Abstract
Background
C reactive protein (CRP) kinetics have recently been suggested as predictive biomarkers for the efficacy of immune checkpoint inhibitor (ICI) therapy in selected cancer types. The aim of this study was to characterize early CRP kinetics as a tumor-agnostic biomarker for ICI treatment outcomes.
Methods
In this multicenter retrospective cohort study, two independent cohorts of patients with various cancer types undergoing palliative ICI treatment at Austrian academic centers served as the discovery (n=562) and validation cohort (n=474). Four different patterns of CRP kinetics in the first 3 months of ICI therapy were defined (CRP-flare responders, CRP-responders, CRP non-responders, patients with all-normal CRP). Objective response rate (ORR), progression-free survival (PFS) and overall survival (OS) were defined as coprimary endpoints. Univariable and multivariable logistic regression, landmark analysis and Cox regression including CRP kinetics as time-dependent variable were performed.
Results
The ORR in patients with all-normal CRP, CRP responders, CRP flare-responders and CRP non-responders was 41%, 38%, 31% and 12%, respectively. The median OS and PFS estimates were 24.5 months (95% CI 18.5 to not reached) and 8.2 months (95% CI 5.9 to 12.0) in patients with all-normal CRP, 16.1 months (95% CI 12.6 to 19-8) and 6.1 months (95% CI 4.9 to 7.2) in CRP-responders, 14.0 months (95% CI 8.5 to 19.4) and 5.7 months (95% CI 4.1 to 8.5) in CRP flare-responders and 8.1 months (95% CI 5.8 to 9.9) and 2.3 months (95% CI 2.2 to 2.8) in CRP non-responders (log-rank p for PFS and OS<0.001). These findings prevailed in multivariable analysis and could be fully confirmed in our validation cohort. Pooled subgroup analysis suggested a consistent predictive significance of early CRP kinetics for treatment efficacy and outcome independent of cancer type.
Conclusion
Early CRP kinetics represent a tumor-agnostic predictor for treatment response, progression risk and mortality in patients with cancer undergoing ICI therapy.
Keywords: Biomarkers, Tumor; Immune Checkpoint Inhibitors
WHAT IS ALREADY KNOWN ON THIS TOPIC
Early kinetics of C reactive protein (CRP) within the first 3 months of treatment with immune checkpoint inhibitors (ICIs) have been linked to treatment efficacy and risk of disease progression in selected cancer types. In detail, patients showing a rise in CRP values followed by a decline below baseline (CRP flare-responders) as well as patients with declining CRP values below 30% of baseline on treatment initiation (CRP responders) showed superior response and survival rates as compared to patients not fitting these criteria (CRP non-responders).
WHAT THIS STUDY ADDS
This is the first study to characterize early CRP kinetics upon ICI treatment as a tumor agnostic biomarker for treatment response, progression risk and survival in two large and independent cohorts including a total number of 1036 patients with different cancer types.
This study refines the previously proposed CRP kinetics model by identifying a distinct pattern of CRP kinetics in patients who had CRP values consistently below the upper limit of normal throughout the first three months of ICI therapy. These patients with‘all time normal CRP’ had a particularly favorable prognosis indicated by the longest survival.
This is the first study on this matter to account for immortal time bias by treating CRP kinetics as time dependent variables. Neglecting the time-dependent nature of CRP response group definition leads to a significant overestimation of survival times in the subgroups of patients with CRP flare response and CRP response. Our analysis fully accounts for immortal time bias, which significantly underlines the validity of the results.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study demonstrates that early kinetics of CRP after start of ICI therapy represent a robust biomarker for treatment response, progression risk and survival in patients undergoing ICI therapy across various cancer types. Given, its broad availability, low costs and high reproducibility CRP kinetics might serve as a simple but highly valuable biomarker for the assessment and monitoring of ICI response and might be particularly valuable for early identification of patients with primary treatment resistance and rapid disease progression. In a patient subgroup with permanently elevated or steadily increasing CRP values, intensified clinical monitoring and early radiographic response assessment could be prompted.
Introduction
The implementation of immune checkpoint inhibitor (ICI) therapy based on PD-(L)1 and CTLA4 blockade has revolutionized cancer care in the last decade and came along with a remarkable survival benefit in patients with different solid malignancies.1–4
Despite these promising results, there is an urgent need for robust biomarkers to predict treatment response and prognosis and to monitor ICI treatment benefit.5 6 So far, microsatellite instability and tumor mutation burden (TMB) represent the only established tumor agnostic biomarkers for ICI treatment selection.7 8 However, also the predictive accuracy of the TMB could only be validated in a subset of cancer types.9
Consequently, the identification of predictive biomarkers independent of tumor entity are an unmet clinical need. Recent studies suggested persisting systemic inflammation may be an adverse prognostic marker during ICI therapy,10 which is reliably indicated by elevated levels of C reactive protein (CRP).11 Previously, high CRP levels at treatment initiation of ICI therapy were shown to be associated with lower response rates and detrimental survival across cancer entities.11 12 Moreover, we could demonstrate that longitudinal CRP trajectories during ICI treatment successfully predict disease progression risk in patients with non-small cell lung cancer (NSCLC).11 Fukuda et al 13 defined three groups of early CRP kinetics within the first 3 months of ICI treatment based on the percentage change of CRP from baseline in a cohort of metastatic renal cell carcinoma (mRCC) patients, and demonstrated, that patients who showed an initial flare in CRP levels or had a decrease in CRP over time had a higher probability of treatment response and more favorable survival outcomes than patients with no CRP decline. Since then, this concept has been validated in tumor type-specific studies of patients with mRCC, NSCLC and urothelial carcinoma.14–17 Yet, this proposed model does not account for clinically non-significant changes in CRP levels, such as, for instance, changes within the range of normal CRP values. This may lead to improper classification of CRP response patterns. Further, the tumor-agnostic predictive utility of early CRP kinetics has not been studied yet.
Consequently, the aim of this study was to characterize early CRP kinetics as a tumor agnostic biomarker for treatment efficacy, progression risk and survival in patients with cancer undergoing ICI treatment and refine the previously proposed CRP kinetics prediction model.
Materials and methods
Study design and patient cohort
In this multicenter cohort study, two independent cohorts of patients with solid malignancies undergoing palliative ICI treatment were included. The first cohort, serving as discovery cohort, comprised 562 patients with cancer consecutively treated at the Medical University of Graz, Austria (Department of Internal Medicine, Division of Oncology and Division of Pulmonology as well as Department of Dermatology) and at the State Hospital of Feldkirch, Austria between January 2015 and November 2021. Starting from September 2019, data of these patients were recruited prospectively into an online registry called (AUsTrian Registry for Immune CHEckpoint inhibitors), patients before that date were retrospectively included.
In our second cohort (validation cohort), 474 consecutive patients with cancer who received palliative ICI treatment at the Medical University of Vienna, Austria between February 2011 and December 2018 were retrospectively included.18
Patients who had missing CRP values at the time point of ICI treatment initiation, which was defined as the study baseline, were excluded from the study in both cohorts.
Clinicopathological parameters were retrieved from the electronic database systems as well as from paper chart documentation of the participating hospitals, respectively. Assessed parameters included age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, tumor type, ICI agent, Charlson comorbidity index, cancer stage, baseline CRP and the occurrence of infections during the first 3 months of ICI treatment. Further, all available CRP levels (in mg/L) within 3 months after ICI treatment initiation were evaluated.
As proposed previously by Fukuda et al, patients were classified into three different CRP-response groups according to individual early CRP kinetics during the first 3 months after ICI initiation.13 ‘CRP flare-response’ was defined as at least doubling of baseline CRP values (≥100% increase) within 1 month after treatment start, followed by a drop in CRP below baseline within the consecutive 2 months. ‘CRP response’was defined as a decrease in CRP levels of at least 30% from baseline within 3 months. All other patients, including those with only one longitudinal CRP readout (n=21 in the discovery and n=74 in the validation cohort), were classified as CRP non-responders. In a second step, we refined patient stratification by implementing a fourth group of patients characterized by CRP levels consistently below the upper limit of normal (ULN) throughout the first 3 months (patients with all-time normal CRP). The ULN was set at a CRP level of ≤5 mg/L according to the established laboratory reference values of the participating hospitals.
Coprimary endpoints were defined as the objective response rate (ORR), the progression-free survival (PFS), and overall survival (OS). ORR was defined as the proportion (%) of patients having a complete remission or partial remission defined by the in-house radiologists in analogy to immune-related response evaluation criteria in solid tumors.19 In case of death prior radiographic response assessment, the best treatment response was defined as progressive disease. PFS was defined as the time (in months) from ICI treatment start until cancer progression or death from any cause, whatever came first. OS was defined as the time (in months) from ICI treatment start to death of any cause. Dates of death were accurately obtained from the Austrian social security database.
Statistical analyses
All statistical analyses were performed using Stata for Windows V.16.1 (StataCorp).
χ2 tests, t-tests and Kruskal-Wallis tests were used to assess associations between CRP-response groups and baseline clinicopathological characteristics in both cohorts. To assess the predictive potential of CRP response groups toward the ORR, we implemented univariable and multivariable logistic regression models. PFS and OS functions for CRP response groups were computed with Kaplan-Meier analysis and compared with log-rank tests. By definition, on analyzing longitudinal kinetics of CRP levels, varying degrees of immortal time bias arise, as a patient needs to live for, for example, at least 3 months to be classified as CRP flare-responder while the classification into the CRP non-responder group can occur at an earlier time point of follow-up. This artificially and differentially affects survival times and represents an important potential confounder. Therefore, the association of CRP kinetics with outcomes was analyzed by landmark analysis with the landmark date set empirically at 40 days of follow-up (=median time to definition of flare response). PFS and OS curves from landmark analyses were compared with Mantel-Byar tests. Further, immortal time bias in time-to-event regression was controlled by fitting univariable and multivariable Cox proportional hazards models including CRP response categories as time-dependent variables.
For multivariable logistic and Cox regression modeling, we only considered variables which were univariably associated with the outcome at the 5% level (ie, p<0.05). In addition, a sensitivity analysis including age, tumor entity and stage a treatment start a priori in the multivariable analyses was performed. Follow-up was truncated at 5 years for all survival outcomes in the Cox regression models. For the univariable and multivariable implementation of baseline CRP in logistic regression and Cox models, CRP at baseline was included as a dichotomized variable (high vs low CRP) using an empirical cut-off at the 50th percentile in the absence of validated cut-offs in this setting. Missing baseline covariables (ECOG performance status, tumor stage at ICI treatment start and treatment line) were accounted for by multiple imputation models using chained equations with 100 (m=100) imputations for each missing variable. Outcomes and longitudinal CRP values were not imputed. The full analysis code is available on reasonable request.
Results
Cohort description
Overall, 562 patients with solid malignancies undergoing palliative ICI treatment between January 2015 and November 2021 represented the discovery cohort (table 1). Of these patients, 432 received palliative ICI treatment at the Medical University of Graz, Austria and 130 patients were treated at the State Hospital of Feldkirch, Austria. The most frequent tumor types were NSCLC (n=231, 41.1%), melanoma (n=73, 13%), and RCC (n=46, 8.2% (online supplemental table 1), and most patients (94%) suffered from stage IV disease when ICI treatment was initiated. Further, 474 patients who received palliative ICI treatment at the Medical University of Vienna, Austria between February 2011 and December 2018 represented the validation cohort (table 1).18 Significant differences in terms of key baseline characteristics were observed between the two study cohorts. Patients in the validation cohort among others, were more likely to have metastatic melanoma, had better ECOG performance status and less comorbidities at ICI therapy start.
Table 1.
Descriptive characteristics (summary table) of the study population
| Discovery cohort (n=562) | Validation cohort (n=474) | ||||
| n (%miss.) |
Summary measure | n (%miss.) | Summary measure | P value | |
| Sex | 562 (0%) |
474 (0%) | 0.479 | ||
| Male | 350 (62.3%) | 285 (60.1%) | |||
| Female | 212 (37.7%) | 189 (39.9%) | |||
| Age (years) | 66.3 (IQR 58.4–72.7) | 474 (0%) | 64 (IQR 53–72) | <0.001 | |
| ECOG | 455 (19%) | 387 (18.4%) | <0.001 | ||
| ECOG 0 | 59 (10.5%) | 274 (57.8%) | |||
| ECOG >0 | 396 (70.5%) | 113 (23.8%) | |||
| Charlson Index | 562 (0%) | 10 (IQR 10–13) | 474 (0%) | 8 (IQR 7–9) | <0.001 |
| Palliative at diagnosis | 562 (0%) | 285 (50.7%) | NA | NA | |
| Cancer types | 562 (0%) | 474 (0%) | <0.001 | ||
| NSCLC | 231 (41.1%) | 126 (26.6%) | |||
| Melanoma | 95 (16.9%) | 173 (36.5%) | |||
| RCC | 73 (13%) | 38 (8%) | |||
| UC | 46 (8.2%) | 21 (4.4%) | |||
| Other | 117 (20.8%) | 116 (24.5%) | |||
| Tumor stage at ICI start | 555 (1.25%) | 435 (8.2%) | <0.001 | ||
| II+III | 25 (4.4%) | 45 (9.5%) | |||
| IV | 530 (94.3%) | 390 (82.3%) | |||
| No of metastatic sites | 562 (0%) | 1(IQR 1–2) | NA | NA | |
| High baseline CRP | 562 (0%) | 280 (55.2%) | 435 (8.2%) | 227 (47.9%) | 0.517 |
| ICI | |||||
| Treatment line | 559 (0.5%) |
474 (0%) | 0.001 | ||
| 1st line | 250 (44.5%) | 164 (34.6%) | |||
| 2nd line or higher | 183 (55%) | 310 (65.4%) | |||
| ICI agent | 562 (0%) | 474 (0%) | <0.001 | ||
| Nivolumab | 234 (41.6%) | 204 (43%) | |||
| Pembrolizumab | 248 (44.1%) | 187 (39.5%) | |||
| Atezolizumab | 30 (5.3%) | 11 (2.3%) | |||
| Durvalumab | 4 (0.7%) | 0 (0%) | |||
| Ipilimumab | 10 (1.8%) | 44 (9.3%) | |||
| Avelumab | 0 (0%) | 4 (0.8%) | |||
| Nivolumab/ipilimumab | 36 (6.4%) | 24 (5.1%) | |||
| Additional treatments | 562 (0%) | 474 (0%) | |||
| Chemotherapy | 53 (9.4%) | 17 (2.6%) | <0.001 | ||
| Radiotherapy | 12 (2.1%) | 81 (17.1%) | <0.001 | ||
| Targeted therapy | 11 (2.0%) | 46 (9.7%) | <0.001 | ||
| Infection during first 3 months of ICI treatment | 417 (25.8%) | ||||
| yes | 42 (7.5%) | ||||
| no | 375 (66.73%) | ||||
| CRP response (original model) |
562 (0%) | 474 (0%) | |||
| CRP non-responder | 249 (44%) | 266 (56.1%) | 0.001 | ||
| CRP responder | 242 (43%) | 157 (33.1%) | |||
| CRP flare-responder | 71 (13%) | 51 (10.8%) | |||
| CRP response (refined model) |
562 (0%) | 474 (0%) | |||
| CRP non-responder | 226 (40.2%) | 204 (43%) | <0.001 | ||
| CRP responder | 209 (37.2%) | 129 (27.2%) | |||
| CRP flare-responder | 69 (12.4%) | 48 (10.1%) | |||
| All-normal CRP | 58 (10.3%) | 93 (19.6%) | |||
P values indicate differences between the study cohorts.
CRP, C reactive protein; ECOG, Eastern Cooperative Oncology Group performance score; ICI, immune checkpoint inhibitor; NA, not available; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; UC, urothelial carcinoma.
jitc-2023-007765supp001.pdf (370.8KB, pdf)
In the discovery cohort, n=3652 CRP values obtained within the first 3 months of ICI treatment were included in the analysis with a median of 4 CRP values per patient (minimum 1, maximum 21). In the validation cohort, a total of n=3527 CRP values were analyzed, with a median of 5 CRP values per patient (minimum 1, maximum 50). At baseline, median CRP levels were 14.4 mg/L (IQR 4.3–50.1 mg/L) in the discovery cohort and 11.3 mg/dL (IQR 03.4–42.9 mg/dL) in the validation cohort. According to the definition of Fukuda et al, 13 patients were classified as CRP non-responders (n=249, 44%), CRP-responders (n=242, 43%) and CRP-flare responders (n=71, 13%), respectively. Proportions were similar in the validation cohort (n=266, 56.1%; n=157, 33.1%; n=51, 10.8%), respectively.
The overall ORR was 27% in the discovery cohort and 32% in the validation cohort. Median PFS and OS estimates were 4.6 months (95% CI 4.0 to 5.2) and 10.0 months (95% CI 8.6 to 11.6) in the discovery and 5.3 months (95% CI 4.0 to 7.1) and 25.0 months (95% CI 19.9 to not reached) in the validation cohort, respectively. Importantly, there was no significant difference in the distribution of CRP response groups in patients with and without infections in the first 3 months of treatment (χ2 p=0.081).
Multicancer validation of early CRP kinetics as biomarkers for ICI response and survival
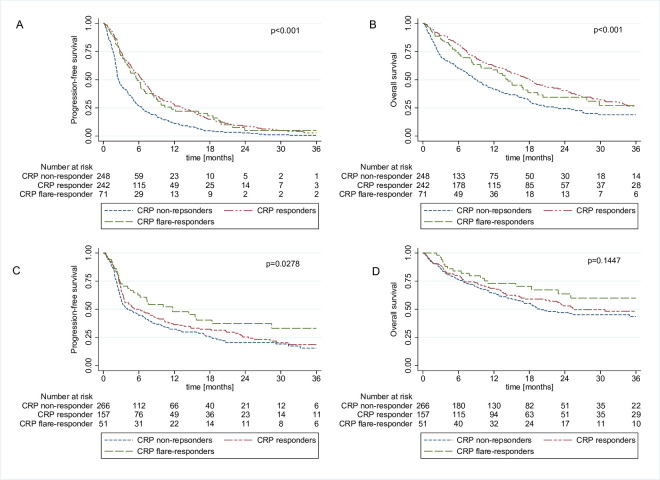
In univariable logistic regression models evaluating the impact of early CRP kinetics on treatment response, CRP responders and CRP flare-responders had significantly higher odds of response than CRP non-responders (table 2). These associations prevailed on multivariable adjustment for age, baseline CRP, cancer type and treatment line in both study cohort (table 2, online supplemental table 2). Kaplan-Meier analysis showed significantly shorter PFS and OS in CRP non-responders as compared with CRP responders and flare-responders (figure 1A–B). In our discovery cohort, this association prevailed after accounting for immortal time bias within landmark analyses (online supplemental figure 1A–B). Median time to definition as CRP flare-responders was 41.5 days, thus 40 days was considered as a reasonable pragmatic cut-off for landmark analysis for all further analysis. Further confirmation for a consistent association between early CRP kinetics and PFS and OS outcomes emerged from univariable and multivariable Cox proportional hazard models which treated CRP kinetic groups as time-dependent variables indicating longer PFS (table 3) and OS (table 4) in patients with CRP flare response and CRP response.
Table 2.
Univariable and multivariable logistic regression models for the original and refined model for early CRP kinetics
| Original model | Refined model | |||||
| Variable | Univariable analysis | Multivariable analysis | Multivariable analysis | |||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (continuously coded) | 1.028 (1.003 to 1.034) | 0.022 | 1.025 (1.006 to 1.045) | 0.011 | 1.023 (1.004 to 1.043) | 0.031 |
| Sex Male Female |
1 (reference) 0.771 (0.528 to 1.126) |
0.178 | ||||
| ECOG ECOG 1 ECOG>1 |
1 (reference) 0.690 (0.395 to 1.204) |
0.191 | ||||
| Charlson Index | 0.995 (0.889 to 1.026) | 0.209 | ||||
| Baseline CRP Low (<median) High (≥median) |
1 (reference) 0.676 (0.465 to 0.983) |
0.040 | 1 (reference) 0.584 (0.386 to 0.886) |
0.011 | 1 (reference) 0.631 (0.401 to 0.993) |
0.046 |
| Cancer type NSCLC Melanoma RCC UC Other |
1 (reference) 1.270 (0.761 to 2.120) 1.078 (0.606 to 1.918) 0.526 (0.233 to 1.188) 0.714 (0.424 to 1.203) |
0.361 0.797 0.122 0.206 |
||||
| Metastatic sites | 0.988 (0.831 to 1.175) | 0.896 | ||||
| Stage at ICI start II+III IV |
1 (reference) 0.284 (0.126 to 0.641) |
0.002 | 1 (reference) 0.319 (0.130 to 1.780) |
0.012 | 1 (reference) 0.294 (0.121 to 0.716) |
0.007 |
| Treatment line 1st line 2nd line or higher |
1 (reference) 0.448 (0.307 to 0.655) |
<0.001 | 1 (reference) 0.500 (0.333 to 0.751) |
0.001 | 1 (reference) 0.496 (0.330 to 0.744) |
0.001 |
| Additional treatments No additional treatment Radiotherapy Chemotherapy Targeted therapy |
1 (reference) 1.413 (0.418 to 4.774) 1.454 (0.795 to 2.658) 2.356 (0.707 to 7.852) |
0.577 0.224 0.163 |
||||
| CRP response CRP non-responder CRP responder CRP flare-responder |
1 (reference) 4.230 (2.714 to 6.593) 3.030 (1.639 to 5.603) |
<0.001
<0.001 |
1 (reference) 4.803 (3.001 to 7.687) 3.026 (1.603 to 5.714) |
<0.001
0.001 |
||
| CRP response CRP non-responder CRP responder CRP flare-responder All-normal CRP |
1 (reference) 4.385 (2.702 to 7.117) 3.310 (1.741 to 6.294) 4.992 (2.592 to 9.614) |
<0.001
<0.001 <0.001 |
1 (reference) 5.024 (3.006 to 8.398) 3.246 (1.672 to 6.301) 3.937 (1.955 to 7.929) |
<0.001
0.001 <0.001 |
||
significant p-values are highlighted in bold
CRP, C reactive protein; ECOG, Eastern Cooperative Oncology Group performance score; ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; UC, urothelial carcinoma.
Figure 1.
Kaplan-Meier curves showing progression-free survival and overall survival according to the previously established three group CRP response model by Fukuda et al 13 for the discovery cohort (A, B) and the validation cohort (C, D). CRP, C reactive protein.
Table 3.
Univariable and multivariable Cox regression models for PFS for the original and refined model for early CRP kinetics
| Original model | Refined model | |||||
| Variable | Univariable analysis | Multivariable analysis | Multivariable analysis | |||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (continuously coded) | 0.99 (0.99 to 1.00) | 0.096 | ||||
| Sex Male Female |
1 (reference) 1.13 (0.94 to 1.35) |
0.202 | ||||
| ECOG ECOG 1 ECOG>1 |
1 (reference) 1.50 (1.12 to 2.00) |
0.007 | 1 (reference) 1.28 (0.95 to 1.73) |
0.103 | 1 (reference) 1.32 (0.98 to 1.78) |
0.067 |
| Charlson Index | 1.00 (0.97 to 1.03) | 0.936 | ||||
| Baseline CRP Low (<median) High (≥median) |
1 (reference) 1.46 (1.22 to 1.75) |
<0.001 | 1 (reference) 1.65 (1.36 to 2.01) |
<0.001 | 1 (reference) 1.80 (1.46 to 2.22) |
<0.001 |
| Cancer type NSCLC Melanoma RCC UC Other |
1 (reference) 0.90 (0.70 to 1.16) 0.73 (0.55 to 0.98) 1.37 (0.99 to 1.89) 1.13 (0.89 to 1.44) |
0.426 0.035 0.057 0.315 |
1 (reference) 1.03 (0.79 to 1.34) 0.63 (0.47 to 0.85) 1.37 (0.99 to 1.90) 1.19 (0.94 to 1.53) |
0.838 0.002 0.057 0.152 |
1 (reference) 1.04 (0.80 to 1.35) 0.66 (0.49 to 0.89) 1.29 (0.93 to 1.79) 1.23 (0.96 to 1.57) |
0.784 0.006 0.126 0.103 |
| Metastatic sites | 1.08 (0.99 to 1.17) | 0.076 | ||||
| Stage at ICI start II+III IV |
1 (reference) 1.57 (1.00 to 2.49) |
0.053 | ||||
| Treatment line 1st line 2nd line or higher |
1 (reference) 1.20 (1.00 to 1.44) |
0.050 | ||||
| Additional treatments No additional treatment Radiotherapy Chemotherapy Targeted therapy |
1 (reference) 1.051 (0.592 to 1.868) 1.063 (0.777 to 1.453) 0.442 (0.183 to 1.067) |
0.864 0.703 0.070 |
||||
| CRP response CRP non-responder CRP responder CRP flare-responder |
1 (reference) 0.63 (0.51 to 0.77) 0.68 (0.51 to 0.92) |
<0.001
0.013 |
1 (reference) 0.52 (0.42 to 0.65) 0.62 (0.46 to 0.84) |
<0.001
0.002 |
||
| CRP response CRP non-responder CRP responder CRP flare-responder All-normal CRP |
1 (reference) 0.59 (0.48 to 0.73) 0.66 (0.49 to 0.89) 0.56 (0.40 to 0.78) |
<0.001
0.006 0.001 |
1 (reference) 0.48 (0.38 to 0.59) 0.63 (0.46 to 0.85) 0.74 (0.52 to 1.05) |
<0.001
0.003 0.096 |
||
significant p-values are highlighted in bold
CRP, C reactive protein; ECOG, Eastern Cooperative Oncology Group performance score; ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; PFS, progression-free survival; UC, urothelial carcinoma.
Table 4.
Univariable and multivariable Cox regression models for OS for the original and refined model for early CRP kinetics
| Original model | Refined model | |||||
| Variable | Univariable analysis | Multivariable analysis | Multivariable analysis | |||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (continuously coded) | 1.00 (0.99 to 1.01) | 0.385 | ||||
| Sex Male Female |
1 (reference) 1.08 (0.87 to 1.34) |
0.477 | ||||
| ECOG ECOG 1 ECOG>1 |
1 (reference) 2.07 (1.39 to 3.10) |
<0.001 | 1 (reference) 1.70 (1.13 to 2.59) |
0.010 | 1 (reference) 1.74 (1.16 to 2.60) |
0.008 |
| Charlson Index | 1.10 (1.06 to 1.14) | <0.001 | 1.07 (1.03 to 1.11) | <0.001 | 1.08 (1.03 to 1.12) | <0.001 |
| Baseline CRP Low (<median) High (≥median) |
1 (reference) 1.79 (1.45 to 2.20) |
<0.001 | 1 (reference) 1.87 (1.49 to 2.36) |
<0.001 | 1 (reference) 2.01 (1.58 to 2.57) |
<0.001 |
| Cancer type NSCLC Melanoma RCC UC Other |
1 (reference) 0.76 (0.57 to 1.02) 0.46 (0.31 to 0.69) 1.66 (1.14 to 2.41) 1.11 (0.84 to 1.47) |
0.071 <0.001 0.008 0.462 |
1 (reference) 0.83 (0.61 to 1.12) 0.41 (0.27 to 0.63) 1.77 (1.21 to 2.59) 1.14 (0.86 to 1.53) |
0.219 <0.001 0.003 0.363 |
1 (reference) 0.84 (0.62 to 1.13) 0.42 (0.28 to 0.62) 1.66 (1.13 to 2.43) 1.14 (0.86 to 1.53) |
0.250 <0.001 0.009 0.365 |
| Metastatic sites | 1.21 (1.10 to 1.33) | <0.001 | 1.18 (1.06 to 1.32) | 0.003 | 1.19 (1.07 to 1.33) | 0.001 |
| Stage at ICI start II+III IV |
1 (reference) 2.03 (1.08 to 3.80) |
0.028 | 1 (reference) 1.63 (0.84 to 3.16) |
0.151 | 1 (reference) 1.61 (0.83 to 3.13) |
0.159 |
| Treatment line 1st line 2nd line or higher |
1 (reference) 1.14 (0.92 to 1.41) |
0.315 | ||||
| Additional treatments no additional treatment Radiotherapy Chemotherapy Targeted therapy |
1 (reference) 1.341 (0.714 to 2.520) 1.065 (0.735 to 1.545) 0.251 (0.062 to 1.008) |
0.361 0.738 0.051 |
||||
| CRP response CRP non-responder CRP responder CRP flare-responder |
1 (reference) 0.73 (0.58 to 0.92) 0.85 (0.60 to 1.19) |
0.008
0.339 |
1 (reference) 0.57 (0.45 to 0.73) 0.68 (0.48 to 0.96) |
<0.001
0.029 |
||
| CRP response CRP non-responder CRP responder CRP flare-responder All-normal CRP |
1 (reference) 0.64 (0.50 to 0.81) 0.75 (0.54 to 1.06) 0.46 (0.31 to 0.69) |
<0.001
0.104 <0.001 |
1 (reference) 0.46 (0.35 to 0.59) 0.62 (0.44 to 0.88) 0.66 (0.43 to 1.01) |
<0.001
0.007 0.053 |
||
significant p-values are highlighted in bold
CRP, C reactive protein; ECOG, Eastern Cooperative Oncology Group performance score; ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; OS, overall survival; RCC, renal cell carcinoma; UC, urothelial carcinoma.
We sought to validate these findings in the validation cohort, in which a significant survival difference between the three CRP response groups could only be shown for PFS but not for OS in Kaplan-Meier and Landmark analysis (figure 1C–D, online supplemental figure 1C–D) and multivariable Cox regression analysis (online supplemental table 3 and online supplemental table 4).
Refined CRP kinetics model accounting for patients with all-normal CRP
In total, n=58 (10.3%) patients of the discovery cohort had CRP levels consistently below the ULN throughout the first 3 months of ICI treatment. Of those, n=23, n=33 and n=2 patients would have otherwise been classified as CRP non-responders, CRP-responders and CRP flare-responders according to the preciously established model of early CRP kinetics by Fukuda et al,13 respectively. Likewise, n=93 (19.6%) patients in the validation cohort had CRP values below the ULN during the first 3 months of ICI treatment, and thus were considered as patients with all-normal CRP, of which n=62, n=28 and n=3 patients would have otherwise been classified as CRP non-responders, CRP responders and CRP flare-responders, respectively. Patients with all-normal CRP were more likely to have better ECOG performance status, receive ICI in a first-line treatment setting and have melanoma and less likely to have NSCLC (online supplemental table 5). On reclassifying patients with all-normal CRP, a four-group CRP kinetics model was subsequentially defined. In the discovery cohort, n=58 (10.3%), n=209 (37.2%), n=69 (12.4%) and n=226 (40.2) were classified as patients with all-normal CRP, CRP-responders, CRP flare-responders and CRP non-responders, respectively. Distributions were slightly different in the validation cohort (table 1).
Predictive and prognostic accuracy of refined classification of early CRP kinetics
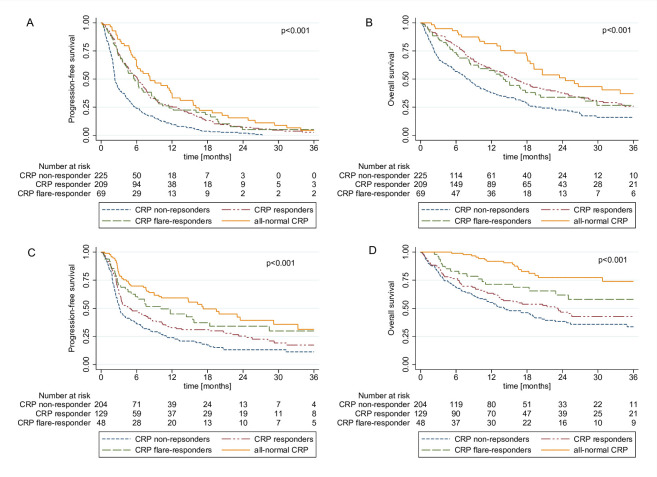
Univariable and multivariable logistic regression analysis of the refined CRP response stratification revealed that in addition to CRP responders and CRP flare-responders, patients with all-normal CRP showed significantly higher odds of treatment response compared with CRP non-responders (table 2). In detail, the ORR was 41%, 38%, 31% and 12% in patients with all-normal CRP, CRP responders, CRP flare-responders and CRP non-responders, respectively. These findings could be confirmed in the validation cohort, although the association between all-normal CRP and ORR weakened after multivariable adjusting for baseline CRP (online supplemental table 2).
In addition, Kaplan-Meier analysis revealed significantly increased PFS and OS in patients with all-normal CRP, CRP responders and CRP flare responders (figure 2) a finding which was confirmed in landmark analysis (online supplemental figure 2A–B) and Cox proportional hazards model (table 3). In detail, median PFS was 8.2 (5.9–12.0) months in patients with all-normal CRP, 6.1 months (95% CI 4.9 to 7.2) in CRP responders, 5.7 months (95% CI 4.1 to 8.5) in CRP flare-responders and 2.3 months (95% CI 2.2 to 2.8) in CRP non-responders. Median OS estimates of these four groups were 24.5 months (95% CI 18.5 to not reached), 16.1 months (95% CI 12.6 to 19.8), 14.0 months (95% CI 8.5 to 19.4) and 8.1 months (95% CI 5.8 to 9.9), respectively.
Figure 2.
Kaplan-Meier curves showing progression-free survival and overall survival according to our refined four group CRP kinetics model including patients with CRP levels consistently below the ULN throughout the first three treatment months of ICI treatment (all-normal CRP responder) for the discovery cohort (A, B) and the validation cohort (C, D). CRP, C reactive protein; ICI, immune checkpoint inhibitor; ULN, upper limit of normal.
After adjusting for potential confounders including baseline CRP the association between the all-normal CRP group and the survival endpoints slightly weakened, whereas the other CRP response groups fully prevailed as significant predictors (tables 3 and 4). Importantly, since by definition, patients in the all-normal CRP group had to have baseline CRP levels below the ULN, a very strong correlation between low baseline CRP and the all-normal CRP group was observed (β regression coefficient for continuous baseline CRP as dependent variable and all-normal CRP as predictor variable=−37.74, 95% CI −52.92 to −22.56, p<0.001). To account for this collinearity an exploratory analysis excluding baseline CRP from the multivariable model was performed. Consequently, PFS (HR 0.60, 95% CI 0.43 to 0.84, p=0.003) and OS estimates (HR 0.52, 95% CI 0.24 to 0.78, p=0.002) were significantly longer in patients with all-normal CRP compared with the reference group of CRP non-responders (online supplemental tables 6 and 7).
In addition, a sensitivity analysis further adjusting for age, tumor entity, and cancer stage (not significantly associated with outcome in univariable analyses, but clinically established prognostic markers) within the multivariable models for ORR, PFS, and OS fully confirmed CRP response groups as significant predictors of outcome (online supplemental tables 8,9 and 10).
As for our validation cohort, CRP kinetics were significantly and independently associated with PFS and OS in both, landmark analysis as well as univariable and multivariable Cox regression analysis (online supplemental figure 2C–D), (online supplemental table 3), and (online supplemental table 4).
Subgroup analysis of novel CRP kinetics prediction model stratified by tumor type
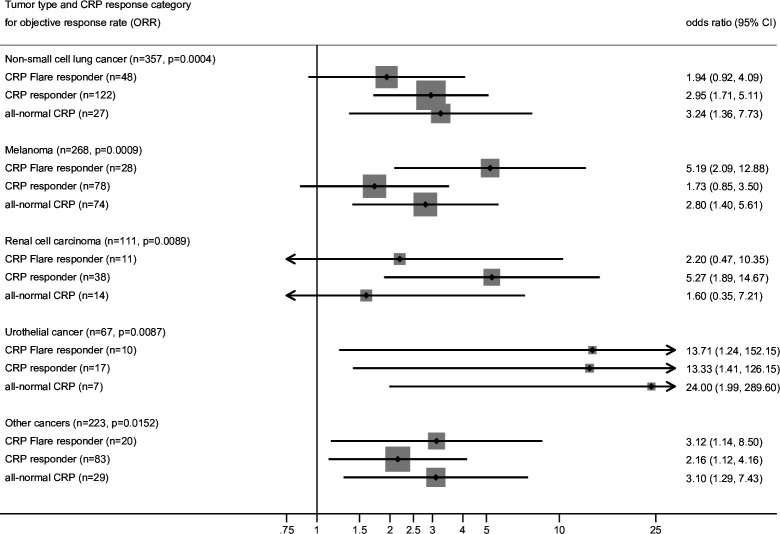
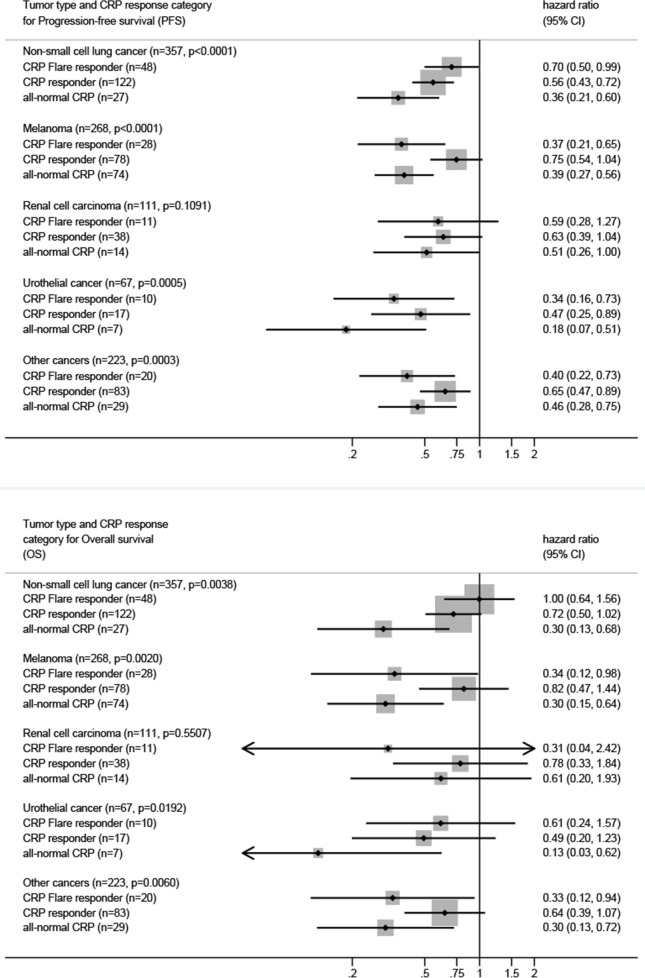
Finally, an exploratory subgroup analysis of our novel CRP response prediction model stratified by tumor type was performed for all three coprimary endpoints ORR, PFS and OS. For this analysis, the discovery and validation cohort were pooled to increase statistical power. Consistently, CRP responders, CRP flare-responders and patients with all-normal CRP had increased odds of response (figure 3) and longer PFS and OS (figure 4) compared with CRP non-responders across all cancer types.
Figure 3.
Logistic regression for ORR stratified by tumor type for the refined four group CRP kinetics model. P values in the sub headers indicate χ2 P for CRP. CRP, C reactive protein.
Figure 4.
Cox regression for PFS and OS stratified by tumor type for the refined four group CRP kinetics model. P values in the sub headers indicate χ2 P for CRP. CRP, C reactive protein.
Discussion
In this study, we could demonstrate the prognostic and predictive significance of early CRP kinetics for cancer-agnostic prediction of therapy response and mortality in patients treated with ICIs in two large and independent multi-cancer cohorts. Notably, by observing a distinct pattern of CRP kinetics in patients who had CRP values consistently below the ULN throughout the first 3 months of ICI therapy (ie, all-normal CRP) we could refine the previously proposed CRP kinetics prediction model for ICI efficacy.
Alongside CRP-responders and CRP flare-responders also patients with all-normal CRP showed superior response rates and survival outcomes as compared with CRP non-responders. These results indicate that longitudinal measurements of CRP represent a valuable tool to predict and monitor ICI therapy efficacy.
Fukuda et al 13 analyzed early CRP kinetics in 42 patients with mRCC undergoing treatment with nivolumab and defined three groups of early CRP kinetics based on the percentage change of CRP from baseline. In their analysis, patients showing a CRP flare response or CRP response in the first 3 months of ICI treatment had significantly higher odds of response and more favorable survival outcomes as compared with CRP non-responders.13 This model was later validated by Klümper et al 17 in cohorts of mRCC, metastatic UC and advanced NSCLC patients receiving ICI treatment.14–16 In this study, we thus hypothesized that early CRP kinetics might serve as a tumor-agnostic biomarker for ICI efficacy. To test this hypothesis, we evaluated a total of 1036 patients with various tumor types treated with ICI therapy in two academic centers in Austria within a validation study design. To the best of our knowledge, this is by far the largest study on this matter. In a first step, we aimed to investigate the prognostic and predictive accuracy of the previously described CRP response patterns model by Fukuda et al 13 in our pan-cancer cohort. We fully confirmed a significant association between the CRP response patterns and our three study endpoints in our discovery cohort, and partly confirmed these findings in the validation cohort. Importantly, the tested model by Fukuda et al assigns patients to the CRP response groups (ie, CRP responder, CRP flare-responder and CRP non-responder) by the percentage change from baseline CRP values in the first 3 months after treatment initiation. Although this model accurately reflects clinically relevant CRP changes in patients with elevated baseline CRP, its group distinction in patients with normal baseline CRP may be improved. For instance, a patient with a baseline CRP of 2 mg/L and consecutive CRP measurements of 3 mg/L would be classified as a CRP non-responder. We thus hypothesized that patients with CRP measurements consistently below the ULN (ie, 5 mg/L) throughout the first 3 months of ICI treatment represent a distinct CRP response group with favorable prognosis. Consequently, 10% of patients in our discovery cohort and 21% of patients in our validation cohort were reclassified as all-normal CRP, of which a large proportion would have otherwise been classified as CRP non-responders who have been previously shown to have a poor prognosis. This four-CRP-group model showed improved discrimination of response rates, disease progression risk, and death. In detail, we observed that patients with all-normal CRP had fourfold higher odds for treatment response than CRP non-responders and had the numerically longest PFS and OS. As anticipated, all-normal CRP was highly correlated with low baseline CRP levels, which have been previously linked to favorable prognosis in patients with cancer.11 20 This collinearity led to enlarged confidence intervals and higher p values of the association between all-normal CRP and the endpoints under study in both the discovery and validation cohort. Excluding baseline CRP from our multivariable regression models and thus accounting for this collinearity demonstrated a strong and statistically significant association between all-normal CRP and the study endpoints. Alongside, patients with all-normal CRP also CRP responders and CPR flare-responders had significantly higher response rates and better prognosis than CRP non-responders. In detail, the median PFS in CRP non-responders, which comprised 40% of our study population was only 2.3 months with an ORR of 10%. This underlines that patients with elevated CRP levels at baseline, who do not show any kind of early CRP response (ie, CRP non-responders) after ICI start have a very low chance of response and should thus be closely monitored for radiographic disease progression.
Although our two study cohorts significantly differed in terms of clinically relevant patient characteristics such as tumor type, EOCG performance status and line of ICI treatment allocation, the prognostic potential of our model could be fully confirmed in our validation cohort, which further strengthens its general validity. An added strength of this study is that we rigorously accounted for immortal time bias. Due to the time-dependent nature of CRP response group definitions, unaccounted immortal time bias leads to an overestimation of the favorable prognostic impact of being in any of the CRP response groups.21 Importantly, after fully controlling for this bias by implementing landmark analysis and time-dependent regression models, the four CRP kinetic groups remained strongly and significantly associated with the outcomes under study.
Finally, we aimed to elucidate whether the impact of CRP kinetics on ICI treatment response and survival outcomes differed across tumor types. Although the relatively small sample size in the respective cancer-type subgroups led to enlarged CIs, patients with all-normal CRP, CRP responders and CRP flare-responders consistently showed increased odds of treatment response as well as longer PFS and OS compared with CRP non-responders across all tumor entities under study. The impact of CRP kinetic group on outcomes was strongest in patients with urothelial cancer, which is in line with previous studies that have suggested CRP as a proxy marker for cancer induced systematic inflammation and tumor activity in this tumor entity.22
Although CRP kinetics are assessed post-treatment start, and therefore, cannot be used for upfront prediction of ICI efficacy, our study findings suggest that monitoring of CRP kinetics after ICI initiation might be particularly valuable for early identification of patients (ie, CRP non-responders) with very high risk of primary treatment resistance and rapid disease progression. In this patient subgroup with permanently elevated or steadily increasing CRP values, intensified clinical monitoring and early radiographic response assessment should be prompted. Previous studies further suggest a potential role of CRP kinetics to help to distinguish radiographic pseudoprogression, a phenomenon caused by immune cell invasion into the tumor lesions that is reported to occur in approximately 10% of patients treated with ICI,23 from actual disease progression.15
CRP is an acute phase protein and a well-established surrogate marker of inflammatory conditions such as cancer induced inflammation. Recently, cancer-related systemic and local inflammation in the tumor microenvironment have been proposed to play a crucial role in the development of resistance to ICI treatment.24 As such, we can conceive a strong immunological rationale that persistently elevated CRP levels after ICI treatment initiation (ie, CRP non-responder) reflect chronic cancer induced-inflammatory processes that are linked with an immunosuppressive tumor microenvironment.25 Importantly, the elucidation of the exact mechanisms involved in CRP-flare phenomena in the context of immunotherapy still warrants further investigation in functional studies.
Some limitations of our study must be discussed. First, due to the mostly retrospective study design, selection bias cannot be entirely excluded. However, the multicenter study design and validation of our results in two large independent all-comer cohorts may reduce the risk of selection bias by a significant extent, which ultimately represents a strength of our study. Second, CRP values were retrospectively assessed within routine clinical practice and not collected following a prospective, predefined protocol. Thus, patients experiencing CRP flare response or CRP response between two measurements may not have been detected. Third, information on infections and consequent antibiotics use within the first three treatment months were partly missing due to the retrospective study design. However, no significant association between the presence of infections within the first 3 months of ICI therapy and the CRP response groups was observed.
Conclusion
This study demonstrates that early kinetics of CRP after start of ICI therapy represents a robust predictive and prognostic biomarker for treatment response, progression risk and survival in patients undergoing ICI therapy across various cancer types. Given, its broad availability, low costs and high reproducibility CRP kinetics might serve as a simple but highly valuable biomarker for the assessment and monitoring of ICI therapy benefit and might be particularly valuable for early identification of patients with primary treatment resistance.
Footnotes
Contributors: Conceptualization: DAB, FM and JMR; Methodology: DAB, FP and JMR; statistical analysis: DAB and FP; Investigation: all authors; data contribution: all authors, Writing–original draft: DAB, FP and JMR; Writing–review and editing: all authors; Visualization: DAB, FP; Supervision: AT and JMR.
Funding: This research was supported by a research grant from AstraZeneca GmbH,Bristol-Myers Squibb GesmbH (BMS), Merck Sharp & Dohme Ges.m.b.H., Roche Austria and Sanofi-aventis. The hypothesis of this study was not suggested by the the funding body, which had no role in the design, analysis, and publication of this study. MP gratefully acknowledges financial support from the Austrian Federal Ministry for Digital and Economic Affairs, the Austrian National Foundation for Research, Technology and Development and the Christian Doppler Research Association.
Competing interests: DAB has received travel/congress support from EISAI, Lilly, Bristol-Myers Squibb, MSD; honoraria for consulting or advisory boards from Roche, EISAI, MSD; honoraria for lectures from Ipsen unrelated to the submitted work. FM received has received honoraria for advisory boards from Servier and Bristol-Myers Squibb. LK has received honoraria for travel/congress support and consulting/advisory roles from Bristol Myers Squibb (BMS), Merck Sharp & Dome (MSD), Novartis, Pierre Fabre and Sanofi Aventis unrelated to the submitted work. ER stated the following conflicts of interest: Honoraria: Amgen, BMS, Delcath, MSD, Merck, Novartis, Pierre Fabre, Sanofi. Consulting or advisory role: Amgen, BMS, MSD, Merck, Novartis, Pierre Fabre, Sanofi. Speakers’ bureau: Amgen, BMS, MSD, Merck, Novartis, Pierre Fabre, Sanofi. Former Site PI for Medical University of Graz (last three years): Amgen, BMS, Curevac, Incyte, MSD, Merck, Novartis, Pierre Fabre, Roche. To date Site PI for Medical University of Graz: Delcath. Steering Committee: Novartis. Travel reimbursements: Amgen, BMS, MSD, Merck, Novartis, Pierre Fabre, Sanofi. Stock (under US$10,000): Roche. Honorary member of the Austrian Cancer Aid and the Austrian Cancer Aid/StyriaMP (Matthias Preusser) has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra, Gan & Lee Pharmaceuticals, Servier. MP declares no conflict of interest related to this work. JMR has received honoraria for lectures from BMS and MSD.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and the study was approved by the IRB of the Medical University of Graz (No. 31-357 ex 18/19) and by the ethics committee of the Medical University of Vienna, Austria (No. 2213/2019). Written informed consent was obtained from all prospectively enrolled patients. Participants gave informed consent to participate in the study before taking part.
References
- 1. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus Ipilimumab in advanced Melanoma. N Engl J Med 2013;369:122–33. 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med 2018;379:2040–51. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 5. Darvin P, Toor SM, Sasidharan Nair V, et al. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 2018;50:1–11. 10.1038/s12276-018-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor Immunotherapy. Nat Rev Cancer 2019;19:133–50. 10.1038/s41568-019-0116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. NEnglJMed 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with Pembrolizumab: prospective biomarker analysis of the Multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353–65. 10.1016/S1470-2045(20)30445-9 [DOI] [PubMed] [Google Scholar]
- 9. McGrail DJ, Pilié PG, Rashid NU, et al. High tumor Mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol 2021;32:661–72. 10.1016/j.annonc.2021.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kauffmann-Guerrero D, Kahnert K, Kiefl R, et al. Systemic inflammation and pro-inflammatory cytokine profile predict response to checkpoint inhibitor treatment in NSCLC: a prospective study. Sci Rep 2021;11. 10.1038/s41598-021-90397-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riedl JM, Barth DA, Brueckl WM, et al. C-reactive protein (CRP) levels in immune checkpoint inhibitor response and progression in advanced non-small cell lung cancer: a bi-center study. Cancers (Basel) 2020;12. 10.3390/cancers12082319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roussel E, Kinget L, Verbiest A, et al. C-reactive protein and neutrophil-lymphocyte ratio are Prognostic in metastatic clear-cell renal cell carcinoma patients treated with Nivolumab. Urol Oncol 2021;39:239. 10.1016/j.urolonc.2020.12.020 [DOI] [PubMed] [Google Scholar]
- 13. Fukuda S, Saito K, Yasuda Y, et al. Impact of C-reactive protein flare-response on Oncological outcomes in patients with metastatic renal cell carcinoma treated with Nivolumab. J Immunother Cancer 2021;9:e001564. 10.1136/jitc-2020-001564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klümper N, Saal J, Berner F, et al. C reactive protein flare predicts response to Checkpoint inhibitor treatment in non-small cell lung cancer. J Immunother Cancer 2022;10:e004024. 10.1136/jitc-2021-004024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klümper N, Sikic D, Saal J, et al. C-reactive protein flare predicts response to anti-PD-(L)1 immune Checkpoint blockade in metastatic urothelial carcinoma. Eur J Cancer 2022;167:13–22. 10.1016/j.ejca.2022.02.022 [DOI] [PubMed] [Google Scholar]
- 16. Tomisaki I, Harada M, Tokutsu K, et al. Impact of C-reactive protein flare response in patients with advanced urothelial carcinoma who received Pembrolizumab. In Vivo 2021;35:3563–8. 10.21873/invivo.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klümper N, Schmucker P, Hahn O, et al. C-reactive protein flare-response predicts long-term efficacy to first-line anti-PD-1-based combination therapy in metastatic renal cell carcinoma. Clin Transl Immunology 2021;10. 10.1002/cti2.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moik F, Chan W-SE, Wiedemann S, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune Checkpoint inhibitor therapy. Blood 2021;137:1669–78. 10.1182/blood.2020007878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing Immunotherapeutics. Lancet Oncol 2017;18:e143–52. 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riedl JM, Posch F, Moik F, et al. Inflammatory biomarkers in metastatic colorectal cancer: prognostic and predictive role beyond the first line setting. Oncotarget 2017;8:96048–61. 10.18632/oncotarget.21647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int 2018;31:125–30. 10.1111/tri.13081 [DOI] [PubMed] [Google Scholar]
- 22. Yuk HD, Ku JH. Role of systemic inflammatory response markers in urothelial carcinoma. Front Oncol 2020;10. 10.3389/fonc.2020.01473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borcoman E, Nandikolla A, Long G, et al. Patterns of response and progression to Immunotherapy. Am Soc Clin Oncol Educ Book 2018;38:169–78. 10.1200/EDBK_200643 [DOI] [PubMed] [Google Scholar]
- 24. Sui Q, Zhang X, Chen C, et al. Inflammation promotes resistance to immune checkpoint inhibitors in high microsatellite instability colorectal cancer. Nat Commun 2022;13. 10.1038/s41467-022-35096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 2021;6:263. 10.1038/s41392-021-00658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-007765supp001.pdf (370.8KB, pdf)
Data Availability Statement
Data are available on reasonable request.