Abstract
Introduction
Over half of the populations with knee osteoarthritis (OA) have obesity. These individuals have many other shared metabolic risk factors. Metformin is a safe, inexpensive, well-tolerated drug that has pleiotropic effects, including structural protection, anti-inflammatory and analgesic effects in OA, specifically the knee. The aim of this randomised, double-blind, placebo-controlled trial is to determine whether metformin reduces knee pain over 6 months in individuals with symptomatic knee OA who are overweight or obese.
Methods and analysis
One hundred and two participants with symptomatic knee OA and overweight or obesity will be recruited from the community in Melbourne, Australia, and randomly allocated in a 1:1 ratio to receive either metformin 2 g or identical placebo daily for 6 months. The primary outcome is reduction of knee pain [assessed by 100 mm Visual Analogue Scale (VAS)] at 6 months. The secondary outcomes are OMERACT-OARSI (Outcome Measures in Rheumatology-Osteoarthritis Research Society International) responder criteria [Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain, function and participant’s global assessment (VAS)] at 6 months; change in knee pain, stiffness, function using WOMAC at 6 months and quality of life at 6 months. Adverse events will be recorded. The primary analysis will be by intention to treat, including all participants in their randomised groups.
Ethics and dissemination
Ethics approval has been obtained from the Alfred Hospital Ethics Committee (708/20) and Monash University Human Research Ethics Committee (28498). Written informed consent will be obtained from all the participants. The findings will be disseminated through peer-review publications and conference presentations.
Trial registration number
ACTRN12621000710820 .
Keywords: Obesity, Knee, Randomized Controlled Trial
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study is a randomised, double-blind, placebo-controlled trial.
This study will provide high-quality evidence to address whether metformin has an analgesic effect over 6 months in individuals with symptomatic knee osteoarthritis with overweight or obesity.
The generalisability of this study result will be limited to those without diabetes or those not requiring antihyperglycaemic therapy.
Introduction
Osteoarthritis (OA) is a leading cause of global disability, resulting in 19 million years lived with disability in 2019.1 There is no approved disease-modifying treatment for OA to date. With limited effective therapies, end-stage OA is treated with total joint replacement, estimated to cost about US$10 billion/year in the USA2 and over $A1 billion/year in Australia.3 Despite being a multifactorial disease, management of OA has taken a ‘one-size-fits-all’ approach without considering the different pathological pathways and OA phenotypes, resulting in poor patient outcomes. One distinctive knee OA phenotype is the obese phenotype,4 5 mediated by inflammatory and metabolic mechanisms.6 Over 50% of knee OA patients have obesity.7 Given obesity and obesity-related metabolic factors (hyperglycaemia, dyslipidaemia, hypertension) are all risk factors for knee OA,6 8 drugs targeting obesity and its associated inflammatory and metabolic abnormalities have the potential to affect the pathogenesis of knee OA.
Metformin is a safe, inexpensive, well-tolerated oral biguanide, which is not only widely used for treatment of type 2 diabetes for over 60 years, but also has a long history of safe use in non-diabetic populations.9 10 Additional to its glucose lowering effects, metformin modulates metabolic factors, resulting in at least 2–3 kg of weight loss11 12 and reduced inflammation and plasma lipids.9 10 A recent systematic review of animal and human studies showed metformin has structural protective, anti-inflammatory and analgesic effects for OA, specifically for the knee.13 These pleiotropic effects of metformin are mainly driven by the activation of AMP-activated protein kinase (AMPK) pathway.14–16 Hence, metformin has the potential to reduce pain in those with knee OA and overweight or obesity. This study aims to determine the effect of metformin on reducing knee pain in people with symptomatic knee OA and overweight or obesity.
Methods and analysis
Study design
This is a randomised, double-blind, placebo-controlled trial in people with symptomatic knee OA and overweight or obesity, to determine the effect of metformin 2 g daily versus placebo on reducing knee pain over 6 months.
Hypothesis and objectives
It is hypothesised that metformin, compared with placebo, will (1) reduce knee pain (primary hypothesis); (2) improve clinical outcomes (stiffness, function and health-related quality of life) and that (3) the effect of metformin on knee pain and function will be associated with changes in inflammatory and metabolic biomarkers and/or weight loss. If metformin is proven to be effective, it will provide a safe, low-cost treatment to reduce pain and improve function for people with symptomatic knee OA with concurrent overweight or obesity.
Trial registration and reporting
The trial was registered at the Australian New Zealand Clinical Trials Registry prior to commencing recruitment (ACTRN12621000710820, registered 8 June 2021). The trial reporting will be guided by the Consolidated Standards of Reporting Trials Statement.17
Study setting and participants
Participants with symptomatic knee OA and overweight or obesity will be recruited using a combined strategy including collaboration with medical practitioners and advertisements in social and local media. This single-centre study will be conducted in Melbourne, Australia.
Inclusion criteria
Men and women aged >40 years, with overweight or obesity (body mass index ≥25 kg/m2); (2) Knee pain for at least 6 months with a pain score >40 mm on a 100 mm Visual Analogue Scale (VAS) and (3) Meet the American College of Rheumatology clinical criteria for knee OA.18
Exclusion criteria
(1) Severe radiographic knee OA (Kellgren-Lawrence grade 4) or severe knee pain (on standing >80 mm on a 100 mm VAS); (2) Any inflammatory arthritis including rheumatoid arthritis, psoriatic arthritis, crystal arthritis, spondyloarthritis, connective-tissue disease associated arthritis or reactive arthritis or significant knee injury; (3) Known or newly diagnosed diabetes requiring antihyperglycaemic therapy or previous adverse reaction to metformin; (4) Index knee surgery (arthroscopy or open surgery) in the past year; (5) Index knee intra-articular hyaluronic acid injection in the past 6 months or corticosteroid injection in the past 3 months; (6) Use of any investigational drugs or device within 30 days prior to randomisation; (7) Index knee planned joint replacement or arthroscopy in the next 6 months; (8) Other muscular, joint or neurological condition affecting lower limb function; (9) Acute or chronic renal or liver impairment; (10) Other medical condition precluding study participation or relocation and (11) Women who are pregnant, lactating or trying to become pregnant. Use of menopausal hormone therapy or contraceptive pill will be permitted so long as the dose has been stable for at least 30 days prior to study entry.
Study timeline
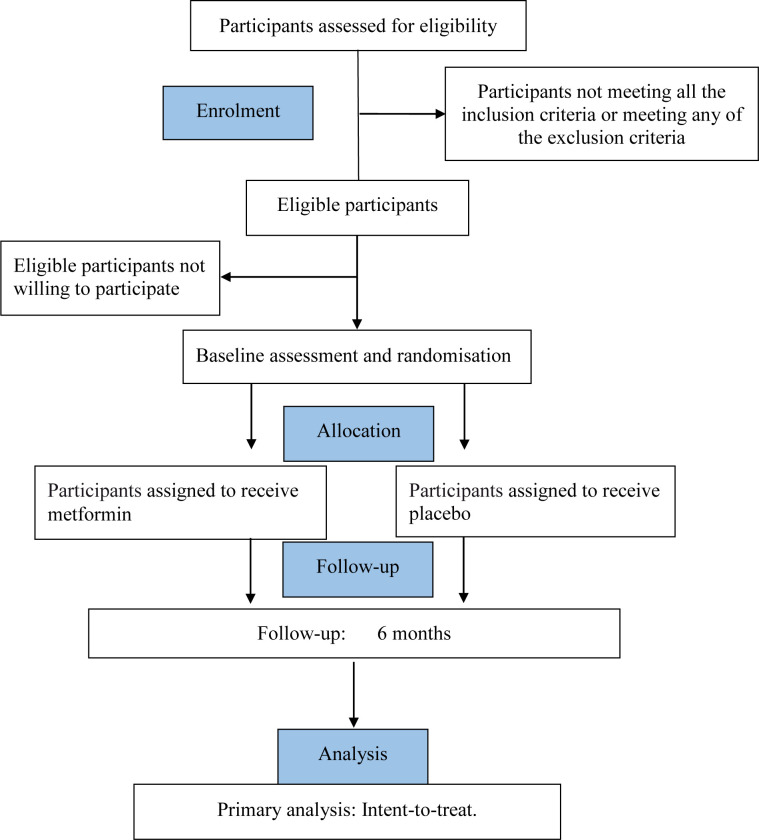
This trial began recruitment on 16 June 2021. It is estimated to finish recruitment on 30 September 2023 and complete the 6-month follow-up and data collection in March 2024. Figure 1 shows trial participation and study procedure.
Figure 1.
Flow chart of trial participation.
Randomisation, allocation concealment and blinding
Allocation of participants in a 1:1 ratio to one of the two groups will be by computer-generated random numbers prepared by a statistician with no involvement in the trial. Block randomisation using random permuted blocks of sizes 4 and 6 will be performed. The use of a central automated allocation procedure with security in place will ensure the allocation cannot be accessed or influenced by any person. Allocation concealment and double blinding will be ensured by: (1) medications being dispensed by Syntro Pharmacy; (2) use of an identical placebo tablet and (3) subjective measures being taken by research assistants blinded to group allocation. Participants, assessors and statisticians will be blinded to group allocation.
Intervention and dosing
All participants will undergo usual care by their treating health practitioners. Eligible participants will be randomly assigned to receive either metformin (up to 2000 mg) once daily or placebo once daily. Treatment with study drug will be initiated at a dose of 500 mg once a day with the evening meal. Over 6 weeks, the dose of study drug will be titrated to 2000 mg once daily (or placebo once daily) to minimise gastrointestinal side effects.
Safety
Any adverse events or serious adverse event will be reported during the study. Blood tests will occur at screening. No data safety monitoring board is required as this agent is approved by Therapeutic Goods Administration with a well-known safety profile.19 Unblinding participants due to side effects of metformin was not an issue in a previous clinical trial.12
Compliance
Compliance with trial medication will be assessed at 6 months by pill count. Study staff will phone participants in the middle between study visits to monitor medication adherence. Monthly telephone contact for the first 5 months will be conducted to address any concerns, as well as following up knee pain outcome (VAS). This will help to mitigate non-compliance.
Concomitant medication
To maintain the pragmatic nature of the trial, there are no restrictions with regard to concomitant medications, including glucosamine, chondroitin, non-steroidal anti-inflammatory drugs and opioids analgesics, which will be allowed during the trial and be recorded by questionnaire at all visits. Patients will be asked to keep medications as stable as possible and use paracetamol as rescue medication.
Study procedures
Participants will be screened via phone by questionnaire before attending the screening visit via telehealth. There will be two study visits (onsite or telehealth): screening/baseline and month 6, as shown in table 1. At screening, participants will complete questionnaires, have a knee X-ray and blood tests (renal and liver function, plasma glucose and lipids, vitamin B12 and inflammatory biomarkers (C reactive protein, tumour necrosis factor, interleukin-1 and interleukin 6)) to ensure inclusion criteria are met and exclusion criteria are absent. The index knee will be defined as having symptomatic OA. If both knees are symptomatic and eligible based on VAS pain, the one with higher VAS pain score will be used; if both knees are symptomatic with the same pain level, the one with least severe radiographic OA (joint space narrowing) will be used; if both knees have the same pain level and radiographic severity of OA, the dominant knee will be used. Physical examinations and questionnaires will be performed at months 0 and 6. The same researchers, blinded to treatment allocation, will measure all clinical variables, administer questionnaires, monitor compliance and record adverse events. Participants are able to withdraw at any time during the trial; the time and reasons will be recorded. If participants withdraw from the study, they will be requested to complete questionnaires (posted to the participants with a return envelope).
Table 1.
Schedule of assessments
| Screening | Double-blind period | |||
| Screening/ baseline assessment | Randomisation | 1–5 months | 6 months | |
| Study visit (onsite or telehealth) | X | X | ||
| Telephone interview (monthly) | X | |||
| Informed consent | X | |||
| Knee X-ray | X | |||
| Blood test | X | |||
| Medical history/conditions | X | X | ||
| Medication | X | X | ||
| Employment and education | X | |||
| Smoking and alcohol | X | |||
| Questionnaires | ||||
| Knee VAS | X | X | X | |
| WOMAC | X | X | ||
| PainDETECT | X | X | ||
| Hand VAS | X | X | X | |
| Multisite pain | X | X | ||
| AQoL | X | X | ||
| IPAQ | X | X | ||
| Physical examination | ||||
| Height, weight* | X | X | ||
| Compliance and safety (adverse events) | X | X | ||
| Dispense medication | X | |||
*Height and weight will be self-reported if the visit is via telehealth.
AQoL, assessment of quality of life; IPAQ, international physical activity questionnaire; VAS, Visual Analogue Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Primary outcome
The primary outcome is pain reduction at 6 months, measured by change in VAS knee pain from baseline to 6 months (follow-up VAS pain score—baseline score). Knee pain will be measured at baseline and monthly follow-up using a 100 mm VAS by asking ‘on this line, where would you rate your knee pain over the last 7 days?’ with terminal descriptors ‘no pain’ (score 0) and ‘worst imaginable pain’ (score 100).
Secondary outcomes
OMERACT-OARSI responder criteria
This will be used to define a responder based on improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and function and the participant’s global assessment20 at 6 months. Participant’s global assessment will be evaluated by 100 mm VAS.21
Change in knee pain, stiffness and function
Knee pain, stiffness and function will be assessed using the WOMAC22 at baseline and 6 months.
Health-related quality of life
This will be measured using the Assessment of Quality of Life23 at baseline and 6 months.
Other measures
Descriptive data
Data regarding age, gender, height, weight, duration of symptoms, employment, medical history, medication use, education level, smoking, alcohol consumption will be collected using a questionnaire at baseline.
PainDETECT
PainDETECT is a validated questionnaire used to assess pain sensitisation in OA24 and will be assessed at baseline and 6 months.
Physical activity
Physical activity will be measured using the International Physical Activity Questionnaire25 short version at baseline and 6 months.
Hand VAS
Pain reduction in hands will be measured at baseline, then monthly for 6 months, according to the OARSI recommendations for the design and conduct of clinical trials for hand OA, which recommend the use of single question pain VAS.26
Multisite pain
The presence and levels of multisite musculoskeletal pain will be assessed at baseline and 6 months using a questionnaire.
Adverse events
These will be measured in a log-book by the blinded assessor at each follow-up.
Biochemical parameters
General (cell counts, liver and renal function), inflammatory biomarkers (C reactive protein, erythrocyte sedimentation rate, interleukin-6, tumour necrosis factor), plasma glucose and lipids, and vitamin B12 will be measured at baseline and 6 months.
Knee X-ray
X-ray of the study knee (weight-bearing anteroposterior view) will be scored using Kellgren-Lawrence grade. Our intraobserver and interobserver reliability is 0.93 and 0.86 for osteophytes, 0.93 and 0.85 for joint space narrowing, respectively.27
Sample size calculation
The primary outcome is change in VAS knee pain over 6 months. The mean VAS pain was 55 mm (out of 100 mm) in our previous knee OA clinical trial with similar eligibility criteria.28 29 Using the control group data, we assume a between-participant SD of change in VAS pain of 24 mm. With 41 participants per arm, the study will have 80% power to detect a 15 mm difference in VAS pain between the intervention and control groups which is the minimum clinically important difference to be detected in OA trials,21 alpha 0.05, two-sided significance. Based on our previous knee OA trials,28 30–32 with a conservative assumption of 20% lost to follow-up, we will recruit 102 participants (51 in each arm of the study).
Statistical analyses
Intention-to-treat analyses of primary and secondary outcomes will be presented, including all participants in their randomised groups. Comparisons between randomised groups of change in knee pain at 6 months will be analysed using analysis of covariance (ANCOVA), adjusting for baseline value for knee pain outcomes. Differences in knee pain trajectories over 6 months will be examined using linear mixed-effects models with baseline value as the covariate, fixed factors for treatment, time and treatment×time interaction, and with an autoregressive (1) covariance matrix for repeated measures of individuals over time. Sensitivity analyses will be conducted for clinically important imbalances in baseline factors using multiple linear regression, or mixed models regression, as appropriate for the outcome measures. Multiple imputation of missing follow-up measures will be carried out as a sensitivity analysis when the percentage of missing data exceeds 5%. Subgroup analyses will be performed to examine whether the difference in outcomes between randomised groups varied based on sex, knee pain level and radiographic severity of knee OA. Analyses of treatment efficacy will be done by blinding individuals at the time of any protocol deviation and developing a model for the probability of deviation, followed by analyses using only the uncensored individuals where the weights are the inverse probability of censoring. This produces estimates of treatment effect as if there was full compliance with the protocol in this randomised controlled trial and is far preferable to per-protocol analyses based on (unweighted) observed compliance.33
Data integrity and management
All data will be collected using Monash Research Electronic Data Capture (REDCap). Paper copies of questionnaires (if participants prefer to complete the questionnaires on hard copy) will be stored in locked filing cabinets, with restricted access. Electronic data will be stored in REDCap, and exported to a password-protected server after data collection, separating the identifying and non-identifying information. The codes linking data to identifying participant information will be kept separately from the study data, under password protection and with restricted access.
Due to the COVID-19, we will be providing a telehealth option for all clinic visits. This will be done in such a way that will not compromise participant safety or the scientific integrity of the trial. This study uses REDCap for consent and data collection, facilitating telehealth options. For participants who use the telehealth option for the screening/baseline visit, we will seek consent electronically (eConsent). REDCap has a feature that implements consent forms through an online survey which can be accessed on a computer, mobile phone or tablet. The completed eConsent portable document format (PDF)s are stored in REDCap in a file repository under ‘PDF Survey Archive’. Physical examination will not be possible with telehealth option. Thus, height and weight will be self-reported.
Patient and public involvement
This study was informed by identification of clinical need in patients with OA attending our clinics. The clinical need and approach to translation is informed by the work with Musculoskeletal Australia in systematic reviews of consumers’ needs in OA.34 Once the trial has been published, participants will receive a study newsletter with details of the results which is suitable for a non-specialist audience.
Ethics and dissemination
Ethics approval has been obtained from the Alfred Hospital Ethics Committee (708/20) and Monash University Human Research Ethics Committee (28498). Written informed consent will be obtained from all the participants (online supplemental document). Trial results, regardless of statistical significance, will be published in peer-reviewed journals and presented at national and international conferences. On publication of the primary manuscript, participants will be informed of their group allocation and provided with the results.
bmjopen-2023-079489supp001.pdf (182KB, pdf)
Discussion
This randomised controlled trial is conducted to determine whether metformin 2 g daily over 6 months reduces knee pain in participants with symptomatic knee OA and concurrent overweight or obesity. If metformin proves effective in patients with symptomatic knee OA and concurrent overweight or obesity, it will offer an important therapeutic approach for obesity-metabolic syndrome phenotype of knee OA.
There are consistent chondroprotective, immunomodulatory and analgesic effects from metformin in preclinical cell and animal studies.13 In preclinical studies, in addition to chondroprotective and anti-inflammatory effects, metformin was shown to be able to improve pain, such that rats or mice treated with metformin showed increased paw withdraw latency indicative of reduction in pain.13 15 16 35 In human studies, a randomised, double-blind trial showed that the combination of metformin with meloxicam improved knee pain by at least 50% more than meloxicam alone.36 Additionally, one of the metformin’s pleiotropic effects is mild weight loss (~2.5%),37 which is important when tackling the slow insidious weight creep from early to middle adulthood,38–40 particularly when obesity is a well-known risk factor for OA, and for more symptomatic and more progressive knee OA. Slowing weight gain over time not only has been proven to improve knee pain,41 but also was estimated to reduce knee replacement by up to 28.4%.42 As such, metformin has the potential to play an important role in individuals who have knee OA with obesity-metabolic syndrome phenotype.
Studies have shown the beneficial effects of metformin in OA were mainly mediated by activation of the AMPK pathway.13 As a key regulator of energy homeostasis and metabolism, activation of AMPK regulates key downstream enzymes involved in metabolism and transcription factors that regulate gene expression. As such, activation of the AMPK pathway in liver, muscle and adipose results in decreased lipogenesis and increased fatty acid oxidation, explaining some of the pleiotropic effects of metformin in improving metabolic profiles.10
The study has several strengths. It is a randomised, double-blind, placebo-controlled trial which will provide high-quality evidence to address the aim of this study. Nevertheless, our study population is limited to those without a valid indication for metformin use, as it would be unethical to withhold metformin with a clinical indication, specifically people with diabetes, thus limiting the generalisability of the study results. The diabetic population is known to have more obesity and concurrent metabolic syndrome,43 and it is likely that those with diabetes and knee OA who will be excluded from this study, are the populations at greatest need for metformin, which may underestimate the potential effect of metformin in this study.
In summary, knee OA, specifically the obesity-metabolic syndrome phenotype, has limited effective treatment options. This study will provide high-quality evidence to determine whether metformin reduces knee pain in people with symptomatic knee OA with overweight or obesity over 6 months, with major clinical and public health importance for a potentially effective treatment option for knee OA to reduce knee pain and disease burden.
Supplementary Material
Acknowledgments
Ms Molly Bond, Mr Noor Abid, Mr Ashish Dinesh Nair, Dr Benjamin Sutu, Dr Talia Igel, Dr Luigi Zolio and Dr Rushab Shah have been involved in the coordination and/or execution of this study.
Footnotes
YZL and YW contributed equally.
Contributors: Conception and design: YW, DMU, AW and FC. Study execution and data acquisition: YL, YW, MME, AW and FC. Drafting of the manuscript: YL, YW and FC. Critical revision of the manuscript for important intellectual content and approval of the final manuscript: YW, DMU, MME, AW, SH and FC. Obtaining of funding: FC.
Funding: The study is funded by an Investigator Grant from the National Health and Medical Research Council of Australia (NHMRC APP1194829). YL is the recipient of National Health and Medical Research Council (NHMRC) Clinical Postgraduate Scholarship (#1133903) and Royal Australasian College of Physicians Woolcock Scholarship. YW is the recipient of NHMRC Translating Research into Practice Fellowship (APP1168185). DMU is a recipient of an NHMRC/Medical Research Future Fund (MRFF) Career Development Fellowship (Clinical Level 2 #1142809). MME is the recipient of Bangabandhu Science and Technology Fellowship from Ministry of Science and Technology, Government of the People's Republic of Bangladesh. FC is the recipient of NHMRC Investigator Grant (APP1194829).
Disclaimer: The funders of the study had no role in the study design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferket BS, Feldman Z, Zhou J, et al. Impact of total knee replacement practice: cost effectiveness analysis of data from the osteoarthritis initiative. BMJ 2017;356:j1131. 10.1136/bmj.j1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackerman IN, Bohensky MA, Zomer E, et al. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet Disord 2019;20:90. 10.1186/s12891-019-2411-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Esch M, Knoop J, van der Leeden M, et al. Clinical phenotypes in patients with knee osteoarthritis: a study in the Amsterdam osteoarthritis cohort. Osteoarthr Cartil 2015;23:544–9. 10.1016/j.joca.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 5.Knoop J, van der Leeden M, Thorstensson CA, et al. Identification of phenotypes with different clinical outcomes in knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2011;63:1535–42. 10.1002/acr.20571 [DOI] [PubMed] [Google Scholar]
- 6.Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford) 2015;54:588–600. 10.1093/rheumatology/keu464 [DOI] [PubMed] [Google Scholar]
- 7.Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med 2009;121:9–20. 10.3810/pgm.2009.11.2073 [DOI] [PubMed] [Google Scholar]
- 8.Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol 2013;25:114–8. 10.1097/BOR.0b013e32835a9414 [DOI] [PubMed] [Google Scholar]
- 9.Rojas LBA, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr 2013;5. 10.1186/1758-5996-5-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saisho Y. Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets 2015;15:196–205. 10.2174/1871530315666150316124019 [DOI] [PubMed] [Google Scholar]
- 11.Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet 2009;374:1677–86. 10.1016/S0140-6736(09)61457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Worsley R, Jane F, Robinson PJ, et al. Metformin for overweight women at midlife: a double-blind, randomized, controlled trial. Climacteric 2015;18:270–7. 10.3109/13697137.2014.954997 [DOI] [PubMed] [Google Scholar]
- 13.Lim YZ, Wang Y, Estee M, et al. Metformin as a potential disease-modifying drug in osteoarthritis: a systematic review of pre-clinical and human studies. Osteoarthr Cartil 2022;30:1434–42. 10.1016/j.joca.2022.05.005 [DOI] [PubMed] [Google Scholar]
- 14.Li H, Ding X, Terkeltaub R, et al. Exploration of metformin as novel therapy for osteoarthritis: preventing cartilage degeneration and reducing pain behavior. Arthritis Res Ther 2020;22. 10.1186/s13075-020-2129-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Zhang B, Liu W-X, et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann Rheum Dis 2020;79:635–45. 10.1136/annrheumdis-2019-216713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Na HS, Kwon JY, Lee S-Y, et al. Metformin attenuates monosodium-Iodoacetate-induced osteoarthritis via regulation of pain mediators and the autophagy-lysosomal pathway. Cells 2021;10:681. 10.3390/cells10030681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Hopewell S, Schulz KF, et al. Explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. classification of osteoarthritis of the knee. Arthritis Rheumatol 1986;29:1039–49. 10.1002/art.1780290816 [DOI] [PubMed] [Google Scholar]
- 19.Australian Public Assessment Report for Linagliptin / Metformin HCI . Proprietary product name: Trajentamet. Therapeutics goods administration health safety regulation; 2013.
- 20.Pham T, van der Heijde D, Altman RD, et al. OMERACT-OARSI initiative: osteoarthritis research society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr Cartil 2004;12:389–99. 10.1016/j.joca.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 21.Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis 2005;64:29–33. 10.1136/ard.2004.022905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- 23.Osborne RH, Hawthorne G, Lew EA, et al. Quality of life assessment in the community-dwelling elderly: validation of the assessment of quality of life (AQoL) instrument and comparison with the SF-36. J Clin Epidemiol 2003;56:138–47. 10.1016/s0895-4356(02)00601-7 [DOI] [PubMed] [Google Scholar]
- 24.Hochman JR, Gagliese L, Davis AM, et al. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthr Cartil 2011;19:647–54. 10.1016/j.joca.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 25.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 26.Kloppenburg M, Maheu E, Kraus VB, et al. OARSI clinical trials recommendations: design and conduct of clinical trials for hand osteoarthritis. Osteoarthr Cartil 2015;23:772–86. 10.1016/j.joca.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 27.Wluka AE, Ding C, Jones G, et al. The clinical correlates of articular cartilage defects in symptomatic knee osteoarthritis: a prospective study. Rheumatology (Oxford) 2005;44:1311–6. 10.1093/rheumatology/kei018 [DOI] [PubMed] [Google Scholar]
- 28.Bennell KL, Paterson KL, Metcalf BR, et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA 2021;326:2021–30. 10.1001/jama.2021.19415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai G, Laslett LL, Aitken D, et al. Zoledronic acid plus methylprednisolone Versus Zoledronic acid or placebo in symptomatic knee osteoarthritis: a randomized controlled trial. Ther Adv Musculoskelet Dis 2019;11:1759720X19880054. 10.1177/1759720X19880054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai G, Aitken D, Laslett LL, et al. Effect of intravenous zoledronic acid on Tibiofemoral cartilage volume among patients with knee osteoarthritis with bone marrow lesions: a randomized clinical trial. JAMA 2020;323:1456–66. 10.1001/jama.2020.2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Jones G, Hill C, et al. Effect of atorvastatin on knee cartilage volume in patients with symptomatic knee osteoarthritis: results from a randomised placebo-controlled trial. Arthritis Rheumatol 2021;73:2035–43. 10.1002/art.41760 [DOI] [PubMed] [Google Scholar]
- 32.Cai G, Jones G, Cicuttini FM, et al. Study protocol for a randomised controlled trial of diacerein versus placebo to treat knee osteoarthritis with effusion-synovitis (DICKENS). Trials 2022;23:768. 10.1186/s13063-022-06715-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernán MA, Lanoy E, Costagliola D, et al. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol 2006;98:237–42. 10.1111/j.1742-7843.2006.pto_329.x [DOI] [PubMed] [Google Scholar]
- 34.Wluka AE, Chou L, Briggs AM, et al. Understanding the needs of consumers with musculoskeletal conditions: consumers’ perceived needs of health information, health services and other non-medical services: a systematic scoping review. Melbourne: MOVE muscle, bone & joint health, 2016. [Google Scholar]
- 35.Park M-J, Moon S-J, Baek J-A, et al. Metformin augments anti-inflammatory and chondroprotective properties of mesenchymal stem cells in experimental osteoarthritis. J Immunol 2019;203:127–36. 10.4049/jimmunol.1800006 [DOI] [PubMed] [Google Scholar]
- 36.M. Mohammed M, J. Al-Shamma K, A. Jassim N. Evaluation of the clinical use of metformin or pioglitazone in combination with Meloxicam in patients with knee osteoarthritis; using knee injury and osteoarthritis outcome score. IJPS 2014;23:13–23. 10.31351/vol23iss2pp13-23 [DOI] [Google Scholar]
- 37.Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. Lancet 2022;399:259–69. 10.1016/S0140-6736(21)01640-8 [DOI] [PubMed] [Google Scholar]
- 38.Brebal K de M, Silveira J da, Menezes R de, et al. Weight gain and changes in nutritional status of Brazilian adults after 20 years of age: a time-trend analysis (2006-2012). Rev Bras Epidemiol 2020;23. 10.1590/1980-549720200045 [DOI] [PubMed] [Google Scholar]
- 39.Peeters A, Magliano DJ, Backholer K, et al. Changes in the rates of weight and waist circumference gain in Australian adults over time: a longitudinal cohort study. BMJ Open 2014;4:e003667. 10.1136/bmjopen-2013-003667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y, Manson JE, Yuan C, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 2017;318:255–69. 10.1001/jama.2017.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Lombard C, Hussain SM, et al. Effect of a low-intensity, self-management lifestyle intervention on knee pain in community-based young to middle-aged rural women: a cluster randomised controlled trial. Arthritis Res Ther 2018;20. 10.1186/s13075-018-1572-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hussain SM, Ackerman IN, Wang Y, et al. Trajectories of body mass index from early adulthood to late Midlife and incidence of total knee arthroplasty for osteoarthritis: findings from a prospective cohort study. Osteoarthr Cartil 2023;31:397–405. 10.1016/j.joca.2022.11.013 [DOI] [PubMed] [Google Scholar]
- 43.Katsiki N, Anagnostis P, Kotsa K, et al. Obesity, metabolic syndrome and the risk of microvascular complications in patients with diabetes mellitus. Curr Pharm Des 2019;25:2051–9. 10.2174/1381612825666190708192134 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-079489supp001.pdf (182KB, pdf)