Abstract
Background
Targeted temperature management (TTM) is a recommended therapy for postcardiac arrest patients. Hyperthermia worsened the patient outcome, and overcooling increased the incidence of complications; therefore, a high-quality TTM is required. The target temperature tended to be modified worldwide after the TTM trial in 2013. Our institute modified the target temperature to 35°C in 2017. This study aimed to compare the conventional and modified protocols, assess the relationship between target temperature deviation and patient outcomes, and identify the factors influencing temperature deviation.
Methods
This single-centre, retrospective, observational study included adult out-of-hospital cardiac arrest patients who underwent TTM between April 2013 and October 2019. We compared the conventional and modified protocol groups to evaluate the difference in the background characteristics and details on TTM. Subsequently, we assessed the relationship of deviation (>±0.5°C, >37°C, or<33°C) rates from the target temperature with mortality and neurological outcomes. We assessed the factors that influenced the deviation from the target temperature.
Results
Temperature deviation was frequently observed in the conventional protocol group (p=0.012), and the modified protocol group required higher doses of neuromuscular blocking agents (NMBAs) during TTM (p=0.016). Other background data, completion of protocol, incidence of complications, mortality and rate of favourable neurological outcomes were not significantly different. The performance rate of TTM was significantly higher in the modified group than in the conventional protocol group (p<0.001). Temperature deviation did not have an impact on the outcomes. Age, sex, body surface area, NMBA doses and type of cooling device were the factors influencing temperature deviation.
Conclusions
A target temperature of 35°C might be acceptable and easily attainable if shivering of the patients was well controlled using NMBAs. Temperature deviation did not have an impact on outcomes. The identified factors influencing deviation from target temperature might be useful for ensuring a high-quality TTM.
Keywords: Out-of-Hospital Cardiac Arrest; Heart Arrest; Death, Sudden, Cardiac; Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
A target temperature of 32–36℃ was acceptable after a targeted temperature management (TTM) trial in 2017.
In the postcardiac arrest patients who underwent TTM, hyperthermia worsened the patient outcome, and overcooling increased the incidence of complications; therefore, a high-quality TTM is required.
WHAT THIS STUDY ADDS
A target temperature modification from 34°C or 36°C to 35°C increased the performance rate of TTM.
Age, sex, body surface area, neuromuscular blocking agents doses and type of cooling device were the factors influencing temperature deviation (>±0.5°C from target temperature, >37°C, or<33°C).
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
A modified target temperature of 35°C was lower than the hurdle for adopting TTM and tended to avoid temperature deviation.
The identified factors influencing deviation from target temperature might be useful for ensuring a high-quality TTM.
Introduction
The International Liaison Committee on Resuscitation1 and 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care2 stated that targeted temperature management (TTM) should be performed in patients with unconscious postcardiac arrest syndrome (PCAS) with an initial shockable and non-shockable rhythm. A target temperature of 32–36℃ for at least 24 hours is recommended. Nielsen et al conducted a TTM trial and reported that hypothermia at a target temperature of 33°C did not confer a benefit compared with a target temperature of 36°C.3 After the TTM trial, several physicians modified the TTM practice, including the target temperature.4–8 In December 2017, our institute also changed the target temperature from 34°C or 36°C to 35°C. Although a target temperature of 35°C is uncommon, it was assumed that the safety margin to prevent the complications of temperature deviation was broad. Both hyperthermia and overcooling during TTM can cause complications and have some disadvantages. In a recent study, a target temperature of 37°C was proven to worsen the neurological outcomes and increase the mortality rate even if the temperature was strictly controlled.9 As regards overcooling, coagulopathy is seldom or slightly observed at a target temperature of 33°C but increased at a target temperature of <33°C,9–13 whereas the blood coagulation system was not influenced at a target temperature of 35°C.11 12 Moreover, arrhythmias developed at body temperatures of 28–32°C.12 14 15 Some studies referred that a target temperature of 35°C can be safely induced.11 12
After the TTM trial publication, modification of the TTM protocol poses a risk as the efficacy of TTM would be underestimated, and a higher target temperature would be misconstrued as unnecessary during strict temperature management.8 14 Additionally, the TTM performance rate decreased4 5 7 8; moreover, maintaining a higher target temperature without deviations is difficult to strictly manage.8 14 16
Although a few evidence proved that temperature deviation during TTM worsened the neurological outcomes,17–19 some studies suggested that maintaining a precise target temperature during TTM would be ideal.14 16 18 Polderman and Herold stated that only minor temperature deviations within 0.2–0.5°C should be permitted during TTM.20
In this study, the differences in patients’ characteristics, TTM course and the applicability before and after the protocol modification were compared. Then, we assessed whether temperature deviations during TTM contribute to the worsening of patients’ outcomes and determine the factors that influence temperature deviation to improve the quality of TTM for patients with PCAS.
Methods
Study design and setting
This single-centre, retrospective, observational study was conducted in a tertiary emergency and critical care centre of a university hospital. Due to its retrospective nature, information regarding the conduct of this study was published on the hospital’s homepage in lieu of acquiring informed consent statements.
We included adult patients (≥18 years of age) who were treated with TTM after experiencing out-of-hospital cardiac arrest (OHCA) and were admitted to the intensive care unit (ICU) of the emergency department between April 2013 and October 2019. The TTM protocol was modified in December 2017. In both protocols, all patients whose motor component of Glasgow Coma Scale (GCS) was 6, who did not receive treatment in our department, who had a whole brain ischaemia detected on CT scan after return of spontaneous circulation (ROSC), and who discontinued the treatment were excluded.
Patients who were under the modified TTM protocol with a target temperature of 35°C (modified protocol group) were compared with those who were under the conventional TTM protocol with a target temperature of 34°C or 36°C (conventional protocol group) to evaluate the differences in their background characteristics, details on TTM, performance rate of TTM, completion rate of TTM and incidence of complications during TTM. The initiation of cooling using any kind of cooling device was considered as the performance of TTM. Completion of TTM was defined as striving to maintain the target temperature for at least 24 hours after achieving the target temperature. The complications of TTM included unstable haemodynamics (eg, bradycardia, hypotension, and arrhythmia), bleeding and electrolyte disturbances that required medication or intervention. Achievement of target temperature was defined as the time point where a temperature within 0.5°C from the target temperature was achieved. The performance rate was calculated by dividing the number of patients who were under TTM by the total number of patients with OHCA who were transported to our emergency room during the study period.
The relationship between the deviation rate from the target temperature and the mortality rate and neurological outcomes was evaluated. A temperature deviation of 0.5°C from the target temperature was regarded as a deviation from the target temperature. Temperatures >37°C and <33°C were defined as severe high and low deviations, respectively, regardless of the target temperature. The mortality rates and neurological outcomes at 30 days post-ROSC were evaluated. The neurological outcomes were assessed using the cerebral performance category (CPC) scores. CPC scores of 1–2 were defined as favourable, while CPC scores of 3–5 were considered as unfavourable.
The factors that influenced temperature deviation were identified. The patients’ characteristics (eg, age, sex, body weight and body surface area (BSA)), prehospital factors (eg, witness of collapse, bystander cardiopulmonary resuscitation (CPR), initial rhythm, prehospital ROSC and cause of arrest), and measurement and treatment performed after hospital arrival (eg, motor component of GCS, extracorporeal CPR, coronary angiography, ROSC to target temperature time, sedatives and neuromuscular blocking agents (NMBAs) used during TTM) were the candidate factors that causes deviation from the target temperature.
Patient care
The target temperature (34°C or 36°C) for the conventional protocol was selected by the attending doctor. Target temperature was maintained longer than 24 hours in both protocols. No drastic differences were observed in patient care provided in both the conventional and modified TTM protocols, except for the target temperature.
If a target temperature of 34°C or 35°C was selected, cooling was initiated in the emergency room immediately after ROSC, with the injection of 4°C crystalloid and the subsequent use of cooling devices once the patient was admitted to the ICU. If the target temperature was 36°C, cooling and warming were initiated if necessary in the emergency room, and temperature management devices were used after admission to the ICU.
The period for maintaining the target temperature was at least 24 hours, and the rewarming rate was no more than 0.25°C/hour, regardless of the target temperature, except for the 36°C protocol.
The Medi-Therm III (Gaymar Industries, New York, USA), Arctic San (Medivance, Louisville, USA), Thermoguard (ZOLL Circulation, San Jose, USA), and heater exchanger for extracorporeal membrane oxygenation (ECMO, Terumo Corporation, Tokyo, Japan) were used as cooling devices depending on the situation. The patients’ body temperature was measured via the inserted thermometer bladder catheter. All patients were continuously administered with NMBAs (vecuronium bromide or rocuronium) and sedatives (midazolam or propofol) until completion of rewarming, while analgesics (fentanyl) usually continued after rewarming. The medications or interventions used for the management of complications, including antiepileptic or osmouretic drugs, were decided by the attending physicians based on the individual patient’s clinical status.
After rewarming, NMBAs and sedatives were immediately discontinued to promote reawakening, and the body temperature was maintained below 37.5°C using ice packs, antipyretics, or a cooling device when temperature control was difficult. Brain CT, MRI and electroencephalography (EEG) examinations were performed as required for neurological assessment after TTM. When a patient was considered unlikely to recover consciousness, evidenced by the disappearance of the brainstem reflection, global brain ischaemia on CT scan, a flat-lined EEG, or significant abnormalities in the results of other examinations, the attending physician discussed the ‘do not attempt resuscitation’ procedures with the patient’s kin; then, the induction of new treatment was abandoned or withheld on reaching an agreement.
Data collection
Information including patients’ characteristics, prehospital data, treatment received in hospital, 30-day mortality rates and neurological outcomes were retrieved from the emergency medical team and hospital medical records.
Data on the temperature (288 points) measured per patient within the 24-hour period of TTM with a 5-min interval were collected. If TTM was discontinued due to death, complications, or other reasons, only the temperature data until abandonment of TTM were collected. All available temperature records of patients with missing data were collected. The deviation rate was obtained by dividing the deviated numbers by the observed temperature points. Temperature data of <25°C were excluded as it was not plausible under the condition of temperature control.
Statistical analysis
Categorical variables are expressed as frequencies and percentages, while continuous variables are expressed as medians with IQRs.
The χ2 test for categorical variables and Mann-Whitney U test for non-parametric continuous variables were used to compare the background characteristics and details of TTM between the conventional and modified protocol groups.
To assess the relationship between deviation rate from target temperature and mortality rates and neurological outcomes, the binomial logistic regression analysis was used. Four types of temperature deviations (>+0.5°C or <−0.5°C compared with the target temperature, >37°C, and <33°C) were evaluated. After performing a univariate analysis, a multivariate analysis adjusted for age, sex, witness, bystander CPR, initial rhythm, cause of arrest and time from ROSC to target temperature was performed.
To identify the factors that influence temperature deviation during TTM, a χ2 test was used for each candidate factor; subsequently, multiple regression analysis was performed to specify the factors that influenced the deviation from the target temperature. Age, sex, BSA, cooling devices and NBMA and sedative doses were included as adjustment factors. As different NMBAs (eg, vecuronium bromide and rocuronium bromide) were used depending on the patients’ clinical status, the mean doses per hour was divided into three levels based on the dose stipulated in the medication package insert (vecuronium bromide: <0.02 mg/kg/hour, 0.02–0.04 mg/kg/hour, and 0.04 mg/kg/hour <; rocuronium bromide: <0.1 mg/kg/hour, 0.1–0.2 mg/kg/hour, and 0.2 mg/kg/hour<); the same process was also followed for sedatives (midazolam: <0.03 mg/kg/hour, 0.03–0.06 mg/kg/hour, and 0.06 mg/kg/hour; propofol: <0.3 mg/kg/hour, 0.3–3.0 mg/kg/hour and 3.0 mg/kg/hour<).
All statistical analyses were performed using the SPSS software V.27 (IBM).
Results
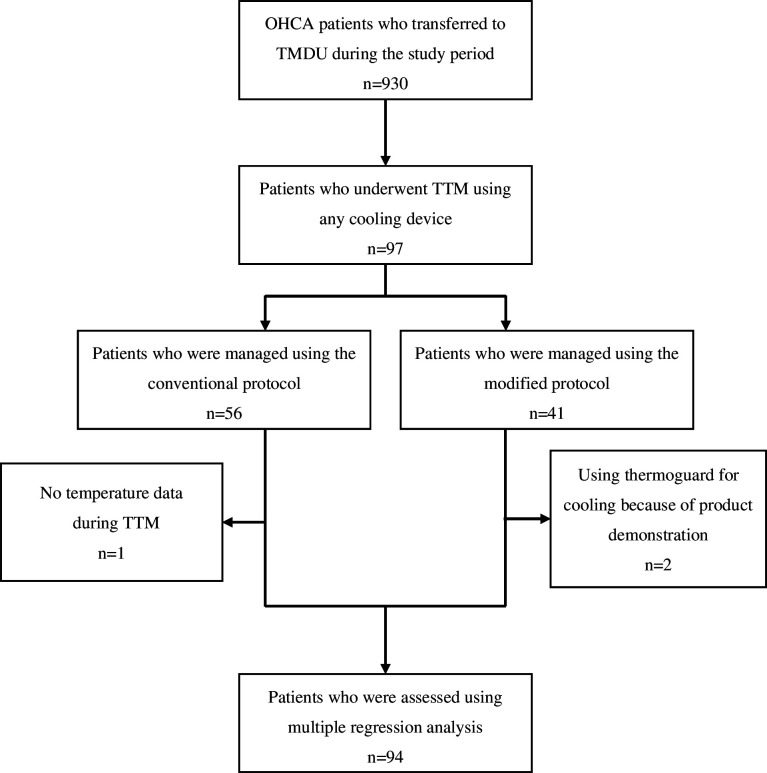
A total of 930 patients with OHCA were identified during the study period (672 patients before protocol modification). TTM was performed in 97 patients during the study period; among them, 56 (57.1%) patients used the conventional protocol (figure 1).
Figure 1.
Process of the inclusion and exclusion of patients. Among the 930 patients with OHCA who transferred to our institution during the study period, 97 underwent TTM. Overall, 55 (57.1%) patients in the conventional protocol group were compared with 41 (42.9%) patients in the modified protocol group. Ninety-four patients were assessed for temperature deviation after excluding those with missing data and those who used an unusual cooling device. OHCA, out-of-hospital cardiac arrest; TMDU, Tokyo Medical and Dental University; TTM, targeted temperature management.
Table 1 shows a comparison of the background characteristics and details of TTM, including protocol completion and incidence of complications, between the conventional and modified protocols. The shockable initial rhythm was frequently observed in the conventional protocol group; however, the difference was not significant (p=0.052). Higher doses of NMBAs were used in the modified protocol after categorising the NMBA doses into three (p=0.016). The completion of protocol, the incidence of complications, the mortality rate and the rate of favourable neurological outcomes were not significantly different between the two groups. Severe deviation (<33.0°C and/or >37.0°C) was frequently observed in the conventional protocol group (p=0.012). The performance rates of TTM were 8.3% and 15.9% before and after protocol modification, respectively, and were significantly different (p<0.001).
Table 1.
Background characteristics of the patients and details on targeted temperature management
| Conventional protocol n=56 |
Modified protocol n=41 |
P value | Total n=97 |
|
| Age median (IQR) | 59 (51–68) | 63 (53–72) | 0.151 | 60 (52–69) |
| Male n (%) | 47 (83.9) | 31 (75.6) | 0.308 | 78 (80.4) |
| Body weight, kg median (IQR) | 68.0 (55.0–74.3) | 68.0 (57.0–77.3) | 0.626 | 68.0 (55.0–76.0) |
| Witness n (%) | 45 (80.4) | 37 (90.2) | 0.183 | 82 (84.5) |
| Bystander CPR n (%) | 36 (54.3) | 25 (61.0) | 0.739 | 61 (62.9) |
| Initial rhythm, shockable n (%) | 37 (66.1) | 19 (46.3) | 0.052 | 56 (57.7) |
| Prehospital ROSC n (%) | 35 (62.5) | 22 (53.7) | 0.382 | 57 (58.8) |
| Cause, cardiogenic n (%) | 40 (71.4) | 29 (70.7) | 0.940 | 69 (71.1) |
| ECPR n (%) | 8 (14.3) | 12 (29.3) | 0.072 | 20 (20.6) |
| CAG after ROSC n (%) | 33 (58.9) | 26 (63.4) | 0.655 | 59 (60.8) |
| ROSC-TT time, min median (IQR) | 418 (267–523) | 340 (233–435) | 0.088 | 363 (240–483) |
| Cooling device n (%) | ||||
| Medi-Therm III | 6 (10.7) | 1 (2.4) | 0.117 | 7 (7.2) |
| Arctic San | 43 (76.8) | 26 (63.4) | 69 (71.1) | |
| Thermoguard | 0 (0.0) | 2 (4.9) | 2 (2.1) | |
| ECMO | 7 (12.5) | 12 (29.3) | 19 (19.6) | |
| Sedatives*, mg median (IQR) | ||||
| Midazolam | 96 (48–128) | 72 (45–96) | NA | 88 (48–120) |
| Propofol | 385 (0–973) | 0 (0–2140) | 200 (0–1255) | |
| Sedative level n (%) | ||||
| 0 (no sedatives) | 5 (8.9) | 4 (9.8) | 0.500 | 9 (9.3) |
| 1 (midazolam<0.03 mg/kg/hour or propofol<0.3 mg/kg/hour) | 8 (14.3) | 6 (14.6) | 14 (14.4) | |
| 2 (midazolam 0.03–0.06 mg/kg/hour or propofol 0.3–3.0 mg/kg/hour | 18 (32.1) | 18 (43.9) | 36 (37.1) | |
| 3 (midazolam 0.06 mg/kg/hour< or propofol 3.0 mg/kg/hour<) | 25 (44.6) | 12 (29.3) | 37 (38.1) | |
| Analgesia*, μg median (IQR) | ||||
| Fentanyl | 480 (67–585) | 480 (480–720) | 0.194 | 480 (240, 720) |
| NMBA*, mg median (IQR) | ||||
| Vecuronium | 62 (8–96) | 96 (18–96) | NA | 64 (8–96) |
| Rocuronium | 0 (0–0) | 480 (358–480) | 480 (358–480) | |
| NMBA level n (%) | ||||
| 0 (No NMBAs) | 11 (19.6) | 5 (12.2) | 0.016 | 16 (16.5) |
| 1 (Vb<0.02 mg/kg/hour or Rb<0.1 mg/kg/hour) | 6 (10.7) | 2 (4.9) | 8 (8.2) | |
| 2 (Vb 0.02–0.04 mg/kg/hour or Rb 0.1–0.2 mg/kg/hour) | 13 (23.2) | 2 (4.9) | 15 (15.5) | |
| 3 (Vb 0.04 mg/kg/hour< or Rb 0.2 mg/kg/hour<) | 26 (46.4) | 31 (75.6) | 57 (58.8) | |
| Deviation from target temperature n(%) | 0.016 | |||
| >±0.5° C† | 44 (80.0) | 36 (87.8) | 0.310 | 80 (83.3) |
| <33.0 °C and/or >37.0 °C† | 19 (34.5) | 5 (12.2) | 0.012 | 24 (25.0) |
| Completion of protocol n (%) | 45 (80.4) | 35 (85.4) | 0.522 | 80 (82.5) |
| Incidence of complications n (%) | 42 (75.0) | 30 (73.2) | 0.839 | 72 (74.2) |
| Survive n (%) | 41 (73.2) | 29 (70.7) | 0.788 | 70 (72.2) |
| Favourable neurological outcome n (%) | 23 (41.1) | 13 (31.7) | 0.346 | 36 (37.1) |
Comparison between the conventional and modified protocol groups.
*Each medicine was presented as a cumulation dose.
†Patients in the conventional protocol group with no temperature data were excluded.
CAG, coronary angiography; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; NMBA, neuromuscular blocking agent; Rb, rocuronium bromide; ROSC, return of spontaneous circulation; Vb, Vecuronium bromide.
The temperature deviation was neither related to mortality nor neurological outcome across all four types of temperature deviations (table 2). The results did not differ after adjustment (data not shown).
Table 2.
Relationship between deviation rate from target temperature and mortality and neurological outcome
| Mortality median (IQR) | P value | Neurological outcome median (IQR) | P value | |
| <−0.5°C than target temperature deviation rate % | 0.122 (0.008–1.941) | 0.136 | 0.077 (0.001–4.788) | 0.224 |
| >0.5°C than target temperature deviation rate % | 1.211 (0.205–7.137) | 0.833 | 0.365 (0.060–2.211) | 0.273 |
| >37°C deviation rate % | 3.933 (0.001–13 957.219) | 0.743 | 2.266 (0.003–1650.819) | 0.808 |
| <33°C deviation rate % | 0.000 (0.000–206126.256) | 0.244 | 0.631 (0.000–1.486E+16) | 0.631 |
Four types of temperature deviations (>0.5°C or <−0.5°C compared with the target temperature, >37°C, and <33°C) were evaluated.
Multiple regression analysis revealed that older age, male sex and higher BSA contributed to the temperature deviation of >−0.5°C, while male sex contributed to the severely low-temperature deviation (table 3). Although the lower NMBA dose and cooling device were influencing factors of severely high-temperature deviation, no other factors influenced a deviation of >0.5°C (table 3).
Table 3.
Factors influencing temperature deviation during targeted temperature management
| Temperature deviation | Factor | P value | B | 95% CI |
| <−0.5°C | Age | 0.002 | 0.003 | 0.001 to 0.005 |
| Sex | <0.001 | −0.149 | −0.224 to −0.074 | |
| BSA | <0.001 | 0.325 | 0.171 to 0.480 | |
| Cooling devices | 0.464 | −0.018 | −0.066 to 0.030 | |
| NMBA level | 0.965 | 0.001 | −0.023 to 0.024 | |
| Sedative level | 0.723 | −0.005 | −0.034 to 0.024 | |
| >+0.5°C | Age | 0.337 | −0.002 | −0.006 to 0.002 |
| Sex | 0.415 | 0.072 | −0.103 to 0.247 | |
| BSA | 0.392 | −0.156 | −0.518 to 0.205 | |
| Cooling devices | 0.529 | 0.036 | −0.077 to 0.149 | |
| NMBA level | 0.275 | −0.030 | −0.084 to 0.024 | |
| Sedative level | 0.803 | −0.008 | −0.076 to 0.059 | |
| <33.0°C | Age | 0.828 | 0.000 | 0.000 to 0.000 |
| Sex | 0.047 | −0.009 | −0.018 to 0.000 | |
| BSA | 0.942 | 0.001 | −0.018 to 0.000 | |
| Cooling devices | 0.585 | 0.002 | −0.004 to 0.007 | |
| NMBA level | 0.806 | 0.000 | −0.003 to 0.002 | |
| Sedative level | 0.720 | 0.001 | −0.003 to 0.004 | |
| >37.0°C | Age | 0.815 | 0.000 | −0.001 to −0.006 |
| Sex | 0.414 | 0.016 | −0.023 to 0.056 | |
| BSA | 0.487 | −0.029 | −0.111 to 0.053 | |
| Cooling devices | 0.046 | −0.026 | −0.052 to −0.001 | |
| NMBA level | 0.004 | −0.018 | −0.031 to −0.006 | |
| Sedative level | 0.575 | 0.004 | −0.011 to 0.020 |
BSA, body surface area; NMBA, neuromuscular blocking agent.
Discussion
We compared the effects of modification of the TTM protocol and identified the factors that influenced the temperature deviations. Severe temperature deviation is likely to occur in the conventional protocol group, while the modified protocol group required higher doses of NMBA during TTM. The performance rate of TTM drastically increased after the modification of the protocol. The temperature deviation did not have an impact on survival and neurologic outcomes. Older age, male sex, higher BSA, lower NMBA dose and type of cooling device were identified as the factors that influence temperature deviation during TTM.
Severe temperature deviation was observed in a target temperature of 34°C and 36°C as these temperatures easily exceeded the upper and lower limit (33°C and 37°C) compared with a target temperature of 35°C. After the TTM trial, several studies indicated that the result of adopting lenient TTM protocols and difficulties in complying with the target temperature4 14 worsened the outcomes of patients with PCAS.5 7 Deye et al reported in their survey that most of the included physicians who changed the TTM protocol chose an intermediate temperature range of 35–36°C due to the adjustability of the target temperature.4 A target temperature of 35°C prevents hyperthermia and overcooling; however, shivering is likely to occur at a temperature of 35°C.14 This might explain the need for a higher dose of NMBAs in the modified protocol.
Following the publication of the TTM trial, several physicians changed their TTM practice, and the performance rate of TTM in patients with PCAS decreased by 6.5%–30%.4 5 7 8 Hence, the patient outcomes tended to worsen; thus, a high-quality temperature management is recommended, regardless of the target temperature. In the current study, the performance rate of TTM improved in the modified protocol group compared with that in the conventional protocol group. Prior to the modification of the protocol, physicians in our institution are allowed to choose either a target temperature of 34°C or 36°C or no TTM at all. After protocol modification, the target temperature was unified to 35°C, and physicians were no longer required to determine the target temperature. This may have contributed to the frequency of shockable initial rhythms observed in the conventional protocol group, despite no significant difference. Physicians often deemed patients with shockable initial rhythms as suitable candidates for TTM.
The result of our study showed that temperature deviation during TTM is not associated with the patient outcome, which is consistent with previous findings.18 19 However, cooling reduces oxygen consumption by approximately 10% per degree decrease in core temperature in critically ill patients with fever, and fever through shivering increases the metabolic rate above the basal levels by sixfold.21 This may potentially exacerbate the neurological outcome in patients with PCAS due to the increased metabolic rate of the brain cell if the temperature deviates to higher levels during TTM. Similarly, overcooling increased the incidence of adverse events, such as coagulopathy, bleeding, electrolyte disturbance and arrhythmia.9–15 TTM trial was also criticised because of the larger deviation from the targeted temperature during TTM.15 22
Ageing was associated with increased vulnerability to environmental temperature change because the mechanisms of vasoconstriction and vasodilation were impaired.23 24 Thus, old age can influence thermoregulation during TTM. Similarly, female reproductive hormones affect thermoregulation in humans,25 and thus sex can be a factor influencing the temperature deviation. Badjatia et al suggested that a resting energy expenditure estimation (REE) during TTM is associated with older age, male sex and higher BSA.26 These factors are corroborated by our study findings. As REE is related to metabolism, it is consistent that these factors contribute to thermoregulatory processes during TTM. NMBAs were administered to stop the occurrence of shivering during TTM, and the association between lower NMBA dose and temperature deviation was reasonable. The insufficient dose of NMBAs can also cause temperature deviation. Previous studies have compared the type of cooling devices. Gel pad cooling was considered to be superior to water blanket cooling due to its strict temperature management27 28; however, the neurological outcome and incidence of complications during TTM in patients with PCAS were not significantly different. Additionally, ECMO helps control the target temperature compared with water blanket.29
Previous studies have compared the protocols of pre-TTM and post-TTM trials in terms of patient outcomes, performance rate and quality of TTM. However, to the best of our knowledge, the present study is the first to specify the factors influencing temperature deviation during TTM, and this information would be useful in improving the TTM quality. Insights from our results could be helpful in complying with the target temperature and maintaining high-quality TTM.
This study had several limitations. First, it was a single-centre retrospective study, and the sample size was small. However, due to the accessibility of the individual medical records of the patients, data on the temperature measured during TTM with a 5-min interval were extracted. Accumulation of cases and further investigation are required. Second, a number of confounders were identified, which were not adjusted for in the current study. Initial rhythm, aetiology of cardiac arrest, time from ROSC to target temperature, pH level and lactate value immediately after ROSC might be candidate factors influencing the temperature deviations. Third, although protocols were established in the pre-TTM and post-TTM trials, the details of patient therapy were not obtained from the attending physician, and thus it could not be homogenised among patients. Moreover, a target temperature of 35°C is uncommon, and the results could not be generalised.
Conclusion
After changing the target temperature to 35°C, a temperature deviation of >37°C or <33°C eventually decreased, although remarkably higher doses of NMBAs were required. The TTM performance rate drastically increased after the modification of the protocol. A target temperature of 35°C might be easily attainable if shivering is well controlled, without impact on patient’s outcome. Older age, male sex, large BSA, low NMBA dose and type of cooling device were identified as factors influencing the deviation from target temperature. Managing these factors might be useful for improving the TTM quality.
Acknowledgments
We thank all the staff of Trauma and Acute Critical Care Medical Center, Tokyo Medical and Dental University Hospital for their support to this study.
Footnotes
Contributors: OK designed the study, analysed the data and was a major contributor to writing the manuscript. OY designed the study and revised the manuscript. All authors participated in the interpretation of data. OK is responsible for the overall content as garantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the Institutional Ethics Review Board of the Tokyo Medical and Dental University Hospital (M2019-327). Due to its retrospective nature, information regarding the conduct of this study was published on the hospital’s homepage in lieu of acquiring informed consent statements.
References
- 1.Soar J, Berg KM, Andersen LW, et al. Adult advanced life support: 2020 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 2020;156:A80–119. 10.1016/j.resuscitation.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: adult basic and advanced life support: 2020 American heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020;142(16_suppl_2):S366–468. 10.1161/CIR.0000000000000916 [DOI] [PubMed] [Google Scholar]
- 3.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013;369:2197–206. 10.1056/NEJMoa1310519 [DOI] [PubMed] [Google Scholar]
- 4.Deye N, Vincent F, Michel P, et al. “Changes in cardiac arrest patients’ temperature management after the 2013 “TTM” trial: results from an international survey”. Ann Intensive Care 2016;6:4. 10.1186/s13613-015-0104-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray JE, Stub D, Bloom JE, et al. Changing target temperature from 33°C to 36°C in the ICU management of out-of-hospital cardiac arrest: A before and after study. Resuscitation 2017;113:39–43. 10.1016/j.resuscitation.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 6.Salter R, Bailey M, Bellomo R, et al. Changes in temperature management of cardiac arrest patients following publication of the target temperature management trial. Crit Care Med 2018;46:1722–30. 10.1097/CCM.0000000000003339 [DOI] [PubMed] [Google Scholar]
- 7.Bradley SM, Liu W, McNally B, et al. Temporal trends in the use of therapeutic hypothermia for out-of-hospital cardiac arrest. JAMA Netw Open 2018;1:e184511. 10.1001/jamanetworkopen.2018.4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abazi L, Awad A, Nordberg P, et al. Long-term survival in out-of-hospital cardiac arrest patients treated with targeted temperature control at 33 °C or 36 °C: A national Registry study. Resuscitation 2019;143:142–7. 10.1016/j.resuscitation.2019.08.029 [DOI] [PubMed] [Google Scholar]
- 9.Lascarrou J-B, Merdji H, Le Gouge A, et al. Targeted temperature management for cardiac arrest with Nonshockable rhythm. N Engl J Med 2019;381:2327–37. 10.1056/NEJMoa1906661 [DOI] [PubMed] [Google Scholar]
- 10.Tokutomi T, Miyagi T, Morimoto K, et al. Effect of hypothermia on serum electrolyte, inflammation, coagulation, and nutritional parameters in patients with severe traumatic brain injury. Neurocrit Care 2004;1:171–82. 10.1385/NCC:1:2:171 [DOI] [PubMed] [Google Scholar]
- 11.Polderman KH. Hypothermia and coagulation. Crit Care 2012;16. 10.1186/cc11278 [DOI] [Google Scholar]
- 12.Tripathy S, Mahapatra AK. Targeted temperature management in brain protection: an evidence-based review. Indian J Anaesth 2015;59:9–14. 10.4103/0019-5049.149442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob M, Hassager C, Bro-Jeppesen J, et al. The effect of targeted temperature management on coagulation parameters and bleeding events after out-of-hospital cardiac arrest of presumed cardiac cause. Resuscitation 2015;96:260–7. 10.1016/j.resuscitation.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 14.Elmer J, Polderman KH. Emergency neurological life support: resuscitation following cardiac arrest. Neurocrit Care 2017;27(Suppl 1):134–43. 10.1007/s12028-017-0457-9 [DOI] [PubMed] [Google Scholar]
- 15.Rittenberger JC, Friess S, Polderman KH. Emergency neurological life support: resuscitation following cardiac arrest. Neurocrit Care 2015;23 Suppl 2:S119–28. 10.1007/s12028-015-0171-4 [DOI] [PubMed] [Google Scholar]
- 16.Taccone FS, Picetti E, Vincent JL. High quality targeted temperature management (TTM) after cardiac arrest. Crit Care 2020;24:6. 10.1186/s13054-019-2721-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nobile L, Lamanna I, Fontana V, et al. Greater temperature variability is not associated with a worse neurological outcome after cardiac arrest. Resuscitation 2015;96:268–74. 10.1016/j.resuscitation.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 18.Nayeri A, Bhatia N, Holmes B, et al. Temperature variability during targeted temperature management is not associated with neurological outcomes following cardiac arrest. Am J Emerg Med 2017;35:889–92. 10.1016/j.ajem.2017.01.058 [DOI] [PubMed] [Google Scholar]
- 19.Cordoza M, Thompson H, Bridges E, et al. Association between target temperature variability and neurologic outcomes for patients receiving targeted temperature management at 36°C after cardiac arrest: A retrospective cohort study. Ther Hypothermia Temp Manag 2021;11:103–9. 10.1089/ther.2020.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polderman KH, Herold I. Therapeutic hypothermia and controlled Normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med 2009;37:1101–20. 10.1097/CCM.0b013e3181962ad5 [DOI] [PubMed] [Google Scholar]
- 21.Young PJ, Saxena M. Fever management in intensive care patients with infections. Crit Care 2014;18:206. 10.1186/cc13773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuroda Y. Erratum to: Neurocritical care update. J Intensive Care 2016;4:49. 10.1186/s40560-016-0168-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Front Biosci 2010;15:718. 10.2741/3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thapa D, Valente J de S, Barrett B, et al. Dysfunctional Trpm8 signalling in the vascular response to environmental cold in ageing. Elife 2021;10:e70153. 10.7554/eLife.70153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charkoudian N, Stachenfeld N. Sex hormone effects on autonomic mechanisms of thermoregulation in humans. Auton Neurosci 2016;196:75–80. 10.1016/j.autneu.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 26.Badjatia N, Haymore J, Voorhees ME, et al. Development of a resting energy expenditure estimation in patients undergoing targeted temperature management with a surface GEL pad temperature Modulating device. Ther Hypothermia Temp Manag 2022;12:38–42. 10.1089/ther.2021.0005 [DOI] [PubMed] [Google Scholar]
- 27.Jung YS, Kim KS, Suh GJ, et al. Comparison between GEL pad cooling device and water blanket during target temperature management in cardiac arrest patients. Acute Crit Care 2018;33:246–51. 10.4266/acc.2018.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keum KT, Kim YH, Lee JH, et al. Comparison of Hydrogel pad and water-circulating blanket cooling methods for targeted temperature management: A propensity score-matched analysis from a prospective Multicentre Registry. Resuscitation 2021;169:78–85. 10.1016/j.resuscitation.2021.10.021 [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Wang Y, Wang R, et al. Clinical observation of different targeted temperature management methods in patients with cardiac arrest. Am J Transl Res 2022;14:2436–42. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.