Abstract
PURPOSE
Human epidermal growth factor receptor 3 (HER3) is broadly expressed in breast cancer; high expression is associated with an adverse prognosis. Patritumab deruxtecan (HER3-DXd) is an investigational HER3-targeted antibody-drug conjugate that is being evaluated as a novel treatment in HER3-expressing advanced breast cancer in the U31402-A-J101 study.
METHODS
Adults with disease progression on previous therapies were eligible. Patients in the dose-escalation, dose-finding, and dose-expansion parts received HER3-DXd 1.6-8.0 mg/kg intravenously once every 3 weeks or one of two alternative dosing regimens. In the dose-escalation part, the primary objectives were to determine the maximum tolerated dose and recommended dose for expansion (RDE). The safety and efficacy of the RDE were assessed during dose expansion.
RESULTS
One hundred eighty-two enrolled patients received ≥1 dose of HER3-DXd. Patients had a median of five previous therapies for advanced disease. Efficacy results are reported across clinical subtypes: hormone receptor–positive (HR+)/human epidermal growth factor receptor 2–negative (HER2-negative) breast cancer (n = 113; objective response rate [ORR], 30.1%; median progression-free survival [mPFS], 7.4 months), triple-negative breast cancer (n = 53; ORR, 22.6%; mPFS, 5.5 months), and HER2-positive breast cancer (n = 14; ORR, 42.9%; mPFS, 11.0 months). Objective responses were observed in cancers with HER3-high and HER3-low membrane expression. Dose-limiting toxicities observed during dose selection were decreased platelet count and elevated aminotransferases. In dose expansion, GI and hematologic toxicities were the most common treatment-emergent adverse events (TEAEs) observed. Grade ≥3 TEAEs were observed in 71.4% of patients, and 9.9% discontinued treatment because of TEAEs. Three grade 3 and one grade 5 treatment-related interstitial lung disease events occurred.
CONCLUSION
HER3-DXd demonstrated a manageable safety profile and durable efficacy in heavily pretreated patients across clinical subtypes. These data warrant further evaluation of HER3-DXd in patients with HER3-expressing metastatic breast cancer.
INTRODUCTION
Human epidermal growth factor receptor 3 (HER3) is highly expressed in breast cancer and other solid tumors,1,2 and high expression is associated with poor prognoses.3-5 Currently, no HER3-directed treatments have been approved for the treatment of any cancer.
CONTEXT
Key Objective
This study aims to evaluate the tolerability and efficacy of an investigational human epidermal growth factor receptor 3 (HER3)–targeted antibody-drug conjugate (ADC; HER3-DXd) in HER3-expressing breast cancer and represents the first comprehensive analysis of HER3-DXd in patients with metastatic breast cancer.
Knowledge Generated
These findings identify a dose range of HER3-DXd with a favorable risk/benefit profile in heavily pretreated patients with poor prognostic features. In addition, these results demonstrate durable antitumor activity and a manageable safety profile across breast cancer subtypes and establish HER3 as a clinical target in breast cancer.
Relevance (K.D. Miller)
-
This is the first study to clearly demonstrate efficacy with a HER3-targeted therapeutic. Whether prior treatment with other ADCs reduces activity is a critical clinical question not addressed in this initial study.*
*Relevance section written by JCO Senior Deputy Editor Kathy D. Miller, MD.
Patritumab deruxtecan (U3-1402; HER3-DXd) is a novel, first-in-class, HER3-directed antibody-drug conjugate (ADC) that is composed of a human immunoglobulin G1 monoclonal antibody to HER3 (patritumab) covalently bonded to an exatecan-derived topoisomerase I inhibitor payload via a stable, tetrapeptide-based, tumor-selective, cleavable linker.6-9 In HER3-expressing xenograft models, HER3-DXd demonstrated antitumor responses across a range of baseline HER3 expression levels10,11 that were sustained over time.11 Moreover, in vitro studies have shown that HER3-DXd inhibits the proliferation of chemotherapy-resistant breast cancer cell lines with upregulated baseline HER3 expression.12
U31402-A-J101 (ClinicalTrials.gov identifier: NCT02980341) is assessing the safety and efficacy of HER3-DXd in patients with HER3-expressing advanced breast cancer. Efficacy and translational findings on the basis of clinical subtypes (hormone receptor–positive [HR+]/human epidermal growth factor receptor 2–negative [HER2–] breast cancer, triple-negative breast cancer [TNBC], and HER2-positive [HER2+] breast cancer) along with safety results by dose for all enrolled patients are reported herein.
METHODS
Trial Design and Patients
This is a global, open-label, three-part, phase I/II trial of HER3-DXd in HER3-expressing advanced breast cancer. Patients were enrolled at centers in the United States and Japan (Data Supplement, Fig S1, online only). In the dose-escalation part, patients received HER3-DXd 1.6, 3.2, 4.8, 6.4, or 8.0 mg/kg once every 3 weeks. The primary objectives were to assess tolerability and determine the maximum tolerated dose. In the dose-finding part, alternative uptitration dosing regimens were evaluated to determine if tolerability might be enhanced. Patients were treated with either 3.2 mg/kg during the first cycle, 4.8 mg/kg during the second cycle, and 6.4 mg/kg once every 3 weeks thereafter or 4.2 mg/kg once every 2 weeks for three cycles followed by 6.4 mg/kg once every 3 weeks for subsequent cycles. The primary objectives were to determine the recommended dose for expansion (RDE) and the safety and efficacy of the alternative dosing regimens. On the basis of these findings, the fixed-dose regimens of 4.8 mg/kg and 6.4 mg/kg once every 3 weeks were selected for dose expansion and evaluated for safety and efficacy; the alternative dosing regimens were not further evaluated. The dose-escalation and dose-finding parts included patients with HER3-high–expressing breast cancer of any clinical subtype. The dose-expansion part included patients with HER3-high– or HER3-low–expressing HR+/HER2– breast cancer or HER3-high–expressing TNBC. A full description of the dosing procedures and study end points is provided in the Data Supplement (Methods). The study was conducted in accordance with the Protocol (online only), the ethical principles of the Declaration of Helsinki, the International Council for Harmonization consolidated Guideline E6 for Good Clinical Practice, and local regulatory requirements. It was approved by the institutional review boards at the participating clinical centers. All patients provided written informed consent.
The study enrolled patients with advanced breast cancer who were intolerant of or whose disease was refractory to standard treatment. Patients must have had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, and those with stable brain metastases were permitted. Previous treatment with an anti-HER3 antibody or an ADC containing a topoisomerase I inhibitor was not permitted. Patients in the dose-finding part and patients with HR+/HER2– breast cancer in the dose-expansion part must have received ≥2 and ≤6 previous chemotherapeutic regimens for breast cancer, including ≥2 for advanced disease with ≥1 previous taxane-containing regimens. Patients with TNBC in the dose-expansion part must have had disease progression after one or two previous chemotherapeutic regimens for advanced breast cancer. A full list of inclusion/exclusion criteria is provided in the Data Supplement (Methods).
HER3 Expression and Eligibility
In the dose-escalation and dose-finding parts, HER3 expression was assessed using immunohistochemistry (IHC) testing and only patients with HER3-high membrane expression were included. HER3-high was defined as an IHC score of 2+ or 3+, and HER3-low was defined as an IHC score of 1+.
Analysis of HER3 expression transitioned to assessment of HER3 membrane expression at 10x magnification and reported from 0% to 100% during the dose-finding and dose-expansion parts. HER3-high was set at a membrane positivity of ≥75%, and HER3-low was set at ≥25% to <75% at 10x magnification. HR+/HER2− tumors must have had HER3-high or HER3-low membrane expression; TNBC tumors must have had HER3-high membrane expression as trial eligibility criteria. Only HER3-high tumors were included in the dose-finding part. A full description of these methods is included in the Data Supplement.
Efficacy, Safety, and Pharmacokinetic Assessments
Tumor responses were evaluated by blinded independent central review (BICR), and separately by the investigator, using RECIST version 1.1. Safety was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. An independent interstitial lung disease (ILD) adjudication committee reviewed all cases of potential ILD/pneumonitis. Pharmacokinetics (PK) was evaluated in patients from all cohorts. A full description of PK assessments is provided in the Data Supplement (Methods).
Statistical Analysis
All statistical analyses were descriptive, with no formal hypothesis testing or formal success criteria. Data were summarized by protocol-defined cohorts in dose-escalation, dose-finding, and dose-expansion parts. Efficacy data were also summarized by clinical subtype in patients treated with a fixed dose of HER3-DXd 1.6, 3.2, 4.8, 6.4, or 8.0 mg/kg once every 3 weeks or either alternative dosing regimen (Data Supplement, Fig S2). Efficacy end points included objective response rate (ORR; confirmed complete responses + partial responses), duration of response (DOR), disease control rate (DCR; confirmed complete responses + partial responses + stable disease), clinical benefit rate (confirmed complete responses + partial responses + stable disease for ≥6 months from the first dose), progression-free survival (PFS), overall survival (OS), and percentage change in the target lesion. A full description of the statistical analyses is provided in the Data Supplement (Methods).
RESULTS
Between December 2016 and August 2021, 235 patients were screened for eligibility and 182 patients were enrolled across dose cohorts (HR+/HER2– [n = 113]; TNBC [n = 53]; HER2+ [n = 14]; and unknown subtype [n = 2]; Data Supplement, Fig S2).
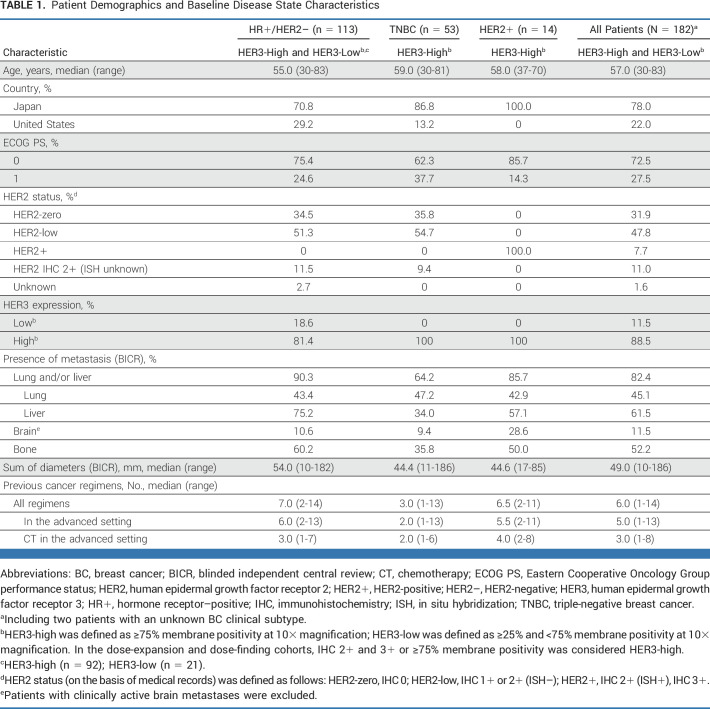
Patient Demographics and Patient Disposition
The median age of patients was 57 years (range, 30-83 years; Table 1; Data Supplement, Table S1). The majority of patients were from Japan (78%). Lung and/or liver metastases were observed in 82.4% of patients, and brain metastases in 11.5% of patients, both assessed by BICR at baseline (Table 1). The patient population was heavily pretreated across clinical subtypes, with a median of 6 (range, 2-13) previous treatments in the advanced setting in patients with HR+/HER2– breast cancer, a median of 2 (range, 1-13) previous treatments in those with TNBC, and a median of 5.5 (range, 2-11) previous treatments in those with HER2+ breast cancer (Table 1). Characteristics of patients with HER2-low (IHC 1+ or IHC 2+, in situ hybridization–negative) and HER2-zero (IHC 0) breast cancer are summarized in Table 1.
TABLE 1.
Patient Demographics and Baseline Disease State Characteristics
At the time of data cutoff (August 16, 2021), the median follow-up was 31.9 months (range, 15-56 months) and the median treatment duration was 5.9 months (range, 0.7-30.6 months) for all patients. Median follow-up and treatment duration for all patients and by clinical subtype are shown in the Data Supplement (Table S2). Most patients (97.8% [178 of 182]) had discontinued treatment as of the data cutoff of August 16, 2021 (Data Supplement, Fig S2 and Table S2). The primary reasons for treatment discontinuation were progressive disease by RECIST 1.1 (73.1% [133 of 182]) and clinical progression (8.2% [15 of 182]). For 8.2% (15 of 182) of patients, the primary reason for discontinuation was adverse events.
Dose Selection
A dose range of 1.6-8.0 mg/kg once every 3 weeks was evaluated during dose selection. Dose-limiting toxicities were observed in four patients—one patient each in the 4.8 mg/kg (11.1% [1 of 9]) and 6.4 mg/kg (7.7% [1 of 11]) cohorts (both with grade 4 decreased platelet count) and two patients in the 8.0 mg/kg (33.3% [2 of 6]) cohort (one patient with grade 4 decreased platelet count, grade 3 increased AST, and grade 3 increased ALT and one patient with grade 3 increased ALT). The highest dose evaluated did not exceed the maximum tolerated dose. Two alternative uptitration dosing regimens were also assessed on the basis of findings from the dose-escalation part. Antitumor activity was observed in both cohorts, with a confirmed ORR of 41.7% in patients treated with the 3.2/4.8/6.6-mg/kg once every 3 weeks regimen (n = 12) and 25.0% in those treated with the 4.2 once every 2 weeks /6.4-mg/kg once every 3 weeks regimen (n = 12; Data Supplement, Table S3). Frequencies of grade ≥3 thrombocytopenia and neutropenia, particularly in the 4.2/6.4-mg/kg alternative dosing regimen, appeared to be numerically lower than those in the fixed-dose regimens (Data Supplement, Table S4). However, generally, higher efficacy was observed with the fixed doses of 4.8 and 6.4 mg/kg once every 3 weeks. On the basis of an overall risk-benefit assessment using all data from the dose-escalation and dose-finding parts, the fixed doses of 4.8 and 6.4 mg/kg once every 3 weeks were selected for dose expansion.
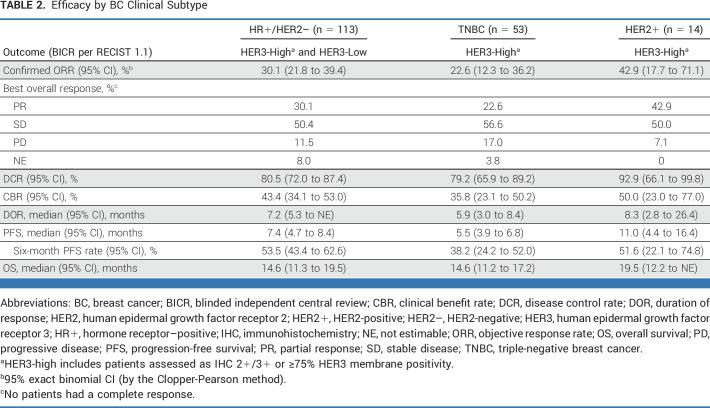
Efficacy
Efficacy results are reported here according to the breast cancer clinical subtype (efficacy results by protocol-defined escalation, alternative dosing regimen, expansion cohorts, and selected fixed-dose regimens for expansion [4.8 and 6.4 mg/kg] are summarized in the Data Supplement, Tables S3 and S5). Antitumor efficacy was observed at all doses tested, except for the lowest dose of 1.6 mg/kg.
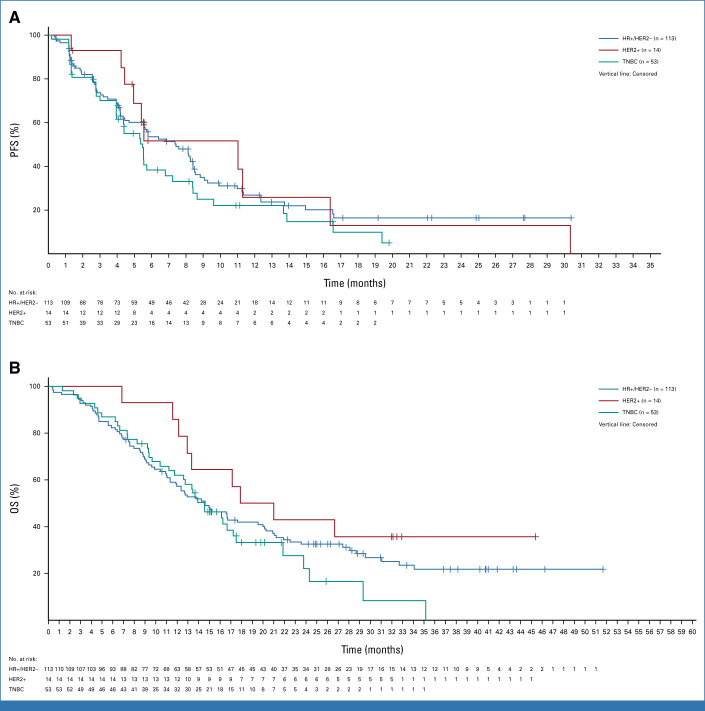
In patients with HR+/HER2– breast cancer (n = 113; all with HER3-high or HER3-low expression), the confirmed ORR by BICR was 30.1% (95% CI, 21.8 to 39.4) and the DCR was 80.5% (95% CI, 72.0 to 87.4). The median DOR was 7.2 months (95% CI, 5.3 months to not estimable [NE]; Table 2). The median PFS was 7.4 months (95% CI, 4.7 to 8.4 months), with a 6-month PFS rate of 53.5% (95% CI, 43.4 to 62.6). The median OS was 14.6 months (95% CI, 11.3 to 19.5 months; Table 2; Fig 1).
TABLE 2.
Efficacy by BC Clinical Subtype
FIG 1.
Kaplan-Meier plots for (A) PFS and (B) OS by breast cancer clinical subtype. HER2+, human epidermal growth factor receptor 2–positive; HER2–, HER2-negative; HR+, hormone receptor–positive; OS, overall survival; PFS, progression-free survival; TNBC, triple-negative breast cancer.
In patients with HR+/HER2– breast cancer, ORR was also evaluated by HER2 status. Of the 113 patients with HR+/HER2– disease, 58 had tumors with HER2-low expression and 39 had HER2-zero expression. The confirmed ORR by BICR was 36.2% (95% CI, 24.0 to 49.9) in patients with HR+/HER2-low breast cancer and 28.2% (95% CI, 15.0 to 44.9) in patients with HR+/HER2-zero disease (Data Supplement, Table S6).
In patients with TNBC (n = 53; all with HER3-high expression), the confirmed ORR by BICR was 22.6% (95% CI, 12.3 to 36.2) and the DCR was 79.2% (95% CI, 65.9 to 89.2). The median DOR was 5.9 months (95% CI, 3.0 to 8.4 months; Table 2). Patients with TNBC had a median PFS of 5.5 months (95% CI, 3.9 to 6.8 months) and a 6-month PFS rate of 38.2% (95% CI, 24.2 to 52.0). The median OS was 14.6 months (95% CI, 11.2 to 17.2 months; Table 2; Fig 1).
In patients with HER2+ breast cancer (n = 14; all with HER3-high expression), the confirmed ORR by BICR was 42.9% (95% CI, 17.1 to 71.1) and the DCR was 92.9% (95% CI, 66.1 to 99.8). The median DOR was 8.3 months (95% CI, 2.8 to 26.4 months; Table 2). Patients with HER2+ breast cancer had a median PFS of 11.0 months (95% CI, 4.4 to 16.4 months) and a 6-month PFS rate of 51.6% (95% CI, 22.1 to 74.8). The median OS was 19.5 months (95% CI, 12.2 months to NE; Table 2; Fig 1).
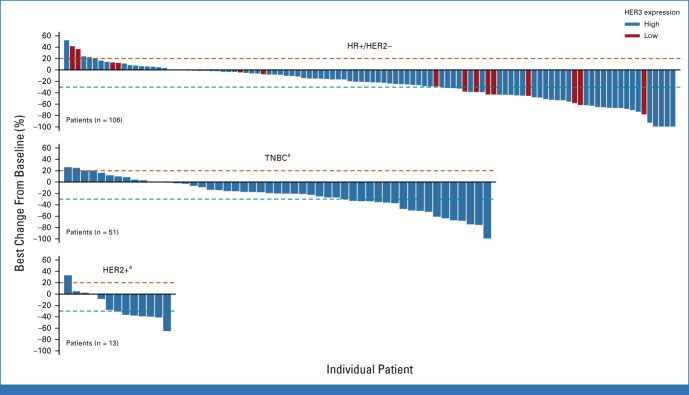
Most patients (80.0%), across clinical subtypes, experienced a reduction in the best percentage change in tumor size from baseline (Fig 2). In patients with HR+/HER2– breast cancer, reduced tumor size was seen in patients with low and high HER3 expression. Among patients with brain metastases at baseline (in all breast cancer subgroups), the confirmed ORR by BICR was 28.6% (95% CI, 11.3 to 52.2; Data Supplement, Table S7). In patients without brain metastases at baseline, a confirmed ORR by BICR of 28.6% (95% CI, 21.7 to 36.2) was observed.
FIG 2.
Best percentage change from baseline in tumor size in patients with HR+/HER2– breast cancer, TNBC, or HER2+ breast cancer. Best percent change from baseline in sum of diameters based on blinded independent central review for all target lesions identified is represented by patient. The patients who have available data for both the baseline and postbaseline tumor assessment visit are included in the analysis. aPatients with TNBC and HER2+ were all HER3-high. HER2+, human epidermal growth factor receptor 2–positive; HER2–, HER2-negative; HER3, human epidermal growth factor receptor 3; HR+, hormone receptor–positive; TNBC, triple-negative breast cancer.
Safety
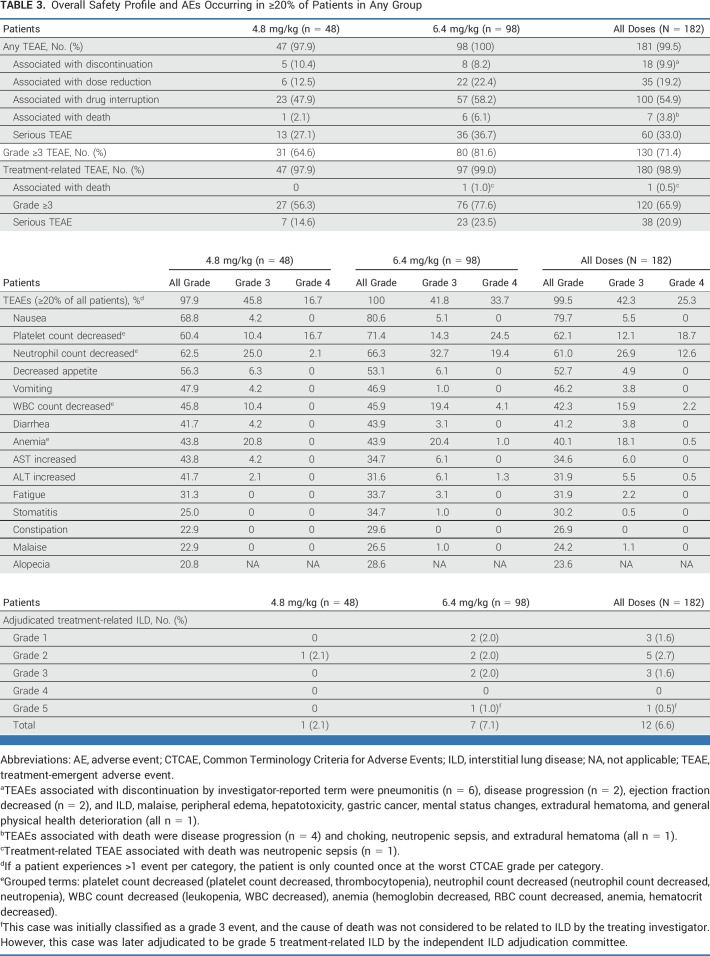
The most common TEAEs in all patients (N = 182) and patients who received HER3-DXd 4.8 mg/kg (n = 48) or 6.4 mg/kg (n = 98) are summarized in Table 3. The most common TEAEs of any grade were nausea in 79.7% of all patients, decreased platelet count in 62.1%, and decreased neutrophil count in 61.0%. Grade ≥3 TEAEs occurred in 71.4% of all patients: 64.6% of patients treated with 4.8 mg/kg and 81.6% of patients treated with 6.4 mg/kg. The most common grade 3 or 4 TEAEs observed in all patients are summarized in Table 3 and included decreased neutrophil count (grade 3, 26.9%; grade 4, 12.6%), decreased platelet count (grade 3, 12.1%; grade 4, 18.7%), anemia (grade 3, 18.1%; grade 4, 0.5%), and decreased WBC count (grade 3, 15.9%; grade 4, 2.2%). Rates of nonhematologic TEAEs were similar at both doses and primarily of low grade. Of the 50 patients (27.5%) who experienced a reduction in dose, 35 (19.2%) experienced a reduction associated with TEAEs; 100 patients (54.9%) had TEAEs associated with dose interruption, and 18 (9.9%) had TEAEs associated with treatment discontinuation. Hematologic AEs were managed by dose delay or reduction, and none were associated with treatment discontinuation. Seven patients (3.8%) had TEAEs associated with death; one was attributed to study treatment (neutropenic sepsis; Table 3).
TABLE 3.
Overall Safety Profile and AEs Occurring in ≥20% of Patients in Any Group
Treatment-related ILD, determined by an independent adjudication committee, occurred in 12 patients (6.6%); the majority of cases were grade 1 or 2. Three grade 3 (1.6%) and one grade 5 (0.5%) treatment-related ILD events occurred (Table 3). The cause of death in one patient who experienced grade 5 ILD according to central adjudication was deemed not related to ILD by the treating investigator. The median time to the onset of treatment-related ILD was 141.5 days (range, 36-584 days).
In the dose-finding part, the most common TEAEs in patients who received the 3.2/4.8/6.4-mg/kg dosing regimen were nausea (100%), decreased neutrophil count (75.0%), and decreased WBC count (50.0%). Decreased platelet count was also observed in 33% of patients. The most common TEAEs observed in patients treated with the 4.2/6.4 mg/kg -dosing regimen were nausea (83.3%), diarrhea (50.0%), and decreased platelet count (41.7%).
Translational/Biomarker Data and PK
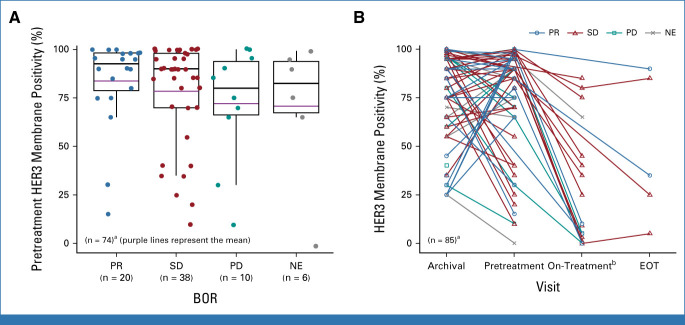
Efficacy, as assessed by best overall response by BICR, was observed in patients with HER3-high and HER3-low pretreatment tumor membrane expression (Fig 3A; Data Supplement, Fig S3A). The percentage of tumor cells with HER3 expression varied between paired archival and pretreatment samples in patients with HR+/HER2– breast cancer. A total of 15 patients had paired baseline and on-treatment biopsies available for HER3 testing. In these patients, HER3 expression decreased in on-treatment biopsies compared with the pretreatment time point, with a mean decrease in HER3 membrane positivity of 72.7% (Fig 3B). A limited number of pretreatment biopsies demonstrated a reduction in HER3 membrane expression below the cutoff of 25% membrane positivity compared with the paired archival tissue sample. Efficacy outcomes in these patients require further investigation. The number of samples from patients with TNBC was too small to assess (Data Supplement, Fig S3B). Notably, durable efficacy was observed in patients with both breast cancer subtypes, including those with HR+/HER2– breast cancer that had decreased HER3 expression observed at the early on-treatment time point.
FIG 3.
Pretreatment HER3 membrane expression by (A) BOR and (B) over time in patients with HR+/HER2– breast cancer. Overall percentage of HER3 membrane positivity was centrally assessed by HER3 immunohistochemistry. aHER3 positivity from patients in the dose-expansion cohorts. bOn-treatment biopsy specimen collected at cycle 2 day 3 or cycle 3 day 3. BOR, best overall response; EOT, end of treatment ; HER2–, human epidermal growth factor receptor 2–negative; HER3, human epidermal growth factor receptor 3; HR+, hormone receptor–positive; NE, not estimable; PD, progressive disease; PR, partial response; SD, stable disease.
Systemic exposure to HER3-DXd and DXd payload increased in a generally dose-dependent manner (Data Supplement, Tables S8 and S9). PK profiles were generally similar between races and countries (data not shown).
DISCUSSION
This article is the first comprehensive analysis of HER3-DXd in patients with advanced breast cancer and demonstrates clinically meaningful antitumor activity in a heavily pretreated patient population across a range of HER3 (HER3-high and HER3-low) expression. The majority of patients had visceral metastases, and 11.5% of patients had brain metastases confirmed by BICR at baseline. Durable antitumor activity was seen across clinical subtypes, and antitumor activity was also observed in patients with HR+ disease who had HER2-low or HER2-zero protein expression. While the 4.8- and 6.4-mg/kg dose groups had a limited number of patients, numerical differences in efficacy were observed between the dose groups. No notable differences in the number of early treatment discontinuations were observed. Potential differences in baseline disease characteristics, such as the number of patients with lung metastases, might have contributed to the varying response rates observed between groups.
Patients enrolled in this study were representative of a heavily pretreated patient population with advanced disease, and the demographics were similar to those in other recent phase III trials evaluating ADCs in patients with HR+/HER2– breast cancer and TNBC.13,14 However, these data may under-represent the frequency of visceral metastases observed in clinical practice because of the selection biases of the controlled trial setting, such as limiting ECOG PS to 0 or 1.
Effective treatment options for this patient population are limited; treatments in the later-line setting are not standardized, and outcomes are suboptimal, particularly for patients with HER2– breast cancer. Although this study had limited patient numbers, an encouraging median OS of 14.6 months was observed in patients with HR+/HER2– breast cancer (median PFS, 7.4 months) and TNBC (median PFS, 5.5 months). ADCs targeting other cell surface receptors are being explored as potential treatments in the later-line setting for patients with HER2– breast cancer. In the TROPiCS-02 trial, sacituzumab govitecan led to improved efficacy in patients with HR+/HER2– breast cancer compared with chemotherapy (median PFS, 5.5 v 4.0 months; median OS, 14.4 v 11.2 months).13 In the phase III ASCENT trial, patients with TNBC also experienced treatment benefit from sacituzumab govitecan compared with physician's choice chemotherapy (median PFS, 4.8 v 1.7 months; median OS, 11.8 v 6.9 months).14 These findings are comparable with HER3-DXd and further support the exploration of ADCs as a treatment paradigm in advanced breast cancer.
Although the number of patients was limited (n = 14), HER3-DXd demonstrated antitumor activity in patients with HER2+ breast cancer. All patients had disease progression while receiving previous anti-HER2 therapy. While HER3 expression has been implicated in mediating resistance to HER2-directed targeted therapy,15 these data suggest that further exploration of HER3-DXd as a potential treatment option after progression on anti-HER2 therapies is warranted.
A decrease in the percentage of tumor cells with HER3 membrane expression was observed early during treatment, potentially as a result of binding of HER3-DXd to the HER3 receptor and subsequent internalization of the HER3/HER3-DXd complex and/or a treatment effect reducing the number of tumor cells with HER3 membrane expression. While this decrease could potentially have been persistent and limited the antitumor efficacy of HER3-DXd, the depth and length of response in a heavily pretreated patient population suggest sustainable antitumor activity of HER3-DXd. However, these data are limited because of the relatively small number of biopsy samples available to assess HER3 expression for each time point throughout the study.
HER3-DXd demonstrated a manageable safety profile, with a low rate of treatment discontinuations associated with adverse events (9.9%). GI and hematologic toxicities were the most common and were generally not associated with any major bleeding events or infection; most cases were considered transient and managed by dose modifications. The rate of adjudicated treatment-related ILD was 6.6%, and the majority of cases were low grade and managed by dose modification following the established management guidelines. Safety results from the dose-finding part suggested that the alternative dosing regimens may be associated with reduced frequencies of grade ≥3 thrombocytopenia and neutropenia; however, the RDE was considered to be in the range of 4.8 to 6.4 mg/kg on the basis of an overall risk-benefit assessment using all available data from the dose-expansion and dose-finding parts.
The results of the U31402-A-J101 trial highlight the therapeutic reach that HER3-DXd may extend across the treatment landscape for metastatic breast cancer. Multiple phase II studies are ongoing to further evaluate HER3-DXd 5.6 mg/kg in the metastatic setting and explore potential biomarkers associated with response (ICARUS-Breast, ClinicalTrials.gov identifier: NCT04965766; BRE-354, ClinicalTrials.gov identifier: NCT04699630). In addition, studies have suggested that one potential mechanism of resistance to current HER2-targeted ADCs is decreased HER2 expression, raising the possibility that such cancers may remain sensitive to HER3-directed agents after progression.16-18 As a result, the phase II BRE-354 study will evaluate HER3-DXd as a treatment option for HER2+ breast cancer that has progressed on trastuzumab deruxtecan. Studies are also ongoing to evaluate HER3-DXd in earlier lines of therapy. Both the window-of-opportunity SOLTI TOT-HER3 (ClinicalTrials.gov identifier: NCT04610528) study and the phase II SOLTI-2103 VALENTINE (ClinicalTrials.gov identifier: NCT05569811) study are investigating the efficacy of neoadjuvant HER3-DXd and its biological effects on the tumor microenvironment.19
In conclusion, HER3-DXd showed clinically meaningful and durable antitumor activity in heavily pretreated patients, across major breast cancer subtypes with high or low HER3 expression. A manageable and acceptable safety profile was observed in this population with adverse prognostic features. Further evaluation of HER3-DXd as a potential treatment option in advanced breast cancer is ongoing.
ACKNOWLEDGMENT
We thank Allison Lytle, PhD, CMPP, and Erinn Gideons, PhD (ArticulateScience, LLC), for medical writing and providing editorial support which were funded by Daiichi Sankyo, Inc.
Ian E. Krop
Employment: Freeline Therapeutics, PureTech
Leadership: Freeline Therapeutics, PureTech
Stock and Other Ownership Interests: Freeline Therapeutics, PureTech
Honoraria: Genentech/Roche, AstraZeneca
Consulting or Advisory Role: Genentech/Roche, Seagen, Daiichi Sankyo, MacroGenics, Merck, AstraZeneca, Novartis
Research Funding: Genentech (Inst), Pfizer (Inst), Macrogenics (Inst)
Norikazu Masuda
Leadership: Japan Breast Cancer Research Group, Japanese Breast Cancer Society
Honoraria: Chugai Pharma, AstraZeneca, Pfizer, Eisai, Lilly Japan, Takeda, Kyowa-Kirin, Novartis, Daiichi Sankyo
Research Funding: Chugai Pharma (Inst), AstraZeneca (Inst), Kyowa Hakko Kirin (Inst), MSD (Inst), Novartis (Inst), Pfizer (Inst), Lilly (Inst), Eisai (Inst), Daiichi Sankyo (Inst), Nippon Kayaku (Inst), Sanofi (Inst), Gilead Sciences (Inst), Ono Pharmaceutical (Inst)
Toru Mukohara
Honoraria: Eisai, Pfizer, Novartis, Chugai Pharma, Lilly Japan, AstraZeneca, Kyowa Kirin, Taiho Pharmaceutical
Consulting or Advisory Role: Eisai, Micin
Research Funding: Sysmex (Inst), Eisai (Inst), MSD (Inst), Pfizer (Inst), Novartis (Inst), Sanofi (Inst), Chugai Pharma (Inst), Daiichi Sankyo/AstraZeneca (Inst), AstraZeneca (Inst), Ono Pharmaceutical (Inst)
Shunji Takahashi
Honoraria: Daiichi Sankyo, Eisai, Bayer, Taiho Pharmaceutical, MSD, Chugai Pharma, Bristol Myers Squibb Japan, Ono Pharmaceutical, Lilly Japan
Consulting or Advisory Role: Bayer
Research Funding: Daiichi Sankyo (Inst), Sanofi (Inst), Eisai (Inst), Bayer (Inst), Taiho Pharmaceutical (Inst), MSD (Inst), Novartis (Inst), Chugai Pharma (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Ono Pharmaceutical (Inst), Pfizer/EMD Serono (Inst), Seagen (Inst), Boehringer Ingelheim (Inst), Amgen (Inst), Astellas Pharma (Inst)
Takahiro Nakayama
Honoraria: Lilly, Eisai, Novartis, Daiichi Sankyo/AstraZeneca, MSD, Sandoz, Pfizer
Kenichi Inoue
Research Funding: MSD (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Pfizer (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Takeda (Inst), Chugai Pharma (Inst), Eisai (Inst), Gilead Sciences (Inst), Novartis (Inst)
Hiroji Iwata
Honoraria: Chugai Pharma, AstraZeneca, Eisai, Pfizer, Daiichi Sankyo, Lilly Japan, Kyowa Hakko Kirin, Taiho Pharmaceutical, MSD
Consulting or Advisory Role: Chugai Pharma, Daiichi Sankyo, Pfizer, AstraZeneca, Lilly Japan, Kyowa Hakko Kirin, Novartis, MSD, Sanofi
Research Funding: MSD (Inst), AstraZeneca (Inst), Kyowa Hakko Kirin (Inst), Daiichi Sankyo (Inst), Chugai Pharma (Inst), Nippon Kayaku (Inst), Lilly Japan (Inst), Novartis (Inst), Bayer (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst), Sanofi (Inst), Amgen (Inst)
Yutaka Yamamoto
Honoraria: Chugai Pharma, AstraZeneca, Novartis, Kyowa Hakko Kirin, Eisai, Lilly Japan, Pfizer, Daiichi Sankyo/UCB Japan, MSD, Taiho Pharmaceutical, Sysmex, Sanofi, Exact Sciences
Consulting or Advisory Role: AstraZeneca, Novartis, Chugai Pharma, Pfizer, Daiichi Sankyo, MSD
Research Funding: MSD Oncology, Daiichi Sankyo, Chugai Pharma, Taiho Pharmaceutical, AstraZeneca, Pfizer, Gilead Sciences, Sanofi
Other Relationship: Japanese Breast Cancer Society, Japan Breast Cancer Research Group
Ricardo H. Alvarez
Employment: Oncology Consultants
Honoraria: Gilead Sciences, Stemline Therapeutics, Boston Biomedical
Speakers' Bureau: Gilead Sciences
Other Relationship: Eisai, Puma Biotechnology, Pfizer
Uncompensated Relationships: Susan G. Komen for the Cure
Tatsuya Toyama
Consulting or Advisory Role: Daiichi Sankyo/UCB Japan
Speakers' Bureau: Lilly, Novartis, Chugai Pharma, Eisai, Pfizer, Kyowa Hakko Kirin, Taiho Pharmaceutical, Nippon Kayaku, AstraZeneca
Research Funding: Eisai (Inst), Pfizer (Inst), Kyowa Hakko Kirin (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), Chugai Pharma (Inst), Nippon Kayaku (Inst), Takeda (Inst)
Masato Takahashi
Honoraria: AstraZeneca, Eisai, Pfizer, Lilly, Daiichi Sankyo/UCB Japan, MSD
Research Funding: Eisai (Inst), Nippon Kayaku (Inst)
Akihiko Osaki
Honoraria: Eisai, Nippon Kayaku, Chugai Pharma, Daiichi Sankyo Co, Ltd, Eli Lilly Japan KK, Pfizer, Kyowa Kirin Co, Ltd
Research Funding: AstraZeneca (Inst), Eisai (Inst), Kyowa Kirin Co, Ltd (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Nippon Kayaku (Inst), Covance Japan Co, Ltd (Inst), LabCorp Japan, G.K. (Inst), MSD K.K. (Inst), Daiichi Sankyo Co, Ltd (Inst), Takeda Pharmaceutical Co, Ltd (Inst), Lilly Japan (Inst), Novartis (Inst), Sanofi (Inst)
Shigehira Saji
Honoraria: Chugai Pharma, Eisai, Taiho Pharmaceutical, Novartis, Pfizer, Kyowa Kirin, Daiichi Sankyo, AstraZeneca, Lilly, MSD, Ono Pharmaceutical, Takeda, Exact Sciences
Consulting or Advisory Role: Kyowa Kirin, Chugai Pharma, Roche/Genentech, Daiichi Sankyo/UCB Japan, MSD
Research Funding: Chugai Pharma (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo/UCB Japan (Inst), MSD (Inst), AstraZeneca (Inst), Sanofi (Inst)
Yasuaki Sagara
Speakers' Bureau: AstraZeneca, Chugai Pharma, Eisai, Pfizer, Lilly, MSD, Daiichi Sankyo/Japan
Joyce O'Shaughnessy
Honoraria: AstraZeneca, Lilly, AbbVie, Celgene, Eisai, Novartis, Pfizer, Agendia, Amgen, Bristol Myers Squibb, Genentech, GRAIL, Immunomedics, HERON, Ipsen, Merck, Myriad Pharmaceuticals, Puma Biotechnology, Roche, Syndax, Sanofi, Samsung, Daiichi Sankyo, Aptitude Health, Bayer, G1 Therapeutics, Gilead Sciences, Halozyme, Nektar, Pharmacyclics, Pierre Fabre, Prime Oncology, Seagen, Taiho Oncology, Takeda, Synthon, Ontada/McKesson
Consulting or Advisory Role: Novartis, Pfizer, Lilly, AbbVie, AstraZeneca, Celgene, Eisai, Agendia, Amgen, Bristol Myers Squibb, Genentech, GRAIL, Immunomedics, HERON, Ipsen, Merck, Myriad Pharmaceuticals, Puma Biotechnology, Roche, Syndax, Sanofi, Samsung, Daiichi Sankyo, Aptitude Health, Bayer, G1 Therapeutics, Gilead Sciences, Halozyme, Nektar, Pharmacyclics, Pierre Fabre, Prime Oncology, Seagen, Taiho Oncology, Takeda, Synthon, Ontada/McKesson
Speakers' Bureau: AstraZeneca, Novartis, Lilly, Pfizer, Seagen
Research Funding: Seagen (Inst)
Travel, Accommodations, Expenses: Celgene, Lilly, Novartis, Pfizer, AbbVie, Agendia, Amgen, Eisai, GRAIL, Ipsen, Myriad Pharmaceuticals, Puma Biotechnology, Seagen, AstraZeneca, Sanofi, Roche
Shoichi Ohwada
Employment: Daiichi Sankyo Co, Ltd
Kumiko Koyama
Employment: Daiichi Sankyo Co, Ltd
Tatsuya Inoue
Employment: Daiichi Sankyo RD Novare
Li Li
Employment: Daiichi Sankyo, Inc
Stock and Other Ownership Interests: Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Patent with BMS
Parul Patel
Employment: Daiichi Sankyo
Stock and Other Ownership Interests: Merck (I), Daiichi Sankyo/Lilly
Travel, Accommodations, Expenses: Daiichi Sankyo/Lilly
Joseph Mostillo
Employment: Daiichi Sankyo, Inc
Yoshimi Tanaka
Employment: Daiichi Sankyo, Inc
Stock and Other Ownership Interests: Daiichi Sankyo, Inc
Travel, Accommodations, Expenses: Daiichi Sankyo, Inc
David W. Sternberg
Employment: Daiichi Sankyo, Inc
Stock and Other Ownership Interests: Daiichi Sankyo, Inc
Dalila Sellami
Employment: Daiichi Sankyo, Inc, Radius Pharmaceutical
Stock and Other Ownership Interests: Daiichi Sankyo, Janssen-Ortho
Kan Yonemori
Honoraria: Eisai, Pfizer, AstraZeneca, Novartis, Taiho Pharmaceutical, Lilly Japan, Daiichi Sankyo/AstraZeneca, Takeda, Fujifilm, Ono Pharmaceutical, Chugai Pharma, MSD Oncology
Consulting or Advisory Role: Chugai Pharma, Ono Pharmaceutical, Novartis, Eisai, OncXerna Therapeutics
Research Funding: Ono Pharmaceutical (Inst), MSD (Inst), Daiichi Sankyo/AstraZeneca (Inst), AstraZeneca/MedImmune (Inst), Taiho Pharmaceutical (Inst), Pfizer (Inst), Novartis (Inst), Takeda (Inst), Chugai Pharma (Inst), Sanofi (Inst), Seagen (Inst), Eisai (Inst), Lilly (Inst), Genmab (Inst), Boehringer Ingelheim (Inst), Kyowa Hakko Kirin (Inst), Haihe Pharmaceutical (Inst), Nippon Kayaku (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2018 ASCO Annual Meeting, Chicago, IL, June 1-5, 2018; the 2018 San Antonio Breast Cancer Symposium, San Antonio, TX, December 4-8, 2018; the European Society for Medical Oncology Breast Cancer, Berlin, Germany, May 2-4, 2019; the 2020 San Antonio Breast Cancer Symposium, virtual, December 8-11, 2020; and the 2022 ASCO Annual Meeting, Chicago, IL, June 3-7, 2022.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Anonymized individual participant data on completed studies and applicable supporting clinical study documents may be available upon request at https://vivli.org/. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of the company and our clinical study subjects. Details on data sharing criteria and the procedure for requesting access can be found at https://vivli.org/ourmember/daiichi-sankyo/.
AUTHOR CONTRIBUTIONS
Conception and design: Ian E. Krop, Takahiro Nakayama, Hiroji Iwata, Tatsuya Toyama, Shigehira Saji, Shoichi Ohwada, David W. Sternberg, Dalila Sellami
Administrative support: Parul Patel, David W. Sternberg
Provision of study materials or patients: Kenichi Inoue, Ricardo H. Alvarez, Akihiko Osaki, Shigehira Saji, Kan Yonemori
Collection and assembly of data: Norikazu Masuda, Toru Mukohara, Shunji Takahashi, Takahiro Nakayama, Kenichi Inoue, Hiroji Iwata, Tatsuya Toyama, Masato Takahashi, Akihiko Osaki, Yasuaki Sagara, Tatsuya Inoue, Li Li, Parul Patel, Joseph Mostillo, David W. Sternberg, Dalila Sellami, Kan Yonemori
Data analysis and interpretation: Ian E. Krop, Norikazu Masuda, Takahiro Nakayama, Kenichi Inoue, Ricardo H. Alvarez, Tatsuya Toyama, Masato Takahashi, Shigehira Saji, Yasuaki Sagara, Shoichi Ohwada, Kumiko Koyama, Tatsuya Inoue, Li Li, Parul Patel, Joseph Mostillo, Yoshimi Tanaka, David W. Sternberg, Dalila Sellami
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patritumab Deruxtecan (HER3-DXd), a Human Epidermal Growth Factor Receptor 3–Directed Antibody-Drug Conjugate, in Patients With Previously Treated Human Epidermal Growth Factor Receptor 3–Expressing Metastatic Breast Cancer: A Multicenter, Phase I/II Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ian E. Krop
Employment: Freeline Therapeutics, PureTech
Leadership: Freeline Therapeutics, PureTech
Stock and Other Ownership Interests: Freeline Therapeutics, PureTech
Honoraria: Genentech/Roche, AstraZeneca
Consulting or Advisory Role: Genentech/Roche, Seagen, Daiichi Sankyo, MacroGenics, Merck, AstraZeneca, Novartis
Research Funding: Genentech (Inst), Pfizer (Inst), Macrogenics (Inst)
Norikazu Masuda
Leadership: Japan Breast Cancer Research Group, Japanese Breast Cancer Society
Honoraria: Chugai Pharma, AstraZeneca, Pfizer, Eisai, Lilly Japan, Takeda, Kyowa-Kirin, Novartis, Daiichi Sankyo
Research Funding: Chugai Pharma (Inst), AstraZeneca (Inst), Kyowa Hakko Kirin (Inst), MSD (Inst), Novartis (Inst), Pfizer (Inst), Lilly (Inst), Eisai (Inst), Daiichi Sankyo (Inst), Nippon Kayaku (Inst), Sanofi (Inst), Gilead Sciences (Inst), Ono Pharmaceutical (Inst)
Toru Mukohara
Honoraria: Eisai, Pfizer, Novartis, Chugai Pharma, Lilly Japan, AstraZeneca, Kyowa Kirin, Taiho Pharmaceutical
Consulting or Advisory Role: Eisai, Micin
Research Funding: Sysmex (Inst), Eisai (Inst), MSD (Inst), Pfizer (Inst), Novartis (Inst), Sanofi (Inst), Chugai Pharma (Inst), Daiichi Sankyo/AstraZeneca (Inst), AstraZeneca (Inst), Ono Pharmaceutical (Inst)
Shunji Takahashi
Honoraria: Daiichi Sankyo, Eisai, Bayer, Taiho Pharmaceutical, MSD, Chugai Pharma, Bristol Myers Squibb Japan, Ono Pharmaceutical, Lilly Japan
Consulting or Advisory Role: Bayer
Research Funding: Daiichi Sankyo (Inst), Sanofi (Inst), Eisai (Inst), Bayer (Inst), Taiho Pharmaceutical (Inst), MSD (Inst), Novartis (Inst), Chugai Pharma (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Ono Pharmaceutical (Inst), Pfizer/EMD Serono (Inst), Seagen (Inst), Boehringer Ingelheim (Inst), Amgen (Inst), Astellas Pharma (Inst)
Takahiro Nakayama
Honoraria: Lilly, Eisai, Novartis, Daiichi Sankyo/AstraZeneca, MSD, Sandoz, Pfizer
Kenichi Inoue
Research Funding: MSD (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Pfizer (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Takeda (Inst), Chugai Pharma (Inst), Eisai (Inst), Gilead Sciences (Inst), Novartis (Inst)
Hiroji Iwata
Honoraria: Chugai Pharma, AstraZeneca, Eisai, Pfizer, Daiichi Sankyo, Lilly Japan, Kyowa Hakko Kirin, Taiho Pharmaceutical, MSD
Consulting or Advisory Role: Chugai Pharma, Daiichi Sankyo, Pfizer, AstraZeneca, Lilly Japan, Kyowa Hakko Kirin, Novartis, MSD, Sanofi
Research Funding: MSD (Inst), AstraZeneca (Inst), Kyowa Hakko Kirin (Inst), Daiichi Sankyo (Inst), Chugai Pharma (Inst), Nippon Kayaku (Inst), Lilly Japan (Inst), Novartis (Inst), Bayer (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst), Sanofi (Inst), Amgen (Inst)
Yutaka Yamamoto
Honoraria: Chugai Pharma, AstraZeneca, Novartis, Kyowa Hakko Kirin, Eisai, Lilly Japan, Pfizer, Daiichi Sankyo/UCB Japan, MSD, Taiho Pharmaceutical, Sysmex, Sanofi, Exact Sciences
Consulting or Advisory Role: AstraZeneca, Novartis, Chugai Pharma, Pfizer, Daiichi Sankyo, MSD
Research Funding: MSD Oncology, Daiichi Sankyo, Chugai Pharma, Taiho Pharmaceutical, AstraZeneca, Pfizer, Gilead Sciences, Sanofi
Other Relationship: Japanese Breast Cancer Society, Japan Breast Cancer Research Group
Ricardo H. Alvarez
Employment: Oncology Consultants
Honoraria: Gilead Sciences, Stemline Therapeutics, Boston Biomedical
Speakers' Bureau: Gilead Sciences
Other Relationship: Eisai, Puma Biotechnology, Pfizer
Uncompensated Relationships: Susan G. Komen for the Cure
Tatsuya Toyama
Consulting or Advisory Role: Daiichi Sankyo/UCB Japan
Speakers' Bureau: Lilly, Novartis, Chugai Pharma, Eisai, Pfizer, Kyowa Hakko Kirin, Taiho Pharmaceutical, Nippon Kayaku, AstraZeneca
Research Funding: Eisai (Inst), Pfizer (Inst), Kyowa Hakko Kirin (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), Chugai Pharma (Inst), Nippon Kayaku (Inst), Takeda (Inst)
Masato Takahashi
Honoraria: AstraZeneca, Eisai, Pfizer, Lilly, Daiichi Sankyo/UCB Japan, MSD
Research Funding: Eisai (Inst), Nippon Kayaku (Inst)
Akihiko Osaki
Honoraria: Eisai, Nippon Kayaku, Chugai Pharma, Daiichi Sankyo Co, Ltd, Eli Lilly Japan KK, Pfizer, Kyowa Kirin Co, Ltd
Research Funding: AstraZeneca (Inst), Eisai (Inst), Kyowa Kirin Co, Ltd (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Nippon Kayaku (Inst), Covance Japan Co, Ltd (Inst), LabCorp Japan, G.K. (Inst), MSD K.K. (Inst), Daiichi Sankyo Co, Ltd (Inst), Takeda Pharmaceutical Co, Ltd (Inst), Lilly Japan (Inst), Novartis (Inst), Sanofi (Inst)
Shigehira Saji
Honoraria: Chugai Pharma, Eisai, Taiho Pharmaceutical, Novartis, Pfizer, Kyowa Kirin, Daiichi Sankyo, AstraZeneca, Lilly, MSD, Ono Pharmaceutical, Takeda, Exact Sciences
Consulting or Advisory Role: Kyowa Kirin, Chugai Pharma, Roche/Genentech, Daiichi Sankyo/UCB Japan, MSD
Research Funding: Chugai Pharma (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo/UCB Japan (Inst), MSD (Inst), AstraZeneca (Inst), Sanofi (Inst)
Yasuaki Sagara
Speakers' Bureau: AstraZeneca, Chugai Pharma, Eisai, Pfizer, Lilly, MSD, Daiichi Sankyo/Japan
Joyce O'Shaughnessy
Honoraria: AstraZeneca, Lilly, AbbVie, Celgene, Eisai, Novartis, Pfizer, Agendia, Amgen, Bristol Myers Squibb, Genentech, GRAIL, Immunomedics, HERON, Ipsen, Merck, Myriad Pharmaceuticals, Puma Biotechnology, Roche, Syndax, Sanofi, Samsung, Daiichi Sankyo, Aptitude Health, Bayer, G1 Therapeutics, Gilead Sciences, Halozyme, Nektar, Pharmacyclics, Pierre Fabre, Prime Oncology, Seagen, Taiho Oncology, Takeda, Synthon, Ontada/McKesson
Consulting or Advisory Role: Novartis, Pfizer, Lilly, AbbVie, AstraZeneca, Celgene, Eisai, Agendia, Amgen, Bristol Myers Squibb, Genentech, GRAIL, Immunomedics, HERON, Ipsen, Merck, Myriad Pharmaceuticals, Puma Biotechnology, Roche, Syndax, Sanofi, Samsung, Daiichi Sankyo, Aptitude Health, Bayer, G1 Therapeutics, Gilead Sciences, Halozyme, Nektar, Pharmacyclics, Pierre Fabre, Prime Oncology, Seagen, Taiho Oncology, Takeda, Synthon, Ontada/McKesson
Speakers' Bureau: AstraZeneca, Novartis, Lilly, Pfizer, Seagen
Research Funding: Seagen (Inst)
Travel, Accommodations, Expenses: Celgene, Lilly, Novartis, Pfizer, AbbVie, Agendia, Amgen, Eisai, GRAIL, Ipsen, Myriad Pharmaceuticals, Puma Biotechnology, Seagen, AstraZeneca, Sanofi, Roche
Shoichi Ohwada
Employment: Daiichi Sankyo Co, Ltd
Kumiko Koyama
Employment: Daiichi Sankyo Co, Ltd
Tatsuya Inoue
Employment: Daiichi Sankyo RD Novare
Li Li
Employment: Daiichi Sankyo, Inc
Stock and Other Ownership Interests: Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Patent with BMS
Parul Patel
Employment: Daiichi Sankyo
Stock and Other Ownership Interests: Merck (I), Daiichi Sankyo/Lilly
Travel, Accommodations, Expenses: Daiichi Sankyo/Lilly
Joseph Mostillo
Employment: Daiichi Sankyo, Inc
Yoshimi Tanaka
Employment: Daiichi Sankyo, Inc
Stock and Other Ownership Interests: Daiichi Sankyo, Inc
Travel, Accommodations, Expenses: Daiichi Sankyo, Inc
David W. Sternberg
Employment: Daiichi Sankyo, Inc
Stock and Other Ownership Interests: Daiichi Sankyo, Inc
Dalila Sellami
Employment: Daiichi Sankyo, Inc, Radius Pharmaceutical
Stock and Other Ownership Interests: Daiichi Sankyo, Janssen-Ortho
Kan Yonemori
Honoraria: Eisai, Pfizer, AstraZeneca, Novartis, Taiho Pharmaceutical, Lilly Japan, Daiichi Sankyo/AstraZeneca, Takeda, Fujifilm, Ono Pharmaceutical, Chugai Pharma, MSD Oncology
Consulting or Advisory Role: Chugai Pharma, Ono Pharmaceutical, Novartis, Eisai, OncXerna Therapeutics
Research Funding: Ono Pharmaceutical (Inst), MSD (Inst), Daiichi Sankyo/AstraZeneca (Inst), AstraZeneca/MedImmune (Inst), Taiho Pharmaceutical (Inst), Pfizer (Inst), Novartis (Inst), Takeda (Inst), Chugai Pharma (Inst), Sanofi (Inst), Seagen (Inst), Eisai (Inst), Lilly (Inst), Genmab (Inst), Boehringer Ingelheim (Inst), Kyowa Hakko Kirin (Inst), Haihe Pharmaceutical (Inst), Nippon Kayaku (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Naidu R, Yadav M, Nair S, et al. : Expression of c-erbB3 protein in primary breast carcinomas. Br J Cancer 78:1385-1390, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travis A, Pinder SE, Robertson JF, et al. : C-erbB-3 in human breast carcinoma: Expression and relation to prognosis and established prognostic indicators. Br J Cancer 74:229-233, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu CG, Masoudi H, Leung S, et al. : HER-3 overexpression is prognostic of reduced breast cancer survival: A study of 4046 patients. Ann Surg 251:1107-1116, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Ocana A, Vera-Badillo F, Seruga B, et al. : HER3 overexpression and survival in solid tumors: A meta-analysis. J Natl Cancer Inst 105:266-273, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Witton CJ, Reeves JR, Going JJ, et al. : Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol 200:290-297, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Ogitani Y, Aida T, Hagihara K, et al. : DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 22:5097-5108, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto Y, Koyama K, Kamai Y, et al. : A novel HER3-targeting antibody-drug conjugate, U3-1402, exhibits potent therapeutic efficacy through the delivery of cytotoxic payload by efficient internalization. Clin Cancer Res 25:7151-7161, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Nakada T, Sugihara K, Jikoh T, et al. : The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull (Tokyo) 67:173-185, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Koganemaru S, Kuboki Y, Koga Y, et al. : U3-1402, a novel HER3-targeting antibody-drug conjugate, for the treatment of colorectal cancer. Mol Cancer Ther 18:2043-2050, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto Y, Kamai Y, Miyamoto M, et al. : HER3-targeting antibody-drug conjugate U3-1402 induces tumor regression in a variety of xenograft models and exerts enhanced antitumor activity by combining with PI3K inhibition. Presented at the American Association for Cancer Research Annual Meeting, Virtual, 2020
- 11.Òdena A, Monserrat L, Brasó-Maristany F, et al. : Identification of biomarkers of response to a novel HER3-directed antibody drug conjugate (ADC) using breast cancer (BC) patient-derived xenografts (PDX) models. Presented at the European Association for Cancer Research Congress, Seville, Spain, June 20-23, 2022
- 12.Kotake T, Suzuki E, Pu F, et al. : Human epidermal growth factor receptor 3 might be a targetable molecule for treatment of chemotherapy resistant breast cancer. Presented at the American Association for Cancer Research Annual Meeting, Atlanta, GA, March 29-April 3, 2019
- 13.Rugo HS, Bardia A, Marme F, et al. : Overall survival results from the phase 3 TROPiCS-02 study of sacituzumab govitecan vs treatment with physician's choice in patients with HR+/HER2- metastatic breast cancer. Presented at the European Society of Medical Oncology Congress, Paris, France, September 9-13, 2022
- 14.Bardia A, Hurvitz SA, Tolaney SM, et al. : Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med 384:1529-1541, 2021 [DOI] [PubMed] [Google Scholar]
- 15.Schlam I, Tarantino P, Tolaney SM: Overcoming resistance to HER2-directed therapies in breast cancer. Cancers (Basel) 14:3996, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosele MF, Lusque A, Dieras VC, et al. : Unraveling the mechanism of action and resistance to trastuzumab deruxtecan (T-DXd): Biomarker analyses from patients from DAISY trial. Presented at the European Society of Medical Oncology Congress, Paris, France, September 9-13, 2022
- 17.Shitara K, Iwata H, Takahashi S, et al. : Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: A dose-expansion, phase 1 study. Lancet Oncol 20:827-836, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Verma S, Miles D, Gianni L, et al. : Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783-1791, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prat A, Falato C, Pare Brunet L, et al. : Patritumab deruxtecan in early-stage HR+/HER2- breast cancer: Final results of the SOLTI TOT-HER3 preoperative trial. Presented at the European Society of Medical Oncology Breast Cancer Congress, Berlin, Germany and online, May 3-5, 2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized individual participant data on completed studies and applicable supporting clinical study documents may be available upon request at https://vivli.org/. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of the company and our clinical study subjects. Details on data sharing criteria and the procedure for requesting access can be found at https://vivli.org/ourmember/daiichi-sankyo/.