Summary:
Advances in the development of robotic systems have recently enabled the use of robotic technology in reconstructive lymphatic surgery. Although the advantages of microsurgical robots must be weighed carefully against the costs, their use may allow for smaller surgical approaches and easier access to anatomically deeper structures or even smaller vessels. We report on a case of a patient with central lymphatic dilation causing abdominal pain and severely reduced physical capacity. Sonography-assisted intranodal injection of indocyanine green allowed for localization of the lymphatic cyst and anastomosis with the left ovarian vein, applying robotic-assisted microsurgery for the first time on the central lymphatic system. Following the successful reconstruction of lymphatic drainage and decompression of the cyst, the patient reported a complete regression of her preoperative symptoms. From a surgical point of view, the Symani Surgical System improved precision and allowed significantly smaller surgical access. Considering the high morbidity and rarity of pathologies of the central lymphatic system, central lymphatic surgery is to date rarely performed. With improved precision and significantly smaller surgical access, robotic-assisted microsurgery has great potential to expand the treatment options for central lymphatic lesions.
Developments in robotic technology have only recently enabled its application in reconstructive lymphatic surgery. Preclinical and clinical studies of independent groups using different robotic systems demonstrate the feasibility of robotic-assisted (super) microsurgical procedures. In 2021, we reported the first-in-human use of the Symani Surgical System (Medical Microinstruments, Inc., Wilmington, Del.) to perform lymphatic reconstruction.1 In addition to a steep learning curve, robot-assisted surgery has been shown to be beneficial in deeper anatomical planes.2,3 In the following, we report on the first-in-human use of a robotic microsurgical system for the reconstruction of central lymphatic flow.
CASE REPORT
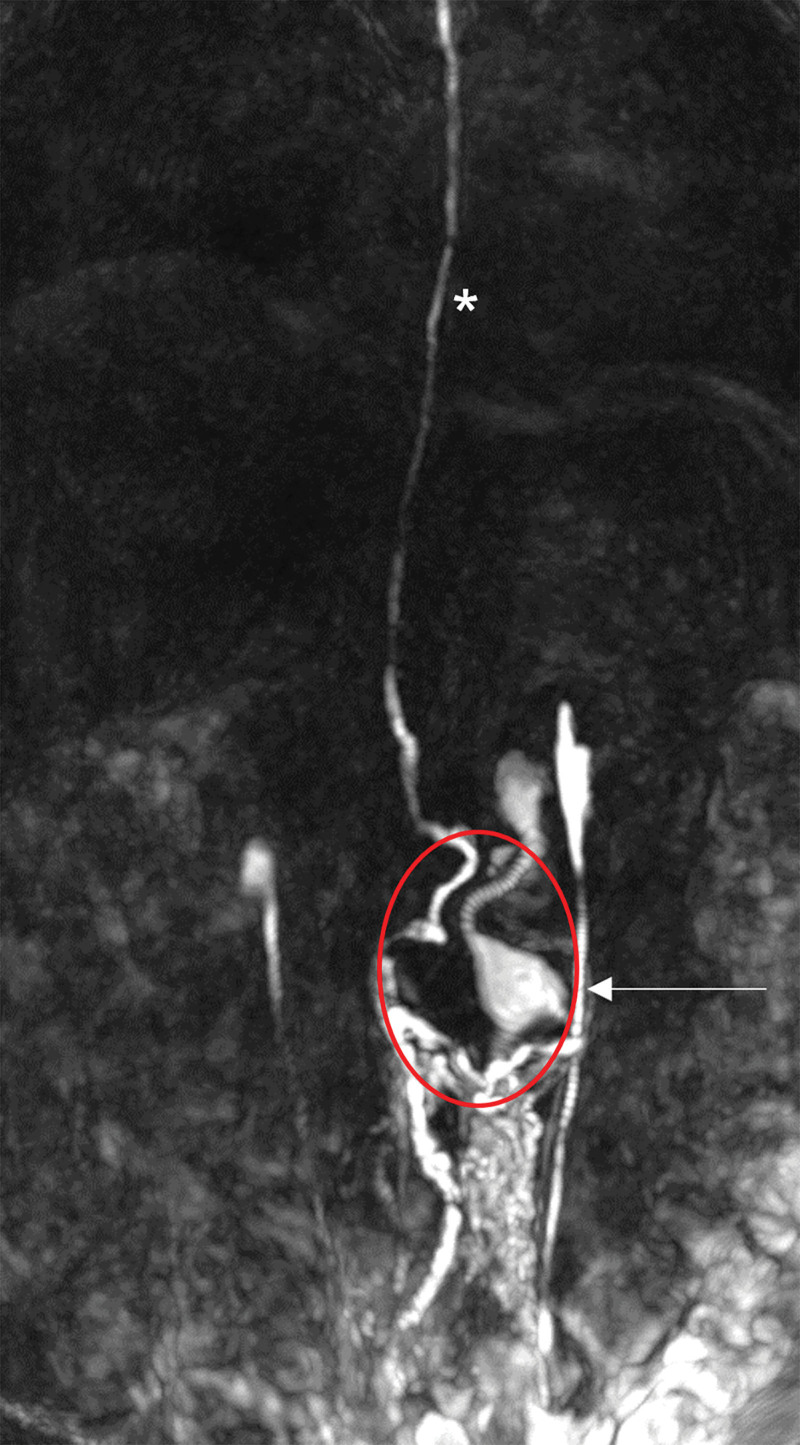
A 47-year-old female patient presented with a long history of pain in her left abdomen, which was pronounced after physical activity. This was accompanied by circulatory reactions and the inability to walk longer distances (Fig. 1). Due to symptom exacerbation, a dynamic MR lymphangiography (Fig. 1) was performed. The diagnostic workup revealed an aneurysmal dilatation of the left lumbar trunks (10 × 4.6 × 4 cm). An attempt to treat the cystic malformation by means of interventional obliteration in another hospital had previously failed.
Fig. 1.
Dynamic MR lymphangiography showed a dilatation of the lumbar trunks (white arrow). Flow into the thoracic duct was not obstructed (white asterisk).
After a multidisciplinary evaluation, a further interventional therapeutic approach was excluded due to the risk of complete obstruction of the lymphatic pathways feeding into the lymphatic dilatation. Instead, surgical anastomosis of this cystic malformation to the left ovarian vein was planned to divert the accumulating lymphatic fluid back into the venous system.
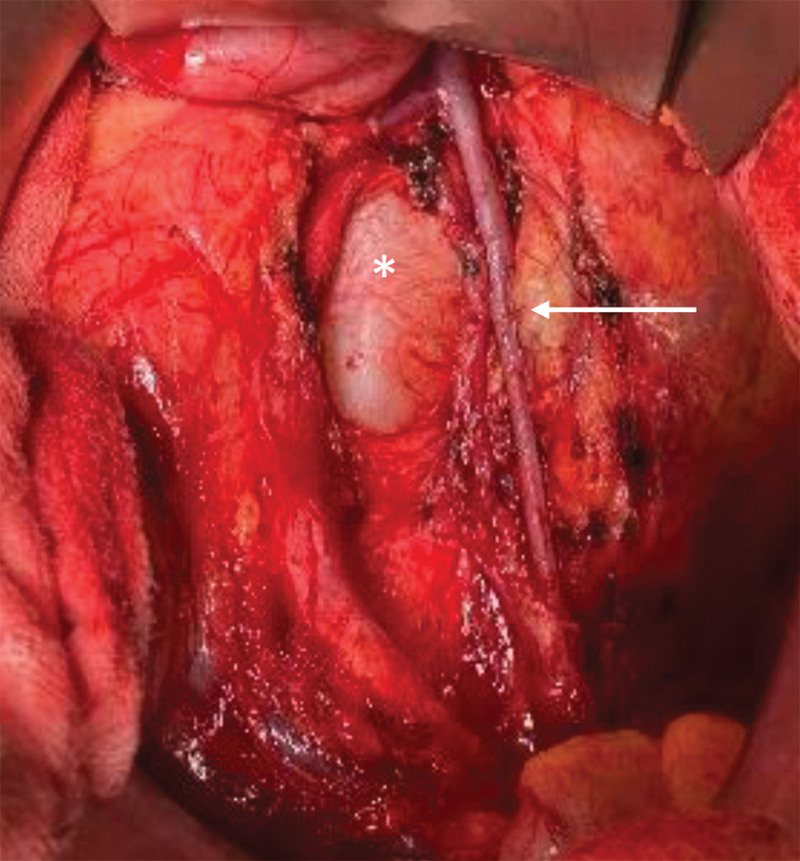
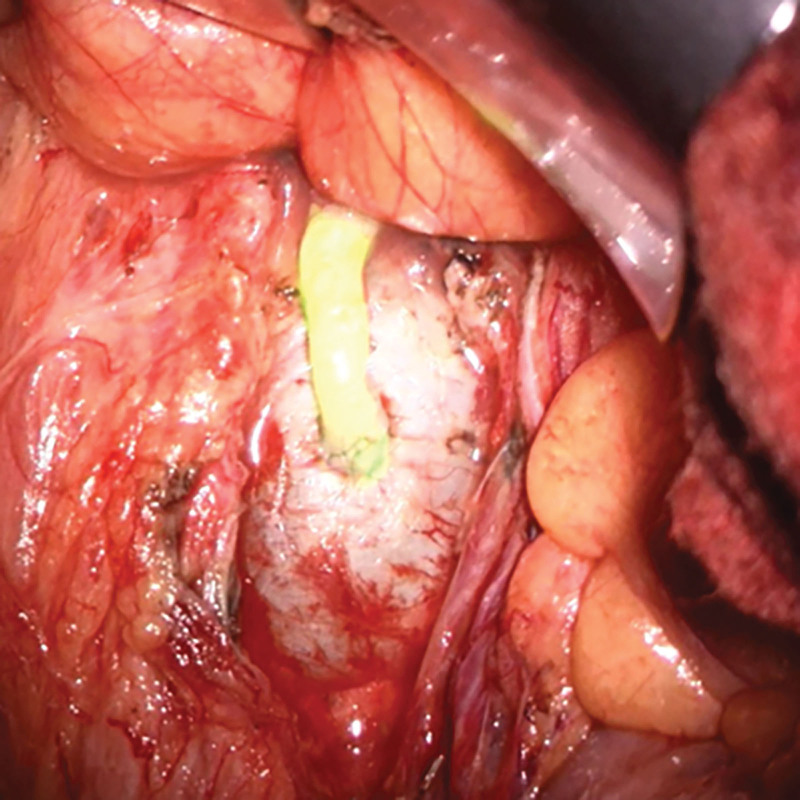
Before surgery, sonography-assisted intranodal injection of indocyanine green (ICG) at both groins was performed. (See figure 1, Supplemental Digital Content 1, which shows the technique of sonographic intranodal injection: puncture of a groin lymph node, http://links.lww.com/PRSGO/C946.) The lymph node was visualized with an 18-MHz probe hockey stick (LOGIQ E9; GE HealthCare Technologies Inc., Chicago, Ill.). The needle was placed into the cortical-medullary junction, and 1.5 mL of ICG was injected. Microbubbles contained within the ICG solution can be visualized in the lymphatic vessels by ultrasound to confirm accurate injection.4,5 The visceral surgery team gained laparoscopic access to the left retroperitoneum by mobilizing the descending and sigmoid colon. After visualization of the left renal vein and the confluence with the left ovarian vein, the dilated lymphatic system was exposed. Subsequently, a limited (15 cm) laparotomy was performed. A near-infrared camera was then used to localize the ICG-enriched lymphatic cyst (Fig. 2). (See figure 2, Supplemental Digital Content 2, which shows identification of the ICG-positive lymphatic cyst in the retroperitoneum using a near-infrared camera, http://links.lww.com/PRSGO/C947.) Afterward, the roof of the lymphatic cyst (visible 4.6 × 4.0 cm) and the adjacent left ovarian vein were prepared for anastomosis (Fig. 2). Pressure on the cyst was relieved by a puncture. The Symani Surgical System was used to perform the surgical anastomosis with an 8-0 suture in a remote manner in combination with a 3D exoscope integrated into an optical microscope (KINEVO 900; Carl Zeiss AG, Oberkochen, Germany) (Fig. 3). (See video 1 [online], which shows robotic-assisted anastomosis of the left ovarian vein to the aneurysmal dilatation of the lumbar trunks.) After opening the clamps, patency of the anastomosis was confirmed by the flow of ICG-containing lymphatic fluid from the cyst into the ovarian vein (Fig. 3).
Fig. 2.
Macroscopic view of the central lymphatic dilatation (white asterisk) and adjacent left ovarian vein measuring 4 mm (white arrow).
Fig. 3.
Successful robotically assisted anastomosis between the aneurysmal dilatation and left ovarian vein. The near-infrared overlay mode showed ICG flow over the anastomosis which confirmed its patency and antegrade flow into the vein.
Video 1. shows robotic-assisted anastomosis of the left ovarian vein to the aneurysmal dilatation of the lumbar truncs.
The patient reported a complete regression of abdominal pain almost immediately after surgery and increased physical strength. At the last follow-up, 3 months postoperatively, the patient was asymptomatic and reported a significantly increased walking distance (several hours) without any episodes of fainting (Fig. 4). The postoperative MR lymphangiography (Fig. 4) showed an early drainage of the lymphatic fluid from the aneurysmal dilatation into the ovarian vein, proving the patency of the anastomosis.
Fig. 4.
MR lymphangiography 3 months postoperatively: coronary GRE-MRI (LAVA Flex) MIP following transnodal gadolinium application in an inguinal lymph node on both sides. The cystic paravertebral lesion (white dotted line) is partially filled with contrast (white outlined arrowhead). The ovarian vein (white arrow) is partially filled with contrast and drains into the renal vein (white-filled arrowheads).
DISCUSSION
We report on the first use of a robotic system for successful anastomosis between the retroperitoneal central lymphatic system and the left ovarian vein to reconstruct lymphatic flow. A current review of the literature revealed 15 articles on thoracic duct (TD) vein anastomosis, including 11 case reports and four case series. In the past, we have successfully treated both congenital lymphatic anomalies and acquired lesions of the central lymphatic system by TD anastomosis in the abdomen or neck depending on the underlying cause.6–9
So far, the Symani Surgical System has been used, particularly for lympho-venous anastomoses and vascularized lymph node transfers.1 Besides, we have used different types of free flaps, autologous breast reconstruction, and dynamic reconstruction of facial paralysis and epineural coaptations. It has also been used by other authors to suture partial corneal grafts in an ex vivo porcine model.2,10 However, its benefits must be weighed against the costs. Consumables for one robot-assisted surgery currently amount to 2000–2500 CHF in Switzerland. Additional costs include implementation and maintenance costs. We have found that robot-assisted surgery is particularly beneficial in deeper anatomical planes difficult to reach with conventional microsurgical instruments. Added precision, in particular in structures less than 1 mm, is of particular value. Furthermore, the Symani Surgical System can easily be teleoperated using a 3D exoscope, which provides enough space for a second team of surgeons to operate in parallel at a nearby anatomic region and also improves the surgeon’s ergonomic position and endurance performance.3 We have previously shown that the time required to perform anastomoses with a robotic surgical system quickly decreases after implementation.2
Current limitations of the system include fixed angles with respect to the position of the two robotic arms relative to each other; that is, the more the arms are lowered, the greater the distance between them on the skin level. However, technical refinements of the robotic system involving flexible arms may resolve this in the future.
To our knowledge, this is the first anastomosis on the central lymphatic system performed with the robotic system. Of note, the robotic technology allowed reconstruction of the retroperitoneal central lymphatics via limited laparotomy. Considering the high morbidity and rarity of pathologies of the central lymphatic system, surgery should be performed only after careful planning with utmost precision. In this context, the Symani Surgical System not only improved precision but also allowed significantly smaller surgical access and thus a smaller final scar. Based on this, robotic-assisted microsurgery shows great potential to expand the reconstructive treatment options for central lymphatic anomalies.
DISCLOSURE
Dr. Lindenblatt acts as a scientific consultant and scientific advisor for Medical Microinstruments (MMI). The other authors have no financial interest to declare.
Supplementary Material
Footnotes
Published online 19 December 2023.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Lindenblatt N, Grunherz L, Wang A, et al. Early experience using a new robotic microsurgical system for lymphatic surgery. Plast Reconstr Surg Glob Open. 2022;10:e4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbon C, Grunherz L, Uyulmaz S, et al. Exploring the learning curve of a new robotic microsurgical system for microsurgery. JPRAS Open. 2022;34:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinzierl A, Barbon C, Gousopoulos E, et al. Benefits of robotic-assisted lymphatic microsurgery in deep anatomical planes. JPRAS Open. 2023;37:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagenpfeil J, Kupczyk PA, Henkel A, et al. Ultrasound-guided needle positioning for nodal dynamic contrast-enhanced MR lymphangiography. Sci Rep. 2022;12:3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itkin M, Nadolski GJ. Modern techniques of lymphangiography and interventions: current status and future development. Cardiovasc Intervent Radiol. 2018;41:366–376. [DOI] [PubMed] [Google Scholar]
- 6.Rosenauer F. Side-to-side anastomosis of the thoracic duct and the internal jugular vein. Chirurg. 1953;24:476–477. [PubMed] [Google Scholar]
- 7.Grünherz L, Lindenblatt N. Central lymphatic surgery. Plast Aesthet Res. 2023;10:20. [Google Scholar]
- 8.Lindenblatt N, Gutschow CA, Vetter D, et al. Lympho-venous anastomosis for the treatment of congenital and acquired lesions of the central lymphatic system: a multidisciplinary treatment approach. Eur J Plast Surg. 2022;45:841–849. [Google Scholar]
- 9.Lindenblatt N, Puippe G, Broglie MA, et al. Lymphovenous anastomosis for the treatment of thoracic duct lesion: a case report and systematic review of literature. Ann Plast Surg. 2020;84:402–408. [DOI] [PubMed] [Google Scholar]
- 10.Grunherz L, Gousopoulos E, Barbon C, et al. Robotics in plastic surgery. Chirurgie (Heidelb). 2023;94:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.