Abstract
Asthma is a common condition in children. This review describes the evidence from the literature and international asthma guidelines for using fractional exhaled nitric oxide (FENO) in the diagnosis and monitoring of childhood asthma. The accuracy of FENO measuring devices could be further improved, the difference in FENO results between devices are equivalent to what is considered a clinically important difference. For diagnosing asthma no guideline currently recommends FENO is used as the first test, but many recommend FENO as part of a series of tests. A cut-off of 35 ppb is widely recommended as being supportive of an asthma diagnosis, but evidence from children at risk of asthma suggests that a lower threshold of 25 ppb may be more appropriate. Nine randomised clinical trials including 1885 children have added FENO to usual asthma care and find that exacerbations are reduced when care is guided by FENO (OR for exacerbation compared to usual care 0.77, 95% CI 0.62–0.94). What is not clear is what cut-off(s) of FENO should be used to trigger a change in treatment. After 30 years of intensive research there is not sufficient evidence to recommend FENO for routine diagnosing and monitoring asthma in children.
Educational aims
To give the reader an overview of literature that supports and does not support the role of FENO in diagnosing asthma in children.
To give the reader an overview of literature that supports and does not support the role of FENO in monitoring asthma in children.
To give the reader an understanding of the role of FENO in international guidelines for diagnosing and monitoring asthma in children.
Tweetable abstract
Guidelines recommend FENO as a second-line test for diagnosing asthma in children. FENO-guided treatment is associated with reduced odds of exacerbation, but it is unclear how FENO should be incorporated into pathways of asthma care. https://bit.ly/3roh6hG
Introduction
Childhood asthma is a condition with complex underlying mechanisms and is characterised by symptoms of cough and wheeze and difficulty in breathing [1]. The aetiology of asthma remains uncertain and seems likely to involve interactions between genetic and environmental factors, resulting in a spectrum of different clinical presentations with common symptoms and which respond to similar treatment [2]. Asthma affects millions of children around the world [3, 4], causes morbidity by disrupting sleep and limiting activities, and can be life-threatening when symptoms are severe during an asthma exacerbation. There is no cure for asthma, but there are effective treatments to prevent symptoms and exacerbations. Despite being so common, there is no universally accepted definition of asthma and no single test for diagnosing or monitoring symptoms.
Childhood-onset asthma is associated with diagnoses of eczema, hayfever and food allergies, and individuals with childhood-onset asthma characteristically have an immune system where T-helper type 2 (Th2) cells [5] predominate. These Th2 cells preferentially release mediators, including interleukin (IL)-4, IL-5 and IL-13, which are implicated in causing the pathological findings seen in the airways of children with asthma, e.g. airway eosinophilia.
Airway eosinophilia is considered a key feature of childhood asthma, and therefore a potential objective outcome which could support asthma diagnosis, monitoring and treatment. Airway eosinophilia is determined from airway biopsy or from induced sputum, but neither of these is readily available in children, which severely limits their application to routine clinical practice. Fractional exhaled nitric oxide (FENO) is positively correlated to airway eosinophilia in both airway mucosal biopsies [6] and induced sputum [7–9], can be measured in the clinical setting, without discomfort to the patient, results are available within a minute and all at a reasonable price [10].
Nitric oxide (NO) is a diatomic molecule which was considered as an air pollutant until the 1980s when it was recognised as having multiple key roles in both health and illness, most notably as being synonymous with “endothelial derived relaxing factor” and explaining why glycerine trinitrate was an effective treatment for angina. By the time NO was voted molecule of the year in 1992 it was seen as being key to multiple roles in health and disease including causing vasodilation, smooth muscle hypertrophy and reducing platelet adhesion. A family of enzymes called nitric oxide synthases (NOS) produce NO; constitutional NOS produces NO for physiological purposes and NO associated with diseases such as asthma arises from the action of inducible NOS [11]. Gaseous NO was identified in exhaled breath in 1993 [12], prompting 30 years of research into the potential use of FENO in asthma diagnosis, monitoring and treatment.
Measuring FENO
Exhaled NO concentrations are inversely proportional to expiratory flow and by consensus are measured at 50 mL·s−1 [13]. The flow dependence of FENO has been used to derive alveolar NO and central airway NO flux; these flow-dependent parameters are not discussed further in this article and the interested reader is referred elsewhere [14]. Despite standardisation of flow to measure FENO, there are marked differences in FENO measurements when analysers made by different manufacturers are used by the same person [15], meaning that measurements from different analysers are not directly comparable.
FENO analysers have visual/audio aids to encourage the patient to exhale at the correct flow; this is particularly useful in paediatrics. FENO can only be measured passively in infants [16], but children aged ≥5 years can exhale into apparatus for FENO measurements to be obtained [17]. The apparatus is very transportable and this allows FENO measurements to be made in children in community and hospital settings [17, 18].
This article reviews the current evidence for FENO being a useful tool to diagnose and monitor asthma in children aged ≥5 years. A related article reviews the relevant literature for the role of spirometry in diagnosing and monitoring asthma in this age group [19].
Exhaled NO to diagnose asthma
What is the evidence that FENO may help diagnose asthma?
For >25 years, children with asthma have been known to have higher FENO concentrations compared to children without asthma [20]. However, the relationship between asthma and elevated FENO is not exclusive [21]; for example children without asthma but who have allergy and/or bronchial hyperresponsiveness also have elevated FENO [22]. There are many non-asthma-related and non-allergy-related factors associated with elevated FENO values [13, 23] including increasing age [13] and height [13], ingestion of food or drink containing arginine [24] and caffeine [25]. FENO values vary through the day (in adults this diurnal variation may be clinically useful [26]) and are reported to differ between ethnic groups [27]. Exposures that may be associated with reduced FENO include upper respiratory tract infection, active and passive smoking [13] and doing spirometry [28]. Many of the associations described here are not replicated in all populations and the magnitude of difference in FENO is generally small, e.g. <5 ppb, meaning that these individual factors may not be relevant to clinical interpretation. However, these factors might combine to have a “clinically meaningful” effect in FENO values, e.g. make FENO values “high” or result in a “significantly different” change in repeated FENO measurements.
The best level of evidence for FENO as a diagnostic tool comes from a small number of studies where children with undifferentiated respiratory symptoms had FENO measured and then were categorised as either having asthma or not having asthma. A recent European Respiratory Society (ERS) task force [29] identified five studies with this design and found that there was a relationship between increasing FENO concentrations and increasing likelihood of asthma; additionally, decreasing FENO concentrations were associated with a decreasing likelihood of asthma.
What cut-off value in FENO defines abnormality?
There is no consistency in which cut-off should be used when considering with a diagnosis of asthma in children. The most commonly used values of <20 ppb being “normal” and >35 ppb being “elevated” [13] comes from normative data within a cross-sectional whole-population study [30]. This cut-off of >35 ppb is defined as positive by both UK asthma guidelines [31, 32] and also the National Asthma Education and Prevention Program (United States of America) [33]. The American Thoracic Society (ATS) states that values >35 ppb identify a child whose symptoms are steroid responsive [34]. The assumption that a cut-off based on normative data within the general population can be extrapolated to a cut-off for diagnosing asthma is not unreasonable, but elevated FENO is present in children without asthma [21, 22]. The previously mentioned ERS task force used data from children presenting with possible asthma symptoms and found that values >25 ppb had best sensitivity and specificity for diagnosing asthma and values <19 ppb for excluding an asthma diagnosis [29].
While FENO is usually expressed in parts per billion, attempts have been made to standardise concentrations, similar to percentage predicted or z-score values of forced expiratory volume in 1 s (FEV1). One group standardised FENO for height using data from two UK populations and found that concentrations above the 90th centile (equivalent to 25 ppb in a 10-year-old boy of average height) has 59% sensitivity, 96% specificity, 97% positive predictive value and 50% negative predicted value [35].
Recognising that factors such as age, sex, height and ethnicity are associated with different FENO concentrations, it would be desirable to have predicted normal values for FENO, similar to those that we have for spirometry. An ERS task force has attempted to apply Global Lung Initiative methodology [36] to produce individualised “norms” for FENO, but has found it was not able to derive age-, gender- and ethnic-specific normative values [37]. This was because the variation in FENO measurements between centres contributing data was too wide, even when a subgroup of centres who used the same device was considered. In the meantime, the cut-off values of >25 ppb and >35 ppb remain the most pragmatic way of determining “high” or “low” values. In summary, there is no agreed cut-off value of FENO to support a diagnosis of asthma in children. All guidelines emphasise that asthma is a clinical diagnosis which is primarily based on the presence of episodic cough and wheeze and difficulty in breathing, and that one (or ideally two) objective tests can usefully support a diagnosis (or help exclude a diagnosis). So the absence of an agreed cut-off does not prevent a diagnosis being made. A very high or very low FENO can be useful to clinicians, but both can occur in children with asthma and without asthma.
Where does FENO sit in the diagnostic hierarchy?
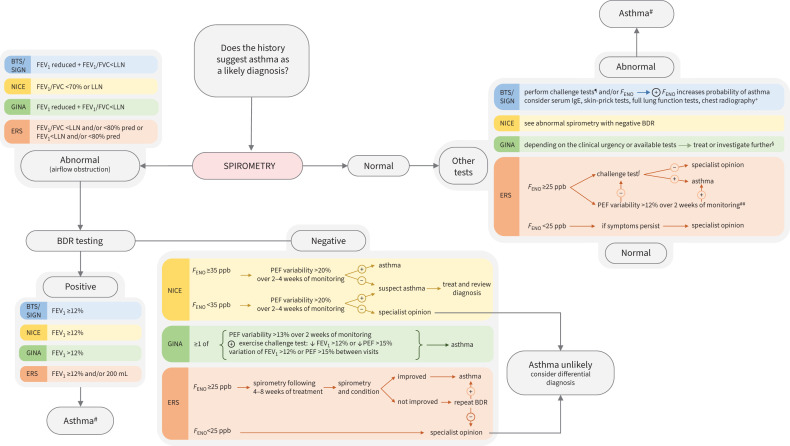
Due to the lack of precision of FENO for asthma, no guideline recommends FENO as the first-line diagnostic test. Figure 1 shows that spirometry is the first-line test recommended by all guidelines. FENO is recommended by the ERS task force [29] and the UK National Institute for Health and Care Excellence [32] where lung function is normal. FENO is also recommended for diagnosis by the Scottish Intercollegiate Guidelines Network/British Thoracic Society [31] after lung function testing and bronchodilator reversibility, if there is an intermediate probability of asthma. The Global Initiative for Asthma says that “FENO has not been established as useful for ruling in or ruling out a diagnosis of asthma…it is also elevated in non-asthma conditions” [1].
FIGURE 1.
A summary of the place of spirometry in diagnostic algorithms from current guidelines. BTS: British Thoracic Society; SIGN: Scottish Intercollegiate Guidelines Network; NICE: National Institute for Health and Care Excellence; GINA: Global Initiative for Asthma; ERS: European Respiratory Society; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; LLN: lower limit of normal; FENO: fractional exhaled nitric oxide; BDR: bronchodilator responsiveness; PEF: peak expiratory flow; ⊕: positive; ⊝: negative. #: BTS/SIGN uses high probability/intermediate/low probabilities of asthma as part of the diagnostic algorithm. A patient in the high-probability category has an increased probability of having asthma if spirometry shows airflow obstruction reversible to bronchodilator; a child who is in the intermediate category can have asthma diagnosis confirmed if spirometry is abnormal and has significant responsiveness to bronchodilator; ¶: direct challenge tests (methacholine challenge test positive if provocative concentration causing a 20% fall in FEV1 ≤8 mg·mL−1; mannitol test positive if fall in FEV1 ≥15% at cumulative dose of ≤635 mg), indirect challenge test (exercise challenge positive range: 8–20% fall in FEV1); +: indicated for children with severe disease or clinical suspicion of other conditions; §: GINA guideline provides two options in case the patient is on controller treatment and requires asthma diagnosis confirmation: in case there are variable respiratory symptoms but spirometry is normal, the recommendation is to consider repeating spirometry either when withholding bronchodilator or the patient is symptomatic. In case there are lesser respiratory symptoms and spirometry is normal, bronchodilator testing can be repeated when symptomatic or when temporarily stopping bronchodilator; ƒ: direct bronchial challenge test with methacholine or indirect bronchial challenge tests by using either a treadmill, bike or both; ##: PEF variability would be an inferior choice to challenge tests and could be used if challenge tests are not available.
How do these guidelines perform in real life?
A study of 514 children aged 5–17 years where FENO was measured and in whom an asthma diagnosis was subsequently given in 357 has reported sensitivities and specificities for different FENO cut-offs (table 1) [38]. A second whole-population study of 630 13–16-year-old children, including 34 on regular inhaled corticosteroids (ICS) has also reported the relationship between increasing FENO cut-offs for diagnosed asthma (table 1) [39]. Table 1 presents sensitivities and specificities of different FENO cut-offs for asthma from three different sources [29, 38, 39].
TABLE 1.
Sensitivity and specificity of fractional exhaled nitric oxide (FENO) for diagnosing asthma from three studies
| FENO ppb | Sensitivity % | Specificity % | |
| Murray et al. [39] | ≥24# | 63# | 73# |
| ≥35 | 52 | 83 | |
| ≥40 | 46 | 87 | |
| De Jong et al. [38] | ≥20 | 52 | 77 |
| ≥21 | 50 | 80 | |
| ≥23 | 48 | 87 | |
| ≥25# | 46# | 88# | |
| ERS task force [29] | >15 | 75 | 61 |
| >20 | 58 | 68 | |
| >25# | 57# | 81# | |
| >30 | 44 | 79 | |
| >35 | 36 | 81 |
The study by Murray et al. [39] was based on a population not selected for asthma, whereas the remaining studies used data from children referred to hospital with possible asthma. ERS: European Respiratory Society. #: at values of 24 or 25 ppb, the sensitivity of FENO is lower and the specificity is higher for children at a higher risk of asthma compared to children in the general population.
At cut-off values of >24 or >25 ppb, the sensitivity (i.e. precision for ruling out asthma) of FENO is lower for children at a higher risk of asthma [29, 38] and the specificity (i.e. precision for ruling in asthma) is higher compared to children selected from the general population [39] (table 1). Applying a cut-off of >35 ppb, the sensitivity of FENO remains lower in children at higher risk of asthma [29] compared to a group not selected for asthma [39], but the specificity is the same (table 1). This suggests that a lower cut-off (e.g. >25 ppb) than the currently recommended concentration of >35 ppb may be more useful in children where a diagnosis of asthma is being considered.
Why might FENO be useful to monitor asthma?
What is the evidence?
There is persuasive evidence that FENO should be a useful measurement to support monitoring of asthma, including making treatment decisions, in order to reduce exacerbations.
1) FENO concentrations rose after reducing ICS dose in a study of 40 children [40]: the results suggest that odds of ICS reduction being successful were six times higher when FENO was ≥22 ppb compared to <22 ppb.
2) FENO concentrations rose before symptoms relapsed after stopping ICS in one study of 40 children [41]; a FENO of ≥49 ppb has the best combination of sensitivity and specificity for predicting relapse. This finding was not replicated in a second study of 32 children [42].
3) FENO concentrations fall after treatment with oral corticosteroids for an asthma exacerbation [43, 44].
4) FENO concentrations fell by 35% in 11 children after commencing treatment with leukotriene receptor antagonist (LTRA) and rose when LTRA was stopped [45].
5) FENO levels fell by 40% after starting anti-IgE biological treatment [46].
To balance this convincing evidence, there are some results which need to be considered when evaluating the role of FENO in monitoring asthma:
1) Combined data from eight randomised clinical trials found no evidence that FENO-guided asthma treatment improves asthma control [47].
2) A trial of 55 children found no evidence that sputum eosinophil count (the physiological characteristic which FENO is a proxy for) was a useful guide for asthma treatment in children [48].
3) FENO values remain high in some individuals despite high-dose ICS treatment [49].
4) FENO is not sensitive to ICS adherence in children. One study of 130 children found no relationship between FENO values and adherence to ICS adherence [50], and a second, of 93 children with severe asthma, found a weak relationship between poor ICS adherence and elevated FENO [51].
5) One study of 1112 children, using data from six clinical trials whose intervention was to add FENO to usual care to guide asthma treatment, found that rising FENO over a 3-month period was not associated with risk of subsequent exacerbations [52].
6) At the time of writing there are no clinical trials which have described the use of FENO in children within a primary care setting.
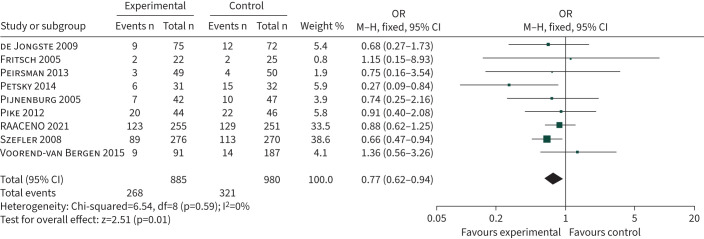
Despite any concerns about the role of FENO in guiding asthma treatment in children, a Cochrane review of 1279 participants from eight clinical trials found that the odds ratio for asthma exacerbation was 0.62 (95% CI 0.49–0.80) when care was delivered by symptoms plus FENO compared to symptoms only [53]. When data from a ninth recently published trial [54] are added to the Cochrane analysis, the odds ratio for exacerbation is 0.77 (95% CI 0.62–0.92), as shown in figure 2. There is no evidence that FENO-guided treatment improves asthma control [53]. The mechanism for FENO reducing exacerbations is likely to involve optimal titration of the child's ICS dose (equivalent to an increase in ICS dose of 60–100 μg budesonide equivalent) [23, 55].
FIGURE 2.
Forest plot comparing the number of participants in randomised clinical trials who had an asthma attack requiring treatment with oral steroids. Participants were randomised to asthma treatment guided by fractional exhaled nitric oxide plus standard care (symptoms±forced expiratory volume in 1 s) or standard care only. RAACENO: Reducing Asthma Attacks in Children Using Exhaled Nitric Oxide; M–H: Maentel–Haenszel; df: degrees of freedom.
Fielding et al. [56] combined original data from six of the eight clinical trials cited in the Cochrane review [53] and explored whether FENO-guided treatment was more effective in certain subgroups of children. The individual patient data (IPD) analysis included information from 1112 children and found that FENO-guided treatment was associated with reduced exacerbations among those not on LTRA treatment (OR 0.68, 95% CI 0.49–0.94) [56]. Among children who were initially controlled, the odds ratio for losing control during follow-up when treatment was guided by FENO compared to standard treatment were reduced for those who were not treated with LTRA (OR 0.70, 95% CI 0.49–1.00) and who were not obese (OR 0.69, 95% CI 0.48–0.99) [56]. These findings suggest that treatment with LTRA may mask the relationship between FENO and ICS treatment. There was no evidence that FENO-guided treatment was more or less effective in improving asthma outcomes in other subgroups stratified by obesity, high/low ICS dose, atopy or ethnicity [56].
How should change in FENO values be expressed?
The distribution of FENO values is not normally distributed; a population median FENO value is considerably lower than the mean value. The implication of this is that expressing change in FENO on a linear scale is less meaningful, especially at higher concentrations; for example, a 10-ppb rise may be more relevant if the original concentration was 10 ppb, compared to 50 ppb. The consensus is that change in FENO should be expressed as absolute terms where values are <50 ppb and as a percentage change at higher values [13]. The IPD analysis mentioned previously found that a percentage change, but not absolute change, in FENO was associated with odds for losing control in future [52].
What is a significant change in FENO?
The ATS guideline [13] recommends that a clinically relevant change is 10 ppb where concentrations are <50 ppb and 20% at higher concentrations. These changes are similar to the magnitude of difference between measurements when one person uses two different FENO devices [15], and this emphasises the importance of using the same device when comparing change in FENO values. One study of 178 children, including 47 with asthma, measured FENO at 2-month intervals over a year and found that FENO concentrations could rise by 100% over 2–4 months, independent of asthma and regardless of the initial concentration [57]. In a second study, a subgroup of 665 children in the previously mentioned IPD were identified as having controlled asthma and unchanged ICS treatment over a 3-month period [58]. In this subgroup with “stable asthma”, the interquartile range (IQR) for a change in FENO for those with an initial FENO <50 ppb was −7 to +9 ppb, meaning that in only ~50% of individuals FENO remained within 10 ppb of initial concentration. For children with “stable asthma” and an initial FENO of ≥50 ppb, the IQR for percentage change was between −33% and +51% [58]. These results cautiously support the ATS statement for FENO values <50 ppb, but suggest that the cut-off for values ≥50 ppb might be considerably more than 20%.
There have been nine clinical trials which have intervened by adding FENO to usual care, and table 2 describes the FENO cut-off values used in these trials. The cut-off values of FENO which triggered a change in treatment differ, some studies use a single value of between 12 and 30 ppb or a 50% change; one used different cut-offs for certain phenotypes while others had different cut-offs for stepping-up or -down treatment. Table 2 describes how the design of these studies differed in several aspects, including:
variable (but usually small) sample size;
many studies included only atopic children;
one study measured FENO on a daily basis, while others measured FENO at intervals between 6 weeks and 4 months;
the studies used different FENO cut-offs to trigger different treatment steps;
some studies also used changes in FEV1 when considering treatment change in both arms of the trial.
TABLE 2.
Methodological details of randomised clinical trials which have intervened with fractional exhaled nitric oxide (FENO) to guide asthma treatment
| First author, year [reference] | Primary outcome(s) | Age years | Participants | Atopy as inclusion criterion? | FEV1 <80% also used in treatment algorithm? | FENO cut-off(s) (ppb) used to step-up/-down treatment | How often was FENO measured after initial assessment? | What did the trial find? (FENO treatment compared to standard care) |
| de Jongste, 2009 [59] | Symptom-free days |
FENO group 11.6±2.6 Control group 11.8±4.3 |
151 | Yes | No | 20 for 6–10-year-olds 25 for older children |
Daily measurements over 3 months | No difference in outcomes |
| Fritsch, 2006 [60] | FEV1 | 11.5±3.1 | 47 | Yes | Yes | 20 | 6, 12, 18 and 24 weeks | Higher mid-expiratory flow, higher dose of ICS |
| Peirsman, 2014 [61] | Symptom-free days | 10.7±2.1 | 99 | Yes | Yes | 20 | 3, 6, 9 and 12 months | Reduced exacerbations, increased LTRA and ICS dose No difference in primary outcome |
| Petsky, 2015 [62] | Exacerbations | 10.0±3.2 | 63 | No | No | 10 for nonatopic 12 with 1 PSPT 20 for >1 PSPT |
1, 2, 3, 4, 6, 8, 10 and 12 months | Reduced exacerbation, increased ICS dose |
| Pijnenburg, 2005 [63] | Cumulative ICS dose | 12.3±2.8 | 85 | No | No | 30 | 3, 6, 9 and 12 months | Reduced FENO and bronchial hyperresponsiveness No increase in ICS dose |
| Pike, 2013 [64] | ICS dose and exacerbation frequency | 10.9±2.6 | 90 | No | No | ≤15 and ≥25 | 2, 4, 6, 8, 10 and 12 months | No differences in outcomes |
| Szefler, 2008 [65] | Days with asthma symptoms | 14.4±2.1 | 546 | Yes | Yes | 20, 30 and 40 | 6, 14, 22, 30, 38 and 46 weeks | Reduced exacerbations, increased ICS dose No difference in primary outcome |
| Turner, 2022 [54] | Exacerbation | 10.1±2.6 | 509 | No | No | >50% change | 3, 6, 9 and 12 months | No differences in outcomes |
| Verini, 2010 [66] | Exacerbation, symptoms and therapy score |
FENO group 10.7±2.4 Control group 11.3±2.1 |
64 | Yes | No | 12 | 6 and 12 months | The FENO group were less likely to have an exacerbation at 6 and 12 months |
| Voorend-van Bergen, 2015 [67] | Proportion of symptom-free days | 10.2±3.0 | 181# | Yes | No | 20 and 50 | 4, 8 and 12 months | Increased asthma control, but not the primary outcome |
Data are presented as mean±sd or n, unless otherwise stated. FEV1: forced expiratory volume in 1 s; ICS: inhaled corticosteroid; LTRA: leukotriene receptor antagonist; PSPT: positive skin-prick test. #: although 272 children were included in the study, the 91 participants randomised to a web-based intervention did not have FENO measurements and are not included here
The many heterogeneities in the design of these studies may explain the inconsistent results for reduction in exacerbations. Importantly, studies that used the same FENO cut-off (20 ppb) [59–61] did not have the same outcomes. Additionally, the four studies that found that the addition of FENO to usual care was associated with reduced exacerbations [61, 62, 65, 66] used different FENO cut-offs. These observations suggest that there is no ideal FENO cut-off for use in monitoring childhood asthma.
Looking ahead
A key priority is for the industry to provide more accurate devices (ideally with much smaller inter-user variability). A universally accepted definition of asthma would be welcome.
For diagnosis, FENO cut-offs from clinically relevant populations (i.e. children with possible asthma) should be applied to diagnostic algorithms which include symptoms and other objective markers, e.g. FEV1. Diagnostic uncertainty for asthma in children aged <5 years is very common and a method to measure FENO in this age group for incorporation into a diagnostic algorithm would be welcome. When you see a child with asthma in the next few weeks, a “low” FENO value (<20 ppb [13] or <19 ppb [29]) in a steroid-naïve child with low or intermediate likelihood of asthma based on symptoms may be helpful in excluding a diagnosis of asthma, and/or support a plan of watchful waiting before considering a diagnosis at a later date. A “high” FENO value (>35 ppb [13] or >25 ppb [29]) in isolation is of uncertain relevance and needs to be interpreted in the context of symptoms and FEV1.
For monitoring asthma, we know that there is good evidence that adding FENO measurements every 2–3 months to usual care leads to a modest reduction in asthma attacks (figure 2), but there is no clear indication of exactly how to integrate FENO into to usual care, e.g. what change in FENO is clinically meaningful and what treatment should be altered in light of changing FENO concentrations? We know that there is no consistently reported subgroup of children in whom FENO is particularly suitable (or unsuitable) for monitoring. One guideline suggests that FENO may be useful in specialist asthma clinics, but this is not based on evidence [31]. What is not known is what benefit may come from using a combination of both FENO and FEV1 to guide asthma treatment: FENO-guided asthma treatment is associated with increased FEV1 [55], so there may be synergy between FENO- and FEV1-guided asthma treatment. An important limitation to research aimed at using objective markers to guide asthma treatment is that there are few treatment options at present: once a child is on ICS, LTRA and long-acting β-agonists, the step-up options for participants in different arms of a clinical trials are the same, i.e. increase ICS dose. So in tomorrow's asthma clinic, regardless of any FENO result, you are still highly likely to step-up treatment in a child with poorly controlled symptoms (assuming they are compliant) and you are likely to step-down treatment in the spring and summer months for a child who has not needed their reliever for 3 months. At the time of writing, there is insufficient evidence to suggest that treatment decisions can be informed by FENO concentrations in identical twins with intermediate symptom control who are on the same treatment and have FENO values of 20 and 30 ppb.
Take-home message
Even the greatest advocates for FENO cannot be certain that FENO should be routinely be used for diagnosing and monitoring childhood asthma. Equally, FENO's greatest critics cannot deny that there is persuasive evidence for its use in some aspects of care (figure 2). We do know that there is currently insufficient evidence to recommend FENO for routine diagnosis and monitoring of asthma in children; clinical decisions should not be made solely on a FENO result. Although data from nine randomised clinical trials suggest that adding FENO to usual asthma is associated with a reduction in exacerbations, it is not unclear what cut-off(s) should be used to trigger a change in treatment. So at this point in time, it is neither myth nor maxim that FENO should be used for diagnosing and monitoring childhood asthma.
Self-evaluation questions
- Which of the following statements is most correct?
- Age, height and active smoking are factors unrelated to asthma that are known to affect FENO measurements.
- Age, height, active smoking and dietary factors are exposures unrelated to asthma that are known to affect FENO measurements.
- Age, height, active smoking, dietary factors and ethnicity are exposures unrelated to asthma that are known to affect FENO measurements.
- Age, height, active smoking, dietary factors, ethnicity and asthma treatment are exposures unrelated to asthma that are known to affect FENO measurements.
- Which of the following statements is correct?
- Reliable FENO measurements can be made in all children.
- Reliable FENO measurements can be made in the majority of children aged >5 years.
- FENO measurements made on apparatus made by different manufacturers can be reliably compared.
- By convention, change in FENO measurements is expressed as a percentage change.
- Which of the following statements regarding diagnosing asthma is most correct?
- A FENO concentration of >35 ppb is diagnostic of asthma.
- A FENO concentration of >25 ppb is diagnostic of asthma.
- All international guidelines recommend that FENO should be measured in children aged ≥5 years.
- No international guideline recommends FENO as the initial test for diagnosing asthma.
- For monitoring asthma in children, which of the following statements is correct?
- There is evidence from nine randomised controlled trials that the addition of FENO to usual care improves the odds for having controlled asthma symptoms in children.
- There is evidence from nine randomised controlled trials that the addition of FENO to usual care reduces the odds of having an asthma exacerbation.
- A rise in FENO of 10 ppb over 3 months in a child with asthma treated with ICS and whose asthma symptoms have intermediate control suggests that a LABA should be introduced.
- A rise in FENO of 10 ppb over 3 months in a child with asthma treated with inhaled corticosteroids suggests that they are not adherent with their treatment.
Suggested answers
c. Asthma treatment (stated in d) is an asthma-related exposure.
b. Differences in FENO measurements between devices are comparable to what is considered a clinically meaningful difference. Change in FENO can be expressed as both absolute change and percentage change.
d.
b.
Footnotes
Conflict of interest: S. Turner received FENO measurement apparatus from Circassia for a clinical trial at no cost between 2016 and 2018. None of the other authors has a conflict of interest to declare.
References
- 1.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2023. https://ginasthma.org/wp-content/uploads/2023/07/GINA-2023-Full-report-23_07_06-WMS.pdf. Date last accessed: 14 October 2023.
- 2.Bush A, Hedlin G, Carlsen K, et al. . Severe childhood asthma: a common international approach? Lancet 2008; 372: 1019–1021. doi: 10.1016/S0140-6736(08)61422-1 [DOI] [PubMed] [Google Scholar]
- 3.Asher MI, Keil U, Anderson HR, et al. . International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995; 8: 483–491. doi: 10.1183/09031936.95.08030483 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention . Asthma Surveillance in the United States, 2001–2021. 2023. www.cdc.gov/asthma/Asthma-Prevalence-US-2023-508.pdf. Date last accessed: 14 October 2023.
- 5.Krusche J, Basse S, Schaub B. Role of early life immune regulation in asthma development. Semin Immunopathol 2020; 42: 29–42. doi: 10.1007/s00281-019-00774-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne DN, Adcock IM, Wilson NM, et al. . Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med 2001; 164: 1376–1381. doi: 10.1164/ajrccm.164.8.2101145 [DOI] [PubMed] [Google Scholar]
- 7.Pontin J, Blaylock MG, Walsh GM, et al. . Sputum eosinophil apoptotic rate is positively correlated to exhaled nitric oxide in children. Pediatr Pulmonol 2008; 43: 1130–1134. doi: 10.1002/ppul.20921 [DOI] [PubMed] [Google Scholar]
- 8.Warke TJ, Fitch PS, Brown V, et al. . Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax 2002; 57: 383–387. doi: 10.1136/thorax.57.5.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piacentini GL, Bodini A, Costella S, et al. . Exhaled nitric oxide and sputum eosinophil markers of inflammation in asthmatic children. Eur Respir J 1999; 13: 1386–1390. doi: 10.1183/09031936.99.13613919 [DOI] [PubMed] [Google Scholar]
- 10.Karrasch S, Linde K, Rucker G, et al. . Accuracy of FENO for diagnosing asthma: a systematic review. Thorax 2017; 72: 109–116. doi: 10.1136/thoraxjnl-2016-208704 [DOI] [PubMed] [Google Scholar]
- 11.Ricciardolo FLM. Multiple roles of nitric oxide in the airways. Thorax 2003; 58: 175–182. doi: 10.1136/thorax.58.2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borland C, Cox Y, Higenbottam T. Measurement of exhaled nitric oxide in man. Thorax 1993; 48: 1160–1162. doi: 10.1136/thx.48.11.1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dweik RA, Boggs PB, Erzurum SC, et al. . An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011; 184: 602–615. doi: 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paraskakis E, Brindicci C, Fleming L, et al. . Measurement of bronchial and alveolar nitric oxide production in normal children and children with asthma. Am J Respir Crit Care Med 2006; 174: 260–267. doi: 10.1164/rccm.200506-962OC [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Health and Care Excellence (NICE) . Measuring Fractional Exhaled Nitric Oxide Concentration in Asthma: NIOX MINO, NIOX VERO and NObreath. 2014. www.nice.org.uk/guidance/dg12. Date last accessed: 7 January 2015. Date last updated: 2 April 2014.
- 16.Franklin PJ, Turner SW, Mutch RC, et al. . Comparison of single-breath and tidal breathing exhaled nitric oxide levels in infants. Eur Respir J 2004; 23: 369–372. doi: 10.1183/09031936.04.00084604 [DOI] [PubMed] [Google Scholar]
- 17.Napier E, Turner SW. Methodological issues related to exhaled nitric oxide measurement in children aged four to six years. Pediatr Pulmonol 2005; 40: 97–104. doi: 10.1002/ppul.20249 [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Lo DK, Beardsmore C, et al. . Implementing spirometry and fractional exhaled nitric oxide testing in childhood asthma management in UK primary care: an observational study to examine training and implementation cost and impact on patient's health use and outcome. Arch Dis Child 2022; 107: 21–25. doi: 10.1136/archdischild-2020-319310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onisor MO, Turner S. Routine FEV1 measurement is essential in diagnosis and monitoring of childhood asthma: myth or maxim? Breathe 2023; 19: 230048. doi: 10.1183/20734735.0048-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson BV, Sears S, Woods J, et al. . Expired nitric oxide as a marker for childhood asthma. J Pediatr 1997; 130: 423–427. doi: 10.1016/S0022-3476(97)70204-X [DOI] [PubMed] [Google Scholar]
- 21.Silvestri M, Sabatini F, Spallarossa D, et al. . Exhaled nitric oxide levels in non-allergic and allergic mono- or polysensitised children with asthma. Thorax 2001; 56: 857–862. doi: 10.1136/thorax.56.11.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin PJ, Turner SW, Le Souëf PN, et al. . Exhaled nitric oxide and asthma: complex interactions between atopy, airway responsiveness, and symptoms in a community population of children. Thorax 2003; 58: 1048–1052. doi: 10.1136/thorax.58.12.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner S. Exhaled nitric oxide and the management of childhood asthma – yet another promising biomarker “has been” or a misunderstood gem. Paediatr Respir Rev 2015; 16: 88–96. doi: 10.1016/j.prrv.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 24.Abuzayan I, Turner SW. Changes in exhaled nitric oxide after ingestion of l-arginine in children: a pilot study. Pediatr Pulmonol 2010; 45: 236–240. doi: 10.1002/ppul.21313 [DOI] [PubMed] [Google Scholar]
- 25.Abuzayan I, Paraskavi U, Turner SW. Changes to exhaled nitric oxide in asthmatic children after drinking a caffeine-containing cola drink. Pediatr Pulmonol 2010; 45: 1228–1232. doi: 10.1002/ppul.21313 [DOI] [PubMed] [Google Scholar]
- 26.Saito J, Gibeon D, Macedo P, et al. . Domiciliary diurnal variation of exhaled nitric oxide fraction for asthma control. Eur Respir J 2014; 43: 474–484. doi: 10.1183/09031936.00048513 [DOI] [PubMed] [Google Scholar]
- 27.Blake TL, Chang AB, Chatfield MD, et al. . Does ethnicity influence fractional exhaled nitric oxide in healthy individuals? A systematic review. Chest 2017; 152: 40–50. doi: 10.1016/j.chest.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 28.Barreto M, Villa MP, Montesano M, et al. . Reduced exhaled nitric oxide in children after testing of maximal expiratory pressures. Pediatr Pulmonol 2006; 41: 141–145. doi: 10.1002/ppul.20358 [DOI] [PubMed] [Google Scholar]
- 29.Gaillard EA, Kuehni CE, Turner S, et al. . European Respiratory Society clinical practice guidelines for the diagnosis of asthma in children aged 5-16 years. Eur Respir J 2021; 58: 2004173. doi: 10.1183/13993003.04173-2020 [DOI] [PubMed] [Google Scholar]
- 30.Buchvald F, Hermansen MN, Nielsen KG, et al. . Exhaled nitric oxide predicts exercise-induced bronchoconstriction in asthmatic school children. Chest 2005; 128: 1964–1967. doi: 10.1378/chest.128.4.1964 [DOI] [PubMed] [Google Scholar]
- 31.Scottish Intercollegiate Guidelines Network (SIGN) , British Thoracic Society (BTS) . SIGN 158: British Guideline on the Management of Asthma. Edinburgh, Scottish Intercollegiate Guidelines Network, 2019. [Google Scholar]
- 32.National Institute for Health and Care Excellence (NICE) . Asthma: Diagnosis, Monitoring and Chronic Asthma Management. 2017. https://www.nice.org.uk/guidance/ng80. Date last updated: 22 March 2021.
- 33.Cloutier MM, Baptist AP, Blake KV, et al. . 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee expert panel working group. J Allergy Clin Immunol 2020; 146: 1217–1270. doi: 10.1016/j.jaci.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khatri SB, Iaccarino JM, Barochia A, et al. . Use of fractional exhaled nitric oxide to guide the treatment of asthma: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2021; 204: e97–e109. doi: 10.1164/rccm.202109-2093ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang R, Fowler SJ, Turner SW, et al. . Defining the normal range of fractional exhaled nitric oxide in children: one size does not fit all. ERJ Open Res 2022; 8: 00319-2022. doi: 10.1183/23120541.00319-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quanjer PH, Stanojevic S, Cole TJ, et al. . Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Högman M, Bowerman C, Chavez L, et al. . ERS technical standard: Global Lung Function Initiative reference values for exhaled nitric oxide (FENO). Eur Respir J 2023; in press [ 10.1183/13993003.00370-2023]. 10.1183/13993003.00370-2023. [DOI] [PubMed] [Google Scholar]
- 38.de Jong CCM, Pedersen ESL, Mozun R, et al. . Diagnosis of asthma in children: findings from the Swiss Paediatric Airway Cohort. Eur Respir J 2020; 56: 2000132. doi: 10.1183/13993003.00132-2020 [DOI] [PubMed] [Google Scholar]
- 39.Murray C, Foden P, Lowe L, et al. . Diagnosis of asthma in symptomatic children based on measures of lung function: an analysis of data from a population-based birth cohort study. Lancet Child Adolesc Health 2017; 1: 114–123. doi: 10.1016/S2352-4642(17)30008-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zacharasiewicz A, Wilson N, Lex C, et al. . Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med 2005; 171: 1077–1082. doi: 10.1164/rccm.200409-1242OC [DOI] [PubMed] [Google Scholar]
- 41.Pijnenburg MW, Hofhuis W, Hop WC, et al. . Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax 2005; 60: 215–218. doi: 10.1136/thx.2004.023374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cabral ALB, Vollmer WM, Barbirotto RM, et al. . Exhaled nitric oxide as a predictor of exacerbation in children with moderate-to-severe asthma: a prospective, 5–month study. Ann Allergy Asthma Immunol 2009; 103: 206–211. doi: 10.1016/S1081-1206(10)60183-4 [DOI] [PubMed] [Google Scholar]
- 43.Baraldi E, Dario C, Ongaro R, et al. . Exhaled nitric oxide concentrations during treatment of wheezing exacerbation in infants and young children. Am J Respir Crit Care Med 1999; 159: 1284–1288. doi: 10.1164/ajrccm.159.4.9807084 [DOI] [PubMed] [Google Scholar]
- 44.Comberiati P, Peroni D, Malka-Rais J, et al. . Fractional exhaled nitric oxide response to oral corticosteroids in children with mild-to-moderate asthma: influence of race. Ann Allergy Asthma Immunol 2020; 125: 440–446. doi: 10.1016/j.anai.2020.06.036 [DOI] [PubMed] [Google Scholar]
- 45.Montuschi P, Mondino C, Koch P, et al. . Effects of montelukast treatment and withdrawal on fractional exhaled nitric oxide and lung function in children with asthma. Chest 2007; 132: 1876–1881. doi: 10.1378/chest.07-1587 [DOI] [PubMed] [Google Scholar]
- 46.Szefler SJ, Casale TB, Haselkorn T, et al. . Treatment benefit with omalizumab in children by indicators of asthma severity. J Allergy Clin Immunol Pract 2020; 8: 2673–2680. doi: 10.1016/j.jaip.2020.03.033 [DOI] [PubMed] [Google Scholar]
- 47.Petsky HL, Cates CJ, Lasserson TJ, et al. . A systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils). Thorax 2012; 67: 199–208. doi: 10.1136/thx.2010.135574 [DOI] [PubMed] [Google Scholar]
- 48.Fleming L, Bush A. Use of sputum eosinophil counts to guide management in children with severe asthma. Thorax 2012; 67: 1015–1016. doi: 10.1136/thx.2010.156836 [DOI] [PubMed] [Google Scholar]
- 49.Buchvald F, Eiberg H, Bisgaard H. Heterogeneity of FENO response to inhaled steroid in asthmatic children. Clin Exp Allergy 2003; 33: 1735–1740. doi: 10.1111/j.1365-2222.2003.01822.x [DOI] [PubMed] [Google Scholar]
- 50.Paracha R, Lo DKH, Montgomery U, et al. . Asthma medication adherence and exacerbations and lung function in children managed in Leicester primary care. NPJ Prim Care Respir Med 2023; 33: 12. doi: 10.1038/s41533-022-00323-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jochmann A, Artusio L, Jamalzadeh A, et al. . Electronic monitoring of adherence to inhaled corticosteroids: an essential tool in identifying severe asthma in children. Eur Respir J 2017; 50: 1700910. doi: 10.1183/13993003.00910-2017 [DOI] [PubMed] [Google Scholar]
- 52.Fielding S, Pijnenburg M, de Jongste JC, et al. . Change in FEV1 and FENO measurements as predictors of future asthma outcomes in children. Chest 2019; 155: 331–341. doi: 10.1016/j.chest.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petsky HL, Kew KM, Chang AB. Exhaled nitric oxide levels to guide treatment for children with asthma. Cochrane Database Syst Rev 2016; 11: CD011439. doi: 10.1002/14651858.CD011439.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner S, Cotton S, Wood J, et al. Reducing asthma attacks in children using exhaled nitric oxide (RAACENO) as a biomarker to inform treatment strategy: a multicentre, parallel, randomised, controlled, phase 3 trial. Lancet Respir Med 2022; 10: 584–592. 10.1016/S2213-2600(21)00486-0 [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Tan X, Li Q. Effectiveness of fractional exhaled nitric oxide for asthma management in children: a systematic review and meta-analysis. Pediatr Pulmonol 2020; 55: 1936–1945. doi: 10.1002/ppul.24898 [DOI] [PubMed] [Google Scholar]
- 56.Fielding S, Pijnenburg MW, de Jongste JC, et al. . Does treatment guided by exhaled nitric oxide fraction improve outcomes in subgroups of children with asthma? Eur Respir J 2020; 55: 1901879. doi: 10.1183/13993003.01879-2019 [DOI] [PubMed] [Google Scholar]
- 57.Cutts R, Turner S. Longitudinal measurements of exhaled nitric oxide in children – what is a significant change in FENO? Pediatr Allergy Immunol 2013; 24: 540–548. doi: 10.1111/pai.12101 [DOI] [PubMed] [Google Scholar]
- 58.Fielding S, Pijnenburg M, de Jongste J, et al. . What is a clinically meaningful change in exhaled nitric oxide for children with asthma? Pediatr Pulmonol 2020; 55: 599–606. doi: 10.1002/ppul.24630 [DOI] [PubMed] [Google Scholar]
- 59.de Jongste JC, Carraro S, Hop WC, et al. . Daily telemonitoring of exhaled nitric oxide and symptoms in the treatment of childhood asthma. Am J Respir Crit Care Med 2009; 179: 93–97. doi: 10.1164/rccm.200807-1010OC [DOI] [PubMed] [Google Scholar]
- 60.Fritsch M, Uxa S, Horak FJ, et al. . Exhaled nitric oxide in the management of childhood asthma: a prospective 6-months study. Pediatr Pulmonol 2006; 41: 855–862. doi: 10.1002/ppul.20455 [DOI] [PubMed] [Google Scholar]
- 61.Peirsman EJ, Carvelli TJ, Hage PY, et al. . Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol 2014; 49: 624–631. doi: 10.1002/ppul.22873 [DOI] [PubMed] [Google Scholar]
- 62.Petsky HL, Li AM, Au CT, et al. . Management based on exhaled nitric oxide levels adjusted for atopy reduces asthma exacerbations in children: a dual centre randomized controlled trial. Pediatr Pulmonol 2015; 50: 535–543. doi: 10.1002/ppul.23064 [DOI] [PubMed] [Google Scholar]
- 63.Pijnenburg MW, Bakker EM, Hop WC, et al. . Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med 2005; 172: 831–836. doi: 10.1164/rccm.200503-458OC [DOI] [PubMed] [Google Scholar]
- 64.Pike K, Selby A, Price S, et al. . Exhaled nitric oxide monitoring does not reduce exacerbation frequency or inhaled corticosteroid dose in paediatric asthma: a randomised controlled trial. Clin Respir J 2013; 7: 204–213. doi: 10.1111/j.1752-699X.2012.00306.x [DOI] [PubMed] [Google Scholar]
- 65.Szefler SJ, Mitchell H, Sorkness CA, et al. . Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet 2008; 372: 1065–1072. doi: 10.1016/S0140-6736(08)61448-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verini M, Consilvio NP, Di Pillo S, et al. . FENO as a marker of airways inflammation: the possible implications in childhood asthma management. J Allergy 2010; 2010: 691425. doi: 10.1155/2010/691425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voorend-van Bergen S, Vaessen-Verberne AA, Brackel HJ, et al. . Monitoring strategies in children with asthma: a randomised controlled trial. Thorax 2015; 70: 543–550. doi: 10.1136/thoraxjnl-2014-206161 [DOI] [PubMed] [Google Scholar]