Abstract
Introduction
Postoperative delirium is a prominent and clinically important complication in older adults after coronary artery bypass grafting (CABG) surgery, resulting in prolonged hospital stay, long-term cognitive impairment and increased morbidity and mortality. Many studies have shown that cerebral desaturation is associated with increased risk of postoperative delirium during on-pump cardiac surgery. However, few studies have focused on the effect of optimising regional cerebral oxygen saturation (rSO2) on postoperative delirium during off-pump CABG. The purpose of this study is to investigate whether intraoperative anaesthetic management based on percutaneous cerebral oximetry monitoring decreases the incidence of postoperative delirium in older adults undergoing off-pump CABG.
Methods
This single-centre randomised controlled trial will randomly assign 200 patients to the intervention group or the control group at a ratio of 1:1. The patients in the intervention group will be observed by percutaneous cerebral oximetry monitoring that the desaturation (a drop of more than 20% from baseline value or rSO2 less than 55% for >60 consecutive seconds at either probe) during the procedure triggered the intervention strategies, while the cerebral oximetry data of the control group will be hidden from the clinical team and patients will be anaesthetised by the usual anaesthetic management. The primary outcome will be the incidence of postoperative delirium during the first 7 days after off-pump CABG. Delirium will be comprehensively evaluated by the combination of the Richmond Agitation Sedation Scale and the Confusion Assessment Method for the intensive care unit. The secondary outcomes will include the incidence of postoperative acute kidney injury and myocardial infarction during the hospital stay, as well as the intensive care unit and hospital length of stay.
Ethics and dissemination
This study was approved by the Ethics Committee of the Chinese Academy of Medical Sciences, Fuwai Hospital (No 2022–1824). Written informed consent will be obtained from each patient or their legal representatives before enrolment. The results of this trial will be published in an international peer-reviewed scientific journal.
Trial registration number
ChiCTR2300068537.
Keywords: anaesthesia in cardiology, aged, health services for the aged, delirium & cognitive disorders
Strengths and limitations of this study.
The prospective, randomised and controlled trial design will be used in this study to assess the incidence of postoperative delirium in older adults undergoing off-pump coronary artery bypass grafting (CABG).
We will apply standardised methods in combination with the Richmond Agitation Sedation Scale and the Confusion Assessment Method for the intensive care unit to assess postoperative delirium in older adults.
The anaesthetic management guided by cerebral oximetry monitoring will be performed during off-pump CABG surgery due to intraoperative haemodynamic fluctuations, and no further attention will be given to the postoperative cerebral oximetry desaturation and the impact of long-term cognitive impairment.
This trial will exclude some high-risk patients, such as those with severe carotid artery stenosis or left ventricular ejection fraction <30%, due to the consideration of heterogeneity.
This is a single-centre study; therefore, the generalisability may not be extrapolated.
Introduction
Postoperative delirium (POD) following coronary artery bypass grafting (CABG) is a prominent and clinically important complication with a rate of 20–50% of elderly surgical patients.1–5 Elderly patients are likely to suffer from age-related diseases, such as cardiovascular diseases, hypertension, diabetes or organ dysfunction, and are more prone to postoperative neurological disorders.5–7 POD is associated with prolonged hospital stay, long-term cognitive impairment, as well as increased morbidity and mortality.2 8 9 The prevention of POD has been considered a public health priority by the UK’s National Institute for Health and Care Excellence, the American Geriatric Society and the American College of Surgeons.10–12
Regional cerebral oxygen saturation (rSO2), measured at the microvascular level by near-infrared spectroscopy (NIRS), can be used as a practical parameter to evaluate the cerebral oxygen supply/demand imbalance with a high risk of cerebrovascular complications.13–15 Based on its unique features including the absolute values and variation of rSO2, the NIRS has obtained increased usage in goal-directed optimisation of treatment to improve the prognosis of neurological function.16 17 In a prospective study concerning cardiac surgery, patients with intraoperative rSO2 values maintained above 60% had better the results of postoperative memory.18 Another randomised trial published by Colak et al has reported that maintaining rSO2 of >50% (absolute value) or >80% (relative to baseline value) could reduce cognitive impairment after CABG.19 A recent meta-analysis and review has shown that intraoperative anaesthetic practice guided by percutaneous cerebral oximetry monitoring was associated with reduced POD incidence and improved postoperative outcomes in cardiac surgical patients.20
Severe inflammatory response, hypoperfusion and microembolisation associated with cardiopulmonary bypass are considered to be the pathogenesis of cerebral oxygen imbalance and lead to subsequent delirium in patients undergoing CABG surgery.21–23 Accumulating evidence has shown that maintenance of ‘adequate’ blood pressure, avoidance of perioperative hypoxia or off-pump cardiac surgery could be correlated with rSO2 and used to optimise cerebral oxygenation and improve cognitive outcome.24 25 With the development of medical technology and the increase in life expectancy, more and more elderly high-risk patients will have cardiac surgery and off-pump CABG is preferred by surgeons with fewer postoperative neurological complications and faster recovery.26 Older adults with off-pump CABG are prone to significant increases in haemodynamic fluctuations due to surgical procedures, especially the anastomosis of vascular graft, which can lead to persistent hypotension and hypoperfusion. The ventricle compressed by the fixator may lead to a reduced cardiac output, which can decrease the perfusion pressure of the brain. Intraoperative hypotension has historically been thought to be the cause of this ischaemic imbalance, resulting in reduced oxygen supply and associated with ischaemia-reperfusion injury to the heart, brain and kidney.24 27 In addition, the increase of intraoperative venous pressure in patients with ventricular dysfunction may affect the reflux of cerebral venous blood volume to the right atrium, resulting in an increase in the proportion of cerebral venous blood flow and a decrease in intraoperative rSO2.28 A retrospective study including 1439 patients found that intraoperative rSO2 reduction was associated with an increased risk of POD. For the prediction of POD, the cut-off value of intraoperative rSO2 was 55% for the older adults who underwent off-pump CABG.29 To our knowledge, there are no unified standards for the optimal anaesthetic practice based on optimising cerebral oxygenation during off-pump CABG among older adults.
The purpose of this study is to investigate whether intraoperative anaesthetic management based on percutaneous cerebral oximetry monitoring decreases the incidence of POD in older adults underwent off-pump CABG and provides a neuroprotective effect.
Methods
Study design
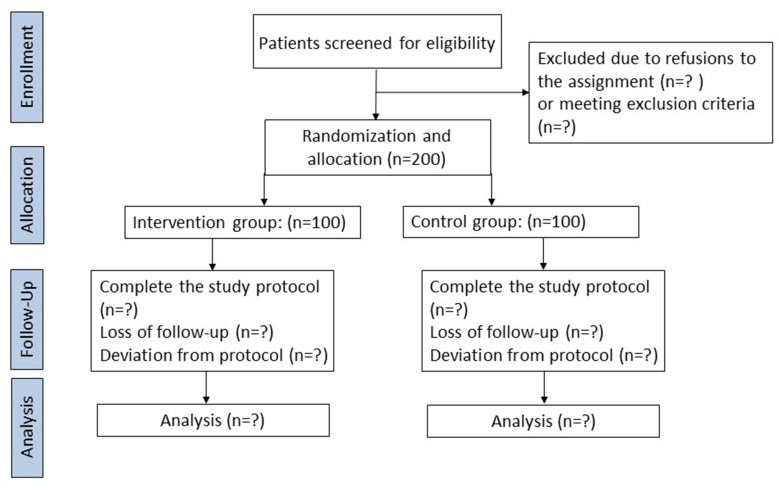
This study is a prospective, single-centre, randomised, controlled trial. It is designed to allocate patients in an intended 1:1 allocation ratio to explore the neuroprotective effect of intraoperative anaesthetic practice based on percutaneous cerebral oximetry monitoring on postoperative delirium in older adults undergoing off-pump CABG. Figure 1 shows the flow chart of the trial. This protocol provides all component items described in the Standard Protocol Items: according to the Recommendations for Interventional Trials (SPIRIT) checklist.30 Table 1 demonstrates the SPIRIT including the schedule of enrolment, interventions and assessments of the trial.
Figure 1.
Study flow chart.
Table 1.
Standard Protocol Items: Recommendations for Interventional Trials schedule for enrolment, interventions and assessments
| Study period | |||||||
| Time point | Enrolment | Allocation | Post-allocation | Close-out | |||
| The day before surgery | Day of surgery | T1 | T2 | T3 | T4 | 7 days after surgery | |
| Enrolment: | |||||||
| Eligibility screen | X | ||||||
| Informed consent | X | ||||||
| Allocation | X | ||||||
| Interventions: | |||||||
| Cerebral oximetry guided anaesthetic management |

|
||||||
| Usual anaesthetic care |

|
||||||
| Assessments: | |||||||
| Baseline variables | X | X | |||||
| Haemodynamic parameters | X | X | X | X | X | ||
| Follow-up variables | X | ||||||
T1, before anaesthetic induction; T2, after anaesthetic induction; T3, during surgery; T4, after surgery.
Study location
We will conduct this trial at Fuwai Hospital, Chinese Academy of Medical Sciences, which is a tertiary academic hospital in China.
Study population
Adult patients (age ≥65 years) undergoing off-pump CABG will be screened for inclusion in this study.
Patients who meet the following criteria will be excluded:
A previous history of cardiac surgery or emergency surgery.
Combined surgery such as simultaneous CABG mixed percutaneous coronary intervention.
History of schizophrenia, Parkinson’s disease, epilepsy, myasthenia gravis or severe dementia (severe cognitive dysfunction based on Mini-Mental State Examination score <24 points).
Lack of cooperation due to linguistic difficulties and other reasons.
History of brain trauma or neurosurgery.
Left ventricular ejection fraction <30%.
Severe liver dysfunction (Child-Pugh grade C), renal failure (requiring kidney replacement therapy) or severe carotid artery stenosis.
Patient recruitment and perioperative data collection
In this clinical trial, 200 eligible patients scheduled to undergo elective off-pump CABG will be recruited from 1 May 2023 to 31 December 2024. A member of the research team will visit the patients the day before the surgery and explain the study’s potential risks and advantages to the patients. Written informed consent will be obtained from each patient or their legal representatives before surgery (online supplemental appendix).
bmjopen-2023-076419supp001.pdf (247KB, pdf)
We will gather the demographic data (age, gender, body mass index, Mini-Mental State Examination score and educational level), comorbidity, laboratory tests (platelet, haemoglobin, aspartate aminotransferase, alanine aminotransferase, cardiac troponin I and creatinine levels), history of surgeries, surgery duration, cerebral oximetry data and intraoperative haemodynamic parameters (blood pressure, heart rate and cardiac output).
Baseline rSO2 and incidence of desaturation from cerebral oximetry will be collected. Bilateral NIRS sensors (INVOS 5100C, cerebral/somatic oximeter; Medtronic) will be placed above the eyebrow on both sides of the forehead to monitor the rSO2. For baseline oximetry, the average rSO2 value before anaesthetic induction for 2 min without any medication will be computed for each probe. The incidence of desaturation was defined as the proportion of patients who experienced at least one episode of desaturation (a drop of more than 20% from baseline value or rSO2 less than 55% for >60 consecutive seconds at either side).
Randomisation and blinding
An independent statistician will generate a computerised sequence of 200 allocation codes and then be sealed in envelopes to ensure concealment. Eligible participants will be randomised at a 1:1 ratio to the intervention group or the control group. The study participants, surgeons, intensive care unit (ICU) physicians and an independent follow-up staff team will be blinded to randomisation and group allocation. The anaesthesiologist will collect data and perform cerebral oximetry monitoring without being blinded to the group assignments. The blinding will be unmasked to surgeons and ICU physicians immediately if the participants suffered cardiac arrest or ventricular fibrillation during the procedure, resulting in conversion from off-pump to cardiopulmonary bypass. These situations will be documented in the case report forms (CRFs). The follow-up staff team will receive formal training in delirium assessment and be completely masked to the group allocation to conduct delirium assessment. The follow-up staff team will be divided into two groups who will perform the assessment separately. Delirium will be diagnosed when the two groups reach an agreement.
Interventions protocol
For all participants using bilateral NIRS, the baseline rSO2 value (≥55% for enrolled patients) will be computed for each probe while patients will inhale room air without any premedication before anaesthetic induction. In the intervention group, the cerebral oximetry data will be displayed openly to the anaesthesiologist. The criterion for intervention was defined as a drop of more than 20% from baseline value or rSO2 less than 55% for >60 consecutive seconds at either probe during the procedure. In the occurrence of intraoperative cerebral desaturation, clinical intervention strategies will be implemented, which is a strategy studied by Deschamps et al31 to improve cerebral rSO2. Verifying probe position, increasing blood pressure to within 20% of baseline, enhancing the fraction of inspired oxygen (FiO2), adjusting respiratory parameters to increase end-tidal CO2 levels within normal ranges (35–45 mm Hg), optimisation of the fluid volume therapy (haemoglobin levels <90 g/L will warrant consideration of a red blood cell transfusion), improving ventricular function in the presence of low cardiac output, or eliminating hypothermia (35–37°C) will be implemented as the in-sequence programme. In the control group, the cerebral oximetry data will be concealed from the perioperative team and recorded by the machine during surgery. The anaesthetic protocol will be standardised in our institute. The perioperative haemodynamic stability of the patients will be maintained by adjusting the depth of the anaesthesia and applying vasoactive drugs to get a target of mean arterial pressure in the range of 60–100 mm Hg.
General anaesthesia and postoperative analgesia protocol
Cerebral oximetry monitoring will be initiated in the operating room before anaesthetic induction and recorded continuously during surgery for all participants by bilateral NIRS. Baseline rSO2 value will be obtained for the first 2 min while patients will inhale room air without any premedication. General anaesthesia will be induced by midazolam, etomidate, cisatracurium and sufentanil intravenously and maintained with infusion of sufentanil, cisatracurium and propofol in all patients. The body temperature will be kept between 35°C and 37°C. For the bispectral index Vista monitor, a disposable BIS sensor (Covidien, Colorado, USA) will be applied to the patient’s forehead maintaining between 40 and 60 during surgery. Mechanical ventilation parameters will be set to a tidal volume of 8–10 mL/kg, respiratory rate of 12–14/min, FiO2 less than 60% and the target pressure of end-tidal CO2 in the 35–45 mm Hg range and pulse oxygen saturation (SpO2)≥95%. If SpO2 remains below 95% and does not improve with the treatment of positive end-exhalation pressure ventilation, a higher FiO2 will be delivered. The participants will be transferred to the ICU for postoperative care. All participants will be equipped with a patient-controlled intravenous analgesia pump (total volume of 100 mL saline, background infusion rate 2 mL/hours, bolus 2 mL and lockout time 10 min) which will contain 100 µg sufentanil, 20 mg dezocine and 5 mg tropisetron. Furthermore, intravenous sufentanil, dezocine or oral oxycodone-acetaminophen (oxycodone hydrochloride 5 mg and acetaminophen 325 mg) will be used to relieve pain in patients with postoperative Numerical Rating Scale pain assessment greater than 4 points.
Outcome assessment
The primary outcome will be the incidence of POD during the first 7 days after off-pump CABG. Delirium will be evaluated only once on day 1 after surgery, and twice daily (08:00–12:00 and 18:00–20:00) from days 2–7 after surgery by an independent follow-up staff team who will be blinded to group assignment using the Richmond Agitation Sedation Scale (RASS), and the Confusion Assessment Method for ICU (CAM-ICU).32–34 The sedation status will be first assessed through RASS with a score ranging from −5 to +4. When the RASS score ≥–3, delirium will be evaluated. The following indexes: (1) acute onset of a mental status change or fluctuating level of consciousness, (2) inattention, (3) disorganised thinking and (4) an altered degree of consciousness, are the four main components of the CAM-ICU. Delirium will be diagnosed as (1) and (2) present, plus (3), (4) or both.
The secondary outcomes will include the incidence of postoperative acute kidney injury (AKI) and myocardial infarction (MI) during the hospital stay, as well as the length of ICU and hospital stay.
AKI will be defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria.35 The ratio of peak serum creatinine level to the preoperative serum creatinine level on each postoperative day will be used to define the change in renal function. The patient will be considered to have mild AKI if the highest ratio attained is 1.5 to 1.9, moderate AKI if the highest ratio is 2.0 to 2.9 and severe AKI if the serum creatinine level is ≥4.0 mg/dL, the peak ratio is ≥3.0 or renal replacement therapy is required.
The definition of CABG-related MI will be based on the fourth universal definition of MI.36 In patients with elevated cardiac troponin (cTn) values >10 times the 99th percentile upper reference limit in patients with normal baseline cTn values. In addition, one of the following elements is required: (1) development of new pathologic Q waves in an electrocardiograph leads; (2) new graft occlusion or new native coronary artery occlusion recorded by angiography; (3) imaging evidence for new surviving myocardial loss or new regional wall motion abnormalities consistent with ischaemic aetiology.
Data monitoring and quality assurance
Two qualified investigators will be responsible for data collection and accuracy verification. The original data will be recorded in the CRFs and maintained confidential. All assessments will be conducted by investigators blinded to the group allocation. The Ethics Committee of the Chinese Academy of Medical Sciences, Fuwai Hospital will complete the responsibility of monitoring the quality of the data. The data management and statistical analysis will be performed by the Biostatistics Department of the Chinese Academy of Medical Sciences, Fuwai Hospital.
Harms
A serious adverse event (SAE) for this study will be defined as any adverse medical event or unintended symptom associated with the study intervention and result in any of the following complications: life-threatening condition or mortality, cardiocerebral vascular events or serious disability. All related adverse events that meet the criteria for SAE will be recorded and reported to the ethics committee as part of the annual report. Adverse events will be the responsibility of the principal investigator. If there is clinical suspicion that the intervention is harmful, the study will be suspended. We will provide free clinical treatment as compensation.
Sample size estimation
For the primary outcome, the prevalence of POD in older adults undergoing off-pump CABG surgery was approximately 35% based on the data from our institution.37 According to a previous meta-analysis, intraoperative anaesthetic practice based on optimising cerebral oxygenation during cardiac surgery could reduce the incidence of POD by approximately 50%.20 According to power analysis (a type I error rate of 5% and a power of 0.8), 192 participants (96 for each group) will be needed for the study (PASS V.11.0, NCSS, Utah, USA). Considering a 5% dropout rate, we will enrol 200 participants in this study.
Statistical methods
The data with a normal distribution will be expressed as mean±SD and compared by independent sample t-tests. The data of non-normal distribution will be expressed as median (IQR) and compared by Mann-Whitney U tests. Categorical data will be described as numbers or percentages and analysed using χ2 or Fisher’s exact test. Kaplan-Meier survival analysis will be applied to the time-to-event data. We will perform the intention-to-treat analysis on the primary outcome according to group allocation. Sensitivity analysis will be used to examine missing data after it has been imputed using the worst-case imputation scenarios. A p value of <0.05 will be considered statistically significant. SPSS V.26.0 software (IBM, New York, USA) will be used for statistical analyses.
Patient and public involvement
None.
Ethics and dissemination
The final study V.2.0 was approved by the Ethics Committee of the Chinese Academy of Medical Sciences, Fuwai Hospital, China, on 11 January 2023 (No 2022–1824) and was registered at the Chinese Clinical Trial Registry on 22 February 2023. Written informed consent will be obtained from each patient or their legal representatives before enrolment (online supplemental appendix). The results of this trial will be published in an international peer-reviewed scientific journal.
Supplementary Material
Footnotes
Contributors: LT, CZ and SY: Substantial contribution to the conception and design of the work, and manuscript drafting. LT, HW, LJ, YJ and HZ conducted the study. All authors were involved in drafting and revision of intellectual content in the manuscript and approved the final version to be published.
Funding: Supported by National High-Level Hospital Clinical Research Funding (2022 GSP-QN-16).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Schenning KJ, Deiner SG. Postoperative delirium in the geriatric patient. Anesthesiol Clin 2015;33:505–16. 10.1016/j.anclin.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults . Postoperative delirium in older adults: best practice statement from the American Geriatrics society. J Am Coll Surg 2015;220:136–48. [DOI] [PubMed] [Google Scholar]
- 3.Daiello LA, Racine AM, Yun Gou R, et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology 2019;131:477–91. 10.1097/ALN.0000000000002729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greaves D, Psaltis PJ, Ross TJ, et al. Cognitive outcomes following coronary artery bypass Grafting: A systematic review and meta-analysis of 91,829 patients. Int J Cardiol 2019;289:43–9. 10.1016/j.ijcard.2019.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Şahan C, Sungur Z, Çamcı E, et al. Effects of cerebral oxygen changes during coronary bypass surgery on postoperative cognitive dysfunction in elderly patients: a pilot study. Braz J Anesthesiol 2018;68:142–8. 10.1016/j.bjan.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greaves D, Psaltis PJ, Davis DHJ, et al. Risk factors for delirium and cognitive decline following coronary artery bypass Grafting surgery: A systematic review and meta-analysis. J Am Heart Assoc 2020;9:e017275. 10.1161/JAHA.120.017275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szylińska A, Rotter I, Listewnik M, et al. Postoperative delirium in patients with chronic obstructive pulmonary disease after coronary artery bypass Grafting. Medicina (Kaunas) 2020;56:342. 10.3390/medicina56070342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Au E, Saripella A, et al. Postoperative outcomes in older surgical patients with preoperative cognitive impairment: A systematic review and meta-analysis. J Clin Anesth 2022;80:S0952-8180(22)00241-0. 10.1016/j.jclinane.2022.110883 [DOI] [PubMed] [Google Scholar]
- 9.Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of delirium and other major complications on outcomes after elective surgery in older adults. JAMA Surg 2015;150:1134–40. 10.1001/jamasurg.2015.2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohanty S, Rosenthal RA, Russell MM, et al. Optimal perioperative management of the geriatric patient: A best practices guideline from the American college of Surgeons NSQIP and the American Geriatrics society. J Am Coll Surg 2016;222:930–47. 10.1016/j.jamcollsurg.2015.12.026 [DOI] [PubMed] [Google Scholar]
- 11.O’Mahony R, Murthy L, Akunne A, et al. Synopsis of the National Institute for health and clinical excellence guideline for prevention of delirium. Ann Intern Med 2011;154:746–51. 10.7326/0003-4819-154-11-201106070-00006 [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence (NICE) . Delirium: prevention, diagnosis and management in hospital and long-term care. London, 2023. [PubMed] [Google Scholar]
- 13.Murkin JM. Cerebral Oximetry: monitoring the brain as the index organ. Anesthesiology 2011;114:12–3. 10.1097/ALN.0b013e3181fef5d2 [DOI] [PubMed] [Google Scholar]
- 14.Yu Y, Zhang K, Zhang L, et al. Cerebral near-infrared spectroscopy (NIRS) for perioperative monitoring of brain oxygenation in children and adults. Cochrane Database Syst Rev 2018;1:CD010947. 10.1002/14651858.CD010947.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheeren TWL, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. J Clin Monit Comput 2012;26:279–87. 10.1007/s10877-012-9348-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jo YY, Shim JK, Soh S, et al. Association between cerebral oxygen saturation with outcome in cardiac surgery: brain as an index organ. J Clin Med 2020;9:840. 10.3390/jcm9030840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serraino GF, Murphy GJ. Effects of cerebral near-infrared spectroscopy on the outcome of patients undergoing cardiac surgery: a systematic review of randomised trials. BMJ Open 2017;7:e016613. 10.1136/bmjopen-2017-016613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uysal S, Lin HM, Trinh M, et al. Optimizing cerebral oxygenation in cardiac surgery: A randomized controlled trial examining Neurocognitive and perioperative outcomes. J Thorac Cardiovasc Surg 2020;159:943–53. 10.1016/j.jtcvs.2019.03.036 [DOI] [PubMed] [Google Scholar]
- 19.Colak Z, Borojevic M, Bogovic A, et al. Influence of intraoperative cerebral Oximetry monitoring on Neurocognitive function after coronary artery bypass surgery: a randomized, prospective study. Eur J Cardiothorac Surg 2015;47:447–54. 10.1093/ejcts/ezu193 [DOI] [PubMed] [Google Scholar]
- 20.Tian LJ, Yuan S, Zhou CH, et al. The effect of intraoperative cerebral Oximetry monitoring on postoperative cognitive dysfunction and ICU stay in adult patients undergoing cardiac surgery: an updated systematic review and meta-analysis. Front Cardiovasc Med 2021;8:814313. 10.3389/fcvm.2021.814313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gosselt AN, Slooter AJ, Boere PR, et al. Risk factors for delirium after on-pump cardiac surgery: a systematic review. Crit Care 2015;19:346. 10.1186/s13054-015-1060-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez MG, Pandharipande P, Morse J, et al. Intraoperative cerebral oxygenation, oxidative injury, and delirium following cardiac surgery. Free Radic Biol Med 2017;103:192–8. 10.1016/j.freeradbiomed.2016.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inčiūra D, Antuševas A, Aladaitis A, et al. Near-infrared spectroscopy as a Predictor of cerebral ischaemia during carotid Endarterectomy in awake patients. Vascular 2020;28:301–8. 10.1177/1708538119893830 [DOI] [PubMed] [Google Scholar]
- 24.Diegeler A, Börgermann J, Kappert U, et al. Off-pump versus on-pump coronary-artery bypass Grafting in elderly patients. N Engl J Med 2013;368:1189–98. 10.1056/NEJMoa1211666 [DOI] [PubMed] [Google Scholar]
- 25.Czok M, Pluta MP, Putowski Z, et al. Postoperative Neurocognitive disorders in cardiac surgery: investigating the role of intraoperative hypotension. Int J Environ Res Public Health 2021;18:786. 10.3390/ijerph18020786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominici C, Salsano A, Nenna A, et al. Neurological outcomes after on-pump vs off-pump CABG in patients with cerebrovascular disease. J Card Surg 2019;34:941–7. 10.1111/jocs.14158 [DOI] [PubMed] [Google Scholar]
- 27.Ko SH, Song JW, Shim JK, et al. Low intraoperative cerebral oxygen saturation is associated with acute kidney injury after off-pump coronary artery bypass. J Clin Med 2023;12:359. 10.3390/jcm12010359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez MJ, Corredera A, Martinez-Orgado J, et al. Cerebral blood flow velocity and oxygenation correlate predominantly with right ventricular function in cooled neonates with moderate-severe hypoxic-ischemic encephalopathy. Eur J Pediatr 2020;179:1609–18. 10.1007/s00431-020-03657-w [DOI] [PubMed] [Google Scholar]
- 29.Lim L, Nam K, Lee S, et al. The relationship between intraoperative cerebral Oximetry and postoperative delirium in patients undergoing off-pump coronary artery bypass graft surgery: a retrospective study. BMC Anesthesiol 2020;20:285. 10.1186/s12871-020-01180-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deschamps A, Hall R, Grocott H, et al. Cerebral Oximetry monitoring to maintain normal cerebral oxygen saturation during high-risk cardiac surgery: A randomized controlled feasibility trial. Anesthesiology 2016;124:826–36. 10.1097/ALN.0000000000001029 [DOI] [PubMed] [Google Scholar]
- 32.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338–44. 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 33.Wang HB, Jia Y, Zhang CB, et al. A randomised controlled trial of dexmedetomidine for delirium in adults undergoing heart valve surgery. Anaesthesia 2023;78:571–6. 10.1111/anae.15983 [DOI] [PubMed] [Google Scholar]
- 34.Turan A, Duncan A, Leung S, et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Lancet 2020;396:177–85. 10.1016/S0140-6736(20)30631-0 [DOI] [PubMed] [Google Scholar]
- 35.Kellum JA, Lameire N, for the KDIGO AKI Guideline Work Group . Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care 2013;17:204. 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237–69. 10.1093/eurheartj/ehy462 [DOI] [PubMed] [Google Scholar]
- 37.Pei FB, Wei JH, Cheng ZJ, et al. Delirium after on-pump or off-pump coronary artery bypass Grafting. Chinese Journal of Cardiovascular Research 2021;19:718–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-076419supp001.pdf (247KB, pdf)