Abstract
Snakebite envenoming is a significant global health challenge, and for over a century, traditional plasma-derived antivenoms from hyperimmunized animals have been the primary treatment against this infliction. However, these antivenoms have several inherent limitations, including the risk of causing adverse reactions when administered to patients, batch-to-batch variation, and high production costs. To address these issues and improve treatment outcomes, the development of new types of antivenoms is crucial. During this development, key aspects such as improved clinical efficacy, enhanced safety profiles, and greater affordability should be in focus. To achieve these goals, modern biotechnological methods can be applied to the discovery and development of therapeutic agents that can neutralize medically important toxins from multiple snake species. This review highlights some of these agents, including monoclonal antibodies, nanobodies, and selected small molecules, that can achieve broad toxin neutralization, have favorable safety profiles, and can be produced on a large scale with standardized manufacturing processes. Considering the inherent strengths and limitations related to the pharmacokinetics of these different agents, a combination of them might be beneficial in the development of new types of antivenom products with improved therapeutic properties. While the implementation of new therapies requires time, it is foreseeable that the application of biotechnological advancements represents a promising trajectory toward the development of improved therapies for snakebite envenoming. As research and development continue to advance, these new products could emerge as the mainstay treatment in the future.
Keywords: Antivenom, Next-generation antivenom, Recombinant antivenom, Antivenom development, Snakebite envenoming, Small molecule toxin inhibitors, Toxin-neutralization
Background
Snakebite envenoming represents a persistent and significant global health challenge, with an annual global incidence estimated to be between 1.8 - 2.7 million envenomings, leading to approximately 81,000 - 138,000 fatalities and many more indefinable debilitating consequences [1-3]. Despite the longstanding history of human exposure to snakebite envenomings [2], antivenoms consisting of plasma-derived antibodies (or fragments thereof) from hyperimmunized animals have been the primary treatment option since they were first developed by Albert Calmette, Césaire Phisalix, and Gabriel Bertrand in the late 19th century [4, 5]. The proven efficacy of these antivenoms in preventing fatalities [6] has led to their recognition as essential medicines by the World Health Organization (WHO) [7]. Consequently, antivenoms have been integrated into standard treatment guidelines for snakebite envenoming that are implemented worldwide [8], resulting in serum institutes having set up large-scale production of these products in many countries. Nevertheless, traditional antivenoms come with several drawbacks related to their heterologous nature and production method. In particular, the non-human proteins present in antivenoms (including the antibodies themselves) may trigger immunogenic reactions in snakebite victims, such as serum sickness or anaphylaxis, and many antivenom products suffer from batch-to-batch variation and a relatively low content (and/or unbalanced composition) of therapeutically relevant antibodies [9, 10]. Moreover, although paraspecificity (binding to structural similar antigens/toxins that were not included in the immunization mixture) can occur, antivenoms are typically mostly effective against the venom(s) from the snake species that they have been raised against [11, 12]. Finally, the laborious and low-throughput manufacturing process used to produce antivenoms results in several issues. These include a risk of incorporating impurities of animal origin and the potential for vertical transmission of diseases even after the purification process. Additionally, the relatively high cost of goods that antivenoms have may impose a significant economic burden on snakebite victims and/or healthcare systems in low-income areas with a high incidence of snakebite envenoming [13]. This latter aspect, in particular, is critical for the deployment of antivenoms, as most snakebite victims worldwide are found amongst poor, rural populations [3, 14].
While the clinical management of snakebite envenoming is multifaceted and involves not only medical intervention but also logistics, training, diagnostics, and economic considerations, antivenoms that can neutralize snake venoms remain a cornerstone of modern envenoming therapy [15, 16]. Given the persistent obstacles of traditional antivenoms, there is a need to develop new types of antivenom products that are safer, more effective, and affordable [2, 17]. While antivenom researchers and manufacturers have made strides in optimizing product efficacy, safety, stability, and neutralization capacity across species [18-20], new technological advances now offer an opportunity for rethinking how antivenom products could ideally be developed and manufactured to improve this important type of medicines. In this review, we discuss key aspects that should be considered before engaging in the development of new types of antivenom products, present various molecular formats that might be utilized in these, and mention recent technological advancements that have the potential to improve the development process. Other clinically important aspects surrounding optimal envenoming therapy, including timely and correct administration of appropriate doses, treatment of secondary effects and symptoms, and optimal care and nursing of patients, can be found elsewhere [3, 16].
Requirements for new types of antivenom products
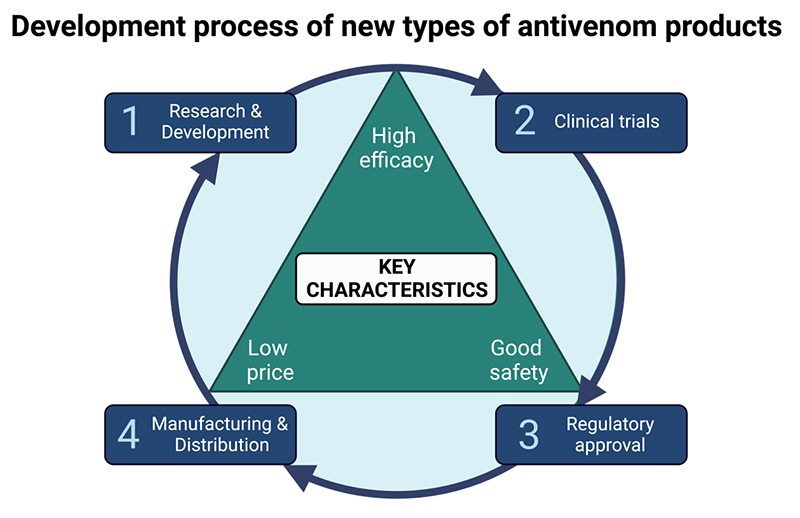
When developing new types of antivenom products, a comprehensive approach spanning from bench to bedside needs to be taken (Figure 1). First and foremost, new types of antivenoms need to demonstrate improved clinical efficacy compared to traditional antivenoms [9, 19]. The efficacy of traditional antivenoms relies on the antibodies generated through the immune response of the production animals against the toxins in the venom(s) used for their immunization [21-23]. However, venoms are complex mixtures containing multiple toxins with varying functions, abundance, toxicity, size, and immunogenicity [19, 24]. As a result, not all medically important toxins in the venom(s) elicit a sufficiently strong immune response in the animals to trigger the production of neutralizing antibodies, consequently limiting the neutralizing capacity of the resulting antivenom(s) against some toxins [25-28]. Therefore, a thorough understanding of snake venom complexity is necessary to help guide the development of an antivenom product that can eliminate the venom from the patient’s body and ideally neutralize all medically relevant toxins [24]. To this end, particularly toxicovenomics, which combines venomics and toxicity studies of individual venom components, can be used to identify the medically most important toxins that require neutralization [29-31]. Having this knowledge provides the opportunity to isolate or recombinantly express and use these toxins as targets in rational drug discovery campaigns to find toxin-neutralizing agents that can be used to formulate new types of antivenom products [32-35].
Figure 1. Key considerations for antivenom development. The development of new types of antivenom products requires careful consideration of key characteristics, including high efficacy, favorable safety profiles, and affordability. These critical aspects should be addressed throughout the product development process, starting from conception and extending to manufacturing. Comprehensive clinical assessments and rigorous approval processes are indispensable to ensure the efficacy and safety of antivenom products. Simultaneously, cost-effectiveness plays a pivotal role in ensuring the accessibility of antivenoms in regions where they are needed. It is thus essential to have the end product in mind when developing new types of antivenom products, as well as to have a holistic overview of the process. The figure was created with BioRender.com.
In addition to being more efficacious in terms of neutralizing capacity, new types of antivenom products should preferably be polyvalent, i.e., be able to neutralize the venoms of multiple snake species, similar to many traditional antivenoms [36]. To minimize the required number of toxin-neutralizing agents needed in an antivenom product while still effectively neutralizing all medically important toxins of several snake species, a carefully designed mixture of broadly neutralizing agents targeting multiple (similar) toxins can be utilized [37-43]. Within antibody research, one strategy to discover such broadly neutralizing agents against multiple toxins involves the use of phage display technology [42-45]. This technology allows antibody fragments to be discovered in vitro against, in principle, any target toxin regardless of its immunogenicity and toxicity [43, 46-48] and can be set up in a way that facilitates the discovery of cross-reactive antibodies capable of binding multiple similar toxins [42, 43, 45]. Another approach for the discovery of cross-reactive neutralizing agents involves the use of recombinantly produced consensus toxins. These artificial toxins are designed to resemble an average sequence of several related toxins and can be used for immunization [49, 50] or as target antigens during phage display-based discovery campaigns [49, 51]. Lastly, cross-reactive, high-affinity neutralizing agents can be discovered or optimized by applying recent advances within the field of artificial intelligence, next-generation sequencing, and machine learning [52, 53]. The combination of these techniques presents an opportunity to either design novel high-affinity neutralizing agents or improve existing neutralizing agents both in terms of affinity and/or cross-reactivity in silico [54], which could help speed up and improve the development process for new types of antivenoms.
Beyond the neutralization of all medically relevant toxins, the clinical efficacy of antivenom products is further influenced by the interplay between their pharmacokinetic properties (which include how they are administered, distributed, metabolized, and eventually eliminated from the body) and the toxicokinetics of venoms [55]. The toxicokinetics of whole venoms are typically complex due to the presence of multiple toxins with varying kinetic properties, which can include fast systemic distribution, rapid deep tissue penetration, slow elimination from the body [56], and the gradual release of toxins from the bite site and into circulation over time (known as the depot effect) [57, 58]. While the use of smaller therapeutic agents might facilitate rapid tissue penetration and organ access, larger molecules tend to remain in circulation for longer periods [59]. Therefore, when selecting the most optimal format(s) for the therapeutic agents in an antivenom product, it is important to consider their inherent pharmacokinetic properties to ensure that these fit the toxicokinetics of the target venom(s). This entails that toxins must be neutralized before their toxic effects become too detrimental to the victim and that the antivenom has a sufficient duration of action to neutralize toxins that may leave the bite site and enter circulation a long time after the bite occurred. To achieve this, a combination of different therapeutic formats in a single antivenom product may be necessary, as no single therapeutic agent is superior across all properties [55].
Alongside efficacy, a good safety profile is essential for new types of antivenom products. Today, due to the risk of severe adverse reactions, traditional antivenoms are typically administered only after the onset of clinical manifestations [60]. This delay in administration allows the injected toxins to further exert their toxic effects, which can lead to patient distress, prolonged hospital stays, and irreversible complications, such as tissue necrosis [61, 62]. Therefore, to achieve timely intervention and mitigate the risk of adverse reactions, the products must be free from contaminations, possess very low immunogenicity, and show no off-target effects, which refer to unintended and undesired interactions between the therapeutic agents in the antivenom and non-target molecules (such as host proteins). To improve the safety profiles, removing the reliance on animal-derived components should be a priority for the development of new types of antivenoms. For this, an opportunity may lie in the utilization of recombinant expression systems (bacteria, yeast, and mammalian cells) to produce protein-based binding molecules [63, 64], or in the chemical synthesis of, for example, small molecule inhibitors [3].
To evaluate the efficacy and safety of antivenoms, a comprehensive preclinical and clinical assessment is essential. For preclinical assessment, the current recommendation by the WHO is to evaluate the ability of antivenoms to neutralize venom-induced lethality in rodent models that involve pre-incubation of venom and antivenom for 30 minutes before being injected [65]. To more closely mimic a real-life snakebite scenario, rescue assays, where the animal is exposed to the toxin or venom before administration of the antivenom, can be conducted. While it is more challenging to perform such assays in a standardized manner, rescue assays enable a more thorough assessment of the therapeutic utility of an antivenom product, including its efficacy, pharmacokinetics, and pharmacodynamics, as well as providing a better insight into the toxicokinetics of the venom [66, 67]. Moreover, conducting supplementary in vitro assays to evaluate the neutralization of venom-induced pathologies, such as hematotoxicity, cytotoxicity, and neurotoxicity, allows for a more comprehensive preclinical assessment beyond lethality alone and can be used to reduce the number of animals needed for experiments [65, 68-72].
After a thorough preclinical evaluation, new antivenom products should undergo a well-designed clinical assessment. Surprisingly, this has not been done for many traditional antivenoms [73] since clinical trials are not mandatory to obtain approval for plasma-derived antivenoms and their use in clinical settings [28]. Ideally, clinical trials for new (and traditional) types of antivenom products should be prospective, comparative, interventional, and well-conducted to confirm safety and efficacy in humans [73-75]. Beyond the design of the clinical trials themselves, a swift and efficient approval process for all types of antivenom products would be beneficial, as this could facilitate timely access to more effective and safer therapeutic alternatives for patients. To accelerate the approval processes, common guidelines between different national regulatory agencies and simplified regulatory pathways, such as a shortened route for biological products, could be implemented [76]. Another approach to expedite the approval process could involve repurposing drugs that have already undergone clinical trials for other indications than snakebite envenoming to potentially bypass the need for some of the early clinical trials (such as phase 1 trials on healthy volunteers) [77].
Lastly, when developing new types of antivenom products, it is important to estimate their final market price, considering that affordability is a key factor for therapies that are to be deployed in low-income regions [13, 78]. To ultimately reduce overall costs related to both development and manufacturing, the development of new types of antivenom products should employ versatile and standardized approaches during the discovery process as well as scalable production technologies during the manufacturing [79]. For example, by applying in vitro display technologies for the discovery of recombinant antibodies or antibody fragments and by employing microbial or mammalian expression systems for large-scale production, an antivenom with lower costs than traditional antivenoms could potentially be manufactured [40, 80]. Alternatively, development costs, especially those related to the discovery of new toxin-neutralizing molecules and running clinical trials, can be reduced by utilizing repurposed medications with established pharmacokinetic properties and safety profiles [81, 82]. Moreover, expanding the market by developing polyvalent antivenom products that can be used across larger regions and against multiple snake species could potentially enable manufacturers to achieve higher production volumes and better economies of scale [13, 83, 84]. Consequently, this may help make antivenom products more affordable for victims and healthcare systems [85], addressing the importance of affordability in regions with limited resources.
Relevant toxin-neutralizing agents for new types of antivenom products
Various therapeutic agents can be considered for the development of effective, safe, and affordable antivenom products, each with their own set of advantages and disadvantages (Figure 2). While polyclonal antibodies, including immunoglobulin G (IgG) antibodies and their fragments, are common therapeutic formats used in traditional antivenoms [4, 5], the conventional method of immunizing animals with whole venom(s) presents challenges in generating antibodies against some specific toxins and results in end products with low therapeutic contents [9, 10]. Consequently, there is an interest in more specific therapies based on common types of antibodies, such as monoclonal IgG antibodies and antibody fragments, which are well-validated classes of therapeutic agents already used for multiple indications, such as autoimmune diseases, cancers, and infections [86-90]. Besides their proven efficacy as therapeutics, recombinantly produced monoclonal IgG antibodies have also been in focus in snakebite envenoming research due to their good safety profiles, long half-lives in circulation (typically around three weeks) [91], and the growing evidence of their effectiveness in neutralizing both specific toxins and whole venom-induced lethality in vivo [38, 43, 92]. By utilizing strategies for the discovery of broadly neutralizing antibodies and combining such antibodies in carefully designed oligoclonal mixtures, an antivenom targeting multiple similar and dissimilar toxins found in the venoms of various snake species can likely be achieved [42-44]. In addition to monoclonal IgG antibodies, which consist of two light-chains and two heavy-chains (Figure 3), smaller antibody fragments comprised of only one domain, called single-domain antibodies (sdAbs), are being investigated for potential use as toxin-neutralizing agents. Among these, there is a particular focus on variable heavy-chain domains (VHHs or nanobodies) [46], which are variable domains derived from heavy-chain-only antibodies naturally found in camelids. VHHs can have comparable toxin-neutralization capacities to IgGs [93] and possess a long flexible loop in their antigen-binding site, which allows them to bind to buried epitopes within protein structures that are typically inaccessible to antibodies [94]. The much smaller size of VHHs (and sdAbs in general) compared to IgGs (15 kDa versus 150 kDa) might enable these smaller fragments to penetrate deep tissue faster than IgGs [95] (Figure 3). Furthermore, VHHs offer the advantages of high solubility and high thermal stability, withstanding temperatures of 60-80 °C, and often have the potential to refold into an active form upon thermally induced denaturation [95, 96].
Figure 2. Different antibody formats that have been investigated for their utility in neutralizing snake toxins. (A) Schematic representations of an immunoglobulin G (IgG) antibody, a heavy-chain-only antibody (HCAb), and a variable heavy-chain domain (VHH). IgG antibodies are composed of four polypeptide chains: two identical heavy chains (H) and two identical light chains (L), forming a flexible Y-shaped structure. Each chain contains a variable (V) region and one or more constant (C) region(s). In contrast, camelids produce a unique type of antibodies, consisting of only heavy chains known as HCAbs. When expressed alone, the variable domains of HCAbs are referred to as VHHs and are notably smaller in size compared to the IgG. (B) The crystal structure of a representative VHH (PDB ID 4PPT). The complementarity-determining regions (CDRs), particularly the long flexible loop of CDR3, are highly variable and crucial for antigen binding. CDRs 1, 2, and 3 are indicated in red, yellow, and blue, respectively. The figure was created with BioRender.com.
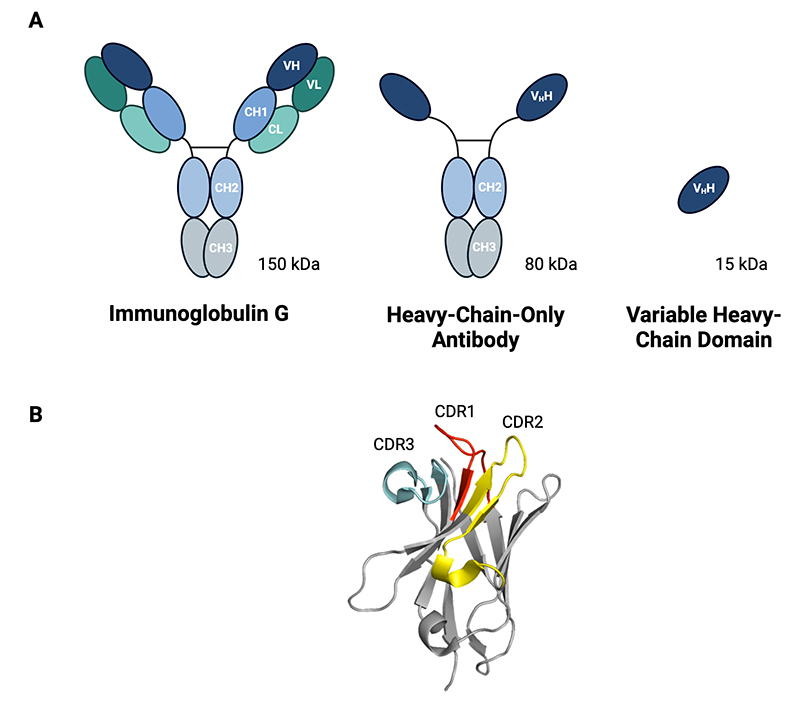
Figure 3. Characteristics of selected therapeutic agents that could potentially be used for the development of new types of antivenom products, including their structures, molecular weights, and important therapeutic properties. The Harvey balls visually illustrate the theoretical favorability of each property, represented by the dark blue area, and based on the professional, yet arguably subjective, expert evaluation by the authors. It is essential to acknowledge that the Harvey balls are meant to capture the general characteristics of each type of molecular scaffold/class and may not apply to specific molecular agents within each class, which could have properties that deviate from the mean. Versatility indicates the ability to neutralize different types of toxins, while engineerability refers to the ease of modifying molecules from the class to achieve specific properties or functions, such as half-life extension. Specificity refers to the ability to selectively interact with a specific target, thereby minimizing off-target effects (unintended interactions that could lead to undesired effects). Half-life represents the time it takes for half of the administered therapeutic agent to be cleared from the body. Tissue penetration denotes the capability of effectively reaching and binding to target toxins in tissues, including deep tissues and various organs. Shelf-life indicates how long an agent maintains its activity under cool storage conditions. Affordability highlights the cost-effectiveness of the therapeutic agent. Safety is deliberately not included, although it is an essential therapeutic property, as this should be evaluated on a case-by-case basis for each specific molecule in a class. The figures were created with BioRender.com, and the structure of Varespladib corresponds to PubChem CID 155815. The size of the molecules is not drawn to scale.
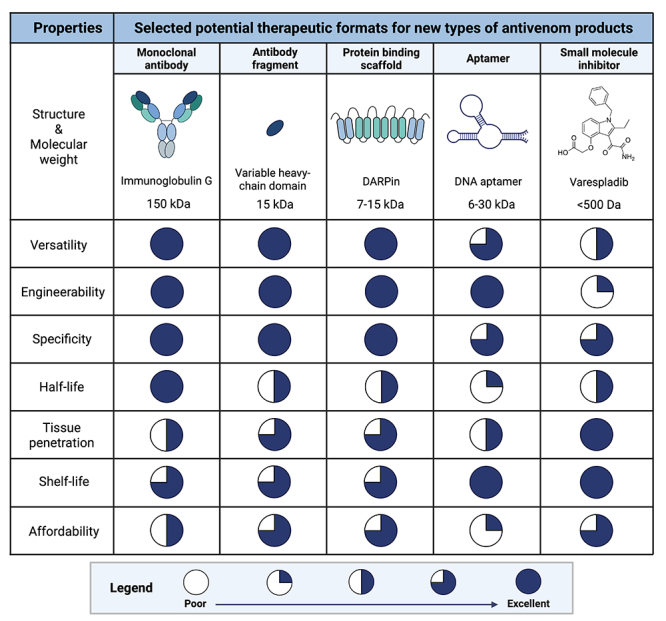
Monoclonal antibody-based therapeutics will likely offer improved safety profiles compared to plasma-derived antibodies as they enable the formulation of an end product with a high therapeutic content, thus allowing for the administration of much lower doses compared to traditional antivenoms. In addition, monoclonal antibodies can be designed to be more compatible with the human immune system than heterologous polyclonal antibodies. This can be achieved by developing them as fully human antibodies, where the entire molecule is of human origin, or as humanized antibodies, where the non-human complementarity-determining regions in the variable domains responsible for antigen binding are incorporated into a human antibody framework. Depending on their animal origin, non-human antibodies or antibody fragments can also possess low immunogenicity even without any engineering, as is the case with VHHs from camelids. The typical low immunogenicity of VHHs partially arises from their much smaller size compared to IgGs, resulting in a lower amount of protein needed for neutralization [97], as well as their high solubility (that makes them less prone to aggregation). Additionally, their high sequence homology to human antibodies often makes humanization unnecessary [98, 99]. To obtain fully human antibodies, in vitro discovery strategies, such as phage display technology [100], or in vivo discovery strategies, including the use of transgenic animals, such as transgenic mice that have been genetically engineered to produce human antibodies, can be used [101].
Monoclonal antibodies can be further engineered to have specific pharmacokinetic properties. One example involves the extension of the half-life of IgGs in circulation through mutations in the crystallizable fragment (Fc) to increase their affinity to the neonatal Fc receptor (FcRn), which protects the antibodies from lysosomal degradation and enhances their recycling properties [102]. Likewise, the binding between the Fc domain and FcRn can be exploited to prolong the inherently short half-life of VHHs in circulation (about 1.5 hours) [103] by fusing the VHHs with a human Fc domain or by making a bivalent construct combining a toxin-binding VHH with an albumin binding VHH, as albumin also binds to FcRn [95, 104-106]. In addition, the half-life of VHHs can be extended by increasing the molecular weight above the renal filtration threshold, for example, by assembling them into multimers [107] or by fusing them with larger molecules that have long half-lives, such as albumin [108-110].
To keep the production cost of monoclonal antibodies low, established and validated large-scale manufacturing setups of widely used monoclonal antibody-based therapeutics can be utilized [111]. Moreover, employing antibody fragments with simple structures, such as sdAbs, can likely further reduce the production cost, as these can be expressed using microbial expression systems, which upon optimization can reach very high titers and high productivity during fermentation [112-115]. Finally, the overall cost of the final antivenom products could potentially be reduced by implementing more stable formats to eliminate the expenses associated with temperature-controlled supply chains during product distribution [116].
Apart from antibodies and fragments thereof, non-antibody-based therapeutic agents are being considered by some research groups for the development of new types of antivenom products [117]. Examples of such molecules are protein binding scaffolds, which are synthetic peptide frameworks, and aptamers, which are short single-stranded oligonucleotides [118, 119]. Similar to antibodies, these molecules allow for the design and discovery of constructs that have high affinity and specificity to a wide spectrum of targets, including toxic and non-immunogenic ones [120, 121]. As an example, recent studies have shown that aptamers can be designed to neutralize toxins from some important snake toxin families, such as phospholipases A2 (PLA2s), neurotoxins, and cytotoxins [122-124]. Similarly, it has been demonstrated that designed ankyrin repeat proteins (DARPins), a specific type of protein binding scaffold that mimics and enhances the functionality of natural ankyrin repeat proteins involved in protein-protein interactions [125], can be used to neutralize toxins [125-127]. This suggests that they can be used for the development of antivenom products [119, 125]. In addition to their demonstrated capabilities of being able to neutralize selected toxins, these non-antibody-based molecules share several similar advantageous characteristics with VHHs, including their high thermal stability [119, 128, 129] and small sizes (7 to 15 kDa for protein binding scaffolds and 6 to 30 kDa for aptamers), which could allow for rapid tissue penetration [119, 121, 130]. Furthermore, both molecules can be manufactured on a large scale [129, 131, 132], although the actual cost of producing large quantities of aptamers remains elusive, and we as authors are skeptical about whether this is on par with the cost of manufacture of antibodies and other proteins [9]. Typically, aptamers can be chemically synthesized from a library of different nucleic acid sequences. Protein scaffolds, such as DARPins, generally do not need mammalian cells for production and can be efficiently expressed in bacterial or fungal expression systems due to the absence of disulfide bonds and lack of required post-translational modifications in these molecules [40, 132]. Regarding safety, clinical data from a few U.S. Food and Drug Administration (FDA)-approved products based on protein binding scaffolds [131, 133-135] and one aptamer product [136, 137] indicate that these types of scaffolds can have a relatively good safety profile and show low immunogenicity, although their general safety remains inconclusive and necessitates further clinical data [121, 138]. Similar to other low molecular weight molecules, both protein binding scaffolds and aptamers carry the drawback of a short circulatory half-life [139]. In addition to this, unmodified aptamers are unstable under physiological conditions due to their general susceptibility to hydrolysis by ubiquitous nucleases in human serum [120], which can quickly shorten their half-life, sometimes even down to the orders of seconds for single-stranded hydroxy nucleic acid or ribonucleic acid aptamers [140]. To extend the half-life of these two types of therapeutic agents, strategies that increase their sizes, such as conjugating them with polyethylene glycol (PEG) or albumin, similar to sdAbs, can be employed [131, 141-146]. However, it is important to consider that modifications made to these molecules may significantly affect their overall manufacturing complexity and cost. This concern is particularly pronounced for aptamers, as the application of such half-life extension technologies would necessitate additional steps in the manufacturing process, including chemical synthesis/conjugation and/or recombinant expression. In some instances, the use of such half-life extension technologies can also compromise the efficacy, pharmacokinetics, and safety profiles of the therapeutic agents [142, 145, 147, 148]. While protein binding scaffolds could potentially find utility in snakebite envenoming therapies, we find it challenging to envision how their benefits exceed those of antibody-based therapeutic agents [101, 149], although we acknowledge that, with improved in vitro discovery approaches, it may one day be possible that protein binding scaffolds become a competitive alternative [150]. In contrast, it seems highly speculative that aptamers will find utility as therapeutics in clinical snakebite management, given the inherent developability liabilities and the number of therapeutic drawbacks of this molecular scaffold [140].
Another therapeutic modality to consider for the development of new types of antivenom products is small molecule inhibitors [37]. These molecules typically function by binding to the active site of enzymatic toxins, which potentially enables them to inhibit an entire class or family of toxins that share similar active sites. In contrast to biological drugs, many small molecule inhibitors are typically not susceptible to enzymatic degradation in the gastrointestinal tract and can often be administered orally (sometimes as prodrugs) [81]. In some settings, this route of delivery can be beneficial since it enables easy administration (if the patient is conscious and not an infant) before hospital arrival, which may allow for an earlier start of treatment for patients living far away from a hospital or clinic. Early administration is particularly crucial when targeting toxins that rapidly distribute in circulation and penetrate deep tissues [77, 151] and toxins that cause irreversible damage [152]. Once administered, some small molecule inhibitors may rapidly distribute in the body and can reach the active sites of enzymes, including those that are typically difficult to access by larger molecules. Because small molecule inhibitors are produced through chemical synthesis and often can be manufactured in large quantities via cost-effective and validated methods, they could be a promising option for the development of more affordable antivenom products [153]. However, it is important to note that different small molecule inhibitors require different manufacturing processes [154], which might make production facilities less versatile compared to those used for the production of standard protein formats, such as antibodies. It is also crucial to highlight that repurposing small molecule inhibitors, which have already gone through clinical development, differs significantly from attempting to discover novel inhibitors. Small molecule drug discovery faces very high attrition rates, where drug candidates frequently fail to advance through subsequent stages of development [155] compared to, for example, standard antibody formats [79, 156].
To date, several of the small molecule inhibitors that have been evaluated for their ability to neutralize snake toxins are repurposed pharmaceuticals that were initially developed for other indications [157, 158]. For instance, Varespladib, originally developed for ulcerative colitis, rheumatoid arthritis, asthma, sepsis, and acute coronary syndrome [159], has been shown to inhibit the toxic effects of snake PLA2s [159, 160]. Similarly, snake venom metalloproteinase inhibitors initially developed for cancer therapy, such as batimastat and marimastat [161-165], and metal ion chelators, such as ethylenediaminetetraacetic acid (EDTA) and 2,3-dimercapto-1-propanesulfonic acid, have shown promise for being repurposed for treatment of snakebite envenoming [84, 166]. Although clinical efficacy data is pending, repurposing these or similar molecules that have already been proven safe in early clinical trials may reduce the development risk and timeline for therapeutic agents entering the clinic [77], as it may circumvent the need to perform clinical phase I safety studies and the need to initiate an entirely new resource-intensive discovery and development process [81].
Although several antibody and non-antibody-based therapeutic agents discussed in this review are still in the early stages of investigation and require further validation of both efficacy and safety, some of them have demonstrated promising results in preclinical studies, indicating their potential to be included in new types of antivenom products [59, 117, 119]. By combining various types of therapeutic agents that comprise different characteristics and target different venom toxins, along with knowledge about venom complexity and toxicokinetics, we believe that it may become feasible to develop new types of antivenom products that can neutralize (all) medically relevant toxins and are tailored to possess favorable pharmacokinetic profiles.
Conclusion
To overcome some of the challenges associated with traditional antivenoms, there is a need to develop new types of antivenom products that possess better efficacy and safety while being affordable at the same time. To achieve this, modern biotechnological methods could be applied in the development of new therapeutic agents with different neutralizing capacities and pharmacokinetic profiles. By combining various therapeutic agents, it is possible to develop new types of antivenom products that are broadly neutralizing, safe, quality-assured, and cost-effective to produce on a large scale using standardized manufacturing platforms. So far, monoclonal antibodies and antibody fragments stand out as the most promising types of versatile molecular scaffolds that possess these attributes. Meanwhile, selected small molecule inhibitors repurposed from other drug development programs may find utility in some cases (against specific toxin families), as their established manufacture and former clinical assessment may fast-track them through the regulatory approval process. However, acknowledging the time-consuming undertaking of developing, evaluating, and obtaining regulatory approval for new antivenom products, traditional antivenoms will likely remain a therapeutic cornerstone within the treatment of snakebites for now. In the meantime, alternative therapeutic options might also see the light of day, such as fortifying existing antivenoms with, e.g., repurposed small molecule inhibitors, monoclonal antibodies, or fragments thereof. Nevertheless, the application of biotechnological innovations and close collaborations between researchers, engineers, clinicians, regulatory agencies, and funders across multiple countries could potentially deliver new types of envenoming therapies to snakebite victims worldwide.
Abbreviations
CDR: Complementarity-determining region; DARPin: Designed ankyrin repeat protein; EDTA: Ethylenediaminetetraacetic acid; Fc: Crystallizable fragment; FcRn: Neonatal Fc receptor; FDA: Food and Drug Administration; IgG: Immunoglobulin G; PLA2: Phospholipase A2; sdAb: Single-domain antibody; VHH: Variable heavy-chain domain; WHO: World Health Organization.
Acknowledgments
Figures in this manuscript were created with BioRender.com.
Footnotes
Availability of data and materials: Not applicable.
Funding: ST is supported by a scholarship from the Anandamahidol Foundation under the Royal Patronage of His Majesty King Bhumibol Adulyadej of Thailand. AHL is supported by a grant from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program [850974], a grant from the Villum Foundation [00025302], and a grant from Wellcome [221702/Z/20/Z].
Ethics approval: Not applicable
Consent for publication: Not applicable.
References
- Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, Saviolli L, Lalloo DG, Silva HJ. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5(11):e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nat Rev Dis Primers. 2017;3(1):1–21. doi: 10.1038/nrdp.2017.79. [DOI] [PubMed] [Google Scholar]
- Warrell DA, Williams DJ. Clinical aspects of snakebite envenoming and its treatment in low-resource settings. Lancet. 2023;401(10385):1382–1398. doi: 10.1016/S0140-6736(23)00002-8. [DOI] [PubMed] [Google Scholar]
- Calmette A. L’immunisation artificelle des animaux contre le venin des serpents et la therapeutic experimentale de morsures venimeuses. C R Soc Biol. 1894;46:120–124. [Google Scholar]
- Pucca MB, Cerni FA, Janke R, Bermúdez-Méndez E, Ledsgaard L, Barbosa JE, Laustsen AH. History of envenoming therapy and current perspectives. Front Immunol. 2019;10:1598. doi: 10.3389/fimmu.2019.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell DA. Snake bite. Lancet. 2010;375(9708):77–88. doi: 10.1016/S0140-6736(09)61754-2. [DOI] [PubMed] [Google Scholar]
- WHO Expert Committee on the Selection of Essential Drugs. World Health Organization . The selection of essential drugs: report of a WHO expert committee. 1977. meeting held in Geneva from 17 to 21 October 1977. [Google Scholar]
- Williams DJ, Faiz MA, Abela-Ridder B, Ainsworth S, Bulfone TC, Nickerson AD, Habib AG, Junghanss T, Fan HW, Turner M, Harrison RA, Warrell DA. Strategy for a globally coordinated response to a priority neglected tropical disease: snakebite envenoming. PLoS Negl Trop Dis. 2019;13(2):e0007059. doi: 10.1371/journal.pntd.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen C, Ledsgaard L, Dehli RI, Ahmadi S, Sørensen CV, Laustsen AH. Engineering and design considerations for next-generation snakebite antivenoms. Toxicon. 2019;167:67–75. doi: 10.1016/j.toxicon.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Segura A, Herrera M, Villalta M, Vargas M, Gutiérrez JM, Leon G. Assessment of snake antivenom purity by comparing physicochemical and immunochemical methods. Biologicals. 2013;41(2):93–97. doi: 10.1016/j.biologicals.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Ledsgaard L, Jenkins TP, Davidsen K, Krause KE, Martos-Esteban A, Engmark M, Andersen MR, Lund O, Laustsen AH. Antibody cross-reactivity in antivenom research. Toxins. 2018;10(10):393. doi: 10.3390/toxins10100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandesha VD, Darshan B, Tejas C, Girish KS, Kempaiah K. A comparative cross-reactivity and paraspecific neutralization study on Hypnale hypnale, Echis carinatus, and Daboia russelii monovalent and therapeutic polyvalent anti-venoms. PLoS Negl Trop Dis. 2022;16(3):e0010292. doi: 10.1371/journal.pntd.0010292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potet J, Beran D, Ray N, Alcoba G, Habib AG, Iliyasu G, Waldmann B, Ralph R, Faiz MA, Monteiro WM, Sachett JAG, di Fabio JL, Cortés MLA, Brown N, Williams D. Access to antivenoms in the developing world: a multidisciplinary analysis. Toxicon X. 2021;12:100086. doi: 10.1016/j.toxcx.2021.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RA, Hargreaves A, Wagstaff SC, Faragher B, Lalloo DG. Snake envenoming: a disease of poverty. PLoS Negl Trop Dis. 2009;3(12):e569. doi: 10.1371/journal.pntd.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen C, Jürgensen JA, Føns S, Haack AM, Friis RUW, Dam SH, Bush SP, White J, Laustsen AH. Snakebite Envenoming Diagnosis and Diagnostics. Front Immunol. 2021;12:661457. doi: 10.3389/fimmu.2021.661457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza M, Knudsen C, Gnanathasan CA, Monteiro W, Lewin MR, Laustsen AH, Habib AG. Clinical management of snakebite envenoming: Future perspectives. Toxicon X. 2021;11:100079. doi: 10.1016/j.toxcx.2021.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DJ, Gutiérrez JM, Calvete JJ, Wuster W, Ratanabanangkoon K, Paiva O, Brown NI, Casewell NR, Harrison RA, Rowley PD, O’Shea M, Jensen SD, Winkel KD, Warrell DA. Ending the drought: new strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J Proteomics. 2011;74(9):1735–1767. doi: 10.1016/j.jprot.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Leon G, Vargas M, Segura A, Herrera M, Villalta M, Sanchez A, Solano G, Gómez A, Sánches M, Estrada R, Gutiérrez JM. Current technology for the industrial manufacture of snake antivenoms. Toxicon. 2018;151:63–73. doi: 10.1016/j.toxicon.2018.06.084. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Méndez E, Fuglsang-Madsen A, Føns S, Lomonte B, Gutiérrez JM, Laustsen AH. Innovative immunization strategies for antivenom development. Toxins. 2018;10(11) doi: 10.3390/toxins10110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez JM, Lomonte B, Sanz L, Calvete JJ, Pla D. Immunological profile of antivenoms: preclinical analysis of the efficacy of a polyspecific antivenom through antivenomics and neutralization assays. J Proteomics. 2014;105:340–350. doi: 10.1016/j.jprot.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Fernández-Quintero ML, Ljungars A, Waibl F, Greiff V, Andersen JT, Gjølberg TT, Jenkins TP, Voldborg BG, Grav LM, Kumar S, Georges G, Kettenberger H, Liedl KR, Tessier PM, McCafferty J, Laustsen AH. Assessing developability early in the discovery process for novel biologics. MAbs. 2023;15(1):2171248. doi: 10.1080/19420862.2023.2171248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunez J, Fernandez J, Lomonte B, Angulo Y, Sanz L, Perez A, Calvete JJ, Gutiérrez JM. Antivenomics of Atropoides mexicanus and Atropoides picadoi snake venoms: Relationship to the neutralization of toxic and enzymatic activities. J Venom Res. 2010;1:8–17. [PMC free article] [PubMed] [Google Scholar]
- Lomonte B, Escolano J, Fernández J, Sanz L, Angulo Y, Gutiérrez JM, Calvete JJ. Snake venomics and antivenomics of the arboreal neotropical pitvipers Bothriechis lateralis and Bothriechis schlegelii. J Proteome Res. 2008;7(6):2445–2457. doi: 10.1021/pr8000139. [DOI] [PubMed] [Google Scholar]
- Casewell NR, Jackson TNW, Laustsen AH, Sunagar K. Causes and consequences of snake venom variation. Trends Pharmacol Sci. 2020;41(8):570–581. doi: 10.1016/j.tips.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi ME, Marini S, Condo SG, Giardina B. Antibodies against small molecules. Ann Ist Super Sanita. 1991;27(1):139–143. [PubMed] [Google Scholar]
- Chan YW, Tan CH, Heh CH, Tan KY. An immunoinformatic approach to assessing the immunogenic capacity of alpha-neurotoxins in elapid snake venoms. Front Pharmacol. 2023;14:1143437. doi: 10.3389/fphar.2023.1143437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery NM, Santana HM, Rego CMA, Lopes JA, Silva MDS, Ferreira AA, Reis VP, Paloschi MV, Serrath SN, Bastos JSF, Silva CP, Magalhães JGS, Cruz LF, Setubal SS, Zuliani JP. Bothrops jararacussu snake venom decreases CD1d, CD83, and CD86 expression on bone marrow-derived dendritic cells. Immunol Lett. 2023;262:7–17. doi: 10.1016/j.imlet.2023.08.003. [DOI] [PubMed] [Google Scholar]
- Ainsworth S, Menzies SK, Casewell NR, Harrison RA. An analysis of preclinical efficacy testing of antivenoms for sub-Saharan Africa: inadequate independent scrutiny and poor-quality reporting are barriers to improving snakebite treatment and management. PLoS Negl Trop Dis. 2020;14(8):e0008579. doi: 10.1371/journal.pntd.0008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CH. Snake Venomics: fundamentals, recent updates, and a look to the next decade. Toxins. 2022;14(4) doi: 10.3390/toxins14040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete JJ, Juarez P, Sanz L. Snake venomics. Strategy and applications. J Mass Spectrom. 2007;42(11):1405–1414. doi: 10.1002/jms.1242. [DOI] [PubMed] [Google Scholar]
- Lomonte B, Calvete JJ. Strategies in 'snake venomics' aiming at an integrative view of compositional, functional, and immunological characteristics of venoms. J Venom Anim Toxins Incl Trop Dis. 2017;23:26. doi: 10.1186/s40409-017-0117-8. Epub 20170428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen AH, Lohse B, Lomonte B, Engmark M, Gutiérrez JM. Selecting key toxins for focused development of elapid snake antivenoms and inhibitors guided by a toxicity score. Toxicon. 2015;104:43–45. doi: 10.1016/j.toxicon.2015.07.334. [DOI] [PubMed] [Google Scholar]
- Ainsworth S, Petras D, Engmark M, Sussmuth RD, Whiteley G, Albulescu LO, Kazandjian TD, Wagstaff SC, Rowley P, Wuster W, Dorrestein PC, Arias AS, Gutiérrez JM, Harrison RA, Casewell NR, Calvete JJ. The medical threat of mamba envenoming in sub-Saharan Africa revealed by genus-wide analysis of venom composition, toxicity and antivenomics profiling of available antivenoms. J Proteomics. 2018;172:173–189. doi: 10.1016/j.jprot.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Rimbault C, Knudsen PD, Damsbo A, Boddum K, Ali H, Hackney CM, Ellgaard L, Bohn MF, Laustsen AH. A single-chain variable fragment selected against a conformational epitope of a recombinantly produced snake toxin using phage display. N Biotechnol. 2023;76:23–32. doi: 10.1016/j.nbt.2023.04.002. [DOI] [PubMed] [Google Scholar]
- Rivera-de-Torre E, Rimbault C, Jenkins TP, Sorensen CV, Damsbo A, Saez NJ, Duhoo Y, Hackney CM, Ellgaard L, Laustsen AH. Strategies for heterologous expression, synthesis, and purification of animal venom toxins. Front Bioeng Biotechnol. 2022;9:811905. doi: 10.3389/fbioe.2021.811905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Regional Office for Africa. Guidelines for the prevention and clinical management of snakebite in Africa. Brazzaville: World Health Organization. Regional Office for Africa; 2010. [Google Scholar]
- Knudsen C, Laustsen AH. Recent advances in next generation snakebite antivenoms. Trop Med Infect Dis. 2018;3(2):Epub 20180415. doi: 10.3390/tropicalmed3020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen AH, Karatt-Vellatt A, Masters EW, Arias AS, Pus U, Knudsen C, Oscoz S, Slavny P, Griffiths DT, Luther AM, Leah RA, Lindholm M, Lomonte B, Gutiérrez JM, McCafferty J. In vivo neutralization of dendrotoxin-mediated neurotoxicity of black mamba venom by oligoclonal human IgG antibodies. Nat Commun. 2018;9(1):3928. doi: 10.1038/s41467-018-06086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albulescu LO, Xie C, Ainsworth S, Alsolaiss J, Crittenden E, Dawson CA, Softley R, Bartlett KE, Harrison RA, Kool J, Casewell NR. A therapeutic combination of two small molecule toxin inhibitors provides broad preclinical efficacy against viper snakebite. Nat Commun. 2020;11(1):6094. doi: 10.1038/s41467-020-19981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TP, Laustsen AH. Cost of manufacturing for recombinant snakebite antivenoms. Front Bioeng Biotechnol. 2020;8:703. doi: 10.3389/fbioe.2020.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen AH. In: Handbook of venoms and toxins of reptiles 2ed. Mackessy SP, editor. Boca Raton: CRC Press; 2021. Antivenom in the age of recombinant DNA technology. [Google Scholar]
- Ahmadi S, Pucca MB, Jürgensen JA, Janke R, Ledsgaard L, Schoof EM, Sorensen CV, Çaliskan F, Laustsen AH. An in vitro methodology for discovering broadly-neutralizing monoclonal antibodies. Sci Rep. 2020;10(1):10765. doi: 10.1038/s41598-020-67654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledsgaard L, Wade J, Jenkins TP, Boddum K, Oganesyan I, Harrison JA, Villar P, Leah RA, Zenobi R, Schoffelen S, Voldborg B, Ljungars A, McCafferty J, Lomonte B, Gutiérrez JM, Laustsen AH, Karrat-Vellatt A. Discovery and optimization of a broadly-neutralizing human monoclonal antibody against long-chain alpha-neurotoxins from snakes. Nat Commun. 2023;14(1):682. doi: 10.1038/s41467-023-36393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen CV, Almeida JR, Bohn MF, Rivera-de-Torre E, Schoffelen S, Voldborg BG, Ljungars A, Vaiyapuri S, Laustsen AH. Discovery of a human monoclonal antibody that cross-neutralizes venom phospholipase A2s from three different snake genera. Toxicon. 2023;234:107307. doi: 10.1016/j.toxicon.2023.107307. [DOI] [PubMed] [Google Scholar]
- Sørensen CV, Ledsgaard L, Wildenauer HHK, Dahl CH, Ebersole TW, Bohn MF, Anne Ljungars, Jenkins TP, Laustsen AH. Cross-reactivity trends when selecting scFv antibodies against snake toxins using a phage display-based cross-panning strategy. Sci Rep. 2023;13(1):10181. doi: 10.1038/s41598-023-37056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard G, Meyers AJ, McLean MD, Arbabi-Ghahroudi M, MacKenzie R, Hall JC. In vivo neutralization of alpha-cobratoxin with high-affinity llama single-domain antibodies (VHHs) and a VHH-Fc antibody. PLoS One. 2013;8(7):e69495. doi: 10.1371/journal.pone.0069495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledsgaard L, Ljungars A, Rimbault C, Sorensen CV, Tulika T, Wade J, Wouters Y, McCafferty J, Laustsen AH. Advances in antibody phage display technology. Drug Discov Today. 2022;27(8):2151–2169. doi: 10.1016/j.drudis.2022.05.002. [DOI] [PubMed] [Google Scholar]
- Prado ND, Pereira SS, da Silva MP, Morais MS, Kayano AM, Moreira-Dill LS, Luiz MB, Zanchi FB, Fuly AL, Huacca MEF, Fernandes CF, Calderon LA, Zuliani JP, Silva LHP, Soares AM, Stabeli RG, Fernandes CFC. Inhibition of the myotoxicity induced by Bothrops jararacussu venom and isolated phospholipases A2 by specific camelid single-domain antibody fragments. PLoS One. 2016;11(3):e0151363. doi: 10.1371/journal.pone.0151363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa G, Olvera F, Archundia IG, Lomonte B, Alagón A, Corzo G. Horse immunization with short-chain consensus α-neurotoxin generates antibodies against broad spectrum of elapid venomous species. Nat Commun. 2019;10(1):3642. doi: 10.1038/s41467-019-11639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa G, Corrales-García LL, Rodriguez-Ruiz X, López-Vera E, Corzo G. Short-chain consensus alpha-neurotoxin: a synthetic 60-mer peptide with generic traits and enhanced immunogenic properties. Amino Acids. 2018;50(7):885–895. doi: 10.1007/s00726-018-2556-0. [DOI] [PubMed] [Google Scholar]
- Rivera-de-Torre E, Lamparidou S, Bohn M, Kazemi SM, Laustsen AH. Discovery of broadly-neutralizing antibodies against brown recluse spider and Gadim scorpion sphingomyelinases using consensus toxins as antigens. bioRxiv. 2023 doi: 10.1101/2023.04.17.537284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Srivastava D, Sahu M, Tiwari S, Ambasta RK, Kumar P. Artificial intelligence to deep learning: machine intelligence approach for drug discovery. Mol Divers. 2021;25(3):1315–1360. doi: 10.1007/s11030-021-10217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Yoon A, Lee S, Kim S, Han J, Chung J. Next-generation sequencing enables the discovery of more diverse positive clones from a phage-displayed antibody library. Exp Mol Med. 2017;49(3):e308-e. doi: 10.1038/emm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen AH, Greiff V, Karatt-Vellatt A, Muyldermans S, Jenkins TP. Animal immunization, in vitro display technologies, and machine learning for antibody discovery. Trends Biotechnol. 2021;39(12):1263–1273. doi: 10.1016/j.tibtech.2021.03.003. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Leon G, Lomonte B. Pharmacokinetic-pharmacodynamic relationships of immunoglobulin therapy for envenomation. Clin Pharmacokinet. 2003;42(8):721–741. doi: 10.2165/00003088-200342080-00002. [DOI] [PubMed] [Google Scholar]
- Sanhajariya S, Duffull SB, Isbister GK. Pharmacokinetics of snake venom. Toxins. 2018;10(2):73. doi: 10.3390/toxins10020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audebert F, Urtizberea M, Sabouraud A, Scherrmann JM, Bon C. Pharmacokinetics of Vipera aspis venom after experimental envenomation in rabbits. J Pharmacol Exp Ther. 1994;268(3):1512–1517. [PubMed] [Google Scholar]
- Paniagua D, Vergara I, Boyer L, Alagón A. In: Snake Venoms. Inagaki H, Vogel C-W, Mukherjee AK, Rahmy TR, Gopalakrishnakone P, editors. Dordrecht; Springer Netherlands: 2017. Role of lymphatic system on snake venom absorption; pp. 453–474. [Google Scholar]
- Laustsen AH, Maria Gutierrez J, Knudsen C, Johansen KH, Bermudez-Mendez E, Cerni FA, Jurgensen JA, Ledsgaard L, Martos-Esteban A, Ohlenschaeger M, Pus U, Andersen MR, Lomont B, Engmark M, Pucca MB. Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon. 2018;146:151–175. doi: 10.1016/j.toxicon.2018.03.004. [DOI] [PubMed] [Google Scholar]
- de Silva HA, Ryan NM, de Silva HJ. Adverse reactions to snake antivenom, and their prevention and treatment. Br J Clin Pharmacol. 2016;81(3):446–452. doi: 10.1111/bcp.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbister GK. Antivenom availability, delays and use in Australia. Toxicon X. 2022;17:100145. doi: 10.1016/j.toxcx.2022.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A, Hlusicka J, Siribaddana N, Waiddyanatha S, Pilapitiya S, Weerawansa P, Lokunarangoda N, Thalgaspitiya S, Siribaddana S, Isbister GK. Time delays in treatment of snakebite patients in rural Sri Lanka and the need for rapid diagnostic tests. PLoS Negl Trop Dis. 2020;14(11):e0008914. doi: 10.1371/journal.pntd.0008914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel A, Hust M, Schirrmann T. Expression of recombinant antibodies. Front Immunol. 2013;4(217) doi: 10.3389/fimmu.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen DC, Krummen L. Recombinant protein expression for therapeutic applications. Curr Opin Biotechnol. 2002;13(2):117–117. doi: 10.1016/s0958-1669(02)00300-2. [DOI] [PubMed] [Google Scholar]
- World Health Organization Annex 5. Guidelines for the production, control and regulation of snake antivenom immunoglobulins Replacement of Annex 2 of WHO Technical Report Series, No. 964. WHO Tech Rep Ser. 2017:197–388. [Google Scholar]
- Knudsen C, Casewell NR, Lomonte B, Gutiérrez JM, Vaiyapuri S, Laustsen AH. Novel snakebite therapeutics must be tested in appropriate rescue models to robustly assess their preclinical efficacy. Toxins. 2020;12(9):528. doi: 10.3390/toxins12090528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen CV, Julián Fernández J, Adams AC, Wildenauer HHK, Schoffelen S, Ledsgaard L. Antibody-dependent enhancement of toxicity of myotoxin II from Bothrops asper. Nat Commun. Forthcoming. 2023 doi: 10.1038/s41467-023-42624-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez JM, Solano G, Pla D, Herrera M, Segura A, Vargas M, Villalta M, Sánchez A, Sanz L, Lomonte B, León G, Calvete JJ. Preclinical evaluation of the efficacy of antivenoms for snakebite envenoming: state-of-the-art and challenges ahead. Toxins. 2017;9(5):163. doi: 10.3390/toxins9050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez JM, Vargas M, Segura A, Herrera M, Villalta M, Solano G, Sánchez A, Herrera C, León G. In vitro tests for assessing the neutralizing ability of snake antivenoms: toward the 3Rs principles. Front Immunol. 2020;11:617429. doi: 10.3389/fimmu.2020.617429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi S, Pachis ST, Kalogeropoulos K, McGeoghan F, Canbay V, Hall SR, Crittenden EP, Dawson CA, Bartlett KE, Gutiérrez JM, Casewell NR, dem Keller UA, Laustsen AH. Proteomics and histological assessment of an organotypic model of human skin following exposure to Naja nigricollis venom. Toxicon. 2022;220:106955. doi: 10.1016/j.toxicon.2022.106955. [DOI] [PubMed] [Google Scholar]
- Ahmadi S, Benard-Valle M, Boddum K, Cardoso FC, King GF, Laustsen AH, Ljungars A. From squid giant axon to automated patch-clamp: electrophysiology in venom and antivenom research. Front Pharmacol. 2023;14:1249336. doi: 10.3389/fphar.2023.1249336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RN, Clare RH, Ledsgaard L, Nys M, Kool J, Laustsen AH, Ulens C, Casewell NR. An in vitro assay to investigate venom neurotoxin activity on muscle-type nicotinic acetylcholine receptor activation and for the discovery of toxin-inhibitory molecules. Biochem Pharmacol. 2023;216:115758. doi: 10.1016/j.bcp.2023.115758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potet J, Smith J, McIver L. Reviewing evidence of the clinical effectiveness of commercially available antivenoms in sub-Saharan Africa identifies the need for a multi-centre, multi-antivenom clinical trial. PLoS Negl Trop Dis. 2019;13(6):e0007551. doi: 10.1371/journal.pntd.0007551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman GA. Drugs, devices, and the FDA: Part 1: An overview of approval processes for drugs. JACC Basic Transl Sci. 2016;1(3):170–179. doi: 10.1016/j.jacbts.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SR. Fundamentals of clinical trial design. J Exp Stroke Transl Med. 2010;3(1):19–27. doi: 10.6030/1939-067x-3.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti L, Breckenridge A, Hoekman J, Leufkens H, Lumpkin M, McAuslane N. Accelerating access to new medicines: current status of facilitated regulatory pathways used by emerging regulatory authorities. J Public Health Policy. 2016;37(3):315–333. doi: 10.1057/jphp.2016.8. [DOI] [PubMed] [Google Scholar]
- Clare RH, Hall SR, Patel RN, Casewell NR. Small molecule drug discovery for neglected tropical snakebite. Trends Pharmacol Sci. 2021;42(5):340–353. doi: 10.1016/j.tips.2021.02.005. [DOI] [PubMed] [Google Scholar]
- Theakston RD, Warrell DA. Crisis in snake antivenom supply for Africa. Lancet. 2000;356(9247):2104. doi: 10.1016/s0140-6736(05)74319-1. [DOI] [PubMed] [Google Scholar]
- Laustsen AH, Dorrestijn N. Integrating engineering, manufacturing, and regulatory considerations in the development of novel antivenoms. Toxins. 2018;10(8):309. doi: 10.3390/toxins10080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HF, Ma J Fau, Winter C, Winter C Fau, Bayer R, Bayer R. Recovery and purification process development for monoclonal antibody production. (1942-0870 (Electronic)) [DOI] [PMC free article] [PubMed]
- Bulfone TC, Samuel SP, Bickler PE, Lewin MR. Developing small molecule therapeutics for the initial and adjunctive treatment of snakebite. J Trop Med. 2018;2018:4320175. doi: 10.1155/2018/4320175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzari U, Fernandes PA, Mukherjee AK. Advances in the therapeutic application of small-Molecule inhibitors and repurposed drugs against snakebite. J Med Chem. 2021;64(19):13938–13979. doi: 10.1021/acs.jmedchem.1c00266. [DOI] [PubMed] [Google Scholar]
- World Health Organization Expert Committee on Biological Standardization . WHO Expert Committee on Biological Standardization, sixty-seventh report. Geneva: World Health Organization; 2017. [Google Scholar]
- Ainsworth S, Slagboom J, Alomran N, Pla D, Alhamdi Y, King SI, Bolton FMS, Gutiérrez JM, Vonk FJ, Toh CH, Calvete JJ, Kool J, Harrison RA, Casewell NR. The paraspecific neutralisation of snake venom induced coagulopathy by antivenoms. Commun Biol. 2018;1:34. doi: 10.1038/s42003-018-0039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner HC, Toor J, Hollingsworth TD, Anderson RM. Economic evaluations of mass drug administration: The importance of economies of scale and scope. Clin Infect Dis. 2018;66(8):1298–1303. doi: 10.1093/cid/cix1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Putte LB, Atkins C, Malaise M, Sany J, Russell AS, van Riel PL, Settas L, Bijlsma JW, Todesco S, Dougados M, Nash P, Emery P, Walter N, Kaul M, Fischkoff S, Kupper H. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63(5):508–516. doi: 10.1136/ard.2003.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- Pirkalkhoran S, Grabowska WR, Kashkoli HH, Mirhassani R, Guiliano D, Dolphin C, Khalili H. Bioengineering of antibody fragments: challenges and opportunities. Bioengineering. 2023;10(2):122. doi: 10.3390/bioengineering10020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Sun Y, Liang X, Gu X, Ning J, Xu Y, Chen S, Pan L. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct Target Ther. 2022;7(1):39. doi: 10.1038/s41392-021-00868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen AH. How can monoclonal antibodies be harnessed against neglected tropical diseases and other infectious diseases? Expert Opin Drug Discov. 2019;14(11):1103–1112. doi: 10.1080/17460441.2019.1646723. [DOI] [PubMed] [Google Scholar]
- Kontermann RE. Half-life extended biotherapeutics. Expert Opin Biol Ther. 2016;16(7):903–915. doi: 10.1517/14712598.2016.1165661. [DOI] [PubMed] [Google Scholar]
- Ledsgaard L, Laustsen AH, Pus U, Wade J, Villar P, Boddum K, Slavny P, Masters EW, Arias AS, Oscoz S, Griffiths DT, Luther AM, Lindholm M, Leah RA, Moller MS, Ali H, McCafferty J, Lomonte B, Gutiérrez JM, Karatt-Vellatt A. In vitro discovery of a human monoclonal antibody that neutralizes lethality of cobra snake venom. MAbs. 2022;14(1):2085536. doi: 10.1080/19420862.2022.2085536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, John TR, Kaiser II. Specificity and binding affinity of an anti-crotoxin combinatorial antibody selected from a phage-displayed library. Biochem Pharmacol. 1995;50(12):1969–1977. doi: 10.1016/0006-2952(95)02095-0. [DOI] [PubMed] [Google Scholar]
- Desmyter A, Transue TR, Ghahroudi MA, Thi MH, Poortmans F, Hamers R, Muyldermans S, Wyns L. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat Struct Biol. 1996;3(9):803–811. doi: 10.1038/nsb0996-803. [DOI] [PubMed] [Google Scholar]
- Bannas P, Hambach J, Koch-Nolte F. Nanobodies and nanobody-based human heavy chain antibodies as antitumor therapeutics. Front Immunol. 2017;8:1603. doi: 10.3389/fimmu.2017.01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- Ljungars A, Laustsen AH. Neutralization capacity of recombinant antivenoms based on monoclonal antibodies and nanobodies. Toxicon. 2023;222:106991. doi: 10.1016/j.toxicon.2022.106991. [DOI] [PubMed] [Google Scholar]
- Rossotti MA, Belanger K, Henry KA, Tanha J. Immunogenicity and humanization of single-domain antibodies. FEBS J. 2022;289(14):4304–4327. doi: 10.1111/febs.15809. [DOI] [PubMed] [Google Scholar]
- Klarenbeek A, El Mazouari K, Desmyter A, Blanchetot C, Hultberg A, de Jonge N, Roovers RC, Cambillau C, Spinelli S, Del-Favero J, Verrips T, Haard HJ, Achour I. Camelid Ig V genes reveal significant human homology not seen in therapeutic target genes, providing for a powerful therapeutic antibody platform. MAbs. 2015;7(4):693–706. doi: 10.1080/19420862.2015.1046648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaleh MA, Alsaab HO, Mahmoud AB, Alkayyal AA, Jones ML, Mahler SM, Hashem AM. Phage display derived monoclonal antibodies: from bench to bedside. Front Immunol. 2020;11(1986) doi: 10.3389/fimmu.2020.01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, Wu HC. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1. doi: 10.1186/s12929-019-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Ishii-Watabe A, Tada M, Kobayashi T, Kanayasu-Toyoda T, Kawanishi T, Yamaguchi T. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol. 2010;184(4):1968–1976. doi: 10.4049/jimmunol.0903296. [DOI] [PubMed] [Google Scholar]
- Cortez-Retamozo V, Lauwereys M, Hassanzadeh Gh G, Gobert M, Conrath K, Muyldermans S, De Baetselier P, Revets H. Efficient tumor targeting by single-domain antibody fragments of camels. Int J Cancer. 2002;98(3):456–462. doi: 10.1002/ijc.10212. [DOI] [PubMed] [Google Scholar]
- Wu Y, Jiang S, Ying T. Single-domain antibodies as therapeutics against human viral diseases. Front Immunol. 2017;8:1802. doi: 10.3389/fimmu.2017.01802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CJ, Sette A, Cox C, Di Fiore B, Wyre C, Sydoruk D, Yadin D, Hayes P, Stelter S, Bartlett PD, Zuazo M, Garcia-Granda MJ, Beneditti G, Fiaska S, Birkett NR, Teng Y, Enever C, Arasanz H, Bocanegra A, Chocarro L, Fernandez G, Vera R, Archer B, Osuch I, Pierce AJ. The multi-specific VH-based Humabody CB213 co-targets PD1 and LAG3 on T cells to promote anti-tumour activity. Br J Cancer. 2022;126(8):1168–1177. doi: 10.1038/s41416-021-01684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury C, Brooks CL, Carter DC, Robinson JM, Anderson CL. Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry. 2006;45(15):4983–4990. doi: 10.1021/bi052628y. [DOI] [PubMed] [Google Scholar]
- Wade J, Rimbault C, Ali H, Ledsgaard L, Rivera-de-Torre E, Abou Hachem M, Boddum K, Mirza N, Bohn MF, Sakya SA, Ruso-Julve F, Andersen JT, Laustsen AH. Generation of multivalent nanobody-based proteins with improved neutralization of long alpha-neurotoxins from elapid snakes. Bioconjug Chem. 2022;33(8):1494–1504. doi: 10.1021/acs.bioconjchem.2c00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Faassen H, Ryan S, Henry KA, Raphael S, Yang Q, Rossotti MA, Brunette E, Jiang S, Haqqani AS, Sulea T, Mackenzie CR, Tanha J, Hussack G. Serum albumin-binding VHHs with variable pH sensitivities enable tailored half-life extension of biologics. FASEB J. 2020;34(6):8155–8171. doi: 10.1096/fj.201903231R. [DOI] [PubMed] [Google Scholar]
- Mandrup OA, Ong SC, Lykkemark S, Dinesen A, Rudnik-Jansen I, Dagnæs-Hansen NF, Andersen JT, Alvarez-Vallina L, Howard KA. Programmable half-life and anti-tumour effects of bispecific T-cell engager-albumin fusions with tuned FcRn affinity. Commun Biol. 2021;4(1):310. doi: 10.1038/s42003-021-01790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijink BM, Laeremans T, Budde M, Stigter-van Walsum M, Dreier T, de Haard HJ, Leemans CR, van Dongen GAMS. Improved tumor targeting of anti-epidermal growth factor receptor nanobodies through albumin binding: taking advantage of modular nanobody technology. Mol Cancer Ther. 2008;7(8):2288–2297. doi: 10.1158/1535-7163.MCT-07-2384. [DOI] [PubMed] [Google Scholar]
- Saylor C, Dadachova E, Casadevall A. Monoclonal antibody-based therapies for microbial diseases. Vaccine. 2009;27:G38–G46. doi: 10.1016/j.vaccine.2009.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaadi Y, Jouneghani FF, Janani S, Rahbarizadeh F. A comprehensive comparison between camelid nanobodies and single chain variable fragments. Biomark Res. 2021;9(1):87. doi: 10.1186/s40364-021-00332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbabi-Ghahroudi M, Tanha J, MacKenzie R. Prokaryotic expression of antibodies. Cancer Metastasis Rev. 2005;24(4):501–519. doi: 10.1007/s10555-005-6193-1. [DOI] [PubMed] [Google Scholar]
- Jovčevska I, Muyldermans S. The therapeutic potential of nanobodies. Biodrugs. 2020;34(1):11–26. doi: 10.1007/s40259-019-00392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marco A. Recombinant expression of nanobodies and nanobody-derived immunoreagents. Protein Expr Purif. 2020;172:105645. doi: 10.1016/j.pep.2020.105645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin M, Conrath K, Van Meirhaeghe A, Meersman F, Heremans K, Frenken LG, Muyldermans S, Wyns L, Matagne A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002;11(3):500–515. doi: 10.1110/ps.34602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoët S, De Waard M. Diagnostic and therapeutic value of aptamers in envenomation cases. Int J Mol Sci. 2020;21(10):3565. doi: 10.3390/ijms21103565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhin AV, Tarantul VZ, Gening LV. Aptamers: problems, solutions, and prospects. Acta Naturae. 2013;5(4):34–43. [PMC free article] [PubMed] [Google Scholar]
- Jenkins TP, Fryer T, Dehli RI, Jurgensen JA, Fuglsang-Madsen A, Fons S, Laustsen AH. Toxin neutralization using alternative binding proteins. Toxins. 2019;11(1):53. doi: 10.3390/toxins11010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9(7):537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017;16(3):181–202. doi: 10.1038/nrd.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi A, Doley R. Neutralization of Daboxin P activities by rationally designed aptamers. Toxicon. 2021;203:93–103. doi: 10.1016/j.toxicon.2021.09.026. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Tsai CY, Hu WP, Chang LS. DNA aptamers against Taiwan banded krait alpha-bungarotoxin recognize Taiwan cobra cardiotoxins. Toxins. 2016;8(3):66. doi: 10.3390/toxins8030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alomran N, Chinnappan R, Alsolaiss J, Casewell NR, Zourob M. Exploring the utility of ssDNA aptamers directed against snake venom toxins as new therapeutics for snakebite envenoming. Toxins. 2022;14(7):469. doi: 10.3390/toxins14070469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plückthun A. Designed ankyrin repeat proteins (DARPins): Binding proteins for research, diagnostics, and therapy. Annu Rev Pharmacol Toxicol. 2015;55:489–511. doi: 10.1146/annurev-pharmtox-010611-134654. [DOI] [PubMed] [Google Scholar]
- Peng Z, Simeon R, Mitchell SB, Zhang J, Feng H, Chen Z. Designed ankyrin repeat protein (DARPin) neutralizers of TcdB from Clostridium difficile ribotype 027. mSphere. 2019;4(5):e00596-19. doi: 10.1128/mSphere.00596-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeon R, Jiang M, Chamoun-Emanuelli AM, Yu H, Zhang Y, Meng R, Peng Z, Jakana J, Zhang J, Feng H, Chen Z. Selection and characterization of ultrahigh potency designed ankyrin repeat protein inhibitors of C. difficile toxin B. PLoS Biol. 2019;17(6):e3000311. doi: 10.1371/journal.pbio.3000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipunova VO, Kolesnikova OA, Kotelnikova PA, Soloviev VD, Popov AA, Proshkina GM, Nikitin MP, Deyev SM. Comparative evaluation of engineered polypeptide scaffolds in HER2-targeting magnetic nanocarrier delivery. ACS Omega. 2021;6(24):16000–16008. doi: 10.1021/acsomega.1c01811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscito A, DeRosa MC. Small-molecule binding aptamers: selection strategies, characterization, and applications. Front Chem. 2016;4:14. doi: 10.3389/fchem.2016.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer M, Skerra A. Engineered protein scaffolds as next-generation antibody therapeutics. Curr Opin Chem Biol. 2009;13(3):245–255. doi: 10.1016/j.cbpa.2009.04.627. [DOI] [PubMed] [Google Scholar]
- Vazquez-Lombardi R, Phan TG, Zimmermann C, Lowe D, Jermutus L, Christ D. Challenges and opportunities for non-antibody scaffold drugs. Drug Discov Today. 2015;20(10):1271–1283. doi: 10.1016/j.drudis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Škrlec K, Štrukelj B, Berlec A. Non-immunoglobulin scaffolds: a focus on their targets. Trends Biotechnol. 2015;33(7):408–418. doi: 10.1016/j.tibtech.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Simeon R, Chen Z. In vitro-engineered non-antibody protein therapeutics. Protein Cell. 2018;9(1):3–14. doi: 10.1007/s13238-017-0386-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Yang YP, Dikici E, Deo SK, Daunert S. Beyond antibodies as binding partners: the role of antibody mimetics in bioanalysis. Annu Rev Anal Chem (Palo Alto Calif) 2017;10(1):293–320. doi: 10.1146/annurev-anchem-061516-045205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosse RJ, Rothe A, Power BE. A new generation of protein display scaffolds for molecular recognition. Protein Sci. 2006;15(1):14–27. doi: 10.1110/ps.051817606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimjee SM, Sullenger BA. Therapeutic aptamers: evolving to find their clinical niche. Curr Med Chem. 2020;27(25):4181–4193. doi: 10.2174/0929867326666191001125101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic KD, Gilbert JC, Jilma B. Pharmacokinetics, pharmacodynamics and safety of aptamers. Adv Drug Del Rev. 2018;134:36–50. doi: 10.1016/j.addr.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Xiao X, Li H, Zhao L, Zhang Y, Liu Z. Oligonucleotide aptamers: recent advances in their screening, molecular conformation and therapeutic applications. Biomed Pharmacother. 2021;143:112232. doi: 10.1016/j.biopha.2021.112232. [DOI] [PubMed] [Google Scholar]
- Li Z, Krippendorff BF, Sharma S, Walz AC, Lave T, Shah DK. Influence of molecular size on tissue distribution of antibody fragments. MAbs. 2016;8(1):113–119. doi: 10.1080/19420862.2015.1111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RR, Sullenger BA, Rusconi CP. Developing aptamers into therapeutics. J Clin Invest. 2000;106(8):929–934. doi: 10.1172/JCI11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasut G, Veronese FM. State of the art in PEGylation: the great versatility achieved after forty years of research. J Control Release. 2012;161(2):461–472. doi: 10.1016/j.jconrel.2011.10.037. [DOI] [PubMed] [Google Scholar]
- Ebrahimi SB, Samanta D. Engineering protein-based therapeutics through structural and chemical design. Nat Commun. 2023;14(1):2411. doi: 10.1038/s41467-023-38039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni S, Zhuo Z, Pan Y, Yu Y, Li F, Liu J, Wang L, Wu X, Li D, Wan Y, Zhang L, Yang Z, Zhang BT, Lu A, Zhang G. Recent progress in aptamer discoveries and modifications for therapeutic applications. ACS Appl Mater Interfaces. 2021;13(8):9500–9519. doi: 10.1021/acsami.0c05750. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Chan DWH, Ma Y, Lu A, Yu S, Zhang B, Zhang G. Strategies for developing long-lasting therapeutic nucleic acid aptamer targeting circulating protein: the present and the future. Front Cell Dev Biol. 2022;10:1048148. doi: 10.3389/fcell.2022.1048148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekladious I, Colson YL, Grinstaff MW. Polymer-drug conjugate therapeutics: advances, insights and prospects. Nat Rev Drug Discov. 2019;18(4):273–294. doi: 10.1038/s41573-018-0005-0. [DOI] [PubMed] [Google Scholar]
- Zaman R, Islam RA, Ibnat N, Othman I, Zaini A, Lee CY, Chowdhury EH. Current strategies in extending half-lives of therapeutic proteins. J Control Release. 2019;301:176–189. doi: 10.1016/j.jconrel.2019.02.016. [DOI] [PubMed] [Google Scholar]
- Swayze EE, Siwkowski AM, Wancewicz EV, Migawa MT, Wyrzykiewicz TK, Hung G, Monia BP, Bennett CF. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007;35(2):687–700. doi: 10.1093/nar/gkl1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoff AM, Mehran R, Povsic TJ, Zelenkofske SL, Huang Z, Armstrong PW, Steg PG, Bode C, Cohen MG, Buller C, Laanmets P, Valgimigli M, Marandi T, Fridrich V, Cantor WJ, Merkely B, Lopez-Sendon J, Cornel JH, Kasprzak JD, Aschermann M, Guetta V, Morais J, Sinnaeve PR, Huber K, Stables R, Sellers MA, Borgman M. Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention (REGULATE-PCI): a randomised clinical trial. Lancet. 2016;387(10016):349–356. doi: 10.1016/S0140-6736(15)00515-2. [DOI] [PubMed] [Google Scholar]
- Kaplon H, Crescioli S, Chenoweth A, Visweswaraiah J, Reichert JM. Antibodies to watch in 2023. MAbs. 2023;15(1):2153410. doi: 10.1080/19420862.2022.2153410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA. Exploring alternative antibody scaffolds: Antibody fragments and antibody mimics for targeted drug delivery. Drug Discov Today Technol. 2018;30:35–46. doi: 10.1016/j.ddtec.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Kini RM, Sidhu SS, Laustsen AH. Biosynthetic oligoclonal antivenom (BOA) for snakebite and next-generation treatments for snakebite victims. Toxins. 2018;10(12) doi: 10.3390/toxins10120534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven RH, Sean AR, Edouard C, Charlotte AD, Keirah EB, Adam PW, Laura-Oana A, Jeroen K, Gutiérrez JM, Casewell NR. Repurposed drugs and their combinations prevent morbidity-inducing dermonecrosis caused by diverse cytotoxic snake venoms. bioRxiv. 2022 doi: 10.1101/2022.05.20.492855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Albulescu LO, Bittenbinder MA, Somsen GW, Vonk FJ, Casewell NR, Kool J. Neutralizing effects of small molecule inhibitors and metal chelators on coagulopathic viperinae snake venom toxins. Biomedicines. 2020;8(9):297. doi: 10.3390/biomedicines8090297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JW, Blair DJ, Burke MD. Toward generalization of iterative small molecule synthesis. Nat Rev Chem. 2018;2(2):0115. doi: 10.1038/s41570-018-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammolli F, Righetto L, Abrignani S, Pani L, Pelicci PG, Rabosio E. The endless frontier? The recent increase of R&D productivity in pharmaceuticals. J Transl Med. 2020;18(1):162. doi: 10.1186/s12967-020-02313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9(3):203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, Kastelein JJ, Schwartz GG, Bash D, Rosenson RS, Cavender MA. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311(3):252–262. doi: 10.1001/jama.2013.282836. [DOI] [PubMed] [Google Scholar]
- Winer A, Adams S, Mignatti P. Matrix metalloproteinase inhibitors in cancer therapy: Turning past failures into future successes. Mol Cancer Ther. 2018;17(6):1147–1155. doi: 10.1158/1535-7163.MCT-17-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin MR, Carter RW, Matteo IA, Samuel SP, Rao S, Fry BG, Bickler PE. Varespladib in the treatment of snakebite envenoming: development history and preclinical evidence supporting advancement to clinical trials in patients bitten by venomous snakes. Toxins. 2022;14(11):783. doi: 10.3390/toxins14110783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M, Samuel S, Merkel J, Bickler P. Varespladib (LY315920) appears to be a potent, broad-spectrum, inhibitor of snake venom phospholipase A2 and a possible pre-referral treatment for envenomation. Toxins. 2016;8(9):248. doi: 10.3390/toxins8090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layfield HJ, Williams HF, Ravishankar D, Mehmi A, Sonavane M, Salim A, Vaiyapuri R, Lakshminarayanan K, Vallance TM, Bicknell AB, Trim SA, Patel K, Vaiyapuri S. Repurposing cancer drugs batimastat and marimastat to inhibit the activity of a group I metalloprotease from the venom of the western diamondback rattlesnake, Crotalus atrox. Toxins. 2020;12(5):309. doi: 10.3390/toxins12050309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante T, Franceschi A, Rucavado A, Gutierrez JM. Effectiveness of batimastat, a synthetic inhibitor of matrix metalloproteinases, in neutralizing local tissue damage induced by BaP1, a hemorrhagic metalloproteinase from the venom of the snake Bothrops asper. Biochem Pharmacol. 2000;60(2):269–274. doi: 10.1016/s0006-2952(00)00302-6. [DOI] [PubMed] [Google Scholar]
- Bottomley KM, Johnson WH, Walter DS. Matrix metalloproteinase inhibitors in arthritis. J Enzyme Inhib. 1998;13(2):79–101. doi: 10.3109/14756369809035829. [DOI] [PubMed] [Google Scholar]
- Arias AS, Rucavado A, Gutiérrez JM. Peptidomimetic hydroxamate metalloproteinase inhibitors abrogate local and systemic toxicity induced by Echis ocellatus (saw-scaled) snake venom. Toxicon. 2017;132:40–49. doi: 10.1016/j.toxicon.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93(3):178–193. doi: 10.1093/jnci/93.3.178. [DOI] [PubMed] [Google Scholar]
- Albulescu LO, Hale MS, Ainsworth S, Alsolaiss J, Crittenden E, Calvete JJ, Evans C, Wilkinson MC, Harrison RA, Kool J, Casewell NR. Preclinical validation of a repurposed metal chelator as an early-intervention therapeutic for hemotoxic snakebite. Sci Transl Med. 2020;12(542):eaay8314. doi: 10.1126/scitranslmed.aay8314. [DOI] [PMC free article] [PubMed] [Google Scholar]


