Abstract
Introduction:
Endogenous endophthalmitis-related Klebsiella pyogenic liver abscess is a rare complication of metastatic infection. In most cases, visual acuity results are often impaired, even blind, and even with aggressive treatment with topical antibiotics, the final results are unsatisfactory. The objective of this study is to retrospectively based on medical records to describe clinical features, risk factors, and visual outcomes of patients with endogenous endophthalmitis-related pyogenic liver abscesses.
Methods:
We reported a case series of 12 endogenous endophthalmitis-related pyogenic liver abscess patients from March 2021 to 2023. All cases of endogenous endophthalmitis were diagnosed at admission or during the hospital stay.
Results:
From the medical records of 588 pyogenic liver abscess patients, we found 12 cases of endogenous endophthalmitis with 2.0%. The result showed a mean age of 61.5 ± 12.0 (41–78), diabetes mellitus (7 of 12), right lobe (7 of 12), single abscess (9 of 12), and the mean largest abscess diameter of 5.8 ± 1.7 cm (3.3–9). All patients had ocular symptoms such as eye pain (9 of 12), pus discharge (3 of 12), hypopyon (1 of 12), swollen eyelids (2 of 12), and corneal edema (2 of 12), pyogenic liver abscess before endogenous endophthalmitis (10 of 12), the median interval between endogenous endophthalmitis and pyogenic liver abscess 6.1 ± 1.9 days, ocular symptoms before diagnosis endogenous endophthalmitis 4.4 ± 2.3 days. All affected eyes were injected intravitreously with ceftazidime, amikacin, and vancomycin. Two patients underwent evisceration.
Conclusions:
Endogenous endophthalmitis has permanent morbidity, reducing visual acuity, poor quality of life, and lacks the warning signs, so it is essential for early detection of symptoms and referral to ophthalmologists.
Keywords: Pyogenic liver abscess, endophthalmitis, Klebsiella pneumoniae
Introduction
In Southeast Asia, Klebsiella pneumoniae is an emerging agent causing a pyogenic liver abscess (PLA). 1 In Taiwan and Korea, approximately 80% of cases of PLA are caused by K. pneumoniae (KPLA). 2 K. pneumoniae infection often causes sepsis, causing widespread disease in organs such as the lungs, meninges, and eyes. On the other hand, endogenous endophthalmitis (EE) is a rare complication involving infection inside the eye related to vitreous, aqueous humor. It is a consequence of infection spreading in the bloodstream. 3 In a meta-analysis study, the incidence of EE complications in patients with KPLA is 4.5%. 1 Although the patient had clinical features of PLA, such as fever, and right upper quadrant pain, ocular complications are often signs that require the patient to go to the doctor. Therefore, in most cases, visual acuity (VA) results are often impaired, even blind, and even with aggressive treatment with topical antibiotics, the final results are not satisfactory. 4 In some cases, evisceration is necessary to reduce the risk of infection in the other eye. The objective of this study is to retrospectively based on medical records to describe clinical features, risk factors, and visual outcomes of patients with EE-PLA.
Methods
This study is a retrospective analysis of medical records from PLA patients who were hospitalized between March 2021 and 2023. Only records with complete information on PLA symptoms, positive ultrasound or CT scan results showing an intrahepatic fluid mass, positive blood culture, or positive abscess aspiration were included. Patients were classified as having EE if they met the following criteria: (1) clinical features such as eye pain, redness, and pus discharged; (2) blood culture or intraocular culture or liver abscess fluid positive or evidence of pus in pathology, (3) excluding others etiology of EE: ophthalmic surgery, corneal ulcers from trauma within 1 year of diagnosis. During their hospital stay, all patients diagnosed with EE received treatment through vitreous injection. Visual outcomes were evaluated at the time of patient discharge. Medical records with incomplete information were excluded, as well as patients diagnosed with EE but without evidence of PLA.
We described the demographic, diagnosis sequence, PLA and EE clinical features, laboratory tests, microorganisms, therapeutic antibiotics, and visual outcomes. The time course had three critical points: (1) at the time of diagnosis of pyogenic liver abscess, (2) at the onset of ocular symptoms, and (3) at the time of diagnosis and intervention of endophthalmitis. All patients started receiving antibiotic treatment while their abscess, blood, and vitreous samples were cultured. Visual acuity was evaluated based on Daiber et al. which consisted of counting fingers (CF), hand motion (HM), light perception (LP), and no light perception (NLP). 5
Written informed consent was obtained from all subjects before the study.
Statistical analysis
Descriptive statistics were performed. We calculated frequencies, means, and standard deviations (SDs).
Ethics approval
Ethical approval was waived by the Institutional Review Board of Bach Mai Hospital (BM-2022-192) due to the case series.
Results
From the medical records of 588 PLA patients, we found 12 cases of EE-related PLA with a rate of 2.0%. The mean age was 61.5 ± 12.0 (41–78), three times more males than females (nine males and three females). Seven of the 12 patients had diabetes mellitus. Based on the results of ultrasound and computed tomography, the specific location was in the right lobe (7 of 12), single abscess (9 of 12), and the mean largest abscess diameter was 5.8 ± 1.7 cm (3.3 9) (Table 1). Laboratory tests showed that the average values of aspartate aminotransferase (AST), alanine transaminase (ALT), total bilirubin (Bil), white blood cells (WBC), C-reactive protein (CRP), and procalcitonin (PCT) were 87.6 ± 133.0 U/l, 94.0 ± 126.2 U/l, 29.5 ± 25.5 U/l, 18.2 ± 7.5 G/l, 78.6 ± 119.2 mg/l, and 40.3 ± 27.6 ng/ml, respectively (Table 1).
Table 1.
Demographic, clinical features, and laboratory tests of EE-related PLA patients.
| Patient No | Age, gender | Underlying disease | Location of abscess | Number of abscesses | Size of the abscess (cm) | AST | ALT | Bil | WBC | CRP | PCT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/51 | None | Right lobe | Single | 7.0 | 35 | 77 | 21 | 23.1 | 22.9 | 19.8 |
| 2 | M/63 | DM, HT, MI | Right lobe | Single | 6.8 | 39 | 29 | 6 | 17.9 | 19.3 | 67.9 |
| 3 | M/41 | DM | Left lobe | Single | 4.5 | 25 | 41 | 14 | 11.2 | 368.3 | 3.3 |
| 4 | M/78 | None | Right lobe | Single | 9.0 | 475 | 466 | 86 | 18.8 | 29.8 | 59.3 |
| 5 | M/61 | None | Right lobe | Single | 4.7 | 37 | 26 | 10 | 16.7 | 125.6 | — |
| 6 | F/65 | None | Right lobe | Single | 5.0 | 45 | 49 | 8 | 34.0 | 189.7 | 59.6 |
| 7 | M/43 | DM | Right lobe | Single | 6.3 | 188 | 150 | 54 | 19.2 | — | 67.9 |
| 8 | F/72 | DM | Left lobe | Single | 7.3 | 13 | 26 | 23 | 15.0 | 3.8 | 4.4 |
| 9 | M/75 | None | Left lobe | Multiple | 6.7 | 131 | 148 | 51 | 7.6 | — | 40.0 |
| 10 | M/72 | DM | Left lobe | Single | 3.3 | 14 | 12 | 55 | 14.9 | 9.7 | — |
| 11 | M/56 | DM | Right lobe | Multiple | 3.7 | 16 | 20 | 12 | 7.4 | 0.7 | — |
| 12 | F/61 | DM | Left lobe | Multiple | 4.7 | 33 | 84 | 14 | 19.1 | 15.7 | — |
M: male; F: female; DM: diabetes mellitus; HT: hypertension; MI: myocardial infarction; AP: abdominal pain; size of the abscess, the maximum length of the abscess; Bil: total bilirubin; AST: aspartate aminotransferase; ALT: alanine transaminase; WBCs: white blood cells; CRP: C-reactive protein; PCT: procalcitonin.
At the time of examination, all patients had ocular symptoms such as eye pain (9 of 12), pus discharge (3 of 12), hypopyon (1 of 12), swollen eyelids (2 of 12), and corneal edema (2 of 12) (Table 2). Figure 1 showed the eye involvement and pyogenic liver abscess of patient one. Ten patients had been diagnosed with PLA before EE (Table 2). The median interval between EE and PLA was 6.1 ± 1.9 days. The mean interval between ocular symptoms of diagnosis EE was 4.4 ± 2.3 days.
Table 2.
Clinical eye features and microorganisms of EE-related PLA patients.
| Patient No | Sequence of diagnosis | Eye affected | Symptoms | Interval times (days) | Causative agent | Microorganism | Antibiotic | ||
|---|---|---|---|---|---|---|---|---|---|
| Abscess | Blood | Vitreous | |||||||
| 1 | EE→PLA | OS, OD | Eyes pain, left eye hypopyon | 5 | K. pneumoniae | + | + | − | Sensitivity |
| 2 | PLA→EE | OS | Eye pain, pus discharge | 10 | K. pneumoniae | + | − | − | Sensitivity |
| 3 | PLA→EE | OD | Eye pain | 5 | K. pneumoniae | + | − | − | Sensitivity |
| 4 | PLA→EE | OS, OD | Eyes pain, pus discharge (OS), corneal edema (OS) | 8 | K. pneumoniae | + | − | − | Sensitivity |
| 5 | PLA→EE | OD | Eye pain | 6 | K. pneumoniae | + | − | − | Sensitivity |
| 6 | PLA→EE | OD | Eye pain | 7 | K. pneumoniae | + | − | − | Sensitivity |
| 7 | PLA→EE | OS, OD | Swollen eyelids (OS), pus discharge (OS) | 3 | K. pneumoniae | + | + | + | Sensitivity |
| 8 | PLA→EE | OS | Eye pain, corneal edema, | 5 | K. pneumoniae | + | − | − | Sensitivity only carbapenem |
| 9 | PLA→EE | OD | Eye pain | 4 | K. pneumoniae | + | − | − | Sensitivity |
| 10 | EE→PLA | OD | Eye pain | 7 | Negative | − | − | − | — |
| 11 | PLA→EE | OS | Swollen eyelids | 7 | Negative | − | − | − | — |
| 12 | PLA→EE | OS | Eye pain | 6 | E. coli | − | + | − | Resistance azithromycin, cefuroxime |
EE: endogenous endophthalmitis; PLA: pyogenic liver abscess; OS: left eye; OD: right eye; Interval times: times of interval between EE and PLA; +: positive; −: negative.
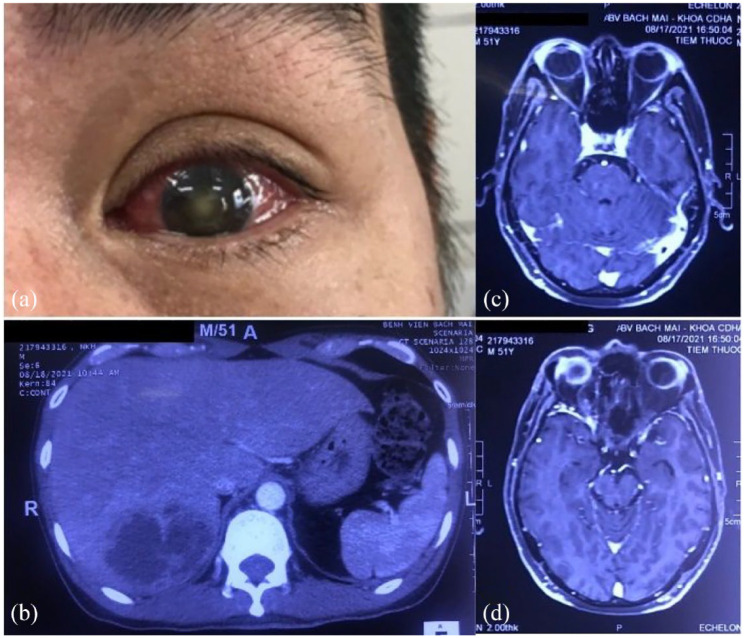
Figure 1.
Eye involvement and pyogenic liver abscess of patient one. (a) OS involvement. (b) Contrast-enhanced abdominal computerized tomography (CT) scanning showed hepatomegaly with 19.5 cm. The posterior parenchymal segment adjacent to the diaphragm has heterogeneous hypoechoic foci of size 70 × 55 × 50 mm, cortical enhancement, central liquefaction, and perfusion disturbances in the liver parenchyma around the lesion. (c and d) On magnetic resonance imaging, there is a component of increased signal on T1W, decreased T2W, limited diffusion, and no enhancement after injection. Infiltrate the orbital soft tissue around the eyeball and the lateral rectus muscle on OS, enhancing after injection.
In all, 12 patients with 15 involved eyes had two OS, four OD, and three cases with both eyes (Table 3). After that, all affected eyes were injected intravitreously with ceftazidime, amikacin, and vancomycin. Most damaged eyes worsened or did not change after treatments. Notably, two eyes underwent evisceration. Three eyes had improved better. The visual outcomes and ocular treatment responses are shown in Table 3.
Table 3.
Treatment and visual outcomes of EE-related PLA patients.
| Patient No | Eye affected | Initial VA (OS/OD) | Final VA (OS/OD) | Intravitreally injected antibiotic | Respond of treatment | Operation |
|---|---|---|---|---|---|---|
| 1 | OS, OD | PL/HM | NPL/CF 10 cm | VAN (OS, 4 times), VAN (OD, 1 time) | Worse (OS), Better (OD) | None |
| 2 | OS | NPL/0.2 | NPL/0.2 | CEF (OS, 3 times) | No change | None |
| 3 | OD | 0.6/HM | 0.6/PL | CEF (OD, 1 time), VAN (OD, 2 times) | No change | None |
| 4 | OS, OD | PL/PL | NPL/PL | AMK (OS, 3 times) | Worse | None |
| 5 | OD | 0.2/HM | 0.2/HM | CEF (OD, 3 times) | No change | None |
| 6 | OD | 0.2/NPL | 0.2/NPL | CEF (OD, 3 times) | No change | None |
| 7 | OS, OD | NPL/0.7 | NPL/0.7 | AMK (OS, 2 times) | No change | None |
| 8 | OS | PL/0.2 | NPL/0.2 | CEF (OS, 3 times) | Worse | Enucleation |
| 9 | OD | 0.2/0.1 | 0.2/NPL | CEF (OD, 3 times) | Worse | Enucleation |
| 10 | OD | 0.1/NPL | 0.28/NPL | Vancomycin (OD, 3 times) | Better | None |
| 11 | OS | 0.2/NPL | 0.25/NPL | Ceftazidim (OS, 3 times) | No change | None |
| 12 | OS | CF1.5 m/0.28 | 0.13/0.2 | Ceftazidim (OS, 3 times) | Better | None |
VA: visual acuity; OS: left eye; OD: right eye; HM: hand motion; CF: counting finger vision; NPL: no perception light; PL: perception light; VAN: vancomycin; CEF: ceftazidime; AMK: amikacin.
Discussion
EE is an uncommon complication of PLA. However, meta-analysis research showed that EE was commonly associated with KPLA with an estimated prevalence of 4.5% (95% CI 2.4%–8.2%). 1 The authors suggested that the appearance of a highly virulent Klebsiella strain with mucosal affinity was the cause of this complication.6,7 In our study, there were only nine KPLA patients (Table 1). K. pneumoniae is found in the intestinal microbiota of healthy individuals and can travel to the liver via the portal vein or intestinal epithelial cells. Recent studies have shown an increased occurrence of K. pneumoniae in stool samples from people of Asian ancestry compared with other races. 2 Hypervirulent K. pneumoniae occurs in more than 80% of PLA cases in South Asia and is strongly associated with risk factors such as right liver abscess, size > 5 cm, and diabetes mellitus. 2 These risk factors were also observed to account for a high proportion in our case series. In addition, high blood glucose levels inhibit the functions of neutrophils, including adhesion, chemotaxis, phagocytosis, and bactericidal ability, thereby reducing the ability to kill bacteria, increasing the severity of the infection and the risk of vascular complications also aggravating the infection.1,2
Most of the patients had ocular symptoms: eye pain, blurred vision, swelling as well as redness, and pus discharge; two patients had symptoms on average 3 days after admission (Table 2). All patients were treated in other hospitals with carbapenem antibiotics, third-generation cephalosporins, and aminoglycosides. However, their condition did not improve, so they were admitted to our hospital. The most likely explanation for late diagnosis is patients presenting to a hospital late when the infection had spread, the ocular symptoms were apparent, and there is decreased or loss of VA. Timing of ocular complications is also essential. Our case series diagnosed all cases late, at least 3 days after the first visual presentation. Correia et al. 8 showed that early treatment within 2 days from the onset of eye symptoms such as red eyes and eye pain would help improve visual outcomes for patients.
The goal of the treatment of EE is to try to preserve the VA. Regarding the treatment method, all patients received carbapenem antibiotics, third-generation cephalosporins combined with aminoglycosides, and percutaneous catheter drainage (Table 3). Although the infection and liver abscess improved, the ocular damage was virtually irreversible. According to Correia et al., 8 eye damage caused by K. pneumoniae is reversible. In our study, only one patient maintained his ocular acuity after treatment, and even two underwent evisceration surgery. One of two these patients are 72-year-old male patient, the test results showed that the K. pneumoniae was only sensitive to carbapenem and resistant to the remaining antibiotics, despite being treated with intraocular injection with ceftazidime three times but the results of the left VA move from perception light to no perception light. The other case is a 75-year-old male patient with no previous medical conditions, treated in a hospital 4 days before; during the examination, the right eye was swollen and blurred and treated with antibiotics but no improvement. Some authors recommend that patients with PLA with blurred vision, pain, and red eyes should be examined by an ophthalmologist immediately for treatment with intraocular antibiotic injections in the early stages, which will improve eyesight.4,9,10 Patients with PLA should also undergo an abdominal ultrasound to detect liver abscesses. In addition, the antibiotic indication depends on culture results from blood, liver abscesses, and vitreous.
Our study has many limitations as it is a retrospective analysis of medical records with a small sample size. However, patients with PLA who are elderly, diabetic, or have K. pneumoniae infections are at higher risk for developing EE. Therefore, it is necessary to combine percutaneous catheter drainage and evaluation of the liver abscess and assessment, early detection of the ocular damage, and combined treatment with an intravitreal antibiotic injection to reduce mortality and morbidity and preserve VA. In addition, for patients with a liver abscess caused by K. pneumoniae, physicians should pay attention to the patient’s vision and eye-related symptoms to detect endophthalmitis early.
Conclusion
Endogenous endophthalmitis could be an uncommon complication of PLA, which has permanent morbidity, reducing visual acuity, poor quality of life, and lack the warning signs. Therefore, it is essential for early detection of the symptoms and referral to ophthalmologists.
Acknowledgments
The authors wish to thank the Gastroenterology and Hepatology Center and the Department of Ophthalmology, Bach Mai Hospital, for their assistance during the time in-hospital observation of these patients.
Footnotes
Author’s contribution: Long Cong Nguyen, Thuy Thi-Ngoc Pham, Tinh Nghe Nguyen, and Hung Ngoc Pham designed the study and collected and analyzed the data. Hieu Van Nguyen helped with data collection and analysis. Diep Thi-Minh Luu, Ngoc Minh Nguyen, Son Truong Nguyen, and Ha Thi-Ngoc Doan contributed to the study design and helped interpret the results. All authors contributed to writing the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval for this study was waived by the Institutional Review Board of Bach Mai Hospital with the number BM-2022-192.
Informed consent: Written informed consent was obtained from all subjects before the study.
Trial registration: Not applicable.
ORCID iDs: Long Cong Nguyen  https://orcid.org/0000-0002-5275-9014
https://orcid.org/0000-0002-5275-9014
Thuy Thi-Ngoc Pham  https://orcid.org/0009-0000-5278-8782
https://orcid.org/0009-0000-5278-8782
Diep Thi-Minh Luu  https://orcid.org/0009-0002-7203-6419
https://orcid.org/0009-0002-7203-6419
Tinh Nghe Nguyen  https://orcid.org/0009-0009-9119-4798
https://orcid.org/0009-0009-9119-4798
Ngoc Minh Nguyen  https://orcid.org/0009-0002-9820-5540
https://orcid.org/0009-0002-9820-5540
Hung Ngoc Pham  https://orcid.org/0009-0009-0781-1299
https://orcid.org/0009-0009-0781-1299
Ha Thi-Ngoc Doan  https://orcid.org/0000-0002-8184-7750
https://orcid.org/0000-0002-8184-7750
Son Truong Nguyen  https://orcid.org/0009-0006-9929-9090
https://orcid.org/0009-0006-9929-9090
Hieu Van Nguyen  https://orcid.org/0000-0002-0948-3630
https://orcid.org/0000-0002-0948-3630
References
- 1. Hussain I, Ishrat S, Ho DCW, et al. Endogenous endophthalmitis in Klebsiella pneumoniae pyogenic liver abscess: systematic review and meta-analysis. Int J Infect Dis 2020; 101: 259–268. [DOI] [PubMed] [Google Scholar]
- 2. Jun JB. Klebsiella pneumoniae liver abscess. Infect Chemother 2018; 50: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen Y-H, Li Y-H, Lin Y-J, et al. Prognostic factors and visual outcomes of pyogenic liver abscess-related endogenous Klebsiella pneumoniae endophthalmitis: a 20-year retrospective review. Sci Rep 2019; 9: 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung CY, Wong ES, Liu CCH, et al. Clinical features and prognostic factors of Klebsiella endophthalmitis—10-year experience in an endemic region. Eye 2017; 31: 1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daiber HF, Gnugnoli DM. Visual acuity. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC., 2023. Salah Aboubakr, Amal Abu-Ghosh. [Google Scholar]
- 6. Baekby M, Hegedüs N, Sandahl TD, et al. Hypervirulent Klebsiella pneumoniae K1 liver abscess and endogenous endophthalmitis in a Caucasian man. Clin Case Rep 2018; 6: 1618–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doshi S, Forbes JD, Mubareka S, et al. Disseminated hypervirulent Klebsiella pneumoniae causing endophthalmitis, and lung and liver abscesses. CMAJ 2022; 194: E645–E648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Correia C, Lopes S, Mendes S, et al. Endogenous endophthalmitis and liver abscess: a metastatic infection or a coincidence? GE Port J Gastroenterol 2022; 29: 426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danielescu C, Anton N, Stanca HT, et al. Endogenous endophthalmitis: a review of case series published between 2011 and 2020. J Ophthalmol 2020; 2020: 8869590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Losie JA, Lam JC, Gregson DB, et al. Epidemiology and risk factors for pyogenic liver abscess in the Calgary Health Zone revisited: a population-based study. BMC Infect Dis 2021; 21: 939. [DOI] [PMC free article] [PubMed] [Google Scholar]