Abstract
No pleural intervention in a patient with confirmed malignant pleural effusion (MPE) prolongs life, but even the recommended interventions for diagnosis and palliation can be costly and therefore unavailable in large parts of the world. However, there is good evidence to guide clinicians working in low- and middle-income countries on the most cost-effective and clinically effective strategies for the diagnosis and management of MPE. Transthoracic ultrasound-guided closed pleural biopsy is a safe method of pleural biopsy with a diagnostic yield approaching that of thoracoscopy. With the use of pleural fluid cytology and ultrasound-guided biopsy, ≥90% of cases can be diagnosed. Cases with an associated mass lesion are best suited to an ultrasound-guided fine needle aspiration with/without core needle biopsy. Those with diffuse pleural thickening and/or nodularity should have an Abrams needle (<1 cm thickening) or core needle (≥1 cm thickening) biopsy of the area of interest. Those with insignificant pleural thickening should have an ultrasound-guided Abrams needle biopsy close to the diaphragm. The goals of management are to alleviate dyspnoea, prevent re-accumulation of the pleural effusion and minimise re-admissions to hospital. As the most cost-effective strategy, we suggest early use of indwelling pleural catheters with daily drainage for 14 days, followed by talc pleurodesis if the lung expands. The insertion of an intercostal drain with talc slurry is an alternative strategy which is noninferior to thoracoscopy with talc poudrage.
Educational aims
To provide clinicians practising in resource-constrained settings with a practical evidence-based approach to the diagnosis and management of malignant pleural effusions.
To explain how to perform an ultrasound-guided closed pleural biopsy.
To explain the cost-effective use of indwelling pleural catheters.
Tweetable abstract
Most malignant pleural effusions can be diagnosed with a combination of thoracentesis and closed pleural biopsy with reusable needles and basic ultrasound guidance. Management with indwelling pleural catheters is cost-effective with simple workarounds. https://bit.ly/3PMBuBk
Introduction
A malignant pleural effusion (MPE) occurs when pleural fluid accumulates because of malignant cells in the pleura. These may arrive at the pleural surface through haematogenous or lymphatic spread from the primary cancer; may invade the pleura directly from a tumour in the adjacent lung; or may arise as primary tumours of the mesothelial cells [1]. The incidence of MPEs in low- and middle-income countries (LMICs) is not known. Limited access to diagnostic procedures and pathology services probably results in underdiagnosis and underreporting of the true burden [2]. However, the global incidence and death rate from respiratory tract malignancies in regions of lower socioeconomic status is increasing [3]. As lung cancer is one of the commonest causes of MPE (in fact, the commonest cause of MPE in our own centre in sub-Saharan Africa), this also suggests that the incidence of MPE is rising in LMICs [4]. Therefore, the need for guidance on the diagnosis and management of MPE in regions with limited resources is increasing too.
There are several international guidelines and review articles which address this topic, but these are almost invariably written with the assumption that all technologies needed to provide the current best evidence-based practice are available [5–8]. In reality, this is untrue for the majority of the world [9]. Despite the challenges, there is good evidence to guide clinicians working in these countries.
The main aim of this review is to provide clinicians practicing in resource-constrained settings with a practical, yet evidence-based approach, to both the diagnosis and management of MPE. We focus specifically on the most cost-effective and efficient use of ultrasound-guided closed pleural biopsy and indwelling pleural catheters.
Diagnostic approach
Imaging
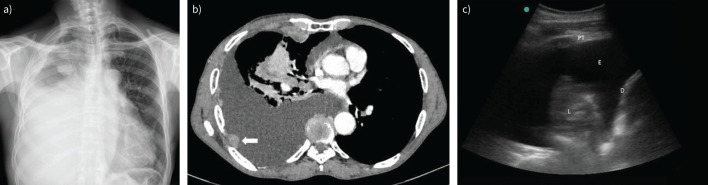
While imaging cannot provide a definitive diagnosis of MPE, the information gained is invaluable in formulating an accurate differential, directing investigations and staging the malignancy. In most LMICs there is extremely limited access (if any) to advanced imaging such as computed tomography (CT), magnetic resonance imaging and positron emission tomography (PET)-CT. Clinicians most often rely on chest radiographs, but even then may have to wait days for the result. Moderate to large free-flowing nonloculated pleural effusions can be visualised on an anteroposterior chest radiograph, with as little as 50 mL of fluid visible on a lateral radiograph [10]. Findings such as crowding of ribs, lung volume loss, an obvious lung mass, circumferential lobulated pleural thickening and a shifted mediastinum suggest a malignant cause of any associated effusion (figure 1a). The mediastinum may be shifted to the opposite side by a large effusion or mass, may remain in the midline or may be shifted toward the malignant mass if it obstructs a bronchus and causes lung collapse, or mediastinal infiltration [10].
FIGURE 1.
Typical imaging findings in a malignant pleural effusion. a) A chest radiograph of a large right-sided malignant pleural effusion with an associated mass in the right upper zone; b) an axial slice from thoracic computed tomography (CT) in a patient with a large right-sided malignant pleural effusion, showing a thickened abnormal pleural with a distinct pleural-based mass lesion amenable to biopsy (arrow); c) a thoracic ultrasound image of large malignant effusion (E) with a markedly thickened parietal pleura (pleural thickening (PT)). Atelectatic lung (L) is seen in the effusion, and the highly echogenic diaphragmatic pleura (D) on the right.
Transthoracic ultrasound should be more readily available than other imaging modalities, as it is routinely used in antenatal medicine and emergency medicine. A basic low frequency (2–5 MHz) curvilinear probe with two-dimensional B-mode ultrasound image is more sensitive than chest radiography for detecting a pleural effusion. If a high-frequency linear probe is available, it can be used for a high-resolution image of the pleural surface, which may show findings strongly suggesting malignancy (figure 1c) [11, 12]. These include parietal pleural thickening >10 mm, diaphragmatic nodularity or thickening of >7 mm, visceral pleural thickening (only seen with a low-frequency probe when the effusion is moderate to large), pleural nodularity/irregularity and an adjacent lung mass [10, 11].
Contrast-enhanced thoracic CT remains the gold standard in evaluating MPE, and should be performed if available. It had a sensitivity and specificity of 97% and 89%, respectively, in one head-to-head comparison with thoracic ultrasound, which had a sensitivity and specificity of 79% and 100%, respectively; the latter mostly driven by pleural nodularity [13, 14]. It is the preferred way of visualising malignant pleural involvement, which usually looks thick or nodular, and may have underlying pulmonary masses or nodules (figure 1b). In addition, it is the best way to evaluate for coexistent liver or adrenal metastasis, although ultrasound may be of help here too [10]. In the case of mesothelioma, circumferential or mediastinal pleural thickening and involvement of the interlobar fissures, which is only visible on the CT, may be an important clue to the diagnosis [15].
If available, PET-CT can be used to detect pleural metastases as a guide to the biopsy site and to monitor treatment response. However, false positive findings can occur from other inflammatory conditions of the pleura, especially in settings with a high burden of communicable diseases [16].
Thoracentesis with pleural fluid analysis
The role of cytology in the diagnosis of MPE is perhaps underappreciated. A recent systematic review found the overall diagnostic sensitivity of pleural fluid cytology for MPE to be 58%, with the highest sensitivity for lung adenocarcinoma (84%), but low sensitivity for squamous cell carcinoma (24%) and mesothelioma (29%) [17]. Immunohistochemical staining dramatically improves our ability to accurately identify a malignancy from a fine-needle aspiration when the pleura is markedly thickened or there is a discrete pleural mass lesion [18]. The controversial exception may be mesothelioma, for which current guidelines still require histology for diagnosis and accurate subtyping [15, 19].
There are a few ways to optimise the yield of pleural fluid cytology. The 2022 guidelines from the British Thoracic Society strongly recommend sending a volume of ≥25 mL; ideally 25–50 mL in suspected MPE [20]. The correct handling of the specimen is important, and ideally it should be processed within 2 h. If not possible, cellular integrity may be preserved for up to 72 h with refrigeration at 2–8°C [21]. Furthermore, centrifuging the fluid sample and obtaining a cellblock for haematoxylin and eosin staining and immunohistochemistry should be done routinely to improve the overall diagnosis and establish the origin of metastatic cancers [18].
Performing a second thoracentesis where the first has failed to diagnose a pleural exudate is discouraged. The additional yield is only 7–17%, and repeat aspirations risk introducing infection into the effusion [22]. However, if the initial volume aspirated was small (<25 mL) and the suspected tumour type is one associated with a higher likelihood of cytological diagnosis from pleural fluid (such as adenocarcinoma), then re-aspiration under aseptic conditions could be considered [20].
It must be acknowledged that cytology is rarely sufficient to identify the molecular markers required to guide targeted immunotherapy. However, considering the current exorbitant cost of these therapies, this is not likely to impact diagnostic pathways in LMICs.
Image-guided closed pleural biopsy
Unguided or “blind” closed pleural biopsy has no place in current practice. The procedure has a high risk of organ injury; only yields a diagnosis of MPE in 47% of cases; and only increases the diagnostic yield over pleural fluid cytology by 7–27% [22, 23]. However, image-guided pleural biopsies provide a reasonable alternative for obtaining pleural tissue with minimal complications, especially in an underresourced setting [12]. The reported sensitivity in MPE varies slightly between studies, but is generally ∼87%; sometimes >95% when there is a gross pleural abnormality [20, 24, 25].
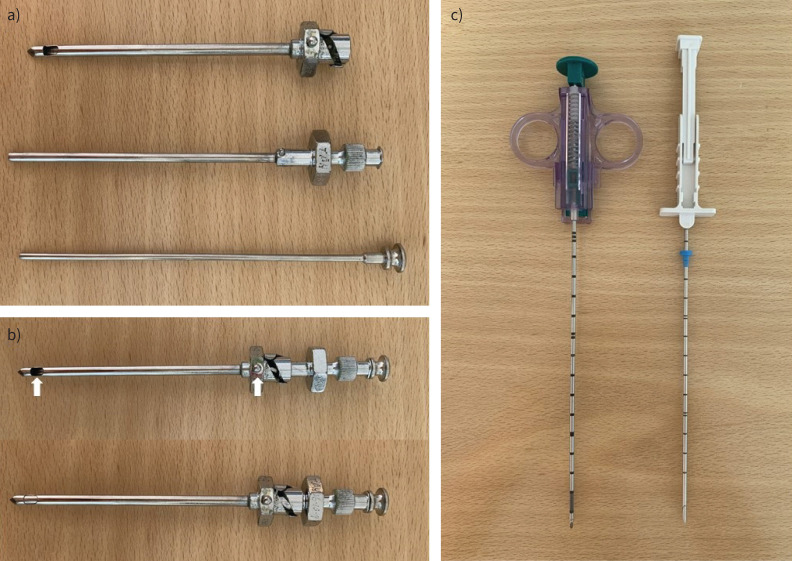
Various dedicated pleural biopsy needles are available. The Abrams or reverse-bevel needle (figure 2a and b) has been used since as far back as the 1950s and is still preferred in many LMICs, as it is reusable and lasts decades [26]. It is a large-bore metal needle, which consists of two cylinders with a stylet. The outer cylinder acts as a short trocar to puncture the chest wall through a small incision, with the fine-edged notch on the side used to hook pleura. The cutting edge of the inner cylinder is used to shear off a piece of tissue of up to 5 mm in diameter. Since the late 1980s, other needles (not specifically designed for pleural tissue sampling) have been validated, particularly core-cutting needles like the Tru-Cut needle (figure 2c) [27]. These were originally designed to be manually deployed, but numerous automated core needles are commercially available. Unfortunately, they are generally single use, which increases the cost of the procedure.
FIGURE 2.
Needles for image-guided closed pleural biopsy. a) The traditional reusable Abrams pleural biopsy needle; the three parts of the needle are shown (from top to bottom): the outer cylinder, the inner cylinder with cutting edge, and the stylet. b) The assembled Abrams needle in the open position with the notch indicator on the grip in line with the specimen open notch (arrows). Below this is the needle in the closed position, as the inner cylinder has been rotated down into the outer cylinder. c) Typical core-cutting needles: on the left, an automated needle which will trigger a closing action after being primed; on the right, a manual needle.
A recent systematic review and meta-analysis found that there was no significant difference between the yield of CT- and ultrasound-guided biopsies in the diagnosis of pleural lesions [28]. Considering that ultrasound has no radiation exposure and is easily performed at the bedside, the authors concluded that ultrasound-guided biopsies should be the preferred approach in the presence of adequate skills. Only the most basic ultrasound equipment is needed to guide a biopsy in the presence of an effusion. A curvilinear probe on the “abdomen” preset, exactly as is used for obstetric services, is sufficient. If available, a linear probe for high-resolution scanning of the pleural surface is recommended when there is a small effusion or no effusion, and colour Doppler ultrasound to identify the intercostal vessels. Additionally, ultrasound allows the immediate detection of complications [12].
Most importantly, the biopsy needs to be performed in a safe and controlled environment where optimal patient positioning is possible [12]. It must be done with a full aseptic technique. So, the ideal environment is an elective theatre or clean procedure room. The patient should be seated upright, with their legs supported by a step, and their arms supported in front of them to lift the scapulae off the posterior chest wall. Once the patient is positioned properly and comfortable, the biopsy area is identified using the ultrasound, and marked on the patient's skin. Then the local anaesthetic agent is instilled. This may simply be 1% lignocaine given directly to the skin, subcutaneous tissues and intercostal structures over the planned biopsy site. Alternatively, a rib block can be done, which is very effective.
Techniques used for ultrasound-guided biopsy include the “free-hand” or “image-assisted” technique, and the “real-time” technique [29]. Most commonly, the image-assisted technique is used. The most suitable biopsy area is identified and marked, then the ultrasound probe is put down and the biopsy performed. In this case it is critical to assess the depth, correct plane and angle of the biopsy needle while the ultrasound is in use. A safe zone must be measured, to avoid injury to visceral structures, the diaphragm and intercostal neurovascular bundles [30]. The most suitable biopsy area is pleura that is thickened, nodular or a clearly visualised pleural mass. If there is no overtly abnormal pleura, the biopsy site should be as close as possible to the diaphragm and midline, as this is where most of the malignant deposits will occur. The real-time technique involves a second person holding the ultrasound probe in position throughout the procedure so that the needle is observed in real-time. This can be done with the needle in the long or short axis of the probe, although having the whole length of the needle visible during the procedure is preferred [29]. There are commercially available attachments for the ultrasound probe designed to hold a core needle, which may allow a single user to perform real-time ultrasound guidance [11].
The biopsy procedure is different depending on the needle used. An Abrams needle should be slowly inserted through a small skin incision until within the pleural space, angled infero-laterally (away from the usual position of the neurovascular bundle), until the specimen notch is against the pleura. The needle is opened by twisting the grip of the outer cylinder. When pushed against the pleura and closed, a piece of pleura will be sheared off inside the notch. The needle is then removed. A core needle also enters the skin through a small incision and is angled lengthwise against the pleura. The cutting sheath is then either manually closed over the specimen notch, or having been “primed”, triggered to close automatically. The clinician must stabilise the needle when the cutting sheath is closed, as the needle tends to push back [30].
The yield of ultrasound-guided biopsy is ∼84% for all pathologies; possibly a bit lower (∼70%) for malignant effusions [20]. Important factors which improve the yield of ultrasound-guided biopsy include a larger needle gauge, thicker pleura and nodular pleura [31]. The number of samples taken in one procedure also matters: we recommend that at least six cores be sent for the highest likelihood of a diagnosis [32, 33].
The most common complication of a closed pleural biopsy is a pneumothorax. When image guidance is used, pneumothorax is more often caused by air moving into the pleural space through the needle than by visceral pleura and lung injury. Fortunately, only a minor proportion of these patients will require intervention to manage this complication. Other rare complications include haemothorax (<2%), local haemorrhage and haematoma (<1%), site pain (1–15%) and transient fever (<1%) [22]. Life-threatening haemorrhage is a potential risk, which is most often due to injury of an intercostal vessel. The risk of introducing infection into the pleural space is theoretical, with data suggesting that the only preventative measure needed is an aseptic technique [34]. Procedure tract metastasis has a variable incidence between 4% and 24%. It is more common in mesothelioma, and more likely with larger bore procedures, with the highest incidence reported in surgical biopsies [35, 36].
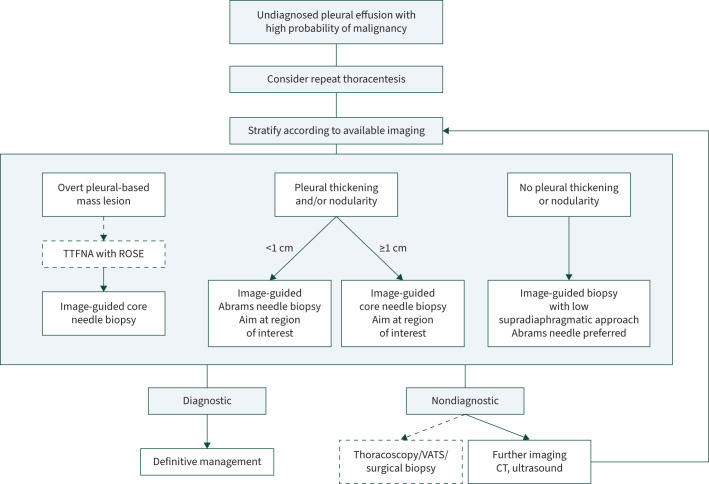
In a prospective study preformed at our institution, we validated a previously suggested algorithm to guide both the biopsy technique and device based on pleural imaging, and reported a 90% diagnostic yield for all diagnoses, including malignancy [25]. Patients were stratified into three groups: 1) those with an associated mass lesion; 2) those with diffuse pleural thickening (>10 mm) and/or nodularity; and 3) those with insignificant pleural thickening (irrespective of the size of the effusion). Where there was an overt pleural-based mass lesion, transthoracic fine needle aspiration (TTFNA) cytology with cell block was performed with rapid on-site evaluation (ROSE) of specimens by an experienced pathologist. In cases where ROSE showed insufficient material, Tru-Cut core biopsies (six passes) were performed. ROSE allowed immediate feedback from the pathologist to the clinician performing the TTFNA, so that if needed, a biopsy could be done during the same visit. In cases where there was uniform pleural thickening of <1–2 cm in depth, we used an Abrams needle closed biopsy with ultrasound guidance. In cases of marked pleural thickening of >1–2 cm depth, we proceeded with a core needle for biopsy. In the case of insignificant pleural thickening, an Abrams needle was performed in basal region of the pleural surface as identified by ultrasound.
Considering that ROSE and cytopathology services are not available in many LMICs, we propose an alternative ultrasound-based algorithm, summarised in figure 3.
FIGURE 3.
A practical approach to the diagnosis of a malignant pleural effusion in a severely resource-constrained setting. Transthoracic fine-needle aspiration (TTFNA) with rapid on-site evaluation (ROSE) by a pathologist may not be available, in which case an image-guided biopsy would the first investigation after thoracentesis in an effusion with an overt pleural-based mass lesion. Where thoracoscopy, video-assisted thoracoscopic surgery (VATS) and surgical biopsy are unavailable, then we suggest obtaining either computed tomography (CT) or ultrasound, depending on what has already been done. Then stratify according to this imaging and repeat the biopsy with a new target. Figure created using BioRender.com.
Medical/surgical thoracoscopy and video-assisted thoracoscopic surgery
Where available, undiagnosed cases should be referred for either medical or surgical thoracoscopy or video-assisted thoracoscopic surgery (VATS), which provides a higher diagnostic yield of ∼98% [37]. However, most countries in sub-Saharan Africa have either no or extremely limited access to any form of thoracoscopy.
Medical thoracoscopy, or pleuroscopy, is done under conscious sedation through a single port in a spontaneously breathing patient, usually in a bronchoscopy suite. In contrast, traditional VATS needs three chest ports and general anaesthesia. While both procedures allow for biopsy of visually abnormal parietal pleura, adhesiolysis and pleurodesis, VATS is associated with longer hospital stay (median 5.8–10.7 days) and inpatient mortality (1–8%) [38]. Recently, techniques have improved to uniportal VATS as well as VATS under conscious sedation, blurring the boundaries with medical pleuroscopy [39].
Management of MPE
Principles
It is important to realise that no pleural intervention prolongs life, and that in most cases, interventions are palliative. The aims therefore are to improve quality of life (especially minimising dyspnoea), avoid unnecessary and potentially harmful pleural procedures, and to avoid need for repeated healthcare visits, in a patient-centred approach.
Prognostic scoring models have been developed to predict mortality in MPE and may aid in deciding which palliative procedure should be performed. Validated scores include the LENT score which classifies patients as low, intermediate or high risk of death at 1, 3 and 6 months, using four simple characteristics: the pleural fluid lactate dehydrogenase, Eastern Cooperative Oncology Group (ECOG) performance status, pleural neutrophil/lymphocyte ratio and tumour type [40]. More recently, the PROMISE score was developed to predict 3-month mortality. This score uses eight variables: ECOG status, tumour type, previous chemo- or radiotherapy, haemoglobin, C-reactive protein, white blood cell count and pleural tissue inhibitor of metalloproteinases 1 concentration [41]. In mesothelioma, the Brims decision tree is clinically useful and incorporates weight loss, performance status, laboratory markers and underlying histology [42].
Ultimately, many factors will influence choice of definitive management offered, including the prognosis, performance status, size/recurrence rate, personal preferences, tumour involvement of the underlying lung, and, importantly in LMICs, the availability of local expertise and resources.
Therapeutic thoracentesis
Initial large-volume thoracentesis is useful in evaluating whether the patient has symptomatic relief with drainage and would benefit from definitive pleural intervention. It can also give an indication of the underlying expandability of the lung, and therefore if pleurodesis would be possible. Recent evidence suggests that 55% of patients will require another definitive procedure, of which >50% will require it within 14 days of the initial large-volume thoracentesis [43].
Nondefinitive management of MPE with only therapeutic thoracentesis is associated with increased risk of re-admission to hospital, with an almost 20% in-hospital mortality on readmission [44]. This can add significantly to hospital costs and presents an important barrier to home-based palliative care [45, 46]. In patients with an anticipated survival of <1–2 weeks, this may still be a viable option [47].
Intercostal drainage and pleurodesis
The choice of definitive drainage method and whether to do a pleurodesis will depend on the ability of the underlying lung to expand. Nonexpandable lung is caused by encasement of the lung by pleural thickening, or proximal endobronchial obstruction with distal lung collapse [6]. Up to 30% of patients with MPE do not have re-expansion of the lung. This may not be suspected, even when thoracoscopy is performed [47]. Pleurodesis will not be possible if the lung does not expand, although the patient may still get symptomatic relief with drainage of fluid. For these patients indwelling pleural catheters (IPC) are considered the drainage method of choice.
Pleurodesis remains the primary management strategy in most patients with MPE and expandable lung to prevent re-accumulation of fluid. The optimal agent for pleurodesis, supported by large prospective studies, is sterile large-particle graded talc. Recent evidence has confirmed that the risk of acute respiratory distress syndrome is negligible with large-particle talc, as it is not systemically absorbed [48]. Delivery methods include talc poudrage during thoracoscopy, and talc slurry through an intercostal drain or IPC. The method does not have a major influence on the effectiveness of the talc [49, 50]. Therefore, the method of delivery will depend on local infrastructure and expertise available, the patient's clinical condition, their preference and whether an intercostal drain was already inserted.
Traditional management is to admit the patient to hospital with an intercostal drain in situ for 4–7 days, then instil the talc slurry through the drain when there is pleural apposition demonstrated on imaging, or there is <150 mL fluid drained per day [38]. The intercostal drain is clamped for 1 h and then removed completely. Recently, bedside thoracic ultrasound has been used successfully in place of chest radiograph to assess pleural apposition, resulting in a shorter hospital stay [51]. With this method, successful pleurodesis at 1 month is achieved in 70–90% of cases, but the success rate decreases over time [5, 6, 49]. There is no evidence that a large-bore intercostal drain is superior to small-bore drains in pleurodesis success rates. Moreover, small-bore drains are associated with fewer complications [52]. The unfortunate reality of this method of pleurodesis is that the long inpatient stay may represent a substantial proportion of the patient's remaining life [40].
Indwelling pleural catheter with or without pleurodesis
There is now a global shift toward ambulatory or outpatient management with the use of an IPC, and we have found this strategy to be particularly useful in our own setting.
An IPC is a fenestrated small-bore silicone catheter that is tunnelled subcutaneously, with a valve at the external part that prevents entry of air. This allows periodic drainage of the pleural fluid either at the patient's convenience, or according to prescription. The procedure is done as an outpatient under local anaesthesia. IPCs relieve dyspnoea and improve quality of life with the same efficacy as talc slurry pleurodesis [20]. In addition, IPCs reduce the need for further pleural interventions. Randomised controlled trials assessing the effectiveness of IPCs are shown in table 1.
TABLE 1.
Major studies on the utility of indwelling pleural catheters (IPCs) in the management of malignant pleural effusions
| Study (year) [ref.] | Design | Main positive findings | Secondary findings |
| TIME 2 (2012) [53] | Unblinded randomised: IPC (n=52) versus ICD with talc pleurodesis (n=54) | IPC group: Improvement in dyspnoea in the IPC group at 6 months (p=0.01) Initial hospitalisation shorter (p<0.001) Less need for further interventions (p=0.03) |
No significant difference in: Dyspnoea after 42 days QoL |
| ASAP (2016) [54] | Randomised: IPC daily drainage (n=73) versus standard drainage (alternative days, n=76) | Autopleurodesis Greater with daily drainage (47% versus 24%, p=0.003) Median time to autopleurodesis shorter with daily drainage (54 versus 90 days) |
No significant difference in: Rate of adverse events QoL Patient satisfaction |
| AMPLE (2017) [55] | Open-label, randomised: IPC (n=74) versus talc pleurodesis (n=72) |
Hospitalisation days from treatment to death Median (IQR) 10.0 (3–17) in IPC group versus 12.0 (7–21) days in pleurodesis group (p=0.03) Fewer additional pleural procedures in IPC group |
No significant difference in: Improvements in breathlessness QoL |
| AMPLE II (2018) [56] | Open-label randomised: IPC daily (n=43) versus symptom-guided drainage (n=44) |
Daily drainage group: No difference in breathlessness scores Autopleurodesis more likely at 3 (37.2% versus 11.4%, p=0.0049) and 6 months (44.2% versus 15.9%, p=0.004) Better patient-reported QoL measures |
No significant difference in: Rate of adverse events Pain scores Days in hospital Mortality |
| IPC-Plus (2018) [57] | Randomised (only if no trapped lung at 10 days after IPC): 4 g of talc slurry (n=69) versus placebo (n=70) |
Talc group: Pleurodesis 43% in the talc group versus 23% (p=0.008) Better QoL and symptom control |
No significant difference in: Rate of adverse events Effusion size and complexity Number of inpatient days Mortality |
ICD: intercostal drain; QoL: quality of life; IQR: interquartile range.
Spontaneous pleurodesis occurs in up to 45% of patients. Instilling talc slurry through the IPC improves the pleurodesis efficacy, facilitating earlier IPC removal [57, 58]. Daily drainage of pleural fluid through the IPC is associated with a higher rate of spontaneous pleurodesis and earlier removal of the IPC, although pleurodesis success rates remain <50%. In addition, there is no marked difference in patient-centred outcomes such as quality of life, with more aggressive daily drainage compared to symptom-driven drainage [54]. A customised drainage schedule for patients can be set, depending on patient preferences, how often family members or home-based care workers are available to assist with drainage, the desire for pleurodesis in the case of expandable lung, and the underlying cost of consumables [48]. Ultrasound can be used to assess suitability for pleurodesis, as the presence of lung sliding indicates pleural apposition, with no need for further imaging. Talc can then be instilled through the IPC and, if successful, the IPC may be removed, alleviating the discomfort of daily drainage.
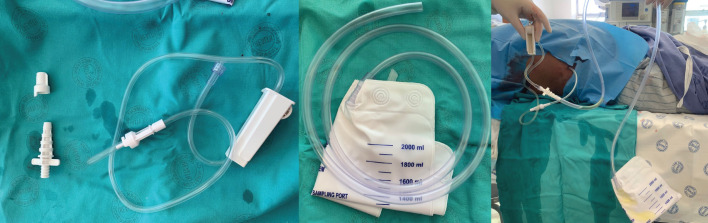
In our institution the main limitation to the use of IPCs is the cost of the drainage kit. Cost analysis has shown that where the expected survival is <14 weeks, IPC is more cost-effective than other methods of drainage and pleurodesis. When the survival extends to ≥1 year, the cost of IPC and intercostal drain with pleurodesis are almost equivalent [59]. The cost of an IPC set in our intuition is EUR270. We have found that we can reduce the cost significantly by replacing the reusable drainage kit with a standard urine catheter bag (figure 4). During the coronavirus disease 2019 pandemic, inpatient services were severely truncated by unavailability of beds. During that time many centres which previously used talc slurry through an intercostal drain as their intervention of choice switched to an IPC-first strategy by necessity. In our institution this practice continues, as the benefits cannot be ignored.
FIGURE 4.
An indwelling pleural catheter (IPC) with alternative drainage option. Shown is the equipment supplied in the typical IPC pack for attachment to the single-use drainage bottles, and how these can be attached to a conventional urine catheter bag instead.
The risk of local insertion site or pleural infection is the most common complication of IPC use. A recent large meta-analysis found an overall infection rate of 6.7% in IPCs used for MPE [60]. The rate of local infection (1.3% wound infection or cellulitis) was similar to that of infection in the pleural space (1.9% pleural infection or empyema). These infections were effectively managed with antibiotics, and very rarely required the removal of the IPC [60]. Importantly, there is no increase in infection in those receiving antineoplastic therapy (chemotherapy and/or biologic) with an IPC in situ, even when immunocompromised [61]. Therefore, there is no need to delay IPC insertion in patients who are going to receive chemotherapy. The rate of infection is minimised by simple measures such as maintaining an aseptic field during IPC insertion, educating patients on the use of IPC and regularly changing wound dressings and drainage bags [46]. Prophylactic antibiotics at the time of insertion can also be considered, though there are no large trials to confirm this [62].
Other complications of IPC are catheter malfunction (1.1%), obstruction (1.5%) and tract metastasis (0–26% depending on tumour type) [60, 63]. Nondrainage may signal successful pleurodesis or indicate catheter malfunction. If blockage of the IPC is caused by fibrin septations, instillation of fibrinolytics can improve drainage [64]. If necessary, tract metastasis can receive radiotherapy [65].
There are no available data on the rate of IPC complications in LMICs. Anecdotally, we have not seen a higher rate of infectious or other complications than that quoted in the literature. However, establishing this as fact is important, as the many barriers to healthcare access in LMICs may theoretically increase complications, and deter clinicians in these regions from using IPCs.
Suggested approach to nonsurgical management
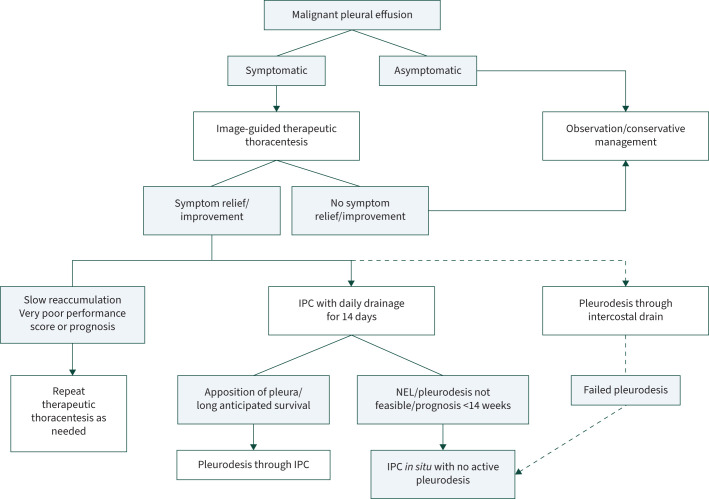
Most patients with MPE can be definitively managed without hospital admission. Patients who have an extremely poor prognosis or who experience little or no symptomatic improvement after a therapeutic aspiration generally do not require a definitive intervention (figure 5). As we have shown, an IPC is arguably the cheapest option for those who do experience significant improvement, and aggressive daily drainage for 14 days is suggested. If apposition of the pleura can be achieved, talc pleurodesis is advised. In this setting, talc slurry through an intercostal drain is an acceptable alternative. For those with a nonexpandable lung or failed pleurodesis, an IPC is realistically the only nonsurgical treatment option.
FIGURE 5.
A practical approach to the management of a malignant pleural effusion in a severely resource constrained setting. Where the patient experiences and improvement in symptoms after thoracentesis and has a reasonable prognosis, an indwelling pleural catheter (IPC) is the preferred intervention. Talc pleurodesis may be done through the IPC, unless there is a nonexpandable lung (NEL), or the life expectancy is <14 weeks. If no IPC is available, an intercostal drain with talc pleurodesis is an acceptable alternative. Figure created using BioRender.com.
Other management options
VATS pleurodesis is generally unavailable in LMICs; provides only incremental benefit over talc slurry through an intercostal drain; and is associated with increased risk, increased length of hospital stay, with increased costs. Patients with nonexpandable lung may need surgical decortication before they can have a pleurodesis procedure, or alternatively a pleuroperitoneal shunt procedure. However, an IPC may also be used in this situation, at a lower cost, without hospital admission, and with a lower complication rate.
Extrapleural pneumonectomy was previously performed in malignant mesothelioma, but is not advised as it carries a high operative risk and has been found to shorten survival [38].
Conclusion
As clinicians working in one of the better-resourced centres in sub-Saharan Africa, we are aware that most of our LMIC colleagues are under even greater constraints than us. However, the techniques for diagnosis and management of MPE that we have presented in this review are the most cost-effective ways to implement the current best practice according to the evidence. We strongly advocate that clinicians seek access to both ultrasound and IPCs (without the expensive consumables), as a means to providing early and accurate diagnosis, and ambulatory care that improves the patient's quality of life.
Key points
There is ample evidence on management of malignant pleural effusions that can be applied in low- and middle-income countries.
Pleural fluid cytology and ultrasound-guided biopsy is safe and has a combined diagnostic yield ≥90% in malignant pleural effusions.
Early use of indwelling pleural catheters, with daily drainage for 14 days is advised, followed by talc pleurodesis if the lung expands.
The insertion of an intercostal drain with talc slurry is an effective alternative strategy, but requires hospital admission.
Self-evaluation questions
1. Which of these features on ultrasound imaging is associated with a high risk of malignant pleural effusion?
a) Absence of lung sliding, pleural calcification, and pleural thickening.
b) Visceral pleural thickening, multiple septations, and echogenic material in the effusion.
c) Pleural nodularity, visceral pleural thickening, diaphragmatic nodules.
d) Absence of B lines, enhanced A lines, absence of lung sliding.
2. What is the recommended volume of pleural fluid to send for cytology?
a) ≥10 mL, ideally 10–25 mL.
b) ≥25 mL, ideally 25–50 mL.
c) ≥50 mL, ideally 50–100 mL.
d) >100 mL.
3. Which of the following statements is TRUE of image-guided closed pleural biopsy?
a) The diagnostic yield of closed pleural biopsy in malignant effusions is increased by ∼40% with the use of image guidance.
b) Computed tomography guidance is superior to ultrasound guidance.
c) The Abrams needle is a single-use pleural biopsy needle.
d) Image-guided closed pleural biopsy has a high rate of pneumothorax which generally needs to be managed with an intercostal drain.
4. Which of the following are the most important aims in the management of a malignant pleural effusion? Choose the correct combination of aims.
a) To relieve dyspnoea and improve the quality of life.
b) To cure the malignancy.
c) To avoid unnecessary and potentially harmful pleural procedures.
d) To prolong life.
e) To avoid repeated healthcare visits.
Suggested answers
1. c.
2. b.
3. a.
4. a, c and e.
Footnotes
Conflict of interest: J.A. Shaw reports receiving an honorarium from AstraZeneca, outside the submitted work. E.H. Louw has nothing to disclose. C.F.N. Koegelenberg reports receiving an honorarium from GSK and AstraZeneca, outside the submitted work.
References
- 1.Rodrîguez-Panadero F, Borderas Naranjo F, López Mejîas J. Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J 1989; 2: 366–369. doi: 10.1183/09031936.93.02040366 [DOI] [PubMed] [Google Scholar]
- 2.Adeoye PO, Johnson WR, Desalu OO, et al. . Etiology, clinical characteristics, and management of pleural effusion in Ilorin, Nigeria. Niger Med J 2017; 58: 76–80. doi: 10.4103/0300-1652.219349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebrahimi H, Aryan Z, Saeedi Moghaddam S, et al. . Global, regional, and national burden of respiratory tract cancers and associated risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Respir Med 2021; 9: 1030–1049. doi: 10.1016/S2213-2600(21)00164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koegelenberg CFN, Bennji SM, Boer E, et al. . The current aetiology of malignant pleural effusion in the Western Cape Province, South Africa. S Afr Med J 2018; 108: 275–277. doi: 10.7196/SAMJ.2018.v108i4.12936 [DOI] [PubMed] [Google Scholar]
- 5.Roberts ME, Neville E, Berrisford RG, et al. . Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65: Suppl. 2, ii32–ii40. doi: 10.1136/thx.2010.136994 [DOI] [PubMed] [Google Scholar]
- 6.Bibby AC, Dorn P, Psallidas I, et al. . ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J 2018; 52: 1800349. doi: 10.1183/13993003.00349-2018 [DOI] [PubMed] [Google Scholar]
- 7.Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. . Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med 2018; 198: 839–849. doi: 10.1164/rccm.201807-1415ST [DOI] [PubMed] [Google Scholar]
- 8.Scarci M, Caruana E, Bertolaccini L, et al. . Current practices in the management of malignant pleural effusions: a survey among members of the European Society of Thoracic Surgeons. Interact Cardiovasc Thorac Surg 2017; 24: 414–417. doi: 10.1093/icvts/ivw373 [DOI] [PubMed] [Google Scholar]
- 9.Prydz EB, Wadhwa D. Classifying Countries by Income. 2019. https://datatopics.worldbank.org/world-development-indicators/stories/the-classification-of-countries-by-income.html. Date last updated: 9 September 2019.
- 10.Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc 2008; 83: 235–250. doi: 10.1016/S0025-6196(11)60848-3 [DOI] [PubMed] [Google Scholar]
- 11.Koegelenberg CFN, Diacon AH. Image-guided pleural biopsy. Curr Opin Pulm Med 2013; 19: 368–373. doi: 10.1097/MCP.0b013e32835f4c23 [DOI] [PubMed] [Google Scholar]
- 12.Corcoran JP, Tazi-Mezalek R, Maldonado F, et al. . State of the art thoracic ultrasound: intervention and therapeutics. Thorax 2017; 72: 840–849. doi: 10.1136/thoraxjnl-2016-209340 [DOI] [PubMed] [Google Scholar]
- 13.Shiroshita A, Nozaki S, Tanaka Y, et al. . Thoracic ultrasound for malignant pleural effusion: a systematic review and meta-analysis. ERJ Open Res 2020; 6: 00464-2020. doi: 10.1183/23120541.00464-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qureshi NR, Rahman NM, Gleeson FV. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax 2009; 64: 139–143. doi: 10.1136/thx.2008.100545 [DOI] [PubMed] [Google Scholar]
- 15.Scherpereel A, Opitz I, Berghmans T, et al. . ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur Respir J 2020; 55: 1900953. doi: 10.1183/13993003.00953-2019 [DOI] [PubMed] [Google Scholar]
- 16.Fjaellegaard K, Koefod Petersen J, Reuter S, et al. . Positron emission tomography-computed tomography (PET-CT) in suspected malignant pleural effusion. An updated systematic review and meta-analysis. Lung Cancer 2021; 162: 106–118. doi: 10.1016/j.lungcan.2021.10.018 [DOI] [PubMed] [Google Scholar]
- 17.Kassirian S, Hinton SN, Cuninghame S, et al. . Diagnostic sensitivity of pleural fluid cytology in malignant pleural effusions: systematic review and meta-analysis. Thorax 2023; 78: 32–40. doi: 10.1136/thoraxjnl-2021-217959 [DOI] [PubMed] [Google Scholar]
- 18.Bhanvadia VM, Santwani PM, Vachhani JH. Analysis of diagnostic value of cytological smear method versus cell block method in body fluid cytology: study of 150 cases. Ethiop J Health Sci 2014; 24: 125–131. doi: 10.4314/ejhs.v24i2.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolhouse I, Bishop L, Darlison L, et al. . British Thoracic Society Guideline for the investigation and management of malignant pleural mesothelioma. Thorax 2018; 73: i1–i30. doi: 10.1136/thoraxjnl-2017-211321 [DOI] [PubMed] [Google Scholar]
- 20.Roberts ME, Rahman NM, Maskell NA, et al. . British Thoracic Society Guideline for pleural disease. Thorax 2023; 78: s1–s42. doi: 10.1136/thorax-2022-219784 [DOI] [PubMed] [Google Scholar]
- 21.Antonangelo L, Vargas FS, Acencio MMP, et al. . Effect of temperature and storage time on cellular analysis of fresh pleural fluid samples. Cytopathology 2012; 23: 103–107. doi: 10.1111/j.1365-2303.2011.00863.x [DOI] [PubMed] [Google Scholar]
- 22.Hooper C, Lee YCG, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65: Suppl. 2, ii4–ii17. doi: 10.1136/thx.2010.136978 [DOI] [PubMed] [Google Scholar]
- 23.Maskell NA, Gleeson FV, Davies RJO. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet 2003; 361: 1326–1330. doi: 10.1016/S0140-6736(03)13079-6 [DOI] [PubMed] [Google Scholar]
- 24.Sconfienza LM, Mauri G, Grossi F, et al. . Pleural and peripheral lung lesions: comparison of US-and CT-guided biopsy. Radiology 2013; 266: 930–935. doi: 10.1148/radiol.12112077 [DOI] [PubMed] [Google Scholar]
- 25.Koegelenberg CFN, Irusen EM, von Groote-Bidlingmaier F, et al. . The utility of ultrasound-guided thoracentesis and pleural biopsy in undiagnosed pleural exudates. Thorax 2015; 70: 995–997. doi: 10.1136/thoraxjnl-2014-206567 [DOI] [PubMed] [Google Scholar]
- 26.Abrams L. A pleural-biopsy punch. Lancet 1958; 271: 30–31. doi: 10.1016/S0140-6736(58)92521-2 [DOI] [PubMed] [Google Scholar]
- 27.McLeod DT, Ternouth I, Nkanza N. Comparison of the Tru-cut biopsy needle with the Abrams punch for pleural biopsy. Thorax 1989; 44: 794–796. doi: 10.1136/thx.44.10.794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mei F, Bonifazi M, Rota M, et al. . Diagnostic yield and safety of image-guided pleural biopsy: a systematic review and meta-analysis. Respiration 2021; 100: 77–87. doi: 10.1159/000511626 [DOI] [PubMed] [Google Scholar]
- 29.Koegelenberg CFN, von Groote-Bidlingmaier F, Bolliger CT. Transthoracic ultrasonography for the respiratory physician. Respiration 2012; 84: 337–350. doi: 10.1159/000339997 [DOI] [PubMed] [Google Scholar]
- 30.Shaw JA, Irusen EM, Koegelenberg CFN. Pleural interventions: closed pleural biopsy. In: Janes SM, ed. BT-Encyclopedia of Respiratory Medicine. 2nd Edn. Oxford, Academic Press, 2022; pp. 566–577. Doi: 10.1016/B978-0-12-801238-3.11310-8 [DOI] [Google Scholar]
- 31.Zhang Y, Tang J, Zhou X, et al. . Ultrasound-guided pleural cutting needle biopsy: accuracy and factors influencing diagnostic yield. J Thorac Dis 2018; 10: 3244–3252. doi: 10.21037/jtd.2018.05.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallifax RJ, Corcoran JP, Ahmed A, et al. . Physician-based ultrasound-guided biopsy for diagnosing pleural disease. Chest 2014; 146: 1001–1006. doi: 10.1378/chest.14-0299 [DOI] [PubMed] [Google Scholar]
- 33.Asciak R, Rahman NM. Malignant pleural effusion: from diagnostics to therapeutics. Clin Chest Med 2018; 39: 181–193. doi: 10.1016/j.ccm.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 34.Shaw JA, Koegelenberg CFN. Debugging: is routine antimicrobial prophylaxis indicated in medical thoracoscopy? Respiration 2017; 94: 176–177. doi: 10.1159/000477819 [DOI] [PubMed] [Google Scholar]
- 35.Agarwal PP, Seely JM, Matzinger FR, et al. . Pleural mesothelioma: sensitivity and incidence of needle track seeding after image-guided biopsy versus surgical biopsy. Radiology 2006; 241: 589–594. doi: 10.1148/radiol.2412051020 [DOI] [PubMed] [Google Scholar]
- 36.Metintas M, Ak G, Parspour S, et al. . Local recurrence of tumor at sites of intervention in malignant pleural mesothelioma. Lung Cancer 2008; 61: 255–261. doi: 10.1016/j.lungcan.2007.12.022 [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Zayas G, Molina S, Ost DE. Sensitivity and complications of thoracentesis and thoracoscopy: a meta-analysis. Eur Respir Rev 2022; 31: 220053. doi: 10.1183/16000617.0053-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koegelenberg CFN, Shaw JA, Irusen EM, et al. . Contemporary best practice in the management of malignant pleural effusion. Ther Adv Respir Dis 2018; 12: 1753466618785098. doi: 10.1177/1753466618785098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali MS, Light RW, Maldonado F. Pleuroscopy or video-assisted thoracoscopic surgery for exudative pleural effusion: a comparative overview. J Thorac Dis 2019; 11: 3207–3216. doi: 10.21037/jtd.2019.03.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clive AO, Kahan BC, Hooper CE, et al. . Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014; 69: 1098–1104. doi: 10.1136/thoraxjnl-2014-205285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Psallidas I, Kanellakis NI, Gerry S, et al. . Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): a multicohort analysis. Lancet Oncol 2018; 19: 930–939. doi: 10.1016/S1470-2045(18)30294-8 [DOI] [PubMed] [Google Scholar]
- 42.Brims FJH, Meniawy TM, Duffus I, et al. . A novel clinical prediction model for prognosis in malignant pleural mesothelioma using decision tree analysis. J Thorac Oncol 2016; 11: 573–582. doi: 10.1016/j.jtho.2015.12.108 [DOI] [PubMed] [Google Scholar]
- 43.Ost DE, Niu J, Zhao H, et al. . Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest 2018; 153: 438–452. doi: 10.1016/j.chest.2017.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell MA, Dhaliwal I, Mulpuru S, et al. . Early readmission to hospital in patients with cancer with malignant pleural effusions: analysis of the nationwide readmissions database. Chest 2020; 157: 435–445. doi: 10.1016/j.chest.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 45.Mummadi SR, Stoller JK, Lopez R, et al. . Epidemiology of adult pleural disease in the United States. Chest 2021; 160: 1534–1551. doi: 10.1016/j.chest.2021.05.026 [DOI] [PubMed] [Google Scholar]
- 46.Alwakeel AJ, Shieh B, Gonzalez AV, et al. . Impact of a pleural care program on the management of patients with malignant pleural effusions. J Bronchology Interv Pulmonol 2022; 30: 122–128. doi: 10.1097/LBR.0000000000000907 [DOI] [PubMed] [Google Scholar]
- 47.Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378: 740–751. doi: 10.1056/NEJMra1403503 [DOI] [PubMed] [Google Scholar]
- 48.Bashour SI, Mankidy BJ, Lazarus DR. Update on the diagnosis and management of malignant pleural effusions. Respir Med 2022; 196: 106802. doi: 10.1016/j.rmed.2022.106802 [DOI] [PubMed] [Google Scholar]
- 49.Bhatnagar R, Piotrowska HEG, Laskawiec-Szkonter M, et al. . Effect of thoracoscopic talc poudrage vs talc slurry via chest tube on pleurodesis failure rate among patients with malignant pleural effusions: a randomized clinical trial. JAMA 2020; 323: 60–69. doi: 10.1001/jama.2019.19997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dipper A, Jones HE, Bhatnagar R, et al. . Interventions for the management of malignant pleural effusions: an updated network meta-analysis. Eur Respir Rev 2021; 30: 210025. doi: 10.1183/16000617.0025-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Psallidas I, Hassan M, Yousuf A, et al. . Role of thoracic ultrasonography in pleurodesis pathways for malignant pleural effusions (SIMPLE): an open-label, randomised controlled trial. Lancet Respir Med 2022; 10: 139–148. doi: 10.1016/S2213-2600(21)00353-2 [DOI] [PubMed] [Google Scholar]
- 52.Thethi I, Ramirez S, Shen W, et al. . Effect of chest tube size on pleurodesis efficacy in malignant pleural effusion: a meta-analysis of randomized controlled trials. J Thorac Dis 2018; 10: 355–362. doi: 10.21037/jtd.2017.11.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davies HE, Mishra EK, Kahan BC, et al. . Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307: 2383–2389. doi: 10.1001/jama.2012.5535 [DOI] [PubMed] [Google Scholar]
- 54.Wahidi MM, Reddy C, Yarmus L, et al. . Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. The ASAP trial. Am J Respir Crit Care Med 2017; 195: 1050–1057. doi: 10.1164/rccm.201607-1404OC [DOI] [PubMed] [Google Scholar]
- 55.Thomas R, Fysh ETH, Smith NA, et al. . Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA 2017; 318: 1903–1912. doi: 10.1001/jama.2017.17426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muruganandan S, Azzopardi M, Fitzgerald DB, et al. . Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med 2018; 6: 671–680. doi: 10.1016/S2213-2600(18)30288-1 [DOI] [PubMed] [Google Scholar]
- 57.Bhatnagar R, Keenan EK, Morley AJ, et al. . Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med 2018; 378: 1313–1322. doi: 10.1056/NEJMoa1716883 [DOI] [PubMed] [Google Scholar]
- 58.Dipper A, Bhatnagar R, Maskell N. Outpatient talc administration via indwelling pleural catheters for malignant effusions. Curr Opin Pulm Med 2019; 25: 380–383. doi: 10.1097/MCP.0000000000000587 [DOI] [PubMed] [Google Scholar]
- 59.Penz ED, Mishra EK, Davies HE, et al. . Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest 2014; 146: 991–1000. doi: 10.1378/chest.13-2481 [DOI] [PubMed] [Google Scholar]
- 60.Wang S, Zhang R, Wan C, et al. . Incidence of complications from indwelling pleural catheter for pleural effusion: a meta-analysis. Clin Transl Sci 2023; 16: 104–117. doi: 10.1111/cts.13430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilshire CL, Chang SC, Gilbert CR, et al. . Association between tunneled pleural catheter use and infection in patients immunosuppressed from antineoplastic therapy. A multicenter study. Ann Am Thorac Soc 2021; 18: 606–612. doi: 10.1513/AnnalsATS.202007-886OC [DOI] [PubMed] [Google Scholar]
- 62.Gilbert CR, Lee HJ, Akulian JA, et al. . A quality improvement intervention to reduce indwelling tunneled pleural catheter infection rates. Ann Am Thorac Soc 2015; 12: 847–853. doi: 10.1513/AnnalsATS.201411-511OC [DOI] [PubMed] [Google Scholar]
- 63.Mitchell MA, Li P, Pease C, et al. . Catheter tract metastasis in mesothelioma patients with indwelling pleural catheters: a retrospective cohort study. Respiration 2019; 97: 428–435. doi: 10.1159/000494500 [DOI] [PubMed] [Google Scholar]
- 64.Schwalk AJ, Ost DE. Indwelling pleural catheters. Clin Chest Med 2021; 42: 739–750. doi: 10.1016/j.ccm.2021.08.009 [DOI] [PubMed] [Google Scholar]
- 65.Thomas R, Budgeon CA, Kuok YJ, et al. . Catheter tract metastasis associated with indwelling pleural catheters. Chest 2014; 146: 557–562. doi: 10.1378/chest.13-3057 [DOI] [PubMed] [Google Scholar]