)-dichlorprop, (S)-dichlorprop, and 2,4-D uptake was also observed in the presence of nigericin (Table 4), a K+ ionophore that catalyzes an electroneutral K+-H+ exchange and thus selectively dissipates the ΔpH in the presence of high K+ concentrations (9). Valinomycin, another potassium ionophore which can selectively dissipate the Δψ in the presence of high concentrations of K+ (9), showed little effect on uptake of (R)-dichlorprop, (S)-dichlorprop, and 2,4-D. On the other hand, valinomycin and nigericin in combination reduced the transport of all three substrates to values from 0.4 to 10.9% of the control values (Table 4). In combination, these two ionophores function as an uncoupler (9). Although the ATPase inhibitor N,N′-dicyclohexylcarbodiimine (DCCD) reduced uptake of (R)-dichlorprop, (S)-dichlorprop, and 2,4-D to some extent, no correlation between uptake activity and internal ATP concentration was found. For instance, the ATP content of (S)-dichlorprop-grown cells treated with nigericin was 83% of the control, whereas uptake of (S)-dichlorprop was almost completely inhibited (Table 4). Moreover, transport of (S)-dichlorprop was completely inhibited by the uncouplers CCCP, CCFP and DNP, whereas the ATP concentrations were still in the range of 50 to 73% of the control (Table 4).
TABLE 4.
Effects of inhibitors on the uptake of (R)-dichlorprop, (S)-dichlorprop, and 2,4-D and on the intracellular ATP concentration in S. herbicidovorans MHa
| Addition | % of control
|
|||||
|---|---|---|---|---|---|---|
| Cells grown on (R)-dichlorprop
|
Cells grown on (S)-dichlorprop
|
Cells grown on 2,4-D
|
||||
| Uptake of (R)-dichlorprop | Internal ATP concn | Uptake of (S)-dichlorprop | Internal ATP concn | Uptake of 2,4-D | Internal ATP concn | |
| CCCP (50 μM) | 7.4 | 47 | 0.0 | 50 | 3.8 | 61 |
| CCFP (50 μM) | 0.8 | 56 | 0.0 | 64 | 1.4 | 60 |
| DNP (500 μM) | 11.9 | 44 | 4.3 | 73 | 7.1 | 61 |
| Valinomycin (100 μM) | 39.3 | 49 | 77.6 | 88 | 92.5 | 97 |
| Nigericin (100 μM) | 0.0 | 61 | 1.5 | 83 | 6.8 | 47 |
| Valinomycin plus nigericin (100 μM each) | 7.0 | 73 | 10.9 | 78 | 0.4 | 65 |
| DCCD (50 μM) | 21.0 | 34 | 9.3 | 19 | 37.5 | 29 |
Cells grown on (R)-dichlorprop, (S)-dichlorprop, and 2,4-D were washed and resuspended in 20 mM phosphate buffer (pH 6.5). After incubation for 5 min at 30°C in the presence or absence of an inhibitor, initial uptake rates {with 60 μM [14C](R)-dichlorprop, 115 μM [14C](S)-dichlorprop and 30 μM [14C]2,4-D} and intracellular ATP concentrations were determined. In the absence of an inhibitor (control), the initial uptake rates were 7.1 ± 0.5, 5.5 ± 0.1, and 4.2 ± 0.5 nmol min−1 mg of protein−1 for (R)-dichlorprop, (S)-dichlorprop, and 2,4-D, respectively, and the internal ATP concentration was 1.4 ± 0.3 mM for all substrates; these values were taken as 100%.
DISCUSSION
Growth characteristics.
Racemic mixtures of the chiral herbicide dichlorprop were degraded in an enantioselective manner by S. herbicidovorans MH (Fig. 1B). The growth patterns of strain MH incubated with (R)-, (S)-, and (RS)-mecoprop, a structurally related compound, were essentially similar, with preferential degradation of the (S) enantiomer (24). The growth rates with dichlorprop were in the same range, and the growth yields were nearly identical to those obtained with mecoprop (Table 1). Hence, the two enantiomers of these compounds were equally well suited as a carbon and energy source for this bacterium. These results are in contrast to findings with Alcaligenes denitrificans, which exclusively degraded (R)-mecoprop (19, 20), and with laboratory soil systems with low concentrations of mecoprop, where extensive enantiomerization was observed (2, 14). Strain MH was also able to degrade the (achiral) 2,4-D to completion, with maximum growth rates that were higher than those with the enantiomers of dichlorprop and mecoprop (Table 1).
Induction and specificity of uptake.
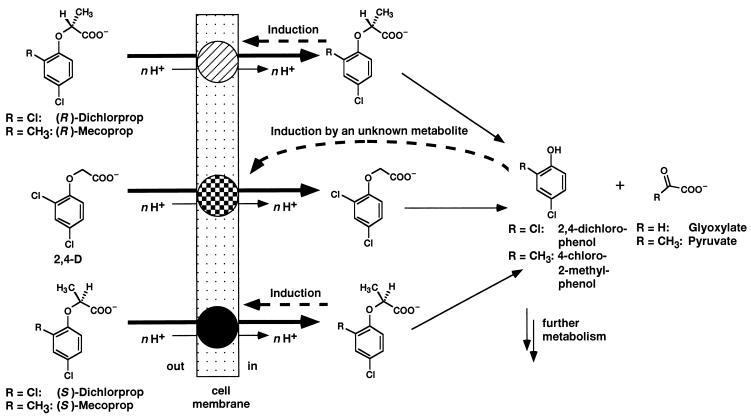
Our data clearly indicate that the uptake of (R)-dichlorprop, (S)-dichlorprop, and 2,4-D was inducible. The data presented in Fig. 2 clearly demonstrate that the uptake of dichlorprop was enantioselective and that there must be a separate uptake system for each enantiomer. These results substantiate the view that uptake of both enantiomers was carrier mediated, as (chiral) transport proteins can be expected to bind enantiomers in a selective manner (6). d- and l-xylose were transported at different rates by the yeast Rhodotorula gracilis, a fact that was used to illustrate the substrate specificity of carrier-mediated transport systems (6, 7). Because cells grown on 2,4-D could not take up (R)-dichlorprop and (S)-dichlorprop and because uptake of (R)-dichlorprop and (S)-dichlorprop by (R)- and (S)-dichlorprop-grown cells, respectively, was only slightly reduced by 2,4-D (Table 2), we suggest that there is a specific transport system for 2,4-D. The first common metabolite in the degradation of dichlorprop and 2,4-D by strain MH is 2,4-dichlorophenol (17), which is further metabolized via the modified ortho pathway (8). Regulation of transport systems by downstream metabolites of the ortho pathway was reported by Nichols and Harwood (16). β-Ketoadipate, a common metabolite of benzoate and 4-hydroxybenzoate degradation by P. putida, induces the 4-hydroxybenzoate transporter, whereas benzoate represses it. These findings made clear why P. putida cells preferentially degrade benzoate over 4-hydroxybenzoate in a mixture of both compounds (16). Filer and Harker demonstrated that dichloromuconate is the inducing agent of the 2,4-D pathway in R. eutropha JMP134 (3). The model for induction of the uptake of phenoxyalkanoic acids is presented in Fig. 4. The suggestion that 2,4-D uptake of strain MH was induced by a common metabolite of 2,4-D and dichlorprop degradation agrees well with our data but needs more elaborate experiments for definitive proof. On the other hand, the inducing agents of the (R)-dichlorprop and (S)-dichlorprop carriers must be (R)-dichlorprop and (S)-dichlorprop, respectively. This is a necessary consequence of the induction pattern of the (R)-dichlorprop and (S)-dichlorprop carriers (Fig. 2A and B), because, except for the first step, (R)- and (S)-dichlorprop are degraded identically (17). From the data presented in Table 2, we conclude that (R)-mecoprop was transported by the (R)-dichlorprop carrier and that (S)-mecoprop was transported by the (S)-dichlorprop carrier. This shows that the transport systems were specific with respect to the substitution and the configuration at the stereogenic center (C-2 atom of the alkanoic side chain) and rather unspecific with respect to the aromatic moiety of the phenoxyalkanoic acids.
FIG. 4.
Model proposed for the uptake of (R)- and (S)-dichlorprop, (R)- and (S)-mecoprop, and 2,4-D by S. herbicidovorans MH. Evidence is provided that strain MH has three inducible uptake systems: one for (R)-dichlorprop and (R)-mecoprop, one for (S)-dichlorprop and (S)-mecoprop, and a third for 2,4-D. Studies with inhibitors of energy metabolism indicate that the proton gradient is the driving force and that the uptake of these phenoxyalkanoic acids proceeds in symport together with protons. The number of protons (n) transported together with the phenoxyalkanoic acids was equal or larger than 1.
Kinetics of uptake.
The rates of uptake of 2,4-D-grown cells in response to increasing 2,4-D concentrations were in accordance with saturation kinetics (Fig. 3C). Saturation kinetics was also observed for the transport of (R)-dichlorprop and (S)-dichlorprop into (R)-dichlorprop- and (S)-dichlorprop-grown cells, respectively, in response to increasing substrate concentrations (Fig. 3A and B). Saturation kinetics is often interpreted as an indicator for carrier-mediated transport for which the uptake rate increases with increasing substrate concentration up to a maximal velocity, at which the carrier is saturated (6). The apparent affinity constants and the maximal velocities for transport of (R)-dichlorprop, (S)-dichlorprop, and 2,4-D, are similar to those reported for uptake of 4-toluene sulfonate by C. testosteroni T-2 (11) and 1 to 2 orders of magnitude higher than those reported for the transport of benzoic acid into Alcaligenes denitrificans (13) and 4-hydroxyphenylacetic acid into Klebsiella pneumoniae (1). Saturation kinetics was also reported for adsorption of 2,4-D to the cell wall of Pseudomonas fluorescens, a strain that does not metabolize 2,4-D (21). But as adsorption is neither inducible nor sensitive to metabolic inhibitors, we excluded it as an important contributor to the observed saturation kinetics. Biological membranes are good diffusion barriers for charged molecules, but they are not completely impermeable. It is generally assumed that the diffusion of deprotonated (negatively charged) organic acids is low compared with diffusion of the protonated (neutral) acid (6). The uptake assays with strain MH were performed at pH 6.5, at which more than 99.9% of the 2,4-D molecules are deprotonated (pKa of 2,4-D is 2.6 [22]). Therefore, we assume that diffusion of the protonated species of 2,4-D was negligible. To assess the impact of unspecific transport of phenoxyalkanoic acids, we examined the 2,4-D uptake by succinate-grown cells. A linear relationship between 2,4-D concentration and uptake rate was found for these cells (Fig. 3C), indicating that 2,4-D uptake of succinate-grown cells might be due to diffusion of 2,4-D into the cells. But it cannot be excluded that succinate-grown cells are partially induced for uptake of 2,4-D, (R)-dichlorprop, and (S)-dichlorprop. In induction experiments (Fig. 2), it was evident that (R)- and (S)-dichlorprop uptake was higher with succinate-grown cells (Fig. 2D) than with 2,4-D-grown cells (Fig. 2C). Therefore, we report apparent uptake rates and did not correct the values for these influences. Furthermore, these influences were smaller than the standard deviation of the measurements in the concentration range that was used for most experiments (0.7 to 300 μM). We assume that diffusion of dichlorprop was also of minor importance, as dichlorprop and 2,4-D have similar pKa values (pKa of dichlorprop is 3.0 [22]).
Carrier-mediated transport.
The apparent accumulation factors were obtained in the presence of catabolic activity (Table 3). Therefore, they cannot be used as an argument for active transport. However, as laid out below, they are proof for the existence of carrier-mediated transport for 2,4-D, (R)-dichlorprop, and (S)-dichlorprop. Cells grown on complex medium and on succinate constitutively express dioxygenase activities for the degradation of 2,4-D and (S)-dichlorprop (17). But succinate-grown cells accumulated significantly smaller amounts of 2,4-D or (S)-dichlorprop than (S)-dichlorprop-grown cells. Furthermore, 2,4-D-grown cells, which contain dioxygenase activity toward (S)-dichlorprop, did not accumulate radioactivity from this latter chemical. Growth on 2,4-D and (S)-dichlorprop triggers much higher accumulation of 2,4-D and (S)-dichlorprop, respectively, than growth on other substrates (Table 3). These results clearly demonstrate that metabolic substrate depletion together with passive diffusion does not account for the accumulation of radioactivity in strain MH.
Facilitated diffusion or active transport?
Two types of carrier-mediated transport systems can be distinguished: facilitated diffusion and active transport (6). The accumulation of compounds against the concentration gradient and the dependence on metabolic energy (i.e., ATP or proton motive force) are characteristic for active transport systems. To study if transport of 2,4-D, (R)-dichlorprop, and (S)-dichlorprop depended on metabolic energy, we tested the effect of different inhibitors of energy metabolism on the uptake rate (Table 4). Evidence in favor of an ATP-driven transport mechanism could not be obtained. In fact, the inhibitor studies and the lack of correlation between transport activity and ATP pools argue against such a mechanism. On the other hand, uptake of (R)-dichlorprop, (S)-dichlorprop, and 2,4-D was strongly inhibited by all uncouplers that were tested and by a combination of valinomycin and nigericin (Table 4). These results suggest that the proton motive force Δp drives the uptake of 2,4-D, (R)-dichlorprop, and (S)-dichlorprop. Inhibition experiments with valinomycin and nigericin gave evidence that the ΔpH rather than the Δψ was necessary for transport, as nigericin completely inhibited uptake of 2,4-D, (R)-dichlorprop, and (S)-dichlorprop. In contrast, valinomycin only had a small effect. These results support the conclusion that the uptake of all three compounds was driven by the proton gradient rather than by ATP or by the electrical potential. Therefore, we propose that the uptake of phenoxyalkanoic acids by S. herbicidovorans MH proceeded in symport with one or more protons (Fig. 4). More detailed investigations are needed for a full understanding of these uptake systems.
By combining the results of previous studies (17, 24) with those in this paper, we demonstrate that the selective degradation of the enantiomers of mecoprop (24) and dichlorprop by the soil isolate S. herbicidovorans MH was due not only to enantioselective metabolism (17) but also to enantioselective uptake. To the best of our knowledge, this is the first report of enantioselective uptake of environmentally relevant chiral xenobiotics by bacteria.
ACKNOWLEDGMENTS
We are grateful to Kathrin Nickel, Hauke Harms, and Sol Resnick for critical reading of the manuscript.
REFERENCES
- 1.Allende J L, Gibello A, Martin M, Garrido-Pertierra A. Transport of 4-hydroxyphenylacetic acid in Klebsiella pneumoniae. Arch Biochem Biophys. 1992;292:583–588. doi: 10.1016/0003-9861(92)90034-t. [DOI] [PubMed] [Google Scholar]
- 2.Buser H-R, Müller M D. Conversion reactions of various phenoxyalkanoic acid herbicides in soil. 2. Elucidation of the enantiomerization process of chiral phenoxy acids from incubation in a D2O/soil system. Environ Sci Technol. 1997;31:1960–1967. [Google Scholar]
- 3.Filer K, Harker A R. Identification of the inducing agent of the 2,4-dichlorophenoxyacetic acid pathway encoded by plasmid pJP4. Appl Environ Microbiol. 1997;63:317–320. doi: 10.1128/aem.63.1.317-320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groenewegen P E J, Driessen A J M, Konings W N, de Bont J A M. Energy-dependent uptake of 4-chlorobenzoate in the coryneform bacterium NTB-1. J Bacteriol. 1990;172:419–423. doi: 10.1128/jb.172.1.419-423.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harwood C S, Nichols N N, Kim M-K, Ditty J L, Parales R E. Identification of the pcaRKF gene cluster from Pseudomonas putida: involvement in chemotaxis, biodegradation, and transport of 4-hydroxybenzoate. J Bacteriol. 1994;176:6479–6488. doi: 10.1128/jb.176.21.6479-6488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Höfer M, Hoggett J G. Transport across biological membranes. Boston, Mass: Pitman Publishing Ltd.; 1981. [Google Scholar]
- 7.Höfer M, Kotyk A. Tight coupling of monosaccharide transport and metabolism in Rhodotorula gracilis. Folia Microbiol. 1968;13:197–204. doi: 10.1007/BF02871034. [DOI] [PubMed] [Google Scholar]
- 8.Horvath M, Ditzelmüller G, Loidl M, Streichsbier F. Isolation and characterization of a 2-(2,4-dichlorophenoxy)propionic acid-degrading soil bacterium. Appl Microbiol Biotechnol. 1990;33:213–216. doi: 10.1007/BF00176527. [DOI] [PubMed] [Google Scholar]
- 9.Konings W N, Hellingwerf K J, Robillard G T. Transport across bacterial membranes. In: Bonting S L, de Pont J J H H M, editors. Membrane transport. Vol. 2. Amsterdam, The Netherlands: Elsevier/North-Holland Biomedical Press; 1981. pp. 257–283. [Google Scholar]
- 10.Leveau J H J, Zehnder A J B, van der Meer J R. The tfdK gene product facilitates the uptake of 2,4-dichlorophenoxyacetate by Ralstonia eutropha JMP134(pJP4) J Bacteriol. 1998;180:2237–2243. doi: 10.1128/jb.180.8.2237-2243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locher H H, Poolman B, Cook A M, Konings W N. Uptake of 4-toluene sulfonate by Comamonas testosteroni T-2. J Bacteriol. 1993;175:1075–1080. doi: 10.1128/jb.175.4.1075-1080.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.Miguez C B, Greer C W, Ingram J M, MacLeod R A. Uptake of benzoic acid and chloro-substituted benzoic acids by Alcaligenes denitrificans BRI 3010 and BRI 6011. Appl Environ Microbiol. 1995;61:4152–4159. doi: 10.1128/aem.61.12.4152-4159.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller M D, Buser H-R. Conversion reactions of various phenoxyalkanoic acid herbicides in soil. 1. Enantiomerization and enantioselective degradation of the chiral 2-phenoxypropionic acid herbicides. Environ Sci Technol. 1997;31:1953–1959. [Google Scholar]
- 15.Nichols N N, Harwood C S. PcaK, a high-affinity permease for the aromatic compounds 4-hydroxybenzoate and protocatechuate from Pseudomonas putida. J Bacteriol. 1997;179:5056–5061. doi: 10.1128/jb.179.16.5056-5061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichols N N, Harwood C S. Repression of 4-hydroxybenzoate transport and degradation by benzoate: a new layer of regulatory control in the Pseudomonas putida β-ketoadipate pathway. J Bacteriol. 1995;177:7033–7040. doi: 10.1128/jb.177.24.7033-7040.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nickel K, Suter M J-F, Kohler H-P E. Involvement of two α-ketoglutarate-dependent dioxygenases in enantioselective degradation of (R)- and (S)-mecoprop by Sphingomonas herbicidovorans MH. J Bacteriol. 1997;179:6674–6679. doi: 10.1128/jb.179.21.6674-6679.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poolman B, Smid E J, Konings W N. Kinetic properties of a phosphate-bond-driven glutamate-glutamine transport system in Streptococcus lactis and Streptococcus cremoris. J Bacteriol. 1987;169:2755–2761. doi: 10.1128/jb.169.6.2755-2761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tett V A, Willets A J, Lappin-Scott H M. Enantioselective degradation of the herbicide mecoprop [2-(2-methyl-4-chlorophenoxy)propionic acid] by mixed and pure bacterial cultures. FEMS Microbiol Ecol. 1994;14:191–200. [Google Scholar]
- 20.Tett V A, Willetts A J, Lappin-Scott H M. Biodegradation of the chlorophenoxy herbicide (R)-(+)-mecoprop by Alcaligenes denitrificans. Biodegradation. 1997;8:43–52. [Google Scholar]
- 21.Wedemeyer G. Uptake of 2,4-dichlorophenoxyacetic acid by Pseudomonas fluorescens. Appl Microbiol. 1966;14:486–491. doi: 10.1128/am.14.4.486-491.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worthing C R, Hance R J. The pesticide manual—a world compendium. 9th ed. Farnham, Great Britain: The British Crop Protection Council; 1991. [Google Scholar]
- 23.Zipper C. Ph.D. dissertation. Zürich, Switzerland: Swiss Federal Institute of Technology; 1998. [Google Scholar]
- 24.Zipper C, Nickel K, Angst W, Kohler H-P E. Complete microbial degradation of both enantiomers of the chiral herbicide mecoprop [(RS)-2-(4-chloro-2-methylphenoxy)propionic acid] in an enantioselective manner by Sphingomonas herbicidovorans sp. nov. Appl Environ Microbiol. 1996;62:4318–4322. doi: 10.1128/aem.62.12.4318-4322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]