Abstract
Yersinia pestis expresses a set of secreted proteins called Yops and the bifunctional LcrV, which has both regulatory and antihost functions. Yops and LcrV expression and the activity of the type III mechanism for their secretion are coordinately regulated by environmental signals such as Ca2+ concentration and eukaryotic cell contact. In vitro, Yops and LcrV are secreted into the culture medium in the absence of Ca2+ as part of the low-Ca2+ response (LCR). The LCR is induced in a tissue culture model by contact with eukaryotic cells that results in Yop translocation into cells and subsequent cytotoxicity. The secretion mechanism is believed to indirectly regulate expression of lcrV and yop operons by controlling the intracellular concentration of a secreted negative regulator. LcrG, a secretion-regulatory protein, is thought to block secretion of Yops and LcrV, possibly at the inner face of the inner membrane. A recent model proposes that when the LCR is induced, the increased expression of LcrV yields an excess of LcrV relative to LcrG, and this is sufficient for LcrV to bind LcrG and unblock secretion. To test this LcrG titration model, LcrG and LcrV were expressed alone or together in a newly constructed lcrG deletion strain, a ΔlcrG2 mutant, of Y. pestis that produces low levels of LcrV and constitutively expresses and secretes Yops. Overexpression of LcrG in this mutant background was able to block secretion and depress expression of Yops in the presence of Ca2+ and to dramatically decrease Yop expression and secretion in growth medium lacking Ca2+. Overexpression of both LcrG and LcrV in the ΔlcrG2 strain restored wild-type levels of Yop expression and Ca2+ control of Yop secretion. Surprisingly, when HeLa cells were infected with the ΔlcrG2 strain, no cytotoxicity was apparent and translocation of Yops was abolished. This correlated with an altered distribution of YopB as measured by accessibility to trypsin. These effects were not due to the absence of LcrG, because they were alleviated by restoration of LcrV expression and secretion alone. LcrV itself was found to enter HeLa cells in a nonpolarized manner. These studies supported the LcrG titration model of LcrV’s regulatory effect at the level of Yop secretion and revealed a further role of LcrV in the deployment of YopB, which in turn is essential for the vectorial translocation of Yops into eukaryotic cells.
Yersinia pestis, the causative agent of plague, and the enteropathogenic yersiniae Y. pseudotuberculosis and Y. enterocolitica have homologous low-Ca2+ response (LCR) virulence plasmids that encode a set of secreted virulence proteins and the type III Ysc mechanism for secretion and partitioning of these proteins to their sites of action (25). The secreted proteins include ∼11 Yops (Yersinia outer proteins; one of these is called YpkA) and the V antigen, LcrV. The expression of the Yops and the Ysc components is subject to thermal induction mediated by the activator LcrF. At 37°C, additional regulation determines the extent to which induction of Yop and LcrV expression will occur and whether the Ysc mechanism will be activated for Yop and LcrV secretion. In vitro, millimolar concentrations of Ca2+ maintain a partially induced level of expression and essentially no secretion. In the absence of Ca2+, maximal expression and secretion occur; this is the response for which this regulatory system is designated LCR. Y. pestis, and to lesser degrees the enteropathogenic yersiniae, show a growth response that correlates with the extent of yop expression in vitro. Maximal induction by incubation at 37°C in the absence of Ca2+ is accompanied by an orderly cessation of growth called restriction (25). If Ca2+ is present, the yersiniae grow normally (without restriction). This growth component of the LCR likely is an in vitro phenomenon (10) and is not known to occur in vivo, but it is a useful marker for the degree of LCR induction in in vitro studies. The absence of Ca2+ appears to mimic an unidentified signal that yersiniae receive when they are adherent to a eukaryotic cell, except that the resulting secretion is localized to the site of contact between the bacterium and the cell (27). In addition to induction of yop expression and secretion of Yops to the bacterial surface, at least four Yops (YopE, YopH, YopM, and YpkA) are vectorially targeted into the eukaryotic cell at the contact site. Three Yops, YopB, YopD, and YopK, have been shown to function in this targeting process. The membrane-interactive YopB may create a pore through which Yops are conducted, and YopK appears to regulate the size of the pore (6, 12, 15). Inside the eukaryotic cell, the Yops derange cellular signaling and cytoskeletal functions necessary for host defense responses such as phagocytosis (6, 7). A visual marker for Yop targeting is the rounding up of the eukaryotic cell due to YopE-elicited depolymerization of F-actin (cytotoxicity [6]).
A widely accepted model hypothesizes that the control of yop transcription is linked to the ability to secrete and target Yops by means of a secreted negative regulator (6, 42). A candidate for this regulator is the secreted protein LcrQ (also called YscM) (6, 27, 30). LcrQ’s mode of action is not established, and there likely are additional components to the negative regulatory pathway. One of these is YopD, as LcrQ requires the presence of YopD to have its negative regulatory effect (42). YopD also is necessary for Yops targeting into eukaryotic cells, but its mechanism of action is not known (14, 31, 38). YopD’s involvement in negative regulation suggests that downregulation in the LCR is inversely linked not only to the ability to secrete but also to the ability to carry out the ultimate function of the system, targeting of Yops (42).
The activity of the Ysc mechanism is regulated by LcrE (also called YopN), which is believed to act at the bacterial surface as a Ca2+ sensor (5, 9), and LcrG, which has been proposed to act at the cytoplasmic face of the inner membrane (24). LcrE and LcrG are necessary for secretion to be blocked at 37°C under noninductive conditions (presence of Ca2+ and absence of cell contact). Y. pestis mutants defective for either LcrE or LcrG maximally express and secrete Yops and enter growth restriction regardless of the presence of Ca2+ (a phenotype termed Ca2+ blind) (9, 25, 28, 36).
LcrG function appears to be modulated by LcrV. LcrV is a secreted antihost component with direct immunomodulatory effects (19–21). LcrV also has a positive regulatory role in the LCR (2, 29) by acting within the bacterial cell to counteract negative regulation (37). This effect of LcrV was recently hypothesized to occur at the level of secretion of the LCR-negative regulator, where LcrV would promote secretion by binding LcrG (24). LcrV forms a stable complex with LcrG within the bacterial cytosol, and when maximal LCR induction occurs there is an excess of LcrV compared to LcrG (24). Formation of an LcrG-LcrV complex might titrate LcrG away from the Ysc and unblock secretion, thereby permitting secretion of LcrQ and the consequent upregulation of yop expression (24).
This LcrG titration model predicts that a determining factor for achieving full upregulation of Yop secretion is the ratio of LcrV to LcrG. In the presence of Ca2+ (and absence of cell contact), there is only a low concentration of LcrV, which would be insufficient to tie up a significant amount of LcrG, and LcrG can function to block secretion. Upon destabilization of the LcrE-imposed secretion block by the absence of Ca2+ in vitro or by cell contact, some secretion of LcrQ occurs and more LcrV begins to be made. As the intracellular LcrV concentration builds, it could titrate LcrG away from the Ysc and stabilize the full activation of the Ysc mechanism.
In the study described here, this LcrG titration model for LcrV’s regulatory mechanism was supported by findings from in vitro experiments where different relative amounts of LcrV and LcrG were expressed. Surprisingly, the extension of these tests to infected eukaryotic cells revealed another dimension to LcrV’s function: LcrV is necessary for the deployment of YopB and hence for Yops targeting. This places LcrV in the role of mediating an extended inductive arm of the LCR that links translocation through secretion to induction of yop expression.
MATERIALS AND METHODS
Bacterial strains, eukaryotic cell lines, and growth conditions.
Y. pestis and Escherichia coli strains used are listed in Table 1. For genetic manipulations (e.g., transformation and isolation of plasmid DNA), Y. pestis strains were grown in heart infusion broth (HIB) or on tryptose blood agar base medium (TBA; Difco Laboratories, Detroit, Mich.) at 26°C, and E. coli strains were grown in LB broth or agar (18) as appropriate at 37°C. Streptomycin (100 μg/ml), ampicillin (100 μg/ml), kanamycin (50 μg/ml), and tetracycline (15 μg/ml) (all from Sigma Chemical, St. Louis, Mo.) were used to supplement the various media as required. TBA-TSS was used for counterselection during allelic exchange and was prepared by modifying TSS agar (3, 17) as follows: 400 ml of TBA was supplemented with 25 mg of chlortetracycline (Sigma) and autoclaved; 5 g of NaH2PO4 · H2O in 100 ml of H2O was autoclaved separately and added, along with 6 mg of fusaric acid (Sigma) dissolved in 1 ml of dimethyl formamide and 2.5 ml of sterile 20 mM ZnCl2, to the TBA after cooling to 45°C. Growth of Y. pestis for physiological studies was conducted in a defined medium, TMH, as previously described (39). Briefly, Y. pestis cultures were grown in exponential phase at 26°C with shaking at 200 rpm for about eight generations. Final cultures for harvesting were initiated at 26°C at an A620 of ∼0.1. When the A620 reached ∼0.2, the temperature was shifted to 37°C, and incubation was continued for 4 or 6 h before harvesting of cells. The epithelium-derived HeLa cell line was maintained in RPMI 1640 (Gibco-BRL, Gaithersburg, Md.) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; Gibco-BRL) (RPMI-FBS) at 37°C with a 5% CO2 atmosphere. For partitioning experiments that measured the postsecretion distribution of Yops in the culture medium and into HeLa cells, RPMI-FBS was replaced with Leibovitz’s L15 medium (L15; Gibco-BRL) lacking FBS.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant properties | Source or reference |
|---|---|---|
| Bacterial strains | ||
| Y. pestis | ||
| KIM8 | pCD1 (Lcr+) pMT1 Pla− | Laboratory stock |
| KIM8-3002 | KIM8 Smr | This study |
| KIM5-3001.5 | Smr pCD1 ΔlcrG (aa 39–53) pPCP1 pMT1 | 36 |
| KIM8-3002.6 | KIM8-3002 ΔlcrG2 (aa 5–95) | This study |
| KIM5-3131 | pCD1 yopK::MudI1734 (YopK− YopL− Kmr Lac+) pPCP1 (Pla+) pMT1 | 40 |
| KIM5-3131.4 | KIM5-3131 ΔlcrG2 (aa 5–95) | This study |
| E. coli | ||
| DH5α | φ80dlacZ ΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− rK+) phoA supE44 thi-1 gyrA96 relA1 | Gibco-BRL |
| DH5α (λpir) | DH5α λpir | Laboratory stock |
| Plasmids | ||
| pLD55 | oriRR6Kγ, lacZα, Apr, Tcr | 17 |
| pMNΔlcrG2 | pLD55 carrying ΔlcrG2 for allelic exchange; insert contains lcrD′ lcrR ΔlcrG2 lcrV lcrH′ | This study |
| pBAD18 | araBADp cloning vector, Apr | 11 |
| pBAD18-Kan | araBADp cloning vector, Kmr | 11 |
| pAraG18 | pBAD18 + lcrG | This study |
| pAraV18 | pBAD18 + lcrV | This study |
| pAraGV18 | pBAD18 + lcrGV | This study |
| pAraG18K | pBAD18-Kan + lcrG | 24 |
| pAraV18K | pBAD18-Kan + lcrV | 24 |
| pAraGV18K | pBAD18-Kan + lcrGV | 24 |
| pTRCM.2 | pTrc99a + yopM | 28 |
DNA methods and plasmid constructions.
Plasmid DNA was isolated by using a QiaPrep Spin kit (Qiagen Inc., Studio City, Calif.). Cloning methods were essentially as described previously (32). DNA fragments were isolated from agarose gels, and PCR fragments were purified by using the appropriate QiaQuick DNA purification kit (Qiagen). Electroporation of DNA into Y. pestis was done as described previously (26). Transformation of DNA into E. coli was done by using either the calcium-manganese-based transformation protocol or the frozen storage-based transformation protocol as described previously (13). Plasmids used in this study are described in Table 1.
Plasmids pAraG18, pAraV18, and pAraGV18 were constructed by cloning EcoRI-cleaved PCR products into EcoRI- and SmaI-cleaved pBAD18 (11). Primers used were AraG-Start (5′ GGA ATT CAG GAG GAA ACG ATG AAG TCT TCC CAT TTT GAT 3′) and AraG-Stop (5′ CGC GGA TCC TTA AAT AAT TTG CCC TCG 3′) to make pAraG18, AraV-Start (5′ GGA ATT CAG GAG GAA ACG ATG ATT AGA GCC TAC GAA 3′) and AraV-Stop (5′ CGC GGA TCC TTA TCA TTT ACC AGA CGT GTC 3′) to make pAraV18, and AraG-Start and AraV-Stop to make pAraGV18. The DNA was amplified using Vent DNA polymerase (New England Biolabs, Beverly, Mass.) with 30 cycles of 94°C for 15 s, 55°C for 15 s, and 72°C for 1 min, carried out in a GeneAmp PCR System 2400 thermocycler (Perkin-Elmer, Foster City, Calif.). pMNΔlcrG2 was constructed by ligating a BamHI-digested PCR product [containing an engineered deletion in lcrG2 that removed amino acids 5 through 95 of LcrG, designated ΔlcrG2 (aa 5–95)] into BamHI- and SmaI-digested pLD55. The insert in pMNΔlcrG2 begins in lcrD (1,060 bp upstream of the start of lcrG) and progresses through lcrR, incorporating the lcrG deletion, which removes all but the first four codons and the stop codon for lcrG, passing through lcrV, and ending just past the start for lcrH (1,305 bp downstream of the start of lcrG). The insert was constructed by using PCR as follows. Primers ΔlcrG-US (5′ CGC GGA TCC GCT ATC TGC TCG AAC AGA 3′) and ΔlcrG-BeginII, flanking the deletion in lcrG (5′ CGT AGG CTC TAA TCA TAT TAG GAA GAC TTC ATA ATC TAC C 3′), were used to amplify the region upstream; primers ΔlcrG-END, complementary to ΔlcrG-BeginII (5′ GGT AGA TTA TGA AGT CTT CCT AAT ATG ATT AGA GCC TAC G 3′), and ΔlcrG-DSII (5′ GAT ATC AGT GTC TGT CGT CTC TTG 3′) were used to amplify the region downstream of lcrG, using the conditions described above for construction of the pAra plasmids. The upstream fragment and the downstream fragment were combined, and primers ΔlcrG-US and ΔlcrG-DSII were used to amplify the final deletion construct, using Vent DNA polymerase with a cycling profile consisting of five cycles of 94°C for 15 s, 45°C for 15 s, and 72°C for 2 min, followed by 30 cycles of 94°C for 15 s, 55°C for 15 s, and 72°C for 2 min.
Strain constructions.
Y. pestis KIM8-3002 was isolated as a spontaneous streptomycin-resistant (Smr) mutant of KIM8. Ten milliliters of an overnight culture of Y. pestis KIM8 was concentrated and plated onto TBA-streptomycin and incubated at 26°C until Smr colonies appeared (∼5 days). Smr colonies were streak purified and verified for an appropriate LCR growth phenotype at 37°C in TMH and confirmed as putative rpsL mutants by complementation to streptomycin sensitivity with E. coli rpsL. Y. pestis KIM8-3002.6 (ΔlcrG2) and KIM5-3131.4 (YopK− YopL− ΔlcrG2) were constructed by allelic exchange of the ΔlcrG2 allele, carried on the suicide plasmid pMNΔlcrG2, for the wild-type copy of lcrG by using a modification of a method described by Metcalf et al. (17). pMNΔlcrG2 was electroporated into Y. pestis KIM8-3002 and KIM5-3131, and ampicillin-resistant (Apr) colonies were selected on TBA-ampicillin. Apr colonies were then streak purified on TBA-ampicillin-tetracycline to isolate bacteria with a single crossover event that had integrated pMNΔlcrG2 into the LCR plasmid, pCD1. Four Apr Tc-resistant (Tcr) colonies were then streaked onto nonselective medium (TBA) to allow accumulation of segregants within colonies. Four colonies from each of those four plates (16 colonies in total) were streaked onto TBA-TSS agar to counterselect against Tcr bacteria. After 5 to 7 days of growth on TBA-TSS, putative Tc-sensitive (Tcs) colonies were streaked onto nonselective medium and onto TBA-ampicillin and TBA-tetracycline to confirm loss of the plasmid markers. Aps Tcs colonies were screened for replacement of lcrG with ΔlcrG2 by using PCR analysis with primers ΔlcrG2-US and ΔlcrG2-DSII. The phenotype of the lcrG deletion strains was confirmed by growth in TMH at 37°C as described above.
Cell fractionation.
Bacterial cells were chilled to 4°C after growth, harvested by centrifugation (5 min at 20,800 × g) at 4°C, and washed once in cold phosphate-buffered saline (PBS; 135 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4 [pH 7.4]). Bacterial whole-cell extracts were prepared by resuspending washed bacterial cells in ice-cold PBS and precipitating total proteins overnight on ice with 10% (vol/vol) trichloroacetic acid (TCA). Secreted proteins were recovered from the bacterial growth medium following harvest of the bacteria by centrifuging (20,800 × g for 5 min at 4°C) the spent medium a second time and transferring the supernatant to a clean tube. Total secreted protein was collected from the medium by precipitation overnight on ice with 10% (vol/vol) TCA. The TCA-precipitated proteins were pelleted by centrifugation (20,800 × g at 4°C) for 30 min in a microcentrifuge and resuspended in 2× sodium dodecyl sulfate (SDS) sample buffer (125 mM Tris [pH 6.8], 20% [vol/vol] glycerol, 4% [wt/vol] SDS, 200 mM dithiothreitol) (1).
Contact hemolysis assay.
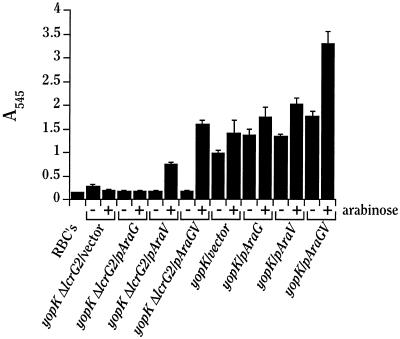
The ability of Y. pestis to lyse erythrocytes (RBCs) was determined as described previously (12, 33). Y. pestis strains to be tested were grown in TMH as described above except that the overnight cultures were diluted to an A620 of ∼0.3 and shifted to 37°C after 1 h. After cultures had been growing at 37°C for 2 to 3 h, bacteria were harvested and resuspended in 37°C PBS to a density of 50 A620 · ml (which corresponds to ∼2.5 × 1010 bacteria/ml). While the bacteria were growing, sheep RBCs were prepared for the assay. Chilled (4°C) sheep blood in Alsever’s solution (Colorado Serum Co., Denver, Colo.) was centrifuged at 1,000 × g at room temperature (RT) for 10 min to pellet the RBCs. The RBCs were then washed twice in cold (4°C) PBS and resuspended in cold PBS to ∼4 × 109 cells/ml. Contact hemolysis assays were performed in quadruplicate in 96-well microtiter dishes by combining 50 μl of RBCs and 50 μl of bacterial suspension and centrifuging at 1,000 × g at RT for 10 min to ensure contact between bacteria and RBCs. After centrifugation, the plates were incubated at 37°C for 3.5 h. Following incubation, 150 μl of cold PBS was added to the liquid in the wells to resuspend the pelleted cells. Next, the RBCs and bacteria were centrifuged at 1,000 × g for 10 min. Finally, 100 μl of supernatant from each assay was transferred to a clean microtiter dish, and the A570 was read by using a Molecular Devices (Sunnyvale, Calif.) υmax microplate reader to determine variation among the quadruplicate samples. Following the measurement at 570 nm, the quadruplicates were pooled and the A545 (peak absorbance for hemoglobin) was measured with a Spectronic Genesys 5 spectrophotometer (Spectronic Instruments, Rochester, N.Y.). Values reported in Fig. 5 represent the average A545 readings from the four pooled assays; error bars represent the percent standard errors derived from the A570 measurements.
FIG. 5.
LcrV is required for contact hemolysis. Y. pestis KIM5-3131 (yopK) and KIM5-3131.4 (yopK ΔlcrG2), both containing pBAD18 (vector), pAraG18, pAraV18, and pAraGV18 were mixed with sheep RBCs in PBS, centrifuged to ensure contact, and incubated at 37°C for 3.5 h. Following incubation, the contents of the wells were diluted 2.5-fold with PBS, the cells were then pelleted onto the wells, and the A545 was measured for the cell-free supernatant in a spectrophotometer to evaluate the extent of RBC lysis.
Infection assays.
Prior to infection, eukaryotic cells were subcultured into 35-mm-diameter six-well tissue culture plates in RPMI-FBS and incubated at 37°C in a 5% CO2 atmosphere for roughly 72 h or to a density of 5 × 105 to 8 × 105 cells per well. Cells were washed twice with warm L15 lacking FBS immediately prior to infection. Bacteria were cultivated at 26°C in HIB and harvested at an A620 of ∼1.0. Arabinose was added to 0.2% (wt/vol) 30 to 60 min prior to harvest for strains harboring constructs with inducible promoters. Bacteria (at a multiplicity of infection of 10) were added directly to prewarmed medium (containing arabinose if appropriate) already in the wells of the six-well plates. Plates were then centrifuged at 200 × g at RT for 5 min to achieve contact between bacteria and target cells and incubated at 37°C with humidification for 4 h. After infection, one replicate well per infecting strain was treated for 5 min at 37°C with 100 μg of trypsin per ml. Protease inhibitors (Pefabloc, leupeptin, and aprotinin; Boehringer Mannheim Biochemicals, Indianapolis, Ind.) were added to 20 μg/ml each to stop the trypsin treatment, and the cultures were harvested and fractionated as follows. The tissue culture medium was removed from wells, passed through 0.2-μm-pore-size filters to remove any yersiniae, and subsequently treated overnight on ice with TCA at 10% (vol/vol) to precipitate secreted proteins. The proteins were recovered by centrifugation at 4°C at 20,800 × g for 30 min. Infected cells were washed twice with RT PBS and lysed by treatment with ice-cold H2O containing protease inhibitors (Pefabloc, leupeptin, and aprotinin) at 2 μg/ml each. The lysed cell samples were then centrifuged at 4°C at 20,800 × g for 15 min. The supernatant, corresponding to the eukaryotic cell soluble fraction, was removed, and proteins were precipitated overnight on ice with 10% (vol/vol) TCA. The TCA-precipitated proteins from the culture medium, the HeLa cell soluble fraction, and the pelleted debris of the lysed HeLa cells plus adherent yersiniae were solubilized in 2× SDS sample buffer.
Protein electrophoresis and immunodetection.
Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE), using 10, 12, or 13.5% (wt/vol) polyacrylamide gels as indicated, according to the method of Laemmli (16). Samples were boiled 3 to 5 min prior to being loaded on the gels. Lanes in which subcellular fractions (whole-cell, soluble, or secreted proteins) are compared were loaded so as to contain amounts of the fractions derived from the same volume of original culture. Proteins separated by SDS-PAGE were transferred to Immobilon-P membranes (Millipore Corp., Bedford, Mass.), using carbonate transfer buffer (pH 9.9) (36). Specific proteins (LcrG, LcrQ, LcrV, YopB, YopD, YopE, and YopM) were visualized on the membranes by using the following rabbit polyclonal antibodies (indicated by the prefix “α”) specific for the proteins at the indicated dilutions: His-tagged LcrV (1:20,000; α-HTV [24]), YopE (1:40,000; α-YopE; gift of G. Plano, University of Miami), glutathione S-transferase (GST)-tagged LcrG (1:40,000; α-GST-G [24]), His-tagged YopD (1:40,000; α-HT-YopD [42]), YopB (1:3,000; α-YopB; gift of Å. Forsberg, National Defence Research Establishment, Umeå, Sweden), YopM (1:20,000; α-YopM [22]), and GST-tagged LcrQ (1:10,000; α-GST-LcrQ [42]). Detection was by alkaline phosphatase conjugated to secondary antibodies (goat anti-rabbit immunoglobulin G, whole molecule; Sigma), assayed by nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP; Gibco/BRL) immunostaining.
RESULTS
LcrG functions as a secretion block in a ΔlcrG2 background.
The initial goal of this study was to test our LcrG titration model for LcrV’s function by characterizing the Yop expression and secretion of Y. pestis strains expressing different ratios of LcrV and LcrG. The ΔlcrG2 allele deleting all but the first four codons and the stop codon of lcrG was designed to provide a strain background lacking the entire LcrG protein. An important consequence of this deletion was that the ribosome-binding site (RBS) for the downstream lcrV gene was deleted. The deletion of lcrV’s RBS was predicted to greatly decrease the amount of LcrV expressed in strains not having it supplied in trans.
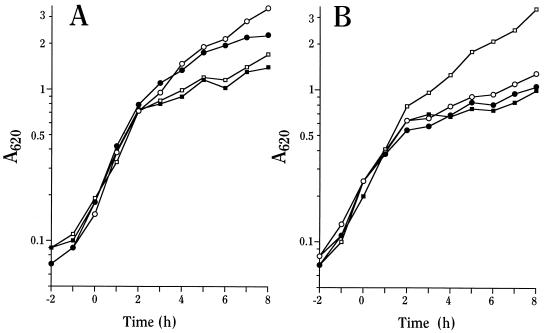
pBAD18-Kan (as a negative control) and plasmids expressing LcrG, LcrV, or LcrG and LcrV (LcrG-LcrV) (all under control of the arabinose [Ara]-inducible araBAD promoter) were introduced into Y. pestis KIM8-3002.6 (ΔlcrG2), and the growth phenotypes were determined in a defined medium, TMH, in the presence and the absence of Ca2+. Sufficient Ara for full induction was added upon subculture to an A620 of 0.1 at 26°C. The ΔlcrG2 mutant containing vector alone entered growth restriction whether Ca2+ was present or not (Fig. 1A; Ca2+-blind phenotype), a phenotype seen for a previously characterized mutant having a partial deletion of lcrG (36). Induction of LcrG expression resulted in an unusual, essentially Ca2+-independent, intermediate growth phenotype suggestive of a constitutively downregulated state of the LCR: the mutant containing pAraG18K grew in the presence of Ca2+ as expected for complementation with LcrG but also showed increased growth in the absence of Ca2+ (Fig. 1A). Growth restriction occurred at an A620 of ∼2 rather than ∼1 for the noncomplemented strain with vector alone and the parent Y. pestis KIM8-3002 (data not shown). Induction of LcrV alone had no effect on the growth of Y. pestis KIM8-3002.6 (ΔlcrG2) (Fig. 1B), as expected if LcrV acts to modify the function of LcrG (24), which is missing in this strain. Overexpression of LcrG-LcrV resulted in restoration of growth characteristic of wild-type Y. pestis (Fig. 1B and data not shown). These results show that LcrG can function to suppress growth restriction in the absence of Ca2+ and that this effect can be relieved by increased LcrV expression.
FIG. 1.
Overexpression of LcrG in Y. pestis ΔlcrG2 produces a novel growth phenotype. Y. pestis KIM8-3002.6 (ΔlcrG2) containing plasmids pBAD18-Kan (vector control; A, squares), pAraG18K (LcrG; A, circles), pAraV18K (LcrV; B, circles), and pAraGV18K (LcrG-LcrV; B, squares) was grown in the defined medium TMH at 37°C with (open symbols) or without (closed symbols) Ca2+. Ara (0.2% [wt/vol]) was added to the cultures prior to the temperature shift, and the A620 of the cultures was monitored hourly.
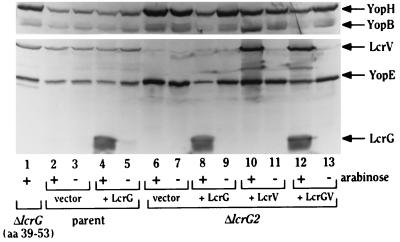
Restriction of growth by yersiniae normally reflects LCR induction and is accompanied by increased expression and secretion of Yops into the culture medium. To determine the effect of overexpressing LcrG, LcrV, or LcrG-LcrV on Yop abundance in the bacteria and on secretion, bacteria harvested from a growth experiment at 4 h (Fig. 2A) or 6 h (data not shown) after temperature shift were examined for YopB, YopD, YopE, YopH, YopM, LcrG, LcrQ, and LcrV in whole-cell fractions and cell-free culture medium (Fig. 2A). As expected from its Ca2+-blind (LCR-induced) growth phenotype, Y. pestis KIM8-3002.6 (ΔlcrG2) was derepressed for Yop expression whether Ca2+ was present or not compared to the wild type (Fig. 2A, Whole cells; compare lanes 1 and 2 with lanes 5 and 6), constitutively secreted Yops (Fig. 2A, Culture supernatants, lanes 5 and 6), and the negative regulator, LcrQ. Interestingly, the abundance of YopB and YopD was moderately diminished, and secretion of YopB and YopD was decreased compared to the wild-type level (Fig. 2A, Culture supernatants, lane 2, 5, and 6). This mutant made no LcrG and made reduced amounts of LcrV, due to the deletion of the lcrV RBS, compared to the wild type (Fig. 2A, Whole cells; compare lanes 1 and 2 with lanes 5 and 6). Overexpression of LcrG in Y. pestis KIM8-3002.6 (ΔlcrG2) decreased Yop expression in both the presence and absence of Ca2+, as expected from its essentially Ca2+-independent growth phenotype (Fig. 2A, Whole cells, lanes 7 and 8). LcrG overexpression blocked secretion of Yops in the presence of Ca2+ (Fig. 2A, Culture supernatants, lane 7). Secretion was not completely blocked by overexpressed LcrG in the absence of Ca2+ but was dramatically decreased (Fig. 2A, Culture supernatants, lane 8); this leaky secretion block was most likely due to the loss of LcrE function that is presumed to occur in the absence of Ca2+. The weak secretion was more evident at 6 h (data not shown) than at 4 h (Fig. 2A, Culture supernatants). As expected, overexpression of LcrV in the ΔlcrG2 mutant had no effect on expression or secretion of YopE, -H, and -M but did largely restore the wild-type abundance and secretion of YopB and -D (Fig. 2A, Whole cells, lanes 9 and 10, and Culture supernatants, lanes 9 and 10). Co-overexpression of LcrG-LcrV in Y. pestis KIM8-3002.6 (ΔlcrG2) resulted in restoration of wild-type control of Yop expression and secretion, consistent with the restoration of the wild-type growth phenotype: there was decreased expression and no secretion when Ca2+ was present and increased expression and secretion when Ca2+ was absent (Fig. 2A, Whole cells, lanes 11 and 12, and Culture supernatants, lanes 11 and 12). The only exception was some secretion of LcrV in the presence of Ca2+, which we find happens when an LCR secreted protein is strongly overexpressed (Fig. 2A, Culture supernatants, lane 11). Decreased secretion of Yops at 37°C in response to overexpressing LcrG in a background that makes little LcrV is evidence supporting the idea that LcrG’s primary function is to block secretion. Overexpression of LcrG in the parent Y. pestis background had no effect on expression or secretion of Yops (Fig. 2A); accordingly, to unmask the phenotype due to LcrG overexpression, it was important to have the lower LcrV expression of the ΔlcrG2 mutant. In the ΔlcrG2 background, the effect of LcrG was overcome by concomitant overexpression of LcrV, and this result supports the hypothesis that LcrV’s role in secretion is to counteract the LcrG secretion block. Overall, these data support our previously proposed model (24) for induction of Yop secretion in the LCR.
FIG. 2.
LcrG functions to block secretion in a ΔlcrG2 background, and LcrV counteracts LcrG’s secretion block. (A) Y. pestis KIM8-3002 containing plasmids pBAD18-Kan (vector; lanes 1 and 2) and pAraG18K (+ LcrG; lanes 3 and 4) and Y. pestis KIM8-3002.6 (ΔlcrG2) containing plasmids pBAD18-Kan (vector; lanes 5 and 6), pAraG18K (+ LcrG; lanes 7 and 8), pAraV18K (+ LcrV; lanes 9 and 10), and pAraGV18K (+ LcrGV; lanes 11 and 12) were grown in TMH at 37°C with (lanes 1, 3, 5, 7, 9, and 11) or without (lanes 2, 4, 6, 8, 10, and 12) Ca2+. Arabinose (0.2% [wt/vol]) was added to the cultures prior to the temperature shift to 37°C to induce expression of LcrG, LcrV, or LcrG-LcrV from the plasmids. (B) Y. pestis KIM8-3002 (parent; lanes 1 and 2), Y. pestis KIM8-3002 containing plasmids pBAD18-Kan and pTrcM.2 (vector; lanes 3 and 4) and pAraG18K and pTrcM.2 (+ LcrG; lanes 5 to 7), and Y. pestis KIM8-3002.6 (ΔlcrG2) containing plasmids pBAD18-Kan and pTrcM.2 (vector; lanes 8 and 9), pAraG18K and pTrcM.2 (+ LcrG; lanes 10 to 12), pAraV18K and pTrcM.2 (+ LcrV; lanes 13 and 14), and pAraGV18K and pTrcM.2 (+ LcrGV; lanes 15 and 16) were grown in TMH at 37°C with (lanes 1, 5, and 10) or without (lanes 2 to 4, 6 to 9, and 11 to 16) Ca2+. Arabinose (0.2% [wt/vol]; lanes 1, 2, 3, 5, 6, 8, 10, 11, 13, and 15) was added to the cultures prior to the temperature shift to 37°C to induce expression of LcrG, LcrV, or LcrG-LcrV from the plasmids. The trc promoter on pTrcM.2 is leaky in Y. pestis and provides sufficient expression of YopM for this experiment without induction by isopropylthiogalactopyranoside. Cultures from both experiments were harvested after 4 h of growth, and a sample of each was fractionated into whole-cell (Whole cells) and culture medium (Culture supernatants) fractions. Portions corresponding to 0.02 A620 · ml were separated by SDS-PAGE in a 12% polyacrylamide gel and analyzed by immunoblotting for the presence of YopM, YopE, LcrG, and LcrV with an antiserum cocktail of α-YopM, α-YopE, α-GST-G, and α-HTV. Samples corresponding to 0.04 A620 · ml were separated by SDS-PAGE in a 12% polyacrylamide gel and analyzed in an immunoblot by probing with an antibody cocktail of α-GST-LcrQ and α-YopB (which also recognizes YopH) for the presence of LcrQ, YopB, and YopH followed by probing for the presence of YopD with α-HT-YopD. All proteins were visualized by immunostaining with NBT-BCIP after treatment with alkaline phosphatase-conjugated secondary antibodies.
Nonetheless, these data did not rule out a direct effect of LcrG on Yop expression with consequently decreased amounts of Yops being secreted. To examine if LcrG was directly affecting secretion as our model proposes, we expressed YopM from the trc promoter, to provide an LCR-independent source of YopM, in Y. pestis KIM8-3002 (parent) containing the vector, pBAD18-Kan, or pAraG18K and in Y. pestis KIM8-3002.6 (ΔlcrG2) containing pBAD18-Kan, pAraG18K, pAraV18K, or pAraGV18K. The resulting strains were grown in TMH lacking Ca2+ in the presence or absence of Ara. Strains overexpressing LcrG were also grown in the presence of Ca2+ and Ara to assess blockage of secretion by LcrG in the Y. pestis ΔlcrG2 strain KIM8-3002.6. Four hours after the cultures were shifted to 37°C, samples of each culture were harvested and separated into whole-cell and medium fractions (Fig. 2B). Analysis of these fractions for YopM, LcrV, YopE, and LcrG revealed that even when YopM was supplied in trans by an LCR-independent promoter, LcrG could prevent its secretion in both the presence and absence of Ca2+ when LcrG was induced with Ara (Fig. 2B, Culture supernatants, lanes 10 and 11). As before (Fig. 2A), induction of LcrG expression by Ara caused decreased expression of YopE and YopM in comparison to when this was strain grown without Ara (Fig. 2B, Whole cells, lanes 10 and 11 compared to lane 12) and to when it lacked added LcrG (Fig. 2B, Whole cells, lanes 10 and 11 compared to lanes 8 and 9). (Some decrease in YopM levels is expected when LcrG is overexpressed because the yopM copy on pCD1 is still subject to LCR control). This experiment shows that LcrG has a direct effect at the level of Yop secretion control, and the data are consistent with the previously postulated role of LcrG functioning at the level of secretion near LcrE (24, 36, 37).
LcrV is required for Y. pestis-induced HeLa cell cytotoxicity.
We extended our characterization of LcrV and LcrG function to a tissue culture model of infection, where the ability of Y. pestis to induce cytotoxicity in HeLa cells served as a screen for the ability of LcrG overexpression to prevent Yop targeting. We wondered if this more natural situation with locally activated secretion might allow the effect of LcrG overexpression to be seen even better than when bacteria were grown in TMH at 37°C lacking Ca2+, which causes such strong induction of the LCR that overexpression of LcrG in Y. pestis KIM8-3002.6 (ΔlcrG2) was unable to completely abolish Yop secretion. To determine if the LcrG-imposed secretion block would be manifested as failure to vectorially target Yops, HeLa cells were infected with Y. pestis KIM8-3002 (wild type for the LCR) carrying pBAD18-Kan (cloning vector) or pAraG18K, and the same Y. pestis KIM8-3002.6 (ΔlcrG2)-derived strains used in the previous experiment in the presence or absence of Ara, as well as a previously described LcrG− strain of Y. pestis, KIM5-3001.5 [ΔlcrG (aa 39–53)] (36), which expresses and secretes high levels of LcrV. After 4 h of infection, cytotoxicity (i.e., rounding up of cells) was evaluated by microscopic examination of the infected cell cultures (Fig. 3). Following photography of the cell cultures, the entire cultures (cells, bacteria, and medium) were harvested and analyzed by immunoblot analysis to determine the LCR expression phenotypes of the strains in this setting and examine expression of YopB and YopD (required for Yop targeting), YopE (induces cell rounding), YopH (contributes to cell rounding), and the Ara-induced LcrG and LcrV (Fig. 4). The parent Y. pestis KIM8-3002 and a derivative of the parent overexpressing LcrG were both cytotoxic (Fig. 3 and data not shown), and there was no effect of LcrG overexpression on expression of YopE or YopH (Fig. 4, lanes 2 to 5). Also, the lcrG strain of Y. pestis KIM5-3001.5, which constitutively expresses and secretes LcrV, demonstrated cytoxicity for HeLa cells (Fig. 3). In contrast, the mutant Y. pestis KIM8-3002.6 (ΔlcrG2) did not induce cytoxicity (Fig. 3), although this strain was strongly induced for YopE and YopH expression (Fig. 4, lanes 6 and 7). Cytotoxicity was not restored by complementation with LcrG (Fig. 3), which decreased the expression of YopE and YopH in the tissue culture model (Fig. 4, lane 8) as it did in vitro. The only demonstrated difference between the lcrG strains of Y. pestis KIM5-3001.5 [ΔlcrG (aa 39–53)] and KIM8-3002.6 (ΔlcrG2) (other than the presence of the Pla-encoding pPCP1 plasmid, which has no effect on cytotoxicity [8]) was the failure of KIM8-3002.6 to secrete LcrV. Consequently, cytotoxicity was restored to the ΔlcrG2 mutant when LcrV was overexpressed (Fig. 3). As was the case in vitro, LcrV overexpression had no effect on the strong induction of YopE and YopH due to the lcrG2 mutation (Fig. 4, lane 10). Expression of LcrG-LcrV also resulted in cytotoxicity (Fig. 3) as well as restoration of wild-type levels of YopE and YopH expression, consistent with the in vitro findings of a restored wild-type LCR phenotype in this strain (Fig. 4, lane 12).
FIG. 3.
LcrV is required for Y. pestis-induced cytoxicity of HeLa cells. Y. pestis KIM8-3002 containing pBAD18-Kan, Y. pestis KIM5-3001.5 [ΔlcrG (aa 39–53)], KIM8-3002.6 (ΔlcrG2) containing pBAD18-Kan, and KIM8-3002.6 (ΔlcrG2) containing pAraG18K, pAraV18K, and pAraGV18K were used to infect HeLa cells at a multiplicity of infection of 10 in the presence (+ ara) or absence (− ara) of Ara (0.2% [wt/vol]) to induce expression of LcrG and/or LcrV from the plasmids. After 4 h of infection, the cultures were viewed by phase-contrast microscopy to evaluate cytotoxicity and photographed with a green filter.
FIG. 4.
Yop expression in infected HeLa cells mirrors expression seen in vitro. Y. pestis KIM5-3001.5 [ΔlcrG (aa 39–53); lane 1], Y. pestis KIM8-3002 (parent) with pBAD18-Kan (vector; lanes 2 and 3) and pAraG18K (+ LcrG; lanes 4 and 5), and KIM8-3002.6 (ΔlcrG2) with pBAD18-Kan (vector; lanes 6 and 7), pAraG18K (+ LcrG; lanes 8 and 9), pAraV18K (+ LcrV; lanes 10 and 11), and pAraGV18K, (+ LcrGV; lanes 12 and 13) were used to infect HeLa cells in the presence (lanes 1, 2, 4, 6, 8, 10, and 12) or absence (lanes 3, 5, 7, 9, 11, and 13) of Ara (0.2% [wt/vol]) to induce expression of LcrG and/or LcrV from the plasmids. After 4 h, the entire culture (medium, HeLa cells, and bacteria) was removed and precipitated with 10% (vol/vol) TCA. Protein samples representing 30% of the culture were separated by SDS-PAGE in a 10% polyacrylamide gel and analyzed by immunoblotting with α-YopB for the presence of YopB and YopH (YopH is detected by α-YopB). Protein samples representing 20% of the culture were separated by SDS-PAGE in a second polyacrylamide gel (13.5%) and analyzed by immunoblotting for the presence of YopE, LcrG, and LcrV with an antiserum cocktail of α-YopE, α-GST-G, and α-HTV. All proteins were visualized by immunostaining with NBT-BCIP after treatment with alkaline phosphatase-conjugated secondary antibodies.
Because LcrG and LcrV were both induced by Ara in the cell culture infections (Fig. 4, lanes 4, 8, 10, and 12), the failure of LcrG to complement the ΔlcrG2 cytotoxicity lesion was not due to the failure of Ara to induce in cell culture. YopB, -E, and -H were induced in the ΔlcrG2 mutant (Fig. 4, lanes 6 and 7) compared to the wild type (Fig. 4, lanes 2 to 5) and depressed in the presence of excess LcrG (Fig. 4, lane 8). These results support the idea that the lack of cytotoxicity by ΔlcrG2 Y. pestis was related to the low level of LcrV expression or the apparent lack of LcrV secretion in this strain.
The differences in cytotoxicity among the strains in the experiments of Fig. 3 and 4 were not related to changes in YopE or YopH expression, as the cytotoxic ΔlcrG2 Y. pestis carrying an LcrV expression plasmid (Fig. 4, lane 10) showed the same level of YopE and YopH expression as the noncytotoxic ΔlcrG2 mutant (Fig. 4, lanes 6 and 7). The presence of an increased LcrV level in ΔlcrG2 Y. pestis did correlate with an increase in overall amount of YopB in the culture; however, this was not necessary for the restoration of cytotoxicity, as other cytotoxic strains such as parent, ΔlcrG (aa 39–53), and ΔlcrG2 with an LcrG-LcrV expression plasmid did not have the same elevated levels of YopB. These data suggest that instead, LcrV’s main role in cytotoxicity could be related to an effect of LcrV on YopB function or secretion. This is consistent with the in vitro finding that restoration of LcrV expression and secretion to the ΔlcrG2 mutant restored secretion as well as increased the abundance of YopB and YopD (Fig. 2).
LcrV is required for contact hemolysis.
To help elucidate the mechanism behind LcrV-dependent cytotoxicity, the role of LcrV in contact-dependent hemolysis was examined. Multiple Yop− or YopK− mutants of Y. pseudotuberculosis have been shown to lyse RBCs in a YopB-dependent interaction (12, 15). To examine ΔlcrG2-containing Y. pestis strains for contact hemolysis, the ΔlcrG2 allele was moved into a YopK− strain of Y. pestis by allelic exchange. This strain, Y. pestis KIM5-3131.4 (yopK ΔlcrG2), was then electroporated with a set of plasmids carrying LcrG, LcrV, or LcrG-LcrV (all under Ara control) or with the cloning vector alone. The parent strain, Y. pestis KIM5-3131 (yopK), was able to lyse RBCs, showing that Y. pestis, like Y. pseudotuberculosis, could mediate contact hemolysis (Fig. 5). The ΔlcrG2 derivative of Y. pestis KIM5-3131, Y. pestis KIM5-3131.4, and the same strain overexpressing LcrG were unable to lyse RBCs. However, overexpression of LcrV in the yopK ΔlcrG2 background partially restored the ability to lyse RBCs. Overexpression of LcrG-LcrV was able to fully restore contact hemolysis. These results show that LcrV is required for contact hemolysis of RBCs, a function that has been shown to be YopB dependent (12, 15). The results of this hemolysis experiment combined with the decreased secretion of YopB seen in ΔlcrG2 Y. pestis and the positive effects of strong LcrV expression in this mutant on net abundance and secretion of YopB suggest that LcrV is necessary, directly or indirectly, for YopB secretion and/or function. Interestingly, expression of both LcrG and LcrV in trans in addition to that from the genes on pCD1 in yopK Y. pestis KIM5-3131 resulted in hemolysis stronger than that caused by the yopK strain with normal LcrG and LcrV levels (Fig. 5). This finding suggests that both LcrG and LcrV may have a role in controlling the hemolytic activity of YopB.
LcrV enters HeLa cells and is required for translocation of Yops.
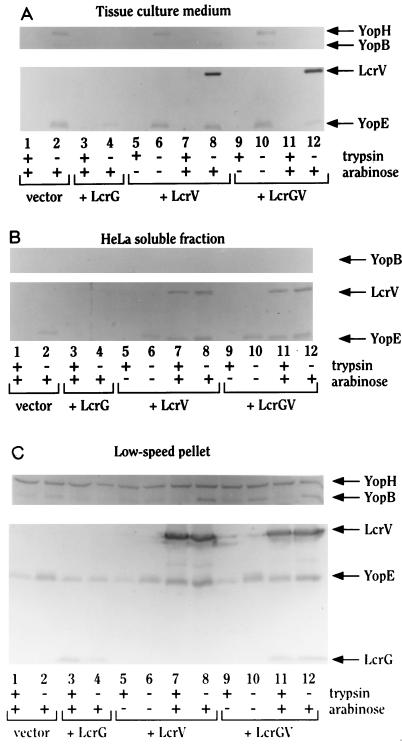
Because LcrV was required for cytotoxicity of HeLa cells and lysis of RBCs, we confirmed that LcrV was in fact necessary for Yop translocation into HeLa cells and also examined LcrV’s fate, by using the series of strains having different levels of expression of LcrV and LcrG. For this analysis, we prepared immunoblots of fractions of infected HeLa cell cultures. Some cultures were treated briefly with trypsin prior to lysis, and trypsin resistance of secreted proteins in the resulting HeLa cell soluble fraction was taken as evidence of cytoplasmic localization of the proteins. YopE, which has been shown to be targeted into eukaryotic cells, was used as a marker for targeted Yops and was found in the cytoplasmic fraction of infected HeLa cells in control experiments with the parent Y. pestis KIM8-3002 (data not shown). As predicted from the previous experiments, the noncytotoxic Y. pestis KIM8-3002.6 (ΔlcrG2) was unable to target YopE into eukaryotic cells (Fig. 6B, lanes 1 and 2) but did secrete YopE into the tissue culture medium (Fig. 6A, lanes 1 and 2). Overexpression of LcrG in Y. pestis KIM8-3002.6 (ΔlcrG2) did not alter the inability of this strain to target YopE in either the absence (data not shown) or the presence of Ara, but it did significantly decrease YopE secretion into the tissue culture medium and decrease YopE expression (Fig. 6, lanes 3 and 4). In the strain containing the LcrV plasmid, induction of LcrV with ara had no effect on YopE expression (Fig. 6, lanes 5 to 8) but did result in YopE targeting (Fig. 6B, lanes 7 and 8). In contrast to wild-type Y. pestis, secretion by this strain was not polarized; i.e., YopE was both secreted into the tissue culture medium and targeted into HeLa cells. Surprisingly, LcrV itself was also found to enter eukaryotic cells in a nonpolarized manner (Fig. 6A and B, lanes 7, 8, 11, and 12). As with the strain containing the LcrV-only plasmid, induction with Ara was required to see YopE targeting in the strain containing LcrG-LcrV expressed from the araBAD promoter (Fig. 6B, lanes 9 to 12). However, the induction of lcrGV allowed the near restoration of polarized character to the targeting of YopE. In contrast, LcrV entry into HeLa cells still was not polarized (Fig. 6A and B, lanes 9 to 12), even in the parent Y. pestis (8). These results demonstrate that LcrV itself most likely enters eukaryotic cells as well as being secreted into the tissue culture medium and that LcrV is required for targeting of YopE (Fig. 6) and presumably other effector Yops. The findings also show that LcrG is required for YopE targeting to be polarized even though it is not required for translocation of Yops into eukaryotic cells.
FIG. 6.
LcrV enters HeLa cells and is required for the translocation of YopE into HeLa cells and the deployment (surface accessibility) of YopB. Y. pestis KIM8-3002.6 (ΔlcrG2) with pBAD18-Kan (vector; lanes 1 and 2), pAraG18K (+ LcrG; lanes 3 and 4), pAraV18K (+ LcrV; lanes 5 to 8), and pAraGV18K, (+ LcrGV; lanes 9 to 12) were used to infect HeLa cells in the presence (lanes 1 to 4, 7, 8, 11, and 12) or absence (lanes 5, 6, 9, and 10) of Ara (0.2% [wt/vol]) to induce expression of LcrG and/or LcrV from the plasmids. After 4 h, trypsin (100 μg/ml; lanes 1, 3, 5, 7, 9, and 11) was added to a duplicate culture for each strain to assess the protease resistance of LcrG, YopE, LcrV, YopB, and YopH in the cell-free tissue culture medium fraction (A), the HeLa cell soluble fraction (B), and the low-speed pellet (C; pellet containing yersiniae, obtained after centrifugation of the H2O-lysed HeLa cells). Protein samples representing 10% of the culture were separated by SDS-PAGE in 12% polyacrylamide gels and analyzed with duplicate immunoblots. One blot was analyzed for the presence of YopE, LcrG, and LcrV with an antiserum cocktail of α-YopE, α-GST-G, and α-HTV. The duplicate blot was analyzed with α-YopB for the presence of YopB and YopH (YopH is detected on some immunoblots by α-YopB). All proteins were visualized by immunostaining with NBT-BCIP after treatment with alkaline phosphatase-conjugated secondary antibodies.
The localization of LcrG was also examined in these experiments. LcrG was detected only in the low-speed pellet obtained after lysis of the infected HeLa cells and probably was located within the bacterial cells in that pellet (Fig. 6C, lanes 3, 4, 11, and 12). However, LcrG that could be present in the tissue culture medium would not be detectable in these experiments, as the filtration of the medium would remove LcrG (LcrG adheres to the membrane used).
A final interesting result from this experiment was obtained by examining YopB localization in these fractions. YopB was not detected in the soluble fraction of HeLa cells, and only small amounts were seen in the tissue culture medium from some of the strains examined (Fig. 6A and B). Most of the YopB detected was in the low-speed pellet containing bacteria and debris from the lysed HeLa cells (Fig. 6C). In this fraction, the amount of YopB appeared to be higher in situations where translocation was occurring, i.e., Y. pestis KIM8-3002.6 (ΔlcrG2) harboring pAraV18K or pAraGV18K, both induced with Ara (Fig. 6C, lanes 8 and 12), and in uninduced Y. pestis KIM8-3002.6 (ΔlcrG2) harboring pAraGV18K (Fig. 6C, lane 11). Interestingly, the increased amount of YopB seen in strains targeting Yops [Y. pestis KIM8-3002.6 (ΔlcrG2) harboring pAraV18K or pAraGV18K, both induced with ara] was trypsin sensitive (Fig. 6C, lanes 8 and 12 compared to lanes 7 and 11). This result suggests that LcrV affects the deployment of YopB at the interface between the bacterial and eukaryotic cells.
DISCUSSION
In this study, we tested the hypothesis that a high ratio of LcrV to LcrG would titrate LcrG’s effect on the Ysc type III secretion system, so as to stabilize an unblocked state of the Ysc and permit maximal Yop and LcrV secretion to occur (24). We created a set of Y. pestis strains having different levels of LcrV and LcrG expression in a new, ΔlcrG2 Y. pestis mutant background. A previously created lcrG mutant (36) was unsuitable for this study, because that mutant has a partial deletion of lcrG and still makes a truncated LcrG product that can weakly interact with LcrV (data not shown). We wanted to eliminate this complexity by making a complete deletion of lcrG. The deletion that we made also deleted the RBS of lcrV, resulting in a strain with very low expression of LcrV. A prediction from the LcrG titration model was that overexpression of LcrG should result in a blockage of secretion. However, when LcrG was overexpressed in wild-type Y. pestis, it was unable even to decrease, much less block, secretion (Fig. 2). We believe that this most likely was due to the very high level of lcrV expression that occurs upon LCR induction in the parent Y. pestis. LcrV is a secreted virulence protein that is made in great excess over LcrG upon induction (24), presumably to provide protein for its direct virulence function as well as for its regulatory role. Accordingly, for our test of the model, we needed a strain that did not overexpress LcrV upon LCR induction, and the ΔlcrG2 mutant provided that condition. The characterization of this strain and of derivatives expressing LcrG, LcrV, and both LcrG and LcrV from an inducible promoter provided evidence in support of the hypothesis as well as new insight into the role of LcrV in the LCR.
Overexpression of LcrG in ΔlcrG2 Y. pestis caused an unusual, nearly Ca2+-independent phenotype. An analogous phenotype was seen in a previously described mutant which has a high ratio of LcrG to LcrV due to a nonpolar deletion within lcrV (Y. pestis KIM5-3241.2 [29]). In contrast to wild-type Y. pestis, both the LcrV− Y. pestis strain and the ΔlcrG2 Y. pestis strain overexpressing LcrG showed decreased expression and secretion of Yops in the absence of Ca2+. This finding is consistent with the idea that LcrG exerts a secretion-blocking effect on the LCR when the amount of LcrV is low. The secretion block would limit secretion of the negative regulator LcrQ as well as of Yops, causing their decreased expression as well as secretion. Providing LcrV expression in addition to LcrG reversed these effects of LcrG in the ΔlcrG2 mutant, and as would be expected, expressing LcrV in the LcrV− mutant (which has a normal lcrG gene) also restored the wild-type control over Yop expression and secretion (data not shown and reference 37). These findings support the idea that the increased amount of LcrV titrates LcrG and counteracts LcrG’s effect on the Ysc.
In the tissue culture infection model, overexpression of LcrG in the absence of high LcrV levels also caused a partial blockage of YopE secretion into the tissue culture medium and of YopE targeting into HeLa cells. Furthermore, as seen in vitro, increasing the level of LcrV in the bacterial cells also expressing LcrG overcame the effect of overexpressing LcrG and restored polarized secretion of Yops; i.e., tight coupling between secretion and targeting of Yops into HeLa cells was reestablished. Importantly, however, LcrV alone did not restore polarization to the secretion response. Therefore, LcrG is necessary in addition to LcrE (4) for the polarization of secretion. Because both of these proteins regulate Ysc activity in vitro, we speculate that their effects in the tissue culture infection system are likely to occur at the level of secretion control rather than reflecting a direct involvement of LcrG or LcrE in the actual process of translocation into eukaryotic cells. Our data enlarge upon the view that contact induction of secretion activates only the secretion channels at the site of contact with the eukaryotic cell: LcrG as well as LcrE is necessary to maintain the closed states of the other Ysc channels in the bacterium.
In the tissue culture system, we did not see the complete blockage of Yop secretion that we had thought might occur in a situation where activation of the Ysc is local and LcrG is being overexpressed in the presence of low amounts of LcrV. Again, this likely reflects the need for both LcrE and LcrG to control secretion. We find that in vitro, overexpression of LcrG has no effect in an lcrE ΔlcrG2 double mutant of Y. pestis, showing that an lcrE mutation is epistatic to the ΔlcrG2 mutation (23), once again suggesting that LcrE and LcrG function in the same pathway, i.e., blocking of secretion. LcrE has been shown to be surface localized in the yersiniae and is thought to function as a sensor for the loss of Ca2+ and for cell contact (4, 9). Induction by cell contact is believed to result in the loss of LcrE’s secretion-blocking function (6). Our data suggest that LcrE also is necessary for LcrG’s blocking function when the LCR is induced by cell contact.
Our experiments revealed that LcrV is required for translocation of Yops into HeLa cells. The finding that ΔlcrG2 Y. pestis was not cytotoxic for HeLa cells was surprising, as other studies in our lab had shown that other Ca2+-blind strains such as LcrE− Y. pestis and another lcrG strain, Y. pestis KIM5-3001.5 [ΔlcrG (aa 39–53)], were cytotoxic, even though they showed nonpolarized targeting of Yops (35). We found that strong expression of LcrV was necessary for Y. pestis to lyse RBCs, an assay that reflects YopB function (12, 15). This finding suggested that the low level of LcrV expression and absence of detectable LcrV secretion in ΔlcrG2 Y. pestis were affecting YopB in some way. The abundance of YopB showed a modest decrease in ΔlcrG2 yersiniae grown in defined medium (Fig. 2), and YopB secretion was significantly decreased (Fig. 2). Both of these effects were largely counteracted by expression of LcrV in trans from pAraV18K or pAraGV18K. However, YopB expression in whole infected HeLa cell cultures did not correlate strictly with the level of LcrV expression (Fig. 4). There appeared to be an increase in the amount of YopB in the Yersinia-containing low-speed pellet obtained from HeLa cell lysis when the ΔlcrG2 Y. pestis was expressing LcrV, but this was lessened when LcrG was also provided. Nevertheless, even with an unnaturally large amount of LcrG present, the bacteria were strongly cytotoxic, because essentially all of the YopB that was secreted was focused at the site of contact between yersiniae and eukaryotic cells. These findings suggest the hypothesis that LcrV somehow facilitates the expression (or stability) and secretion of YopB; LcrG in great excess may partially disrupt this effect when secretion is polarized as it is in the HeLa infection.
Recently Sarker et al. constructed an lcrV deletion mutant of Y. enterocolitica and reported that LcrV was required for secretion of YopB and YopD and that LcrV could interact with both YopB and YopD in E. coli (34). While we could not confirm that LcrV is absolutely essential for YopB and YopD secretion, as the strains used in this study all made at least a small amount of LcrV, the result of Sarker et al. is compatible with our conclusion that YopB secretion is modulated by LcrV. However, their nonpolar LcrV− mutant is complex, as the deletion that they made extended into downstream sycD (also called lcrH), whose product is necessary for the stability of YopD (41). YopD in turn is necessary for LcrQ to have its negative regulatory effect (42). Although Sarker et al. did show that providing sycD in trans did not restore secretion of YopB and YopD to their mutant, they did not demonstrate that the truncated sycD did not produce an interfering product. The secretion profile of Yops other than YopB and YopD in their lcrV sycD strain complemented with sycD was complex. The levels of secreted YopE and YopH were decreased compared to the uncomplemented strain and wild type, which is consistent with our data, but YopM and LcrE (YopN) seemed to be increased in amounts. We did verify that in our ΔlcrG2 mutant, there was no variation in expression of LcrH/SycD that correlated with YopB and YopD expression and secretion (data not shown). Further work is required to resolve the discrepancy between our study and that of Sarker et al.
Interestingly, in the HeLa cell infections, YopB was trypsin accessible only in cultures where the yersiniae were targeting Yops (i.e., strains expressing and secreting LcrV) (Fig. 6). We speculate that the trypsin accessibility of YopB indicates a change in YopB localization when LcrV is strongly expressed and secreted. LcrV may be necessary not only for optimal YopB expression and secretion but also for the proper deployment of YopB, which in turn is required for Yop targeting into eukaryotic cells. LcrV might be involved directly or indirectly in constructing the translocation apparatus at the interface between the bacterial and eukaryotic cells.
We did not see trypsin accessibility of YopB in the low-speed pellet, nor did we see Yop targeting into HeLa cells, when the infecting strain was the uninduced ΔlcrG2 Y. pestis containing pAraGV18K, which did not secrete LcrV. Further, YopE targeting into HeLa cells was decreased in Y. pestis expressing poorly secreted LcrV proteins with small internal deletions [ΔlcrV (aa 25–40) and ΔlcrV (aa 108–125) (37)], and there was no Yop targeting by a strain making a nonsecreted LcrV that lacks its N-terminal 67 residues, even though this strain expresses and secretes Yops (data not shown). These findings suggest the possibility that LcrV secretion is important for targeting of Yops; however, it also is possible that an intact N terminus is necessary for LcrV to promote Yop targeting: this domain was defective in all three mutant LcrV proteins.
Our tissue culture infection experiments revealed two interesting features of LcrV’s own partitioning upon contact with eukaryotic cells. We found that some LcrV entered HeLa cells. This entry appears to be by a mechanism different from that used by Yops and will be described elsewhere (8). We also found that LcrV’s secretion was always nonpolarized: even when YopE was tightly vectorially targeted into HeLa cells, a significant amount of LcrV was released into the tissue culture medium. This finding is consistent with LcrV’s postulated role as an antihost protein with direct immunomodulatory effects (19–21). Secreted LcrV might reach host cells by a paracrine route and have a broadly local or even systemic effect during an infection.
Our findings in this study link the processes of secretion and Yop targeting through LcrV’s activities of preventing LcrG’s secretion-blocking activity and of deploying YopB. The complex lcrGVH yopBD operon serves partitioning as well as regulatory functions for the LCR by targeting Yops to their sites of action (LcrV, YopB, and YopD) in addition to regulating the activity of the Ysc mechanism in response to environmental inputs (LcrG and LcrV). The LCR appears to be designed to tightly coordinate all steps in Yop expression and deployment, from transcription of Yops to their secretion and to their translocation, all in response to contact with a eukaryotic cell. We recently speculated that downregulation of yop expression reflected the operation of such an extended pathway, where YopD acts as a pivotal multifunctional protein, necessary both for Yop translocation and for LcrQ’s negative regulatory effect at the level of transcription (42). Our findings in the present study suggest that a similar extended pathway may exist for LCR induction, where LcrV serves as the pivotal multifunctional protein, necessary for secretion of the negative regulator LcrQ as well as Yops, and possibly is required for constructing a structure that targets secreted Yops into eukaryotic cells.
ACKNOWLEDGMENTS
This study was supported by Public Health Service grant AI21017. M.L.N. was supported for a portion of this study by Public Health Service National Research Service Award AI09854.
We gratefully acknowledge Gregory Plano (University of Miami) for the gift of α-YopE and Åke Forsberg (National Defence Research Establishment, Umeå, Sweden) for the gift of α-YopB.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 2.Bergman T, Häkansson S, Forsberg Å, Norlander L, Macellaro A, Bäckman A, Bölin I, Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol. 1991;173:1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bochner B R, Huang H, Schieven G L, Ames B N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980;143:926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boland A, Sory M-P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelis G, Vanootegem J-C, Sluiters C. Transcription of the yop regulon form Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb Pathog. 1987;2:367–379. doi: 10.1016/0882-4010(87)90078-7. [DOI] [PubMed] [Google Scholar]
- 6.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 7.Fällman M, Persson C, Wolf-Watz H. Yersinia proteins that target host signalling pathways. J Clin Invest. 1997;99:1153–1157. doi: 10.1172/JCI119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields, K. A., and S. C. Straley. Unpublished data.
- 9.Forsberg Å, Viitanen A-M, Skurnik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 10.Fowler J M, Brubaker R R. Physiological basis of the low calcium response in Yersinia pestis. Infect Immun. 1994;62:5234–5241. doi: 10.1128/iai.62.12.5234-5241.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Håkansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homblé F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D, Jessee J, Bloom F R. Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol. 1991;204:63–113. doi: 10.1016/0076-6879(91)04006-a. [DOI] [PubMed] [Google Scholar]
- 14.Hartland E L, Green S P, Phillips W A, Robins-Browne R M. Essential role of YopD in inhibition of the respiratory burst of macrophages by Yersinia enterocolitica. Infect Immun. 1994;62:4445–4453. doi: 10.1128/iai.62.10.4445-4453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmström A, Pettersson J, Rosqvist R, Håkansson S, Tafazoli F, Fällman M, Magnusson K-E, Wolf-Watz H, Forsberg Å. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Metcalf W W, Jiang W, Daniels L L, Kim S-K, Haldimann A, Wanner B L. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Plasmid. 1996;35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 18.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 19.Nakajima R, Brubaker R R. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61:23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima R, Motin V L, Brubaker R R. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nedialkov Y A, Motin V L, Brubaker R R. Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect Immun. 1997;65:1196–1203. doi: 10.1128/iai.65.4.1196-1203.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemeth J, Straley S C. Effect of Yersinia pestis YopM on experimental plague. Infect Immun. 1997;65:924–930. doi: 10.1128/iai.65.3.924-930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilles, M. L., and S. C. Straley. Unpublished data.
- 24.Nilles M L, Williams A W, Skrzypek E, Straley S C. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J Bacteriol. 1997;179:1307–1316. doi: 10.1128/jb.179.4.1307-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry R D, Fetherson J D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry R D, Pendrak M, Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990;172:5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettersson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson K E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 28.Plano G V, Straley S C. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J Bacteriol. 1995;177:3843–3854. doi: 10.1128/jb.177.13.3843-3854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price S B, Cowan C, Perry R D, Straley S C. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2+-dependent growth and maximal expression of low-Ca2+ response virulence genes. J Bacteriol. 1991;173:2649–2657. doi: 10.1128/jb.173.8.2649-2657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rimpiläinen M, Forsberg Å, Wolf-watz H. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis, shows extensive homology to YopH. J Bacteriol. 1992;174:3355–3363. doi: 10.1128/jb.174.10.3355-3363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Sansonetti P J, Ryter A, Clerc P, Maurelli A T, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986;51:461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarker M R, Neyt C, Stainier I, Cornelis G R. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998;180:1207–1214. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skrzypek, E., C. Cowan, and S. C. Straley. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Submitted for publication. [DOI] [PubMed]
- 36.Skrzypek E, Straley S C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J Bacteriol. 1993;175:3520–3528. doi: 10.1128/jb.175.11.3520-3528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skrzypek E, Straley S C. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J Bacteriol. 1995;177:2530–2542. doi: 10.1128/jb.177.9.2530-2542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sory M-P, Cornelis G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 39.Straley S C, Bowmer W S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straley S C, Cibull M L. Differential clearance and host-pathogen interactions of YopE− and YopK− YopL−Yersinia pestis in BALB/c mice. Infect Immun. 1989;57:1200–1210. doi: 10.1128/iai.57.4.1200-1210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wattiau P, Bernier B, Deslée P, Michiels T, Cornelis G R. Individual chaperones required for Yop secretion by Yersinia. Proc Natl Acad Sci USA. 1994;91:10493–10497. doi: 10.1073/pnas.91.22.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams A W, Straley S C. YopD of Yersinia pestis plays a role in the negative regulation of the low-calcium response in addition to its role in the translocation of Yops. J Bacteriol. 1998;180:350–358. doi: 10.1128/jb.180.2.350-358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]