Abstract
Background
Guidelines for patients with atrial fibrillation (AF) at high thromboembolic risk recommend oral anticoagulants (OACs) for preventing stroke and systemic embolism (SE). The reasons for guideline non-adherence are still unclear.
Aim
The aim is to identify clinical, demographic and non-patient characteristics associated with withholding OAC in patients with AF at high stroke risk.
Methods
Patients in the Global Anticoagulant Registry in the FIELD-AF, newly diagnosed with AF between March 2010 and August 2016, and with CHA2DS2-VASc Score≥2 (excluding sex), were grouped by OAC treatment at enrolment. Factors associated with OAC non-use were analysed by multivariable logistic regression.
Results
Of 40 416 eligible patients, 12 126 (30.0%) did not receive OACs at baseline. Globally, OAC prescription increased over time, from 60.4% in 2010–2011 to 74.7% in 2015–2016. Country of enrolment was the major predictor for OAC withholding (χ2−df=2576). Clinical predictors of OAC non-use included type of AF (χ2−df=404), history of bleeding (χ2−df=263) and vascular disease (χ2−df=99). OACs were used most frequently around the age of 75 years and decreasingly with younger as well as older age beyond 75 years (χ2−df=148). Non-cardiologists (χ2−df=201) and emergency room physicians (χ2−df=14) were less likely to prescribe OACs. OAC prescription correlated positively with country health expenditure.
Conclusions
Approximately one out of three AF patients did not receive OAC, while eligible according to the guidelines. Country of enrolment was the major determinant of anticoagulation strategy, while higher country health expenditure was associated with lower likelihood of withholding anticoagulation.
Keywords: atrial fibrillation, delivery of health care, quality of health care, stroke, myocardial infarction
WHAT IS ALREADY KNOWN ON THIS TOPIC
Oral anticoagulants (OACs) are recommended for preventing stroke and systemic embolism in patients with atrial fibrillation (AF) and at high risk of thromboembolism. Previous studies identified common patient-level and physician-level barriers, as well as region-specific system-level barrier, to oral anticoagulant use but their relative importance has been unclear.
WHAT THIS STUDY ADDS
The likelihood of an eligible patient not receiving OAC treatment was associated mainly with country and country health expenditure and, independently, far less with patient-specific or care-specific factors.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The study highlights the importance of country-specific and socioeconomic factors for AF patients receiving guideline-recommended anticoagulation.
Introduction
Stroke is a leading cause of morbidity and mortality worldwide. Atrial fibrillation (AF), which affects approximately 2% of the population, is associated with a fivefold increased risk of ischaemic stroke.1 Depending on the presence of risk factors, the annual incidence of stroke or systemic embolism (SE) is approximately 5% in AF patients not receiving anticoagulation.2
Evidence-based guidelines recommend the use of OAC in AF patients at a high risk of stroke/SE, with more recent guidelines recommending non-vitamin K oral anticoagulants (NOACs) over vitamin K antagonists.3–6 But although prescriptions have increased globally since the introduction of NOACs, significant variability was reported across geographic regions.7–11 Importantly, OACs continue to be underused in many countries.12 Oral anticoagulants (OACs) should be used judiciously because they increase the risk of bleeding. Clinical guidelines therefore recommend the use of stroke risk prediction scores (eg, CHA2DS2-VASc Score) to provide individualised treatment.3–6 Although validated stroke risk prediction models are practical for everyday clinical application, limitations exist. I.e., CHA2DS2-VASc includes several well-known risk factors for stroke but does not incorporate additional patient characteristics such as echocardiographic and other imaging findings, or smoking, sleep apnoea, and hypertrophic cardiomyopathy which may influence treatment outcomes.13 Furthermore, dichotomisation of continuous risk factors (eg, blood pressure, age) within the scores can lead to a misestimation of risk when factor values are close to the cut-off threshold. Bleeding risk scores, such as HAS-BLED,14 are meant to alert physicians to bleeding risks which could, in turn, lead to modification of treatments, additional care and support to avoid bleeding. However, patients with high HAS-BLED scores often also have a high CHA2DS2-VASc Score.15 Since these patients frequently do not receive OAC, it is possible that high bleed risk is regarded as a contraindication to anticoagulation, even in the group who would profit most from OAC use. Limitations such as these are likely to result in suboptimal treatment decisions.
Undertreatment of eligible patients despite evidence-based benefits is a problem which is well documented for Europe and North America.16–22 Here, we investigated factors that might contribute to non-use of OAC in countries with different demographics, income and healthcare systems by modelling the likelihood of eligible patients not receiving OAC.
Methods
Study design and participants
The Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF) is a prospective, observational, international study. Briefly, patients were recruited from 1215 sites in 35 countries, in 5 consecutive cohorts between March 2010 and August 2016.23 Individuals aged≥18 years, with new-onset non-valvular AF (diagnosed within the previous 6 weeks according to standard local procedures), and with at least one investigator-determined risk factor for stroke, were eligible for inclusion. Patients with a transient reversible cause of AF24 such as hyperthyroidism, or for whom follow-up was unlikely, were excluded. In the present analysis from the GARFIELD-AF registry, only patients with a CHA2DS2-VASc Score≥2 (excluding sex) were selected. Patients were followed up for a minimum of 2 years from enrolment. For this study, follow-up was censored at 2 years. Investigators obtained patient data from the medical record and patient interview. Investigators recorded the required data in a study-specific case record form (CRF), and a web-based system was used to collect CRF data.
Statistical analysis
Patients were categorised according to OAC use at enrolment, that is, no OAC use versus any vitamin K antagonist or non-vitamin NOAC (ie, dabigatran, apixaban, rivaroxaban or edoxaban). Descriptive statistics were expressed as median and IQRs for continuous variables, and absolute frequencies and percentages for categorical variables. Multivariable logistic regression analysis was performed using a prespecified set of covariates to determine factors associated with OAC non-use at baseline (online supplemental table S1). More specifically, three models were generated to establish associations with treatment decision: model 1 considered demographic patient characteristics, medical and cardiovascular history, lifestyle factors, vital signs, type of AF and care setting at diagnosis. An additional factor for model 2 was country of enrolment (‘country’). For model 3, country-based expenditure on health per capita was included, expressed in international dollars at purchasing power parity (PPP).25 In brief, PPPs are the rates of currency conversion that equalise the purchasing power of different currencies by eliminating the differences in price levels between countries. This indicator, having in a common currency and adjusted for price relatives, allows for meaningful cross-country comparisons. Each country has a unique value for health expenditure per year. Expenditure values for the years in which each country enrolled patients into GARFIELD-AF were averaged to provide one estimate per country of the ‘country health expenditure’. All patient demographic and clinical variables reflect information collected at the time of enrolment.
openhrt-2023-002506supp001.pdf (622.2KB, pdf)
Logistic least absolute shrinkage and selection operator regression determined predictors of receiving OAC based on data collected at enrolment. The relationship of the identified factors and the likelihood of OAC withholding is expressed by means of ORs and corresponding CI. The significance of a test diminishes as the number of categories (and thus df for the test) increases for a factor. As the numbers of categories varied from mostly 2 to up to 35, their relative importance was calculated as Wald χ2–df.
The linearity assumption was evaluated for each continuous measure by applying restricted cubic splines. Multiple imputation26 was applied to account for missing values and the obtained ORs represent the combinations from five imputed datasets. Statistical significance was assumed for a two-tailed probability level<0.05. Statistical analyses were performed using SAS Enterprise Guide V.8.2. The manuscript was drafted according to Strengthening the Reporting of Observational Studies in Epidemiology guidelines for observational studies.
Results
Study population
The study flow diagram is shown in online supplemental figure S1. The enrolment period was March 2010–August 2016, with the final data cut-off on 30 June 2019. Among 52 057 patients enrolled in GARFIELD-AF, we excluded those with CHA2DS2-VASc Score<2 (excluding sex; n=11 018), or without available baseline treatment or follow-up information (n=623). Of the 40 416 patients included in the analysis, 12 126 (30.0%) did not receive OAC therapy at baseline.
Their baseline characteristics are shown in table 1.
Table 1.
Baseline characteristics by baseline anticoagulation*
| Baseline characteristics | OAC treatment | P value † | |
| No (n=12 126) | Yes (n=28 290) | ||
| Sex, n (col %) | |||
| Male | 6490 (53.5) | 15 081 (53.3) | 0.694 |
| Female | 5636 (46.5) | 13 209 (46.7) | |
| Age, median (Q1; Q3), years | 73.0 (66.0; 80.0) | 74.0 (67.0; 80.0) | <0.001 |
| Ethnicity, n (col %) | |||
| White | 6635 (56.3) | 19 096 (69.2) | <0.001 |
| Hispanic/Latino | 874 (7.4) | 1863 (6.7) | |
| Asian | 4045 (34.3) | 6122 (22.2) | |
| Black/mixed/other | 234 (2.0) | 529 (1.9) | |
| BMI, median (Q1; Q3), kg/m² | 26.4 (23.5; 30.1) | 27.2 (24.2; 31.1) | <0.001 |
| Systolic blood pressure, median (Q1; Q3), mm Hg | 132.0 (120.0; 145.0) | 134.0 (120.0; 147.0) | <0.001 |
| Diastolic blood pressure, median (Q1; Q3), mm Hg | 80.0 (70.0; 87.0) | 80.0 (70.0; 89.0) | <0.001 |
| Pulse, median (Q1; Q3), bpm | 82.0 (70.0; 102.0) | 85.0 (71.0; 105.0) | <0.001 |
| Type of atrial fibrillation, n (col %) | |||
| Permanent | 1371 (11.3) | 4312 (15.2) | <0.001 |
| Persistent | 1315 (10.8) | 4757 (16.8) | |
| Paroxysmal | 3402 (28.1) | 7137 (25.2) | |
| Unclassified | 6038 (49.8) | 12 084 (42.7) | |
| Care setting specialty at diagnosis, n (col %) | |||
| Internal medicine/neurology/geriatrics | 2614 (21.6) | 5872 (20.8) | <0.001 |
| Cardiology | 7528 (62.1) | 18 460 (65.3) | |
| Primary care/general practice | 1984 (16.4) | 3958 (14.0) | |
| Care setting location at diagnosis, n (col %) | |||
| Hospital | 7631 (62.9) | 15 593 (55.1) | <0.001 |
| Office/anticoagulation clinic/thrombosis centre | 3204 (26.4) | 9601 (33.9) | |
| Emergency room | 1291 (10.6) | 3096 (10.9) | |
| Medical history, n (col %) | |||
| Heart failure | 3430 (28.3) | 7379 (26.1) | <0.001 |
| Acute coronary syndrome | 1955 (16.2) | 3324 (11.8) | <0.001 |
| Vascular disease | 4523 (37.3) | 7698 (27.2) | <0.001 |
| Carotid occlusive disease | 389 (3.2) | 1028 (3.7) | 0.032 |
| VTE | 225 (1.9) | 901 (3.2) | <0.001 |
| Prior to stroke/TIA/SE | 1518 (12.5) | 4179 (14.8) | <0.001 |
| History of bleeding | 578 (4.8) | 553 (2.0) | <0.001 |
| Hypertension | 9854 (81.3) | 23 670 (83.7) | <0.001 |
| Hypercholesterolaemia | 4779 (40.8) | 12 788 (46.4) | <0.001 |
| Diabetes | 3136 (25.9) | 7775 (27.5) | <0.001 |
| Cirrhosis | 93 (0.8) | 125 (0.4) | <0.001 |
| Moderate to severe CKD | 1393 (12.0) | 3546 (12.9) | 0.008 |
| Dementia | 289 (2.4) | 440 (1.6) | <0.001 |
| Heavy alcohol user, n (col %) | 226 (2.2) | 425 (1.8) | 0.009 |
| Current smoker, n (col %) | 1045 (9.5) | 2214 (8.6) | 0.006 |
| Anticoagulant at baseline, n (col %) | |||
| NOAC±AP | – | 11 351 (40.1) | – |
| VKA±AP | – | 16 939 (59.9) | |
| Antiplatelet treatment, n (col %) | 8227 (67.8) | 6580 (23.3) | <0.001 |
| CHA2DS2-VASc Score, median (Q1; Q3) | 4.0 (3.0; 5.0) | 4.0 (3.0; 5.0) | 0.405 |
| HAS-BLED Score‡, median (Q1; Q3) | 2.0 (1.0; 2.0) | 1.0 (1.0; 2.0) | <0.001 |
| GARFIELD-AF Death Score §, median (Q1; Q3) | 4.6 (2.7; 8.2) | 4.8 (2.9; 8.1) | <0.001 |
| GARFIELD-AF Stroke Score ¶, median (Q1; Q3) | 1.4 (1.0; 2.0) | 1.4 (1.0; 1.9) | <0.001 |
| GARFIELD-AF Bleeding Score **, median (Q1; Q3) | 1.8 (1.3; 2.6) | 1.6 (1.2; 2.3) | <0.001 |
*This study analysed initial treatment of AF patients, regardless of the AF type, which might have been confirmed at later visits.
†Calculated using t-test or Wilcoxon-Mann-Whitney for continuous variables, as appropriate and χ2 or Fisher’s exact test for categorical variables, as appropriate.
‡The risk factor ‘Labile INRs’ is not included in the HAS-BLED Score as it is not collected at baseline. As a result, the maximum HAS-BLED Score at baseline is 8 points (not 9).
§Denotes the expected probability of death within 2 years from enrolment. To allow for comparability, the expected probability is computed assuming all patients received NOAC at baseline;.
¶The expected probability of developing a non-haemorrhagic stroke/SE within 2 years from enrolment. To allow for comparability, the expected probability is computed assuming all patients received NOAC at baseline;.
**The expected probability of developing a major bleeding within 2 years from enrolment. To allow for comparability, the expected probability is computed assuming all patients received NOAC at baseline.
AF, atrial fibrillation; AP, antiplatelet treatment; BMI, body mass index; CKD, chronic kidney disease; GARFIELD-AF, Global Anticoagulant Registry in the FIELD-AF; NOAC, non-vitamin K oral anticoagulant; OAC, oral anticoagulant; SE, systemic embolism; TIA, transient ischaemic attack; VKA, vitamin K antagonist treatment; VTE, venous thromboembolism.
Compared with those who received OAC, OAC non-users were more often of Asian ethnicity and diagnosed in an emergency room setting. OAC non-users also had higher HAS-BLED14 and GARFIELD-AF bleeding scores27 compared with OAC users. Moreover, OAC non-users had a lower prevalence of previous stroke, transient ischaemic attack (TIA) or SE and venous thromboembolism, and a higher prevalence of vascular disease, acute coronary syndrome, dementia and previous bleeding. The most commonly used antiplatelet drugs (AP) were aspirin (~80%), ADP receptor/P2Y12 inhibitors (~20%) and other Cox inhibitors (~10% of patients), irrespective of concomitant OAC therapy. A patient might take more than one type of AP. A comparison of the baseline characteristics of OAC-treated patients receiving vitamin K antagonist (VKA) versus NOAC is shown in online supplemental table S2.
Use of OAC and physician’s explanations for withholding
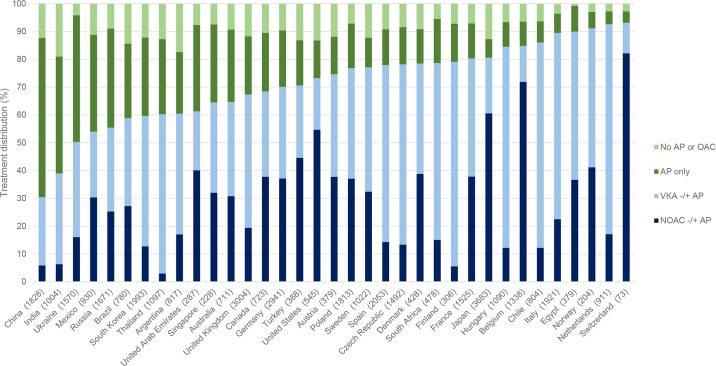
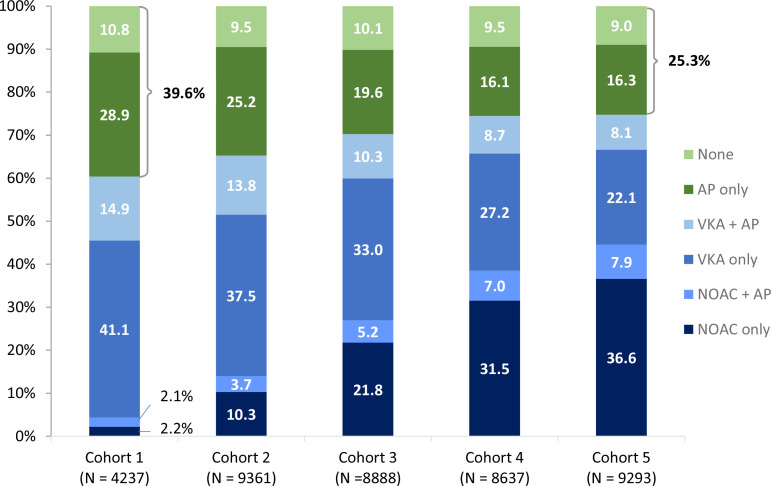
Of all countries in GARFIELD-AF, China and India had the lowest rates of OAC use (≤40%), followed by Ukraine, Mexico, Russia, Brazil and South Korea (figure 1). At the same time, these countries had some of the highest proportions of patients receiving AP alone. Globally, the proportions of patients not receiving OAC at baseline decreased over time from 39.6% in cohort 1 (enrolment period 2010–2011) to 25.3% in cohort 5 (enrolment period 2015–2016). Overall, the proportion of patients on no antithrombotic therapy remained relatively unchanged over time (10.8% in 2010–2011; 9.0% in 2015–2016), but we observed a decline from 28.9% to 16.3% in patients receiving AP therapy only (figure 2), and differences in trends between countries (online supplemental table S3).
Figure 1.
Distribution of treatments at baseline by country. Each column illustrates the proportion of eligible patients (CHA2DS2-VASc Score≥2) across all cohorts in the named country who received either an NOAC (dark blue), a VKA (light blue), only AP therapy (dark green) and no AP or OAC (light green). Countries are sorted from left to right in order of increasing OAC use (the combined blue colours). The total numbers of patients from each country are shown in brackets after the country name. AP, antiplatelet treatment; NOAC, non-vitamin K oral anticoagulant; OAC, oral anticoagulation; VKA, vitamin K antagonist treatment.
Figure 2.
Distribution of baseline treatment by cohort of enrolment in patients eligible for OAC treatment. Blue colours: proportion of patients receiving OAC, green colours: proportion of patients not given OAC treatment. The periods for enrolment were: 2010–2011 (cohort 1), 2011–2013 (cohort 2), 2013–2014 (cohort 3), 2014–2015 (cohort 4), 2014–2015 (cohort 4). AP, antiplatelet treatment; NOAC, non-vitamin K oral anticoagulant; OAC, oral anticoagulation; VKA, vitamin K antagonist treatment.
In 7370 (60.8%) of the 12 126 OAC-untreated patients, the main reason for withholding OAC was documented by the treating physician. Commonly cited reasons were high bleeding risk or previous bleeding event (14.8%), patient choice (12.8%) and low stroke risk (9.8%; despite a CHA2DS2-VASc≥2 excluding sex). The distribution of the reasons given for withholding OAC remained relatively stable throughout the five cohorts of enrolment (data not shown).
Predictors of OAC withholding
Three models were developed starting with clinical and demographic factors (model 1), then adding either country of enrolment (model 2), or the countries’ yearly average health expenditure per person over the enrolment period (model 3). The included predictors and their relative significance, calculated as the Wald χ2–df, are shown in table 2.
Table 2.
Components of the models for predicting withholding OAC with corresponding Wald χ2–df and model C-statistic
| Variable | Wald χ2–df | ||
| Model 1 | Model 2 | Model 3 | |
| Country | – | 2576 | – |
| Country health expenditure * | – | – | 832 |
| Cohort | 518 | 569 | 623 |
| Type of AF | 393 | 404 | 489 |
| History of bleeding | 266 | 263 | 272 |
| Care setting specialty | 422 | 201 | 441 |
| Age | 154 | 148 | 143 |
| Vascular disease | 521 | 99 | 324 |
| Prior to stroke/TIA/SE | 96 | 88 | 86 |
| VTE | 44 | 42 | 38 |
| Dementia | 21 | 40 | 26 |
| BMI | 24 | 36 | 21 |
| Cirrhosis | 13 | 21 | 14 |
| Hypertension | 5 | 16 | 20 |
| Race/ethnicity | 665 | 14 | 304 |
| Care setting location | 156 | 14 | 22 |
| Pulse | 44 | 11 | 28 |
| Hypercholesterolaemia | 45 | 7 | 35 |
| Moderate to severe CKD | 11 | 4 | 7 |
| Diabetes | 10 | 3 | 9 |
| Sex | 9 | 0 | 0 |
| C-statistic | 0.674 | 0.737 | 0.697 |
Model 2 includes all the variables selected in model 1 with the addition of country information. Model 3 includes all the variables selected in model 1 with the addition of country’s average health expenditure per person.
*Health expenditure, purchasing power parity (current international US$) represents the country’s average for the period the country enrolled patients in GARFIELD-AF.
AF, atrial fibrillation; BMI, body mass index; CKD, chronic kidney disease; DF, df; GARFIELD-AF, Global Anticoagulant Registry in the FIELD-AF; SE, systemic embolism; TIA, transient ischaemic attack; VTE, venous thromboembolism.
Cohort number (corresponding to period of enrolment) was the most significant predictor in model 1, and second most significant predictor in models 2 and 3. Country health expenditure was the most significant of all factors in model 3 (χ2−df=832), but did not contain as much information as ‘country’ itself in model 2 (χ2−df=2576). Model 2 (c-index=0.737) was more accurate in predicting treatment decision than model 1 (c-index=0.674) or model 3 (c-index=0.697).
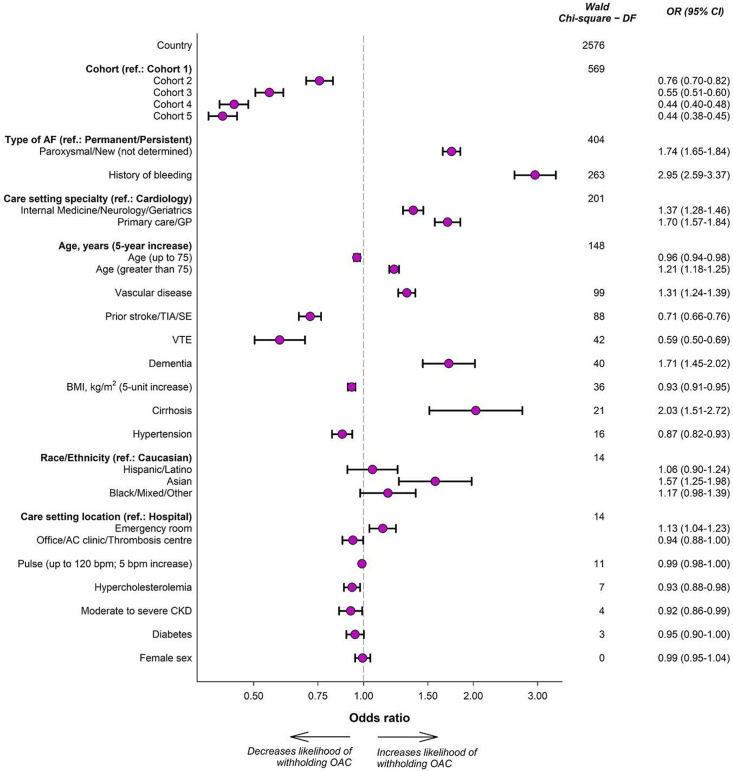
Figure 3 shows ORs and relative significance of the predictors in model 2. The most significant clinical associations were type of AF (χ2−df=404; OR for paroxysmal/new onset vs permanent/persistent=1.74; CI=1.65 to 1.84) and history of bleeding (χ2−df=263; OR=2.95; CI=2.59 to 3.37). History of vascular disease (χ2−df=99; OR=1.31; CI=1.24 to 1.39), history of stroke/TIA/SE (χ2−df=88; OR=0.71; CI=0.66 to 0.76), history of venous thrombosis (χ2−df=42; OR=0.59; CI=0.50 to 0.69), dementia (χ2−df=40; OR=1.71; CI=1.45 to 2.02) and cirrhosis (χ2−df=21; OR=2.03; CI=1.51 to 2.72) were additional factors associated with withholding OAC. The likelihood of withholding OACs decreased with increasing age up to age 75 and increased with increasing age in older individuals (χ2−df=148). Non-cardiologists (χ2−df=201) and physicians in emergency room hospital settings (χ2−df=14) were less likely to prescribe OAC.
Figure 3.
Components of the model predicting withholding of OAC. Associations refer to the model with the inclusion of country information (model 2). ‘Country’ represents the ‘country’ variable, rather than any of the 35 individual countries. Age and BMI are continuous; their ORs illustrate the increased likelihood of withholding OAC for every 5 units increase (eg, going from age 45 to 50 or age 80 to 85). AF, atrial fibrillation; BMI, body mass index; CKD, chronic kidney disease; GP, general practitioner; OAC, oral anticoagulation; SE, systemic embolism; TIA, transient ischaemic attack; VTE, venous thromboembolism.
To test whether the introduction of NOACs modified the risk profile, we repeated the model in cohorts 3–5 only (online supplemental table S4). ‘Country’ remained the dominant component, and the order of 8/9 most significant predictors did not change. The exception was ‘cohort’ which moved from the second to the seventh position, due to a relatively small increase of OAC use from cohort 3–5 (figure 2).
Components of model 3 included country health expenditure information (online supplemental figure S2). Health expenditure per person, averaged across the years of patient enrolment, was the most significant predictor in this model. The corresponding OR (OR 0.79, 95% CI 0.78 to 0.80) indicates that a country health expenditure increase of US$1000 per person is associated to a 21% lower likelihood of withholding OAC in patients eligible for anticoagulation. The relationship between OAC use and health expenditure (averaged across the years of patient enrolment) appeared linear over the range of health expenditure in the included countries (from US$187 to US$8779 per person, data not shown). In our univariable analysis across countries, OAC use, either alone or in combination with AP, correlated positively with average health expenditure per person (online supplemental figure S3).
Discussion
Our key finding is that country and health expenditure were far more significant predictors than ethnicity, demographic and clinical factors, or the period of cohort enrolment. The developed model including country of enrolment as variable had good predictive ability (c-statistic 0.737), whereas the two other models omitting this information performed moderately (c-statistic 0.679 and 0.674, respectively). It therefore appears that country of enrolment was a better predictor than the countries’ health expenditure. The reasons warrant further investigation, but could include the existence of additional country-specific factors, independent of health expenditure. The Global Anticoagulation Roundtable reported that patient-level and physician-level barriers were common across the globe, while system-level barrier had a greater degree of regional variation. Among the latter were under-representation in studies of safety and efficacy, limited use of medical records, anticoagulation management dominated by haematology, socioreligious considerations (Middle East), large differences in access and care between private and public insurance (Latin America), and higher risks of OAC-related bleeding and intercranial haemorrhage (East Asia).28
Of note, patients were recruited for GARFIELD-AF during a time when NOACs were becoming more widely used due to their favourable harm/benefit profile and ease of administration compared with VKAs. This resulted in an overall increase of OAC use in the later cohorts, despite a decline in VKA prescriptions. Also declining was the proportion of patients treated with AP only, as reported previously.7 29 Our findings suggest that country and health expenditure influence prescribing antithrombotic practices. Although beyond the scope of our analysis, this might be due, at least in part, to the higher costs of NOACs which is likely an important barrier for their use in low income countries. For example, a recent Chinese study found that self-paying and duration of AF for five or more years were negatively associated with OAC use, regardless of the risk of stroke.30 Access to specialist advice and free NOAC treatment through a community dwelling Atrial Fibrillation Special Clinic significantly increased OAC use among high-risk patients.31 We did indeed observe a relationship across countries between OAC use and average health expenditure per person. However, a model with country instead of health expenditure as additional component was more accurate, suggesting that the precise factors contributing to intercountry differences remain to be identified. Our results reinforce that the healthcare context is an important consideration when implementing of evidence into practice.
The enrolling physicians were asked to report the strongest reason why no OAC was given to a patient. Frequently named were a perceived high risk of bleeding and low risk of stroke. This is in contrast to the predicted risks of non-anticoagulated patients in this study, all of whom had a CHA2DS2-VASc Score≥2, and only 3.4% had a HAS-BLED Score>3 at baseline. According to their physicians, 9% of patients were deprived of OAC because they were already taking an antiplatelet drug, which is inferior to OAC for stroke prevention.32 Fall risk accounted for 6% of the patients who were not anticoagulated despite major educational efforts to reassure physicians that stroke prevention outweighs the risk of from falling.33 Differences in the perception of risks and benefits between medical specialities might contribute to the relative reluctance of physicians in primary care compared with cardiologists to initiate OAC treatment.10 In addition, 13% of patients chose not to take OAC, which could have been due to adverse effects, personal costs or sociocultural factors.8 34
We and others previously reported withholding of OAC in 25%–30% of patients,17 or off-label prescription of lower doses,35 36 despite data supporting the efficacy and safety of OAC in AF. Moreover, the results of this study are in keeping with prior studies across the globe demonstrating that prior bleeding37 38 and concurrent vascular disease, usually treated with antiplatelet therapy,38 39 are strong risk factors for not using OACs in eligible patients.38 39 Misperceptions regarding the efficacy of aspirin are a major reason for underutilising OAC.40 A study performed in 2013 found that approximately 35% of AF patients on AP had no obvious indication for their use. Bleeding rates were significantly higher in patients on OAC plus aspirin compared with those on OAC alone.41 Several studies also showed that patients with dementia and alcohol or drug abuse were less likely to receive OAC.37 42
OAC use in our patients peaked around 75 years and decreased both with younger and older age. Similarly, a report from the GLORIA-AF (Global Registry on Long‐Term Oral Antithrombotic Treatment in Patients With Atrial Fibrillation) global registry found a slightly higher frequency of OAC treatment in patients aged 75–84 years compared with both younger and older patients.43 In contrast to a meta-analysis of observational studies by Baczek et al,37 we did not find that the presence of renal disease was associated with a decreased likelihood of receiving OAC. However, substantial statistical heterogeneity existed in the meta-analysis. This could reflect variability in the populations studied, the methods used, or definitions of renal impairment, among other factors. Importantly, our study showed that non-patient specific factors, namely time of enrolment and country, were the most significant predictors of OAC treatment in AF patients at a high risk of stroke and SE. Similar observations were made for choice of NOAC versus VKA in AF patients not selected by CHA2DS2-VASc Score.44
Clinical predictors of OAC non-use not only differ with respect to their associated morbidity and mortality, but can be perceived differently by patients and physicians.45–49 Some studies suggest that patients may have a higher risk tolerance for bleeding than stroke, whereas physicians may overestimate bleeding risk when making decisions about OACs.48 50 Therefore, shared decision-making with AF patients including individualised discussions about the potential benefits and harms of OAC for stroke prevention should be advocated.
A limitation of this study is that enrolment in GARFIELD-AF was completed in 2016 when many countries were still in the process of adopting the new guidelines for OAC use. However, recent studies have shown that OACs continue to be underused particularly in Asian countries.12 Our analysis was limited to the examination of antithrombotic agents chosen by treating physicians for the initial treatment of newly diagnosed AF, and did not consider dosing, time on treatment, or possible changes in treatments over time. Moreover, because treatment was not randomly assigned, unobserved baseline confounding cannot be excluded and our inferences should not be interpreted as causal. We asked physicians for the reasons of their treatment choices, but did not collect data on whether a multidisciplinary team approach had been taken, or whether patients had been involved in the decision-making. Newer guidelines for the treatment of AF recommend these procedures, but the impact of this on NOAC use has not been investigated.
Conclusion
In summary, the present analysis of a large international prospective cohort of AF patients confirmed that OAC use was increasing globally over time over the period 2010–2016. Nevertheless, 30% of patients who would be expected to benefit from OAC did not receive them. Non-patient specific factors were the most powerful predictors of OAC non-use, including the country in which the patient was treated, country health expenditure and specialty of clinician managing the patient.
The study highlights the importance of country-specific and socioeconomic factors for AF patients receiving OAC treatment. We hope that its results will stimulate further research and discussion, leading to policy changes that improve patient access to appropriate stroke prevention worldwide.
Acknowledgments
We would like to thank the physicians, nurses and patients involved in the GARFIELD-AF registry and Thomas Weissensteiner (Thrombosis Research Institute, London, UK) for help with writing the manuscript.
Footnotes
Twitter: @DebSiegal, @antithrombosis
Contributors: DS: conceptualisation, writing—original draft and writing—review/editing. FHV, AC-M, BJG, SG, AGGT and PA: conceptualisation and writing—review/editing. SV: methodology and formal analysis. JC and KAAF: conceptualisation, writing—review/editing and supervision. KP: conceptualisation, methodology, formal analysis and writing—review/editing. DS accepts responsibility as the guarantor of this study.
Funding: This work was supported by the Thrombosis Research Institute (London, UK). DS is supported by a Tier 2 Canada Research Chair in Anticoagulant Management of Cardiovascular Disease.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Requests for patient level data can be made to SV, head of statistics at the Thrombosis Research Institute (svirdone@tri-london.ac.uk). These requests should include a protocol summary and a summary of the statistical analysis plan. The request will be reviewed by the data sharing committee for approval and next steps will be discussed with the requestor.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. The GARFIELD-AF protocol was approved by independent ethics committees and/or hospital-based institutional review boards at hundreds of sites worldwide. A complete list can be made available on request. The study was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization, Good Pharmaco epidemiological and Clinical Practice Guidelines, and local regulatory requirements. Participants gave informed consent to participate in the study before taking part.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–8. 10.1161/01.str.22.8.983 [DOI] [PubMed] [Google Scholar]
- 2.Quinn GR, Severdija ON, Chang Y, et al. Wide variation in reported rates of stroke across cohorts of patients with atrial fibrillation. Circulation 2017;135:208–19. 10.1161/CIRCULATIONAHA.116.024057 [DOI] [PubMed] [Google Scholar]
- 3.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 4.Wolfes J, Ellermann C, Frommeyer G, et al. Evidence-based treatment of atrial fibrillation around the globe: comparison of the latest ESC, AHA/ACC/HRS, and CCS guidelines on the management of atrial fibrillation. Rev Cardiovasc Med 2022;23:56. 10.31083/j.rcm2302056 [DOI] [PubMed] [Google Scholar]
- 5.January CT, Wann LS, et al. , Writing Group Members . AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society. Heart Rhythm 2019;16:e66–93. 10.1016/j.hrthm.2019.01.024 [DOI] [PubMed] [Google Scholar]
- 6.Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation. Chest 2018;154:1121–201. 10.1016/j.chest.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 7.Steinberg BA, Gao H, Shrader P, et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J 2017;194:132–40. 10.1016/j.ahj.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 8.Kozieł M, Teutsch C, Bayer V, et al. Changes in anticoagulant prescription patterns over time for patients with atrial fibrillation around the world. J Arrhythm 2021;37:990–1006. 10.1002/joa3.12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox KAA, Virdone S, Bassand J-P, et al. Do baseline characteristics and treatments account for geographical disparities in the outcomes of patients with newly diagnosed atrial fibrillation? The prospective GARFIELD-AF Registry. BMJ Open 2022;12:e049933. 10.1136/bmjopen-2021-049933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayer V, Kotalczyk A, Kea B, et al. Global oral anticoagulation use varies by region in patients with recent diagnosis of atrial fibrillation: the GLORIA-AF phase III Registry. J Am Heart Assoc 2022;11:e023907. 10.1161/JAHA.121.023907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazurek M, Huisman MV, Rothman KJ, et al. Regional differences in antithrombotic treatment for atrial fibrillation: insights from the GLORIA-AF phase II Registry. Thromb Haemost 2017;117:2376–88. 10.1160/TH17-08-0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romiti GF, Corica B, Proietti M, et al. Patterns of oral anticoagulant use and outcomes in Asian patients with atrial fibrillation: a post-hoc analysis from the GLORIA-AF Registry. EClinicalMedicine 2023;63:102039. 10.1016/j.eclinm.2023.102039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang L, Zhang L, Guo Y, et al. A review of biomarkers for ischemic stroke evaluation in patients with non-valvular atrial fibrillation. Front Cardiovasc Med 2021;8:682538. 10.3389/fcvm.2021.682538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro heart survey. Chest 2010;138:1093–100. 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 15.Gheorghe GS, Hodorogea AS, Gheorghe ACD, et al. Decision of anticoagulation in nonvalvular atrial fibrillation in the real world in the non-antivitamin K anticoagulants era. Healthcare (Basel) 2022;10:1333. 10.3390/healthcare10071333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallakati A, Lewis WR. Underuse of anticoagulation in patients with atrial fibrillation. Postgrad Med 2016;128:191–200. 10.1080/00325481.2016.1132939 [DOI] [PubMed] [Google Scholar]
- 17.Camm AJ, Accetta G, Ambrosio G, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart 2017;103:307–14. 10.1136/heartjnl-2016-309832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidbuchel H. The search for the tipping point on when to anticoagulate patients with atrial fibrillation. Heart 2017;103:181–3. 10.1136/heartjnl-2016-310325 [DOI] [PubMed] [Google Scholar]
- 19.Hess PL, Kim S, Fonarow GC, et al. Absence of oral anticoagulation and subsequent outcomes among outpatients with atrial fibrillation. Am J Med 2017;130:449–56. 10.1016/j.amjmed.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 20.Turner GM, Calvert M, Feltham MG, et al. Under-prescribing of prevention drugs and primary prevention of stroke and transient ischaemic attack in UK general practice: a retrospective analysis. PLoS Med 2016;13:e1002169. 10.1371/journal.pmed.1002169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yogasundaram H, Dover DC, Hawkins NM, et al. Trends in uptake and adherence to oral anticoagulation for patients with incident atrial fibrillation at high stroke risk across health care settings. J Am Heart Assoc 2022;11:e024868. 10.1161/JAHA.121.024868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao X, Abraham NS, Alexander GC, et al. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc 2016;5:e003074. 10.1161/JAHA.115.003074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakkar AK, Mueller I, Bassand J-P, et al. International longitudinal registry of patients with atrial fibrillation at risk of stroke: global anticoagulant Registry in the FIELD (GARFIELD). Am Heart J 2012;163:13–19. 10.1016/j.ahj.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 24.Cheung CC, Andrade JG. Reversible or provoked atrial fibrillation?: the devil in the details. JACC Clin Electrophysiol 2018;4:563–4. 10.1016/j.jacep.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 25.Bank W. Purchasing power parities and the real size of world economies: a comprehensive report of the 2011 International Comparison Program. The World Bank, 2014. [Google Scholar]
- 26.Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res 2015;4:287–95. 10.6000/1929-6029.2015.04.03.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox KAA, Virdone S, Pieper KS, et al. GARFIELD-AF risk score for mortality, stroke, and bleeding within 2 years in patients with atrial fibrillation. Eur Heart J Qual Care Clin Outcomes 2022;8:214–27. 10.1093/ehjqcco/qcab028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes GD, Piazza G, Global Anticoagulation Roundtable Working Group . Barriers to stroke prevention in atrial fibrillation: insights from the global anticoagulation roundtable. Int J Cardiol Heart Vasc 2022;42:101096. 10.1016/j.ijcha.2022.101096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrignani MG, Lombardo A, Braschi A, et al. Time trends in antithrombotic therapy prescription patterns: real-world monocentric study in hospitalized patients with atrial fibrillation. World J Cardiol 2022;14:576–98. 10.4330/wjc.v14.i11.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu T, Yang H-L, Gu L, et al. Current status and factors influencing oral anticoagulant therapy among patients with non-valvular atrial fibrillation in Jiangsu province, China: a multi-center, cross-sectional study. BMC Cardiovasc Disord 2020;20:22. 10.1186/s12872-020-01330-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau KM, Leung TF, Li YC, et al. The effectiveness of atrial fibrillation special clinic on oral anticoagulant use for high risk atrial fibrillation patients managed in the community. BMC Prim Care 2023;24:48. 10.1186/s12875-023-02004-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang JT, Chen KP, Zhang S. Efficacy and safety of oral anticoagulants versus aspirin for patients with atrial fibrillation: a meta-analysis. Medicine (Baltimore) 2015;94:e409. 10.1097/MD.0000000000000409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanah L, Kabashneh S, Alkassis S, et al. Use of anticoagulants in patients with non-valvular atrial fibrillation who are at risk of falls. Cureus 2020;12:e10336. 10.7759/cureus.10336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apenteng P, Virdone S, Camm J, et al. Determinants and clinical outcomes of patients who refused anticoagulation: findings from the global GARFIELD-AF Registry. Open Heart 2023;10:e002275. 10.1136/openhrt-2023-002275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinberg BA, Shrader P, Thomas L, et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol 2016;68:2597–604. 10.1016/j.jacc.2016.09.966 [DOI] [PubMed] [Google Scholar]
- 36.Arbel R, Sergienko R, Hammerman A, et al. Effectiveness and safety of off-label dose-reduced direct oral anticoagulants in atrial fibrillation. Am J Med 2019;132:847–55. 10.1016/j.amjmed.2019.01.025 [DOI] [PubMed] [Google Scholar]
- 37.Baczek VL, Chen WT, Kluger J, et al. Predictors of warfarin use in atrial fibrillation in the United States: a systematic review and meta-analysis. BMC Fam Pract 2012;13:5. 10.1186/1471-2296-13-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savarese G, Sartipy U, Friberg L, et al. Reasons for and consequences of oral anticoagulant Underuse in atrial fibrillation with heart failure. Heart 2018;104:1093–100. 10.1136/heartjnl-2017-312720 [DOI] [PubMed] [Google Scholar]
- 39.Lip GYH, Laroche C, Dan G-A, et al. Real-world' Antithrombotic treatment in atrial fibrillation: the EORP-AF pilot survey. Am J Med 2014;127:519–29. 10.1016/j.amjmed.2013.12.022 [DOI] [PubMed] [Google Scholar]
- 40.Ben Freedman S, Gersh BJ, Lip GYH. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant Underutilization in atrial fibrillation. Eur Heart J 2015;36:653–6. 10.1093/eurheartj/ehu494 [DOI] [PubMed] [Google Scholar]
- 41.Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the outcomes Registry for better informed treatment of atrial fibrillation (ORBIT-AF) Registry. Circulation 2013;128:721–8. 10.1161/CIRCULATIONAHA.113.002927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia X, Wang L, Lin T, et al. Barriers to prescribing oral anticoagulants to Inpatients aged 80 years and older with Nonvalvular atrial fibrillation: a cross-sectional study. BMC Geriatr 2022;22:263. 10.1186/s12877-022-02965-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazurek M, Halperin JL, Huisman MV, et al. Antithrombotic treatment for newly diagnosed atrial fibrillation in relation to patient age: the GLORIA-AF Registry programme. Europace 2020;22:47–57. 10.1093/europace/euz278 [DOI] [PubMed] [Google Scholar]
- 44.Haas S, Camm AJ, Bassand J-P, et al. Predictors of NOAC versus VKA use for stroke prevention in patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Am Heart J 2019;213:35–46. 10.1016/j.ahj.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 45.MacLean S, Mulla S, Akl EA, et al. Patient values and preferences in decision making for Antithrombotic therapy: a systematic review: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e1S–e23S. 10.1378/chest.11-2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegal DM, Healey JS. Shared decision-making for anticoagulation in atrial fibrillation: do physicians really listen. Can J Cardiol 2020;36:459–61. 10.1016/j.cjca.2019.11.008 [DOI] [PubMed] [Google Scholar]
- 47.Loewen PS, Ji AT, Kapanen A, et al. Patient values and preferences for antithrombotic therapy in atrial fibrillation. Thromb Haemost 2017;117:1007–22. 10.1160/TH16-10-0787 [DOI] [PubMed] [Google Scholar]
- 48.Alonso-Coello P, Montori VM, Díaz MG, et al. Values and preferences for oral antithrombotic therapy in patients with atrial fibrillation: physician and patient perspectives. Health Expect 2015;18:2318–27. 10.1111/hex.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinto CA, Chua GN, Bridges JFP, et al. Comparing patient preferences for antithrombotic treatment during the acute and chronic phases of myocardial infarction: a discrete-choice experiment. Patient 2022;15:255–66. 10.1007/s40271-021-00548-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devereaux PJ, Anderson DR, Gardner MJ, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ 2001;323:1218–22. 10.1136/bmj.323.7323.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dixon JR. THE international conference on harmonization good clinical practice guideline. Quality Assurance 1999;6:65–74. 10.1080/105294199277860 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2023-002506supp001.pdf (622.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Requests for patient level data can be made to SV, head of statistics at the Thrombosis Research Institute (svirdone@tri-london.ac.uk). These requests should include a protocol summary and a summary of the statistical analysis plan. The request will be reviewed by the data sharing committee for approval and next steps will be discussed with the requestor.