Abstract
No standardised and universal treatment is available for antisynthetase syndrome. In particular, there is an unmet need for a single efficient treatment acting on its various manifestations, including interstitial lung disease, myositis and polyarthritis.
We describe the cases of two patients with multiple and severe manifestations, including joint, muscular and lung involvement, both refractory to various treatments, including rituximab, who demonstrated significant improvement of all their manifestations, including joint, muscular and lung diseases on tocilizumab. The response was also long-lasting, with both patients still being in full remission after >10 years of treatment.
Our experience shows that interleukin-6 inhibition could be a very effective treatment option in antisynthetase syndrome, with efficacy on a wide spectrum of manifestations.
Keywords: dermatomyositis, biological therapy, pulmonary fibrosis
WHAT IS ALREADY KNOWN ON THIS TOPIC.
There is no standardised and universal treatment available for antisynthetase syndrome, and no single efficient treatment acting on its various manifestations, including interstitial lung disease, myositis and polyarthritis.
Mycophenolate mofetil, calcineurin inhibitors, cyclophosphamide and rituximab have been advocated for cases with severe interstitial lung disease, while Janus kinase inhibitors and even CD19 chimeric antigen receptor-T cells have been used in refractory cases.
WHAT THIS STUDY ADDS
We report that interleukin-6 inhibition could be a very effective, long-term treatment option in refractory antisynthetase syndrome, with efficacy on a wide spectrum of manifestations including interstitial lung disease.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Tocilizumab could be an efficacious alternative treatment option for various manifestations of refractory antisynthetase syndrome.
Introduction
Inflammatory myopathies (IM) represent a heterogeneous group of disorders for which classification criteria can now be revised, using the presence of well-defined and specific autoantibodies.1
Antisynthetase syndrome (ASS) is characterised by the presence of autoantibodies against aminoacyl-transfer RNA (tRNA) synthetases. Anti-Jo-1, targeting the histidyl-tRNA synthetase, is the most common aminoacyl-tRNA synthetase autoantibody, representing about 20% of patients with IM, while the alanyl (PL-12), threonyl (PL-7), glycyl (EJ) or isoleucyl (OJ) tRNA synthetases-targeting antibodies are much rarer (1%–5% of IM), even though they are nowadays readily detected by commercially available immunoblot essays. Interestingly, tRNA synthetases specificity appears to be associated with different organ involvement patterns and clinical features with anti-PL7 and anti-PL-12 diseases characterised by more severe lung involvement, while anti-Jo-1 antibodies are typically associated with more severe muscle involvement.2
Despite the fact that the inflammatory infiltrate present in the muscle can vary greatly, all IM are nowadays still treated using a similar approach, mainly based on the use of highly dosed corticosteroids, conventional disease-modifying antirheumatic drugs (DMARDs) (mostly azathioprine and methotrexate) and eventually intravenous immunoglobulins. Mycophenolate mofetil (MMF), calcineurin inhibitors and cyclophosphamide are usually reserved for cases with severe interstitial lung disease,3 while rituximab has gained attention for the treatment of IM with a number of large case series reports3 and despite mixed results in ASS.4 5 It is nevertheless regarded as the first-line therapy in this indication by some authors.6 Finally, even though joint manifestations are common, they are often regarded as minor with no specific therapeutic recommendation.3 Hydroxychloroquine, methotrexate, rituximab and antitumour necrosis factor (anti-TNF) have been reported to show some benefits on both joint and muscle involvement, whereas anti-TNF are beneficial on joint manifestations but have failed to show any efficacy on muscle.3
Interleukin-6 (IL-6) is one of the various cytokines which seems to play a crucial role in myositis and therefore a potential target in IM. IL-6 has been found to be elevated in sera of patients with myositis, and it exerts a variety of biological activities,7 from B and T cells differentiation to growth factor for myeloma cells.8–10 IL-6 is also a potent hepatocyte inducer driving the acute phase reaction11 and exerts effects on various other cell types from osteoclasts to keratinocytes. Tocilizumab is a well-characterised humanised anti-IL-6R monoclonal antibody registered for the treatment of rheumatoid arthritis and now for giant cell arteritis. Tocilizumab has also been described to be effective in a murine model of polymyositis12 and there are a few reports of effective use in polymyositis13 and overlap syndromes.14 It has also demonstrated benefit in lung involvement, with stabilisation of forced vital capacity in patients with early Scleroderma-associated interstitial lung disease (SSc-ILD) and elevated acute-phase reactants.15 Finally, two case reports from France and the UK demonstrated some value on joint and muscle involvement in patients with anti-Jo-1 myositis.16 17
We describe our long-term experience with tocilizumab in two patients with ASS refractory to all current therapies.
Case reports
Case 1
The first patient is a Hispanic woman aged 60 years from Peru who we first met in 2011. She had been suffering since 2000 from a typical Jo-1 ASS with severe myositis, pulmonary fibrosis, polyarthritis and skin involvement (mechanical hands). Previous treatments with prednisone, hydroxychloroquine, methotrexate, sulfasalazine, ciclosporin, azathioprine and intravenous Ig were unsuccessful, and she was on rituximab, 2×1 g every 6 months since 2008 and prednisone 10–20 mg/day. Despite this treatment, she complained of severe weakness, being unable to stand-up without help or climbing any stairs without a handrail, arthralgia with numerous swollen and tender joints and a 6-hour morning stiffness, as well as profound shortness of breath. Examination revealed major joint involvement with synovitis of the elbows, wrists and hands (metacarpophalangeal and proximal interphalangeal joints (MCPs and PIPs)), mechanical hands with classical skin changes and obvious pulmonary crepitations in the lower half of both lungs, with a cough induced by the slightest inspiratory effort. Proximal muscle strength was clearly abnormal, tested between M3 and M4 in the upper and lower limbs (Medical Research Council Scale for muscle strength: M5—normal, M4—movement against gravity and resistance, M3—movement against gravity over (almost) the full range, M2—movement of the limb but not against gravity, M1—visible contraction without movement of the limb and M0—no visible contraction). Although inflammation appeared to be controlled, with a sedimentation rate of 13 mm/hour and a C reactive protein (CRP) of 8 mg/L (n<5 mg/L), creatine kinase (CK) levels were highly increased, close to 20 000 IU/L (normal range <170 IU/L). The patient was rheumatoid factor negative and antinuclear antibody (ANA) positive at 1/640 with anti-Jo-1 antibodies strongly positive at 150 U (n<20 U, Immunoblot EUROIMMUN) and weak positivity for anti-Ro52 antibodies at 38 U (n<20 U).
Pulmonary function tests confirmed a restrictive ventilatory defect with decreased total lung capacity and diffusing capacity of the lungs for carbon monoxide (DLCO), respectively at 52% and 38% predicted. Chest CT-scan demonstrated honeycomb fibrosis with retraction bronchiectasis, without ground glass lesions. Active myositis was confirmed by MRI and biopsy, the latter showing endomysial fibrosis, lymphocytic infiltrate, necrotising fibres and some macrophages and both CD4 and CD8 endomysial T-lymphocytes in equal proportions.
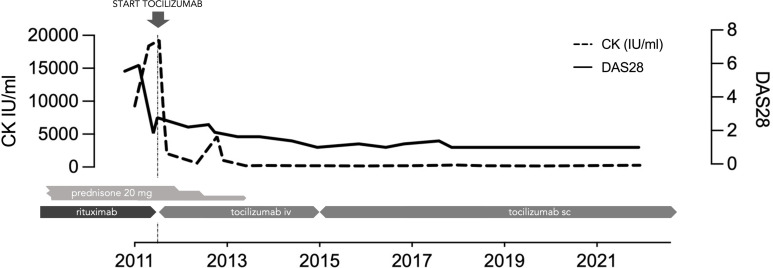
She was started on tocilizumab 8 mg/kg per month with a dramatic and rapid improvement of all symptoms with decreased pain and shortness of breath, major improvement of muscle involvement with normalisation of the strength (M5) and complete remission of joint involvement (figure 1). Laboratory parameters, pulmonary functions and CT confirmed the subjective improvement with a long-lasting response (table 1). Steroids were tapered quickly over the first year of tocilizumab therapy and since then the patient has not had any relapse on tocilizumab in association with a small dose of 10 mg of leflunomide.
Figure 1.
Dramatic improvement in muscle disease after initiation of tocilizumab as demonstrated by normalisation of CK in a refractory disease.
Table 1.
Improvement and stabilisation of lung functions after tocilizumab therapy (in per cent of predicted value)
| Pulmonary function tests | |||||||
| February 2012 | May 2013 | December 2014 | April 2016 | July 2018 | October 2022 | ||
| Patient 1 | |||||||
| TLC | 52 | Start tocilizumab | 73 | 81 | 79 | 96 | 94 |
| FEV1 | 60 | 69 | 74 | 79 | 85 | 80 | |
| DLCO | 38 | 65 | 61 | 75 | Technical problem | 74 | |
| October 2011 | April 2012 | October 2012 | May 2013 | December 2015 | August 2019 | November 2021 | ||
| Patient 2 | ||||||||
| TLC | 121 | 87 | 86 | Start tocilizumab | 104 | 99 | 108 | 96 |
| FEV1 | 99 | 48 | 55 | 56 | 82 | 89 | 94 | |
| DLCO | 97 | 50 | 51 | 87 | 78 | 77 | 74 |
DLCO, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in 1 s; TLC, total lung capacity.
Case 2
The second patient is a young woman aged 21 years initially seen in our department in 2011 for a 2-month history of diffuse arthralgia and myalgia and a 1-week history of severe asymmetrical Raynaud’s phenomenon affecting both hands. Physical examination showed splinter haemorrhages of the second and third digit without ulceration or sclerodactyly, discrete synovitis of the MCP and PIP joints of both hands, but no muscle weakness or lung abnormalities were detected despite multiple clinical evaluation. Blood tests were abnormal with an erythrocyte sedimentation rate (ESR) of 60 mm/hour, a CRP of 98 mg/L (n<5 mg/L), a leucocytosis (14.3 G/L, N 4.0–10.0 G/L) with lymphopenia of 1.144 G/L and a thrombocytosis of 325 G/L (N 150–300 G/L). Other parameters, including CK level (29 IU/L normal range <170 IU/L), were normal. Rheumatoid factor was slightly increased at 14 IU/mL (n<10 IU/mL). ANA were positive at 1/160 with a speckled pattern and anti-SSA antibodies (31 IU (n<20)) were elevated. Dot blot demonstrated positive anti-PL-12 antibodies (50 IU (n<7)), while heart ultrasound, pulmonary function tests and thoraco-abdominal CT were normal. Capillaroscopy demonstrated pathological megacapillaries without any other abnormalities.
For her musculoskeletal involvement, the patient received prednisone (initially 20 mg/day) with azathioprine. This latter had to be promptly interrupted due to intolerance, as for subsequent unsuccessful trials of methotrexate and leflunomide. She also quickly developed frank digital ulcerations and was started first on anticalcic medication, followed by tadalafil, iloprost, bosentan and fluoxetine, which were all very poorly tolerated and inefficient.
Over the first year, she developed progressive shortness of breath and frank polyarthritis. Pulmonary function tests demonstrated some degree of air trapping and a decreased DLCO at 51% predicted, against of 97% 9 months previously, while High Resolution Computed Tomography scan (HRCT) now demonstrated the presence of peripheral subpleural reticular infiltrates indicating incipient fibrosing lesions. MRI of the proximal muscles did not show any sign of inflammation and CK level remained within normal, but further investigations demonstrated some increase in aldolase at 28 IU/L (n<7.6).
At that time, she was started on rituximab (still on 7.5 mg of prednisone). After two cycles of 2 g, she still complained of further myalgia and arthralgia. DLCO remained stable, but sedimentation rate and CRP were increased. She was finally started on tocilizumab monotherapy at the beginning of 2013 at a dose of 8 mg/kg per month.
She demonstrated full resolution of all musculoskeletal symptoms, normalisation of biological parameters and complete and rapid tapering of corticosteroids. Pulmonary function tests also demonstrated progressive improvement with a control showing normal parameters, especially a DLCO of 87% after the initiation of tocilizumab (table 1). Three years later, the patient remains asymptomatic on tocilizumab every 12 weeks, with only a persisting very mild Raynaud’s phenomenon, without any skin ulceration since then. After 6 years of treatment, tocilizumab was stopped and she remained in full clinical remission with normal blood tests and pulmonary lung function tests for a little more than 1 year before presenting with a flare which quickly responded to the reinitiation of tocilizumab. She remained on tocilizumab until a recent pregnancy.
Discussion
ASS should probably be regarded as a distinct and separate nosological entity within the spectrum of inflammatory myositis.1 ASS is related to the presence of different autoantibodies against aminoacyl-tRNA synthetases, autoantibodies associated with phenotypically distinct subgroups within the ASS spectrum associating at various levels some joint, muscle, skin and lung involvement.
There is nowadays no universal treatment for the diverse manifestations of the disease, and often therapeutic agents regarded as effective for one manifestation will be inefficient or inadequate for the others. In severe disease, conventional DMARDs are often insufficient and the use of anti-TNF, MMF, ciclosporin or cyclophosphamide has not allowed us to solve this conundrum. Rituximab has gained interest in this setting and is even regarded as first-line therapy by some,6 but his role in interstitial lung disease remains disputed.3 4 Recently, use of Janus kinase inhibitors and even CD19-targeting chimeric antigen receptor (CAR)-T cells have been published for refractory cases.18 19 Finally, most of those treatments have limited efficacy on the joint manifestations, a common manifestation which is often regarded as secondary despite its high impact on the quality of life of our patients.
We describe our long-term experience with a dramatic response to tocilizumab in two patients with ASS previously refractory to commonly used treatments, even rituximab. Both patients had active lung disease with complete or quasi-complete remission and full preservation of lung functions overtime. In both cases, responses were complete with very rapid and significant improvement in all involvements, including normalisation of strength in the case with severe myositis and resolution of all joint complains in both patients despite previous refractory polyarthritis to multiple therapies. Except for the persistence of asymptomatic residual lung damage on CT-chest in one case and persistent mild Raynaud’s phenomena in the winter in the other case, patients are in full remission on tocilizumab monotherapy.
Confirmation of the effectiveness of tocilizumab in ASS and its different subgroups is needed, but our experience shows that IL-6 inhibition could be an efficacious treatment option on a wide spectrum of manifestations, an alternative certainly cheaper than CD19-targeting CAR-T cells as recently published for a refractory case.19
Acknowledgments
We are very grateful to Anne-Cécile Debrach for help and assistance in editing and proofreading our manuscript.
Footnotes
Contributors: Both authors have contributed to the submitted article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study did not require consent with both patients treated following the Swiss compassionate use guidelines.
References
- 1.Meyer A, Lannes B, Goetz J, et al. Inflammatory myopathies: a new landscape. Jt Bone Spine 2018;85:23–33. 10.1016/j.jbspin.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 2.Pinal-Fernandez I, Casal-Dominguez M, Huapaya JA, et al. A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatology (Oxford) 2017;56:999–1007. 10.1093/rheumatology/kex021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavagna L, Monti S, Caporali R, et al. How I treat idiopathic patients with inflammatory myopathies in the clinical practice. Autoimmun Rev 2017;16:999–1007. 10.1016/j.autrev.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 4.Andersson H, Sem M, Lund MB, et al. Long-term experience with rituximab in anti-synthetase syndrome-related interstitial lung disease. Rheumatology (Oxford) 2015;54:1420–8. 10.1093/rheumatology/kev004 [DOI] [PubMed] [Google Scholar]
- 5.Leclair V, Galindo-Feria AS, Dastmalchi M, et al. Efficacy and safety of rituximab in anti-synthetase antibody positive and negative subjects with idiopathic inflammatory myopathy: a registry-based study. Rheumatology (Oxford) 2019;58:1214–20. 10.1093/rheumatology/key450 [DOI] [PubMed] [Google Scholar]
- 6.Zekić T. Rituximab as the first-line therapy in anti-synthetase syndrome-related interstitial lung disease. Rheumatol Int 2023;43:1015–21. 10.1007/s00296-023-05302-9 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka T, Narazaki M, Ogata A, et al. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin Immunol 2014;26:88–96. 10.1016/j.smim.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 8.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature 2006;441:235–8. 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- 9.Korn T, Bettelli E, Oukka M, et al. IL-17 and Th17 cells. Annu Rev Immunol 2009;27:485–517. 10.1146/annurev.immunol.021908.132710 [DOI] [PubMed] [Google Scholar]
- 10.Suematsu S, Matsuda T, Aozasa K, et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A 1989;86:7547–51. 10.1073/pnas.86.19.7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990;265:621–36. 10.1042/bj2650621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okiyama N, Sugihara T, Iwakura Y, et al. Therapeutic effects of interleukin-6 blockade in a murine model of polymyositis that does not require interleukin-17A. Arthritis Rheum 2009;60:2505–12. 10.1002/art.24689 [DOI] [PubMed] [Google Scholar]
- 13.Narazaki M, Hagihara K, Shima Y, et al. Therapeutic effect of tocilizumab on two patients with polymyositis. Rheumatology (Oxford) 2011;50:1344–6. 10.1093/rheumatology/ker152 [DOI] [PubMed] [Google Scholar]
- 14.Kondo M, Murakawa Y, Matsumura T, et al. A case of overlap syndrome successfully treated with tocilizumab: a hopeful treatment strategy for refractory dermatomyositis? Rheumatology (Oxford) 2014;53:1907–8. 10.1093/rheumatology/keu234 [DOI] [PubMed] [Google Scholar]
- 15.Khanna D, Lin CJF, Furst DE, et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2020;8:963–74. 10.1016/S2213-2600(20)30318-0 [DOI] [PubMed] [Google Scholar]
- 16.Beaumel A, Muis-Pistor O, Tebib J-G, et al. Antisynthetase syndrome treated with tocilizumab. Jt Bone Spine 2016;83:361–2. 10.1016/j.jbspin.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 17.Murphy SM, Lilleker JB, Helliwell P, et al. The successful use of tocilizumab as third-line biologic therapy in a case of refractory anti-synthetase syndrome. Rheumatology (Oxford) 2016;55:2277–8. 10.1093/rheumatology/kew296 [DOI] [PubMed] [Google Scholar]
- 18.Sugino K, Ono H, Saito M, et al. Successful baricitinib treatment of refractory anti-synthetase syndrome associated with interstitial lung disease. Respirol Case Rep 2023;11:e01129. 10.1002/rcr2.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pecher A-C, Hensen L, Klein R, et al. CD19-targeting CAR T cells for myositis and interstitial lung disease associated with antisynthetase syndrome. JAMA 2023;329:2154–62. 10.1001/jama.2023.8753 [DOI] [PMC free article] [PubMed] [Google Scholar]