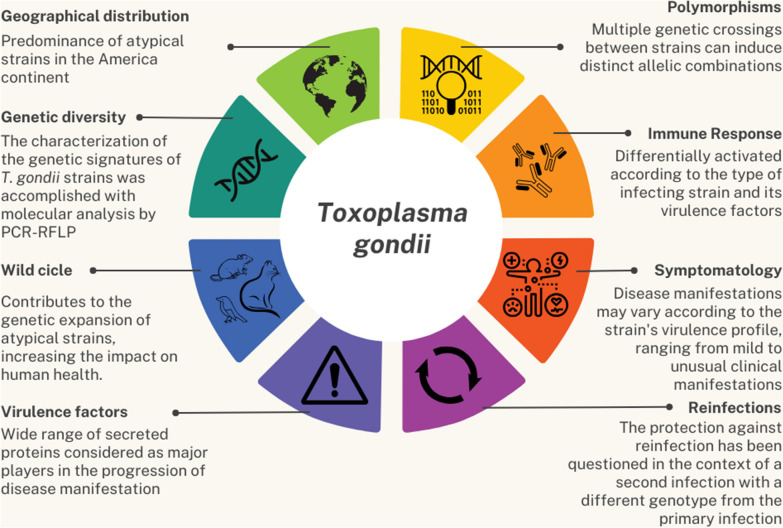
Abstract
Toxoplasma gondii is an intracellular parasite with a worldwide distribution. Toxoplasma gondii infections are of great concern for public health, and their impact is usually most severe in pregnant women and their foetuses, and in immunocompromised individuals. Displaying considerable genetic diversity, T. gondii strains differ widely according to geographical location, with archetypal strains predominantly found in the Northern Hemisphere and non-archetypal (atypical) strains, with highly diverse genotypes, found mainly in South America. In this review, we present an overview of the identification and distribution of non-archetypal strains of T. gondii. Special attention is paid to the strains that have been isolated in Brazil, their interaction with the host immunological response, and their impact on disease outcomes. The genetic differences among the strains are pivotal to the distinct immunological responses that they elicit. These differences arise from polymorphisms of key proteins released by the parasite, which represent important virulence factors. Infection with divergent non-archetypal strains can lead to unusual manifestations of the disease, even in immunocompetent individuals.
Graphical Abstract
Keywords: Atypical strains, Toxoplasmosis, Immunity, Virulence factors
Background
Toxoplasma gondii is an obligate intracellular protozoan parasite that is able to infect a wide range of warm-blooded animals, including humans [1, 2]. The prevalence of T. gondii infection in humans varies widely, and is as high as 90% in some areas of the world [3]. Toxoplasmosis outbreaks have been reported worldwide, with the Americas accounting for almost 74% of reported outbreaks in the past decades. Brazil, with 35% of reported outbreaks [4], is considered a hotspot for toxoplasmosis [5]. In addition to its worldwide distribution and highly diverse host range, T. gondii displays great genetic diversity, which is partly responsible for the variability in infection severity. Toxoplasma gondii strains are traditionally classified as ‘clonal’ or ‘atypical’, according to their genetic characteristics. Recent studies targeting microsatellite sequences showed that although so-called clonal strains share genotypic markers, they differ at the fingerprint level. Because they are not actual clones, the term “archetypal strains” has been proposed instead of clonal strains [6–8]. Thus, in this review we use the terms archetypal strains (types I, II and III genotypes) and non-archetypal strains (new strains, the identification of which is based on divergence in allelic distribution), instead of the terms clonal and atypical strains.
The archetypal strains belong to genotypes I, II, and III, which display less than 1% genetic divergence and are possibly derived from a single genetic crossing [9, 10]; these strains are widespread across the Northern Hemisphere, where they are mainly found in the USA and Europe. Howe and Sibley [9] showed that more than 95% of strains from Europe and North America could be classified as genotype I, II, or III. More recently, it was established that genotypes II and III are dominant in Europe and North America [11]. The diversity of T. gondii found in Europe and the Americas was recently assessed by new phylogenetic methods, which provided new insights into the evolution of T. gondii archetypal and non-archetypal lineages [12]. Recent molecular analysis revealed that the genetic diversity of T. gondii in Mexico is more similar to that observed in South America than in North America [13].
Novel T. gondii genotypes have been designated as atypical, exotic, recombinant, or non-archetypal, based on their allelic composition [14, 15]. An atypical strain has at least one atypical allele in one of the 11 genotyping markers. Strains with mixed profiles, with type I, II or III alleles at different markers, are termed recombinant strains.
Characterization of the genetic signatures of T. gondii archetypal strains is achieved by using the molecular technique polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP). The molecular techniques used to identify and characterize T. gondii genotypes have improved over time, accelerating the discovery of new genotypes [16, 17]. The ability to identify single nucleotide polymorphisms in T. gondii has enabled the identification of new strains, which also allows for a better picture of the parasite’s population genetics. Multilocus PCR–RFLP, microsatellite analysis, and multilocus sequence typing have improved the detection of genetic variations at strain level [16, 17], enabling refined characterization of non-archetypal strains, and elucidation of the population genetics and geographic distribution of T. gondii.
The diversity of T. gondii non-archetypal strains in South America is well recognized, with these strains differing significantly from those observed in the Northern Hemisphere [7, 11, 18]. An analysis of T. gondii’s geographical distribution undertaken almost a decade ago revealed a highly diverse population structure for strains in South America, with 156 distinct genotypes determined for the 646 strains analysed. Of these genotypes, 106 were found exclusively in Brazil [11]. Non-archetypal strains from South America significantly diverge from those of North America and Europe, with the haplogroups showing distinct geographical separation, and a varying number of specific polymorphisms [19].
Considering the great genetic diversity and widespread occurrence of T. gondii genotypes in South America, our review will focus on the main findings from that continent on the identification of non-archetypal strains and their interaction with the host immune system. We will also discuss virulence profiles associated with different genotypes of this parasite and their possible impact on the clinical outcomes of human toxoplasmosis.
Genotyping of non-archetypal strains of T. gondii
A recent study [20] that summarized the occurrence of T. gondii strains in felids worldwide, and included data from North and South America, revealed a marked predominance (~ 74%) of non-archetypal strains in the American continent. In Central and South America, T. gondii non-archetypal strains are abundant in various animal species, and an extremely high genetic diversity has been indicated for this region [19]. Some of these strains are more prevalent and widely distributed in Brazil: BrI (Toxoplasma database (ToxoDB) #6), BrII (#11), BrIII (#8), and BrIV (#17) [21]. The high genetic diversity of T. gondii in Brazil may be associated with several factors, including the great diversity of its hosts there [22]. In this section, we will be covering the main published findings on the identification and genotyping of non-archetypal T. gondii strains in Brazil (Table 1).
Table 1.
Overview of the distribution of Toxoplasma gondii isolates and genotypes in Brazil
| T. gondii ID | Genotype ID (ToxoDB) | Genotyping method | Genotype classification | Isolate source | Localization (Brazilian state) | References |
|---|---|---|---|---|---|---|
| TgCkBr107–116, TgCkBr141–145 | #6, #7, #25, #28, #29, #30, #70, #77, #96, #105 | Nested PCR | 11 Non-archetypal | Free-range chickens | Pará | [121] |
| TgCkBr146–164, TgOvBrRS1–4, TgPkSMBra | #1, #2, #10, #14, #17, #26, #27, #76, #87, #270, #301 | Nested PCR, PCR–RFLP |
1 Archetypal (II), 11 non-archetypal |
Free-range chickens, sheep, pork |
Rio Grande do Sul | [121–123] |
| TgCatBr38–62; TgCatBr64–84; TgCpBr1–36; TgOvBr1–13; TgShBr1–4; TgShBr16–15; TgGtBr1–7, 9–12 | #1–#20, #111, #171, #307 |
Nested PCR; PCR–RFLP |
2 Archetypal (II); 49 non-archetypal; 4 mixed |
Cats, capybaras, sheep | São Paulo | [22–24, 124] |
| TgCkBr210–233, TgCatBrPE01–02 | #2, #3, #59, #60, #61, #62, #146 | PCR–RFLP |
2 Archetypal (II and III), 5 non-archetypal |
Free-range chickens, wild cats |
Fernando de Noronha Island | [125, 126] |
| TgCkBr188–209 | #16, #30, #32, #44, #60, #94–#98 | PCR–RFLP |
10 Non-archetypal, 1 mixed |
Free-range chickens | Mato Grosso do Sul | [127] |
| TgPgBr06–16, TgShBr54, TgShBr124, TgShBr127, TgCkBr284–308, TgCatBr85–89 | #1–#10, #13, #36, #122, #235 | Nested PCR, PCR–RFLP |
14 Non-archetypal, 5 mixed |
Pigs, sheep and free-range chickens, cats |
Bahia | [28, 128–130] |
| TgCTBr1–27, TgWildBrMG1–6 | #8, #11, #13, #36, #41, #67, #108, #162, #206–#212 | PCR–RFLP |
18 Non-archetypal, 1 mixed |
Humans, wild birds | Minas Gerais | [25, 37] |
| TgPgBrRN1–5; TgCkBrRN1–13; TgGtBrRN1; TgGtBr8, 10 | #1–#16, #109, #116, #163 | PCR–RFLP | 8 Non-archetypal | Sheep, goats, pigs and free-range chickens | Rio Grande do Norte | [24, 29] |
| TgCkBr234–250, TgCkBr252–265, TgCkBr267–274, TgCkBr277–281 | #1, #6, #14, #65, #75, #108, #109, #162, #206, #213–#215 | PCR–RFLP | 11 Non-archetypal | Free-range chickens | Espírito Santo | [131] |
| TgCTBral, TgCTBrac, TgCTBrv, TgCtBRca, TgCkBrPr1–18 | #6, #19, #21, #111, #152, #166, #175, #248, #251–#253 | Nested PCR, PCR–RFLP | 12 Non-archetypal |
Humans, free-range chickens |
Paraná | [132, 133] |
| TgHoFBr1, TgMWBr1, TgOncBr1, TgNbaBr1–3, TgCantBr1–3, TgWlpBr1–3, TgSpPBr2, TgLWpBr1, TgSbaBr2 | #6, #11, #175, #195, #196, #231–#239, #241, #246 | PCR–RFLP | 17 Non-archetypal | Wild animals | Mato Grosso, Minas Gerais, Pará, Pernambuco and São Paulo | [134] |
| TgCkBrMA1–5 | #06, #07, #109 | PCR–RFLP | 4 Non-archetypal | Free-range chickens | Maranhão | [135] |
| TgPgBRPB3, 8–14, 16–18, 20–24, 26, 27 | #8, #13, #109, #114, #116, #203, #272–#277 | PCR–RFLP |
11 Non-archetypal, 1 mixed |
Pigs | Paraíba | [136] |
| TgPgBrPI1–16, TgGtBrPI1–9 | #7, #8, #13, #33, #57, #109, #116, #146, #163, #203 | PCR–RFLP | 11 Non-archetypal | Pigs and goats | Piauí | [34] |
| TgCkBrSC1–4 | #10, #26, #53, #278 | PCR–RFLP |
1 Archetypal (I), 3 non-archetypal |
Free-range chickens | Santa Catarina | [137] |
| TgCkAl1–2, TgCTBrAL1 | #146, #162, #279 | PCR–RFLP | 3 Non-archetypal | Free-range chickens, human | Alagoas | [35, 138] |
| TgDgBrMT1–9, TgCatBrMT1, TgPwBrMT1–2, TgCefBrMT1–3, TgBfcuBrMT1, TgAagBrMT1, TgCpBrMT1–2, TgOceBrMT1, TgRhaBrMT1, TgCantBrMT1, TgMduBrMT1, TgLonloBrMT1, TgSAcoBrMT1, TgCpeBrMT1, TgCkBrMT1–51 | #2, #6, #8, #11, #14, #19, #41, #99, #108, #109, #116, #140, #166, #190, #310, #311, #313–#317 | PCR–RFLP |
1 Archetypal (III), 21 non-archetypal, 6 mixed |
Domestic animals, wild animals, humans, free-range chickens | Mato Grosso | [26, 27] |
|
TgCkBrRj2–4, 6, 15, 21, 22, 28; TgCkBr10, 11, 13, 37, 59, 89; TgDgBr6; TgCatBr1 |
#6, #8, #11, #36, #51, #63, #65, #107 | PCR–RFLP | 17 Non-archetypal | Free-range chickens | Rio de Janeiro | [36] |
| TgCkBrGO1–15 | #65 | PCR–RFLP | 1 Non-archetypal | Free-range chickens | Goiás | [139] |
The genotyping analyses were performed using SAG1, SAG2 (5′3′SAG2 and/or alt.SAG2), SAG3, BTUB, dense granule protein 6 (GRA6), c22-8, c29-2, L358, Apico and CS3 loc as the molecular targets
ToxoDB Toxoplasma database, PCR–RFLP polymerase chain reaction-restriction fragment length polymorphism
Mixed genotypes, which indicate the simultaneous presence of two strains in the same host, have been identified in free-range chickens, goats, capybaras, cats, a newborn child [21, 23–29], and in other animals [30–32]. Different strains coexist dynamically in nature and the presence of mixed genotypes of T. gondii in intermediate hosts may facilitate the genetic recombination of the parasite in the gut of a definitive host upon its ingestion of distinct strains [16]. Three main hypotheses have been proposed to explain the existence of mixed infections in a definitive host. First, a mixed infection could be due to an impaired immune response against the superinfecting genotype, allowing the second (or third) genotype to establish a chronic infection. Secondly, the feeding behaviour of felids, which can involve simultaneously (or within a short period of time) preying on various intermediate hosts, offers another opportunity for mixed infections to occur. Thirdly, the ingestion of oocysts containing mixed progeny resulting from a sexual outcross could be a mechanism by which a mixed infection may be introduced into a definitive host. These hypotheses, if supported, could collectively shed light on the intriguing dynamics of mixed infections in the life cycle of T. gondii [33].
Carneiro et al. [25] identified 25 strains, all with non-archetypal genotypes, from the peripheral blood of newborns with congenital toxoplasmosis in the state of Minas Gerais, southeastern Brazil (Table 1). Only two of them were considered avirulent (ToxoDB #8/BrIII, #207), while the others were virulent or of intermediate virulence in experimentally infected mice. The fact that the mouse-virulent strains were isolated from newborns with severe toxoplasmosis and the avirulent strains from asymptomatic children may indicate that there is some correspondence between virulence in mice and disease in humans [25].
In the Central-West region of Brazil there is also a high genetic diversity of T. gondii, and different strains have been isolated from domestic and wild animals, and from humans. A recent study [26] examined 22 strains obtained from nine dogs, one cat, 10 wild animals, and two women in the state of Mato Grosso/Brazil. Multilocus PCR–RFLP revealed 11 T. gondii RFLP genotypes (Table 1), nine of which had been previously described, and two of which were new [26]. More recently, the same authors isolated 51 T. gondii strains from free-range chickens in the state of Mato Grosso [27]. Fifty of the strains were completely genotyped, and were shown to comprise 17 previously described non-archetypal genotypes and five new genotypes. In addition, mixed infections were observed in five of the free-range chickens. In sum, these studies confirm the great diversity of T. gondii strains in animals and humans in Brazil.
Deiró et al. [28] isolated non-archetypal strains of T. gondii from seven naturally infected cats in Bahia, in the Northeast region of Brazil. PCR–RFLP with 11 genetic markers revealed four distinct genotypes. In the genotyping analysis using microsatellite markers, five non-archetypal genotypes were detected in the cats [28].
The occurrence of non-archetypal genotypes in animals slaughtered for human consumption has also been reported in Brazil. Clementino-Andrade et al. [29] identified 19 T. gondii isolates from animals intended for human consumption from the state of Rio Grande do Norte, in the Northeast region of Brazil, of which 17 were classified as virulent based on mice bioassays (ToxoDB #1, #13, #109, #116, #163). Four distinct genotypes were found, including two new ones identified via PCR–RFLP. Rêgo et al. [34] isolated non-archetypal strains of T. gondii from swine and goats raised and slaughtered in the state of Piauí, Northeast region, Brazil. Eleven different genotypes were identified using PCR–RFLP, including a unique one, detected in swine, that had not been previously reported for any other host (Table 1). The 22 other strains belonged to 10 genotypes. In contrast to observations from other states in Brazil, where many of the T. gondii strains examined were reported to be virulent for mice, 72% of the isolates obtained in Piauí were avirulent. Four (16%) displayed intermediate virulence and three (12%) were virulent for mice (ToxoDB #7, #33, #163) [34].
In another study from northeastern Brazil, Santos Silva et al. [35] demonstrated the occurrence of anti-T. gondii antibodies in free-range chickens that were destined for human consumption. Out of 200 blood samples, 72 (36%) were considered positive by indirect immunofluorescence. Two T. gondii strains were isolated, both of which were characterized as non-archetypal and classified as genotypes #146 and #279, with the latter being a new genotype [35].
Casartelli-Alves et al. [36] characterized T. gondii strains obtained from free-range chickens reared in the state of Rio de Janeiro, Brazil. Seventeen non-archetypal genotypes were identified among the 36 T. gondii strains obtained, of which eight had been previously described for different hosts and nine were described for the first time [36].
Non-archetypal genotypes have also been detected in wild animals from Brazil. Rêgo et al. [37] obtained six T. gondii isolates from wild free-range birds rescued in the state of Minas Gerais (Table 1). Five different genotypes were identified by PCR–RFLP, including a new genotype. Of those, three isolates were classified as having intermediate virulence and three as avirulent (ToxoDB #1, #8, #13) for mice. Non-archetypal genotypes detected in free-living wild birds have also been detected in humans, domestic animals, and animals slaughtered for human consumption [37].
Toxoplasma gondii is widespread in South America, and in addition to Brazil, non-archetypal genotypes have been reported in Colombia [38] and Argentina [39, 40]. Pérez-Grisales et al. [44] reviewed 8103 reports of T. gondii in Colombia, of which 86% were from humans and 82% from urban areas. Moré et al. [39] isolated and genotyped T. gondii from serologically positive free-range chickens in Argentina [41]. On one of the farms, they found a non-archetypal genotype that had been previously obtained from chickens in Brazil [42]. They also found the isolate TgCkN21Arg, on another farm, which showed a RFLP pattern identical to that of the Brazilian avirulent archetypal BrIII which had been reported from other species of animals [22–24]. In Argentina, non-archetypal T. gondii genotypes have also been reported as the cause of congenital human toxoplasmosis [40]. Six isolates obtained from umbilical cord blood and placenta were characterized as non-archetypal strains, classified into five genotypes. Of the five genotypes identified from the congenital infection, two had not been previously reported [40], whereas three had been described (ToxoDB #14, #138, and #182) [42, 43]. In sum, these findings indicate the widespread circulation of non-archetypal genotypes in different hosts and geographic regions of South America.
The high diversity of T. gondii strains circulating in Brazil is noteworthy. The identification of both avirulent and virulent strains in animals intended for human consumption underscores the potential health risks of T. gondii, and the need for better monitoring of the food supply chain. Additionally, the detection of non-archetypal genotypes in wild animals, poultry and other birds, and in humans, suggests that they have a wide distribution among various host species. In addition, as many of the studies discussed above were conducted in limited geographical areas and focused on specific host species, the diversity of T. gondii in South America may actually be underestimated. A comprehensive understanding of the genetic diversity of T. gondii, the clinical implications of toxoplasmosis and its potential risk for public health, require more interdisciplinary research and surveillance efforts.
Immunological response to T. gondii infection
The immune response to T. gondii infection has been extensively studied in experimental murine models. Mice Toll-like receptor 11 (TLR11) recognizes T. gondii profilin and, together with TLR12 [44], triggers interleukin (IL)-12 and interferon (IFN)-γ production via the myeloid differentiation primary response 88 protein pathway, the activation of which is essential to elicit an efficient Th1 response against the parasite [45–48].
The innate production of IL-12 and IFN-γ drives the differentiation of Th1 lymphocytes, acting as a positive feedback loop to enhance the inflammatory response and its capacity to control parasite replication [49, 50]. Although the capacity of T. gondii to elicit an efficient IFN-γ-mediated inflammatory response is well established, distinct strains may induce different types of immune responses [51]. In this context, the genotypic characteristics of T. gondii strains, and the differences among them, can determine infection outcomes, based on their ability to up- or downregulate the inflammatory response.
Toxoplasma gondii archetypal strains: virulence factors and immunological activation
Toxoplasma gondii archetypal strains display distinct levels of virulence [52, 53]. In addition, these strains may activate the immune response differently, mainly through the action of rhoptry protein 5 and dense granule-secreted proteins (Table 2), which display significant genetic variation [54].
Table 2.
The main proteins of Toxoplasma gondii identified as virulence factors and their interaction with the immune system
| Proteins | Source | Virulence mechanisms and immunological importance | References |
|---|---|---|---|
| Rhoptry protein 5 (ROP5) | Rhoptries |
Regulation of ROP18 catalytic activity, forming a ROP5/ROP18 complex Blocking of interferon (IFN)-γ-mediated macrophage microbicidal activity Limits antigen presentation in macrophages and dendritic cells |
[58, 140] |
| ROP7 | Interacts with the NACHT domain of NLR family pyrin domain-containing 3 (NLRP3) and promotes inflammasome hyperactivation and induction of interleukin (IL)-1β | [66] | |
| ROP16 |
Phosphorylation of signal transducer and activator of transcription protein 3 (STAT3) and STAT6 and induction of IL-12 and IFN-γ-mediated inflammatory response |
[57, 141] | |
| ROP18 |
Downregulation of the macrophage production of IL-16, TNF-α and IL-12 by phosphorylation of p65 domain of nuclear factor kappa B (NF-κB) Suppression of antigen presentation mechanisms in macrophages and dendritic cells |
[57, 142] | |
| GRA3 | Dense granules | Regulation of the antigen presentation mechanisms in an IFN-γ or cell-independent manner | [57] |
| GRA6 | Regulation of the activation of nuclear factor of activated T cells 4 (NFAT4) in a strain-specific manner, modulating the expression of C-X-C motif chemokine ligand 2 (CXCL2) and C-C motif chemokine ligand 2 (CCL2) and the recruitment of neutrophils | [76] | |
| GRA7 | Interacts with the ROP5/ROP18 complex, binding directly to ROP5 to promote the phosphorylation of immune-regulated guanosine triphosphatase 6 (IRG6) and inactivation of IRG-mediated resistance system | [73] | |
| GRA12 | Confers resistance to IFN-γ-mediated immune response, leading to better parasite survival and establishment of infection in a strain-specific manner | [68, 74] | |
| GRA15 |
Activation of NF-κB via TNF receptor-associated protein 6 (TRAF6) Induction of the inflammasome activation pathway and contributes to IL-1β, IL-12 and TNF-α production |
[52, 67, 69] | |
| GRA24 | Act as an activator of the immune response, modulating IL-12, IL-1β and TNF-α production via mitogen-activated protein kinase (MAPK) activation and the NF-κB pathway, independently of MYD88 innate immune signal transduction adaptor | [52, 75] | |
| GRA25 | Modulation of CCL2 production by macrophages and contributes to efficient parasite survival | [72] |
Secreted rhoptry proteins such as rhoptry protein 16 (ROP16) and ROP18 act as virulence factors in murine models [55, 56]. These proteins have distinct functions depending on the stage of infection. ROP16 is capable of interfering with the signal transducer and activator of transcription protein 3 (STAT3) and STAT6 signalling pathway, leading to the suppression of IL-12 production. ROP18 can suppress antigen presentation in macrophage and dendritic cells of mice, in both an immune-regulated guanosine triphosphatase (IRG)-independent and dependent manner [57, 58]. IRGs are a family of GTPases that play a crucial role in host defence against intracellular pathogens, including T. gondii. These proteins are activated by IFN-γ [59]. Selective recruitment of IRGs to the nascent parasitophorous vacuole membrane (PVM) of susceptible strains leads to destabilization of the vacuole, release of the parasite into the cytoplasm and eventual parasite death in mouse cells [60, 61]. Similar to ROP18, ROP17 targets a common pathway by phosphorylating a member of the IRG family that shows an overlapping function [62]. The phosphorylation ability of ROP18-ROP17 depends on the presence of virulent alleles of pseudokinase ROP5 [63, 64]. In type I parasites, the combination of highly expressed ROP18 and the virulence enhancing ROP5 locus makes the triad of ROP5ROP18ROP17 virulence effectors most potent. Conversely, type II strains exhibit an active ROP18 but an avirulent ROP5 locus. Notably, type III strains, despite possessing the virulence-enhancing ROP5 locus, are entirely avirulent unless highly expressed ROP18 is present [65].
Another important virulence factor is the pseudokinase ROP5, which is particularly significant for the type I strains. The deletion of the ROP5 locus leads to loss of virulence [63]. The virulence profile observed for types I and II (type I is considered to be virulent and type II to be avirulent) can be at least partially explained by the polymorphisms in amino acid sequences from ROP5 [63, 64].
Besides their impacts on mechanisms of antigen-presentation, ROP7, dense granule protein 15 (GRA15), and T. gondii matrix antigen 1 are capable of interfering in the activation of the inflammasome pathway. ROP7 can act as a second signal in the activation of NLR family pyrin domain-containing 3 (NLRP3) inflammasome, and interact with the NACHT domain of NLRP3, stimulating IL-1β and leading to inflammasome hyperactivation [66]. GRA15 and T. gondii matrix antigen 1, both of which belong to the group of proteins produced by the dense granules, are responsible for maintaining a fine balance between activation and dampening of inflammasome activation, respectively [67].
In addition to its role in inducing innate immune responses through the inflammasome pathway, GRA15 has the ability to stimulate IFN-β production via the cGAS/STING pathway [68, 69]. The activation of this signalling cascade enables infected mice to survive infection, as it confers resistance to infection through the control of parasite replication. This is supported by studies showing that mice infected with GRA15 knockout parasites had reduced levels of cytokines such as IFN-β, CXCL10, IFN-γ, and IL-12, followed by higher parasite burden and mortality [68]. Furthermore, GRA15 from type II strains efficiently induces nuclear factor kappa B (NF-κB) activation and its nuclear translocation, contributing to the induction of IFN-γ [70]. It is important to note that there are differences in the parasite-released proteins between strains of the same genotype. For instance, the T. gondii type I RH strain lacks the ability to effectively activate NF-κB due to defective expression of GRA15, leading to lower levels of secreted IL-12. In contrast, the type I GT1 strain can efficiently activate NF-κB, inducing higher amounts of IL-12, by encoding a functional GRA15 [71].
Other GRAs, such as GRA6, GRA7, GRA12, GRA24, and GRA25, interfere in the induction of immune responses, favouring the parasite’s replication and the establishment of the chronic infection stage [72–75]. The activity of these proteins may vary and they can function as important virulence factors, as shown by the significantly reduced mortality observed in mice infected with T. gondii types I and II strains after GRA12 deletion [68]. Furthermore, the protein GRA6 is an important factor in the establishment of strain-specific immunity against T. gondii. Through its ability to induce the activation of NFAT4, and consequently the production of chemokines involved in the recruitment of inflammatory cells such as neutrophils, GRA6 plays a major role in determining the differences in virulence observed among the archetypal genotypes I and II [76].
The activities of strain-specific secreted proteins of T. gondii can explain differences in the severity of infection by known archetypal genotypes, in that these proteins can trigger an early or delayed immune response and interfere in the cell recruitment mechanisms responsible for the parasite’s control [18, 77]. In human infection, genetic variations among T. gondii strains are associated with different levels of disease severity. Highly diverse genotypes of T. gondii were found to be associated with distinct virulence profiles in strains that were mainly from South America [78]. Other geographic differences in disease severity, such as severe ocular toxoplasmosis and increased occurrence of severe congenital toxoplasmosis in areas with a high incidence of non-archetypal strains, have also been shown [79, 80].
While the above-mentioned studies shed light on virulence factors and the immunomodulation mechanisms employed by different T. gondii strains, these mechanisms are complex and multifaceted. For instance, the interactions between parasite-secreted proteins and the host immune response are not fully understood. Additional research is needed to clarify details of these interactions and how they may contribute to disease severity. Despite numerous published studies characterizing the nature of non-archetypal T. gondii strains in South America, few experimental studies have compared the infection dynamics and pathogenicity of these strains with the archetypal strains. Furthermore, there has been limited investigation into the presence of polymorphisms in key virulence factors of non-archetypal T. gondii strains and their immune-modulatory capabilities. Closing these knowledge gaps will be instrumental in advancing our understanding of the biology and pathogenesis of T. gondii.
Toxoplasma gondii non-archetypal strains: virulence factors, immunological activation, and disease outcomes
Secreted proteins described for the T. gondii archetypal strains have also been implicated as virulence factors for non-archetypal strains in mice models [81, 82]. Differences in the virulence of T. gondii non-archetypal strains are also related to polymorphisms in their secreted proteins, such as GRAs and ROPs, which results in their distinct abilities to induce an immune response [80, 83]. Besides the polymorphic nature of ROPs and GRAs, their ability to form protein complexes is key to the prediction of virulence among T. gondii non-archetypal strains. The importance of ROP18 and ROP5 to the virulence of archetypal strains, and that of specific allelic combinations of these two proteins to the virulence of non-archetypal strains in murine models, is acknowledged [84]. Shwab et al. [84] confirmed that the ROP18 type 1 allele (representing a type I archetype) is linked to virulence, while the ROP18 type 2 and 3 alleles (representing type II and III archetypes, respectively) are associated with avirulence. Additionally, they observed that when allele 3 (ROP5) is combined with allele 1 (ROP18), the phenotype is virulent, whereas when combined with allele 3 (ROP18), the phenotype is avirulent [84].
Rego et al. [34] showed that the virulence profile of T. gondii strains obtained from pigs in Brazil was related to the presence of a specific allelic combination of ROP18 and ROP5 (allele 4 for ROP18 and allele 3 for ROP5). Furthermore, virulent T. gondii strains with the same allelic combinations for these proteins were able to induce higher levels of inflammatory cytokines, such as IFN-γ, IL-12, IL-17, and TNF-α, during early infection in mice [85]. However, the allelic profile of ROP5 and ROP18 can change among strains considered to be avirulent or to have intermediate virulence in mice [86].
In a study conducted in Argentina [86], a combination of ROP18 and ROP5 allele types that could be used to predict virulence was detected in a T. gondii strain that was non-lethal to mice. In another study [87], virulent Caribbean and Brazilian strains harboured distinct ROP18 and ROP5 allele types (alleles 3 and 1 for the Caribbean strain and allele 1 and 3 for the Brazilian strain for ROP18 and ROP5, respectively), while avirulent Caribbean and European strains shared the same allelic combination (allele 2 for both loci). The difference in virulence between these distinct T. gondii strains which shared (or did not share) the same combination of ROP18 and ROP5 allele types might have been due to secondary factors that are involved in the parasite’s ability to induce pathology in mice and humans.
The virulence profiles of a diversity of genotypes in mice may be indicative of the severity of toxoplasmosis they cause in humans. Toxoplasma gondii non-archetypal strains obtained from human cases of congenital toxoplasmosis with different levels of severity were able to induce different pathologies in mice, even when the strains were of the same genotype [88]. This suggests that although the classification of genotypes can be helpful in elucidating aspects of their epidemiology and population genetics, it may not fully reflect the biological diversity among strains. Human infections by highly virulent non-archetypal strains can be characterized by differences in their inflammatory stimuli compared to infection caused by archetypal strains. For instance, Colombian patients with ocular toxoplasmosis who were infected by highly heterogeneous strains showed a suppressed IFN-γ response although high levels of TNF-α were induced [89]. The ability of certain strains to downregulate IFN-γ contributes to the host’s reduced capacity to control the parasite’s replication, favouring its dissemination and contributing to tissue damage due to high levels of TNF-α. Thus, the unbalanced immune response triggered by virulent non-archetypal strains may contribute to the development of severe toxoplasmosis. In our recent study [90] comparing chronic murine infection caused by a type II (ME-49) strain and a non-archetypal (CK-2) strain (ToxoDB #163), the non-archetypal strain induced higher levels of IFN-γ and TNF-α in brain tissue compared to the archetypal strain. Additionally, our study revealed that infection with the non-archetypal strain altered the levels of neuroinflammatory mediators and led to the development of behaviour indicative of depression and anxiety, indicating a potential link between persistent inflammation and behavioural changes. For years, serological data have indicated a potential connection between prior T. gondii infection and various psychiatric disorders, including depression [91], schizophrenia [92, 93], bipolar disorders [94], attempted suicide [95] and other conditions [96–98]. It is thus crucial that further research is undertaken to improve our understanding of the mechanisms underlying this potential link. Experimental models can provide valuable insight into the relationship between T. gondii infection and psychiatric disorders, by shedding light on the underlying biological processes. It is also essential to determine if these relationships hold true for human populations. Moreover, clinical studies exploring associations between T. gondii and psychiatric disorders in humans should also examine how genetic variation in the parasite affects the observed outcomes, as this could help to clarify the likely intricate relationship between T. gondii infection and psychiatric disorders.
Although there is little knowledge regarding the exact pathways triggered by the non-archetypal strains, studies have demonstrated the ability of T. gondii secreted factors to induce the inflammatory response. Melo et al. [99] showed that GRA15 from non-archetypal strains can induce the production of IFN-β by macrophages and trigger NF-κB activation, regardless of the allelic composition. Genetic variations among T. gondii non-archetypal strains of the same genotype can drive different immune responses, directly impacting the course of infection in the host. Non-archetypal strains with the same genotype can indeed trigger different immune responses by interacting with different components involved in macrophage activation, and avirulent strain was able to induce alternative activation of macrophages by phosphorylation of STAT6. However, a less virulent strain drove classic macrophage activation by inducing NF-κB activation and IL-12 and TNF-α production [100].
It is assumed that different immunological activation pathways may be involved in disease outcomes of infections with non-archetypal strains of T. gondii with distinct virulence profiles. A study performed in France identified a non-archetypal strain as the causative agent of severe toxoplasmosis in an immunocompetent patient, with lung involvement and the presence of tachyzoites in the bronchoalveolar lavage fluid [101]. Non-archetypal strains have also been identified in cases of congenital toxoplasmosis, and were associated with severe infection [102]. In Egypt, T. gondii seropositivity was found to be significantly higher in children with hydrocephalus and epilepsy; the presence of non-archetypal strains was confirmed by PCR–RFLP [103]. The strain should also be taken into consideration as a potentially important factor in the case of pregnant women, as it may have an impact on fetus survival, as well as on the severity of a newborn’s ocular and/or cerebral impairment [104].
Importantly, the long-held assumption that a previous infection with T. gondii protects the host from subsequent reinfection due to immune memory is now under discussion, with reports of reinfection in humans [105–110] and experimental models [111–113], especially where the second infection is with a different genotype from the primary infection [105, 110, 112–115], as reported for an experimental murine model [116]. In this context, the development of a point-of-care diagnostic test that could distinguish primary from secondary infections would be of value for the monitoring of pregnant women. It is also important to include reinfection as a possible cause in clinical guidelines, with specific prophylactic guidance for pregnant women who are IgG− or IgG+.
Another important matter is the occurrence of a wild cycle of T. gondii, which contributes to the diversity of genotypes that can infect humans, with possibly severe disease manifestations in both immunosuppressed and immunocompetent patients. Severe and unusual cases of toxoplasmosis were reported in Amazonia and named “Amazonian toxoplasmosis” [117, 118]. Demar et al. [119] reported cases of acute toxoplasmosis with visceral complications caused by a single non-archetypal strain (with a unique multilocus genotype) with a high virulence profile in the Amazonian country Suriname, which borders French Guiana and Guyana. In this outbreak, immunocompetent patients presented with multiple organ failure, including signs of respiratory distress, renal insufficiency, and hepatic impairment; there were also some cases of lethal congenital toxoplasmosis [119]. Another, more recent, Amazonian toxoplasmosis outbreak was recorded in French Guiana, in which 37% of 54 patients had confirmed acute toxoplasmosis [120]. The main symptoms were fever, cough, and complications such as hepatic cytolysis. A foetal death caused by a strain belonging to the Amazonian genetic group was also recorded during this outbreak [120].
The wild cycle of T. gondii, which contributes to the expansion of non-archetypal isolates, may increase the impact of toxoplasmosis on public health, regardless of the immunological status of patients. Furthermore, the identification of severe and unusual cases of toxoplasmosis due to infection with highly virulent non-archetypal strains is of great importance for the monitoring of risk factors in transmission areas and possibly for the prediction of outbreaks.
The clinical evolution of toxoplasmosis in adults and children, and in the congenital form, is not completely understood. Nevertheless, it is evident that the host immune response and the diversity of parasite strains are pivotal to the clinical evolution of this disease. The complex interplay between these factors is also influenced by individual host factors, including genetic predisposition and variation in immune environments, which influence disease progression. Due to ongoing advances in molecular genotyping, new T. gondii isolates and genotypes are being constantly identified. This highlights the urgent need for a comprehensive understanding of the impact of strain variability on immune response activation, congenital transmission rates and disease outcomes. Genetic variation in T. gondii may also have implications for treatment success and vaccine development. Investigating these relationships is pivotal to identifying possible patterns of immune response regulation, drug susceptibility, and pathogenicity.
Conclusions
This review highlights the complexity and wide genetic diversity of T. gondii, one of the consequences of which is the classification of its strains into archetypal and non-archetypal types. Despite a growing number of studies that validate the existence of great genotypic diversity among T. gondii strains in South America, where non-archetypal strains significantly differ from those found in North America and Europe, few studies have attempted to further investigate the virulence factors and immunological mechanisms related to infection with non-archetypal strains. This review highlights the intricate relationship between strains of T. gondii and the host immune response, and sheds light on the factors that contribute to the variability in infection severity. It has become increasingly clear that the virulence of these strains is strongly influenced by specific proteins that they secrete, and particularly those secreted by the rhoptry and dense granule organelles, which have distinct roles during infection. Understanding how strain-specific interactions between T. gondii and the host immune system influence infection severity is vital, given the parasite's worldwide distribution and genetic diversity. This knowledge should aid the development of more targeted strategies for the diagnosis, treatment, and prevention of toxoplasmosis, particularly in regions with unique strain profiles. Furthermore, this review shows that T. gondii strains, even within the same genotype, can induce different immune responses and clinical manifestations in both mice models and humans. Variation in the activation of immune pathways may explain the diverse clinical presentation of patients infected with different strains. Geographic differences in strain diversity, such as the emergence of highly virulent non-archetypal strains in Amazonia, are of significance for public health. These highly virulent non-archetypal strains can lead to severe and unusual cases of toxoplasmosis, in both immunosuppressed and immunocompetent individuals. An ability to recognize variation in the disease is thus crucial for the monitoring of transmission risk factors and the prediction of potential outbreaks. Finally, the high heterogeneity of T. gondii strains, particularly in South America, highlights the complexity of toxoplasmosis. Advancements in molecular genotyping have led to the identification of numerous isolates and genotypes, which contribute to unique manifestations of the disease. Understanding the immune responses and inflammatory pathways induced by these diverse strains is essential for predicting disease outcomes and developing effective diagnostic and preventive strategies.
Acknowledgements
We would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Abbreviations
- GRA
Dense granule protein
- IFN
Interferon
- IL
Interleukin
- IRG
Immune-regulated guanosine triphosphatase
- NF-κB
Nuclear factor kappa B
- ROP
Rhoptry protein
- ToxoDB
Toxoplasma database
Author contributions
RMMB wrote, reviewed, and edited the manuscript and tables. GLB and ALB wrote and reviewed the manuscript. LMDM conceptualized, wrote, reviewed, and edited the tables and made the graphical abstract. VFAN, FDT, LLB and RTF reviewed and edited the manuscript.
Funding
LLB and RTF are research fellows at CNPq. RMMB was the recipient of a CAPES PhD scholarship. LMDM is supported by a post-doctoral fellowship from CAPES.
Availability of data and materials
This review is based on published, publicly available data, and did not involve the collection of new data.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Filipe Dantas-Torres is editor-in-chief of Parasites and Vectors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ricardo Toshio Fujiwara, Email: fujiwara@icb.ufmg.br.
Luisa M. D. Magalhães, Email: luisamdmagalhaes@gmail.com
References
- 1.Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol. 1998;28:1019–1024. doi: 10.1016/S0020-7519(98)00023-X. [DOI] [PubMed] [Google Scholar]
- 2.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2002;30:1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegr J, Prandota J, Sovičková M, Israili ZH. Toxoplasmosis—a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS ONE. 2014;9:e90203. doi: 10.1371/journal.pone.0090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto-Ferreira F, Caldart ET, Pasquali AKS, Mitsuka-Breganó R, Freire RL, Navarro IT. Patterns of transmission and sources of infection in outbreaks of human toxoplasmosis. Emerg Infect Dis. 2019;25:2177–2182. doi: 10.3201/eid2512.181565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubey JP. Outbreaks of clinical toxoplasmosis in humans: five decades of personal experience, perspectives and lessons learned. Parasit Vectors. 2021;14:263. doi: 10.1186/s13071-021-04769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ajzenberg D, Collinet F, Mercier A, Vignoles P, Dardé ML. Genotyping of Toxoplasma gondii isolates with 15 microsatellite markers in a single multiplex PCR array. J Clin Microbiol. 2010;48:4641–4645. doi: 10.1128/JCM.01152-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su C, Khan A, Zhou P, Majumdar S, Ajzenberg D, Dardé ML, et al. Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc Natl Acad Sci U S A. 2012;109:5844–5849. doi: 10.1073/pnas.1203190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joeres M, Cardon G, Passebosc-Faure K, Plault N, Fernandez-Escobar M, Hamilton CM, et al. A ring trial to harmonize Toxoplasma gondii microsatellite typing: comparative analysis of results and recommendations for optimization. Eur J Clin Microbiol Infect Dis. 2023;42:803–818. doi: 10.1007/s10096-023-04597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howe DK, David SL. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 10.Su C, Evans D, Cole RH, Kissinger JC, Ajioka JW, Sibley LD. Recent expansion of Toxoplasma through enhanced oral transmission. Science. 2003;299:414–416. doi: 10.1126/science.1078035. [DOI] [PubMed] [Google Scholar]
- 11.Shwab EK, Zhu XQ, Majumdar D, Majumdar D, Pena HFJ, Gennari SM, et al. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology. 2014;141:453–461. doi: 10.1017/S0031182013001844. [DOI] [PubMed] [Google Scholar]
- 12.Galal L, Ariey F, Gouilh MA, Dardé ML, Hamidovic A, Letourneur F, et al. A unique Toxoplasma gondii haplotype accompained the global expansion of cats. Nat Commun. 2022;13:5778. doi: 10.1038/s41467-022-33556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rico-Torres CP, Valenzuela-Moreno LF, Luna-Pasten H, Cedillo-Pelaez C, Correa D, Morales-Salinas E, et al. Genotyping of Toxoplasma gondii isolates from México reveals non-archetypal and potentially virulent strains for mice. Infect Genet Evol. 2023;113:105473. doi: 10.1016/j.meegid.2023.105473. [DOI] [PubMed] [Google Scholar]
- 14.Ajzenberg D, Bañuls AL, Su C, Dumètre A, Demar M, Carme B, et al. Genetic diversity, clonality and sexuality in Toxoplasma gondii. Int J Parasitol. 2004;34:1185–1196. doi: 10.1016/j.ijpara.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Grigg ME, Boothroyd JC. Rapid identification of virulent type I strains of the protozoan pathogen Toxoplasma gondii by PCR-restriction fragment length polymorphism analysis at the B1 gene. J Clin Microbiol. 2001;39:398–400. doi: 10.1128/JCM.39.1.398-400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su C, Zhang X, Dubey JP. Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: a high resolution and simple method for identification of parasites. Int J Parasitol. 2006;36:841–848. doi: 10.1016/j.ijpara.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Su C, Shwab EK, Zhou P, Zhu XQ, Dubey JP. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology. 2010;137:1–11. doi: 10.1017/S0031182009991065. [DOI] [PubMed] [Google Scholar]
- 18.Saeij JPJ, Boyle JP, Boothroyd JC. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 2005;21:476–481. doi: 10.1016/j.pt.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Khan A, Fux B, Su C, Dubey JP, Dardé ML, Ajioka JW, et al. Recent transcontinental sweep of Toxoplasma gondii driven by a single monomorphic chromosome. Proc Natl Acad Sci U S A. 2007;104:14872–17877. doi: 10.1073/pnas.0702356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amouei A, Sarvi S, Sharif M, Aghayan SA, Javidnia J, Mizani A, et al. A systematic review of Toxoplasma gondii genotypes and feline: geographical distribution trends. Transbound Emerg Dis. 2020;67:46–64. doi: 10.1111/tbed.13340. [DOI] [PubMed] [Google Scholar]
- 21.Pena HFJ, Marvulo MFV, Horta MC, Silva MA, Silva JCR, Siqueira DB, et al. Isolation and genetic characterisation of Toxoplasma gondii from a red-handed howler monkey (Alouatta belzebul), a jaguarundi (Puma yagouaroundi), and a black-eared opossum (Didelphis aurita) from Brazil. Vet Parasitol. 2011;175:377–381. doi: 10.1016/j.vetpar.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Pena HFJ, Gennari SM, Dubey JP, Su C. Population structure and mouse-virulence of Toxoplasma gondii in Brazil. Int J Parasitol. 2008;38:561–569. doi: 10.1016/j.ijpara.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Yai LEO, Ragozo AMA, Soares RM, Pena HFJ, Su C, Gennari SM. Genetic diversity among capybara (Hydrochaeris hydrochaeris) isolates of Toxoplasma gondii from Brazil. Vet Parasitol. 2009;162:332–337. doi: 10.1016/j.vetpar.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Ragozo AMA, Pena HFJ, Yai LEO, Su C, Gennari SM. Genetic diversity among Toxoplasma gondii isolates of small ruminants from Brazil: novel genotypes revealed. Vet Parasitol. 2010;170:307–312. doi: 10.1016/j.vetpar.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Carneiro ACAV, Andrade GM, Costa JGL, Pinheiro BV, Vasconcelos-Santos DV, Ferreira AM, et al. Genetic characterization of Toxoplasma gondii revealed highly diverse genotypes for isolates from newborns with congenital toxoplasmosis in southeastern Brazil. J Clin Microbiol. 2013;51:901–907. doi: 10.1128/JCM.02502-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witter R, Pena HFJ, Maia MO, Magalhães AO, Morgado TO, Colodel EM, et al. Isolation and genotyping of Toxoplasma gondii in midwestern Brazil revealed high genetic diversity and new genotypes. Acta Trop. 2020;212:105681. doi: 10.1016/j.actatropica.2020.105681. [DOI] [PubMed] [Google Scholar]
- 27.Witter R, Pena HFJ, Maia MO, Freitas LC, Almeida SLH, Aguar DM, et al. First report on the isolation and genotyping of Toxoplasma gondii strains from free-range chickens in the state of Mato Grosso, midwestern Brazil. Comp Immunol Microbiol Infect Dis. 2022;80:101725. doi: 10.1016/j.cimid.2021.101725. [DOI] [PubMed] [Google Scholar]
- 28.Deiró AGJ, Prado DP, Sousa IP, Rocha DS, Bezerra RA, Gaiotto FA, et al. Presence of atypical genotypes of Toxoplasma gondii isolated from cats in the state of Bahia, Northeast of Brazil. PLoS ONE. 2021;16:e0253630. doi: 10.1371/journal.pone.0253630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clementino Andrade MM, Pinheiro BV, Cunha MM, Carneiro ACAV, Andrade Neto VF, Vitor RWA. New gentotypes of Toxoplasma gondii obtained from farm animals in northeast Brazil. Res Vet Sci. 2013;94:587–589. doi: 10.1016/j.rvsc.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Aspinall TV, Guy EC, Roberts KE, Joynson DHM, Hyde JE, Sims PFG. Molecular evidence for multiple Toxoplasma gondii infections in individual patients in England and Wales: public health implications. Int J Parasitol. 2003;33:97–103. doi: 10.1016/S0020-7519(02)00230-8. [DOI] [PubMed] [Google Scholar]
- 31.Lindstrom I, Sundar N, Lindh J, Kironde F, Kabasa JD, Kwok OCH, et al. Isolation and genotyping of Toxoplasma gondii from Ugandan chickens reveals frequent multiple infections. Parasitology. 2008;135:39–45. doi: 10.1017/S0031182007003654. [DOI] [PubMed] [Google Scholar]
- 32.Pan S, Thompson RCA, Grigg ME, Sundar N, Smith A, Lymbery AJ. Western Australian marsupials are multiply infected with genetically diverse strains of Toxoplasma gondii. PLoS ONE. 2012;7:e45147. doi: 10.1371/journal.pone.0045147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma SK, Sweeny AR, Lovallo MJ, Calero-Bernal R, Kwok OC, Jiang T, et al. Seroprevalence, isolation and co-infection of multiple Toxoplasma gondii strains in individuals bobcats (Lynx rufus) from Mississippi, USA. Int J Parasitol. 2017;47:297–303. doi: 10.1016/j.ijpara.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Rêgo WMF, Costa JGL, Baraviera RCA, Pinto LV, Bessa GL, Lopes REN, et al. Association of ROP18 and ROP5 was efficient as a marker of virulence in atypical isolates of Toxoplasma gondii obtained from pigs and goats in Piauí, Brazil. Vet Parasitol. 2017;247:19–25. doi: 10.1016/j.vetpar.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Silva ACS, de Barros LD, Barros VMC, Alcântara AM, Andrade MR, Garcia JL, et al. Occurrence of atypical and new genotypes of Toxoplasma gondii in free-range chickens intended for human consumption in Brazil. Acta Parasitol. 2020;65:774–778. doi: 10.2478/s11686-020-00194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casartelli-Alves L, Pereira SA, Ferreira LC, Couto RM, Schubach TM, Amendoeira MRR, et al. Genetic and histopathological characterization of Toxoplasma gondii genotypes isolated from free-range chickens reared in the metropolitan region of Rio de Janeiro state, Brazil. Parasitol Res. 2021;120:665–677. doi: 10.1007/s00436-020-07011-9. [DOI] [PubMed] [Google Scholar]
- 37.Rêgo WMF, Costa JGL, Baraviera RCA, Pinto LV, Bessa GL, Lopes REN, et al. Genetic diversity of Toxoplasma gondii isolates obtained from free-living wild birds rescued in southeastern Brazil. Int J Parasitol Parasites Wildl. 2018;7:432–438. doi: 10.1016/j.ijppaw.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortés DA, Aguilar MC, Ríos HA, Rodriguez FJ, Montes KV, Gómez-Marin JR, et al. Severe acute multi-systemic failure with bilateral ocular toxoplasmosis in immunocompetent patients from urban settings in Colombia: case reports. Am J Ophthalmol Case Rep. 2020;18:100661. doi: 10.1016/j.ajoc.2020.100661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moré G, Maksimov P, Pardini L, Herrmann DC, Bacigalupe D, Maksimov A, et al. Toxoplasma gondii infection in sentinel and free-range chickens from Argentina. Vet Parasitol. 2012;184:116–121. doi: 10.1016/j.vetpar.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Pardini L, Bernstein M, Carral LA, Kaufer FJ, Dellarupe A, Gos ML, et al. Congenital human toxoplasmosis caused by non-clonal Toxoplasma gondii genotypes in Argentina. Parasitol Int. 2019;68:48–52. doi: 10.1016/j.parint.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Pérez-Grisales LJ, Cruz-Moncada M, Peláez-Sánchez R, Díaz-Nieto JF. Toxoplasma gondii infection in Colombia with a review of hosts and their ecogeographic distribution. Zoonoses Public Health. 2021;68:38–53. doi: 10.1111/zph.12787. [DOI] [PubMed] [Google Scholar]
- 42.Dubey JP, Velmurugan GV, Chockalingam A, Pena HJF, Oliveira LN, Leifer CA, et al. Genetic diversity of Toxoplasma gondii isolates from chickens from Brazil. Vet Parasitol. 2008;157:299–305. doi: 10.1016/j.vetpar.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barros LD, Taroda A, Zulpo DL, Cunha IAL, Sammi AS, Cardim ST, et al. Caracterização genética de isolados de Toxoplasma gondii de pombos (Zenaida auriculata) no Brasil. Rev Bras Parasitol Vet. 2014;23:443–448. doi: 10.1590/s1984-29612014073. [DOI] [PubMed] [Google Scholar]
- 44.Raetz M, Kibardin A, Sturge CR, Pifer R, Li H, Burstein E, et al. Cooperation of TLR12 and TLR11 in the IRF8-dependent IL-12 response to Toxoplasma gondii profilin. J Immunol. 2013;191:4818–4827. doi: 10.4049/jimmunol.1301301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sturge CR, Yarovinsky F. Complex immune cell interplay in the gamma interferon response during Toxoplasma gondii infection. Infect Immunity. 2014;82:3090–3097. doi: 10.1128/IAI.01722-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 47.Lang C, Groß U, Lüder CGK. Subversion of innate and adaptive immune responses by Toxoplasma gondii. Parasitol Res. 2007;100:191–203. doi: 10.1007/s00436-006-0306-9. [DOI] [PubMed] [Google Scholar]
- 48.Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, et al. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008;3:77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 50.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell-deficient hosts (Toxoplasma gondii/natural killer cells) Proc Natl Acad Sci U S A. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J Immunol. 2004;172:3686–3694. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- 52.Mukhopadhyay D, Arranz-Solís D, Saeij JPJ. Influence of the host and parasite strain on the immune response during Toxoplasma infection. Front Cell Infect Microbiol. 2020;10:580425. doi: 10.3389/fcimb.2020.580425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sibley LD, Boothroyd JC. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992;359:82–85. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Lai BS, Juhas M, Zhang Y. Toxoplasma gondii secretory proteins and their role in invasion and pathogenesis. Microbiol Res. 2019;227:126293. doi: 10.1016/j.micres.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Saeij JPJ, Boyle JP, Coller S, Tylor S, Sibley LD, Brooke-Powell ET, et al. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 57.Hernández-de-los-Ríos A, Murillo-Leon M, Mantilla-Muriel LE, Arenas AF, Vargas-Montes M, Cardona N, et al. Influence of two major Toxoplasma gondii virulence factors (ROP16 and ROP18) on the immune response of peripheral blood mononuclear cells to human toxoplasmosis infection. Front Cell Infect Microbiol. 2019;9:413. doi: 10.3389/fcimb.2019.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rommereim LM, Fox BA, Butler KL, Cantillana V, Taylor GA, Bzik DJ. Rhoptry and dense granule secreted effectors regulate CD8+ T cell recognition of Toxoplasma gondii infected host cells. Front Immunol. 2019;10:2104. doi: 10.3389/fimmu.2019.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor GA, Feng CG, Sher A. Control of IFN-γ-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases) Microbes Infect. 2007;9:1644–1651. doi: 10.1016/j.micinf.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Khaminets A, Hunn JP, Könen-Waisman S, Zhao YO, Preukschat D, Coers J, et al. Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole. Cell Microbiol. 2010;12:939–961. doi: 10.1111/j.1462-5822.2010.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y, Ferguson DJP, Wilson DC, Howard JC, Sibley LD, Yap GS. Virulent Toxoplasma gondii evade immunity-related GTPase (IRG)-mediated parasite vacuole disruption within primed macrophages. J Immunol. 2009;182:3775–3781. doi: 10.4049/jimmunol.0804190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Etheridge RD, Alaganan A, Tang K, Lou HJ, Turk BE, Sibley LD. The Toxoplasma gondii pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe. 2014;15:537–550. doi: 10.1016/j.chom.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Behnke MS, Khan A, Wootton JC, Dubey JP, Tang K, Sibley LD. Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proc Natl Acad Sci U S A. 2011;108:9631–9636. doi: 10.1073/pnas.1015338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reese ML, Zeiner GM, Saeij JPJ, Boothroyd JC, Boyle JP. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci U S A. 2011;108:9625–9630. doi: 10.1073/pnas.1015980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 2012;10:766–778. doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu L, Qi W, Yang G, Yang Y, Wang Y, Zheng L, et al. Toxoplasma gondii rhoptry protein 7 (ROP7) interacts with NLRP3 and promotes inflammasome hyperactivation in THP-1-derived macrophages. Cells. 2022;11:1630. doi: 10.3390/cells11101630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomita T, Guevara RB, Shah LM, Afrifa AY, Weiss LM. Secreted effectors modulating immune responses to Toxoplasma gondii. Life. 2021;11:988. doi: 10.3390/life11090988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang P, Li S, Zhao Y, Zhang B, Li Y, Liu S, et al. The GRA15 protein from Toxoplasma gondii enhances host defense responses by activating the interferon stimulator STING. J Biol Chem. 2019;294:16494–16508. doi: 10.1074/jbc.RA119.009172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ihara F, Fereig RM, Himori Y, Kameyama K, Umeda K, Tanaka S, et al. Toxoplasma gondii dense granule proteins 7, 14, and 15 are involved in modification and control of the immune response mediated via NF-κB pathway. Front Immunol. 2020;11:1709. doi: 10.3389/fimmu.2020.01709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KDC, et al. Strain-specific activation of the NF-κB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med. 2011;208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang N, Farrell A, Niedelman W, Melo M, Lu D, Julien L, et al. Genetic basis for phenotypic differences between different Toxoplasma gondii type I strains. BMC Genomics. 2013;14:467. doi: 10.1186/1471-2164-14-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shastri AJ, Marino ND, Franco M, Lodoen MB, Boothroyd JC. GRA25 is a novel virulence factor of Toxoplasma gondii and influences the host immune response. Infect Immun. 2014;82:2595–2605. doi: 10.1128/IAI.01339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hermanns T, Müller UB, Könen-Waisman S, Howard JC, Steinfeldt T. The Toxoplasma gondii rhoptry protein ROP18 is an Irga6-specific kinase and regulated by the dense granule protein GRA7. Cell Microbiol. 2016;18:244–259. doi: 10.1111/cmi.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fox BA, Guevara RB, Rommereim LM, Falla A, Bellini V, Pètre G, et al. Toxoplasma gondii parasitophorous vacuole membrane-associated dense granule proteins orchestrate chronic infection and GRA12 underpins resistance to host gamma interferon. MBio. 2019;10:e00589–e619. doi: 10.1128/mBio.00589-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mercer HL, Snyder LM, Doherty CM, Fox BA, Bzik DJ, Denkers EY. Toxoplasma gondii dense granule protein GRA24 drives MyD88-independent p38 MAPK activation, IL-12 production and induction of protective immunity. PLoS Pathog. 2020;16:e1008572. doi: 10.1371/journal.ppat.1008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma JS, Sasai M, Ohshima J, Lee Y, Bando H, Takeda K, et al. Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. J Exp Med. 2014;211:2013–2032. doi: 10.1084/jem.20131272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Melo MB, Jensen KDC, Saeij JPJ. Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol. 2011;27:487–495. doi: 10.1016/j.pt.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bessa GL, Vitor RWA, Martins-Duarte ES. Toxoplasma gondii in South America: a differentiated pattern of spread, population structure and clinical manifestations. Parasitol Res. 2021;120:3065–3076. doi: 10.1007/s00436-021-07282-w. [DOI] [PubMed] [Google Scholar]
- 79.Bottós J, Miller RH, Belfort RN, Macedo AC, Belfort-Jr R, UNIFESP Toxoplasmosis Group et al. Bilateral retinochoroiditis caused by an atypical strain of Toxoplasma gondii. Brit J Ophthalmol. 2009;93:1546–1550. doi: 10.1136/bjo.2009.162412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khan A, Taylor S, Ajioka JW, Rosenthal BM, Sibley LD. Selection at a single locus leads to widespread expansion of Toxoplasma gondii lineages that are virulent in mice. PLoS Genet. 2009;5:e10000404. doi: 10.1371/journal.pgen.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen J, Li ZY, Zhou DH, Liu GH, Zhu XQ. Genetic diversity among Toxoplasma gondii strains from different hosts and geographical regions revealed by sequence analysis of GRA5 gene. Parasit Vectors. 2012;5:279. doi: 10.1186/1756-3305-5-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Behnke MS, Khan A, Lauron EJ, Jimah JR, Wang Q, Tolia NH, et al. Rhoptry proteins ROP5 and ROP18 are major murine virulence factors in genetically divergent South American strains of Toxoplasma gondii. PLoS Genet. 2015;11:e1005434. doi: 10.1371/journal.pgen.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niedelman W, Gold DA, Rosowski EE, Sprokholt JK, Lim D, Arenas AF, et al. The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog. 2012;8:e1002784. doi: 10.1371/journal.ppat.1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shwab EK, Jiang T, Pena HFJ, Gennari SM, Dubey JP, Su C. The ROP18 and ROP5 gene allele types are highly predictive of virulence in mice across globally distributed strains of Toxoplasma gondii. Int J Parasitol. 2016;46:141–146. doi: 10.1016/j.ijpara.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 85.Costa JGL, Pinto LV, de Baraviera RCA, Geiger SM, Araújo MSS, Martins-Filho AO, et al. Toxoplasma gondii: cytokine responses in mice reinfected with atypical strains. Exp Parasitol. 2020;218:108006. doi: 10.1016/j.exppara.2020.108006. [DOI] [PubMed] [Google Scholar]
- 86.Bernstein M, Pardini L, Bello Pede Castro B, Unzaga JM, Venturini MC, Moré G. ROP18 and ROP5 alleles combinations are related with virulence of T. gondii isolates from Argentina. Parasitol Int. 2021;83:102328. doi: 10.1016/j.parint.2021.102328. [DOI] [PubMed] [Google Scholar]
- 87.Hamilton CM, Black L, Oliveira S, Burrells A, Bartley PM, Melo RPB, et al. Comparative virulence of Caribbean, Brazilian and European isolates of Toxoplasma gondii. Parasit Vectors. 2019;12:104. doi: 10.1186/s13071-019-3372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pinheiro BV, Noviello MLM, Cunha MM, Tavares AT, Carneiro ACAV, Arantes RME, et al. Pathological changes in acute experimental toxoplasmosis with Toxoplasma gondii strains obtained from human cases of congenital disease. Exp Parasitol. 2015;156:87–94. doi: 10.1016/j.exppara.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 89.De-La-Torre A, Sauer A, Pfaff AW, Bourcier T, Brunet J, Speeg-Schatz C, et al. Severe South American ocular toxoplasmosis is associated with decreased IFN-γ/IL-17a and increased IL-6/IL-13 intraocular levels. PLoS Negl Trop Dis. 2013;7:e2541. doi: 10.1371/journal.pntd.0002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brito RMM, Silva MCM, Vieira-Santos F, Lopes CA, Souza JLN, Bastilho AL, et al. Chronic infection by atypical Toxoplasma gondii strain induces disturbance in microglia population and altered behaviour in mice. Brain Behav Immun Health. 2023;30:100652. doi: 10.1016/j.bbih.2023.100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mahmoud ME, Ihara F, Fereig RM, Nishimura M, Nishikawa Y. Induction of depression-related behaviors by reactivation of chronic Toxoplasma gondii infection in mice. Behav Brain Res. 2016;298:125–133. doi: 10.1016/j.bbr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 92.Elsheikha HM, Zhu XQ. Toxoplasma gondii infection and schizophrenia: an inter-kingdom communication perspective. Curr Opin Infect Dis. 2016;29:311–318. doi: 10.1097/QCO.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 93.Xiao J, Prandovszky E, Kannan G, Plentnikov MV, Dickerson F, Severance EG, et al. Toxoplasma gondii: biological parameters of the connection to schizophrenia. Schizophr Bull. 2018;44:983–992. doi: 10.1093/schbul/sby082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barros JLVM, Barbosa IG, Salem H, Rocha NP, Kummer A, Okusaga OO, et al. Is there any association between Toxoplasma gondii infection and bipolar disorder? A systematic review and meta-analysis. J Affect Disord. 2017;209:59–65. doi: 10.1016/j.jad.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 95.Bak J, Shim SH, Kwon YJ, Lee HY, Kim JS, Yoon H, et al. The association between suicide attempts and Toxoplasma gondii infection. Clin Psychopharmacol Neurosci. 2018;16:95–102. doi: 10.9758/cpn.2018.16.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cook TB, Brenner LA, Cloninger CR, Langenberg P, Igbide A, Giegling I, et al. “Latent” infection with Toxoplasma gondii: association with trait aggression and impulsivity in healthy adults. J Psychiatr Res. 2015;60:87–94. doi: 10.1016/j.jpsychires.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 97.Fabiani S, Pinto B, Bonuccelli U, Bruschi F. Neurobiological studies on the relationship between toxoplasmosis and neuropsychiatric diseases. J Neurol Sci. 2015;351:3–8. doi: 10.1016/j.jns.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 98.Ngoungou EB, Bhalla D, Nzoghe A, Dardé ML, Preux PM. Toxoplasmosis and epilepsy–systematic review and meta-analysis. PLoS Negl Trop Dis. 2015;9:e0003525. doi: 10.1371/journal.pntd.0003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Melo MB, Nguyen QP, Cordeiro C, Hassan MA, Yang N, McKell R, et al. Transcriptional analysis of murine macrophages infected with different Toxoplasma strains identifies novel regulation of host signaling pathways. PLoS Pathog. 2013;9:1–17. doi: 10.1371/journal.ppat.1003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang AM, Shen Q, Li M, Xu XC, Chen H, Cai YH, et al. Comparative studies of macrophage-biased responses in mice to infection with Toxoplasma gondii ToxoDB #9 strains of different virulence isolated from China. Parasit Vectors. 2013;6:308. doi: 10.1186/1756-3305-6-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salvador-Guillouët F, Ajzenberg D, Chaillou-Opitz S, Saint-Paul MC, Dunais B, Dellamonica P, et al. Severe pneumonia during primary infection with an atypical strain of Toxoplasma gondii in an immunocompetent young man. J Infect. 2006;53:e47–e50. doi: 10.1016/j.jinf.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 102.Ajzenberg D, Cogné N, Paris L, Bessières MH, Thulliez P, Filisetti D, et al. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J Infect Dis. 2002;186:684–689. doi: 10.1086/342663. [DOI] [PubMed] [Google Scholar]
- 103.Elzeky SM, Nabih N, Abdel-Magied AA, Abdelmagid DS, Handoussa AE, Hamouda MM. Seroprevalence and genetic characterization of Toxoplasma gondii among children with neurodevelopmental disorders in Egypt. J Trop Med. 2022;2022:2343679. doi: 10.1155/2022/2343679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rico-Torres CP, Vargas-Villavicencio JA, Correa D. Is Toxoplasma gondii type related to clinical outcome in human congenital infection? Systematic and critical review. Eur J Clin Microbiol Infect Dis. 2016;35:1079–1108. doi: 10.1007/s10096-016-2656-2. [DOI] [PubMed] [Google Scholar]
- 105.Dao A, Fortier B, Soete M, Plenat F, Dubremetz JF. Successful reinfection of chronically infected mice by a different Toxoplasma gondii genotype. Int J Parasitol. 2001;31:63–65. doi: 10.1016/S0020-7519(00)00151-X. [DOI] [PubMed] [Google Scholar]
- 106.Gavinet MF, Robert F, Firtion G, Delouvrier E, Hennequin C, Maurin JR, et al. Congenital toxoplasmosis due to maternal reinfection during pregnancy. J Clin Microbiol. 1997;35:1276–1277. doi: 10.1128/jcm.35.5.1276-1277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Valdès V, Legagneur H, Watrin V, Paris L, Hascoët JM. Congenital toxoplasmosis due to maternal reinfection during pregnancy. Arch Pediatr. 2011;18:761–763. doi: 10.1016/j.arcped.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 108.de Aloise DA, Coura-Vital W, Carneiro M, Rodrigues MV, Toscano GAS, Silva RB, et al. Association between ocular toxoplasmosis and APEX1 and MYD88 polymorphism. Acta Trop. 2021;221:106006. doi: 10.1016/j.actatropica.2021.106006. [DOI] [PubMed] [Google Scholar]
- 109.Lebas F, Ducrocq S, Mucignat V, Paris L, Mégier P, Baudon JJ, et al. Congenital toxoplasmosis: infection during pregnancy in an immune and immunocompetent woman. Arch Pediatr. 2004;11:926–928. doi: 10.1016/j.arcped.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 110.Silveira C, Ferreira R, Muccioli C, Nussenblatt R, Belfort R. Toxoplamosis transmitted to a newborn from the mother infected 20 years earlier. Am J Ophthalmol. 2003;136:370–371. doi: 10.1016/S0002-9394(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 111.Freyre A, Falcón J, Mendez J, Correa O, Morgades D, Rodríguez A. An investigation of sterile immunity against toxoplasmosis in rats. Exp Parasitol. 2004;107:14–19. doi: 10.1016/j.exppara.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 112.Dzitko K, Staczek P, Gatkowska J, Dlugonska H. Toxoplasma gondii: serological recognition of reinfection. Exp Parasitol. 2006;112:134–137. doi: 10.1016/j.exppara.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 113.Franco PS, da Silva NM, de Barbosa BF, Gomes AO, Ietta F, Shwab EK, et al. Calomys callosus chronically infected by Toxoplasma gondii clonal type II strain and reinfected by Brazilian strains is not able to prevent vertical transmission. Front Microbiol. 2015;6:181. doi: 10.3389/fmicb.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Elbez-Rubinstein A, Ajzenberg D, Dardé ML, Cohen R, Dumètre A, Year H, et al. Congenital toxoplasmosis and reinfection during pregnancy: case report, strain characterization, experimental model of reinfection, and review. J Infect Dis. 2009;199:280–285. doi: 10.1086/595793. [DOI] [PubMed] [Google Scholar]
- 115.Bessa GL, Costa JGL, Rêgo WMF, Baraviera RCA, Pinto LV, Lopes REN, et al. Tissue dissemination and humoral response after experimental reinfection with atypical Toxoplasma gondii strains obtained from congenital human toxoplasmosis in Brazil. Exp Parasitol. 2019;207:107781. doi: 10.1016/j.exppara.2019.107781. [DOI] [PubMed] [Google Scholar]
- 116.Jensen KDC, Camejo A, Melo MB, Cordeiro C, Julien L, Grotenbreg GM, et al. Toxoplasma gondii superinfection and virulence during secondary infection correlate with the exact ROP5/ROP18 allelic combination. MBio. 2015;6:e02280. doi: 10.1128/mBio.02280-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carme B, Demar M, Ajzenberg D, Dardé ML. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerg Infect Dis. 2009;15:656–658. doi: 10.3201/eid1504.081306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blaizot R, Nabet C, Blanchet D, Martin E, Mercier A, Dardé ML, et al. Pediatric Amazonian toxoplasmosis caused by atypical strains in French Guiana, 2002–2017. J Pediatr Infect Dis. 2019;38:39–42. doi: 10.1097/INF.0000000000002130. [DOI] [PubMed] [Google Scholar]
- 119.Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W, et al. Fatal outbreak of human toxoplasmosis along the Maroni river: epidemiological, clinical, and parasitological aspects. Clin Infect Dis. 2007;45:e88–e95. doi: 10.1086/521246. [DOI] [PubMed] [Google Scholar]
- 120.Blaizot R, Nabet C, Laghoe L, Faivre B, Escotte-Binet S, Djossou F, et al. Outbreak of Amazonian toxoplasmosis: a One Health investigation in a remote Amerindian community. Front Cell Infect Microbiol. 2020;10:401. doi: 10.3389/fcimb.2020.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dubey JP, Sundar N, Gennari SM, Minervino AHH, Farias NAR, Ruas JL, et al. Biologic and genetic comparison of Toxoplasma gondii isolates in free-range chickens from the northern Pará state and the southern state Rio Grande do Sul, Brazil revealed highly diverse and distinct parasite populations. Vet Parasitol. 2007;143:182–188. doi: 10.1016/j.vetpar.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 122.Ramos TS, de Jesus Pena HF, Santos Junior AG, Santos LMJF, Cademartori BG, Oliveira S, et al. Characterization of Toxoplasma gondii isolates from herds of sheep in southern Brazil reveals the archetypal type II genotype and new non-archetypal genotypes. Parasitol Int. 2018;67:59–63. doi: 10.1016/j.parint.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 123.Paraboni MLR, Costa DF, Silveira C, Gava R, Pereira-Chioccola VL, Belfort-Jr R, et al. A new strain of Toxoplasma gondii circulating in southern Brazil. J Parasit Dis. 2020;44:248–252. doi: 10.1007/s12639-019-01155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Silva RC, Langoni H, Su C, Silva AV. Genotypic characterization of Toxoplasma gondii in sheep from Brazilian slaughterhouses: new atypical genotypes and the clonal type II strain identified. Vet Parasitol. 2011;175:173–177. doi: 10.1016/j.vetpar.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 125.Dubey JP, Rajendran C, Costa DGC, Ferreira LR, Kwok OCH, Qu D, et al. New Toxoplasma gondii genotypes isolated from free-range chickens from the Fernando de Noronha, Brazil: unexpected findings. J Parasitol. 2010;96:709–712. doi: 10.1645/GE-2425.1. [DOI] [PubMed] [Google Scholar]
- 126.Melo RPB, Almeida JC, Lima DCV, Pedrosa CM, Magalhães FJR, Alcântara AM, et al. Atypical Toxoplasma gondii genotype in feral cats from the Fernando de Noronha Island, northeastern Brazil. Vet Parasitol. 2016;224:92–95. doi: 10.1016/j.vetpar.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 127.Soares RM, Silveira LH, Silva AV, Ragozo A, Galli S, Lopes EG, et al. Genotyping of Toxoplasma gondii isolates from free range chickens in the Pantanal area of Brazil. Vet Parasitol. 2011;178:29–34. doi: 10.1016/j.vetpar.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 128.Bezerra RA, Carvalho FS, Guimarães LA, Rocha DS, Maciel DM, Wenceslau AA, et al. Genetic characterization of Toxoplasma gondii isolates from pigs intended for human consumption in Brazil. Vet Parasitol. 2012;189:153–161. doi: 10.1016/j.vetpar.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 129.Maciel BM, Moura RLS, Carvalho FS, Costa EA, Albuquerque GR. Identification and genetic characterization of a new Brazilian genotype of Toxoplasma gondii from sheep intended for human consumption. Parasitol Int. 2014;63:567–570. doi: 10.1016/j.parint.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 130.Rocha DS, Nilsson MG, Maciel BM, Pena HFJ, Alves BF, Silva AV, et al. Genetic diversity of Toxoplasma gondii isolates from free-range chickens in Bahia, Brazil. J Parasitol. 2018;104:377–382. doi: 10.1645/18-9. [DOI] [PubMed] [Google Scholar]
- 131.Pena HFJ, Vitaliano SN, Beltrame MAV, Pereira FEL, Gennari SM, Soares RM. PCR-RFLP genotyping of Toxoplasma gondii from chickens from Espírito Santo state, Southeast region, Brazil: new genotypes and a new SAG3 marker allele. Vet Parasitol. 2013;192:111–117. doi: 10.1016/j.vetpar.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 132.Higa LT, Garcia JL, Su C, Rossinid RC, Falavigna-Guilhermee AL. Toxoplasma gondii genotypes isolated from pregnant women with follow-up of infected children in southern Brazil. Trans R Soc Trop Med Hyg. 2014;108:244–246. doi: 10.1093/trstmh/tru014. [DOI] [PubMed] [Google Scholar]
- 133.Vieira FEG, Sasse JP, Minutti AF, Miura AC, Barros LD, Cardim ST, et al. Toxoplasma gondii: prevalence and characterization of new genotypes in free-range chickens from south Brazil. Parasitol Res. 2018;117:681–688. doi: 10.1007/s00436-017-5730-5. [DOI] [PubMed] [Google Scholar]
- 134.Vitaliano SN, Soares HS, Minervino AHH, Santos ALQ, Werther K, Marvulo MFV, et al. Genetic characterization of Toxoplasma gondii from Brazilian wildlife revealed abundant new genotypes. Int J Parasitol Parasites Wildl. 2014;3:276–283. doi: 10.1016/j.ijppaw.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sousa IC, Pena HFJ, Santos LS, Gennari SM, Costa FN. First isolation and genotyping of Toxoplasma gondii from free-range chickens on São Luis island, Maranhão state, Brazil, with a new genotype described. Vet Parasitol. 2016;223:159–164. doi: 10.1016/j.vetpar.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 136.Feitosa TF, Ribeiro Vilela VL, de Almeida-Neto JL, Santos A, Morais DF, et al. High genetic diversity in Toxoplasma gondii isolates from pigs at slaughterhouses in Paraíba state, northeastern Brazil: circulation of new genotypes and Brazilian clonal lineages. Vet Parasitol. 2017;244:76–80. doi: 10.1016/j.vetpar.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 137.Pena HFJ, Alves BF, Soares HS, Oliveira S, Ferreiras MN, Bricarello PA, et al. Free-range chickens from Santa Catarina state, southern Brazil, as asymptomatic intermediate hosts for Toxoplasma gondii clonal type I and typical Brazilian genotypes. Vet Parasitol Reg Stud Reports. 2018;13:55–59. doi: 10.1016/j.vprsr.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 138.Melo RPB, Wanderley FS, Porto WJN, Pedrosa CM, Hamilton CM, Oliveira MHGS, et al. Description of an atypical Toxoplasma gondii isolate from a case of congenital toxoplasmosis in northeastern Brazil. Parasitol Res. 2020;119:2727–2731. doi: 10.1007/s00436-020-06746-9. [DOI] [PubMed] [Google Scholar]
- 139.Rezende HHA, Igreja JASL, Gomes-Júnior AR, Melo JO, Garcia JL, Martins FDC, et al. Molecular characterization of Toxoplasma gondii isolates from free-range chickens reveals new genotypes in Goiânia, Goiás, Brazil. Rev Bras Parasitol Vet. 2021;30:e000321. doi: 10.1590/s1984-29612021029. [DOI] [PubMed] [Google Scholar]
- 140.Behnke MS, Fentress SJ, Mashayekhi M, Li LX, Taylor GA, Sibley LD. The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18. PLoS Pathog. 2012;8:e1002992. doi: 10.1371/journal.ppat.1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Butcher BA, Fox BA, Rommereim LM, Kim SG, Maurer KJ, Yarovinsky F, et al. Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 2011;7:e1002236. doi: 10.1371/journal.ppat.1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Du J, An R, Chen L, Shen Y, Chen Y, Cheng L, et al. Toxoplasma gondii virulence factor ROP18 inhibits the host NK-κB pathway by promoting P65 degradation. J Biol Chem. 2014;289:12578–12592. doi: 10.1074/jbc.M113.544718. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review is based on published, publicly available data, and did not involve the collection of new data.