Abstract
Objectives
This study aimed to evaluate the effectiveness, safety and costs of FreeStyle Libre (FSL) glucose monitoring system for children and adolescents with type 1 diabetes mellitus (T1DM) in Spain.
Design
Prospective, multicentre pre-post study.
Setting
Thirteen Spanish public hospitals recruited patients from January 2019 to March 2020, with a 12-month follow-up.
Participants
156 patients were included.
Primary and secondary outcome measures
Primary: glycated haemoglobin (HbA1c) change. Secondary: severe hypoglycaemic events (self-reported and clinical records), quality of life, diabetes treatment knowledge, treatment satisfaction, adverse events, adherence, sensor usage time and scans. Healthcare resource utilisation was assessed for cost analysis from the National Health System perspective, incorporating direct healthcare costs. Data analysis used mixed regression models with repeated measures. The intervention’s total cost was estimated by multiplying health resource usage with unit costs.
Results
In the whole sample, HbA1c increased significantly (0.32%; 95% CI 0.10% to 0.55%). In the subgroup with baseline HbA1c≥7.5% (n=88), there was a significant reduction at 3 months (−0.46%; 95% CI −0.69% to −0.23%), 6 months (−0.49%; 95% CI −0.73% to −0.25%) and 12 months (−0.43%; 95% CI −0.68% to –0.19%). Well-controlled patients had a significant 12-month worsening (0.32%; 95% CI 0.18% to 0.47%). Self-reported severe hypoglycaemia significantly decreased compared with the previous year for the whole sample (−0.37; 95% CI −0.62 to –0.11). Quality of life and diabetes treatment knowledge showed no significant differences, but satisfaction increased. Adolescents had lower sensor usage time and scans than children. Reduction in HbA1c was significantly associated with device adherence. No serious adverse effects were observed. Data suggest that use of FSL could reduce healthcare resource use (strips and lancets) and costs related to productivity loss.
Conclusions
The use of FSL in young patients with T1DM was associated with a significant reduction in severe hypoglycaemia, and improved HbA1c levels were seen in patients with poor baseline control. Findings suggest cost savings and productivity gains for caregivers. Causal evidence is limited due to the study design. Further research is needed to confirm results and assess risks, especially for patients with lower baseline HbA1c.
Keywords: paediatric endocrinology, public health, health economics
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study provides nationally contextualised real-world scientific evidence on the effectiveness, safety and costs of the flash glucose monitoring systems (FreeStyle Libre (FSL)) indicated for type 1 diabetes in childhood and adolescence in Spain.
The study used a combination of self-reported outcomes, clinical data extracted from electronic health records and device-stored information from the FSL devices, which provides a robust and multifaceted assessment of the outcomes.
The uncontrolled design of the study precludes causal inferences and results from randomised trials are needed to draw definitive conclusions.
The small sample size limits the generalisability and statistical power of the findings.
The cost estimation analysis only considered direct healthcare costs from the Spanish National Health System perspective, and indirect costs were not fully taken into account, which may underestimate the overall economic impact of the intervention.
Introduction
Type 1 diabetes mellitus (T1DM) requires continuous medical monitoring, to reduce the development of vascular complications.1 2 The early onset and chronic character of this condition increase the likelihood of reducing health-related quality of life (HRQoL) and health expectancy among young T1DM people.3 A total of 586 000 children aged under 15 years suffer from T1DM globally.4 In Spain, the incidence is 11.5–27.6/100 000,5 which represents a high cost to society.6
To reduce the risk of short (metabolic) and long-term (vascular) diabetes complications, frequent determination of blood glucose levels is required. Continuous glucose monitoring systems, such as the flash glucose monitoring (FGM) systems, contribute to glycaemia monitoring, as well as to reduce the daily number of fingersticks,7 providing dynamic information to the users about their glucose level. FreeStyle Libre (FSL), developed and marketed in Spain by Abbott Laboratories, has been indicated to measure glucose levels in the interstitial fluid in people aged over 4 years with T1DM. No serious adverse effects related to the use of these devices have been reported. Mild effects consist of skin problems in the area where the sensor is inserted, similar to other FGM.8 9
In randomised trials, the FSL system has been shown to significantly reduce glycated haemoglobin (HbA1c) levels and the frequency of hypoglycaemia in patients with type 2 diabetes mellitus (T2DM), compared with the conventional finger-pricking method.10 In T1DM, most published studies had an uncontrolled design, and meta-analyses have revealed that the use of FSL is associated with significant HbA1c reductions from baseline to the last follow-up.11 12 Approximately, 30% of these studies included children and adolescents, which also led to obtaining significant pre-post HbA1c reductions. To the best of our knowledge, only one randomised trial has evaluated the FSL versus conventional glucose measurement in non-adults (13–20 years) with T1DM,13 showing no significant results on HbA1c or quality of life.13
Spain has a universal public health system, financed by taxes. The system is highly decentralised and the 17 Spanish administrative regions have their own health policy budget, which enables a tailored approach to meet the specific needs and demands of each region. The competences and portfolio of the Spanish Ministry of Health encompass a wide range of responsibilities aimed at ensuring the well-being and health of the population. These include policy development, regulation and oversight of healthcare services, public health initiatives, pharmaceutical regulation, health technology assessment and coordination of emergency responses, among others. The Spanish Network of Health Technology Assessment Agencies of the National Health System (RedETS),14 published a report in 2016,15 later updated in 2017,16 devised by the Canary Islands Health Service Evaluation Department (SESCS),17 about the effectiveness, safety and cost-effectiveness of FSL in patients with T1DM and T2DM. In 2019, the Spanish Ministry of Health decided to fund FSL for adult patients with T1DM,18 and in 2020, the reimbursement was extended to any insulin-dependent patient not diagnosed with T1DM or T2DM.19
Regarding children and adolescents with T1DM, the Spanish Ministry of Health decided to perform a postlaunch evidence generation study to provide real-world information in the Spanish context on the effectiveness, safety, acceptability and potential use barriers, as well as on healthcare resources use and costs, to inform health policy decision-making on a national level in regard to coverage and public funding in these population groups.20 21 This paper reports its results.
Methods
Study design
Prospective, multicentre, pre-post study performed in 13 public hospitals throughout Spain (see online supplemental appendix 1). Patients were recruited between January 2019 and March 2020, with a 12-month follow-up.
bmjopen-2022-071334supp001.pdf (55.6KB, pdf)
Interventions
FSL consists of: (1) an arm sensor that measures and stores interstitial glucose levels, wearable for 14 days22 and (2) a reader that obtains glucose readings from the sensor when placed at a distance between 1 and 4 cm, storing up to 90 days of glucose measures and user-entered notes. The Libre View software and the FSL Link, and LibreLinkUp Apps enable obtaining reports with the daily patterns of glucose levels.
Participants
Patients were eligible for inclusion if they were aged between 4 and 17, had been diagnosed with T1DM for at least 1 year prior to the study, were receiving intensive insulin therapy, required more than six fingersticks per day and provided their informed consent to participate.
We excluded patients who had hypoglycaemia unawareness (judged by the clinician), were currently undergoing systemic corticosteroid treatment for more than 2 weeks within the last 3 months, had previously used or were currently using an FGM device within the last 12 months, were pregnant adolescents, had allergies to device adhesives, were unwilling to participate, lacked the necessary skills to effectively use the technology (patient/caregiver) or failed to provide informed consent.
Setting, logistics and recruitment
The study protocol was devised by SESCS researchers with the assistance of clinical experts from all hospitals taking part, patient association and industry representatives. A centralised information system (Monitoring Studies Information System,MSIS) was developed on the Spanish Ministry of Health’s intranet, accessible both for the clinical researchers responsible for recruitment, clinical examination and data collection, as well as SESCS researchers.
Clinical researchers from hospitals taking part were responsible for recruiting, informing and training both patients and caregivers. They collected self-reported data using various measurement scales and extracted clinical information from the electronic health record (EHR) at baseline, 3, 6 and 12 months. In addition, they retrieved the stored information from the FSL device during the follow-up phase (3, 6 and 12 months) on the MSIS platform. SESCS researchers were responsible for coordinating the project and supervising data collection, monitoring quality assurance and data validation, analyses and reporting.
Interested Spanish autonomous communities designated the hospitals they wished to take part in the study. Thirteen public hospitals were included between January 2019 and May 2020, distributed over eight Spanish autonomous communities.
Endpoints
Effectiveness
The primary endpoint was the change in HbA1c level from baseline to follow-up. Secondary endpoints included: (1) data extracted from the EHR at baseline and 3, 6 and 12 months: number of severe hypoglycaemia events (defined as those that require help from another person), ketoacidosis episodes, number of hospital admissions and mortality and (2) self-reported outcomes evaluated at baseline and at 12 months follow-up, by means of the EuroQoL 5-Dimension - Youth version (EQ-5D-Y) questionnaire23; with five categories, reporting the level of severity, ranging from 1 (‘I have no problems’) to 5 (‘I have a lot of problems’) in terms of mobility, self-care, activities of daily living, pain/discomfort and anxiety/depression. Furthermore, a Visual Analogue Scale (VAS) measured self-perceived general health, ranging from ‘0’ (worst health status) to ‘100’ (best health status).
Knowledge of diabetes treatment was measured by means of a modified version of the questionnaire devised by Mitchell et al.24 This includes 14 items evaluating basic theoretical knowledge about the management of T1DM and its treatment, as well as the patient/caregiver’s self-perceived involvement in self-care. The final score is the sum of correct answers (range 0–14). To measure satisfaction with treatment, we used the six-item Diabetes Treatment Satisfaction Questionnaire.25 Response options range from 0 (very dissatisfied) to 6 (very satisfied) (range 0–36). Another two items measured the perceived frequency of hyperglycaemia and hypoglycaemia on a scale from 0 (never perceived) to 6 (most of the time).
Safety
Patients’ self-reported device-related adverse events were collected at 3, 6 and 12 months of follow-up.
Adherence
To measure device adherence, the following variables were evaluated: (1) number of daily scans, (2) sensor usage time (percentage) and (3) number of sensors used. These data were collected throughout the follow-up phase by means of the information stored in the device.
Use of healthcare resources
Data were extracted from the EHR at baseline and at 12 months of follow-up on: (1) number of hospitalisations, (2) number of clinic visits (endocrinology, nursing, primary care/paediatrics, emergency), (3) number of HbA1c assays, (4) number of test strips and lancets used and (5) absenteeism from work (number of days the caregiver was absent from work due to problems related to the child’s T1DM).
In addition to these measures, information on age, sex, body mass index, time since diagnosis, presence of comorbidities and pubertal stage according to the Tanner scale,26 which classifies patients into five stages ranging from stage 1 (childhood) to 5 (adult), was systematically collected.
Sample size calculation
We estimated a sample size requirement of 43 participants to detect a minimal clinically relevant change in HbA1c of 0.5%,27 assuming 95% confidence level, 80% power, an HbA1c SD of 1, a pre-post correlation of 0.5 (conservative assumption) and a loss rate of 20%. In addition to the main effect in the whole sample, we were also interested in the effect of the intervention on subgroups defined by their baseline HbA1c level (greater or less than 7.5%), and age (<12 vs ≥12 years). However, the analysis of interactions requires larger sample sizes to attain statistical power, which was not feasible within the study’s time limits. Therefore, we aimed to multiply the sample at least by 4 (n=172) to increase the statistical power as much as possible.
Statistical analysis
Means and SD were estimated for continuous variables, and count and percentage for qualitative variables. Baseline characteristics of patients were compared using Student’s t-test, Pearson χ2, Fisher’s exact test or Cochran Q, according to the type of variables.
Mixed regression models with repeated measures were used, adjusting for the interaction between time and baseline HbA1c (dichotomous variable) and age group, time and its main effects. The duration of the disease and the existence of comorbidities were included as covariates. A linear link function was used for continuous dependent variables, a logistic function for dichotomous dependent variables and a Poisson function for count dependent variables. In the models with significant interaction, mixed regression models were performed for each interaction subgroup.
The relationship between adherence to the device and HbA1c reduction was analysed using two mixed linear regression models, whose independent variables were the percentage of time using the sensor (12 months) and the number of monthly scans; basal HbA1c level was introduced as a covariable. Intercept was introduced as a random effect in all models.
For missing values during follow-up, a comparability analysis was conducted between participants lost to follow-up and those who remained, prior to performing multiple imputation by chained equations using Stata V.15.0. The details of this comparability analysis and the imputation model can be found in online supplemental appendix 2.
bmjopen-2022-071334supp002.pdf (71.5KB, pdf)
A level of 0.05 was considered statistically significant. Analyses were performed with the statistical software Stata V.15.028 and SPSS V.20.0.29
Cost estimation
Intervention costs were estimated from the Spanish National Health System (NHS) perspective, including only direct healthcare costs during the 12 months of the study. The healthcare resources collected in this study, together with the corresponding unit costs and their information sources, can be found in online supplemental appendix 3 table A1. Costs were expressed as 2021 euros (€). When necessary, we adjusted for the consumer price index, using the Spanish National Statistics Institute (INE, for its acronym in Spanish)—the INE’s income conversion tool.30
bmjopen-2022-071334supp003.pdf (93.8KB, pdf)
Unit cost of test strips and lancets were estimated with the average costs of information provided by different regional health services of the Spanish NHS. Total costs were estimated by multiplying the collected data on health resources used by their respective unit costs and then added.
Descriptive statistics are presented for total costs aggregated and broken down into: primary care visits (nursing and physicians), emergency visits (hospital and non-hospitals), specialist physicians visits, laboratory tests (HbA1c assay) and monitoring instruments (FSL sensor and test strips and lancets).
Given the nature of the costs and their non-normal nature, CIs were estimated using a non-parametric bootstrapping method.31 Analyses were performed using the statistical software SPSS V.20.029 with the help of Microsoft Excel.
In addition, although the social perspective was not taken into account in this estimate, indirect technology costs were reported using the human capital theory, that is, considering the costs attributed to productivity losses of the parents or caregivers of the child with T1DM before and after 1 year of using the FSL.
To estimate the cost per day of absenteeism, the cost per hour worked in Spain published by the Statistical Office of the European Union (Eurostat)32 was multiplied by the average number of daily working hours worked in Spain published in the INE’s Labour Force Survey (LFS).33
Patient and public involvement
There was no patient involvement in the design of this study. Clinical experts from all participant hospitals, representatives of patient associations and the industry took part in drawing up the protocol. We undertook with healthcare professionals to share the results with them in an easy-to-understand way.
Results
A total of 165 patients were initially registered for the study. However, nine patients were subsequently excluded as they did not meet the study’s inclusion criteria (figure 1). Therefore, the final analysis included a total of 156 patients.
Figure 1.
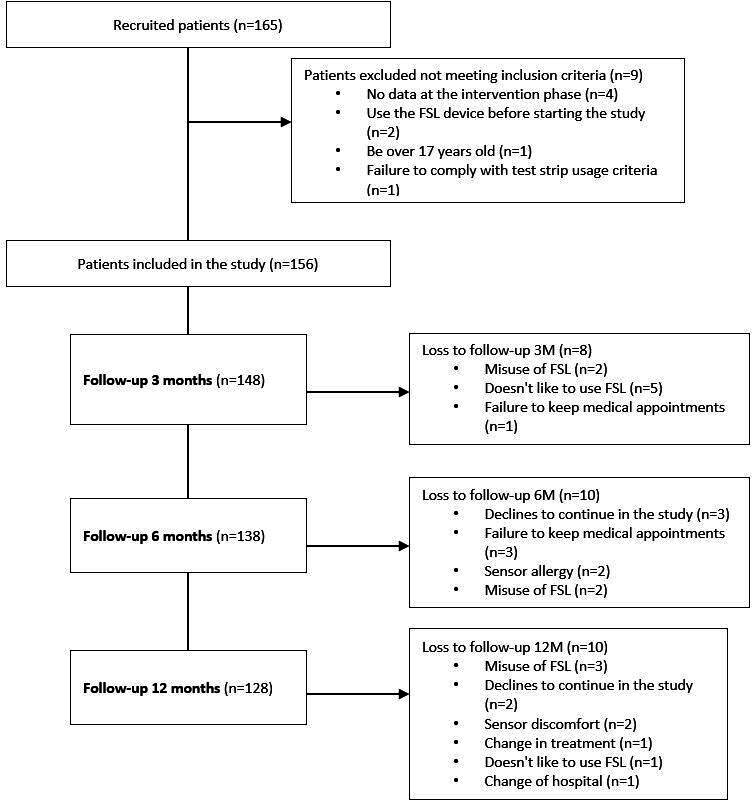
Study flow charts. FSL, FreeStyle Libre.
Patient baseline characteristics are shown in table 1 according to subgroups by level of metabolic control and age. There was a higher percentage of participants in stage 1 and five in the subgroup with worse glycaemic control (p=0.02). In this subgroup, the mean HbA1c value was 8.7%; with 6.8% (p<0.001) in the well-controlled group.
Table 1.
Baseline characteristics of patients according to baseline HbA1c and age groups
| Total (n=156) | HbA1c<7.5% (n=68) | HbA1c≥7.5% (n=88) | P value | <12 years (n=53) | ≥12 years (n=103) | P value | |
| Anthropometric characteristics | |||||||
| Sex (male) n (%) | 86 (55.1) | 35 (51.5) | 51 (58) | 0.419 | 28 (52.8) | 58 (56.3) | 0.679 |
| Age (years), mean (SD) | 12.6 (3.2) | 12.7 (2.84) | 12.49 (3.39) | 0.735 | NA | NA | NA |
| Children<12 years, n (%) | 53 (34) | 21 (30.9) | 32 (36.4) | 0.474 | NA | NA | NA |
| BMI (kg/m2), mean (SD) | 20.3 (4.1) | 20.18 (3.34) | 20.39 (4.54) | 0.754 | NA | NA | NA |
| Pubertal status, n (%) | 0.022 | <0.001 | |||||
| I | 51 (32.7) | 19 (27.9) | 32 (36.4) | 44 (83) | 7 (6.8) | ||
| II | 14 (9.0) | 9 (13.2) | 5 (5.7) | 4 (7.5) | 10 (9.7) | ||
| III | 20 (12.8) | 7 (10.3) | 13 (14.8) | 4 (7.5) | 16 (15.5) | ||
| IV | 23 (14.7) | 16 (23.5) | 7 (8) | 0 (0) | 23 (22.3) | ||
| V | 48 (30.8) | 17 (25) | 31 (35.2) | 1 (1.9) | 47 (45.6) | ||
| Clinical characteristics | |||||||
| Duration of diabetes (years), mean (SD) | 5.65 (3.39) | 5.52 (3.35) | 5.75 (3.44) | 0.671 | 4.06 (2.4) | 6.47 (3.54) | <0.001 |
| HbA1c, mean (SD) | 7.86 (1.36) | 6.82 (0.36) | 8.65 (1.31) | NA | 7.83 (1.17) | 7.87 (1.45) | 0.87 |
| HbA1c<7.5%, n (%) | 68 (43.6) | NA | NA | 21 (39.6) | 47 (45.6) | 0.474 | |
| Presence of comorbidities, n (%) | 50 (32.1) | 27 (39.7) | 23 (26.1) | 0.072 | 17 (32.1) | 33 (32) | 0.996 |
| Comorbidities, n (%) | |||||||
| Asthma | 6 (3.8) | 5 (7.4) | 1 (1.1) | 0.199 | 1 (1.9) | 5 (4.9) | 0.65 |
| Coeliac disease | 8 (5.1) | 6 (8.8) | 2 (2.3) | 0.261 | 5 (9.4) | 3 (2.9) | 0.102 |
| Thyroiditis | 18 (11.5) | 12 (17.6) | 6 (6.8) | 0.178 | 6 (11.3) | 12 (11.7) | 0.941 |
| ADHD | 4 (2.6) | 1 (1.5) | 3 (3.4) | 0.322 | 1 (1.9) | 3 (2.9) | 0.999 |
| Others | 19 (12.2) | 7 (10.3) | 12 (13.6) | 0.057 | 5 (9.4) | 14 (13.6) | 0.369 |
Other comorbidities: allergy, obesity, iron-deficiency anaemia, unilateral anorchia, IgA deficiency, intellectual disability, epilepsy, hypercholesterolaemia, sensorineural hearing loss, migraines, idiopathic hypercalciuria, ovarian teratoma, nephrocalcinosis, psoriasis, allergic rhinitis, vasovagal syncope, Tourette’s syndrome, eating disorder and obsessive–compulsive disorder.
ADHD, attention-deficit/hyperactivity disorder; BMI, body mass index; HbA1c, glycated haemoglobin; NA, not applicable; SD, Standard deviation.
Descriptive statistics obtained at each time point for the total sample and subgroups for each outcome measure can be found in online supplemental appendix 4.
bmjopen-2022-071334supp004.pdf (116.8KB, pdf)
Effectiveness
Glycated haemoglobin
In the entire sample, there was a significant increase in HbA1c at 12 months (with respect to baseline) B=0.32% (95% CI 0.10 to 0.55; p=0.005). The interaction between time and the baseline HbA1c group was statistically significant at 3, 6 and 12 months (p<0.001) (table 2). In the subgroup analysis, participants with baseline HbA1c<7.5% revealed an increase of 0.32% (95% CI 0.18% to 0.47%) in HbA1c at 12 months (with respect to baseline) (p<0.001), without exceeding, on average, the threshold of poor control. Patients with poorly controlled baseline status had a statistically significant reduction in HbA1c at all follow-ups: B=−0.46% (95% CI −0.69% to −0.23%; p<0.001), B=−0.49% (95% CI −0.73% to −0.25%; p<0.001) and B=−0.43% (95% CI −0.68% to −0.19%; p=0.001), at 3, 6 and 12 months, respectively (table 2). On average, this reduction did not attain the threshold of poor control.
Table 2.
Multivariate mixed regression models for HbA1c
| Variable | Total sample (n=156) |
HbA1c<7.5% (n=68) |
HbA1c≥7.5% (n=88) |
|||
| B (95%CI) | P value | B (95% CI) | P value | B (95% CI) | P value | |
| Time | ||||||
| M3 (ref: M0) | 0.03 (−0.18 to 0.24) | 0.765 | 0.03 (−0.09 to 0.16) | 0.611 | −0.46 (−0.69 to −0.23) | <0.001 |
| M6 (ref: M0) | 0.1 (−0.11 to 0.32) | 0.344 | 0.10 (−0.03 to 0.23) | 0.115 | −0.49 (−0.73 to −0.25) | <0.001 |
| M12 (ref: M0) | 0.32 (0.10 to 0.55) | 0.005 | 0.32 (0.18 to 0.47) | <0.001 | −0.43 (−0.68 to −0.19) | 0.001 |
| Duration of T1DM | 0.05 (0.007 to 0.09) | 0.020 | −0.005 (−0.04 to 0.03) | 0.762 | 0.09 (0.02 to 0.15) | 0.011 |
| Presence of comorbidities | −0.10 (−0.39 to 0.18) | 0.477 | 0.09 (−0.13 to 0.30) | 0.439 | −0.22 (−0.70 to 0.26) |
0.372 |
| Age group: ≥12 years (ref: <12 years) | 0.17 (−0.12 to 0.47) | 0.253 | 0.09 (−0.15 to 0.32) | 0.473 | 0.26 (−0.21 to 0.73) | 0.274 |
| Baseline HbA1c group: ≥7.5% (ref: HbA1c<7.5%) | 1.81 (1.50 to 2.13) | <0.001 | ||||
| Time×baseline HbA1c Group (ref: M0 and HbA1c<7.5%) | ||||||
| M3 and HbA1c≥7.5% | −0.49 (−0.78 to −0.21) | <0.001 | ||||
| M6 and HbA1c≥7.5% | −0.59 (−0.88 to −0.29) | <0.001 | ||||
| M12 and HbA1c≥7.5% | −0.76 (−1.05 to −0.46) | <0.001 | ||||
| Intercept | 6.75 (6.41 to 7.09) | <0.001 | 6.73 (6.50 to 6.96) | <0.001 | 8.53 (8.12 to 8.94) | <0.001 |
HbA1c, glycated haemoglobin; M, month; T1DM, type 1 diabetes mellitus.
Severe hypoglycaemic events
The reduction in the number of self-reported events was significant at 12 months β=−0.37 (95% CI −0.62 to −0.11; p=0.004) (online supplemental appendix 5 table 1). Although the interaction with the level of HbA1c at baseline was not statistically significant (p=0.117), the descriptive statistics (online supplemental appendix 4) in patients with controlled HbA1c at baseline show a reduction in the mean number of events; with an increase in the poorly controlled subgroup.
Severe hypoglycaemic (SH) events recorded in the EHR show significantly lower rates compared with self-reported events (online supplemental appendix 4), without significant main or interaction effects (online supplemental appendix 5 table 1). The rate of SH events was significantly higher in the subgroup with poor HbA1c control (p=0.014) (online supplemental appendix 5 table 1).
bmjopen-2022-071334supp005.pdf (105.1KB, pdf)
Diabetic ketoacidosis and other serious adverse events
In the follow-up phase, six mild or moderate ketoacidosis events were recorded at 3 (two), 6 (one) and 12 months (three), respectively; and four serious adverse events at 3 months (two admissions and one episode of ketosis without acidosis due to bubbles in the system); and at 6 months (one admission). No events were observed at 12-month follow-up. No patient died during the follow-up.
Health-related quality of life
At 12 months follow-up, the percentages of severe limitations for mobility, self-care, daily activities, anxiety and depression were similar to baseline values. However, a reduction was observed in the percentage of patients who self-reported pain (online supplemental appendix 4).
VAS score (online supplemental appendix 5) did not show a significant change in the whole sample, and the interaction with baseline HbA1c values was slightly above the statistical significance level (p=0.061). In poorly controlled patients, VAS scores were significantly reduced at 12 months compared with the baseline score B=−6.03 (95% CI −9.66 to −2.41; p=0.001). In the subgroup with good basal metabolic control, no statistically significant findings were observed.
Knowledge of diabetes treatment
There was no significant change in patients’ Knowledge of diabetes treatment, nor a significant interaction with baseline HbA1c. Patients with worse basal metabolic control revealed a significantly lower score compared with well-controlled patients: B=−1.27 (95% CI −1.89 to −0.65; p<0.001) (online supplemental appendix 5 table 1).
Satisfaction with treatment
General satisfaction with treatment significantly increased 3.1 points at 12 months of follow-up (95% CI 0.99 to 5.23; p=0.004) (online supplemental appendix 5 table 1). There were no statistically significant differences in self-perceived hypoglycaemia and hyperglycaemia. For the latter, a higher score of 1.06 points (in a range of 0–6) was observed, in patients with HbA1c≥7.5%, compared with those with good control (95% CI 0.60 to 1.52; p<0.001) (online supplemental appendix 5 table 1).
Safety
Mild adverse events related to the device during follow-up phases had a 3.1% and 6.6% reduction for skin reactions and discomfort or pain, respectively. However, these reductions were not statistically significant (table 3).
Table 3.
Mild adverse effects caused by the sensor
| 3 months (n=150) | 6 months (n=136) | 12 months (n=128) | P value | Differences 12–3 months, % (95%CI) |
|
| Skin reactions, n (%) | 21 (14.0) | 16 (11.8) | 14 (10.9) | 0.542 | −3.1% (−25.2% to 19.0%) |
| Discomfort or pain, n (%) | 17 (11.3) | 13 (9.6) | 6 (4.7) | 0.210 | −6.6% (−29.3% to 16.1%) |
| Other minor events, n (%) | 3 (2.0) | 2 (1.5) | 2 (1.6) | 0.999 | −0.4% (−23.9% to 23.1%) |
Among the other events, there were minor haemorrhages when the sensor was positioned and wounds in the insertion area. In one case, the patient lost consciousness because of the bleeding.
Adherence
Time of sensor use (online supplemental appendix 5 table 2) significantly increased at 6.4% at 12 months of follow-up (95% CI 1.12 to 11.72; p=0.02), compared with 3 months. Longer duration of T1DM (p=0.008), and age older than 12 years (p=0.003), significantly reduced sensor use.
A reduction in the mean number of daily scans at 3 months occurred in poorly controlled patients B=−1.92 (95% CI −3.52 to −0.31; p=0.019). Those aged over 12 underwent an average of four fewer scans than those aged under 12 years B=−3.92 (95% CI −5.4 to −2.43; p<0.001) (online supplemental appendix 5 table 2).
Controlled patients revealed an increase in the mean number of sensors use at 12 months of follow-up B=7 (95% CI 5.85 to 8.06; p<0.001); also increasing in poorly controlled patients by B=1.6 (95% CI 0.48 to 2.7; p=0.005) at 6 months and B=9.4 (95% CI 8.25 to 10.5; p<0.001) at 12 months (online supplemental appendix 5 table 2).
The percentage of time of use was statistically significantly related to a lower HbA1c level at 12 months (B=−0.01; p=0.013), as was the number of scans (B=−0.21; p<0.001).
Cost estimation
The estimated total annual costs per patient are shown in online supplemental appendix 3. Intervention short-term costs from an NHS perspective reveal that specialist visits and test strips and lancets costs account for a significant part of total costs (38% and 41%, respectively), with an average annual cost per patient of €415.48 and €447.25 for specialist visits and strips and lancets, respectively. Regarding the cost of the FSL sensor, it amounts to €43.27 according to information provided by the manufacturer. Taking into account an average number of sensors per patient per year of 26 (considering a sensor half-life of 14 days), the total annual cost of the sensor amounts to €1125 per patient/year. This means that the average total annual costs per patient with the use of FSL amounts to €2204.26 (online supplemental appendix 3).
Total annual costs before and after use of the FSL system can be found in figure 2. All measured costs decreased after use of the device throughout 12 months follow-up, with the most striking difference in costs related to test strips and lancets use, an annual difference of €856.68 per patient.
Figure 2.
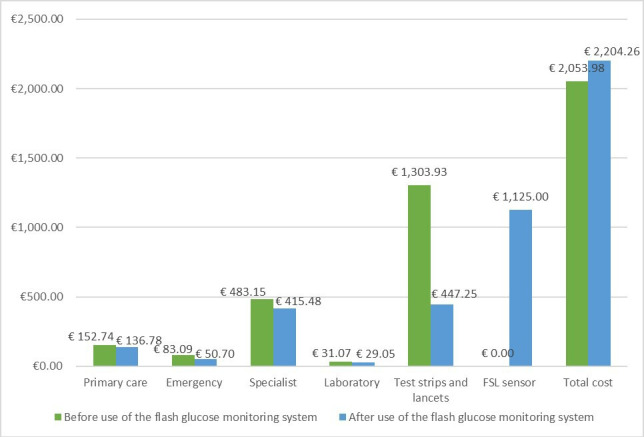
Total annual costs per patient before and after use of the FSL. FSL, FreeStyle Libre.
This information is outlined in online supplemental appendix 3. The annual average number of test strips per patient decreased from 2686.02 strips per year before the use of the FSL, to 883.98 strips per year after its use. The difference in the annual average use of lancets per patient also reduced from 1366.41 before FSL use to 615.94 after its use.
Furthermore, a decrease in total annual costs due to productivity losses of parents/caregivers of minor patients with T1DM was observed after the use of FSL (€545.67 vs €262.73) as shown in online supplemental appendix 3.
Discussion
Glucose monitoring devices can help people with T1DM monitor their glycaemia levels and reduce the frequency and/or severity of acute disease-complication rates, thus improving their HRQoL and life expectancy.34 Two meta-analyses of case series on the effectiveness of the FSL revealed statistically significant HbA1c reductions in children/adolescents with poor HbA1c control (7.5%–9.6%, except two studies with 7.1% and 7.4%) of −0.54% (n=447)35 and −0.29% (n=959),11 although the effect was highly variable across studies. Our study only provides a statistically significant reduction of HbA1c in the group with poor baseline monitoring, (−0.46%, −0.49% and −0.35%), at 3, 6 and 12 months, respectively. On the contrary, patients with basal controlled HbA1c levels revealed a significant 12-month worsening higher than 0.30%. Another case series in Spain (n=145),36 with limited follow-up to 3 months, also detected a reduction in patients with HbA1c≥7.5% (−0.41, p=0.004), and a statistically significant increase in well-monitored patients, that is, a worsening in HbA1c levels (0.23, p=0.03). The uncontrolled design of the study precludes ruling out that this result just reflects a regression to the mean. A recent meta-analysis of randomised controlled trials on the effectiveness of continuous glucose monitoring in people with T1DM showed a significant effect only in studies with mean HbA1c values at baseline >8% (−0.49%).37 However, apart from not being based exclusively on non-adult population, this result is based on meta-analysis and not on the analysis of interactions in the individual studies, and therefore, it is subjected to potential risk of ecological fallacy.
The results also revealed a significant reduction in the number of self-reported SH events for the whole sample (−0.37), but not in the number of patients with at least one event. The interaction effect with baseline HbA1c level was not statistically significant for these two variables (p=0.117 and p=0.108, respectively). The descriptive statistics suggest different subgroup effects, although none was statistically significant. The reduction of self-reported SH events occurred in patients with controlled HbA1c levels at baseline (0.39), whereas in the basally uncontrolled group, an increase was self-reported (0.37 more); together with an important increase in the rate of patients with at least one event (from 26% to 38%). Again, an effect of regression to the mean could be the explanation for this result since, as expected, patients with controlled HbA1c at baseline showed higher SH rates and means. Alternatively, the results on both HbA1c and SH could be reflecting the trade-off faced by patients with T1DM between the reduction in glucose levels and the associated risk of increasing hypoglycaemic events. This interpretation is speculative given the commented methodological limitations of the study, but it would help account for the unexpected significant worsening in self-perceived general health observed in the subgroup of poor baseline HbA1c monitoring. That is, contrary to the HbA1c improvement attained, which has no observable effects on self-perceived HRQoL, suffering an SH event is a salient experience that may impact this self-perception.
Other studies36 have also reported a significant and clinically meaningful improvement in the rate of SH events (from 4.2 to 0.2 events/100 patients-year). However, their results are not reported separately according to basal levels of metabolic control. The largest case series published to date with children and adolescents,38 and with the longest follow-up (12 months), also revealed a statistically significant reduction of SH events (53%, p=0.012) for the whole sample, with no changes in HbA1c.
The interaction of the intervention with the age group (<12 vs ≥12 years) was not statistically significant in any case. However, descriptive statistics reveal different non-significant trends among subgroups, with positive results only for younger participants: −0.26% vs −0.05% (HbA1c), −1.06 vs 0.68 (SH events) and −4.2% vs 10.5% (people with one or more SH). Adolescents revealed significantly lower sensor usage time and scans per day than children, similar to the results observed in previous studies.39–41 Adolescents and young adults face specific challenges and barriers regarding the use of glucose monitoring sensors, such as concerns about self-image and how people perceive them,42 43 differential emotional reactions to diabetes burden44 or a lesser interest in glucose data analysis,45 and therefore, specific strategies might be necessary to increase sensor use in this population.46 Nonetheless, adolescents in our sample showed adequate adherence throughout the study, above 78% of the time at each successive evaluation. Regarding the effectiveness of the FLS in adolescents, the only randomised controlled trial to date included participants aged 13–20 years, with HbA1c≥9.0%,13 and although it found significantly higher satisfaction in the intervention group at 6 months, it did not reveal any statistically significant differences in HbA1c reduction compared with traditional self-monitoring. Therefore, significant uncertainty remains in regard to the effects of FSL in adolescents.
Despite the improvement in the degree of metabolic control that occurred in our study sample of patients with worse baseline HBA1c levels, no statistically significant improvement was observed in their knowledge of diabetes treatment. Device adherence was significantly related to the reduction of HbA1c, a result usually observed in the literature on glucose monitoring devices.39–41 The same can be said about treatment satisfaction,34 47 which improved in the whole sample.
In regard to safety, no serious adverse effects were observed, a result consistent with the literature on glucose monitoring devices in general.9 The number of patients showing mild adverse events at 3 months was reduced at the end of follow-up to 18%, resulting in two losses at 6 months follow-up due to skin reaction to the sensor and another two at 12 months due to discomfort with the sensor.
In terms of cost analysis as observed in the international literature, our results showed that patients with T1DM consume less healthcare resources using FSL.48 Fundamentally, a striking decrease was observed in costs attributed to reactive strips and lancets, where an annual difference of €856.68 per patient was obtained (not including cost of sensor). A decrease in total indirect annual costs due to productivity losses of parents/carers of patients with T1DM was also observed (€545.67 vs €262.73). Despite the savings observed in all cost categories, when the cost of the device is taken into account, there is no potential savings with the use of the FSL. However, this information can be useful for decision-making and negotiating the price of the device.
The main limitation of this study lies in its uncontrolled design, which precludes comparison with an untreated group. Therefore, an inference of causality regarding the introduction of the FLS is not possible, because other factors such as child developmental growth, potential changes in target treatment or insulin administration methods or a regression to the mean could affect the observed changes. A ‘novelty effect’, related to the use of a technological device, could also introduce a motivation bias that could affect self-management habits. Another relevant limitation is the limited sample size to analyse interaction effects, even when we increased the recruited sample fourfold. By the time of study execution, the FSL was already financed and introduced in some hospitals taking part and a large portion of the target population was already using it. This scenario was an important recruitment obstacle to enlarge sample size. Our conclusions to be drawn are, therefore, limited by the low statistical power for interaction analyses and rare events such as severe hypoglycaemia. All these limitations imply a low quality of the evidence.
The start-up of a monitoring study has been used to collect data on the use of resources and make initial estimates of the cost of the intervention. Therefore, our cost analysis was a secondary endpoint and complementary to this study’s primary endpoint and it has limitations. First, our analysis has not taken into account the costs attributable to the possible adverse effects arising from the use of FSL and it has assumed that possible failures of the device will be resolved at no additional cost to the Spanish NHS. Moreover, it was not possible to estimate the costs related to hospitalisation of the patients since the number of days of each hospitalisation was not recorded in this study. However, the extremely low number of total hospitalisations during the monitoring study indicates that including this cost in the estimate would not have produced substantial changes in the results.
To the best of our knowledge, this is the first comparative costs analysis study of FSL use in children and adolescents with T1DM in Spain using observational data in an actual use scenario. Therefore, although a cost-effectiveness analysis could not be performed in this study, due to the absence of a comparator, our results may contribute to inform future cost-effectiveness studies of FSL in Spain.
Conclusion
Our results showed that the use of FSL in young patients with T1DM significantly reduced the rate of SH events, and improved HbA1c levels in patients with poor baseline control. However, future studies should confirm whether these benefits could be at the cost of worsening outcomes in patients with lower HbA1c. No serious adverse events related to FSL were observed. The results also suggest that the use of FSL in young patients with T1DM leads to a decrease in monitoring costs. In addition, the use of FSL reduces costs attributable to lost productivity of parents/caregivers. However, these outcomes correspond to low-quality evidence, mainly due to the study’s uncontrolled design, in addition to the low statistical power in the case of rare complications such as SH.
Based on these results and other information sources (ie, international research and clinical expert advice), the Spanish Ministry of Health has decided to reimburse the FSL for children and adolescents aged 4–17 years with type 1 diabetes who are treated with intensive insulin therapy (multiple daily injections or insulin pump) and require at least six fingerstick blood glucose self-monitoring tests a day.
Supplementary Material
Footnotes
Collaborators: The Health Professional Group included the following members: Amparo González Vergaz (Hospital Universitario Severo Ochoa), Ana María Prado Carro (Complexo Hospitalario Universitario A Coruña), Anunciación Beisti Ortego (Fundación Hospital de Calahorra), Ariadna Campos Martorell (Hospital Universitari Vall d’Hebron), Atilano José Carcavilla Urqui (Hospital Universitario La Paz), Cristina Amparo Del Castillo Villaescusa (Hospital Universitario Dr. Peset), Estela Gil Poch (Complejo Hospitalario Universitario Badajoz), Francisco Javier Arroyo Diez (Complejo Hospitalario Universitario Badajoz), Gemma Novoa Gómez (Complejo Hospitalario de Ourense), Isabel González Casado (Hospital Universitario La Paz), Juncal Martínez Ibáñez (Fundación Hospital de Calahorra), Laura Cuadrado Piqueras (Fundación Hospital de Calahorra), Leticia Reis Iglesias (Complejo Hospitalario de Ourense), Lucia Garzón Lorenzo (Hospital Universitario 12 De Octubre), Luis Salamanca Fresno (Hospital Universitario La Paz), María Asunción Martínez Brocca (Hospital Universitario Virgen Macarena), María Aurea Rodríguez Blanco (Hospital Da Barbanza), María Del Mar Martínez López (Hospital Universitario 12 De Octubre), María Jesús Ferreiro Rodríguez (Complejo Hospitalario de Ourense), María Ruiz del Campo (Hospital San Pedro), Nerea Itza Martín (Hospital Universitario La Paz), Patricia García Navas (Hospital San Pedro), Rebeca García García (Hospital Universitario Central de Asturias).
Contributors: YA-P, AR-S, LP-P and PS-A initiated the study. HG-P did the acquisition of data. HG-P, AR-S, CV-N and YR-F contributed to the analysis and interpretation of data. HG-P did the statistical analyses. HG-P, AR-S, CV-N, YA-P and YR-F wrote the first draft of the manuscript. HG-P, AR-S, YR-F, CV-N, YA-P, LG-P, MAG-B, LP-P and PS-A critically revised the manuscript and approved the final version. The collaborator group participated in the recruitment and data collection. HG-P is responsible for the overall content as the guarantor and accepts full responsibility for the work and/or the conduct of the study, had access to the data and controlled the decision to publish.
Funding: This work was financed by the Spanish Ministry of Health, Consumer Affairs and Social Welfare in the framework of activities developed by the Spanish Network of Agencies for Health Technology Assessment for the Spanish National Health Service.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: The Health Professional Group, Amparo González Vergaz, Ana María Prado Carro, Anunciación Beisti Ortego, Ariadna Campos Martorell, Atilano José Carcavilla Urqui, Cristina Amparo Del Castillo Villaescusa, Estela Gil Poch, Francisco Javier Arroyo Diez, Gemma Novoa Gómez, Isabel González Casado, Juncal Martínez Ibáñez, Laura Cuadrado Piqueras, Leticia Reis Iglesias, Lucia Garzón Lorenzo, Luis Salamanca Fresno, María Asunción Martínez Brocca, María Aurea Rodríguez Blanco, María Del Mar Martínez López, María Jesús Ferreiro Rodríguez, María Ruiz del Campo, Nerea Itza Martín, Patricia García Navas, and Rebeca García García
Data availability statement
Data are available on reasonable request. Data are not publicly available. Data are available upon reasonable request via the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and all participants provided written informed consent. The scientific and ethics committees approved the study protocol (Hospital Universitari Vall d’Hebron, ID-RTF065). Participants gave informed consent to participate in the study before taking part.
References
- 1.Lucier J, Weinstock RS, Doerr C. Diabetes Mellitus Type 1 (Nursing). Treasure Island: StatPearls Publishing, 2023. [Google Scholar]
- 2.Golden SH, Sapir T. Methods for insulin delivery and glucose monitoring in diabetes: summary of a comparative effectiveness review. J Manag Care Pharm 2012;18:S1–17. 10.18553/jmcp.2012.18.s6-A.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miranda Velasco MJ, Domínguez Martín E, Arroyo Díez FJ, et al. Calidad de Vida Relacionada con La Salud en La diabetes mellitus Tipo 1. Anales de Pediatría 2012;77:329–33. 10.1016/j.anpedi.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 4.International Diabetes Federation . IDF diabetes Atlas. 8th Edn. Bruselas, Bélgica. 2017.
- 5.Conde Barreiro S, Rodríguez Rigual M, Bueno Lozano G, et al. Epidemiología de la diabetes mellitus Tipo 1 en Menores de 15 Años en España. Anales de Pediatría 2014;81:189. 10.1016/j.anpedi.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 6.Crespo C, Brosa M, Soria-Juan A, et al. Costes Directos de la diabetes mellitus Y de Sus Complicaciones en España (Estudio SECCAID: Spain estimated cost Ciberdem-Cabimer in diabetes). Avances En Diabetología 2013;29:182–9. 10.1016/j.avdiab.2013.07.007 [DOI] [Google Scholar]
- 7.Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: A review of the technology and clinical use. Diabetes Res Clin Pract 2017;133:178–92. 10.1016/j.diabres.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 8.Marsters BL, Boucher SE, Galland BC, et al. Cutaneous adverse events in a randomized controlled trial of flash glucose monitoring among youth with type 1 diabetes mellitus. Pediatr Diabetes 2020;21:1516–24. 10.1111/pedi.13121 [DOI] [PubMed] [Google Scholar]
- 9.Landau Z, Abiri S, Gruber N, et al. Correction to: use of flash glucose-sensing technology (Freestyle Libre) in youth with type 1 diabetes: awesome study group real-life observational experience. Acta Diabetol 2018;55:1311. 10.1007/s00592-018-1227-7 [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Zhou M, Xu X, et al. Effects of flash glucose monitoring on Glycemic control in participants with diabetes mellitus: A meta-analysis of randomized controlled trials. J Diabetes Complications 2022;36:S1056-8727(22)00226-4. 10.1016/j.jdiacomp.2022.108314 [DOI] [PubMed] [Google Scholar]
- 11.Gordon I, Rutherford C, Makarounas-Kirchmann K, et al. Meta-analysis of average change in laboratory-measured Hba1C among people with type 1 diabetes mellitus using the 14 day flash glucose monitoring system. Diabetes Res Clin Pract 2020;164:S0168-8227(20)30408-3. 10.1016/j.diabres.2020.108158 [DOI] [PubMed] [Google Scholar]
- 12.Castellana M, Parisi C, Di Molfetta S, et al. Efficacy and safety of flash glucose monitoring in patients with type 1 and type 2 diabetes: a systematic review and meta-analysis. BMJ Open Diabetes Res Care 2020;8:e001092. 10.1136/bmjdrc-2019-001092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boucher SE, Gray AR, Wiltshire EJ, et al. Effect of 6 months of flash glucose monitoring in youth with type 1 diabetes and high-risk Glycemic control: A randomized controlled trial. Diabetes Care 2020;43:2388–95. 10.2337/dc20-0613 [DOI] [PubMed] [Google Scholar]
- 14.Serrano-Aguilar P, Asua-Batarrita J, Molina-López MT, et al. The Spanish network of agencies for health technology assessment and services of the national health system (Redets). Int J Technol Assess Health Care 2019;35:176–80. 10.1017/S0266462319000205 [DOI] [PubMed] [Google Scholar]
- 15.Perestelo-Pérez L, Rivero-Santana A, García-Lorenzo B, et al. Efectividad, Seguridad Y coste-Efectividad del Sistema flash de Monitorización de Glucosa en Líquido Intersticial (Freestyle Libre®) para La diabetes mellitus Tipo 1 Y 2. In: Ministerio de Sanidad, Servicios Sociales e Igualdad. Servicio de Evaluación del Servicio Canario de la Salud; 2016. Informes de Evaluación de Tecnologías Sanitarias. [Google Scholar]
- 16.Perestelo-Pérez L, Rivero-Santana A, García-Lorenzo B, et al. Efectividad, Seguridad Y coste-Efectividad del Sistema flash de Monitorización de Glucosa en Líquido Intersticial (Freestyle Libre®) para La diabetes mellitus Tipo 1 Y 2 (Apéndice de Actualización). In: Ministerio de Sanidad, Servicios Sociales e Igualdad. Servicio de Evaluación del Servicio Canario de la Salud; 2017. Informes de Evaluación de Tecnologías Sanitarias. [Google Scholar]
- 17.Servicio de Evaluación del Servicio Canario de la Salud, Available: http://www.sescs.es/
- 18.Resolución de 26 de abril de 2019, de la Dirección General de Cartera Básica de Servicios del Sistema Nacional de Salud y Farmacia, por la que se hace público el acuerdo de la Comisión de prestaciones, aseguramiento y financiación de 5 de noviembre de 2018 y 28 de marzo de 2019, sobre el sistema de monitorización de glucosa mediante sensores (tipo flash) en adultos en la cartera común de servicios del Sistema Nacional de Salud, Available: https://www.sanidad.gob.es/profesionales/prestacionesSanitarias/CarteraDeServicios/ContenidoCS/docs/Resolucionglucosadultos.pdf
- 19.Resolución de 30 de noviembre de 2020, de la Dirección General de Cartera Común de Servicios del Sistema Nacional de Salud y Farmacia, por la que se hace público el acuerdo de la Comisión de prestaciones, aseguramiento y financiación de 14 de julio de 2020 sobre el sistema de monitorización de glucosa mediante sensores (tipo flash) en la cartera común de servicios del Sistema Nacional de Salud, Available: https://www.sanidad.gob.es/profesionales/prestacionesSanitarias/CarteraDeServicios/ContenidoCS/docs/Resol_Flash_2020.pdf
- 20.Serrano-Aguilar P, Gutierrez-Ibarluzea I, Díaz P, et al. Postlaunch evidence-generation studies for medical devices in Spain: the Redets approach to integrate real-world evidence into decision making. Int J Technol Assess Health Care 2021;37:e63. 10.1017/S0266462321000295 [DOI] [PubMed] [Google Scholar]
- 21.Resolución de 28 de agosto de 2018, de la Dirección General de Cartera Básica de Servicios del Sistema Nacional de Salud y Farmacia, por la que se determina el sometimiento del sistema de monitorización de glucosa mediante sensores (tipo flash) a estudio de monitorización y se establecen sus requisitos específicos, Available: https://www.boe.es/boe/dias/2018/09/18/pdfs/BOE-A-2018-12686.pdf
- 22.Abbott Diabetes Care . Manual de Usuario. Freestyle Libre. Sistema flash de Monitorización de Glucosa. Madrid. 2017.
- 23.Wille N, Badia X, Bonsel G, et al. Development of the EQ-5D-Y: A child-friendly version of the EQ-5D. Qual Life Res 2010;19:875–86. 10.1007/s11136-010-9648-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell K, Johnson K, Cullen K, et al. Parental mastery of continuous subcutaneous insulin infusion skills and Glycemic control in youth with Type1 diabetes. Diabetes Technol Ther 2013;15:591–5. 10.1089/dia.2013.0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley C. The diabetes treatment satisfaction questionnaire: DTSQ. In: Bradley C, ed. Handbook of Psychology and Diabetes: a guide to psychological measurement in diabetes research and practice. Chur, Switzerland: Harwood Academic Publishers, 1994: 111–32. [Google Scholar]
- 26.Tanner JM. Growth at Adolescence: With a General Consideration of the Effects of Hereditary and Environmental Factors Upon Growth and Maturation from Birth to Maturity. Oxford: Blackwell Scientific Publications, 1962. [Google Scholar]
- 27.Hayes AJ, Leal J, Gray AM, et al. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom prospective diabetes study: UKPDS 82. Diabetologia 2013;56:1925–33. 10.1007/s00125-013-2940-y [DOI] [PubMed] [Google Scholar]
- 28.StataCorp . Stata statistical software: release 15. College Station, TX: StataCorp LLC, 2017. [Google Scholar]
- 29.IBM Corp . IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp, 2011. [Google Scholar]
- 30.Instituto Nacional de Estadística (INE) . Actualización de rentas con el IPC general (sistema IPC base 2016) para periodos anuales completos. Available: https://www.ine.es/calcula/ [Google Scholar]
- 31.Zhu W. Making Bootstrap statistical inferences: a Tutorial. Res Q Exerc Sport 1997;68:44–55. 10.1080/02701367.1997.10608865 [DOI] [PubMed] [Google Scholar]
- 32.Eurostat . Labour cost levels by NACE Rev. 2 activity. Available: https://ec.europa.eu/eurostat/databrowser/view/lc_lci_lev/default/table?lang=en [Google Scholar]
- 33.Instituto Nacional de Estadística (INE) . Encuesta de Población Activa (EPA). Available: https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176918&menu=ultiDatos&idp=1254735976595 [Google Scholar]
- 34.Ang E, Lee ZX, Moore S, et al. Flash glucose monitoring (FGM): A clinical review on Glycaemic outcomes and impact on quality of life. J Diabetes Complications 2020;34:S1056-8727(19)31341-8. 10.1016/j.jdiacomp.2020.107559 [DOI] [PubMed] [Google Scholar]
- 35.Evans M, Welsh Z, Ells S, et al. The impact of flash glucose monitoring on Glycaemic control as measured by Hba1C: A meta-analysis of clinical trials and real-world observational studies. Diabetes Ther 2020;11:83–95. 10.1007/s13300-019-00720-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leiva-Gea I, Garcia Vázquez J, Liñán Jurado FR, et al. Introduction of flash glucose monitoring in children with type 1 diabetes: experience of a single-centre in Spain. ESPE Abstracts 2019. [Google Scholar]
- 37.Teo E, Hassan N, Tam W, et al. Effectiveness of continuous glucose monitoring in maintaining Glycaemic control among people with type 1 diabetes mellitus: a systematic review of randomised controlled trials and meta-analysis. Diabetologia 2022;65:604–19. 10.1007/s00125-021-05648-4 [DOI] [PubMed] [Google Scholar]
- 38.Messaaoui A, Tenoutasse S, Crenier L. Flash glucose monitoring accepted in daily life of children and adolescents with type 1 diabetes and reduction of severe Hypoglycemia in real-life use. Diabetes Technol Ther 2019;21:329–35. 10.1089/dia.2018.0339 [DOI] [PubMed] [Google Scholar]
- 39.Urakami T, Yoshida K, Kuwabara R, et al. Frequent scanning using flash glucose monitoring contributes to better Glycemic control in children and adolescents with type 1 diabetes. J Diabetes Investig 2022;13:185–90. 10.1111/jdi.13618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lameijer A, Lommerde N, Dunn TC, et al. Flash glucose monitoring in the Netherlands: increased monitoring frequency is associated with improvement of Glycemic parameters. Diabetes Res Clin Pract 2021;177:S0168-8227(21)00257-6. 10.1016/j.diabres.2021.108897 [DOI] [PubMed] [Google Scholar]
- 41.Battelino T, Liabat S, Veeze HJ, et al. Routine use of continuous glucose monitoring in 10 501 people with diabetes mellitus. Diabet Med 2015;32:1568–74. 10.1111/dme.12825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther 2020;22:760–7. 10.1089/dia.2019.0509 [DOI] [PubMed] [Google Scholar]
- 43.Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr 2010;22:405–11. 10.1097/MOP.0b013e32833a46a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patton SR, Clements MA. Psychological reactions associated with continuous glucose monitoring in youth. J Diabetes Sci Technol 2016;10:656–61. 10.1177/1932296816638109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huhn F, Lange K, Jördening M, et al. Real-world use of continuous glucose monitoring systems among adolescents and young adults with type 1 diabetes: reduced burden, but little interest in data analysis. J Diabetes Sci Technol 2023;17:943–50. 10.1177/19322968221081216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prahalad P, Ebekozien O, Alonso GT, et al. Multi-clinic quality improvement initiative increases continuous glucose monitoring use among adolescents and young adults with type 1 diabetes. Clin Diabetes 2021;39:264–71. 10.2337/cd21-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palylyk-Colwell E, Ford C. Flash glucose monitoring system for diabetes. In: CADTH Issues in Emerging Health Technologies. Ottawa: Canadian Agency for Drugs and Technologies in Health, 2017. [PubMed] [Google Scholar]
- 48.Lin R, Brown F, James S, et al. Continuous glucose monitoring: A review of the evidence in type 1 and 2 diabetes mellitus. Diabet Med 2021;38:e14528. 10.1111/dme.14528 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-071334supp001.pdf (55.6KB, pdf)
bmjopen-2022-071334supp002.pdf (71.5KB, pdf)
bmjopen-2022-071334supp003.pdf (93.8KB, pdf)
bmjopen-2022-071334supp004.pdf (116.8KB, pdf)
bmjopen-2022-071334supp005.pdf (105.1KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data are not publicly available. Data are available upon reasonable request via the corresponding author.


