Abstract
PURPOSE
The GMMG-CONCEPT trial investigated isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in transplant-eligible (TE) and transplant-noneligible (TNE) patients with newly diagnosed multiple myeloma (NDMM) with exclusively high-risk disease for whom prospective trials are limited, aiming to induce minimal residual disease (MRD) negativity.
METHODS
This academic, investigator-initiated, multicenter, phase II trial enrolled patients with high-risk NDMM (HRNDMM) defined by mandatory International Staging System stage II/III combined with del17p, t(4;14), t(14;16), or more than three 1q21 copies as high-risk cytogenetic aberrations (HRCAs). Patients received Isa-KRd induction/consolidation and Isa-KR maintenance. TE patients received high-dose melphalan. TNE patients received two additional Isa-KRd cycles postinduction. This prespecified interim analysis (IA) reports the primary end point, MRD negativity (<10−5, next-generation flow), at the end of consolidation. The secondary end point was progression-free survival (PFS).
RESULTS
Among 125 patients with HRNDMM (TE–intention-to-treat [ITT]-IA, 99; TNE-ITT, 26) of the IA population for the primary end point, the median age was 58 (TE-ITT-IA) and 74 (TNE-ITT) years. Del17p was the most common HRCA (TE, 44.4%; TNE, 42.3%); about one third of evaluable TE/TNE patients presented two or more HRCAs, respectively. The trial met its primary end point with MRD negativity rates after consolidation of 67.7% (TE) and 54.2% (TNE) of patients. Eighty-one of 99 TE-ITT-IA patients reached MRD negativity at any time point (81.8%). MRD negativity was sustained for ≥1 year in 62.6% of patients. With a median follow-up of 44 (TE) and 33 (TNE) months, median PFS was not reached in either arm.
CONCLUSION
Isa-KRd effectively induces high rates of sustainable MRD negativity in the difficult-to-treat HRNDMM population, regardless of transplant status, translating into a median PFS that was not yet reached after 44/33 months.
INTRODUCTION
Clinical outcomes in multiple myeloma (MM) have markedly improved over the past decade with the implementation of novel agents and continuous treatment approaches.1-6 Adding anti-CD38 monoclonal antibodies to backbone regimens has led to unprecedented outcomes for transplant-eligible (TE) and transplant-noneligible (TNE) patients.2-4,7 However, outcomes remain dismal for patients with MM with high-risk disease. As was recently reported, patients with higher Second Revision of the International Staging System (R2-ISS) stages III and IV show a median progression-free survival (PFS) of only 30 and 20 months, respectively.8
CONTEXT
Key Objective
What is the impact of using extended quadruplet treatment with isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) on achieving minimal residual disease (MRD) negativity in patients with difficult-to-treat high-risk newly diagnosed (ND) multiple myeloma, regardless of age and transplant eligibility, and how does this translate into progression-free survival (PFS)?
Knowledge Generated
MRD negativity rates after consolidation were 67.7% for transplant-eligible (TE) and 54.2% for transplant-noneligible (TNE) patients, and sustained MRD negativity for ≥1 year was observed in 62.6% and 46.2% (TE and TNE, respectively). Median PFS was not reached in either arm after a median follow-up of 44 (TE) and 33 (TNE) months.
Relevance (S. Lentzsch)
-
Isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRD) is a well-tolerated and very effective induction treatment for newly diagnosed high-risk multiple myeloma, resulting in deep remissions. Future prospective studies need to determine how daratumumab, cyclophosphamide, bortezomib, lenalidomide, dexamethasone (D-CVRD) tested in the OPTIMUM trial or daratumumab, carfilzomib, lenalidomide, dexamethasone (Dara-KRD) tested in the IFM 2018-04 trial compared to the here reported Isa-KRD data in the population of patients with high-risk multiple myeloma.*
*Relevance section written by JCO Associate Editor Suzanne Lentzsch, MD, PhD.
Achievement of minimal residual disease (MRD) negativity is currently the strongest outcome predictor9-12; therefore, inducing and maintaining MRD-negative responses is of particular importance in HRMM. Nonetheless, patients with HR disease have been underrepresented in clinical trials. Most data on HRMM come from subgroup analyses of clinical studies or retrospective/observational studies, with few prospective trials studying only patients with HRMM, which are needed to help determine the optimal treatment strategy for this population.
The phase II GMMG-CONCEPT trial (ClinicalTrials.gov identifier: NCT03104842) was designed to study patients with newly diagnosed (ND) HRMM (TE and TNE) treated with the quadruplet combination of isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in induction/consolidation, followed by triplet maintenance (Isa-KR). The primary objective was to evaluate MRD negativity (<10−5, next-generation flow [NGF]) after consolidation. The secondary objective was to assess PFS.
METHODS
Patients
Adult patients with HR NDMM were eligible, with HR defined by ISS stage II or III combined with ≥1 of the following: del17p (in >10% of purified cells), t(4;14), t(14;16), or more than three 1q21 copies (amplification 1q21 [amp1q21]) as high-risk cytogenetic aberrations (HRCAs); primary plasma cell leukemia was eligible. An Eastern Cooperative Oncology Group performance status of 0-3 and adequate organ function (eg, creatinine clearance ≥30 mL/min) at the time of inclusion were required. To include NDMM with aggressive disease and immediate need for treatment, one prior cycle of any anti-MM therapy was allowed; HRNDMM patients with oligosecretory or nonsecretory disease but measurable bone lesions or extramedullary manifestations were also eligible. All patients provided written informed consent. Detailed inclusion/exclusion criteria are shown in Appendix Table A1 (online only).
Trial Design and Treatment
GMMG-CONCEPT is an academic, investigator-initiated, nonrandomized, multicenter, phase II clinical trial conducted in 17 German sites with two treatment arms according to eligibility for high-dose therapy (HDT). In arm A, TE patients received six cycles of Isa-KRd induction (with stem-cell collection after cycle 3), followed by HDT and autologous stem-cell transplantation (ASCT). Consolidation consisted of four cycles of Isa-KRd, and maintenance was 26 cycles of Isa-KR. Patients noneligible for HDT or age older than 70 years entered arm B with identical induction, consolidation, and maintenance and two additional cycles of Isa-KRd instead of HDT-ASCT.
Induction and consolidation cycles lasted 28 days, with isatuximab 10 mg/kg given intravenously (IV) once daily on days 1 and 15; carfilzomib 36 mg/m2 (IV) once daily on days 1, 2, 8, 9, 15, and 16; lenalidomide 25 mg orally once daily on days 1-21; and weekly dexamethasone 40 mg (orally/IV once daily; 20 mg if older than 75 years). Further specifications and maintenance treatment doses are listed in Appendix 1.
Patients in the first cohort were enrolled from August 2017 to April 2020. The trial was amended in 2021 to recruit a second cohort of TE patients (Appendix 1).
End Points and Assessments
The primary end point is the proportion of patients reaching MRD negativity (<10−5, NGF) at the end of consolidation. Secondary end point is PFS, defined as the time from enrollment to progression or death, whichever occurs first. Tertiary end points include overall response rate (ORR), defined as the proportion of patients with partial responses (PRs) or better; complete response (CR) or better; and overall survival (OS), defined as the time from enrollment to death from any cause.
MRD assessment was mandatory at the end of consolidation and every 6 months thereafter. Before the end of consolidation, MRD assessment was recommended in case of very good PR (VGPR) or better.
MRD assessments from the first pull of bone marrow aspirate were measured in a central laboratory using a standardized and validated eight-color flow panel. MRD results and traditional response assessments are reported according to International Myeloma Working Group (IMWG) consensus criteria.13
Duration of sustained MRD negativity is defined as the time from first MRD-negative until the last MRD-negative or first MRD-positive assessment, depending on what ends the sequence of MRD-negative assessments with no positive in between.
Trial Oversight
The trial was approved by competent authorities and ethics committees and was conducted according to the Good Clinical Practice Guidelines of the International Conference on Harmonisation and the ethical principles described in the 2013 Declaration of Helsinki. The study is registered at ClinicalTrials.gov (identifier: NCT03104842).
University Medical Center Hamburg-Eppendorf is the academic sponsor of this trial and is solely responsible for study design, conduct, data collection, and analysis. A Data Safety Monitoring Board provided external independent oversight of the trial.
Statistical Analysis
Sample size was calculated for primary and secondary end points in arm A and for primary end point in arm B; the details are provided in Appendix 1.
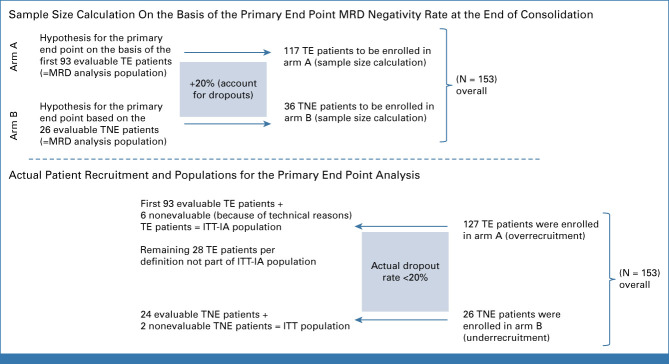
The primary end point (MRD negativity rate at the end of consolidation) was tested on the MRD analysis population, defined by all intention-to-treat (ITT) patients, except those with technical evaluation failures. For arm A, a group sequential design was applied with a single-efficacy interim analysis (IA) on the basis of the first 93 patients of the MRD analysis population (according to trial allocation; Appendix Fig A1) on α = 3.05% and a final analysis on α = 3.0% (Pocock α-spending for cumulative one-sided significance level of 5%), if the test result was not significant at the time of IA. An exact one-sided binomial test was applied to test the null hypothesis (MRD negativity rate ≤50% [arm A] and ≤30% [arm B]).
The secondary end point (PFS) was tested on the ITT-IA population (first 99 ITT patients [Appendix Fig A1]) at the time of IA of the primary end point on a one-sided α = .0001 and the final analysis on α = .0499. The null hypothesis, median PFS ≤25 months, was tested by a one-sample one-sided log-rank test (OSLRT).14 A fixed-sequence testing procedure was used for the primary and secondary end point analyses. For arm B, the null hypothesis (median PFS ≤25 months) was tested by an OSLRT on one-sided α = 5%.
The 95% CI boundaries on the basis of the Wilson method15 are presented for the MRD negativity rates. Kaplan-Meier (KM) estimates and corresponding 95% CIs for survival and IMWG responses were computed for each study arm. Because MRD status changes over time,16 we performed a time-varying Cox regression analysis17 to investigate the prognostic impact of the first achievement of MRD negativity (any time on study) on PFS: Hazard ratios (HRs) and 95% CIs provide an estimate of the risk for PFS from MRD negativity compared with the state of non-negative MRD (MRD-positive, not assessable, or without assessment). The P value was derived by the Wald test. Illustration of survival with respect to MRD status is done using Bernasconi-Simon-Makuch plots.18
As sustained MRD negativity for ≥6 or ≥12 months cannot be achieved during the first 6 or 12 months on study, a landmark analysis providing KM estimates for survival was performed evaluating the difference in PFS from maintenance start comparing patients with sustained MRD negativity with those without until consolidation end. Analogously, we investigated PFS with respect to MRD negativity at the end of consolidation (primary end point) to adjust for potential bias in survival estimates because of nonmandatory MRD assessment before consolidation end.
Safety End Points
Safety analyses were conducted for all ITT patients receiving ≥1 trial medication dose. Medical Dictionary for Regulatory Activities coding version 26.0 was used; in case of multiple occurrences of the same adverse event (AE) term, the maximum grade was reported. Definitions of specific preferred terms are provided in the Appendix (Table A3). The data cutoff was December 1, 2022.
RESULTS
Patients
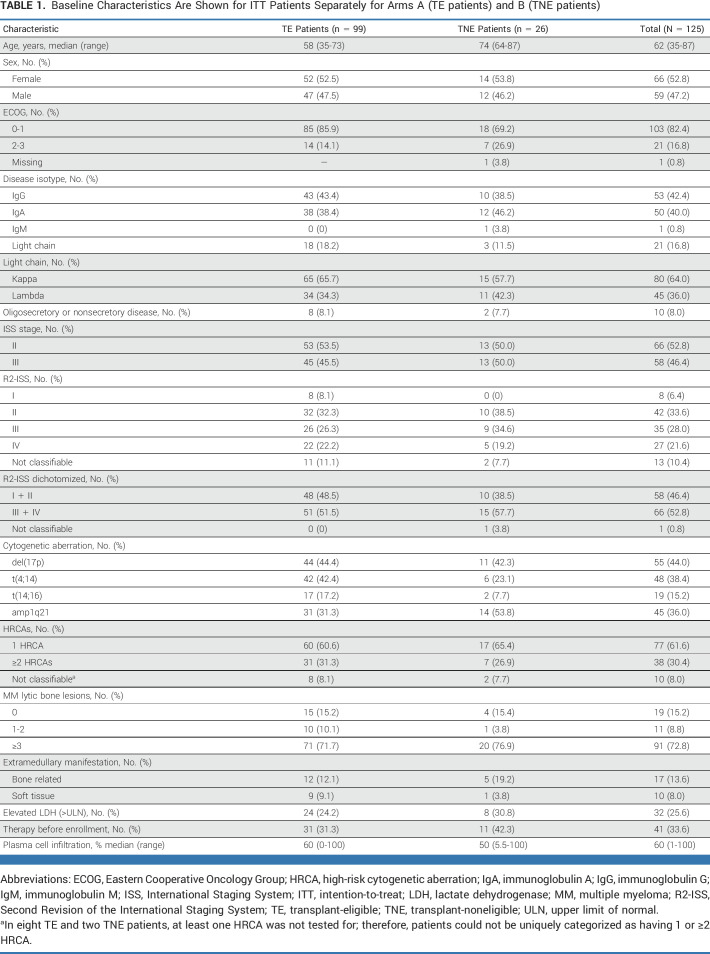
Of 153 patients with HRNDMM enrolled in the first cohort, 127 TE patients entered arm A and 26 TNE patients arm B. According to the trial design, the ITT population for planned IA included 99 TE and all 26 TNE patients (Appendix Fig A1). The median age of TE and TNE patients was 58 (range, 35-73) and 74 (range, 64-87) years, respectively. Fifty-eight patients (46.4%) had ISS stage III; 66 (52.8%) patients were R2-ISS stages III/IV; patient characteristics are depicted in Table 1 (and in Appendix Table A2 for non-ITT-IA patients).
TABLE 1.
Baseline Characteristics Are Shown for ITT Patients Separately for Arms A (TE patients) and B (TNE patients)
Del(17p) was the most common HRCA, followed by t(4;14) and amp1q21. About one third of evaluable patients had ≥2 HRCAs; in eight TE and two TNE patients, ≥1 HRCA was not tested for; therefore, patients could not be uniquely categorized.
Seventy-three of 99 TE and 16 of 26 TNE patients completed consolidation and started maintenance. Patient disposition is shown in Figure 1. The most common reasons for treatment discontinuation through consolidation were disease progression (n = 12) and death (n = 7).
FIG 1.

Patient disposition until the end of consolidation. aOne of the three deaths occurred during mobilization. bAll patients not undergoing HDT + ASCT received two additional cycles of Isa-KRd (analogous to arm B). cNo HDT-ASCT because of investigator decision or patient wish. ASCT, autologous stem-cell transplantation; HDT, high-dose therapy; IA, interim analysis; Isa-KRd, isatuximab, carfilzomib, lenalidomide, and dexamethasone; ITT, intention-to-treat; TE, transplant-eligible; TNE, transplant-noneligible.
IMWG Response and MRD Negativity
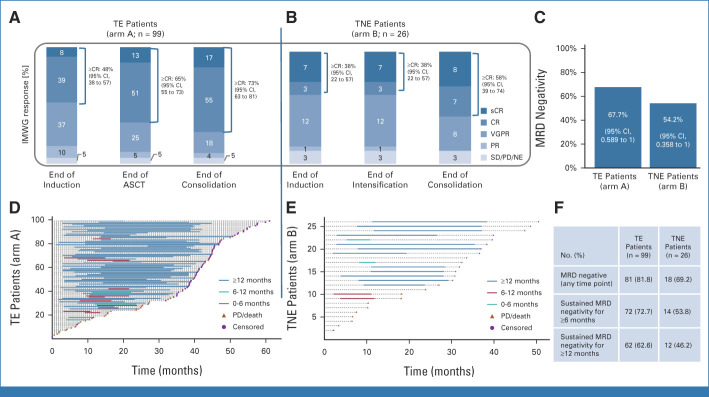
Of 99 TE patients, 72.8% (n = 72) achieved CR/stringent CR (sCR) until the end of consolidation, and 18.2% (n = 18) achieved VGPR, with an ORR of 94.9%. In TNE patients, CR/sCR, VGPR, and ORR rates were 57.7%, 30.8%, and 88.5%, respectively. Responses deepened over time (Figs 2A and 2B).
FIG 2.
Best response according to the IMWG (A and B) according to treatment phase in (A) TE and (B) TNE patients, rates of greater than or equal to CR and 95% CIs are added. (C) MRD negativity status at the end of consolidation (primary end point). 95% CIs refer to the one-sided binomial test result of the primary end point, testing the observed MRD negativity rate against 50% and 30%, respectively. (D and E) Swimmer plots showing achievement and sustainment of MRD negativity (next-generation flow; MRD <10−5) for (D) TE and (E) TNE patients. (F) Sustained MRD negativity for ≥6 months or ≥12 months in both study arms. ASCT, autologous stem-cell transplantation; CR, complete response; IMWG, International Myeloma Working Group; MRD, minimum residual disease; NE, not evaluable; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease; TE, transplant-eligible; TNE, transplant-noneligible; VGPR, very good partial response.
Analysis of the primary end point included 93 TE patients of the IA population and 24 available TNE patients (MRD analysis population; Appendix Fig A1). At consolidation end, 63 (67.7%) TE patients were MRD-negative and three (3.2%) were MRD-positive. Twenty-three (24.7%) patients did not reach consolidation end; four (4.3%) had missing samples. Fifty-three MRD-negative patients were in CR/sCR and 10 in VGPR; MRD-positive patients had VGPR, CR, and sCR (one each).
For TNE patients, 13 (54.2%) were MRD-negative (CR/sCR, 11; VGPR, 2), and 11 (45.8%) did not reach consolidation end. The primary end point of the study was met in both arms (TE, P = .004; TNE, P = .012; Fig 2C); the trial is ongoing per DSMB recommendation.
Of the 99 ITT-IA-TE patients, 81 reached MRD negativity at any time point (81.8%). MRD-negative status was sustained for ≥6 and ≥12 months in 72 and 62 patients, respectively, resulting in sustained MRD negativity rates of 72.7% and 62.6% (Figs 2D and 2F).
The results in TNE patients were slightly lower; 18 (69.2%) patients reached MRD negativity at any time point, with 14 (53.8%) and 12 (46.2%) sustained MRD-negative responses for ≥6 and ≥12 months, respectively (Figs 2E and 2F).
PFS and OS
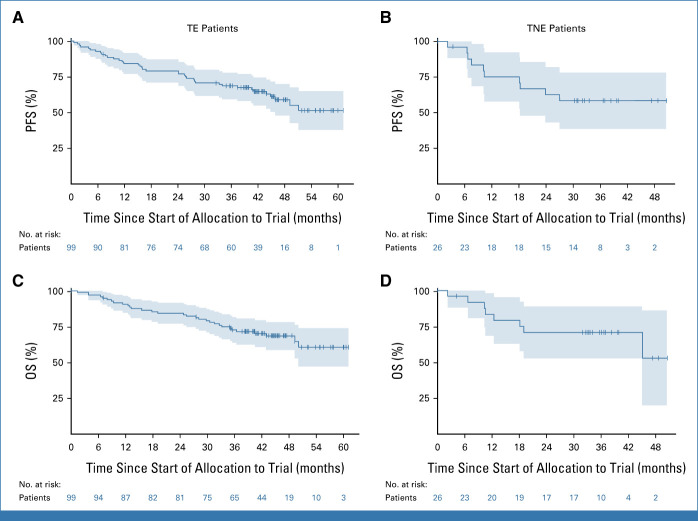
With a median follow-up of 44 (TE) and 33 (TNE) months, median PFS was not reached in either arm (Figs 3A and 3B). The study met its secondary end point of PFS (TE, ZOSLR = 5.75, P < .0001; TNE, ZOSLR = 1.95, P = .0259).
FIG 3.
Kaplan-Meier plots for PFS in (A) TE and (B) TNE patients. OS in (C) TE and (D) TNE patients. OS, overall survival; PFS, progression-free survival; TE, transplant-eligible; TNE, transplant-noneligible.
Respective PFS rates after 1, 2, and 3 years were 86.4% (95% CI, 80.5 to 92.6), 78.3% (95% CI, 71.4 to 85.9), and 68.9% (95% CI, 61.2 to 77.7) for TE patients (Fig 3A) and 75.1% (95% CI, 59.7 to 94.5), 62.6% (95% CI, 46.0 to 85.3), and 58.4% (95% CI, 41.7 to 81.9) for TNE patients (Fig 3B).
Similar to PFS, median (m)OS was not reached in either study arm after 44 (TE) and 35 (TNE) months of follow-up. Respective 1-year and 2-year OS rates of 92% (95% CI, 87.3 to 96.9) and 83.9% (95% CI, 77.7 to 90.6) were observed in TE patients (Fig 3C), and 83.5% (95% CI, 69.6 to 99.7) and 71.0% (95% CI, 55.0 to 91.6) in TNE patients (Fig 3D).
Influence of Different HR Parameters on MRD Status and PFS
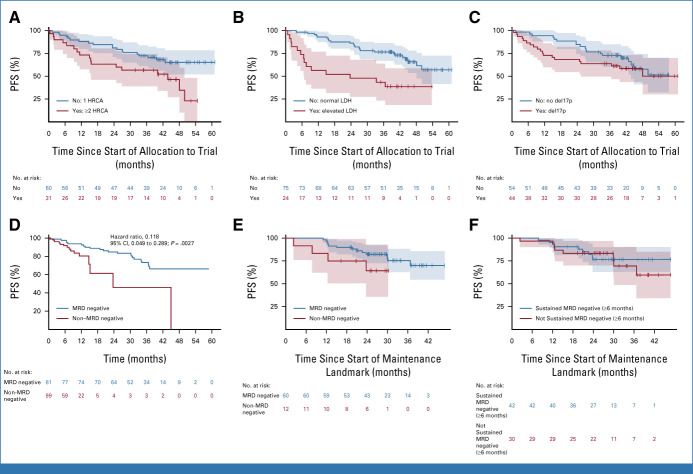
Subgroup analyses regarding various HR features were performed for TE patients for the primary end point in an ITT approach. Subgroups with the lowest MRD negativity rates were patients with an elevated baseline lactate dehydrogenase (LDH; MRD negativity: 45.5% [95% CI, 26.9 to 65.3]), with t(14;16) (56.2% [95% CI, 33.2 to 76.9]), harboring ≥2 HRCAs (57.1% [95% CI, 39.1 to 73.5]) or amp1q21 (58.6% [95% CI, 40.7 to 74.5]). However, MRD negativity was still achieved in approximately half or more of these patients (Appendix Fig A2).
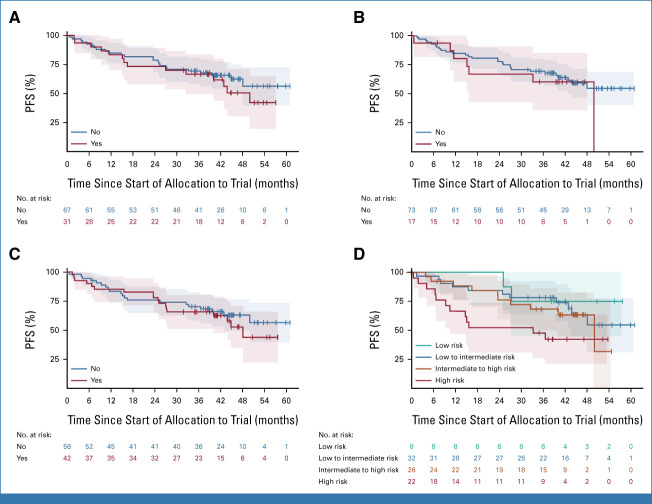
Regarding the impact of these features on survival, univariate Cox regression showed impaired PFS for patients with elevated LDH (HR, 3.18 [95% CI, 1.63 to 6.19]), ≥2 HRCAs (HR, 2.10 [95% CI, 1.09 to 4.04]), del17p (HR, 1.34 [95% CI, 0.71 to 2.54]), or R2-ISS stages III/IV (HR, 2.11 [95% CI, 1.09 to 4.08]; KM estimates Figs 4A-4C, respectively), whereas no major differences were seen for other HRCAs (Appendix Fig A3).
FIG 4.
KM plots for PFS in TE patients according to the presence (red) or absence (blue) of (A) two or more HRCAs, (B) elevated LDH, or (C) del17p. (D) Adjusted Simon-Makuch (Bernasconi-SM) plot for TE patients: estimation of expected PFS under the state of first achievement of MRD negativity on study (blue) compared with the state waiting for MRD negativity (red). Blue curve can be interpreted as the expected PFS of a patient to be known achieving MRD negativity on study (alternatively to be interpreted as the hazard of PD/death under the state of MRD negativity). Red curve can be interpreted as the expected PFS of a patient who is assumed to fail achievement of MRD negativity (alternatively to be interpreted as the hazard of PD/death while waiting for MRD negativity). (E and F) KM plots for PFS from the landmark start of maintenance therapy according to (E) MRD outcome assessed at the end of the consolidation or (F) the achievement of sustained MRD negativity for ≥6 months until the end of consolidation. HRCAs, high-risk cytogenic aberrations; KM, Kaplan-Meier; LDH, lactate dehydrogenase; MRD, minimum residual disease; PD, progressive disease; PFS, progression-free survival; TE, transplant-eligible.
Prognostic Impact of MRD Status on PFS
To investigate the prognostic impact of MRD negativity compared with non-negative MRD state on PFS in TE patients, we performed a time-varying Cox regression analysis, as MRD negativity (might) occur along treatment and compete with progression/death events. Univariate time-varying Cox regression showed a prognostic PFS benefit (HR, 0.118 [95% CI, 0.049 to 0.289]; P = .0027), estimating the risk for PFS events under MRD negativity versus non-MRD negativity (Fig 4D). Multivariable time-varying Cox regression analysis was performed to adjust for the influence of R2-ISS components (Appendix Table A4). To address potential bias in the outcome of MRD assessments before the end of consolidation, a landmark analysis investigating the differences between MRD-negative and MRD–non-negative patients at consolidation end with respect to PFS was performed (HR, 0.44 [95% CI, 0.14 to 1.38]; P = .160; Fig 4E). A landmark analysis from maintenance start was performed to investigate the prognostic impact of sustained MRD negativity ≥6 months on PFS and showed a HR of 0.85 (95% CI, 0.33 to 2.20; P = .738; Fig 4F).
Safety
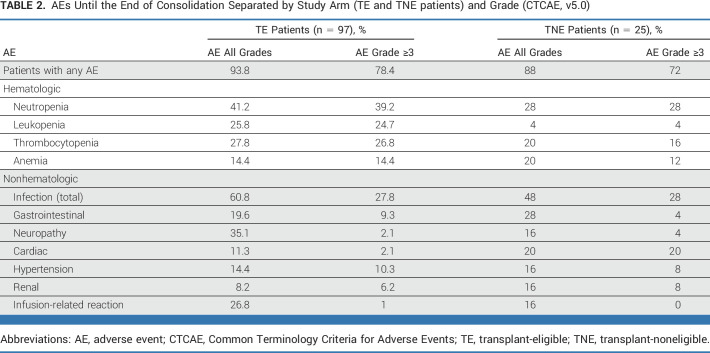
Isa-KRd treatment was tolerable, and side effects were manageable, with a toxicity profile similar to previous reports. Overall, 92.6% of patients experienced at least one AE. AEs of higher Common Terminology Criteria for Adverse Events grades (≥3) were mainly hematologic (neutropenia, 36.9%; thrombocytopenia, 24.6%; leukopenia, 20.5%); infections (mainly respiratory) were the most common higher-grade nonhematologic AEs (27.9%; Table 2; Appendix Tables A5 and A6). Toxicity profiles were comparable between TE and TNE patients, with the exception of more frequent higher-grade cardiac events in TNE patients (TE, n = 2 [1.6%]; TNE, n = 5 [20%]). Six patients (TE, n = 3; TNE, n = 3) discontinued because of treatment-related AEs.
TABLE 2.
AEs Until the End of Consolidation Separated by Study Arm (TE and TNE patients) and Grade (CTCAE, v5.0)
DISCUSSION
Treatment with Isa-KRd in the GMMG-CONCEPT trial led to high MRD negativity rates at any time point of 81.8% and 69.2% in solely HRNDMM TE and TNE patients, respectively. MRD negativity was sustained for ≥1 year in 62.6% (TE) and 46.2% (TNE) of patients and translated into a median PFS that was not reached after 44 (TE) and 33 (TNE) months of follow-up, respectively. To our knowledge, this study was among the first to include only patients with HRMM, without limiting on the basis of age or transplant eligibility, and is also the first to report the use of the quadruplet Isa-KRd in extended induction, consolidation, and Isa-KR maintenance resulting in deep and durable responses in this difficult-to-treat population.
Isa-KRd was tolerable and feasible with a safety profile consistent with similar regimens. In this single-arm trial, there was no evidence that adding isatuximab to the KRd backbone led to increased higher-grade toxicities.
Although HR definitions vary among clinical trials,6,19-21 it was recently shown in the R2-ISS that ISS stages II and III confer the highest risk, along with del(17p) and elevated LDH,8 which is consistent with our findings. R2-ISS stage III patients have an mPFS of 30/19 months (training/validation set), whereas R2-ISS stage IV patients have an mPFS of 20/15 months.8 In our study, over half of patients were R2-ISS stage III/IV, yet mPFS and mOS were not reached after >3 years. Similar promising outcomes for PFS and MRD negativity were recently reported from OPTIMUM/MUKnine (ClinicalTrials.gov identifier: NCT03188172) with daratumumab, cyclophosphamide, bortezomib, lenalidomide, and dexamethasone quintuplet induction in patients with HRNDMM, showing MRD negativity rates of 63.6% after ASCT and a 30-month PFS rate of 77%.21 In OPTIMUM, 27.1% of patients had ISS stage I and 53% harbored ≥2 HRCAs defined as t(4;14)/t(14;16)/t(14;20), del(1p), gain(1q), and del(17p).21 In our trial, no ISS stage I patients were included, and 30.4% had ≥2 HRCAs of t(4;14)/t(14;16), amp(1q), and del(17p). Both OPTIMUM and CONCEPT showed the potency and feasibility of up-front, intensive, multidrug regimens incorporating anti-CD38 antibodies for inducing MRD-negative remission in HR patients.
High MRD negativity rates have been reported by recent NDMM trials incorporating anti-CD38 antibodies in all-comer populations. In the phase II GRIFFIN trial (ClinicalTrials.gov identifier: NCT02874742), 51.0% treated with quadruplet daratumumab, lenalidomide, bortezomib, and dexamethasone (D-RVd) achieved MRD negativity at any point during the study, whereas a 50.0% rate was observed after induction in the phase III GMMG-HD7 trial (ClinicalTrials.gov identifier: NCT03617731) using isatuximab-RVd.7,22
Daratumumab-KRd (D-KRd) was evaluated in the MANHATTAN trial (ClinicalTrials.gov identifier: NCT03290950), resulting in an MRD negativity rate of 71% after eight cycles (thereafter, patients could undergo HDT-ASCT or continue with maintenance),23 leading to National Comprehensive Cancer Network's recommendation of the regimen for TE NDMM.24 Using D-KRd up to 12 cycles but including HDT-ASCT, an 80% MRD negativity rate was described in the MASTER trial (ClinicalTrials.gov identifier: NCT03224507) in a population with 39% ISS stage I and 43% not harboring HRCAs19 versus 81.8% of exclusively HR TE patients in the CONCEPT trial achieving MRD negativity. MASTER also showed that early achievement of MRD negativity was beneficial versus later achievement with regard to MRD resurgence, underlining the importance of effective up-front induction.19
Albeit from a small cohort of TNE patients, the 69.2% MRD negativity rate and mPFS that was not reached after 33 months in CONCEPT compare favorably with the KRd-only arm in the FORTE trial, reporting a 29-month mPFS,20 despite the older age and reduced fitness in CONCEPT. The same holds true for MAIA (ClinicalTrials.gov identifier: NCT02252172) and ALCYONE (ClinicalTrials.gov identifier: NCT02195479) subgroup analyses, with a 41.3% 3-year PFS rate for HRNDMM TNE patients with daratumumab-based regimens (daratumumab-lenalidomide-dexamethasone or daratumumab-bortezomib-melphalan-prednisone)25 compared with a 58.4% 3-year PFS rate in CONCEPT.
Ongoing/upcoming trials focusing on MRD-driven approaches, such as MIDAS (ClinicalTrials.gov identifier: NCT04934475), PERSEUS (ClinicalTrials.gov identifier: NCT03710603), RADAR (ISCRTN46841867), and ADVANCE (ClinicalTrials.gov identifier: NCT04268498), will further elucidate the role of MRD dynamics and possibly tailor treatment, at least for the non-HR patient population.26-29
Although the role of high-dose melphalan in the frontline setting has been questioned, it is still standard for HR patients because of better survival outcomes.6,30,31 In DETERMINATION (ClinicalTrials.gov identifier: NCT01208662), HR patients undergoing HDT-ASCT had an mPFS of 55 months versus 17.1 months for those without6; a subgroup analysis from FORTE confirmed the beneficial PFS effect of KRd + ASCT versus KRd in HR patients.30 As we and others have shown that high rates of MRD negativity and sustained MRD negativity are achieved with extensive quadruplet treatment approaches, it is time to re-evaluate the role of high-dose melphalan, especially considering recent findings of significantly increased mutational burden and detrimental long-term effects of genomic damage.32-35
In the near future, consequent implementation of T-cell engaging strategies in frontline treatment with CAR-T cell application replacing high-dose melphalan or use of bispecific antibodies in maintenance strategies will allow further treatment optimization and are expected to achieve unprecedented high MRD negativity and sustained MRD negativity rates. Results from randomized ongoing trials (eg, CARTITUDE-6 [ClinicalTrials.gov identifier: NCT05257083], MagnetisMM-7 [ClinicalTrials.gov identifier: NCT05317416])36,37 are, therefore, eagerly awaited. To adequately evaluate these concepts for HR patients, particularly, and to better characterize tumor cell subsets, the HR disease definition should be aligned and may incorporate more advanced genomic profiling.
Our phase II, nonrandomized trial is limited by reliance on historical comparators or comparison with ongoing studies with a similar, but never completely equal, study design or population. Owing to the dismal outcomes of this HR population with standard regimens, a two-arm design with a standard-of-care arm was ethically not justifiable. Furthermore, only patients who reached the start of maintenance could be included in the landmark analysis on sustained MRD negativity for ≥6 months, and patients who dropped out before (most commonly because of progressive disease) were not analyzed. Although a PFS benefit for patients being MRD-negative until the end of consolidation can be observed, significance is lacking because of short follow-up. Further investigation of sustained MRD negativity for ≥12 months requires a landmark beyond the start of maintenance and extended follow-up, which was not feasible for this report. The actual rate of ≥2 HRCAs is likely higher than reported because gain1q21 (three copies) was not considered an HRCA and thus not captured, and several patients with noncentral laboratory cytogenetic diagnostics were only tested for some HRCAs.
Taken together, there is now a strong body of evidence for HR disease, supporting use of the most effective multidrug treatment options in induction, followed by HDT-ASCT in TE patients, a prolonged consolidation, and a multidrug maintenance regimen. This approach, as shown in the CONCEPT trial with Isa-KRd, can lead to unprecedented rates of (sustained) MRD negativity and PFS duration in patients with HRNDMM.
ACKNOWLEDGMENT
We are grateful to the patients who consented to participate in this clinical trial and the clinical research teams at all participating centers. We thank all GMMG members and employees who helped to initiate, conduct, and analyze the study; the Coordination Centre for Clinical Trials Heidelberg and participating employees for monitoring the trial; the hematology laboratory with its flow cytometry unit, the FISH laboratory at the Institute of Human Genetics, and the Institute of Pathology at the University Hospital Heidelberg; and the members of the Data Safety Monitoring Board for their support of and input to this study. We owe to Wiebke Kobbe and Carrie-Ann Engel for their administrative support of the trial. The trial was sponsored by the University Medical Center Hamburg-Eppendorf. Study drug and financial support was received from Amgen, Bristol Myers Squibb/Celgene, and Sanofi. Editorial support was provided by Camile Semighini Grubor, PhD, of Envision Pharma Group, funded by Sanofi. Lisa Leypoldt received support from the International Myeloma Society (Young Investigator Award). Lisa Leypoldt receives funding by a scholarship of the German Cancer Aid (Dr Mildred Scheel Postdoktorandenprogramm scholarship by Deutsche Krebshilfe).
APPENDIX 1. ADDITIONAL INFORMATION ON THE AMENDMENT OF THE PROTOCOL
The trial was originally designed to include 117 transplant-eligible (TE) and 36 transplant-noneligible (TNE) patients. Recruitment was completed in April 2020; the median follow-up at that time was 25 months. In an exploratory interim analysis on the first 50 patients, all 46 TE patients showed overall response and the four TNE patients had very good partial response (VGPR) or better (≥VGPR).39 To strengthen the power of the secondary end point of progression-free survival (PFS), an extension of the 117 TE patients to a total of 210 TE patients was considered with a prolongation of the trial duration by 36 months. In addition, an extension of the sample size also enabled detailed subgroup analyses of populations with specific high-risk markers. The Protocol was therefore amended in 2021. The recalculation for the required number of TE patients to be enrolled was done as stated below.
Sample Size Calculation
Arm A: The median PFS in high-risk patients with standard therapy is considered to be (less than) 25 months.40,41 A clinically relevant effect, which should be detected with a probability of at least 80%, is a prolongation of the median PFS compared with standard of care by 6 months to 31 months. A recruitment rate of 5-6 patients per month and a follow-up time of 36 months after inclusion of the last patient were assumed. A sample size of 189 evaluable patients with an expected number of 134 events allows rejection of the null hypothesis (S defining the survival distribution and )—to be translated into median PFS ≤25 months—to detect a median PFS of 31 months with a power of 80%. Thus, a total of 189 evaluable patients are necessary for secondary efficacy analysis; with an assumed rate of 10% screening failure, a total of 210 TE patients need to be enrolled. To enable the evaluation of the primary end point as originally planned on the basis of 93 evaluable TE patients, a group sequential design with one efficacy interim analysis on the basis of 93 evaluable patients and a final analysis on 189 evaluable patients was adapted. The calculation was based on a one-sample log-rank test with a one-sided significance level of 5% assuming exponential survival times.14 With respect to the primary end point, 189 patients provide a cumulative power of 93.8% to detect an increased minimum residual disease (MRD) negativity rate of 50%-62% at a cumulative one-sided significance level of 5%. For arm B, a sample size of 25 evaluable patients allows us to reject the null hypothesis of MRD negativity ≤30% on a one-sided α = 5% with a power of 90% for a MRD negativity rate of 60%.
Because of the allocation to either TE or TNE, the two study arms can be seen as two separate phase II studies; therefore, the primary end point will be tested at an overall two-sided α level of 5% for each of the two study arms.
Additional Information on Treatment Schedule, Dosing, and Administration
Induction and consolidation cycles lasted 28 days, with isatuximab 10 mg/kg given intravenously (IV) once daily on days 1, 8, 15, and 22 during the first cycle and on days 1 and 15 on subsequent cycles; carfilzomib 20 mg/m2 given IV once daily on days 1 and 2 and 36 mg/m2 given IV once daily on days 8, 9, 15, and 16 of the first cycle and 36 mg/m2 given once daily on days 1, 2, 8, 9, 15, and 16 of subsequent cycles (27 mg/m2 throughout the consolidation cycle 1); lenalidomide 25 mg given orally once daily on days 1 through 21 (15 mg throughout consolidation cycle 1); and dexamethasone 40 mg given orally or IV once daily on days 1, 8, 15, and 22 (20 mg for patients age older than 75 years). Maintenance treatment cycles were 28 days and consisted of isatuximab 10 mg/kg given IV once daily on days 1 and 15, carfilzomib 70 mg/m2 given IV once daily on days 1 and 15, and lenalidomide 15 mg given orally once daily on days 1 through 21.
Following a protocol amendment in 2021, carfilzomib dosing was changed to once weekly, with 56 mg/m2 given IV once daily on days 1, 8, and 15 (20 mg/m2 as the first dose of the induction cycle) of the induction and consolidation cycles.
Thromboembolic and herpes zoster prophylaxis were required throughout the trial; antibacterial prophylaxis was mandatory during the first two induction cycles and up to the investigator's discretion thereafter.
Hematopoietic stem-cell mobilization and collection, high-dose therapy with melphalan, and autologous stem-cell transplantation (ASCT) were not formally part of the trial but were done according to the local standard of care at the individual trial sites. Consolidation treatment was to begin 60 days after ASCT.
TABLE A1.
Inclusion and Exclusion Criteria
TABLE A2.
Patient Characteristics of TE Patients Not Part of the Prespecified ITT-IA Population
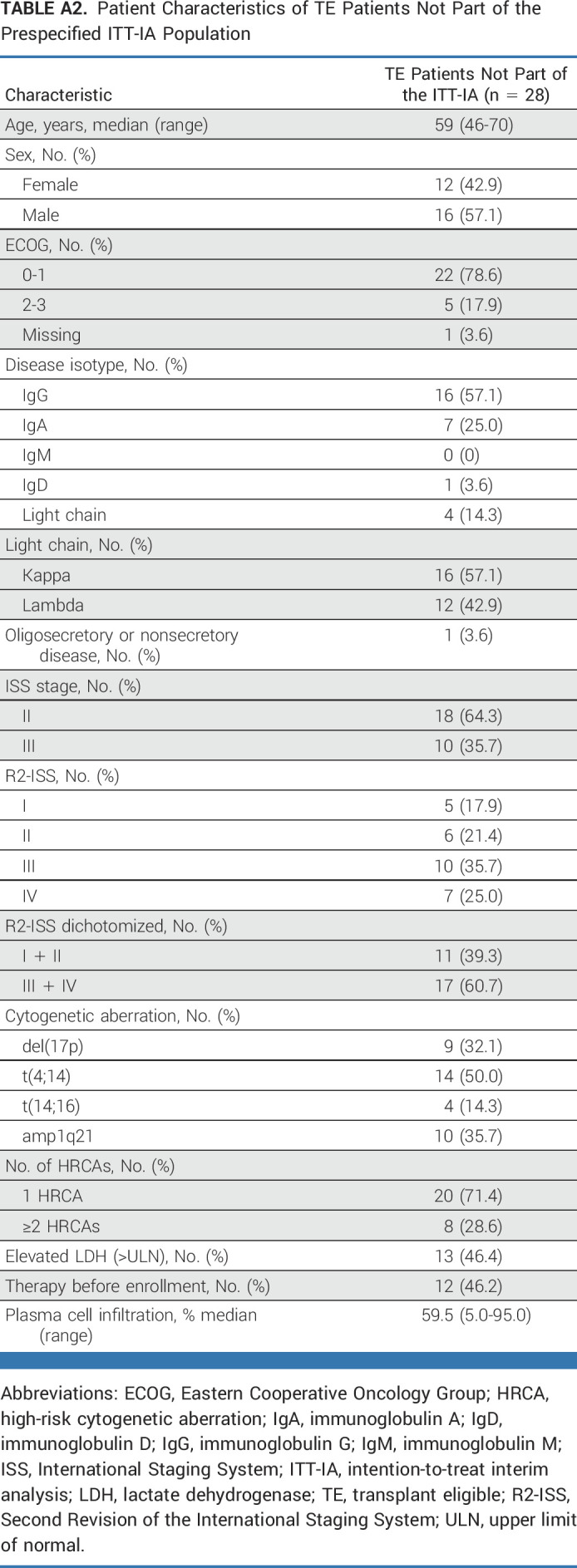
TABLE A3.
Definition of Specific Terms by Preferred Term
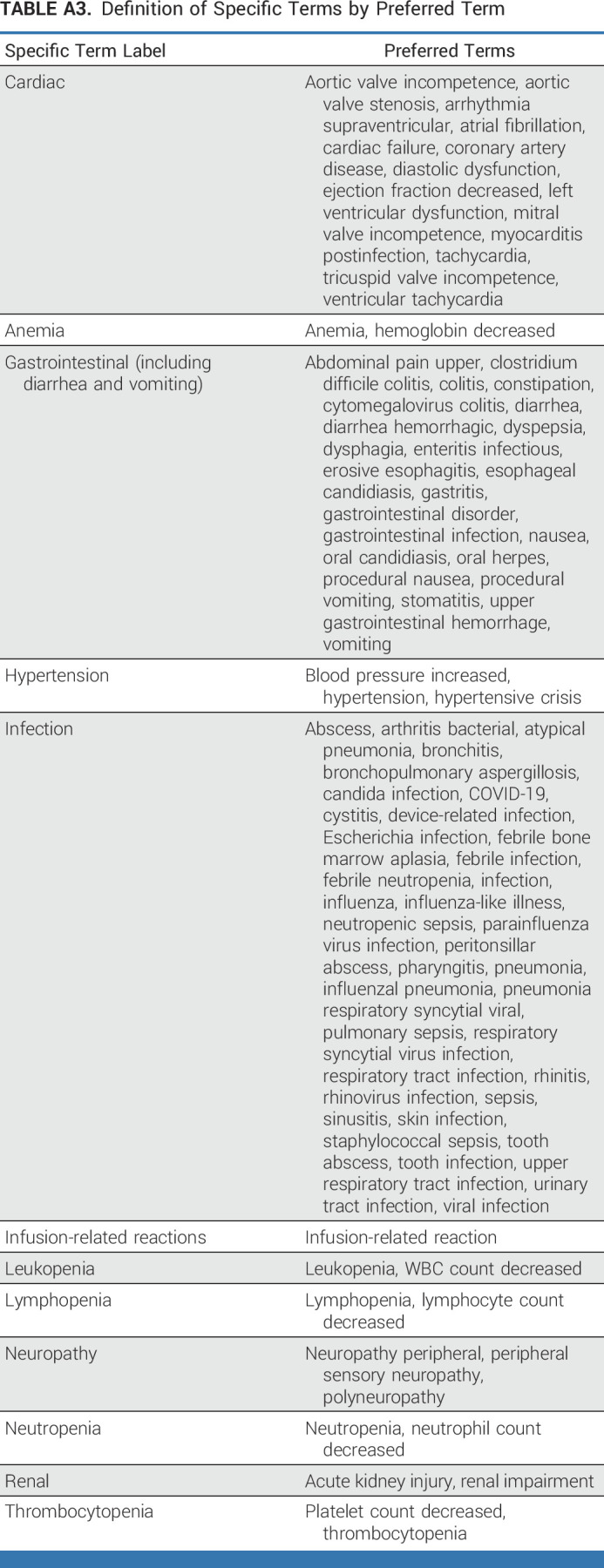
TABLE A4.
Multivariable Time-Varying Cox Regression Analysis to Evaluate the Prognostic Impact of the Achievement of MRD Negativity at Any Time on PFS
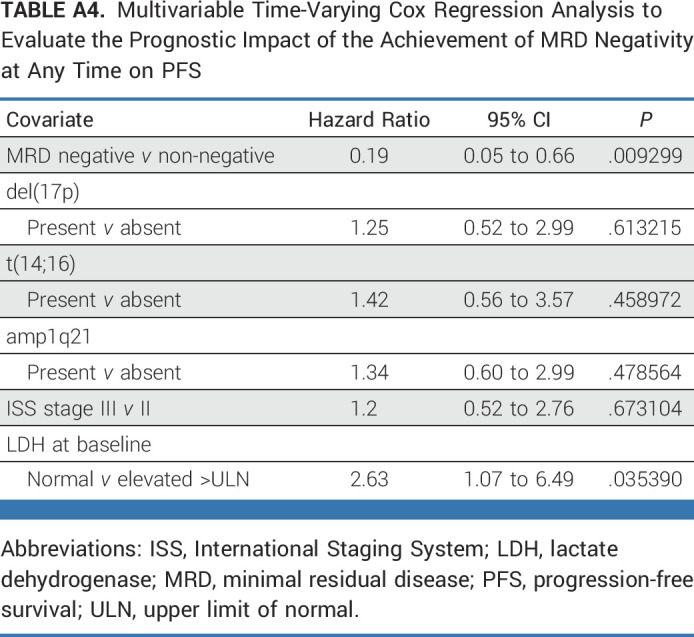
TABLE A5.
AEs Separated by Study Arm (TE and TNE patients) and Grades (CTCAE, vF5.0) Throughout the Whole Trial
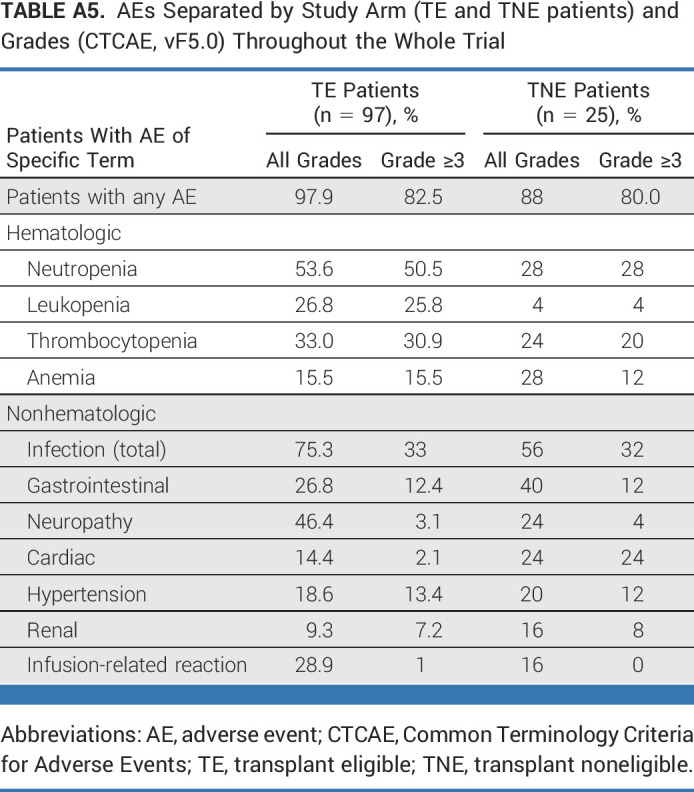
TABLE A6.
Listing of Infectious and Cardiac AEs Separated by Study Arm (TE and TNE patients) and Grades (CTCAE, vF5.0) Until End of Consolidation
FIG A1.
Depiction of analysis populations. IA, interim analysis; ITT, intention-to-treat; MRD, minimum residual disease; TE, transplant-eligible; TNE, transplant-noneligible.
FIG A2.
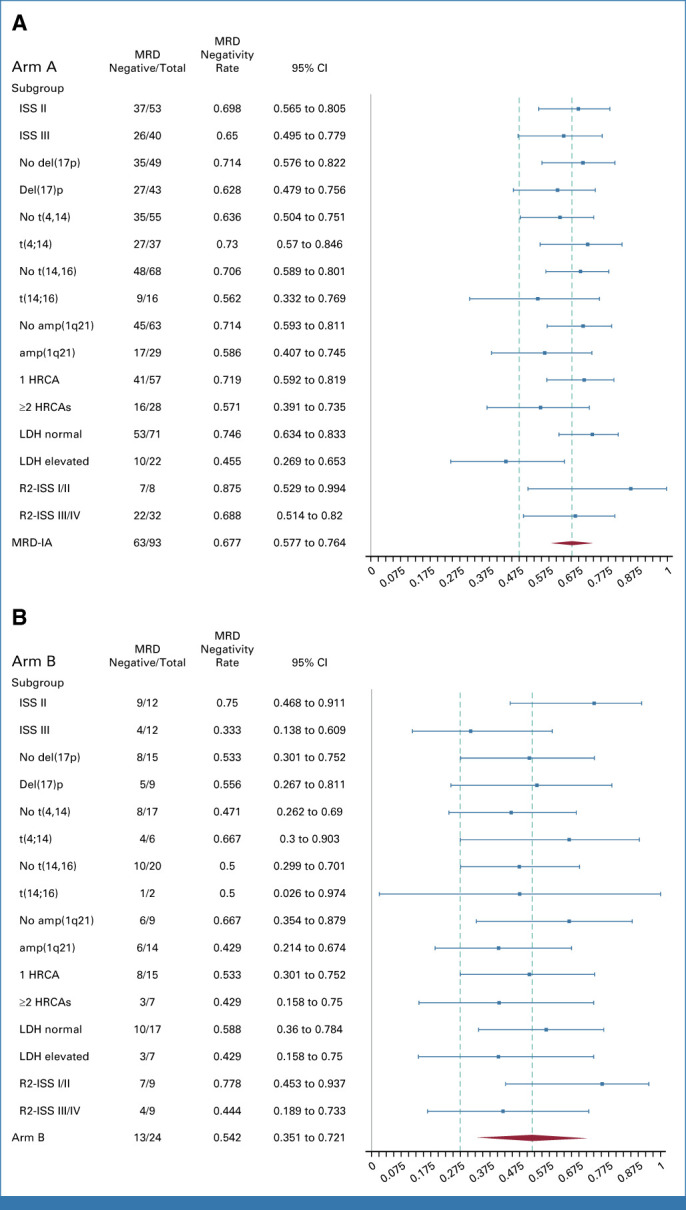
Forest plots displaying achievement of MRD negativity end point according to specific subsets of transplant-eligible (A) and transplant-noneligible (B) patients. HRCA, high-risk cytogenic aberration; ISS, International Staging System; LDH, lactate dehydrogenase; MRD, minimal residual disease; R2-ISS, Second Revision of the International Staging System.
FIG A3.
PFS in transplant-eligible patients according to the presence (red) or absence (blue) of (A) amplification of 1q21, (B) t(14;16), (C) t(4;14), or (D) according to R2-ISS. PFS, progression-free survival; R2-ISS, Second Revision of the International Staging System.
Lisa B. Leypoldt
Honoraria: Janssen, Sanofi
Consulting or Advisory Role: Sanofi, GlaxoSmithKline, Janssen, Bristol Myers Squibb/Celgene
Research Funding: GlaxoSmithKline (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Sanofi, GlaxoSmithKline, AbbVie
Britta Besemer
Honoraria: Janssen-Cilag (Inst), Amgen (Inst), GlaxoSmithKline (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, Amgen (Inst)
Mathias Hänel
Honoraria: Novartis, Sobi, Gilead Sciences, Falk Foundation
Consulting or Advisory Role: Novartis, Bristol Myers Squibb/Celgene, Gilead Sciences, Pfizer, Incyte, Sanofi/Aventis, Roche Pharma AG, Amgen, Sobi, Janssen
Marc S. Raab
Honoraria: AbbVie, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Bristol Myers Squibb/Celgene (Inst), Amgen (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Sanofi (Inst), Pfizer (Inst)
Research Funding: Bristol Myers Squibb/Celgene (Inst), Janssen (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: AbbVie, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Amgen, Janssen, Sanofi, Pfizer
Christoph Mann
Consulting or Advisory Role: Sanofi, BMS GmbH & Co KG, Janssen
Markus Munder
Honoraria: Janssen Oncology, BMS GmbH & Co KG, GlaxoSmithKline, Sanofi
Consulting or Advisory Role: Janssen Oncology, BMS GmbH & Co KG, GlaxoSmithKline, Sanofi, Takeda, Amgen, Stemline Therapeutics
Travel, Accommodations, Expenses: Amgen, Bristol Myers Squibb
Hans Christian Reinhardt
Honoraria: AbbVie, Roche, Novartis
Speakers' Bureau: AbbVie, Roche
Research Funding: Gilead Sciences, AstraZeneca
Travel, Accommodations, Expenses: AbbVie
Axel Nogai
Consulting or Advisory Role: Celgene, Roche, Takeda, Alexion, Janssen, BMS, Sanofi, Amgen, GSK
Research Funding: BMS, Janssen, Celgene
Travel, Accommodations, Expenses: Janssen, Takeda, Amgen
Maike de Wit
Employment: Vivantes Network for Health
Speakers' Bureau: AstraZeneca, Janssen, Sanofi, Pierre Fabre
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), Janssen (Inst), MSD (Inst), Amgen (Inst), Genzyme (Inst), Pfizer (Inst), Takeda (Inst), AbbVie (Inst), MorphoSys (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, AstraZeneca, Pfizer, Janssen
Hans Salwender
Honoraria: Takeda, Chugai Pharma, Janssen, BMS GmbH & Co KG, Amgen, AbbVie, Stemline Therapeutics, Oncopeptides, AstraZeneca, Sanofi, Genzyme, GlaxoSmithKline, Pfizer, Roche
Consulting or Advisory Role: Pfizer, Janssen Oncology, Sanofi, Oncopeptides, GlaxoSmithKline, Amgen, AbbVie, Bristol Myers Squibb/Celgene, Roche, Genzyme, Stemline Therapeutics, AstraZeneca
Travel, Accommodations, Expenses: Amgen, BMS GmbH & Co KG, Janssen, GlaxoSmithKline
Christof Scheid
Honoraria: Amgen, Bristol Myers Squibb/Celgene, Janssen Oncology, Novartis, Pfizer, Takeda, Sanofi/Aventis, GlaxoSmithKline
Consulting or Advisory Role: Amgen, Roche, Janssen Oncology, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Sanofi/Aventis
Research Funding: Janssen Oncology (Inst), Takeda (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb/Celgene, Janssen Oncology, Amgen
Ullrich Graeven
Stock and Other Ownership Interests: Biontech
Honoraria: Boehringer Ingelheim, Amgen, AstraZeneca, Bristol Myers Squibb, MSD Oncology, Sanofi Aventis GmbH, Fujifilm, Novartis, Celltrion, Ipsen
Consulting or Advisory Role: Amgen, MSD Oncology
Research Funding: Ipsen (Inst), Macrogenics (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim, GlaxoSmithKline
Rudolf Peceny
Consulting or Advisory Role: Miltenyi Biomedicine
Travel, Accommodations, Expenses: Janssen-Cilag, RG Ärztefortbildung, JACIE
Peter Staib
Honoraria: AbbVie, Amgen, AstraZeneca, Celgene, BMS GmbH & Co KG, MSD Oncology, Pfizer, Merck Serono, Roche Pharma AG, Novartis
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, BMS GmbH & Co KG, Incyte
Speakers' Bureau: AbbVie, Amgen, AstraZeneca, BMS GmbH & Co KG, Roche Pharma AG, Pfizer, Incyte
Research Funding: AbbVie, Novartis, Celgene, Roche
Travel, Accommodations, Expenses: AbbVie, AstraZeneca
Other Relationship: AbbVie (Inst), Amgen (Inst), BMS GmbH & Co KG (Inst), Eisai Germany (Inst), Incyte (Inst), Ipsen (Inst), Gilead Sciences (Inst), AstraZeneca (Inst), Janssen Oncology (Inst), Lilly Medical (Inst), Merck Serono (Inst), MSD Oncology (Inst), Pfizer (Inst), Roche Pharma AG (Inst), Novartis (Inst), Takeda (Inst)
Annette Dieing
Consulting or Advisory Role: BMSi
Hermann Einsele
Honoraria: Janssen, Amgen, Celgene, Bristol Myers Squibb, Takeda, Novartis, Sanofi, GlaxoSmithKline
Consulting or Advisory Role: Amgen, Celgene, Bristol Myers Squibb, Takeda, Janssen, Novartis, Sanofi, GlaxoSmithKline, Roche
Research Funding: Celgene (Inst), Janssen (Inst), Amgen (Inst), Bristol Myers Squibb, GlaxoSmithKline, Sanofi, Novartis
Travel, Accommodations, Expenses: Amgen, Janssen, Celgene, Bristol Myers Squibb
Michael Hundemer
Employment: MSD
Consulting or Advisory Role: BeiGene (Inst), Becton Dickinson (Inst)
Research Funding: Novartis (Inst), BeiGene (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: BeiGene (Inst)
Carsten Bokemeyer
Honoraria: Merck KGaA, Sanofi, Roche, Bayer, Bristol Myers Squibb, AstraZeneca, Merck Sharp Dohme, Medac, Hexal, Medupdate, I-Med Institute
Consulting or Advisory Role: Sanofi, Bayer Schering Pharma, Merck Sharp & Dohme, AOK Health Insurance, Oncology Drug Consult (ODC), Janssen-Cilag GmbH, BioNTech SE, Lindis Biotech
Research Funding: AbbVie (Inst), ADC Therapeutics (Inst), Agile Therapeutics (Inst), Alexion Pharmaceuticals (Inst), Amgen (Inst), Apellis Pharmaceuticals (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), BerGenBio (Inst), Blueprint Medicines (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Celgene (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Gilead Sciences (Inst), Glycotope GmbH (Inst), GlaxoSmithKline (Inst), Incyte (Inst), IO Biotech (Inst), Isofol Medical (Inst), Janssen-Cilag (Inst), Karyopharm Therapeutics (Inst), Lilly (Inst), Millennium (Inst), MSD (Inst), Nektar (Inst), Novartis (Inst), Rafael Pharmaceuticals (Inst), Roche (Inst), Springworks Therapeutics (Inst), Taiho Pharmaceutical (Inst), Ipsen (Inst), Servier/Pfizer (Inst), immatics (Inst), CPT Cellex Patient Treatment (Inst), Glycostem (Inst), BioNTech SE (Inst)
Travel, Accommodations, Expenses: Merck Serono, Sanofi, Bristol Myers Squibb, Janssen-Cilag, Daiichi Sankyo Europe GmbH
Hartmut Goldschmidt
Honoraria: Janssen-Cilag, Novartis, Bristol Myers Squibb, Chugai Pharma, Sanofi, Amgen, GlaxoSmithKline, Pfizer
Consulting or Advisory Role: Janssen-Cilag (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Adaptive Biotechnologies (Inst), Sanofi (Inst)
Research Funding: Bristol Myers Squibb (Inst), Janssen (Inst), Novartis (Inst), Celgene (Inst), Amgen (Inst), Sanofi (Inst), Takeda (Inst), Molecular Partners (Inst), MSD (Inst), Incyte (Inst), GlycoMimetics Inc (Inst), GlaxoSmithKline (Inst), Heidelberg Pharma (Inst), Roche (Inst), Karyopharm Therapeutics (Inst), Millenium Pharamceuticals (Inst), MorphoSys (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, Sanofi, Amgen, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Novartis, Pfizer
Other Relationship: Amgen (Inst), Celgene/Bristol Myers Squibb (Inst), Chugai Pharma Europe (Inst), Janssen (Inst), Sanofi (Inst), Mundipharma (Inst), Array BioPharma/Pfizer (Inst)
Katja C. Weisel
Honoraria: Amgen, Bristol Myers Squibb, Janssen-Cilag, GlaxoSmithKline, Adaptive Biotechnologies, Karyopharm Therapeutics, Takeda, Sanofi, AbbVie, Novartis, Pfizer, Celgene, Janssen (Inst), Oncopeptides, Roche, Menarini
Consulting or Advisory Role: Amgen, Adaptive Biotechnologies, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen-Cilag, Karyopharm Therapeutics, Sanofi, Takeda, Oncopeptides, Roche, Menarini
Research Funding: Amgen (Inst), Celgene (Inst), Sanofi (Inst), Janssen-Cilag (Inst), Bristol Myers Squibb/Celgene (Inst), GlaxoSmithKline (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Amgen, Celgene, Bristol Myers Squibb, Janssen-Cilag, GlaxoSmithKline, Takeda, Menarini
No other potential conflicts of interest were reported.
See accompanying Editorial, p. 1
PRIOR PRESENTATION
Presented in part at the 64th Annual Meeting of the American Society of Hematology, New Orleans, LA, December 13, 2022.
SUPPORT
Supported in part by Amgen, Bristol Myers Squibb/Celgene, and Sanofi (study drug and funding). The trial was sponsored by University Medical Center Hamburg-Eppendorf.
CLINICAL TRIAL INFORMATION
NCT03104842 (GMMG-CONCEPT)
DATA SHARING STATEMENT
Any requests for individual data should be directed to the corresponding author; only reasonable requests with profound methodological approach will be considered, and approval of both the steering committee of the German-speaking Myeloma Multicenter Group and the Data Safety Monitoring Board must be obtained first.
AUTHOR CONTRIBUTIONS
Conception and design: Lisa B. Leypoldt, Manola Zago, Axel Benner, Diana Tichy, Hartmut Goldschmidt, Katja C. Weisel
Administrative support: Lisa B. Leypoldt, Manola Zago, Hartmut Goldschmidt, Katja C. Weisel
Provision of study materials or patients: Lisa B. Leypoldt, Britta Besemer, Mathias Hänel, Marc S. Raab, Christoph Mann, Markus Munder, Hans Christian Reinhardt, Axel Nogai, Martin Görner, Yon-Dschun Ko, Maike de Wit, Hans Salwender, Christof Scheid, Ullrich Graeven, Rudolf Peceny, Peter Staib, Annette Dieing, Anna Jauch, Carsten Bokemeyer, Hartmut Goldschmidt, Katja C. Weisel
Collection and assembly of data: Lisa B. Leypoldt, Britta Besemer, Mathias Hänel, Marc S. Raab, Christoph Mann, Markus Munder, Hans Christian Reinhardt, Axel Nogai, Martin Görner, Yon-Dschun Ko, Maike de Wit, Hans Salwender, Christof Scheid, Ullrich Graeven, Rudolf Peceny, Peter Staib, Annette Dieing, Anna Jauch, Michael Hundemer, Hartmut Goldschmidt, Katja C. Weisel
Data analysis and interpretation: Lisa B. Leypoldt, Diana Tichy, Britta Besemer, Mathias Hänel, Marc S. Raab, Christoph Mann, Markus Munder, Hans Christian Reinhardt, Axel Nogai, Martin Görner, Yon-Dschun Ko, Maike de Wit, Hans Salwender, Christof Scheid, Ullrich Graeven, Rudolf Peceny, Peter Staib, Annette Dieing, Hermann Einsele, Anna Jauch, Michael Hundemer, Ema Požek, Axel Benner, Carsten Bokemeyer, Hartmut Goldschmidt, Katja C. Weisel
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lisa B. Leypoldt
Honoraria: Janssen, Sanofi
Consulting or Advisory Role: Sanofi, GlaxoSmithKline, Janssen, Bristol Myers Squibb/Celgene
Research Funding: GlaxoSmithKline (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Sanofi, GlaxoSmithKline, AbbVie
Britta Besemer
Honoraria: Janssen-Cilag (Inst), Amgen (Inst), GlaxoSmithKline (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, Amgen (Inst)
Mathias Hänel
Honoraria: Novartis, Sobi, Gilead Sciences, Falk Foundation
Consulting or Advisory Role: Novartis, Bristol Myers Squibb/Celgene, Gilead Sciences, Pfizer, Incyte, Sanofi/Aventis, Roche Pharma AG, Amgen, Sobi, Janssen
Marc S. Raab
Honoraria: AbbVie, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Bristol Myers Squibb/Celgene (Inst), Amgen (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Sanofi (Inst), Pfizer (Inst)
Research Funding: Bristol Myers Squibb/Celgene (Inst), Janssen (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: AbbVie, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Amgen, Janssen, Sanofi, Pfizer
Christoph Mann
Consulting or Advisory Role: Sanofi, BMS GmbH & Co KG, Janssen
Markus Munder
Honoraria: Janssen Oncology, BMS GmbH & Co KG, GlaxoSmithKline, Sanofi
Consulting or Advisory Role: Janssen Oncology, BMS GmbH & Co KG, GlaxoSmithKline, Sanofi, Takeda, Amgen, Stemline Therapeutics
Travel, Accommodations, Expenses: Amgen, Bristol Myers Squibb
Hans Christian Reinhardt
Honoraria: AbbVie, Roche, Novartis
Speakers' Bureau: AbbVie, Roche
Research Funding: Gilead Sciences, AstraZeneca
Travel, Accommodations, Expenses: AbbVie
Axel Nogai
Consulting or Advisory Role: Celgene, Roche, Takeda, Alexion, Janssen, BMS, Sanofi, Amgen, GSK
Research Funding: BMS, Janssen, Celgene
Travel, Accommodations, Expenses: Janssen, Takeda, Amgen
Maike de Wit
Employment: Vivantes Network for Health
Speakers' Bureau: AstraZeneca, Janssen, Sanofi, Pierre Fabre
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), Janssen (Inst), MSD (Inst), Amgen (Inst), Genzyme (Inst), Pfizer (Inst), Takeda (Inst), AbbVie (Inst), MorphoSys (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, AstraZeneca, Pfizer, Janssen
Hans Salwender
Honoraria: Takeda, Chugai Pharma, Janssen, BMS GmbH & Co KG, Amgen, AbbVie, Stemline Therapeutics, Oncopeptides, AstraZeneca, Sanofi, Genzyme, GlaxoSmithKline, Pfizer, Roche
Consulting or Advisory Role: Pfizer, Janssen Oncology, Sanofi, Oncopeptides, GlaxoSmithKline, Amgen, AbbVie, Bristol Myers Squibb/Celgene, Roche, Genzyme, Stemline Therapeutics, AstraZeneca
Travel, Accommodations, Expenses: Amgen, BMS GmbH & Co KG, Janssen, GlaxoSmithKline
Christof Scheid
Honoraria: Amgen, Bristol Myers Squibb/Celgene, Janssen Oncology, Novartis, Pfizer, Takeda, Sanofi/Aventis, GlaxoSmithKline
Consulting or Advisory Role: Amgen, Roche, Janssen Oncology, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Sanofi/Aventis
Research Funding: Janssen Oncology (Inst), Takeda (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb/Celgene, Janssen Oncology, Amgen
Ullrich Graeven
Stock and Other Ownership Interests: Biontech
Honoraria: Boehringer Ingelheim, Amgen, AstraZeneca, Bristol Myers Squibb, MSD Oncology, Sanofi Aventis GmbH, Fujifilm, Novartis, Celltrion, Ipsen
Consulting or Advisory Role: Amgen, MSD Oncology
Research Funding: Ipsen (Inst), Macrogenics (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim, GlaxoSmithKline
Rudolf Peceny
Consulting or Advisory Role: Miltenyi Biomedicine
Travel, Accommodations, Expenses: Janssen-Cilag, RG Ärztefortbildung, JACIE
Peter Staib
Honoraria: AbbVie, Amgen, AstraZeneca, Celgene, BMS GmbH & Co KG, MSD Oncology, Pfizer, Merck Serono, Roche Pharma AG, Novartis
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, BMS GmbH & Co KG, Incyte
Speakers' Bureau: AbbVie, Amgen, AstraZeneca, BMS GmbH & Co KG, Roche Pharma AG, Pfizer, Incyte
Research Funding: AbbVie, Novartis, Celgene, Roche
Travel, Accommodations, Expenses: AbbVie, AstraZeneca
Other Relationship: AbbVie (Inst), Amgen (Inst), BMS GmbH & Co KG (Inst), Eisai Germany (Inst), Incyte (Inst), Ipsen (Inst), Gilead Sciences (Inst), AstraZeneca (Inst), Janssen Oncology (Inst), Lilly Medical (Inst), Merck Serono (Inst), MSD Oncology (Inst), Pfizer (Inst), Roche Pharma AG (Inst), Novartis (Inst), Takeda (Inst)
Annette Dieing
Consulting or Advisory Role: BMSi
Hermann Einsele
Honoraria: Janssen, Amgen, Celgene, Bristol Myers Squibb, Takeda, Novartis, Sanofi, GlaxoSmithKline
Consulting or Advisory Role: Amgen, Celgene, Bristol Myers Squibb, Takeda, Janssen, Novartis, Sanofi, GlaxoSmithKline, Roche
Research Funding: Celgene (Inst), Janssen (Inst), Amgen (Inst), Bristol Myers Squibb, GlaxoSmithKline, Sanofi, Novartis
Travel, Accommodations, Expenses: Amgen, Janssen, Celgene, Bristol Myers Squibb
Michael Hundemer
Employment: MSD
Consulting or Advisory Role: BeiGene (Inst), Becton Dickinson (Inst)
Research Funding: Novartis (Inst), BeiGene (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: BeiGene (Inst)
Carsten Bokemeyer
Honoraria: Merck KGaA, Sanofi, Roche, Bayer, Bristol Myers Squibb, AstraZeneca, Merck Sharp Dohme, Medac, Hexal, Medupdate, I-Med Institute
Consulting or Advisory Role: Sanofi, Bayer Schering Pharma, Merck Sharp & Dohme, AOK Health Insurance, Oncology Drug Consult (ODC), Janssen-Cilag GmbH, BioNTech SE, Lindis Biotech
Research Funding: AbbVie (Inst), ADC Therapeutics (Inst), Agile Therapeutics (Inst), Alexion Pharmaceuticals (Inst), Amgen (Inst), Apellis Pharmaceuticals (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), BerGenBio (Inst), Blueprint Medicines (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Celgene (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Gilead Sciences (Inst), Glycotope GmbH (Inst), GlaxoSmithKline (Inst), Incyte (Inst), IO Biotech (Inst), Isofol Medical (Inst), Janssen-Cilag (Inst), Karyopharm Therapeutics (Inst), Lilly (Inst), Millennium (Inst), MSD (Inst), Nektar (Inst), Novartis (Inst), Rafael Pharmaceuticals (Inst), Roche (Inst), Springworks Therapeutics (Inst), Taiho Pharmaceutical (Inst), Ipsen (Inst), Servier/Pfizer (Inst), immatics (Inst), CPT Cellex Patient Treatment (Inst), Glycostem (Inst), BioNTech SE (Inst)
Travel, Accommodations, Expenses: Merck Serono, Sanofi, Bristol Myers Squibb, Janssen-Cilag, Daiichi Sankyo Europe GmbH
Hartmut Goldschmidt
Honoraria: Janssen-Cilag, Novartis, Bristol Myers Squibb, Chugai Pharma, Sanofi, Amgen, GlaxoSmithKline, Pfizer
Consulting or Advisory Role: Janssen-Cilag (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Adaptive Biotechnologies (Inst), Sanofi (Inst)
Research Funding: Bristol Myers Squibb (Inst), Janssen (Inst), Novartis (Inst), Celgene (Inst), Amgen (Inst), Sanofi (Inst), Takeda (Inst), Molecular Partners (Inst), MSD (Inst), Incyte (Inst), GlycoMimetics Inc (Inst), GlaxoSmithKline (Inst), Heidelberg Pharma (Inst), Roche (Inst), Karyopharm Therapeutics (Inst), Millenium Pharamceuticals (Inst), MorphoSys (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, Sanofi, Amgen, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Novartis, Pfizer
Other Relationship: Amgen (Inst), Celgene/Bristol Myers Squibb (Inst), Chugai Pharma Europe (Inst), Janssen (Inst), Sanofi (Inst), Mundipharma (Inst), Array BioPharma/Pfizer (Inst)
Katja C. Weisel
Honoraria: Amgen, Bristol Myers Squibb, Janssen-Cilag, GlaxoSmithKline, Adaptive Biotechnologies, Karyopharm Therapeutics, Takeda, Sanofi, AbbVie, Novartis, Pfizer, Celgene, Janssen (Inst), Oncopeptides, Roche, Menarini
Consulting or Advisory Role: Amgen, Adaptive Biotechnologies, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen-Cilag, Karyopharm Therapeutics, Sanofi, Takeda, Oncopeptides, Roche, Menarini
Research Funding: Amgen (Inst), Celgene (Inst), Sanofi (Inst), Janssen-Cilag (Inst), Bristol Myers Squibb/Celgene (Inst), GlaxoSmithKline (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Amgen, Celgene, Bristol Myers Squibb, Janssen-Cilag, GlaxoSmithKline, Takeda, Menarini
No other potential conflicts of interest were reported.
REFERENCES
- 1.Attal M, Lauwers-Cances V, Hulin C, et al. : Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 376:1311-1320, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mateos MV, Dimopoulos MA, Cavo M, et al. : Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 378:518-528, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Moreau P, Attal M, Hulin C, et al. : Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 394:29-38, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Facon T, Kumar S, Plesner T, et al. : Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 380:2104-2115, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy PL, Holstein SA, Petrucci MT, et al. : Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: A meta-analysis. J Clin Oncol 35:3279-3289, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson PG, Jacobus SJ, Weller EA, et al. : Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med 387:132-147, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voorhees PM, Kaufman JL, Laubach JP, et al. : Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 136:936-945, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Agostino M, Cairns DA, Lahuerta JJ, et al. : Second revision of the International Staging System (R2-ISS) for overall survival in multiple myeloma: A European Myeloma Network (EMN) report within the HARMONY project. J Clin Oncol 40:3406-3418, 2022 [DOI] [PubMed] [Google Scholar]
- 9.Munshi NC, Avet-Loiseau H, Anderson KC, et al. : A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv 4:5988-5999, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goicoechea I, Puig N, Cedena MT, et al. : Deep MRD profiling defines outcome and unveils different modes of treatment resistance in standard- and high-risk myeloma. Blood 137:49-60, 2021 [DOI] [PubMed] [Google Scholar]
- 11.San-Miguel J, Avet-Loiseau H, Paiva B, et al. : Sustained minimal residual disease negativity in newly diagnosed multiple myeloma and the impact of daratumumab in MAIA and ALCYONE. Blood 139:492-501, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avet-Loiseau H, Ludwig H, Landgren O, et al. : Minimal residual disease status as a surrogate endpoint for progression-free survival in newly diagnosed multiple myeloma studies: A meta-analysis. Clin Lymphoma Myeloma Leuk 20:e30-e37, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Paiva B, Anderson KC, et al. : International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17:e328-e346, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Chen L, Wei J, et al. : Two-stage phase II survival trial design. Pharm Stat 19:214-229, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agresti A, Coull BA: Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 52:119-126, 1998 [Google Scholar]
- 16.Giobbie-Hurder A, Gelber RD, Regan MM: Challenges of guarantee-time bias. J Clin Oncol 31:2963-2969, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therneau T, Grambsch P: Modeling Survival Data: Extending the Cox Model (ed 1). New York, NY, Springer, 2000 [Google Scholar]
- 18.Bernasconi DP, Rebora P, Iacobelli S, et al. : Survival probabilities with time-dependent treatment indicator: Quantities and non-parametric estimators. Stat Med 35:1032-1048, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Costa LJ, Chhabra S, Medvedova E, et al. : Daratumumab, carfilzomib, lenalidomide, and dexamethasone with minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma. J Clin Oncol 40:2901-2912, 2022 [DOI] [PubMed] [Google Scholar]
- 20.Gay F, Musto P, Rota-Scalabrini D, et al. : Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): A randomised, open-label, phase 2 trial. Lancet Oncol 22:1705-1720, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Kaiser MF, Hall A, Walker K, et al. : Daratumumab, cyclophosphamide, bortezomib, lenalidomide, and dexamethasone as induction and extended consolidation improves outcome in ultra-high-risk multiple myeloma. J Clin Oncol 41:3945-3955, 2023 [DOI] [PubMed] [Google Scholar]
- 22.Goldschmidt H, Mai EK, Bertsch U, et al. : Addition of isatuximab to lenalidomide, bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible patients with multiple myeloma (GMMG-HD7): Part 1 of an open-label, multicentre, randomised, active-controlled, phase 3 trial. Lancet Haematol 9:e810-e821, 2022 [DOI] [PubMed] [Google Scholar]
- 23.Landgren O, Hultcrantz M, Diamond B, et al. : Safety and effectiveness of weekly carfilzomib, lenalidomide, dexamethasone, and daratumumab combination therapy for patients with newly diagnosed multiple myeloma: The MANHATTAN nonrandomized clinical trial. JAMA Oncol 7:862-868, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callander NS, Baljevic M, Adekola K, et al. : NCCN Guidelines® insights: Multiple myeloma, version 3.2022: Featured updates to the NCCN Guidelines. J Natl Compr Cancer Netw 20:8-19, 2022 [DOI] [PubMed] [Google Scholar]
- 25.Jakubowiak AJ, Kumar S, Medhekar R, et al. : Daratumumab improves depth of response and progression-free survival in transplant-ineligible, high-risk, newly diagnosed multiple myeloma. Oncologist 27:e589-e596, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Intergroupe Francophone du Myelome; Amgen; Sanofi, et al. : Minimal residual disease adapted strategy, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT04934475
- 27.Sonneveld P, Broijl A, Gay F, et al. : Bortezomib, lenalidomide, and dexamethasone (VRd) ± daratumumab (DARA) in patients (pts) with transplant-eligible (TE) newly diagnosed multiple myeloma (NDMM): A multicenter, randomized, phase III study (PERSEUS). J Clin Oncol 37, 2019. (suppl 15; abstr TPS8055) [Google Scholar]
- 28.Royle KL, Coulson AB, Ramasamy K, et al. : Risk and response adapted therapy following autologous stem cell transplant in patients with newly diagnosed multiple myeloma (RADAR (UK-MRA Myeloma XV) Trial): Study protocol for a phase II/III randomised controlled trial. BMJ Open 12:e063037, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.A study of daratumumab, carfilzomib, lenalidomide, and dexamethasone in patients with newly-diagnosed multiple myeloma. NCT04268498. https://classic.clinicaltrials.gov/show/NCT04268498 [DOI] [PubMed]
- 30.Mina R, Musto P, Rota-Scalabrini D, et al. : Carfilzomib induction, consolidation, and maintenance with or without autologous stem-cell transplantation in patients with newly diagnosed multiple myeloma: Pre-planned cytogenetic subgroup analysis of the randomised, phase 2 FORTE trial. Lancet Oncol 24:64-76, 2023 [DOI] [PubMed] [Google Scholar]
- 31.Sonneveld P, Avet-Loiseau H, Lonial S, et al. : Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 127:2955-2962, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samur MK, Roncador M, Aktas Samur A, et al. : High-dose melphalan treatment significantly increases mutational burden at relapse in multiple myeloma. Blood 141:1724-1736, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diamond B, Ziccheddu B, Maclachlan K, et al. : Tracking the evolution of therapy-related myeloid neoplasms using chemotherapy signatures. Blood 141:2359-2371, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazandjian D, Landgren O: Novel quadruplets and the age of immunotherapies in the treatment of newly diagnosed multiple myeloma. JAMA Oncol 8:1260-1262, 2022 [DOI] [PubMed] [Google Scholar]
- 35.Maura F, Weinhold N, Diamond B, et al. : The mutagenic impact of melphalan in multiple myeloma. Leukemia 35:2145-2150, 2021 [DOI] [PubMed] [Google Scholar]
- 36.A study of daratumumab, bortezomib, lenalidomide and dexamethasone (DVRd) followed by ciltacabtagene autoleucel versus daratumumab, bortezomib, lenalidomide and dexamethasone (DVRd) followed by autologous stem cell transplant (ASCT) in participants with newly diagnosed multiple myeloma. NCT05257083. https://classic.clinicaltrials.gov/show/NCT05257083
- 37.Study with elranatamab versus lenalidomide in patients with newly diagnosed multiple myeloma after transplant. NCT05317416. https://classic.clinicaltrials.gov/show/NCT05317416
- 38.Weisel K, Besemer B, Haenel M, et al. : Isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in patients with high-risk newly diagnosed multiple myeloma: planned interim analysis of the GMMG-CONCEPT trial. Blood 140:1836-1838, 2022 [Google Scholar]
- 39.Leypoldt LB, Besemer B, Asemissen AM, et al. : Isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in front-line treatment of high-risk multiple myeloma: Interim analysis of the GMMG-CONCEPT trial. Leukemia 36:885-888, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avet-Loiseau H, Durie BG, Cavo M, et al. : Combining fluorescent in situ hybridization data with ISS staging improves risk assessment in myeloma: An International Myeloma Working Group collaborative project. Leukemia 27:711-717, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt TM, Barwick BG, Joseph N, et al. : Gain of Chromosome 1q is associated with early progression in multiple myeloma patients treated with lenalidomide, bortezomib, and dexamethasone. Blood Cancer J 9:94, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any requests for individual data should be directed to the corresponding author; only reasonable requests with profound methodological approach will be considered, and approval of both the steering committee of the German-speaking Myeloma Multicenter Group and the Data Safety Monitoring Board must be obtained first.