Abstract
Background
Physical activity was known to be the protective factor against frailty. Technology acceptance is associated with behavioural intention to technology usage. Technology has been effective in promoting healthy behaviour of physical activity. The purposes of this study were to examine the association between physical activity and technology acceptance with frailty and examine the moderation effect of technology acceptance on physical activity and frailty. We hypothesize that 1) physical activity and technology acceptance are associated with frailty, and 2) technology acceptance moderates the association of physical activity with frailty.
Methods
This study employed a cross-sectional design and was conducted in the community settings of Hong Kong in 2021. Eligible participants were old people aged ≥60 and were community-dwelling. Key variables included physical activity measured by Rapid Assessment of Physical Activity (RAPA), social network measured by Lubben Social Network Scale-Six items (LSNS-6); depressive symptoms measured by Patient Health Questionnaire-Nine items (PHQ-9), technology acceptance measured by Senior Technology Acceptance Model-14 items (STAM-14) and frailty measured by Fatigue, Resistance, Ambulation, Illnesses, & Loss of Weight scale (FRAIL). Ordinal logistic regression was employed to test the hypotheses. The moderation effect was examined by introducing an interaction term formed by the multiplication of an independent variable (i.e., physical activity) and a moderating variable (i.e., technology acceptance).
Results
This study recruited 380 eligible participants with a mean age of 66.5 years. Technology acceptance (Beta = − 0.031, p < 0.001, Pseudo-R2 = 0.087) and physical activity (Beta = − 0.182, p = 0.003, Pseudo-R2 = 0.027) were associated with frailty in the unadjusted models. Technology acceptance (Beta = − 0.066, p < 0.001) and physical activity (Beta = − 1.192, p < 0.001) were also associated with frailty in the fully adjusted model (Pseudo-R2 = 0.352). Interaction term formed by the multiplication of technology acceptance and physical activity (Beta = 0.012, p = 0.001) was associated with frailty. Physical activity was significantly associated with frailty in the lower technology acceptance subgroup (Beta = − 0.313, p = 0.002) in the subgroup analysis. However, in the subgroup of higher technology acceptance, the association of physical activity (Beta = 0.104, p = 408) on frailty became positive but not significant.
Conclusions
This study showed that physical activity and technology acceptance were associated with frailty, and technology acceptance moderated the association of physical activity with frailty. This study recommends engaging older adults in physical activity to combat frailty preferentially in those with a lower level of technology acceptance.
Keywords: Frailty, Physical activity, Technology acceptance, Older adults
Introduction
Frailty is defined as a clinical syndrome with multiple causes that is characterized by diminished strength, endurance and reduced physiologic function that increases an individual’s vulnerability to developing increased dependency and/or death [1]. The prevalence of frailty among community-dwelling older adults is different around the world, 10.7% globally [2], 7% in China [3], and 16.6% in Hong Kong S.A.R [4]. Frailty has been linked with poor health outcomes, such as falls and disability, and an increased demand for health care resources [5, 6]. It has also been associated with a substantial increase in negative consequences (e.g., mortality and delirium) in COVID-19 patients [7]. The pooled prevalence of frailty among COVID-19 patients in a meta-analysis was estimated to be 45% [8], which is much higher than a recent meta-analysis which reported an estimated worldwide frailty prevalence of 18% [9]. Frailty is an important at-risk state to be managed in older adults, particularly during the COVID-19 pandemic.
Physical activity has been shown to protect against frailty in older adults [10, 11]. Physical activity is an important factor because it is modifiable and has been found to be significantly associated with frailty in older adults according to a systematic review, though the magnitude of the effect varies across contexts (e.g., types of physical activities) [12]. However, the overwhelming majority of older adults do not meet the minimum physical activity levels needed, according to a study in the United Kingdom [10]. The situation of decreased physical activity worsened under the restricted social distancing measures during the COVID pandemic [13], though it can lower the exposure risk. In Hong Kong, the social distancing measures included the closure of sports and fitness centres, restrictions on the catering business and cessation of mass events [14]. A study (n = 13,503) conducted in 14 countries found that social restrictions significantly reduced physical activity in older adults. In Japan (n = 774), a similar result was obtained. Approximately half of community-dwelling older adults reported declining physical and cognitive fitness during COVID-19 [15]. Evidence showed that decreased physical activity or limitations were also associated with frailty during the COVID-19 pandemic [16, 17]. Therefore, decreased physical activity is a key factor associated with frailty and this situation was further worsened by COVID-19. Nevertheless, frailty is also known to be associated with many factors which may be confounded with the association between physical activity and frailty (e.g., age, gender, education level, financial status, depression, falls, and social network) [18–22]. Identification of effective and modifiable factors associated with decreased physical activity and frailty after considering the effects of confounding factors is essential to develop new interventions to treat frailty.
During the COVID-19 pandemic, digital health interventions were reported to be potentially useful in promoting physical activity in older adults and improving their frailty through home-based interventions and smartphone apps [23]. E-health is also widely used in interventions for promoting physical activity and lifestyle among older adults, such as daily physical activity monitoring in an objective manner, with step counts and time spent [24, 25]. Older adults were informed and connected with the updated preventive behaviours such as social distancing measures and hand washing techniques [26]. 63% of older adults would use technology to communicate with others [27]. Technology acceptance (e.g., perceived usefulness, perceived ease of use) is associated with the behavioural intention of older adults to use technology [28]. Behavioural intention to use is associated with actual usage of technology [29]. As a result, acceptance of technology may play an important role in influencing people’s behaviors toward physical activity, which is known to be associated with frailty.
In the literature, frailty is known to be negatively associated with technology use (e.g., information and communication technologies) [30], but the association between technology acceptance and frailty is unknown. According to a systematic review, physical inactivity is associated with frailty [31]. Given the hypothetical relationships discussed above, empirical evidence demonstrating the relationships between technology acceptance, physical activity, and frailty is lacking. Therefore, we conducted a cross-sectional study to investigate the association between physical activity, technology acceptance and frailty among older adults.
Objectives
This study aims to:
Examine the association between physical activity and technology acceptance with frailty, and
Examine the moderation effect of technology acceptance on physical activity and frailty.
We hypothesize that 1) a higher level of physical activity and a higher level of technology acceptance are associated with a lower level of frailty, and 2) technology acceptance moderates the association of physical activity with frailty.
Methods
Study design
This study employed a cross-sectional design. To ensure clarity of reporting, we followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [32].
Setting
The study was conducted in the community settings of Hong Kong. Data collection was conducted from September 3 2021 to November 29 2021. During this period, Hong Kong was experiencing the COVID-19 pandemic, with a mean daily number of 3.8 new confirmed cases (range = 0–31, median = 3), cumulative 12,430 cases as of November 292,021 [33], in a city with a population of approximately 7.5 million [34]. During this period, many infection control measures and social distancing policies (e.g., wearing a mask in outdoor settings, limiting the number of people co-dinning in one table) were in force [14].
Participants
We adopted a snowball sampling method to invite eligible participants to join the study. We first recruited participants at the Institute of Active Ageing, The Hong Kong Polytechnic University and the Centre of Positive Ageing, The University of Hong Kong. Members of these organizations are aged over 50 years. They regularly participated in a variety of activities organized by the organizations, including community services, training courses, volunteer activities, and research. Subsequently, they invited their friends who were eligible to participate in the survey.
Inclusion criteria
Old adults aged ≥60 years, and
Community-dwelling, which is defined as living in their own homes in the past 6 months before the day of participation in the survey.
Exclusion criteria
Diagnosed dementia, self-reported
Variables
Demographic variables included age, gender, education level, financial status, living condition, history of COVID-19 infection, and severity of COVID-19 in the living area. Physical variables included physical activity and frailty. Psychosocial variables included social network, technology acceptance, and depressive symptoms.
Measurement
An online survey was used to collect data. The online survey was launched on Qualtrics [35], which normally takes 30 minutes to complete. Qualtrics automatically logged each response from the participants. However, this study counted the participants to be valid only if they clicked the “submit” button on the last page to indicate that they had completed the study. The invited participants received a designated hyperlink from the research team. The participants clicked on the link to complete the online survey.
Dependent variable
Frailty was measured by using the Fatigue, Resistance, Ambulation, Illnesses, & Loss of Weight scale (FRAIL). FRAIL comprises five dichotomous items. A total score ranges from 0 to 5. A higher score indicates a higher level of frailty. FRAIL categorizes frailty in three levels by severity (i.e., robust = 0, pre-frail = 1–2, frail = 4–5) [36]. It shares similarities with the Fried Frailty Phenotype [37], and is a screening tool for frailty. Nonetheless, according to a systematic review, FRAIL is a tool that can effectively identify frailty/prefrailty status and quantify frailty status in a graded manner in relation to mortality risk (frailty vs robustness: pooled HR = 3.53, prefrailty vs robustness: polled HR = 1.75) [38]. The Chinese FRAIL scale showed good criterion validity compared with Fried frailty phenotype (area under the curve = 0.91) and good test-retest reliability (ICC = 0.708) [39].
Independent variables
Physical activity was measured by the Rapid Assessment of Physical Activity (RAPA) [40]. RAPA comprises nine dichotomous items (1 = yes, 0 = no). The RAPA classifies physical activity into seven levels by intensity (from 1 = sedentary to 7 = regularly active). A higher score indicates a higher level of physical activity. WHO recommended that healthy older adults should do at least 150–300 minutes of moderate-intensity physical activity or at least 75–150 minutes of vigorous-intensity physical activity, or an equivalent combination of moderate- and vigorous-intensity activity throughout the week [41]. RAPA score below 6 (i.e., “I do 30 minutes or more a day of moderate physical activities, 5 or more days a week”) is interpreted as being physically underactive. It has been validated in older adults that it shows good validity with a good correlation with Community Health Activities Model Program for Seniors (CHAMPS) (r = 0.54) [40].
Technology acceptance was measured by the Senior Technology Acceptance Model-14 items (STAM-14) [42]. STAM-14 comprises 14 items. Each item is rated on a 10-point scale (from 1 = “very unsatisfied” to 10 = “very satisfied”). STAM-14 consists of four constructs: 1) attitudinal beliefs, 2) control beliefs, 3) gerontechnology anxiety, and 4) health. A total score ranges from 14 to 140. A higher score indicates a higher level of acceptance of technology. This Chinese STAM-14 scale was validated by 1012 community-dwelling individuals 55 and older in Hong Kong with good internal consistency (Cronbach’s alpha = 0.817–0.915) and construct validity (AVE = 0.455–0.795) [42].
Covariates
Covariates included age (years), number of falls, gender (1 = male, 2 = female), education level (1 = tertiary, 2 = secondary, and 3 = primary), financial status (a 5-point scale from 1= “much more than enough” to 5= “much less than enough”), co-living status (1 = “live alone”, 2= “live with partner/spouse”, and 3= “live with family/friend”), history of COVID-19 (1 = yes, 2 = no), and severity of COVID-19 in the living area (a 4-point scale from 1 = “not severe” to 4 = “severe”).
Social network was measured by using the Lubben Social Network Scale-Six items (LSNS-6) [43]. LSNS-6 comprises six questions with two constructs of social network sources: family and friends. Each construct consists of three items which measure the size, private conversation and help towards the social network source. Each question is rated on a 6-point scale with a total score ranging from 6 to 36. A high score indicates a higher level of social network. The Chinese version of LSNS-6 was validated and was found to be reliable and valid in predicting late-life suicidality [43] with sufficient internal consistency (Cronbach’s α = 0.832) and the Cronbach’s α were 0.90 and 0.95 for family subscale and friends subscale, respectively.
Depressive symptoms were measured by the Patient Health Questionnaire–Nine items (PHQ-9) [44]. PHQ-9 includes nine items. Each item is measured by a 4-point scale, with a total score ranging from 0 to 27. A higher score indicates a higher level of depressive symptomatology. The Chinese version of the PHQ9 was validated by comparing its scores with the clinical diagnosis of a major depressive episode using the DSM-IV criteria (AUC = 0.95, sensitivity = 0.88, specificity = 0.88) at the cut-off point of 9/10 with good internal consistency (Cronbach’s α = 0.89). The PHQ9 was validated among Chinese older adults aged equal to or over 60 years and found to have good validity (sensitivity = 0.86, specificity = 0.77) for identifying major depression in late life at the cut-off point of 9/10 [45].
Study size
The minimum sample size required for conducting a regression analysis is 104, in addition to the number of predictors in the regression [46]. The association of technology acceptance on frailty and the moderation effect of technology acceptance on physical activity and frailty were never reported in the literature. A meta-analysis of systematic reviews revealed that the relationship between physical activity and frailty is slightly larger than small (i.e., Hedges g = 0.24) [12]. We estimated the sample size according to the effect size of physical activity on frailty. G*Power with the statistical test of linear multiple regression: Fixed model, R2 deviation from zero was used [47]. We assumed that the effect size is slightly larger than small (i.e., f2 = 0.03) [48], α was 0.05, power was 0.08, and the number of predictors was 2 (i.e., physical activity and technology acceptance). The total sample needed was estimated to be 325.
Statistical methods
IBM SPSS Statistics version 26 was used for statistical analysis [49]. All the demographic variables and clinical variables were described using mean with standard deviation or frequency with the percentage depending on their levels of measurement. To test the hypotheses set in the objectives, ordinal logistic regression was employed. The dependent variable was frailty. The independent variables were physical activity and technology acceptance (i.e., objective #1). The moderation effect (i.e., objective #2) was examined by introducing an interaction term formed by the multiplication of an independent variable (i.e., physical activity) and a moderating variable (i.e., technology acceptance) [50]. Demographic [2] (i.e., age, gender) and related clinical variables [4, 51, 52] (i.e., social network and depression) were included as confounding variables because they are known to be associated with frailty in the literature. Models unadjusted and adjusted for confounders were computed separately. Psuedo-R-squared values were computed for unadjusted and adjusted models separately to show the change in model fitness after adding independent variables to the models. A subgroup analysis was conducted to show the moderation effect direction of technology acceptance on the association of physical activity with frailty. Two subgroups (i.e., high technology acceptance and low technology acceptance) were formed and cut off by the median score. The level of significance was set at 0.05. Missing data were replaced by the mean when the missing rate of variables was inconsequential (i.e., 5% or less).
Results
Participants
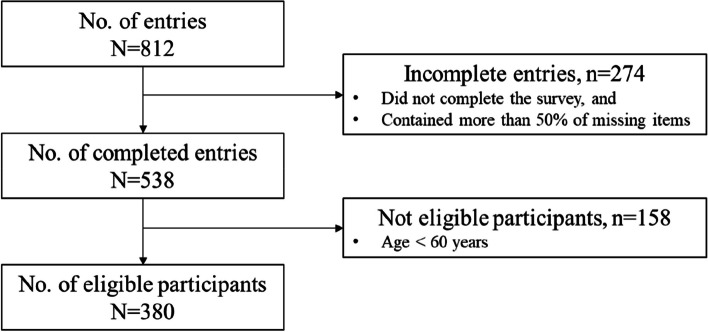
As shown in Fig. 1, 812 older adults attempted the online survey. 274 entries were invalid because the participants filled fewer than half of the total items of the survey and did not click the “submit” button to indicate that they completed the survey. Of the 538 valid inputs, there were 158 inputs not eligible because the age of the participants was lower than 60 years. Finally, there were 380 eligible and valid participants. All participants had a negligible number of missing data (i.e., < 5%), and the missing values were replaced by the mean of the variables.
Fig. 1.
Participant flowchart
Descriptive data
As shown in Table 1, the participants’ mean age was 66.5 (SD = 5.5) years. The mean number of falls in the last 12 months was 0.59 (SD = 1.18). The majority of the participants were female (n = 218, 57.4%), completed secondary school (n = 142, 37.4%), indicated to have enough financial status (n = 194, 51.1%), were living with a partner/spouse (n = 198, 52.1%), had not been infected with COVID-19 (n = 379, 99.7%), and resided in an area where COVID-19 pandemic was not severe (n = 232, 62.1%).
Table 1.
Participants’ demographic and clinical profile
| Variables (N = 380) | Mean (SD) / n (%) |
|---|---|
| Dependent variable | |
| Frailty (FRAIL, possible range 0–5) | 0 (1)a |
| 0 | 249 (65.5) |
| 1 | 78 (20.5) |
| 2 | 36 (9.5) |
| 3 | 16 (4.2) |
| 4 | 1 (0.3) |
| Frailty classification | |
| Robust | 249 (65.5) |
| Pre-frail | 114 (30.0) |
| Frail | 17 (4.5) |
| Independent variables | |
| Physical activity (RAPA, possible range: 0–7) | 4.7 (1.7) |
| Technology acceptance (STAM-14, possible range 14–140] | 94.8 (19.8) |
| Covariates | |
| Age in years | 66.5 (5.5) |
| No. of falls in the last 12 months | 0.59 (1.18) |
| Gender | |
| Male | 162 (42.6) |
| Female | 218 (57.4) |
| Educational level | |
| Tertiary | 127 (33.4) |
| Secondary | 142 (37.4) |
| Primary | 111 (29.2) |
| Financial status | |
| Much more than enough | 12 (3.2) |
| More than enough | 119 (31.3) |
| Enough | 194 (51.1) |
| Not enough | 50 (13.2) |
| Much less than enough | 5 (1.3) |
| Co-living status | |
| Live alone | 56 (14.7) |
| Live with partner/spouse | 198 (52.1) |
| Live with family | 126 (33.2) |
| History of COVID-19 | |
| Yes | 1 (0.3) |
| No | 379 (99.7) |
| Severity of COVID-19 in the living area | |
| Not severe | 232 (62.1) |
| Mild | 120 (31.6) |
| Moderate | 22 (5.8) |
| Severe | 6 (1.6) |
| Social network (LSNS-6, possible range 0-30) | 11.9 (5.6) |
| Depressive symptoms (PHQ-9, possible range 0–27) | 3.4 (3.5) |
COVD-19 Coronavirus Disease of 2019, RAPA Rapid Assessment of Physical Activity scale, LSNS-6 Lubben Social Network Scale-Six items scale, PHQ-9 Patient Health Questionnaire-Nine items scale, STEAM-14 Senior Technology Acceptance Model-14 items scale, FRAIL Fatigue, Resistance, Ambulation, Illnesses, and loss of Weight scale
aThe values represent a median (inter-quartile range)
The mean RAPA score (i.e., physical activity) was 4.7 (SD = 1.7), mean LSNS-6 score (i.e., social network) was 11.9 (SD = 5.6), mean PHQ-9 score (i.e., depression symptoms) was 3.4 (SD = 3.5), mean STAM-14 score (i.e., technology acceptance) was 94.8 (SD = 19.8), and the median FRAIL score (i.e., frailty) was 0 (IQR = 1). The majority of them were classified as robust (n = 249, 65.5%).
Main results
Objective #1
As shown in Table 2, technology acceptance (Beta = − 0.031, p < 0.001, Pseudo-R2 = 0.087) and physical activity (Beta = − 0.182, p = 0.003, Pseudo-R2 = 0.027) were associated with frailty in the unadjusted models. Technology acceptance (Beta = − 0.067, p < 0.001), physical activity (Beta = − 0.895, p = 0.002), and the interaction term of [technology acceptance]*[physical activity] (Beta = 0.009, p = 0.005) were associated with frailty with better fitness (Pseudo-R2 = 0.116) in the adjusted model including factors of technology acceptance, physical activity, and technology acceptance (Beta = − 0.066, p < 0.001), physical activity (Beta = − 1.192, p < 0.001) and the interaction term of [technology acceptance]*[physical activity] (Beta = 0.012, p = 0.001) remained associated with frailty with even better fitness which better explains the phenomena (Pseudo-R2 = 0.352) in the further adjusted model.
Table 2.
Effects of phyical activity and technology acceptance on frailty
| Dependent variable | Unadjusted models | Adjusted model Pseudo-R2 = 0.116 | Further adjusted model#Pseudo-R2 = 0.352 | ||||
|---|---|---|---|---|---|---|---|
| Frailty (FRAIL) | Beta (SE) | p-values | Pseudo-R2 | Beta (SE) | p-values | Beta (SE) | p-values |
| Independent variables | |||||||
| Technology acceptance (STAM) | −0.031 (0.006) | < 0.001* | 0.087 | −0.067 (0.015) | < 0.001* | − 0.066 (0.017) | < 0.001* |
| Physical activity (RAPA) | −0.182 (0.061) | 0.003* | 0.027 | −0.895 (0.287) | 0.002* | −1.192 (0.325) | < 0.001* |
| [Technology acceptance] * [Physical activity] | 0.009 (0.003) | 0.005* | 0.012 (0.004) | 0.001* | |||
*p < 0.05, FRAIL Fatigue, Resistance, Ambulation, Illnesses, & Loss of Weight scale, STAM Senior Technology Acceptance Model, RAPA Rapid Assessment of Physical Activity, COVD-19 Coronavirus Disease of 2019, PHQ-9 Patient Health Questionnaire-nine items, LSNS-6 Lubben Social Network Scale-six items
# The model adjusted for covariates including age, gender, education levels, financial status, co-living status, no. of falls in the last 12 months, COVID-19 severity, depression, and social network
Objective #2
As shown in Table 2, the interaction term formed by the multiplication of technology acceptance and physical activity (Beta = 0.009, p = 0.005) was associated with frailty (Column 2). The interaction term (Beta = 0.012, p = 0.001) remained associated with frailty in the fully adjusted model (Column 3).
As shown in Table 3, in the subgroup analysis, the association of physical activity (Beta = − 0.313, p = 0.002) with frailty in the subgroup of lower technology acceptance was significantly negative. However, in the subgroup of higher technology acceptance, the relationship between physical activity and frailty (Beta = 0.104, p = 0.408) became positive but not significant.
Table 3.
Comparing the effects of physical activity on frailty between subgroups of lower and higher technology acceptance
| Dependent variable | Subgroup 1 (N = 195)a Lower technology acceptance STAM score ≤ 96 (median) | Subgroup 2 (N = 185)a Higher technology acceptance STAM score > 96 (median) |
||
|---|---|---|---|---|
| Frailty (FRAIL) | Beta (SE) | p-values | Beta (SE) | p-values |
| Independent variables | ||||
| Physical activity (RAPA) | −0.313 (0.099) | 0.002* | 0.104 (0.126) | 0.408 |
*p-value< 0.05, STAM Senior Technology Acceptance Model, RAPA Rapid Assessment of Physical Activity
aThe model adjusted for covariates including age, gender, education levels, financial status, co-living status, no. of falls in the last 12 months, COVID-19 severity, depression, and social network
Discussion
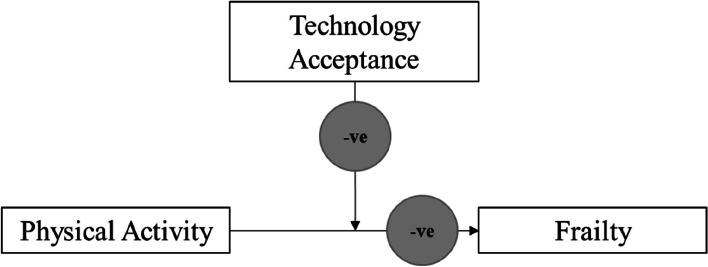
To the best of our knowledge, this is the first study to report the associations between physical activity, technology acceptance and frailty among older adults. There are three key findings which support the hypotheses. First, physical activity is associated with frailty. Second, technology acceptance may be protective against frailty. Third, technology acceptance negatively moderates the association of physical activity with frailty (as shown in Fig. 2). Physical activity’s protective effect on frailty diminishes in people with a higher level of technology acceptance. These findings have a number of ramifications.
Fig. 2.
Moderation effect of technology acceptance on the effect of physical activity on frailty
Physical activity can lower frailty among older adults is well documented in literature [10, 11]. This study yielded a similar. Under the COVID-19 pandemic, because of quarantine and social distancing measures, the level of participation in physical activity of community-dwelling older adults was reported to decline, albeit the fact that they expressed the need to remain physically active [53, 54]. The decline of participation in physical activity imposes the risk of older adults developing frailty, as the findings of this study once again reinforced this risk. Engaging older adults in an adequate amount of physical activity during the COVID-19 pandemic has become a priority public health agenda.
According to the findings of this study, technology acceptance is directly related to frailty. This finding is consistent with a recent study that found frailer people have a lower level of technology acceptance [55]. Recently, many m-health technologies have facilitated older adults with frailty to participate in physical activity in in-door settings and communities close to their homes [56, 57]. These technology-enhanced physical activity interventions are effective and might also be suitable in the context of COVID-19. This study reinforces the use of these technology-enhanced interventions to sustain the participation in physical activity of community-dwelling older adults to prevent the development of frailty. During the COVID-19 pandemic, governments of many countries invested much more in the use of new technologies for the continuation of activities [58]. Further study should also examine if these endeavours have improved technology acceptance in older adults, as well as whether the promotion of technology acceptance may also lead to a reduction of frailty. It could be a novel health promotion policy in the post-COVID era.
One possible explanation is that people with higher levels of technology acceptance may use technology more to engage in more physical activity during COVID-19. According to a systematic review, technology acceptance, particularly behavioral intention to use, is likely to be related to actual usage [29]. Technology usage and technical ability are associated with a higher level of physical activity [59]. For example, older adults use wearable sensors to engage themselves in a higher level of physical activity [20, 24]. According to the evidence, compliance with technology-based exercise is good and can provide a long-term promotion of physical activities [60]. Furthermore, even during COVID-19, when quarantine and social distancing policies were in effect, and older adults’ life-space mobility was limited within their residing areas, the physical activity level of some older adults remained satisfactory, and it was found to be associated with a lower level of sarcopenia [61]. According to the literature, attitude towards technology or actual technological use plays a role in community-dwelling older adults’ physical activity behaviour, which is protective against frailty. This study suggests that promoting technology acceptance among older adults is possibly a public health strategy to combat frailty in the community-dwelling older adults, even during a pandemic when social distancing and infection control policies are in force. Future studies should devise interventional studies to confirm this association.
Another key finding of this study was that technology acceptance negatively moderated the association of physical activity with frailty in older adults. In other words, the protective effect of physical activity on frailty is only prominent among those with a lower level of technology acceptance. The size of the effect of physical activities on frailty in older adults varied across contexts in the literature [12]. This study identified a novel factor moderating the effect of physical activity on frailty in older adults. This contrasted with the findings in the literature that a positive dose-response relationship usually exists on the effect of physical activity on frailty in older adults [62]. The reason could be that the dose-response relationship between physical activity and frailty in people who are frail or robust could be different. The sample of this study is generally less frail, and people who are less frail in this sample are mostly robust. This study showed that frailty is lower in people with a higher level of technology acceptance. These findings may suggest that the protective effect of physical activity on frailty in people with a lower level of frailty is smaller. Another possible reason is that people with a high level of technology acceptance and literacy may have already engaged in more healthy behaviours [63]. Evidence showed that multiple strategies apart from physical activity also protect older adults from frailty (e.g., social and intellectual activities, dietary patterns) [64]. These protective associations may have dominated the associations of physical activity on frailty in the high technology acceptance group. This study, therefore, recommends that further studies should examine the possible negative dose-response relationship of physical activity on frailty in the population of older adults who are less frail, as well as the possible dominations of association by the protective strategies against frailty other than physical activity in the same population. This study also recommends in priority to target older adults who have a lower level of technology acceptance to engage in interventions promoting physical activities because this strategy may yield a larger protective effect against frailty.
This study has a number of limitations. First, the sample of this population is less frail, with a prevalence of frailty of 4.5%, compared to 9.9–13.6% globally, according to a systematic review.67 The findings of this study may only be applicable to a population of older adults who are less frail [2]. The associations observed in this study could only be transferrable to the population of older adults who are less frail. Second, the survey employed a self-filling online approach that recalls bias caused by possible dementia of the participants could be not excluded. Nevertheless, this sample was relatively young, with a mean age of 66.5 years. The prevalence of dementia in this young-old group (i.e., aged 67–69 years) was reported to be low (i.e., below 5%) [65]. Third, RAPA is a crude measure to quantify physical activity, albeit the fact that it has been validated. Further studies should confirm the findings of this study by using a more objective and accurate measurement (e.g., smartphone or accelerometer) [66]. Finally, the data collection period was conducted within the COVID-19 lockdown period. Physical activities which are usually conducted outdoors during the non-pandemic period were largely restricted. This may have limited the variability of the physical activity practised by older adults (i.e., shifted the amount to the lower side). It could have reduced the size of the effect of physical activity on frailty. It could have reduced the size of the effect of physical activity on frailty.
Conclusion
This study showed that physical activity and technology acceptance were associated with frailty, and that technology acceptance moderated the association of physical activity with frailty. Future studies should examine the association of technology promotion with frailty in older adults. This study suggests that engaging older adults in physical activity should be prioritized in those with a low level of technology acceptance.
Acknowledgements
This project is part of an international collaboration on physical activity, technology acceptance, frailty and well-being of older adults in Hong Kong, the United States, Singapore, and Thailand associated with the Aging Among Asia interest group under the Gerontological Society of America (GSA). The present study was based on data collected at the Hong Kong site. We would like to thank all collaborators in all study sites for their contribution during the conceptualization and study design.
Abbreviations
- COVID-19
Coronavirus Disease of 2019
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
Authors’ contributions
RK, VL and JL discussed and developed the hypothesis and methods of the study. RK conducted the statistical analysis. RK and JY made the first draft. RK, VL and JL revised the manuscript. RK, VL, JL and JY read and approved the final version of the manuscript.
Funding
This study was jointly supported by the “Financial Support for Leader of Professional Society/Association at International Level”, The Hong Kong Polytechnic University and Sau Po Centre on Aging, The University of Hong Kong.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to the restrictions involved when obtaining ethical approval for our study, which commit us to share the data only with members of the research team but allow data to be made available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of The Hong Kong Polytechnic University (Ref no.: HSEARS20210315002). The study was explained to all of the participants, and all gave their signed informed consent to take part in this study. Written informed consent and signatures were obtained from all participants in the study and for illiterate participants from their legal guardians/LAR. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morley JE, Vellas B, Van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 3.Wu C, Smit E, Xue Q-L, Odden MC. Prevalence and correlates of frailty among community-dwelling Chinese older adults: the China health and retirement longitudinal study. J Gerontol Ser A. 2018;73(1):102–108. doi: 10.1093/gerona/glx098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu R, Wu W-C, Leung J, Hu SC, Woo J. Frailty and its contributory factors in older adults: a comparison of two Asian regions (Hong Kong and Taiwan) Int J Environ Res Public Health. 2017;14(10):1096. doi: 10.3390/ijerph14101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kojima G. Frailty as a predictor of disabilities among community-dwelling older people: a systematic review and meta-analysis. Disabil Rehabil. 2017;39(19):1897–1908. doi: 10.1080/09638288.2016.1212282. [DOI] [PubMed] [Google Scholar]
- 6.Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 2018;47(2):193–200. doi: 10.1093/ageing/afx162. [DOI] [PubMed] [Google Scholar]
- 7.Dumitrascu F, Branje KE, Hladkowicz ES, Lalu M, McIsaac DI. Association of frailty with outcomes in individuals with COVID-19: a living review and meta-analysis. J Am Geriatr Soc. 2021;69(9):2419–2429. doi: 10.1111/jgs.17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kow CS, Hasan SS. Prevalence of Frailty in Patients with COVID-19: A Meta-Analysis. J Frail Aging. 2021;10(2):189–190. doi: 10.14283/jfa.2020.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Caoimh R, Mannion H, Sezgin D, O'Donovan MR, Liew A, Molloy DW. Non-pharmacological treatments for sleep disturbance in mild cognitive impairment and dementia: a systematic review and meta-analysis. Maturitas. 2019;127:82–94. doi: 10.1016/j.maturitas.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 10.McPhee JS, French DP, Jackson D, Nazroo J, Pendleton N, Degens H. Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology. 2016;17(3):567–580. doi: 10.1007/s10522-016-9641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers NT, Marshall A, Roberts CH, Demakakos P, Steptoe A, Scholes S. Physical activity and trajectories of frailty among older adults: evidence from the English longitudinal study of ageing. PLoS One. 2017;12(2):e0170878. doi: 10.1371/journal.pone.0170878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira JS, Pinheiro MB, Fairhall N, et al. Evidence on physical activity and the prevention of frailty and sarcopenia among older people: a systematic review to inform the World Health Organization physical activity guidelines. J Phys Act Health. 2020;17(12):1247–1258. doi: 10.1123/jpah.2020-0323. [DOI] [PubMed] [Google Scholar]
- 13.Caputo EL, Reichert FF. Studies of physical activity and COVID-19 during the pandemic: a scoping review. J Phys Act Health. 2020;17(12):1275–1284. doi: 10.1123/jpah.2020-0406. [DOI] [PubMed] [Google Scholar]
- 14.Region TGotHKSA. Press release: government further tightens social distancing measures. English Accessed 25 Mar 2022, 2022. https://www.info.gov.hk/gia/general/202202/23/P2022022300760.htm
- 15.Makizako H, Nakai Y, Shiratsuchi D, et al. Perceived declining physical and cognitive fitness during the COVID-19 state of emergency among community-dwelling Japanese old-old adults. Geriatr Gerontol Int. 2021;21(4):364–369. doi: 10.1111/ggi.14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saragih ID, Advani S, Saragih IS, Suarilah I, Susanto I, Lin CJ. Frailty as a mortality predictor in older adults with COVID-19: a systematic review and meta-analysis of cohort studies. Geriatric Nursing. 2021;42(5):983–992. doi: 10.1016/j.gerinurse.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soysal P, Veronese N, Thompson T, et al. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2017;36:78–87. doi: 10.1016/j.arr.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Kwan RYC, Cheung DSK, Lo SKL, et al. Frailty and its association with the Mediterranean diet, life-space, and social participation in community-dwelling older people. Geriatr Nurs. 2019;40(3):320–326. doi: 10.1016/j.gerinurse.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Cheung DSK, Kwan RYC, Wong ASW, et al. Factors associated with improving or worsening the state of frailty: a secondary data analysis of a 5-year longitudinal study. J Nurs Scholarsh. 2020;52(5):515–526. doi: 10.1111/jnu.12588. [DOI] [PubMed] [Google Scholar]
- 21.Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and Meta-analysis. J Am Med Dir Assoc. 2015;16(12):1027–1033. doi: 10.1016/j.jamda.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Szanton SL, Seplaki CL, Thorpe RJ, Jr, Allen JK, Fried LP. Socioeconomic status is associated with frailty: the Women’s health and aging studies. J Epidemiol Community Health. 2010;64(01):63. doi: 10.1136/jech.2008.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah SGS, Nogueras D, van Woerden HC, Kiparoglou V. The COVID-19 pandemic: a pandemic of lockdown loneliness and the role of digital technology. J Med Internet Res. 2020;22(11):e22287. doi: 10.2196/22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonkman NH, van Schooten KS, Maier AB, Pijnappels M. eHealth interventions to promote objectively measured physical activity in community-dwelling older people. Maturitas. 2018;113:32–39. doi: 10.1016/j.maturitas.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Pruchno R. Technology and aging: an evolving partnership. Gerontologist. 2019;59(1):1–5. doi: 10.1093/geront/gny153. [DOI] [PubMed] [Google Scholar]
- 26.Chen AT, Ge S, Cho S, et al. Reactions to COVID-19, information and technology use, and social connectedness among older adults with pre-frailty and frailty. Geriatr Nurs. 2021;42(1):188–195. doi: 10.1016/j.gerinurse.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strutt PA, Johnco CJ, Chen J, et al. Stress and coping in older Australians during COVID-19: health, service utilization, Grandparenting, and technology use. Clin Gerontol. 2022;45(1):106–119. doi: 10.1080/07317115.2021.1884158. [DOI] [PubMed] [Google Scholar]
- 28.Ma Q, Chan AHS, Teh P-L. Insights into older adults’ technology acceptance through Meta-analysis. Int J Hum-Comput Int. 2021;37(11):1049–1062. doi: 10.1080/10447318.2020.1865005. [DOI] [Google Scholar]
- 29.Turner M, Kitchenham B, Brereton P, Charters S, Budgen D. Does the technology acceptance model predict actual use? A systematic literature review. Inf Softw Technol. 2010;52(5):463–479. doi: 10.1016/j.infsof.2009.11.005. [DOI] [Google Scholar]
- 30.Keränen NS, Kangas M, Immonen M, et al. Use of information and communication technologies among older people with and without frailty: a population-based survey. J Med Internet Res. 2017;19(2):e29. doi: 10.2196/jmir.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tolley APL, Ramsey KA, Rojer AGM, Reijnierse EM, Maier AB. Objectively measured physical activity is associated with frailty in community-dwelling older adults: a systematic review. J Clin Epidemiol. 2021;137:218–230. doi: 10.1016/j.jclinepi.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Hannah Ritchie EM, Lucas Rodés-Guirao, Cameron Appel, Charlie Giattino, Esteban Ortiz-Ospina, Joe Hasell, Bobbie Macdonald, Diana Beltekian and Max Roser. Hong Kong: coronavirus pandemic country profile. OurWorldInData. Accessed May 8, 2022. https://ourworldindata.org/coronavirus/country/hong-kong#how-many-tests-are-performed-each-day
- 34.Department CaS. Statistical Products_population the government of the Hong Kong special administrative region updated 1 April, 2022. Accessed 7 May 2022. https://www.censtatd.gov.hk/en/
- 35.Qualtrics. 2020. https://www.qualtrics.com. Accessed 1 July 2022.
- 36.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 38.Kojima G. Frailty defined by FRAIL scale as a predictor of mortality: a systematic review and Meta-analysis. J Am Med Dir Assoc. 2018;19(6):480–483. doi: 10.1016/j.jamda.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Dong L, Qiao X, Tian X, et al. Cross-cultural adaptation and validation of the FRAIL scale in Chinese community-dwelling older adults. J Am Med Dir Assoc. 2018;19(1):12–17. doi: 10.1016/j.jamda.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The rapid assessment of physical activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):A118. [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization. Physical activity. World Health Organization. Accessed 5 Oct, 2022. https://www.who.int/news-room/fact-sheets/detail/physical-activity
- 42.Chen K, Lou VWQ. Measuring senior technology acceptance: development of a brief, 14-item scale. Innov Aging. 2020;4(3):igaa016. doi: 10.1093/geroni/igaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang Q, Sha F, Chan CH, Yip PS. Validation of an abbreviated version of the Lubben social network scale (“LSNS-6”) and its associations with suicidality among older adults in China. PLoS One. 2018;13(8):e0201612. doi: 10.1371/journal.pone.0201612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen S, Chiu H, Xu B, et al. Reliability and validity of the PHQ-9 for screening late-life depression in Chinese primary care. Int J Geriatr Psychiatry. 2010;25(11):1127–1133. doi: 10.1002/gps.2442. [DOI] [PubMed] [Google Scholar]
- 46.Green SB. How many subjects does it take to do a regression analysis. Multivar Behav Res. 1991;26(3):499–510. doi: 10.1207/s15327906mbr2603_7. [DOI] [PubMed] [Google Scholar]
- 47.Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods Instrum Comput. 1996;28(1):1–11. doi: 10.3758/BF03203630. [DOI] [Google Scholar]
- 48.Cohen J. A power primer. Psychol Bull. 1992;112(1):155. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 49.IBM Corp . IBM SPSS statistics for windows, version 26.0. IBM Corp; 2019. [Google Scholar]
- 50.Brambor T, Clark WR, Golder M. Understanding interaction models: improving empirical analyses. Polit Anal. 2006;14(1):63–82. doi: 10.1093/pan/mpi014. [DOI] [Google Scholar]
- 51.Ho LYW, Cheung DSK, Kwan RYC, Wong ASW, Lai CKY. Factors associated with frailty transition at different follow-up intervals: a scoping review. Geriatric Nursing. 2021;42(2):555–565. doi: 10.1016/j.gerinurse.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Gale CR, Westbury L, Cooper C. Social isolation and loneliness as risk factors for the progression of frailty: the English longitudinal study of ageing. Age Ageing. 2017;47(3):392–397. doi: 10.1093/ageing/afx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goethals L, Barth N, Guyot J, Hupin D, Celarier T, Bongue B. Impact of home quarantine on physical activity among older adults living at home during the COVID-19 pandemic: qualitative interview study. JMIR Aging. 2020;3(1):e19007. doi: 10.2196/19007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey L, Ward M, DiCosimo A, et al. Physical and mental health of older people while cocooning during the COVID-19 pandemic. QJM. 2021;114(9):648–653. doi: 10.1093/qjmed/hcab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin HR, Um SR, Yoon HJ, et al. Comprehensive senior technology acceptance model of daily living assistive Technology for Older Adults with Frailty: cross-sectional study. J Med Internet Res. 2023;25:e41935. doi: 10.2196/41935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwan RYC, Liu JYW, Fong KNK, et al. Feasibility and effects of virtual reality motor-cognitive training in community-dwelling older people with cognitive frailty: pilot randomized controlled trial. JMIR Serious Games. 2021;9(3):e28400. doi: 10.2196/28400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwan RY, Lee D, Lee PH, et al. Effects of an mHealth brisk walking intervention on increasing physical activity in older people with cognitive frailty: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2020;8(7):e16596. doi: 10.2196/16596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molleví Bortoló G, Álvarez Valdés J, Nicolas-Sans R. Sustainable, technological, and innovative challenges post Covid-19 in health, economy, and education sectors. Technol Forecast Soc Chang. 2023;190:122424. doi: 10.1016/j.techfore.2023.122424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blažun H, Saranto K, Kokol P, Vošner J. Information and communication technology as a tool for improving physical and social activity of the elderly. NI. 2012;2012:26–26. [PMC free article] [PubMed] [Google Scholar]
- 60.Valenzuela T, Okubo Y, Woodbury A, Lord SR, Delbaere K. Adherence to technology-based exercise programs in older adults: a systematic review. J Geriatr Phys Ther. 2018;41(1):49–61. doi: 10.1519/JPT.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 61.Kwan RYC, Liu JYW, Yin Y-H, et al. Sarcopenia and its association with objectively measured life-space mobility and moderate-to-vigorous physical activity in the oldest-old amid the COVID-19 pandemic when a physical distancing policy is in force. BMC Geriatr. 2022;22(1):250. doi: 10.1186/s12877-022-02861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mañas A, del Pozo-Cruz B, Rodríguez-Gómez I, et al. Dose-response association between physical activity and sedentary time categories on ageing biomarkers. BMC Geriatr. 2019;19(1):270. doi: 10.1186/s12877-019-1284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui G-H, Li S-J, Yin Y-T, et al. The relationship among social capital, eHealth literacy and health behaviours in Chinese elderly people: a cross-sectional study. BMC Public Health. 2021;21(1):45. doi: 10.1186/s12889-020-10037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Lu Y, Li C, et al. Associations of lifestyle activities and a heathy diet with frailty in old age: a community-based study in Singapore. Aging (Albany NY) 2020;12(1):288–308. doi: 10.18632/aging.102615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu Y, Chen Y, Crimmins EM, Zissimopoulos JM. Sex, race, and age differences in prevalence of dementia in Medicare claims and survey data. J Gerontol Ser B. 2020;76(3):596–606. doi: 10.1093/geronb/gbaa083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwan RYC, Liu JYW, Lee D, Tse CYA, Lee PH. A validation study of the use of smartphones and wrist-worn ActiGraphs to measure physical activity at different levels of intensity and step rates in older people. Gait Posture. 2020;82:306–312. doi: 10.1016/j.gaitpost.2020.09.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to the restrictions involved when obtaining ethical approval for our study, which commit us to share the data only with members of the research team but allow data to be made available from the corresponding author upon reasonable request.