Abstract
Background
Existing literature suggests that tertiary lymphatic structure (TLS) is associated with the progression of cancer. However, the prognostic role of TLS in digestive system cancers remains controversial. This meta-analysis aims to synthesize currently available evidence in the association between TLS and the survival of digestive system cancers.
Methods
We systematically searched three digital databases (PubMed, Embase, Web of Science) for articles published from database inception to December 23, 2022. Study selection criteria are based on PECO framework: P (population: patients with digestive system cancers), E (exposure: presence of TLS), C (comparator: absence of TLS), O (outcome: overall survival, OS; recurrence-free survival, RFS; disease-free survival, DFS). The Quality in Prognostic Studies (QUIPS) tool was used to assess risk of bias for included studies. The study protocol was registered with PROSPERO (CRD42023416307).
Results
A total of 25 studies with 6910 patients were included into the final meta-analysis. Random-effects models revealed that the absence of TLS was associated with compromised OS, RFS, and DFS of digestive system cancers, with pooled hazard ratios (HRs) of 1.74 (95% CI: 1.50–2.03), 1.96 (95% CI: 1.58–2.44), and 1.81 (95% CI: 1.49–2.19), respectively. Subgroup analyses disclosed a stronger TLS-survival association for pancreatic cancer, compared with other digestive system cancers.
Conclusion
TLS may be of prognostic significance for digestive system cancers. More original studies are needed to further corroborate this finding.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-11738-w.
Keywords: Tertiary lymphatic structure (TLS), Digestive system cancers, Prognosis, Meta-analysis
Background
Digestive system cancers, including esophageal carcinoma (EC), gastric cancer (GC), colorectal cancer (CRC), hepatocellular carcinoma (HCC), pancreatic cancer (PC), are leading causes of global cancer-related morbidity and mortality. CRC, GC, HCC, and EC take 4 places in the top 10 cancers by incidence [1–4]. Three out of the top five global cancer-related mortality can be ascribed to digestive system cancers [1]. In addition to high morbidity and mortality, the prognosis of digestive system tumors are not optimistic, with overall 5-year survival rates of 11.5%, 20.8%, and 33.3% for PC, HCC, and GC in the US from 2012 to 2018 [5–7]. Exploring meaningful prognostic markers of digestive system cancers are vital for clinical treatment of the patients.
Tertiary lymphoid structure (TLS) is defined as ectopic lymphocyte aggregates in non-lymphoid tissues when chronic inflammation like tumors, autoimmune diseases, and chronic infections arise after birth [8]. TLS includes a T-cell-rich zone containing dendritic cells (DCs) and a B-cell-rich zone containing germinal centers (GCs), surrounded by plasma cells, various lymphocytes freely pass through high endothelial venules (HEVs) [9, 10]. In function, cellular composition, and organization, TLS is similar to secondary lymphoid organs (SLOs). The concept of TLS was first proposed in 1990s [11], in subsequent studies, it has also been referred to as ectopic lymphoid structures (ELS) or tertiary lymphoid organ (TLO) [12, 13].
Controversies exist in the role of TLS in cancer progression. For instance, one study reported that regulatory T cells in tumor-associated TLS can suppress the endogenous immune response against tumors in a genetically engineered mouse model of lung adenocarcinoma [14], another study revealed that TLS formation reduced ovarian tumors growth in mouse model [15]. In recent years, some scholars have begun to investigate the prognostic significance of TLS in cancer patients, and the presence of TLS was found to be associated with a better prognosis in melanoma [16], breast cancer [17], and lung cancer [18]. However, fewer studies on this topic were related to digestive system tumors, with incongruent results [19–22].
Considering existing inconsistencies in the association between TLS and the survival of digestive system cancers, we aim to perform a systematic review and meta-analysis to synthesize currently available evidence.
Methods
Search strategy
This study was performed according to the Preferred Reporting Items for Systematic Evaluation and Meta-Analysis (PRISMA) statement guidelines [23]. We used the PECO [24] framework to clearly frame our study topic: P (population: patients with digestive system cancers), E (exposure: presence of TLS), C (comparator: absence of TLS), O (outcome: overall survival, OS; recurrence-free survival, RFS; disease-free survival, DFS). The study protocol was registered with PROSPERO (CRD42023416307).
We systematically searched three digital databases (PubMed, Embase, Web of Science) for articles published from database inception to December 23, 2022. According to our research theme, the keywords used for searching are closely related to “Tertiary Lymphoid Structure”, “digestive system”, “cancer”, and “prognosis”. A detailed search strategy is presented in the supplementary material (Page 2). This search resulted in an initial check of the titles and abstracts of the articles, followed by full-text review, manual inspection of the reference lists of all relevant papers were also performed to ensure no pertinent studies were missed according to the above strategy.
Inclusion and exclusion criteria
Eligible studies have to meet the following inclusion criteria: (1) Focused on patients with TLS expression in digestive system cancers; (2) TLS was measured according to standard methods; (3) Primary outcome of interest was OS, or RFS, or DFS; (4) Reported complete pathological staging information. Exclusion criteria are as follows: (1) Case reports, animal trials, reviews, or conference abstracts; (2) Investigated TLS in peritumoral tissues; (3) Did not report hazard ratio (HR) or its 95% confidence interval (CI); (4) Focused on only part of the TLS (immune cells, high endothelial vein, etc.) rather than the whole TLS; (5) Overlapping study subjects; (6) Studies published not in English.
Data extraction and quality evaluation
A standard data extraction form has been designed, two investigators (HS, YS) independently extracted the following information from the included studies: first author, year of publication, country of origin, cancer type/site, sample size, disease stage, laboratory methods, enrollment period of patients, follow-up time, criteria or cut-offs for determining TLS, outcome indicators, HR and 95% CI, presence of metastases. For studies reported both univariate and multivariate results, we extracted multivariate results, for studies only reported univariate results, we extracted univariate results, univariate and multivariate results were combined separately.
We used the Quality in Prognostic Studies (QUIPS) tool to assess risk of bias for included studies [25]. The QUIPS tool consists of six bias domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting. There are 3 to 7 different items for each bias domain. The risk of bias for a single study can be rated as low, moderate, or high.
Statistical analysis
Associations between TLS expression and the prognosis of digestive system cancers were evaluated by using pooled HRs from random-effects or fixed-effects models. The I2 was used to assess heterogeneity, usually I2 > 50% and p < 0.05 indicates substantial level of heterogeneity [26]. Sensitivity analyses were performed to test robustness of the combined estimations. Subgroup analysis was performed to estimate heterogeneity introduced by origin of study (China vs. other countries), sample size (< 200 vs. ≥ 200), metastases (yes vs. no), tumor types (ESCC vs. GC vs. CRC vs. HCC vs. PC), and cut-off criteria (presence vs. absence, high vs. low). Funnel plots, Egger’s [27] regression asymmetry test, and Begg’s [28] rank correlation test were used to examine potential publication bias. We use Endnote X9 to filter articles, all statistical analyses were performed using the R software (version 4.2.3), mainly “meta” and “forestploter” packages.
Results
Study selection
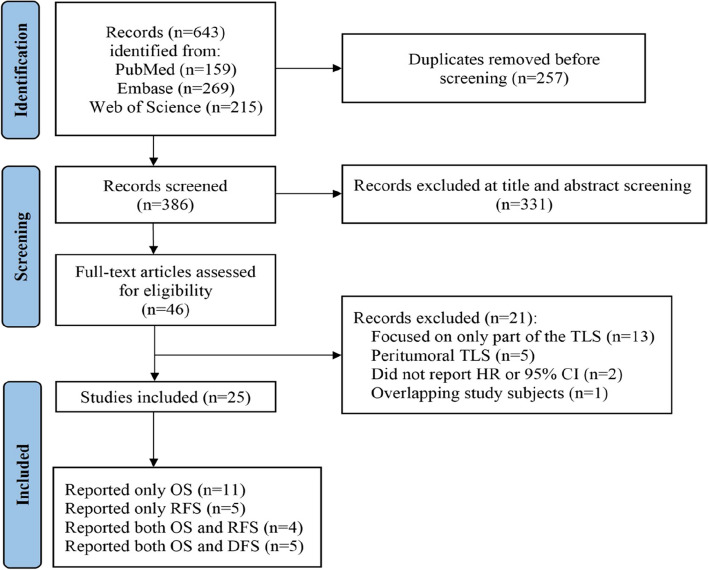
The literature screening process is shown in the PRISMA flowchart presented in Fig. 1. Starting with a total of 643 articles identified from the three databases based on the search strategy, after removing duplicate records, 257 articles remained. After browsing titles and abstracts, we screened out 46 studies that met the inclusion criteria, 21 were further excluded after careful full-text review because of disqualification. Finally, a total of 25 articles were included in the meta-analysis [20, 22, 29–51], including 20 articles reported OS, 9 articles reported RFS, and 5 articles reported DFS.
Fig. 1.
PRISMA flowchart for literature search and study selection
Characteristics of included studies
The risk of bias for all finally included studies in this meta-analysis is moderate or low (see in supplementary material, Table S1). The included studies were all retrospective in nature, their major characteristics were shown in Table 1: most studies (19 in 25) were published in 2020 and after; 19 studies were from Asian countries (China: 14, Japan: 5), 3 studies were from Europe (Finland: 1, France: 1, Italy: 1), 2 studies were from Oceania (Austria), and 1 study was from North America (United States); as to specific types of cancer, 4 studies investigated EC, 8 on GC, 4 on CRC, 5 on HCC, and 4 on PC; sample size ranged from 47 (Shota K, 2019, Japan) to 914 (He WT, 2020, China). The criteria for defining TLS were not identical among included studies, most studies used the HE&IHC methods to determine the presence or absence of TLS, and some studies used the number or density of TLS in the tumor to determine high or low distribution of TLS.
Table 1.
Characteristics of included studies
| Author | Year | Country | Types | Patients(n) | Stage | Enrollment period | Follow-up time | Laboratory | Estimate TLS criteria | Cut-off criteria | Metastases | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deguchi S [29] | 2022 | Japan | ESCC | 236 | 0-IVa | 2014 | median:51.0 month | HE、IHC | GC-TLS density | High vs. Low | No | RFS |
| Li RT [30] | 2022 | China | ESCC | 122 | I-IV | 2017.1–2018.12 | More than 2 years | HE、IHC | TLS | Presence vs. Absence | No | OS, DFS |
| Ling YH [31] | 2022 | China | ESCC | 394 | I-III | 2008–2017 | Median:68.57 month | HE、IHC | TLS | Presence vs. Absence | No | OS, DFS |
| Jiang Q [32] | 2022 | China | GC | 292 | I-IV | 1983–2016 | Until 2020.1 | HE、IHC | Mature TLS | High vs. Low | Yes | OS |
| Kemi N [33] | 2022 | Finland | GC | 721 | I-IV | 2002–2008 | Median: 28.0 month | HE | TLS diameter | High vs. Low | NA | OS |
| Zhan Z [34] | 2022 | China | CRC | 203 | I-IV | 2014.01–2017.07 | Median: 50.0 month | IHC | TLS | High vs. None or Low | Yes | OS, DFS |
| Li JH [35] | 2022 | China | HCC | 150 | I-IV | 2014–2018 | Until 2020.12.30 | HE、IHC、IF | TLS | Positive vs. Negative | Yes | RFS |
| Nie Y [36] | 2022 | China | HCC | 145 | 0-C | 2015–2017 | More than 2 years | HE、IHC | TLS Grade | GradeB + C vs. Absence | Yes | OS |
| Wen SD [37] | 2022 | China | HCC | 126 | A-C | 2013.04–2019.08 | Median: 40.0 month | HE、IHC、IF | TLS density | High vs. Low | Yes | OS |
| Yu JS [38] | 2022 | China | GC | 118 | I-III | 2009–2014 | Until 2019.05 | IHC | TLS | High vs. Low | No | OS, DFS |
| Cheng N [39] | 2021 | China | GC | 846 | I-IV | 2008.12–2019.06 | Mean: 22.1 month | HE、IHC | TLS | Absent vs. Present | NA | OS |
| Yamakoshi Y [40] | 2021 | Japan | GC | 199 | Ib-IV | 2006–2008 | Median: 49.0 month | IHC | TLS (B cell density) | Low vs. High | No | OS |
| Gunderson AJ [41] | 2021 | America | PC | 63 | I-III | NA | NA | HE、IHC | TLS | Presence vs. Absence | No | OS |
| Mori T [42] | 2021 | Japan | GC | 261 | I-IV | 2014–2017 | NA | IHC | TLS | High vs. Low | Yes | OS |
| Zhao YY [43] | 2020 | China | ESCC | 593 | T1 | 2009–2018 | Median: 42.0 month | HE | TLS | Presence vs. Absence | No | OS |
| He WT [44] | 2020 | China | GC | 914 | I-III | 2009–2014 | Until 2018.1 | HE | TLS-SUM | High vs. Low | No | OS |
| Li Q [45] | 2020 | China | GC | 63 | I-III | 2001.01–2013.12 | Until 2011.11.30 | HE | Number of TLS | High vs. Low | No | OS |
| Li H [46] | 2020 | China | HCC | 303 | I-IV | 2009.03–2013.8 | Median: 61.3 month | HE、IHC | TLS | Presence vs. Absence | Yes | OS, RFS |
| Zhang WH [47] | 2020 | China | PC | 182 | I-III | 2006–2018 | Median: 60.0 month | HE、IHC | TLS | Presence vs. Absence | No | OS, RFS |
| Shota K [48] | 2019 | Japan | PC | 47 | 0-IV | 2009.1–2015.12 | Median:24.98 month | IHC | TLO density | High vs. Low | Yes | OS |
| Calderaro J [22] | 2018 | France | HCC | 273 | B-C | 1995–2016 | 2 years after surgery | HE | TLS | Presence vs. Absence | Yes | RFS |
| Posch F [20] | 2017 | Austria | CRC | 109 | II-III | 1996.01–2011.06 | 3 years | IF | Number of TLS | < Q1 vs. ≥ Q1 | No | RFS |
| Schweiger T [49] | 2016 | Austria | CRC | 57 | I-IV | 2009.04–2014.06 | Median: 30.0 month | IHC | TLS | Presence vs. Not present | Yes | OS, RFS |
| Hiraoka N [50] | 2015 | Japan | PC | 308 | Ia-IV | 1990–2004 | Median: 17.6 month | HE、IHC | TLO | Absence vs. Presence | Yes | OS, DFS |
| Di Caro G [51] | 2014 | Italy | CRC | 185 | II-III | 1997.01–2005.11 | 4.71 years | IHC | TLT | < Median vs. ≥ Median | No | RFS |
Abbreviations: ESCC esophageal squamous cell carcinoma, CRC colorectal cancer, GC gastric cancer, HCC hepatocellular carcinoma, PC pancreatic cancer, HE hematoxylin eosin staining, IHC immunohistochemistry, IF immunofluorescence, OS overall survival, RFS relapse-free survival, DFS disease-free survival, TLS tertiary lymphoid structure, iTLS intratumoral tertiary lymphoid structure, TLO tertiary lymphoid organ, GC-TLS germinal center tertiary lymphoid structure, NA not available
TLS with OS of digestive system cancers
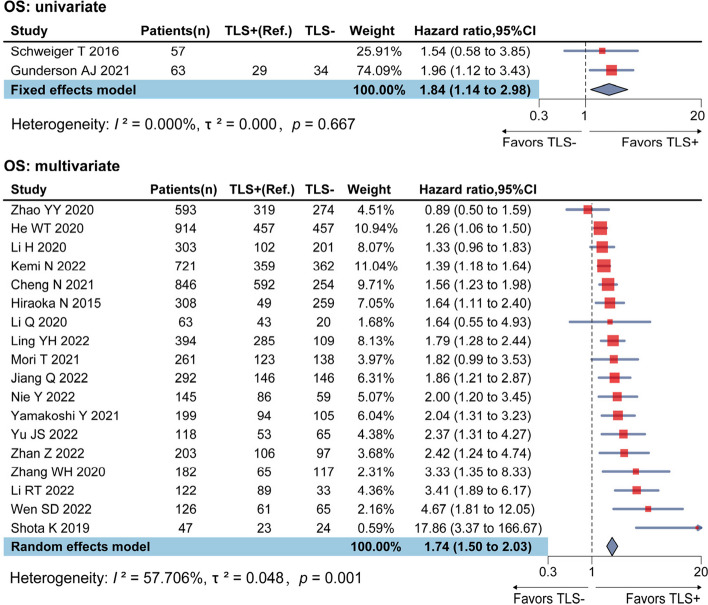
Twenty studies reported HR (95% CI) of TLS on OS, among them, only 2 studies reported univariate analysis results, with insignificant heterogeneity (I2 = 0.00%, p = 0.67), and the combined HR for univariate results was 1.84 (95% CI: 1.14–2.98). Studies (n = 18) reported multivariate analysis results showed a high level of heterogeneity (I2 = 57.71%, p < 0.01), random-effects model yielded a statistically significant combined HR of 1.74 (95% CI: 1.50–2.03), suggesting the absence of TLS was associated with compromised OS for digestive system cancer patients in general (Fig. 2, controlled covariates for multivariate analysis were summarized in Table S2 of supplementary material). Sensitivity analysis using leave-one-out strategy revealed ideal robustness for this combined association (see in supplementary material, Figure S1).
Fig. 2.
Forest plots for the association between TLS and OS in digestive system cancers
Stratified analyses were performed sequentially by using sample size, metastasis, cut-off criteria of TLS, and cancer types. Sample size presented notable influence on the combined HR of OS: compared with studies of smaller sample size (< 200, HR = 2.52, 95% CI: 1.99–3.20), studies of larger sample size (≥ 200) reached a more conservative pooled association (HR = 1.45, 95% CI: 1.31–1.61), although there was no significant difference between tumor types, the combined HR of pancreatic cancer (HR = 3.37, 95% CI: 1.09–10.37) was higher than other malignant tumors (Table 2, Figure S3-6 in supplementary material).
Table 2.
Stratified analyses for the association between TLS and OS of digestive system cancers by key factors
| Subgroup | Number of studies | Pooled results HR(95%CI) | Heterogeneity test | ||
|---|---|---|---|---|---|
| I2(%) | p | ||||
| Sample size | ≥ 200 | 10 | 1.34 (1.31–1.61) | 25.51 | 0.21 |
| < 200 | 8 | 2.52 (1.99–3.20) | 22.85 | 0.25 | |
| Metastasis | No | 8 | 1.82 (1.34–2.48) | 68.981 | < 0.01 |
| Yes | 8 | 1.86 (1.49–2.33) | 48.98 | 0.06 | |
| NA | 2 | 1.44 (1.26–1.65) | 0 | 0.44 | |
| Cut-off criteria | Presence vs. Absence | 8 | 1.69 (1.36–2.10) | 48.15 | 0.05 |
| High vs. Low | 10 | 1.85 (1.45–2.35) | 64.64 | < 0.01 | |
| Types | ESCC | 3 | 1.76 (0.86–3.61) | 80.21 | < 0.01 |
| GC | 8 | 1.54 (1.33–1.79) | 28.80 | 0.20 | |
| CRC | 1 | 2.42 (1.24–4.74) | NA | NA | |
| HCC | 3 | 2.05 (1.08–3.88) | 70.89 | 0.03 | |
| PC | 3 | 3.37 (1.09–10.37) | 71.76 | 0.03 | |
Abbreviations: ESCC esophageal squamous cell carcinoma, CRC colorectal cancer, GC gastric cancer, HCC hepatocellular carcinoma, PC pancreatic cancer, OS overall survival, RFS relapse-free survival, DFS disease-free survival, TLS tertiary lymphoid structure, NA not available
TLS with RFS and DFS of digestive system cancers
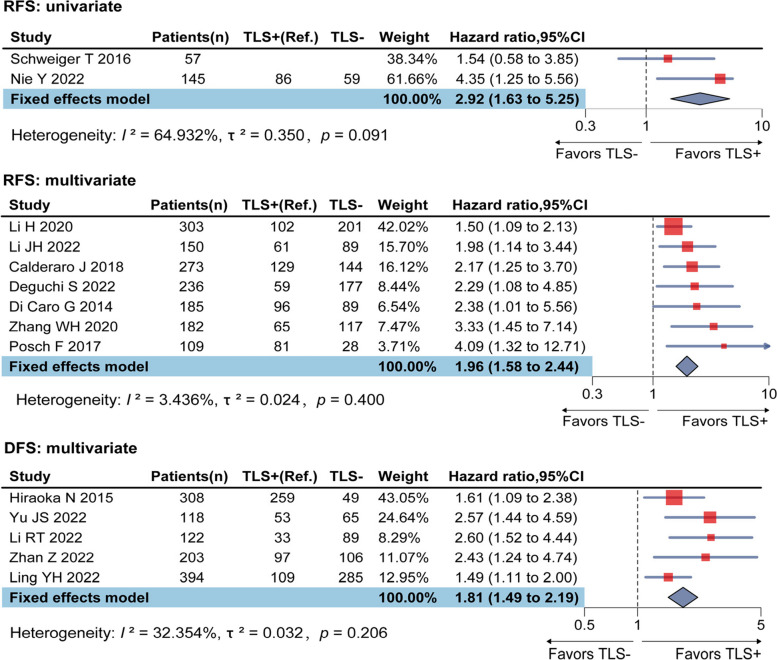
Nine studies reported HR of TLS on RFS: 2 studies reported univariate analysis results, and 7 studies reported multivariate analysis results. The univariate analysis results showed insignificant heterogeneity (I2 = 64.93%, p = 0.09), with a combined HR of 2.92 (95% CI: 1.63–5.25). Heterogeneity for multivariate analysis results were also insignificant (I2 = 3.44%, p = 0.40), fixed-effects model reached a pooled HR of 1.96 (95% CI: 1.58–2.44) (Fig. 3, controlled covariates for multivariate analysis were summarized in Table S2 of supplementary material). Five studies reported HR of TLS on DFS, all used multivariate analysis, with insignificant heterogeneity (I2 = 32.35%, p = 0.21), the pooled HR was statistically significant (HR = 1.81, 95% CI: 1.49–2.19) (Fig. 3, controlled covariates for multivariate analysis were summarized in Table S2 of supplementary material). Sensitivity analysis revealed ideal robustness for included studies of RFS and DFS (see in supplementary material, Figure S2).
Fig. 3.
Forest plots for the associations between TLS and RFS, DFS in digestive system cancers
Publication bias
We used funnel plots with Egger’s and Begg’s tests to detect potential publication bias. Funnel plots of OS and RFS showed that the included studies were not perfectly symmetrical, with significant publication bias as suggested by Begg’s and Egger’s tests (see in supplementary material, Figure S7-8). Funnel plot of DFS showed that the included studies were approximately symmetrical, with insignificant publication bias (see in supplementary material, Figure S9).
Discussion
In this systematic review and meta-analysis, we investigated the association between TLS and the prognosis of patients with digestive system tumors. The synthesized results indicate that based on currently available evidence, the absence of TLS was associated with significantly inferior OS, DFS, and RFS in patients of digestive system cancers. Besides, strength of the association between TLS and OS varied by tumor types, stronger in patients with pancreatic cancer. These important findings suggest that TLS probably plays a role in the prognosis of digestive system tumors, especially for pancreatic cancer.
A successful antitumor immune response requires the presence, activation, and costimulation of all lymphoid components of the immune system, including CD8 + T cells, CD4 + T cells, B cells, and innate lymphocytes. TLS represents a well-organized cluster of tumor-infiltrating lymphocytes and elicits an advanced immune response [52]. Studies have shown that in colorectal cancer, TLS cooperates with tumor-infiltrating T lymphocytes for a coordinated antitumor immune response and predicts a better prognosis [40]. TLS was associated with increased intra-tumoral CD3 + , CD8 + , CD20 + , decreased infiltration of Foxp3 + and CD68 + cells, and predicted better prognosis in early-stage hepatocellular carcinoma [35]. However, studies have also shown that TLS may promote the development of tumors: in a mouse model, TLS that developed in the inflamed liver during hepatitis provided a growth environment for malignant progenitor hepatocytes and was associated with an increased risk of late recurrence and decreased survival [21]. Therefore, it may be reasonable to speculate that inflammation and infection-induced TLS functions differently from cancer-induced TLS, or only intra-tumor TLS is a significant part of the antitumor immune response.
The prognostic propensity of TLS in digestive system cancers has clinical significance. On one hand, for doctors, the detection of TLS may help them preliminarily evaluate mortality risk of the patients. On the other hand, considering the nature of TLS, use of lymphoid chemokines and their drivers may help induce TLS neogenesis, a promising direction for cancer treatment. It has already been possible to induce local TLS in mouse models [10, 53–55]. In gastric adenomas, homeostatic chemokines (including CXCL13, CCLL9 and CCL21) were associated with the formation of TLS [54]. In experimental breast cancer and pancreatic neuroendocrine tumor models, the combination of anti-angiogenesis and anti-PDL1 therapy increased HEV formation and subsequent TLS formation [55]. Adoptive transfer of Hhep-specific CD4 + T cells to Tfh deficient Bcl6fl/flCd4Cre mice restored antitumor immunity and suggested a therapeutic pathway to treat CRC [53].
The significant association between TLS and survival outcomes did not vary much for studies conducted in patients with or without distant metastases, suggesting that TLS may confer similar survival benefit regardless of disease progression. Published studies revealed that TLS was associated with better survival for metastatic patients of lung cancer, ovarian cancer, and cutaneous melanoma [56–58]. Perhaps because the presence of TLS in metastatic sites is a critical factor for tumor-infiltrating lymphocyte levels [56], a crucial factor in anti-tumor immunity which relates to improved prognosis in a variety of solid cancer types [59]. Another important finding would be that the association between TLS and OS was significantly stronger in pancreatic cancer patients. Over 90% pancreatic cancer cases are pancreatic adenocarcinoma (PDAC), one study demonstrated that TLS is almost universal in human PDAC tissue [50], and TLS locates at intratumoral tissue was generally associated with better survival [60]. Moreover, two included studies estimated the association between TLS density and prognosis of gastrointestinal tumors with positive findings [37, 48], suggesting that not only the presence, but also the density of TLS in tumor tissue should be concerned.
This meta-analysis is an exhaustive attempt in synthesizing currently available evidence on the association between TLS and survival in patients with digestive system tumors. The major findings of this study can be consolidated by meticulous literature screening process and strict quality evaluation standard. However, limited number of original studies included, especially for RFS and DFS, hampered effective analysis in discussing possible sources of heterogeneity. Besides, as most of the included studies were originated from 2 Asian countries (China and Japan), the combined estimations of this meta-analysis may suffer from selection bias, more studies should be done in other countries or continents.
Conclusion
In this meta-analysis, we systematically evaluated the prognostic significance of TLS in digestive system cancers. We found that the absence of TLS was in general associated with worse survival, especially for pancreatic cancer patients. More original studies need to be done, particularly in patients outside Asian countries, to further corroborate this suspected beneficial role of TLS in survival of gastrointestinal tumors.
Supplementary Information
Additional file 1: Supplementary Materials. Table S1. Quality assessment details for included studies. Table S2. Summary of controlled covariates in multivariate analyses. Figure S1. Sensitivity analysis for TLS with OS in digestive system cancers patients. Figure S2. Sensitivity analysis for TLS with RFS and DFS in digestive system cancers patients. Figure S3. Forest plots for stratified analysis by sample size in the association between TLS and OS of digestive system cancers. Figure S4. Forest plots for stratified analysis by metastasis state in the association between TLS and OS of digestive system cancers. Figure S5. Forest plots for stratified analysis by cut-off criteria in the association between TLS and OS of digestive system cancers. Figure S6. Forest plots for stratified analysis by tumor types in the association between TLS and OS of digestive system cancers. Figure S7. Funnel plot for publication bias of included studies on the association between TLS and the OS of digestive system cancers. Figure S8. Funnel plot for publication bias of included studies on the association between TLS and the RFS of digestive system cancers. Figure S9. Funnel plot for publication bias of included studies on the association between TLS and the DFS of digestive system cancers.
Authors’ contributions
YX and WC conceived and designed the study. HS, YS, HR, JP, QL, GZ, YH, and SL were responsible for the collection and assembly of data, data analysis, and interpretation. HS and YS were involved in writing the manuscript. YX and WC revised the manuscript. All authors read and approved the final manuscript. HS and YS contributed equally to this work.
Funding
This study was supported by Basic Research Program of Yunnan (202101AT070539), Top Young Talents of Yunnan Ten Thousand Talents Plan (YNWR-QNBJ-2018–286).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Sun and Yuanyu Shi contributed equally as joint first authors.
Contributor Information
Wei Chang, Email: 1397978466@qq.com.
Yuanyuan Xiao, Email: 33225647@qq.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology. 2022;163(3):649–658. doi: 10.1053/j.gastro.2022.05.054. [DOI] [PubMed] [Google Scholar]
- 4.Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338–344. doi: 10.1136/gutjnl-2022-327736. [DOI] [PubMed] [Google Scholar]
- 5.Cancer of the Pancreas - Cancer Stat Facts. SEER. [https://seer.cancer.gov/statfacts/html/pancreas.html]. Accessed 18 Apr 2023.
- 6.Cancer of the Liver and Intrahepatic Bile Duct - Cancer Stat Facts. SEER. [https://seer.cancer.gov/statfacts/html/livibd.html]. Accessed 18 Apr 2023.
- 7.Cancer of the Stomach - Cancer Stat Facts. SEER. [https://seer.cancer.gov/statfacts/html/stomach.html]. Accessed 18 Apr 2023.
- 8.Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science. 2022;375(6576):eabf9419. doi: 10.1126/science.abf9419. [DOI] [PubMed] [Google Scholar]
- 9.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 10.Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 11.Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- 12.Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33(6):297–305. doi: 10.1016/j.it.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6(3):205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 14.Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, et al. Regulatory T Cells in Tumor-Associated Tertiary Lymphoid Structures Suppress Anti-tumor T Cell Responses. Immunity. 2015;43(3):579–590. doi: 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaurio RA, Anadon CM, Lee Costich T, Payne KK, Biswas S, Harro CM, et al. TGF-β-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures. Immunity. 2022;55(1):115–128. doi: 10.1016/j.immuni.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, et al. 12-Chemokine Gene Signature Identifies Lymph Node-like Structures in Melanoma: Potential for Patient Selection for Immunotherapy? Sci Rep. 2012;2:765. doi: 10.1038/srep00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123(7):2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, et al. Dendritic Cells in Tumor-Associated Tertiary Lymphoid Structures Signal a Th1 Cytotoxic Immune Contexture and License the Positive Prognostic Value of Infiltrating CD8+ T Cells. Cancer Res. 2014;74(3):705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 19.Bento DC, Jones E, Junaid S, Tull J, Williams GT, Godkin A, et al. High endothelial venules are rare in colorectal cancers but accumulate in extra-tumoral areas with disease progression. OncoImmunology. 2015;4(3):e974374. doi: 10.4161/2162402X.2014.974374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posch F, Silina K, Leibl S, Mündlein A, Moch H, Siebenhüner A, et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. OncoImmunology. 2018;7(2):e1378844. doi: 10.1080/2162402X.2017.1378844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkin S, Yuan D, Stein I, Taniguchi K, Weber A, Unger K, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol. 2015;16(12):1235–1244. doi: 10.1038/ni.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calderaro J, Petitprez F, Becht E, Laurent A, Hirsch TZ, Rousseau B, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. 2019;70(1):58–65. doi: 10.1016/j.jhep.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, Bossuyt PM, Boutron I, Hoffmann TC, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121(Pt 1):1027–1031. doi: 10.1016/j.envint.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li TJ, Page MJ, et al. Cocbrane Handbook for Systematic Reviews of Interventions, 2nd edition. The Cochrane Collaboration. Available from https://onlinelibrary.wiley.com/doi/book/10.1002/9781119536604. Accessed 20 Sept 2019.
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 29.Deguchi S, Tanaka H, Suzuki S, Natsuki S, Mori T, Miki Y, et al. Clinical relevance of tertiary lymphoid structures in esophageal squamous cell carcinoma. BMC Cancer. 2022;22:699. doi: 10.1186/s12885-022-09777-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li RT, Huang X, Yang W, Wang J, Liang Y, Zhang T, et al. Tertiary lymphoid structures favor outcome in resected esophageal squamous cell carcinoma. J Pathol Clin Res. 2022;8(5):422–435. doi: 10.1002/cjp2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling YH, Zhong J, Weng Z, Lin G, Liu C, Pan C, et al. The prognostic value and molecular properties of tertiary lymphoid structures in oesophageal squamous cell carcinoma. Clin Transl Med. 2022;12(10):e1074. doi: 10.1002/ctm2.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Q, Tian C, Wu H, Min L, Chen H, Chen L, et al. Tertiary lymphoid structure patterns predicted anti-PD1 therapeutic responses in gastric cancer. Chin J Cancer Res. 2022;34(4):365–382. doi: 10.21147/j.issn.1000-9604.2022.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemi N, Ylitalo O, Väyrynen JP, Helminen O, Junttila A, Mrena J, Böhm J, Kauppila JH. Tertiary lymphoid structures and gastric cancer prognosis. APMIS. 2023;131(1):19–25. doi: 10.1111/apm.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhan Z, Shi-Jin L, Yi-Ran Z, Zhi-Long L, Xiao-Xu Z, Hui D, et al. High endothelial venules proportion in tertiary lymphoid structure is a prognostic marker and correlated with anti-tumor immune microenvironment in colorectal cancer. Ann Med. 2023;55(1):114–126. doi: 10.1080/07853890.2022.2153911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li JH, Nie Y, Jia W, Wu W, Song W, Li Y. Effect of Tertiary Lymphoid Structures on Prognosis of Patients with Hepatocellular Carcinoma and Preliminary Exploration of Its Formation Mechanism. Cancers (Basel) 2022;14(20):5157. doi: 10.3390/cancers14205157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nie Y, Fan H, Li J, Lei X, Zhang T, Wang Y, et al. Tertiary lymphoid structures: Associated multiple immune cells and analysis their formation in hepatocellular carcinoma. FASEB J. 2022;36(11):e22586. doi: 10.1096/fj.202200269RR. [DOI] [PubMed] [Google Scholar]
- 37.Wen SD, Chen Y, Hu C, Du X, Xia J, Wang X, et al. Combination of Tertiary Lymphoid Structure and Neutrophil-to-Lymphocyte Ratio Predicts Survival in Patients With Hepatocellular Carcinoma. Front Immunol. 2021;12:788640. doi: 10.3389/fimmu.2021.788640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu JS, Huang WB, Zhang YH, Chen J, Li J, Fu HF, et al. The association of immune cell infiltration and prognostic value of tertiary lymphoid structures in gastric cancer. Neoplasma. 2022;69(4):886–898. doi: 10.4149/neo_2022_220128N123. [DOI] [PubMed] [Google Scholar]
- 39.Cheng N, Li P, Cheng H, Zhao X, Dong M, Zhang Y, et al. Prognostic Value of Tumor-Infiltrating Lymphocytes and Tertiary Lymphoid Structures in Epstein-Barr Virus-Associated and -Negative Gastric Carcinoma. Front Immunol. 2021;12:692859. doi: 10.3389/fimmu.2021.692859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamakoshi Y, Tanaka H, Sakimura C, Mori T, Deguchi S, Yoshii M, et al. Association between the preoperative neutrophil-to-lymphocyte ratio and tertiary lymphoid structures surrounding tumor in gastric cancer. Mol Clin Oncol. 2021;14(4):76. doi: 10.3892/mco.2021.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gunderson AJ, Rajamanickam V, Bui C, Bernard B, Pucilowska J, Ballesteros-Merino C, et al. Germinal center reactions in tertiary lymphoid structures associate with neoantigen burden, humoral immunity and long-term survivorship in pancreatic cancer. Oncoimmunology. 2021;10(1):1900635. [DOI] [PMC free article] [PubMed]
- 42.Mori T, Tanaka H, Deguchi S, Yamakoshi Y, Miki Y, Yoshii M, et al. Clinical efficacy of nivolumab is associated with tertiary lymphoid structures in surgically resected primary tumors of recurrent gastric cancer. PLoS ONE. 2022;17(1):e0262455. doi: 10.1371/journal.pone.0262455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao YY, Xu E, Yang X, Zhang Y, Chen H, Wang Y, Jin M. Tumor infiltrative growth pattern correlates with the immune microenvironment and is an independent factor for lymph node metastasis and prognosis in stage T1 esophageal squamous cell carcinoma. Virchows Arch. 2020;477(3):401–408. doi: 10.1007/s00428-020-02801-z. [DOI] [PubMed] [Google Scholar]
- 44.He WT, Zhang D, Liu H, Chen T, Xie J, Peng L, et al. The High Level of Tertiary Lymphoid Structure Is Correlated With Superior Survival in Patients With Advanced Gastric Cancer. Front Oncol. 2020;10:980. doi: 10.3389/fonc.2020.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q, Zhang D, He W, Chen T, Yan Z, Gao X, et al. CD8+ T cells located in tertiary lymphoid structures are associated with improved prognosis in patients with gastric cancer. Oncol Lett. 2020;20(3):2655–2664. doi: 10.3892/ol.2020.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, Wang J, Liu H, Lan T, Xu L, Wang G, et al. Existence of intratumoral tertiary lymphoid structures is associated with immune cells infiltration and predicts better prognosis in early-stage hepatocellular carcinoma. Aging (Albany NY) 2020;12(4):3451–3472. doi: 10.18632/aging.102821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang WH, Wang WQ, Han X, Gao HL, Xu SS, Li S, et al. Infiltrating pattern and prognostic value of tertiary lymphoid structures in resected non-functional pancreatic neuroendocrine tumors. J Immunother Cancer. 2020;8(2):e001188. doi: 10.1136/jitc-2020-001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuwabara S, Tsuchikawa T, Nakamura T, Hatanaka Y, Hatanaka KC, Sasaki K, et al. Prognostic relevance of tertiary lymphoid organs following neoadjuvant chemoradiotherapy in pancreatic ductal adenocarcinoma. Cancer Sci. 2019;110(6):1853–1862. doi: 10.1111/cas.14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweiger T, Berghoff AS, Glogner C, Glueck O, Rajky O, Traxler D, et al. Tumor-infiltrating lymphocyte subsets and tertiary lymphoid structures in pulmonary metastases from colorectal cancer. Clin Exp Metastasis. 2016;33(7):727–739. doi: 10.1007/s10585-016-9813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112(11):1782–1790. doi: 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014;20(8):2147–2158. doi: 10.1158/1078-0432.CCR-13-2590. [DOI] [PubMed] [Google Scholar]
- 52.Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. 2021;18(4):842–859. doi: 10.1038/s41423-020-00565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Overacre-Delgoffe AE, Bumgarner HJ, Cillo AR, Burr AHP, Tometich JT, Bhattacharjee A, et al. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity. 2021;54(12):2812–2824. doi: 10.1016/j.immuni.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hill DG, Yu L, Gao H, Balic JJ, West A, Oshima H, et al. Hyperactive gp130/STAT3-driven gastric tumourigenesis promotes submucosal tertiary lymphoid structure development. Int J Cancer. 2018;143(1):167–178. doi: 10.1002/ijc.31298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9(385):eaak9679. doi: 10.1126/scitranslmed.aak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee M, Heo SH, Song IH, Rajayi H, Park HS, Park IA, et al. Presence of tertiary lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis. Mod Pathol. 2019;32(1):70–80. doi: 10.1038/s41379-018-0113-8. [DOI] [PubMed] [Google Scholar]
- 57.Montfort A, Pearce O, Maniati E, Vincent BG, Bixby L, Böhm S, et al. A Strong B-cell Response Is Part of the Immune Landscape in Human High-Grade Serous Ovarian Metastases. Clin Cancer Res. 2017;23(1):250–262. doi: 10.1158/1078-0432.CCR-16-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lynch KT, Young SJ, Meneveau MO, Wages NA, Engelhard VH, Slingluff CL, Jr, et al. Heterogeneity in tertiary lymphoid structure B-cells correlates with patient survival in metastatic melanoma. J Immunother Cancer. 2021;9(6):e002273. doi: 10.1136/jitc-2020-002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brummel K, Eerkens AL, de Bruyn M, Nijman HW. Tumour-infiltrating lymphocytes: from prognosis to treatment selection. Br J Cancer. 2023;128(3):451–458. doi: 10.1038/s41416-022-02119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145(5):1121–1132. doi: 10.1053/j.gastro.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Materials. Table S1. Quality assessment details for included studies. Table S2. Summary of controlled covariates in multivariate analyses. Figure S1. Sensitivity analysis for TLS with OS in digestive system cancers patients. Figure S2. Sensitivity analysis for TLS with RFS and DFS in digestive system cancers patients. Figure S3. Forest plots for stratified analysis by sample size in the association between TLS and OS of digestive system cancers. Figure S4. Forest plots for stratified analysis by metastasis state in the association between TLS and OS of digestive system cancers. Figure S5. Forest plots for stratified analysis by cut-off criteria in the association between TLS and OS of digestive system cancers. Figure S6. Forest plots for stratified analysis by tumor types in the association between TLS and OS of digestive system cancers. Figure S7. Funnel plot for publication bias of included studies on the association between TLS and the OS of digestive system cancers. Figure S8. Funnel plot for publication bias of included studies on the association between TLS and the RFS of digestive system cancers. Figure S9. Funnel plot for publication bias of included studies on the association between TLS and the DFS of digestive system cancers.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.