Abstract
Background:
Implant-based reconstruction is the most common method of postmastectomy reconstruction. Many patients require postmastectomy radiation (PMRT). Tissue expanders (TEs), typically inserted as a first stage, have historically been placed subpectorally. More recently, prepectoral reconstruction has gained popularity, but its impact on PMRT is unknown. Prior studies focus on complication rates and aesthetic outcomes. This study examines whether there is a difference in radiation dosimetry among patients undergoing prepectoral versus subpectoral TE reconstruction.
Methods:
Electronic medical records and radiation plans of 50 patients (25 prepectoral, 25 subpectoral) who underwent mastectomy with immediate TE reconstruction at our institution or affiliate site were reviewed. Pectoralis major muscle and chest wall structures were contoured and mean percentage volumes of these structures receiving less than 95%, 100%, and more than 105% target radiation dose were calculated, as were heart and ipsilateral lung doses. Welch two sample t test, Fisher exact test, and Pearson chi-squared tests were performed.
Results:
The groups had comparable patient and tumor characteristics and underwent similar ablative and reconstructive procedures and radiation dosimetry. Subpectoral patients had larger mean areas receiving less than 95% target dose (“cold spots”); prepectoral patients had larger mean areas receiving greater than 105% (“hot spots”) and 100% target doses. There were no differences in chest wall, heart, and lung doses.
Conclusions:
Our results demonstrate an increased mean percentage area of pectoralis cold spots with subpectoral reconstruction and increased area of hot spots and 100% dose delivery to the pectoralis in prepectoral patients. Larger studies should analyze long-term effects of prepectoral reconstruction on radiation dosing and recurrence rates.
Takeaways
Question: Does prepectoral placement of a tissue expander impact the delivery of postmastectomy radiation therapy compared with subpectoral placement?
Findings: This rectrospective cohort study showed that subpectoral patients had larger mean areas receiving less than 95% target dose (“cold spots”); prepectoral patients had larger mean areas receiving greater than 105% (“hot spots”) and 100% target doses. There were no differences in chest wall, heart, and lung doses.
Meaning: Tissue expander position may influence postmastectomy radiation dosimetry.
INTRODUCTION
Implant-based reconstruction is the most common form of postmastectomy breast reconstruction in the United States.1 Implants may be placed deep (subpectoral) or superficial (prepectoral) to the pectoralis muscle. Until recently, subpectoral placement was the most popular approach; however, in recent years, prepectoral reconstruction has been widely adopted. Prepectoral implant-based breast reconstruction has become increasingly popular due to the ability to eliminate animation deformity and reduce discomfort related to disruption of the pectoralis muscle.2 Prepectoral placement has been shown to have equivalent complication rates compared with subpectoral reconstructions, as well as potentially superior aesthetic results after postmastectomy radiation therapy (PMRT).3,4
PMRT reduces local and overall cancer recurrence and/or breast cancer mortality in patients with large tumors, positive margins, or metastasis to axillary lymph nodes or skin.5 In patients with positive axillary lymph nodes, PMRT is typically delivered to the chest wall, supraclavicular and/or axillary fossa, and the internal mammary lymph nodes.5,6 Conventional PMRT dosing is 50–50.4 Gray (Gy) in 1.8–2.0 Gy per fraction (25–28 total fractions) to the chest wall and 45–50 Gy in 1.8–2.0 Gy per fraction (25 total fractions) to regional lymph nodes.7 Radiation to the internal mammary lymph nodes may also be performed.8 Careful mapping is required to deliver the appropriate radiation dose to areas at risk, while minimizing radiation to nearby structures, which can result in cardiovascular disease, pneumonitis, lymphedema, and pulmonary fibrosis.9
Tissue expanders present several theoretical obstacles for radiation planning, such as the degree of inflation, the rigidity of the device, and presence of the magnetic inflation port.10 Prior investigations have identified up to 30% dose reduction in the area around the magnetic port, if the port is not accounted for by the radiation dosimetry.11 Additionally, due to the irregular contour of an expander (eg, from the folds in and prominence of the implant), a radiation dose in the presence of a bolus may not conform as well to the skin or chest wall.
Prior studies on prepectoral reconstruction have focused on the surgical safety and aesthetic outcomes of the procedure.12,13 Notably, prepectoral implant-based reconstruction has been widely adopted without any rigorous investigation of its impact on the delivery of adjuvant radiation therapy. Radiation treatment planning focuses on areas of insufficient dose delivery (“cold spots”), excess dose delivery (“hot spots”), and the amount of radiation delivered to the heart and lungs to optimize oncologic control while minimizing morbidity. This study sought to investigate whether prepectoral tissue expander placement impacted PMRT dosimetry compared with subpectoral placement. Primary endpoints were the presence of cold spots or hot spots in the pectoralis major muscle and/or chest wall within the boundaries targeted by PMRT. Secondary endpoints were the amount of radiation delivered to the heart and lungs, as well as surgical and radiation-related complications.
METHODS
Institutional review board approval (protocol #2021P002856) was obtained, and billing data were used to identify patients who underwent immediate placement of a breast prosthesis at the time of mastectomy (CPT code 19357). The electronic medical records (EMRs) of patients undergoing mastectomy with subpectoral or prepectoral implant-based reconstruction at The Brigham and Women’s Hospital/Dana-Farber Cancer Institute in Boston, Massachusetts followed by PMRT at our institution or one of our Dana-Farber Cancer Institute affiliate sites between 2017 and 2021 were reviewed and 25 patients who underwent subpectoral and 25 patients who underwent prepectoral tissue expander reconstruction were identified. Patients who underwent treatment elsewhere or who had missing data were excluded, as were patients who underwent tissue expander removal before the completion of radiation. Retrospective review of records from the EMR and Eclipse v16.1 radiation planning system (Varian Medical Systems, Palo Alto, Calif.) were used to obtain relevant demographic, clinical and radiation treatment planning data.
The treating radiation oncologists prescribed patients a standard PMRT protocol, consisting of delivery of 42.56 or 50 Gy to the chest wall using opposing tangential fields. When clinically indicated, some patients also received radiation to the supraclavicular and/or axillary lymph nodes. Patients were simulated in the supine position and 96% of prepectoral and 88% of subpectoral patients were simulated using a breath hold technique. Physicists and radiation oncologists intentionally alter plans in designing treatment protocols based on prepectoral or subpectoral positioning, but did account for the magnetic port in all patients to minimize hot and cold spots. The treatment goal was 100% dose delivery to the target area of chest wall and specified regional lymph nodes while minimizing the heart and lung. Skin bolus, nodal radiation, and contralateral tissue expander deflation were at the discretion of the treating radiation oncologist.
Patients who underwent radiation treatment at The Brigham and Women’s Hospital/Dana-Farber Cancer Institute underwent computed tomography (CT) imaging on one of the two scanners: Lightspeed RT16 (GE Medical Systems, Waukesha, Wisc.) or Somatom Confidence (Siemens Medical Solutions USA, Inc., Malvern, Pa.). CT scans were acquired with clinical protocols for breast simulation with either 2.5-mm slice thickness (on the GE Lightspeed RT16 scanner) or 3-mm slice thickness (on the Siemens Somatom Confidence scanner). For patients who underwent radiation at a Dana-Farber Cancer Institute affiliate location, 2.5-mm slices we obtained using the Lightspeed RT16 (GE Medical Systems, Waukesha, Wisc.) and Discovery (GE Medical Systems, Waukesha, Wisc.) scanners. Treatment plans were created in the Eclipse treatment planning system and calculated with the analytical anisotropic algorithm.
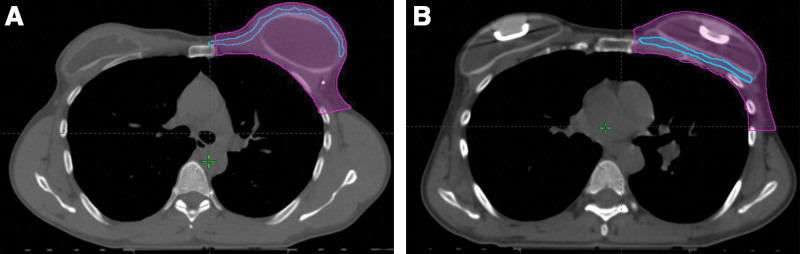
Standard radiation planning does not identify the pectoralis major as distinct from the remainder of the chest wall, so the cross-dimensional area of the pectoralis major was drawn by hand by a plastic surgeon (L.C.H.) retrospectively in the Eclipse treatment planning system. Axial and sagittal cross sectional CT images were examined and the pectoralis major muscle highlighted. Contouring pectoralis major was performed every 0.5 mm in the axial series and every 1 cm in the sagittal series. Figure 1 shows representative images of the contouring performed. Contours were then interpolated and accuracy verified by a second member of the surgical team (J.E.S.), with refinements made as needed.
Fig. 1.
Two examples of the contouring that was performed to calculate chest wall and pectoralis major muscle volumes, using radiation planning CTs. In these axial images, the chest wall area is depicted in magenta and the pectoralis major muscle area is outlined in blue. A, A representative image from a patient who underwent left subpectoral tissue expander reconstruction. The pectoralis major muscle is the thin blue structure running subcutaneously anterior to the fluid-filled tissue expander. B, A representative image from a patient who underwent left prepectoral tissue expander reconstruction. The pectoralis major muscle is the thin blue structure located deep to the fluid-filled tissue expander, just anterior to the ribs. The metallic ports in both tissue expanders are visible at this level.
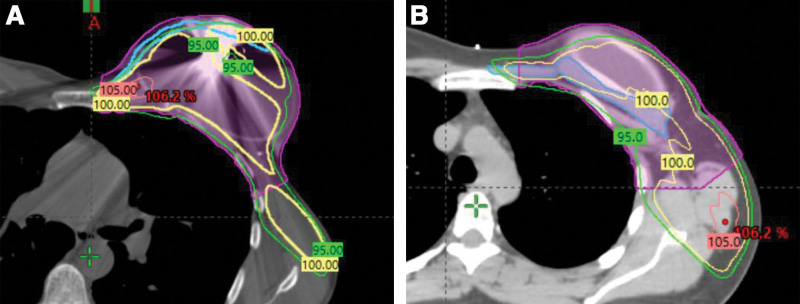
The area of chest wall targeted by PMRT was manually contoured during treatment planning according to boundaries described by the Radiation Therapy Oncology Group guidelines (Fig. 1).14 This area is defined by the following anatomic landmarks: cranially by the inferior border of clavicular head, caudally by the inferior extent of the breast, medially by the costo-sternal junction, laterally by the mid-axillary line, deep by the rib-pleural interface, and superficially by the skin. Contouring for the chest wall area was performed by a radiation oncology physicist or physician. A cold spot was defined as an area receiving less than 95% of the target dose and a hot spot was defined as an area receiving greater than 105% of the target dose (Fig. 2).15 Dose–volume histogram was used to ascertain the percentage of ipsilateral lung volume receiving greater than 20 Gy (V20) and the heart mean dose in Gy. The decision was made to analyze cold spots and hot spots as percentages of a structure’s volume rather than by volume of cold or hot spots alone to neutralize the effect of any difference in pectoralis or chest wall volumes between the two cohorts.
Fig. 2.
Two images demonstrating the areas that received various prescription doses of radiation therapy. The green line outlines an area that received 95% dose; the yellow line, 100% dose; and the pink line, 105% dose. The cold spot for 95% would be the area outside of the green line, and the hotspot would be the area contained within the pink line. A, Various dosage lines on a patient who underwent subpectoral tissue expander placement. The pectoralis major is mapped in blue and a portion of the pectoralis major is visible superficial to the green 95% line, indicating that portion of pectoralis major muscle was part of a cold spot. B, Various dosage lines on a patient who underwent prepectoral tissue expander placement. The pectoralis major is mapped in blue. Nearly all of the pectoralis major muscle is contained within the green 95% dose curve, indicating a minimal area of cold spot.
Categorical variables were summarized using frequency with percentage. Continuous variables were reported as mean and SD. Welch two sample t test, Fisher exact test, and Pearson chi-squared test were performed in case of hypothesis testing status quo. Simple linear regression models were used to assess the bivariable association between each of the outcomes and the set of considered covariates in the study, separately. Multivariable linear regression models were used to identify the association between all the outcomes considered in this study with all the covariates considered in the study. Significance of all hypothesis tests was considered at alpha level of significance less than 5%. All analyses were performed with R software, version 4.1.0.16
RESULTS
The EMRs of 50 patients who underwent immediate tissue expander reconstruction were reviewed (25 prepectoral and 25 subpectoral). Any of eight breast surgeons, eleven plastic surgeons, and eight radiation oncologists were involved in each patient treatment. Baseline demographic and clinical characteristics are depicted in Table 1. There were no significant differences between the prepectoral and subpectoral cohorts with regard to mean age, diagnosis of diabetes, body mass index, smoking status, history of breast surgery, or the receipt of neoadjuvant chemotherapy, endocrine, or anti-Her2 therapies. There were also no statistically significant differences in the two cohorts, based on preoperative clinical stage, postoperative pathologic stage, tumor type, or weight of the mastectomy specimen.
Table 1.
Demographic and Clinical Characteristics of the Prepectoral and Subpectoral Cohorts
| Characteristic | Prepectoral (N = 25)* | Subpectoral (N = 25)* | P † |
|---|---|---|---|
| Age | 44 (7) | 48 (10) | 0.2 |
| Diabetes mellitus | 0 (0%) | 1 (4.0%) | — |
| BMI | 26.0 (4.7) | 26.4 (6.1) | 0.8 |
| Weight class by BMI | 0.3 | ||
| Normal weight (BMI <25) | 13 (52%) | 15 (60%) | |
| Overweight (BMI 25.0–29.9) | 8 (32%) | 3 (12%) | |
| WHO obesity class 1 (30–34.9) | 3 (12%) | 4 (16%) | |
| WHO obesity class 2 (35–39.9) | 1 (4.0%) | 3 (12%) | |
| Smoking status | 0.6 | ||
| Never | 20 (80%) | 19 (76%) | |
| Current | 0 (0%) | 2 (8.0%) | |
| Former | 5 (20%) | 4 (16%) | |
| History of ipsilateral breast surgery | 0.7 | ||
| Yes | 2 (8.0%) | 4 (16%) | |
| No | 23 (92%) | 21 (84%) | |
| Neoadjuvant chemotherapy | 0.3 | ||
| Yes | 13 (52%) | 9 (36%) | |
| No | 12 (48%) | 16 (64%) | |
| Neoadjuvant endocrine therapy | — | ||
| Yes | 2 (8%) | 1 (4%) | |
| No | 23 (92%) | 24 (96%) | |
| Neoadjuvant anti-Her2 therapy | — | ||
| Yes | 22 (88%) | 22 (88%) | |
| No | 3 (12%) | 3 (12%) | |
| Preoperative clinical stage (AJCC) | 0.6 | ||
| Stage 0 | 1 (4%) | 3 (12%) | |
| Stage I | 10 (40%) | 9 (36%) | |
| Stage II | 11 (44%) | 12 (48%) | |
| Stage III | 3 (12.%) | 1 (4%) | |
| Pathologic stage (AJCC) | 0.3 | ||
| Stage 0 | 3 (12%) | 1 (4.0%) | |
| Stage I | 4 (16%) | 3 (12%) | |
| Stage II | 10 (40%) | 17 (68%) | |
| Stage III | 8 (32%) | 4 (16%) | |
| Tumor type | 0.6 | ||
| Invasive ductal | 15 (60%) | 13 (52%) | |
| Invasive lobular | 4 (16%) | 7 (28%) | |
| Invasive carcinoma with ductal and lobular features | 6 (24%) | 5 (20%) | |
| Ipsilateral mastectomy specimen weight (in grams) | 643 (352) | 616 (435) | 0.8 |
No P value reported when data insufficient to carry out a statistical test.
Mean (SD); n (%).
Welch Two Sample t test; Fisher exact test; Pearson chi-squared test.
BMI, body mass index; WHO, World Health Organization; AJCC, American Joint Committee on Cancer.
The surgical and radiation treatment characteristics of the two cohorts are presented in Table 2. Among patients who had undergone bilateral reconstruction, there was a statistically significant greater amount of contralateral tissue expander fill at the time of radiation for the subpectoral cohort compared with the prepectoral cohort (346 mL versus 220 mL, P = 0.047). The remaining surgical characteristics of the two cohorts demonstrated no statistically significant differences, including the type of mastectomies performed, laterality of reconstruction, acellular dermal matrix used, and ipsilateral tissue expander fill volume at the time of radiation. There were no statistically significant differences in radiation treatment characteristics, including laterality, whole breast radiation dose, use of a skin bolus, and utilization of a breath hold technique. The mean pectoralis major muscle volume approached statistical significance but did not reach it (100 cm3 in prepectoral cohort versus 121 cm3 in the subpectoral group, P = 0.053).
Table 2.
Surgical and Radiation Treatment Characteristics of the Two Cohorts
| Characteristic | Prepectoral (N = 25)* | Subpectoral (N = 25)* | P † |
|---|---|---|---|
| Type of LEFT mastectomy performed | 0.7 | ||
| None | 4 (16%) | 3 (12%) | |
| Nipple-sparing mastectomy | 6 (24%) | 4 (16%) | |
| Skin-sparing mastectomy | 13 (52%) | 17 (68%) | |
| Modified radical mastectomy | 2 (8.0%) | 1 (4.0%) | |
| Type of RIGHT mastectomy performed | 0.6 | ||
| None | 5 (20%) | 8 (32%) | |
| Nipple-sparing mastectomy | 5 (20%) | 2 (8.0%) | |
| Skin-sparing mastectomy | 14 (56%) | 14 (56%) | |
| Modified radical mastectomy | 1 (4.0%) | 1 (4.0%) | |
| Laterality of reconstruction | 0.7 | ||
| Left | 5 (20%) | 8 (32%) | |
| Right | 4 (16%) | 3 (12%) | |
| Bilateral | 16 (64%) | 14 (56%) | |
| Acellular dermal matrix used | >0.9 | ||
| Yes | 20 (80%) | 21 (84%) | |
| No | 5 (20%) | 4 (16%) | |
| Ipsilateral tissue expander fill volume at time of radiation (cc) | 462 (164) | 477 (178) | 0.8 |
| Contralateral tissue expander fill volume at time of radiation (in cc), if relevant | 220 (122) | 346 (184) | 0.047 |
| Radiation laterality | >0.9 | ||
| Left | 12 (48%) | 12 (48%) | |
| Right | 13 (52%) | 13 (52%) | |
| Whole breast radiation dose (in Gray) | 0.3 | ||
| 42.56 | 1 (4.0%) | 4 (16%) | |
| 50 | 24 (96%) | 21 (84%) | |
| Chest wall boost given | 0.088 | ||
| Yes | 8 (32%) | 3 (12%) | |
| No | 17 (68%) | 22 (88%) | |
| Skin bolus given | >0.9 | ||
| Yes | 22 (88%) | 23 (92%) | |
| No | 3 (12%) | 2 (8%) | |
| Breath hold technique used | 0.2 | ||
| Yes | 24 (96%) | 20 (80%) | |
| No | 1 (4%) | 5 (10%) | |
| Pectoralis volume (in cm3) | 100 (30) | 121 (46) | 0.053 |
n (%); Mean (SD).
Fisher exact test; Welch Two Sample t test; Pearson chi-squared test
Table 3 summarizes the two cohorts’ surgical and radiation-related complications. The frequency of radiation-related complications, including extensive skin peeling, radiation pneumonitis requiring steroids, and cardiac complications did not differ between cohorts. However, presence of pneumonitis and cardiac complications may be limited by the short follow-up in this study. Likewise, there was no significant difference in reconstructive complications, including infection requiring admission or loss of the reconstruction.
Table 3.
Complications of Surgery and Radiation in the Two Cohorts
| Characteristic | Prepectoral (N = 25)* | Subpectoral (N = 25)* | P † |
|---|---|---|---|
| Complication of radiation | |||
| Yes | |||
| No | |||
| Type of radiation-related complication | — | ||
| None | 21 (84%) | 23 (92%) | |
| Extensive skin peeling | 4 (16%) | 0 (0%) | |
| Radiation pneumonitis requiring steroids | 0 (0%) | 1 (4.0%) | |
| Cardiac complication | 0 (0%) | 1 (4.0%) | |
| Complication of reconstruction | 0.7 | ||
| Yes | 6 (24%) | 5 (20%) | |
| No | 19 (76%) | 20 (80%) | |
| Type of reconstruction-related complication | >0.9 | ||
| None | 19 (76%) | 20 (80%) | |
| Infection requiring admission | 2 (8.0%) | 1 (4.0%) | |
| Loss of reconstruction | 4 (16%) | 4 (16%) |
No P value reported when data insufficient to carry out a statistical test.
n (%).
Fisher exact test; Pearson chi-squared test.
There were significant differences regarding the radiation dose delivered to the pectoralis major muscle (see Table 4). Patients who underwent subpectoral tissue expander reconstruction had a larger mean volume of “cold spots”—those volumes receiving less than 95% of the desired dose—within the pectoralis major muscle (12% mean percentage volume in subpectoral versus 4% prepectoral, P = 0.002). In contrast, patients who underwent prepectoral reconstruction had a larger mean percentage of “hot spots” —those volumes receiving more than 105% of the target dose—delivered to the pectoralis major muscle (36% mean percentage volume prepectoral versus 25% subpectoral, P = 0 .039). Additionally, the pectoralis major muscle in patients with prepectoral reconstruction had larger mean percentage volumes receiving the 100% prescribed dose (91% mean percentage volume prepectoral versus 74% subpectoral, P < 0.001). In contrast, the mean percentage dose delivered to the chest wall was not different between the two cohorts. There was also no difference in the dose delivered to the heart and ipsilateral lung.
Table 4.
Primary Outcomes: Comparison of Dose–Volume Delivery to Pectoralis Major Muscle, Chest Wall, Heart, and Lung
| Outcomes | Prepectoral(N = 25)* | Subpectoral(N = 25)* | P † |
|---|---|---|---|
| Pectoralis major muscle outcomes | |||
| Pectoralis cold spot percentage (muscle volume that received <95% of prescription radiation dose divided by total muscle volume) | 4 (6) | 12 (11) | 0.002 |
| Pectoralis isodose percentage (muscle volume that received ≥100% of prescription radiation dose divided by total muscle volume) | 91 (9) | 74 (17) | <0.001 |
| Pectoralis hot spot percentage (muscle volume that received ≥105% of prescription radiation dose divided by total muscle volume) | 36 (15) | 25 (19) | 0.039 |
| Chest wall outcomes | |||
| Chest wall cold spot percentage (chest wall volume that received <95% of prescription radiation dose divided by total chest wall volume) | 16.0 (4.1) | 16.9 (4.2) | 0.5 |
| Chest wall isodose percentage (chest wall volume that received ≥100% of prescription radiation dose divided by total chest wall volume) | 74.3 (5.2) | 73.3 (5.6) | 0.5 |
| Chest wall hot spot percentage (chest wall volume that received ≥105% of prescription radiation dose divided by total chest wall volume) | 23 (7) | 21 (9) | 0.4 |
| Vital structures | |||
| Ipsilateral lung dose percentage (Goal V20 < 35%) | 29 (6) | 27 (5) | 0.3 |
| Heart mean dose (in Gray) | 1.57 (0.74) | 1.43 (0.71) | 0.5 |
All outcomes listed are percentage, except for heart mean dose.
Mean (SD).
Welch Two Sample t test; Fisher exact test; Pearson chi-squared test.
Multivariate regression analysis showed that subpectoral tissue expander positioning was significantly associated with larger cold spots in the pectoralis major muscle, when controlling for body mass index, pathologic stage, ipsilateral tissue expander fill, and radiation laterality. (See table, Supplemental Digital Content 1, which displays multivariable regression analysis for pectoralis cold spot, which is defined as percentage of pectoralis major muscle volume receiving less than 95% of intended radiation dose. http://links.lww.com/PRSGO/C904.) (See table, Supplemental Digital Content 2, which displays multivariable regression analysis for pectoralis cold spot for 98%, which is defined as percentage of pectoralis major muscle volume receiving less than 98% of intended radiation dose. http://links.lww.com/PRSGO/C905.) Total chest wall dose was also significantly associated with cold spot size, although much less so than position of the tissue expander relative to the pectoralis major muscle. Tissue expander position was a significant factor in cold spot size, whether the cutoff for cold spot was set at 95% (Supplemental Digital Content 1, http://links.lww.com/PRSGO/C904) or 98% (Supplemental Digital Content 2, http://links.lww.com/PRSGO/C905).
DISCUSSION
Tissue expander placement is commonly performed after mastectomy, and a percentage of patients then proceed to PMRT.17 Prepectoral reconstruction has been widely adopted by plastic surgeons despite the absence of data demonstrating equivalence of oncologic outcomes compared with subpectoral reconstruction, and standard practice in radiation oncology has continued without any major consideration given to the position of the tissue expander relative to the pectoralis major. In the current study, we found that patients who underwent subpectoral tissue expander placement had a larger area of cold spots, measured as the percentage of pectoralis major muscle volume receiving less than 95% of the prescribed radiation dose. This is important as 28%–78% of local recurrences occur within the anterior chest wall, consisting of the skin, subcutaneous tissue, and pectoralis musculature.18,19 Due to the small sample size and relatively short-term follow-up available for patients undergoing prepectoral reconstruction future studies are needed to determine the clinical implications of reconstructive technique on locoregional recurrence. We also found the mean percentage volume of the pectoralis muscle receiving 100% of the prescribed dose was greater in the prepectoral than subpectoral tissue expander cohort, and that hot spots were larger in the prepectoral cohort. Our study showed no significant differences in reconstruction or radiation-related complications. However, we focused on clinically symptomatic complications and may not have captured subclinical side-effects of radiation, such as reduction in pulmonary function testing and radiographic changes or symptoms that may have developed in a delayed fashion following completion of radiation therapy.20
There are several potential implications of our findings. First, future radiation planning may want to account for tissue expander positioning relative to the pectoralis major muscle. Second, one relative contraindication to prepectoral reconstruction, namely presence of tumor in close proximity to the pectoralis major muscle, may not be appropriate; in fact, our study suggests that prepectoral positioning may be advantageous when compared with subpectoral positioning of a tissue expander.2
Our finding of greater cold spots in subpectoral tissue expander placements may be attributed to a cold area beneath the skin, which arises due to the incident angle and energy of the tangential radiation beams. In standard radiation planning, the area 3–5 mm deep to the skin is excluded when calculating the clinical target volume during treatment planning.21 We opted to include that superficial 3–5 mm of tissue because the pectoralis major muscle is positioned there when a subpectoral reconstruction is performed. A skin bolus technique may be used to enhance dose delivery to that superficial 3–5 mm of tissue, and the majority of patients in both cohorts in this study received this, but it appears that the skin bolus technique alone did not result in equivalent pectoralis major muscle dose delivery.
The dose at which there is a clinically significant risk of cardiac or pulmonary toxicity has not been precisely quantified. However, a mean heart dose of less than 3 Gy and ipsilateral lung V20 of less than 30%–35% are generally targeted.22 Even seemingly small doses to the heart have been shown to increase the risk of long-term major cardiac complications, with an estimated 7.4% increased risk for every 1 Gy mean dose increment to the heart.23 Clinically significant pneumonitis has been shown to increase up to 36% with a V20 of more than 40%.22 Although a complete review of radiation delivery technology is beyond the scope of this article, three-dimensional conformal radiotherapy, intensity modulated radiotherapy with or without simultaneous integrated boost, arc therapy, and proton therapy have been developed to better target the treatment area and reduce cardiac risks.24 Larger studies with long-term follow-up are needed to determine if rates and severity of cardiopulmonary toxicity vary between patients undergoing prepectoral versus subpectoral reconstruction and radiation treatment.
Prior studies of PMRT following autologous reconstruction, where autologous tissue is positioned superficial to the pectoralis muscle similar to a prepectoral implant, have found mixed results regarding oncologic safety and ability to target the internal mammary lymph nodes, though is generally regarded as safe.25,26 However, autologous tissue is dissimilar to tissue expander, limiting generalizability. Additionally, many of the studies examining the effects of breast reconstruction on the delivery of radiation therapy were conducted using older radiation techniques that did not account for tissue heterogeneity, use CT scan artifact reduction software, or detail the specifics of radiation delivery.
To date, published literature has focused on the technical aspects, surgical safety, and aesthetic outcomes of prepectoral reconstruction.27,28 One retrospective study of 30 patients who underwent prepectoral implant-based reconstruction found that radiation fields were altered by the prepectoral implant location.29 However, this study lacked a comparison group. No study has systematically investigated the oncologic safety of prepectoral reconstruction versus subpectoral reconstruction.
This is the first study to directly compare the impact of prepectoral versus subpectoral tissue expander reconstruction on radiation treatment protocols. Patients were comparable in terms of patient, tumor, surgical, and radiation protocol characteristics. The variety of breast surgeons, plastic surgeons, and radiation oncologists involved in the treatment of the patients in our study is a source of heterogeneity. However, this variability also increases the generalizability of our findings, as many providers were involved in the care of these patients. Limitations of our study include its small sample size and retrospective design. Another potential criticism of our study is that CT planning images are not an adequate proxy for the actual radiation delivered to the chest wall. However, within radiation oncology, these plans are considered representative of the radiation actually delivered.30 These results are specific to the three-dimensional conformational radiation delivery method used at our institution, however, and may not be generalizable to other institutions using other methods of radiation delivery (eg, proton therapy).29 The radiation oncologists at our institution did not specifically adjust treatment planning based on the plane of reconstruction and our findings may differ from institutions with different approaches to radiation planning. Future studies should investigate the generalizability of our findings to institutions using differing radiation techniques.
This study focuses on the presence of “cold” and “hot” spots on the pectoralis muscle and/or chest. We did not categorize in what region of the pectoralis major or chest wall these cold and hot spots were specifically located. Prior studies suggest that the presence of a magnetic port does not significantly influence overall radiation dosimetry when accounted for during planning.10,11 However, larger scale studies should investigate whether cold spots are more prevalent in the area directly behind the magnetic port and the potential impact of this on recurrence (specifically in the pectoralis major muscle) in patients with prepectoral versus subpectoral tissue expanders.
Only patients with tissue expanders in place at the time of radiation were included in this pilot study, and it is unknown whether similar results would be observed in patients with implants in place following either direct-to-implant procedures or implant exchange before radiation delivery. There was no significant difference in the use of acellular dermal matrix between the two groups. However, it is unknown whether the presence or absence of acellular dermal matrix would alter our findings. Ultimately, long-term follow-up is needed to determine the clinical implications of any differences in radiation delivery between the two implant techniques in terms of recurrence rates, morbidity, and overall survival.
CONCLUSIONS
Implant-based reconstruction is the most common form of postmastectomy breast reconstruction in the United States. Although subpectoral placement has traditionally been the most popular approach, prepectoral reconstruction has become increasingly adopted. However, the impact of this technique of PMRT dosimetry has not been studied. We found an increased mean percentage area of pectoralis cold spots with subpectoral reconstruction and increased area of hot spots and 100% dose delivery to the pectoralis in prepectoral patients.
The clinical implications of our study results merit further investigation with larger, multi-institutional studies with long-term follow-up to account for variations in radiation practices, surgical techniques, and expansion protocols. Specifically, future studies should investigate oncologic (eg, cancer recurrence, mortality, and radiation toxicity/side-effects) and reconstructive implications (eg, whether the prepectoral or subpectoral should be preferred in patients with specific tumor features). For instance, contrary to current recommendations, the prepectoral approach may prove preferable in patients with more extensive lymphovascular invasion, inflammatory breast cancer, chest wall invasion, or internal mammary involvement, where higher doses to the pectoralis muscles may be achieved.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 19 December 2023.
Presented at the 2022 American Association of Plastic Surgeons annual meeting, San Diego, Calif.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Breast implant reconstruction: Surgery details, implant types, risks and more. Available at https://www.breastcancer.org/treatment/surgery/reconstruction/types/implants. Updated October 12, 2023. Accessed January 30, 2022.
- 2.Storm-Dickerson T, Sigalove N. Prepectoral breast reconstruction: the breast surgeon’s perspective. Plast Reconstr Surg. 2017;140:43S–48S. [DOI] [PubMed] [Google Scholar]
- 3.Sbitany H. Important considerations for performing prepectoral breast reconstruction. Plast Reconstr Surg. 2017;140:7S–13S. [DOI] [PubMed] [Google Scholar]
- 4.Sinnott CJ, Persing SM, Pronovost M, et al. Impact of postmastectomy radiation therapy in prepectoral versus subpectoral implant-based breast reconstruction. Ann Surg Oncol. 2018;25:2899–2908. [DOI] [PubMed] [Google Scholar]
- 5.Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. Pract Radiat Oncol. 2016;6:e219–e234. [DOI] [PubMed] [Google Scholar]
- 6.See MS, Farhadi J. Radiation therapy and immediate breast reconstruction: novel approaches and evidence base for radiation effects on the reconstructed breast. Clin Plast Surg. 2018;45:13–24. [DOI] [PubMed] [Google Scholar]
- 7.Remick J, Amin NP. Postmastectomy breast cancer radiation therapy [updated Jan 2, 2023]. in: StatPearls. Treasure Island, Fla.: StatPearls publishing. Available at https://www.ncbi.nlm.nih.gov/books/NBK519034/. Accessed August 29, 2021. [PubMed] [Google Scholar]
- 8.Verma V, Vicini F, Tendulkar RD, et al. Role of internal mammary node radiation as a part of modern breast cancer radiation therapy: a systematic review. Int J Radiat Oncol Biol Phys. 2016;95:617–631. [DOI] [PubMed] [Google Scholar]
- 9.Yi A, Kim HH, Shin HJ, et al. Radiation-induced complications after breast cancer radiation therapy: a pictorial review of multimodality imaging findings. Korean J Radiol. 2009;10:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damast S, Beal K, Ballangrud A, et al. Do metallic ports in tissue expanders affect postmastectomy radiation therapy? Int J Radiat Oncol Biol Phys. 2006;66:305–310. [DOI] [PubMed] [Google Scholar]
- 11.Chen SA, Ogunleye T, Dhabbaan A, et al. Impact of internal metallic ports in temporary tissue expanders on postmastectomy radiation dose distribution. Int J Radiat Oncol Biol Phys. 2013;85:630–635. [DOI] [PubMed] [Google Scholar]
- 12.Ter Louw RP, Nahabedian MY. Prepectoral breast reconstruction. Plast Reconstr Surg. 2017;140:51S–59S. [DOI] [PubMed] [Google Scholar]
- 13.Sbitany H, Gomez-Sanchez C, Piper M, et al. Prepectoral breast reconstruction in the setting of postmastectomy radiation therapy: An assessment of clinical outcomes and benefits. Plast Reconstr Surg. 2019;143:10–20. [DOI] [PubMed] [Google Scholar]
- 14.White J, Tai A, Arthur D, et al. Breast cancer atlas for radiation therapy planning: consensus definitions. 2013. Available at https://www.onco-hdf.fr/app/uploads/2019/05/BreastCancerAtlas1.pdf. Accessed October 29, 2023.
- 15.International Commission on Radiation Units and Measurements. Prescribing, recording and reporting photon beam therapy (supplement to ICRU report 50). ICRU report 62 (international commission on radiation units and measurements, Bethesda, MD). 1999.
- 16.The comprehensive R archive network. Available at https://cran.r-project.org/. Accessed April 7, 2022.
- 17.Frasier LL, Holden S, Holden T, et al. Temporal trends in postmastectomy radiation therapy and breast reconstruction associated with changes in national comprehensive cancer network guidelines. JAMA Oncol. 2016;2:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vargo JA, Beriwal S. RTOG chest wall contouring guidelines for post-mastectomy radiation therapy: is it evidence-based? Int J Radiat Oncol Biol Phys. 2015;93:266–267. [DOI] [PubMed] [Google Scholar]
- 19.Lao N, Brackstone M, Formenti SC, et al. Redefining postmastectomy radiation contouring in the era of immediate breast reconstruction: An accurate assessment of local recurrence risk. Clin Transl Radiat Oncol. 2021;29:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alanyali SD, Ceylan N, Haydaroglu A. The organs at risk and radiation tolerance doses. In: Haydaroglu A, Ozyigit G, eds. Principles and Practice of Modern Radiotherapy Techniques in Breast Cancer. New York, N.Y.: Springer; 2013. [Google Scholar]
- 21.Zaorsky NG, Trifiletti DM, Rosenberg J. Breast cancer. In: Trifiletti D, Zaorsky N, eds. Absolute Clinical Radiation Oncology Review. Cham, Switzerland: Springer; 2019. [Google Scholar]
- 22.Horst KC, Kovalchuk N, Marquez C. Postmastectomy radiotherapy with and without reconstruction. In: Bellon J, Wong J, MacDonald S, Ho A, eds. Radiation Therapy Techniques and Treatment Planning for Breast Cancer. Practical Guides in Radiation Oncology. Cham, Switzerland: Springer; 2016. [Google Scholar]
- 23.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. [DOI] [PubMed] [Google Scholar]
- 24.Mondal D, Sharma DN. External beam radiation techniques for breast cancer in the new millennium: new challenging perspectives. J Egypt Natl Canc Inst. 2016;28:211–218. [DOI] [PubMed] [Google Scholar]
- 25.Ohri N, Cordeiro PG, Keam J, et al. Quantifying the impact of immediate reconstruction in postmastectomy radiation: a large, dose-volume histogram-based analysis. Int J Radiat Oncol Biol Phys. 2012;84:e153–e159. [DOI] [PubMed] [Google Scholar]
- 26.Maalouf C, Bou-Merhi J, Karam E, et al. The impact of autologous breast reconstruction using DIEP flap on the oncologic efficacy of radiation therapy. Ann Chir Plast Esthet. 2017;62:630–636. [DOI] [PubMed] [Google Scholar]
- 27.Sigalove S. Prepectoral breast reconstruction and radiotherapy—a closer look. Gland Surg. 2019;8:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urquia LN, Hart AM, Liu DZ, et al. Surgical outcomes in prepectoral breast reconstruction. Plast Reconstr Surg Glob Open. 2020;8:e2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell MP, Wagner J, Butterworth J. Subcutaneous implant-based breast reconstruction, a modern challenge in postmastectomy radiation planning. Pract Radiat Oncol. 2018;8:153–156. [DOI] [PubMed] [Google Scholar]
- 30.Guebert A, Conroy L, Weppler S, et al. Clinical implementation of AXB from AAA for breast: plan quality and subvolume analysis. J Appl Clin Med Phys. 2018;19:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.