Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
The primary analysis of the Early positron emission tomography (ePET) Response–Adapted Treatment in localized Hodgkin Lymphoma H10 Trial demonstrated that in ePET-negative patients, the risk of relapse increased when involved-node radiotherapy (INRT) was omitted and that in ePET-positive patients, switching from doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) to bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPPesc) significantly improved 5-year progression-free survival (PFS). Here, we report the final results of a preplanned analysis at a 10-year follow-up. In the favorable (F) ePET-negative group, the 10-year PFS rates were 98.8% versus 85.4% (hazard ratio [HR], 13.2; 95% CI, 3.1 to 55.8; P value for noninferiority = .9735; difference test P < .0001) in favor of ABVD + INRT; in the unfavorable (U) ePET-negative group, the 10-year PFS rates were 91.4% and 86.5% (HR, 1.52; 95% CI, 0.84 to 2.75; P value for noninferiority = .8577; difference test P = .1628). In ePET-positive patients, the difference in terms of PFS between standard ABVD and intensified BEACOPPesc was no longer statistically significant (HR, 0.67; 95% CI, 0.37 to 1.20; P = .1777). In conclusion, the present long-term analysis confirms that in ePET-negative patients, the omission of INRT is associated with lower 10-year PFS. Instead, in ePET-positive patients, no significant difference between standard and experimental arms emerged although intensification with BEACOPPesc was safe, with no increase in late adverse events, namely, second malignancies.
INTRODUCTION
Efforts to improve outcomes of patients with early-stage Hodgkin lymphoma (HL) and minimize the risk of short- and long-term toxicities have been made. In particular, different positron emission tomography (PET)–adapted strategies have been explored.1-3
Our EORTC/LYSA/FIL H10 trial incorporated an early PET (ePET) response–adapted treatment strategy for both ePET-negative and ePET-positive patients with stage I and II HL. After a median follow-up of 4.5 years, the study showed that when ePET was positive after two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD), switching to bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPPesc) + involved-node radiotherapy (INRT) significantly improved 5-year progression-free survival (PFS). In ePET-negative patients, noninferiority of ABVD alone could not be demonstrated: the risk of relapse increased when INRT was omitted, especially in patients in the favorable (F) group.4,5 Here, we present the results of the preplanned 10-year follow-up analysis.
PATIENTS AND METHODS
Details of the study design have been published previously.4 The study was conducted in compliance with the Declaration of Helsinki and approved by the scientific and ethical committees, and all patients gave written informed consent (ClinicalTrials.gov identifier: NCT00433433). This preplanned analysis focused on late adverse events, overall survival (OS), and PFS. The analyses were conducted on ePET-positive patients, ePET-negative patients with F and U prognosis treated per the initial protocol, and ePET-negative patients treated per safety amendment. Statistical analysis methodology is reported in the Data Supplement (online only).
RESULTS
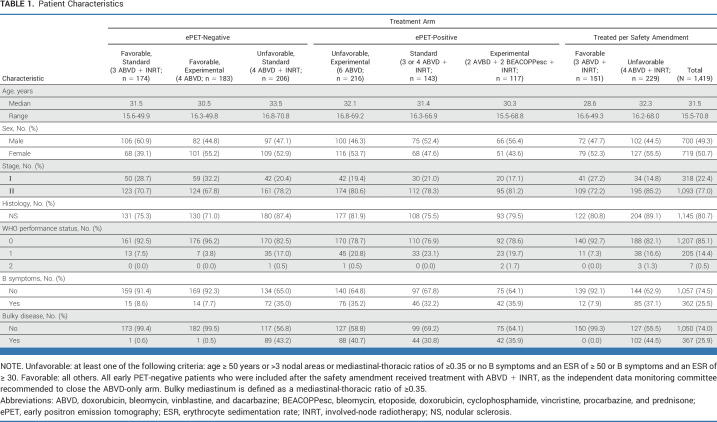
From November 2006 to June 2011, 1,925 patients completed the two ABVD cycles and performed an ePET scan; of these patients, details of 1,419 patients have been updated (Fig 1). Patient characteristics are listed in Table 1.
FIG 1.
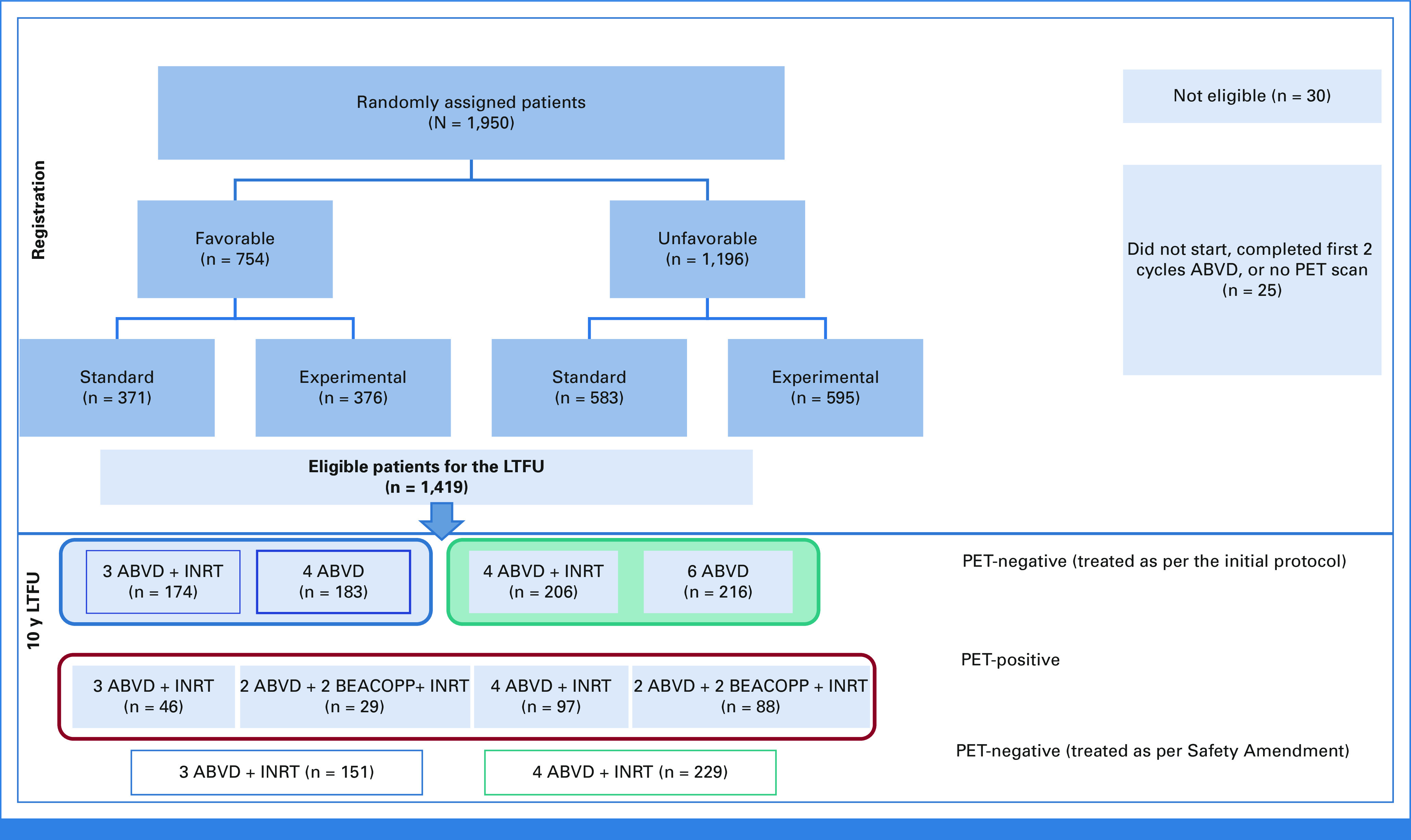
CONSORT diagram. ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; BEACOPPesc, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; INRT, involved-node radiotherapy; LTFU, long-term follow-up; PET, positron emission tomography.
TABLE 1.
Patient Characteristics
After a median follow-up of 9.5 years, 106 progressions or recurrences and 57 deaths were recorded, resulting in 136 events for PFS.
Standard Versus Experimental Arm
Survival analyses were performed in the intention-to-treat population randomly assigned up to the safety amendment (n = 969). The 10-year PFS rates were 91.4% (95% CI, 88.5 to 93.7) and 85.6% (95% CI, 82.0 to 88.5) in the standard versus experimental arm, respectively, with a hazard ratio (HR) of 1.67 (95% CI, 1.13 to 2.44; raw P = .0085; Data Supplement, Fig 1A; adjusted P = .0255). The 10-year OS rates were 95.2% (95% CI, 92.7 to 96.9) and 95.5% (95% CI, 93.0 to 97.1) in the standard versus experimental arm, respectively (P = .6717; Data Supplement, Fig 1B).
ePET-Negative Patients
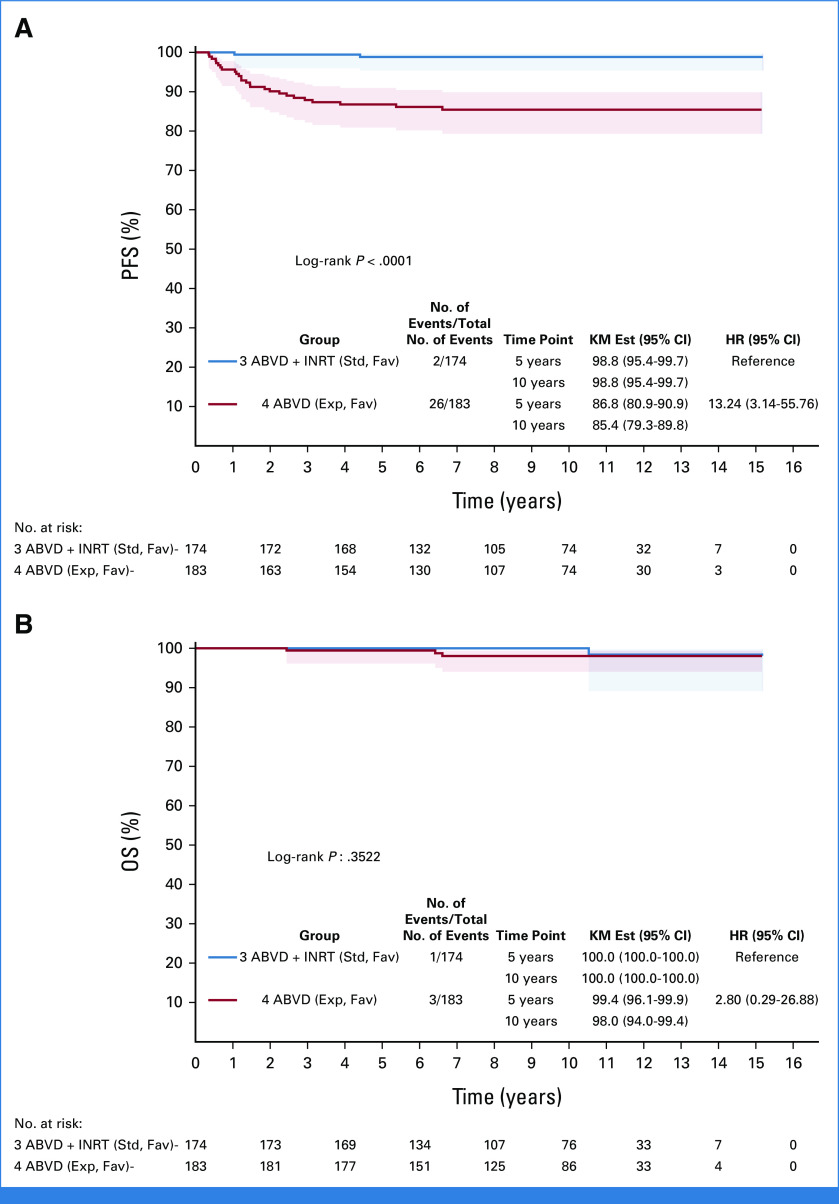
In the F group, a total of 28 events occurred: two patients experienced relapse in the ABVD + INRT arm versus 24 patients who experienced relapse and two patients who died from a cause not related to HL in the ABVD-only arm. Nineteen of 24 relapses (79%) in the ABVD-only arm occurred in previously involved nonirradiated locations. The 10-year PFS rates were 98.8% (95% CI, 95.4 to 99.7) and 85.4% (95% CI, 79.3 to 89.8) in the ABVD + INRT and ABVD-only arms, respectively, with a HR of 13.2 (95% CI, 3.1 to 55.8; noninferiority test [with noninferiority margin HR, 3.2]; P = .9735; difference test P < .0001, Fig 2A).
FIG 2.
(A) Ten-year PFS in the ABVD + INRT and ABVD-only arms; HR, 13.2 (95% CI, 3.1 to 55.8 [noninferiority test with noninferiority margin HR, 3.2; P = .9735]); difference test P < .0001. (B) Ten-year OS; HR, 2.80 (95% CI, 0.29 to 26.9); difference test P = .3522. ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; Exp. Fav, experimental favorable; HR, hazard ratio; INRT, involved-node radiotherapy; KM Est, Kaplan-Meier estimate; OS, overall survival; PFS, progression-free survival; Std. Fav, standard favorable.
There were a total of four deaths in the F group: one in the ABVD + INRT arm and three in the ABVD-only arm. The 10-year OS rates were 100.0% versus 98.0% for the ABVD + INRT and ABVD-only arms, respectively, with a HR of 2.80 (95% CI, 0.29 to 26.9; difference test P = .3522; Fig 2B).
In the U group, a total of 46 events occurred: 11 and 22 patients experienced relapse and seven and six patients died from a cause not related to HL in the standard and experimental arms, respectively. Twenty of the 22 relapses (91%) in the ABVD-only arm occurred in previously involved nonirradiated locations. The 10-year PFS rates were 91.4% (95% CI, 86.4 to 94.5) and 86.5% (95% CI, 81.0 to 90.5) in the standard and experimental arms, respectively, with a HR of 1.52 (95% CI, 0.84 to 2.75; P value for the noninferiority test [with noninferiority margin HR, 2.1]; P = .8577; difference test P = .1628; Data Supplement, Fig 2A).
There were 21 deaths in the U group: 11 in the ABVD + INRT arm and 10 in the ABVD-only arm. The 10-year OS rates were 94.3% versus 94.8%, respectively, with a HR of 0.84 (95% CI, 0.36 to 1.98; difference test P = .6908; Data Supplement, Fig 2B).
ePET-Positive Patients
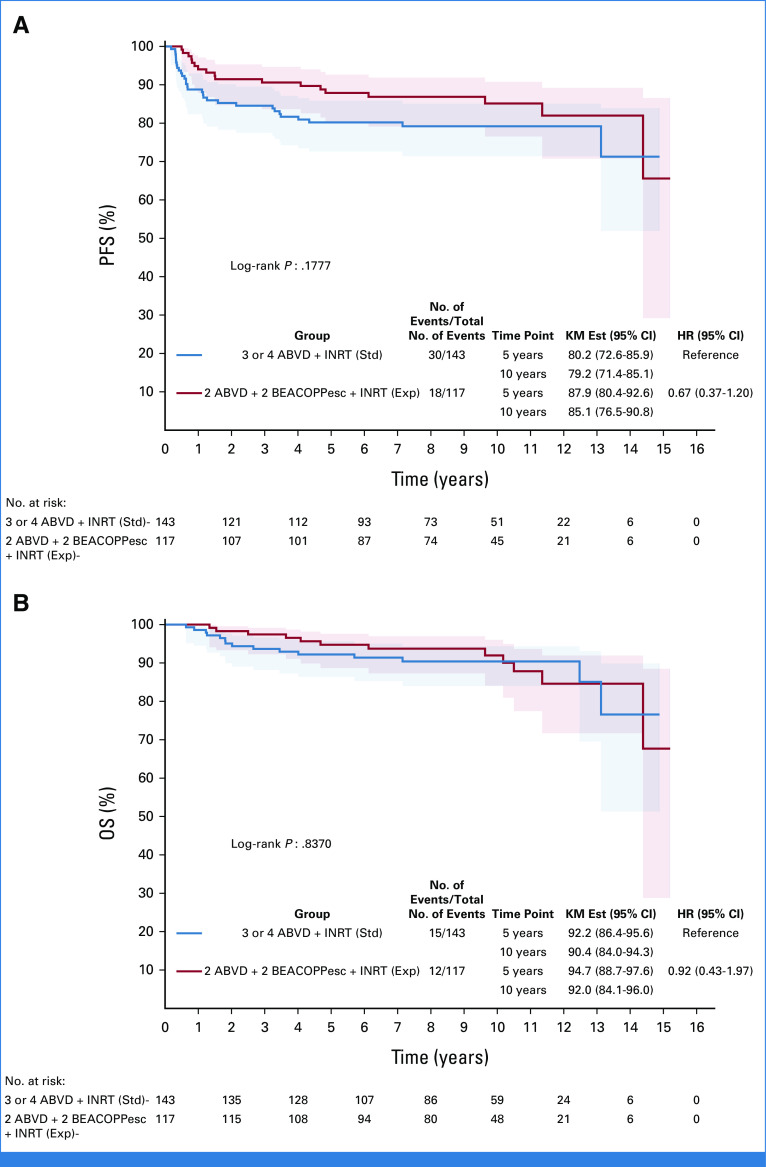
In the ePET-positive group, a total of 48 events occurred for PFS: 24 relapses and 6 deaths not related to HL in the ABVD + INRT arm and 12 relapses and 6 deaths not related to HL in the BEACOPPesc + INRT arm. The 10-year PFS rates were 79.2% (95% CI, 71.4 to 85.1) and 85.1% (95% CI, 76.5 to 90.8) in the ABVD + INRT and BEACOPPesc + INRT arms, respectively, with a HR of 0.67 (95% CI, 0.37 to 1.20; P = .1777; Fig 3A). The 10-year OS rates were 90.4% versus 92.0% for the ABVD + INRT and BEACOPPesc + INRT arms, respectively, with a HR of 0.92 (95% CI, 0.43 to 1.97; P = .8370; Fig 3B).
FIG 3.
(A) Ten-year PFS of early positron emission tomography-positive patients, according to treatment arms: ABVD + INRT versus BEACOPPesc + INRT; P = .1777. (B) Ten-year OS; P = .8370. ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; BEACOPPesc, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; Exp, experimental; HR, hazard ratio; INRT, involved-node radiotherapy; KM Est, Kaplan-Meier estimate; OS, overall survival; PFS, progression-free survival; Std, standard.
Prognostic Value of PET
In the subset of patients randomly assigned to standard treatment, ePET positivity resulted in a worse outcome in terms of PFS (HR, 4.9; 95% CI, 2.9 to 8.2; P < .0001; Data Supplement, Fig 3A) and OS (HR, 4.1; 95% CI, 2.0 to 8.4; P < .0001; Data Supplement, Fig 3B).
Late Adverse Events
A total of 420 patients (30%) experienced late adverse events (Data Supplement, Table 1). In ePET-negative patients, the cumulative incidence rate of late adverse events was similar in the standard and the experimental arms in the F (P = .7077; Data Supplement, Fig 4A) and U (P = .4734; Data Supplement, Fig 4B) groups.
In the ePET-positive group, the incidence rate of late adverse events was 30.7% (95% CI, 22.6 to 39.1) and 33.9% (95% CI, 24.3 to 43.8) in the ABVD + INRT and BEACOPPesc + INRT arms, respectively (P = .7578); 15.9% (95% CI, 9.7 to 23.3) and 14.1% (95% CI, 7.9 to 22.1) for late pulmonary adverse events (P = .6634); 7.7% (95% CI, 3.5 to 14.3) and 9.3% (95% CI, 4.3 to 16.6) for late cardiovascular adverse events (P = .6089); and 4.6% (95% CI, 1.7 to 10.0) and 4.7% (95% CI, 1.5 to 10.7), respectively, for late second malignancies (P = .7375; Data Supplement, Fig 5).
DISCUSSION
The H10 trial was designed to assess the role of PET-adapted treatment in patients with stage I and II HL, offering early intensification of chemotherapy in PET-positive patients and sparing radiotherapy (RT) in PET-negative patients.
At the time of primary analysis, after a median follow-up of 4.5 years, a significant improvement (13.2%) in 5-year PFS was observed in the BEACOPPesc + INRT arm compared with continuation with ABVD + INRT. This finding was considered of immediate clinical relevance.4 Now, with a longer follow-up (9.5 years), it has emerged that switching from ABVD to BEACOPPesc was not associated with a statistically significant better outcome, suggesting that exploring an earlier use of brentuximab vedotin–6-8 or nivolumab9-containing regimens is opportune. However, escalation to two courses of BEACOPPesc resulted safe: the 10-year cumulative incidence of late adverse events, namely, second malignancies, was similar in the ABVD and BEACOPPesc arms.
Our results confirm the excellent overall outcome of ePET-negative patients, either after combined modality treatment (CMT) or after chemotherapy alone. However, CMT resulted in better disease control because omitting RT resulted in more early relapses, mainly affecting the originally involved areas. Fortunately, the low tumor burden at the time of relapse and the efficacy of the timely adoption salvage therapy allowed patients with relapse to achieve a second and durable remission in almost all cases. Instead, the achievement of a negative ePET, as assessed in the H10 trial, seems not to be the ideal tool for identifying those patients who could be spared RT.
In conclusion, this long-term analysis confirmed the previous findings that PET positivity after two cycles of ABVD is associated with a worse outcome, unfortunately not corrected completely by intensification with two cycles of BEACOPPesc.
ACKNOWLEDGMENT
We thank the patients and their families for participation in this study. We also thank Liliana Baila, Sabine de Bedout, Jocelyne Flament, Matthias Karrasch, Emad Shash, and Safaa Ramadan from European Organisation for Research and Treatment of Cancer Headquarters and Monica Bellei and Stefania Badiali from the Fondazione Italiana Linfomi and Anissa Zarour from Assistance Publique-Hospitaux de Paris for their contribution to the present study. The list of participating centers is available in Appendix 1 (online only).
APPENDIX 1. Participating Centers
Belgium: A. Van Hoof, AZ St Jan; R. De Bock, K. Beel, ZNA Middelheim; W.A. Schroyens, UZ Antwerpen; A. Ferrant, Cliniques Universitaires Saint-Luc; A. Bosly, Centre Hospitalier Universitaire (CHU) Dinant Godinne, UCL Namur; S. Vansteenweghen, Centre Hospitalier Regional de la Citadelle; G.E.G. Verhoef, Y. Lievens, UZ Leuven, Campus Gasthuisberg; B. De Prijck, CHU Sart-Tilman; V. Mathieux, Clinique et Maternité Sainte Elisabeth; M. André, Grand Hospital de Charleroi, Site Notre-Dame; P. Mineur, Grand Hospital de Charleroi–Hospitaux Saint-Joseph. Croatia: I. Aurer, University Hospital Rebro. Denmark: L. Specht, Rigshospitalet. France: C. Haouin, Assistance Publique-Hospital de Paris (APHP)–CHU Henri Mondor; M. Aoudjhane, APHP–Hospital Saint Antoine; C. Fruchart, Centre Regional François Baclesse; A. Stamatoullas-Bastard, Centre Henri Becquerel; F. Boue, APHP–Hospital Antoine Beclere; A. Thyss, Centre Antoine Lacassagne, G. Laurent, CHU de Toulouse–Hospital de Purpan; C. Ferme, Institut Gustave Roussy, C. Sebban, Centre Leon Berard; J. Gabarre, APHP–La Pitie ´ Salpetriere; A. Devidas, CH Sud Francilien-Gilles De Corbeil; S. Natarajan-Ame, Hospital Universitaires de Strasbourg-Hautepierre; S. Glaisner, Institut Curie–Hospital Rene Huguenin; F. Morschhauser, Centre Hospitalier Regional Universitaire (CHRU) Lille; D. Jaubert, Hospital Bellevue; R. Delarue, APHP–Hospital Necker; O. Casasnovas, CHU de Dijon; R. Bouabdallah, Institut J. Paoli & I. Calmettes; O. Tournilhac, Nouvel Hospital Estaing; P. Helias, CHRU de Besançon– Hospital Jean Minjoz; M. Fabbro, Institut Regional du Cancer Montpellier; P. Brice, APHP–Hospital Saint- Louis; S. Bologna, CHU de Nancy–Hospital De Brabois; D. Bordessoule, CHU de Limoges–Hospital Dupuytren; B. Corront, CH d'Annecy; S. Castaigne, CH de Versailles–Hospital André Mignot; H. Orfeuvre, CH de Bourg-en-Bresse; O. Reman, CHU de Caen–Hospital Clemenceau; J.P. Marolleau, CHU d'Amiens, Hospital Sud; D. Assouline, Clinique du Mail; C. Kulekci, CH Marc Jacquet; J.C. Eisenmann, CH de Mulhouse, Hospital Emile Muller-Moenchsberg; I. Plantier, CH de Roubaix–Hospital V Provo; L. Detourmignies, CH de Roubaix–Hospital V Provo. Italy: S. Hoahus, Policlinico Universitario A. Gemelli–Universita del Sacro Cuore, Roma; F. Lanza, Ospedale Santa Maria delle Croci, Ravenna; R. Cairoli, Ospedale Niguarda Ca Granda, Milano; A. Ferrario, Ospedale Di Circolo E Fondazione Macchi, Varese; R. Freilone, Azienda Ospedaliera Citta della Salute et della Scienza di Torino–Ospedale Molinette, Torino; F. Merli, S. Luminari, Arcispedale di S. Maria Nuova, Reggio Emilia; M. Martelli, Universita di Roma “La Sapienza”—Clinica Ematologica Università di Roma “La Sapienza”; P.L. Zinzani, Universita di Bologna; S. Galimberti, Azienda Ospedaliera Universitaria Pisana, Pisa; D. Damiani, Azienda Ospedaliera-Universitaria “Santa Maria della Misericordia” di Udine; M. Spina, Centro di Riferimento Oncologico, Aviano; J.M. Ferreri Andres, Ospedale San Raffaele; C. Petti, Istituto Regina Elena/Istituto Fisioterapici Ospitalieri, Milano; S. Bolis, Ospedale San Gerardo, Monza; L. De Fazio, Ospedale Maggiore di Lodi; E. Angelucci, Istituto di Ricovero e Cura a Carattere Scientifico Azienda Ospedaliera Universitaria San Martino “IST” Istituto Nazionale per la Ricerca sul Cancro, Genova; B. Martino, C. Stelitano, Azienda Ospedaliera Bianchi-Melacrino-Morelli. Reggio Calabria; P. De Fabritiis, Ospedale San Eugenio, Roma; F. Leonardi, Ematologia Azienda Ospedaliera di Parma; M. Rossi, Ematologia Ospedale Regionale A. Pugliese, Catanzaro; L. Arcaini, M. Gotti, Policlinico San Matteo, Pavia; G. La Nasa, Ospedale Oncologico A. Businco, Cagliari; E. Pennese, Ospedale Civile Pescara; A. Bari, Azienda Ospedaliero–Universitaria Policlinico di Modena; C. Fozza, Istituto di Ematologia–Universita di Sassari; P. Tosi, Ospedale Infermi di Rimini; M. Massaia, Ematologia Ospedale Santa Croce, Cuneo; C. Foli, Ospedale Civile Ivrea; N. Cascavilla, Ospedale Sollievo Della Sofferenza, San Giovanni Rotondo; R. Musto, Universita Degli Studi–Policlinico, Bari; A. Tucci, A, Re, Ematologia Spedali Civili di Brescia; W. Barcellini, Ematologia Policlinico di Milano; M. Ladetto, Ospedale Civile, Alessandria; G. Polimeno, Ospedale Generale Regionale; Acquaviva delle Fonti (BA); U. Consoli, Ospedale Garibaldi–Universita di Catania; D. Pastore, Ospedale Regionale A. di Summa, Brindisi; A. Santoro, M. Balzarotti, Istituto Clinico Humanitas, Rozzano (MI); M. Aglietta, Fondazione del Piemonte per l'Oncologia–Istitute for Cancer Research and Treatment, Candiolo (TO); A. Fabbri, Policlinico “le Scotte”, Siena; M. Musso, La Maddalena S.P.A., Palermo; D. Mannina, Azienda Ospedaliera Papardo, Messina; M. Martelli, Ematologia Università La Sapienza, Policlinico Umberto I, Roma; A.M. Liberati, Azienda Ospedaliera S. Maria, Terni; A. Tafuria, M.C. Cox, Ematologia Ospedale Sant'Andrea, Roma; G. Gaidano, Amedeo Avogadro University of Eastern Piedmont-Ospedale Maggiore della Carita, Novara; M. Gentile, Ematologia, A.O. di Cosenza; R. Fanin, Ematologia Santa Maria della Misericordia Hospital, Udine; G. Martinelli, Ematologia, Istituto Scientifico Romagnolo per lo Studio et la Cura dei Tumori, Meldola (FC) S. Storti, Fondazione di Ricerca et Cura Giovanni Paolo II–Universita Cattolica del Sacro Cuore, Campobasso; D. Vallisa, Ospedale Civile, Piacenza. The Netherlands: G.J. Goverde, Amphia Ziekenhuis (Molengracht); M. Silbermann, Tergooiziekenhuizen (Blaricum); E.F.M. Posthuma, Reinier De Graaf Gasthuis; H.A.M. Sinnige, Jeroen Bosch Ziekenhuis; M.L.M. Lybeert, Catharina Ziekenhuis; F. Heyning, Medisch Centrum Haaglanden–Antoniushove; F. Peters, Maaslandziekenhuis; J.W. Baars, The Netherlands Cancer Institute–Antoni Van Leeuwenhoeziekenhuis; O.S. Leeksma, Onze Lieve Vrouw Gasthuis; F. Ong, Medisch Spectrum Twente; G.K.S. Jie, Atrium Medisch Centrum; H. Schouten, Academisch Ziekenhuis Maastricht; E.C. Dompeling, Isala Klinieken (Sophia); M.R. Schipperus, HagaZiekenhuis (Leyweg); P.J. Lugtenburg, Erasmus MC Hospital; M.O. Den Boer, Laurentius Ziekenhuis Roermond; P.J. Lugtenburg, Erasmus MC Cancer Institute (Daniel den Hoed); J. Raemaekers, Radboud University Medical Center Nijmegen; A.D.G. Krol, Leiden University Medical Center; G.W. Van Imhoff, University Medical Center Groningen; M.J. Kersten, Academisch Medisch Centrum–Universiteit van Amsterdam; R. Van Der Griend, E.V. Planken, Diaconessenhuis; R. Fijnheer, Meander Medisch Centrum de Lichtenberg; D.H. Biesma, H.R. Koene, St Antonius Ziekenhuis; W. Smit, Radiotherapeutisch Instituut Friesland; P. Joosten, M. Hoogendoorn, Medisch Centrum Leeuwarden-Zuid. Slovakia: A. Vranovsky, National Cancer Institute. Switzerland: G. Berthod, CHU Vaudois-Lausanne.
Alessandro Re
Consulting or Advisory Role: Takeda, Italfarmaco, Incyte
Speakers' Bureau: Take, Takeda
Julien Lazarovici
Consulting or Advisory Role: Takeda
Research Funding: BMS (Inst)
Travel, Accommodations, Expenses: Takeda
Francesco Merli
Consulting or Advisory Role: Janssen, Gilead Sciences, MSD, Takeda, Roche, Novartis, Incyte
Travel, Accommodations, Expenses: Janssen, Gilead Sciences, EUSA Pharma, Celgene, Roche, Takeda
Lena Specht
Honoraria: Kyowa Kirin International
Research Funding: Varian Medical Systems, ViewRay (Inst)
Travel, Accommodations, Expenses: Kyowa Kirin International
Jean-Marc Schiano de Colella
Honoraria: Takeda, Janssen, GlaxoSmithKline, AbbVie
Consulting or Advisory Role: Sanofi
Travel, Accommodations, Expenses: Pfizer, Amgen
Martin Hutchings
Consulting or Advisory Role: Takeda, Roche, Genmab, Janssen, AbbVie
Research Funding: Celgene (Inst), Genmab (Inst), Roche (Inst), Takeda (Inst), Novartis (Inst), Janssen (Inst), Merck (Inst), AbbVie (Inst), AstraZeneca (Inst)
Annibale Versari
Honoraria: GE Healthcare
Travel, Accommodations, Expenses: Novartis
Aspasia Stamatoulas
Honoraria: Takeda
Consulting or Advisory Role: Pfizer, Janssen
Travel, Accommodations, Expenses: Pfizer, Bristol Myers Squibb/Celgene, Takeda
Berthe Aleman
Honoraria: MSD (Inst)
Sanne Tonino
Honoraria: Roche, Takeda
Consulting or Advisory Role: Incyte, Bristol Myers Squibb Foundation
Marc André
Consulting or Advisory Role: Takeda, BMSi, Roche, AbbVie, Novartis, Eli Lilly Benelux
Research Funding: Takeda (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Gilead Sciences, AstraZeneca, Takeda
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 17th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 13-17, 2023.
SUPPORT
This long-term follow-up analysis of the H10 trial (ClinicalTrials.gov identifier: NCT00433433) was supported by European Organisation for Research and Treatment of Cancer (Belgium), LYmphoma Study Association (France), Fondazione Italiana Linfomi (Italy), Fondation Belge contre le Cancer (Belgium), Dutch Cancer Society (the Netherlands), Institut National du Cancer (France), Assistance Publique des Hopitaux de Paris (France), Societe Française de Medecine Nucleaire et Imagerie Moleculaire (France), Associazone Angela Serra (Italy), van Vlissingen Lymfoom Fonds (the Netherlands), and Fondation Mont-Godinne (Belgium).
AUTHOR CONTRIBUTIONS
Conception and design: Catherine Fortpied, Lena Specht, Martin Hutchings, Theodore Girinsky, Umberto Ricardi, John Raemaekers, Marc André
Administrative support: John Raemaekers, Marc André
Provision of study materials or patients: Massimo Federico, Julien Lazarovici, Francesco Merli, Lena Specht, Jean-Marc Schiano de Colella, Martin Hutchings, Aspasia Stamatoulas, Theodore Girinsky, Umberto Ricardi, Berthe Aleman, John Raemaekers, Marc André
Collection and assembly of data: Catherine Fortpied, Yana Stepanishyna, Manuel Gotti, Richard van der Maazen, Caterina Cristinelli, Alessandro Re, Wouter Plattel, Julien Lazarovici, Francesco Merli, Lena Specht, Martin Hutchings, Annibale Versari, Aspasia Stamatoulas, Umberto Ricardi, Berthe Aleman, Bart Meulemans, Sanne Tonino, Marc André
Data analysis and interpretation: Massimo Federico, Catherine Fortpied, Richard van der Maazen, Wouter Plattel, Lena Specht, Jean-Marc Schiano de Colella, Martin Hutchings, Véronique Edeline, Umberto Ricardi, John Raemaekers, Marc André
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Follow-Up of the Response-Adapted Intergroup EORTC/LYSA/FIL H10 Trial for Localized Hodgkin Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Alessandro Re
Consulting or Advisory Role: Takeda, Italfarmaco, Incyte
Speakers' Bureau: Take, Takeda
Julien Lazarovici
Consulting or Advisory Role: Takeda
Research Funding: BMS (Inst)
Travel, Accommodations, Expenses: Takeda
Francesco Merli
Consulting or Advisory Role: Janssen, Gilead Sciences, MSD, Takeda, Roche, Novartis, Incyte
Travel, Accommodations, Expenses: Janssen, Gilead Sciences, EUSA Pharma, Celgene, Roche, Takeda
Lena Specht
Honoraria: Kyowa Kirin International
Research Funding: Varian Medical Systems, ViewRay (Inst)
Travel, Accommodations, Expenses: Kyowa Kirin International
Jean-Marc Schiano de Colella
Honoraria: Takeda, Janssen, GlaxoSmithKline, AbbVie
Consulting or Advisory Role: Sanofi
Travel, Accommodations, Expenses: Pfizer, Amgen
Martin Hutchings
Consulting or Advisory Role: Takeda, Roche, Genmab, Janssen, AbbVie
Research Funding: Celgene (Inst), Genmab (Inst), Roche (Inst), Takeda (Inst), Novartis (Inst), Janssen (Inst), Merck (Inst), AbbVie (Inst), AstraZeneca (Inst)
Annibale Versari
Honoraria: GE Healthcare
Travel, Accommodations, Expenses: Novartis
Aspasia Stamatoulas
Honoraria: Takeda
Consulting or Advisory Role: Pfizer, Janssen
Travel, Accommodations, Expenses: Pfizer, Bristol Myers Squibb/Celgene, Takeda
Berthe Aleman
Honoraria: MSD (Inst)
Sanne Tonino
Honoraria: Roche, Takeda
Consulting or Advisory Role: Incyte, Bristol Myers Squibb Foundation
Marc André
Consulting or Advisory Role: Takeda, BMSi, Roche, AbbVie, Novartis, Eli Lilly Benelux
Research Funding: Takeda (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Gilead Sciences, AstraZeneca, Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1.Radford J, Illidge T, Counsell N, et al. : Results of a trial of PET-directed therapy for early-stage Hodgkin's lymphoma. N Engl J Med 372:1598-1607, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Fuchs M, Goergen H, Kobe C, et al. : Positron emission tomography-guided treatment in early-stage favorable Hodgkin lymphoma: Final results of the International, randomized phase III HD16 trial by the German Hodgkin Study Group. J Clin Oncol 37:2835-2845, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Borchmann P, Plütschow A, Kobe C, et al. : PET-guided omission of radiotherapy in early-stage unfavourable Hodgkin lymphoma (GHSG HD17): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 22:223-234, 2021 [DOI] [PubMed] [Google Scholar]
- 4.Raemaekers J, André M, Federico M, et al. : Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 32:1188-1194, 2014 [DOI] [PubMed] [Google Scholar]
- 5.André M, Girinsky T, Federico M, et al. : Early positron emission tomography response–adapted treatment in stage I and II Hodgkin lymphoma: Final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 35:1786-1798, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Fornecker LM, Lazarovici J, Aurer I, et al. : Brentuximab vedotin plus AVD for first-line treatment of early-stage unfavorable Hodgkin lymphoma (BREACH): A multicenter, open-label, randomized, phase II trial. J Clin Oncol 41:327-335, 2023 [DOI] [PubMed] [Google Scholar]
- 7.Ansell SM, Radford J, Connors JM, et al. : Overall survival with brentuximab vedotin in stage III or IV Hodgkin's lymphoma. N Engl J Med 387:310-320, 2022 [DOI] [PubMed] [Google Scholar]
- 8.Eichenauer DA, Plütschow A, Kreissl S, et al. : Incorporation of brentuximab vedotin into first-line treatment of advanced classical Hodgkin's lymphoma: Final analysis of a phase 2 randomised trial by the German Hodgkin Study Group. Lancet Oncol 18:1680-1687, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Kambhampati S, Herrera AF: Incorporating novel agents into frontline treatment of Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program 2022:706-716, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]