Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
We analyzed long-term results of the response-adapted trial for adult patients with advanced-stage Hodgkin lymphoma. The aim was to confirm noninferiority of treatment de-escalation by omission of bleomycin from doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) for interim fluorodeoxyglucose positron emission tomography (iPET)–negative patients and assess efficacy and long-term safety for iPET-positive patients who underwent treatment intensification with escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone (BEACOPP/BEACOPP14). The median follow-up is 7.3 years. For all patients, the 7-year progression-free survival (PFS) and overall survival (OS) are 78.2% (95% CI, 75.6 to 80.5) and 91.6% (95% CI, 89.7 to 93.2), respectively. The 1.3% difference in 3-year PFS (95% CI, –3.0 to 4.7) between ABVD and doxorubicin, vinblastine, and dacarbazine (AVD) now falls within the predefined noninferiority margin. Among 172 patients with positive iPET, the 7-year PFS was 65.9% (95% CI, 58.1 to 72.6) and the 7-year OS was 83.2% (95% CI, 76.2 to 88.3). The cumulative incidence of second malignancies at 7 years was 5.5% (95% CI, 4.0 to 7.5) for those receiving ABVD/AVD and 2.5% (95% CI, 0.8 to 7.7) for those escalated to BEACOPP. With extended follow-up, these results confirm noninferiority of treatment de-escalation after a negative iPET. Escalation with BEACOPP for iPET-positive patients is effective and safe, with no increase in second malignancies.
INTRODUCTION
When treating patients with classical Hodgkin Lymphoma (HL), it is crucial to balance the intensity of initial treatment with safety.1 For patients with advanced-stage HL, the Response-Adapted Trial (RATHL) investigated whether interim fluorodeoxyglucose positron emission tomography (iPET) scans could guide de-escalation of therapy for patients with a high likelihood of cure after doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and escalation for patients at higher risk of failure.2 The overall results of this approach appeared to be favorable compared with previous studies with ABVD, using more selective chemotherapy and much less radiotherapy. We present the results of longer follow-up, focusing on the late consequences of therapy modulation.
METHODS
Adult patients with newly diagnosed HL (Ann Arbor stages IIB–IV or IIA with bulk or ≥3 involved sites) underwent paired baseline and iPET scans after two cycles of ABVD (PET2). Images were reviewed centrally using the 5-point Deauville scale as negative (1-3) or positive (4-5). Patients with negative scans were randomly assigned to ABVD or AVD, omitting bleomycin, for four further cycles. The hypothesis was to test the noninferiority of 3-year progression-free survival (PFS). Patients with positive scans proceeded to intensification with either escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone (BEACOPP) or BEACOPP-14 selected in advance by center. Radiotherapy (RT) was permitted but not advised for patients with interim-negative scans, irrespective of baseline bulk or residual masses. Written consent was documented for all patients at entry to the trial, and all study procedures were approved by national or local research ethics committees.
The primary end point was PFS 3 years from registration in ABVD and AVD patients and noninferiority with a 5% margin. Secondary end points included overall survival (OS) and the incidence of second malignancies (SMs). PFS and OS were analyzed using Kaplan-Meier survival analysis and Cox regression, with the difference in PFS at 3 years calculated by applying the hazard ratio to the rate in the AVD arm and taking the difference. The cause of death considered deaths because of HL, treatment-related deaths, or both (Hodgkin-related deaths) as well as deaths unrelated to HL or treatment. Time to SM, time to relapse, and time to HL-related death were analyzed using competing risks analysis by the method of Fine and Gray, with non-SM deaths, non–HL-related deaths, and deaths without progression treated as competing risks, respectively. All SMs diagnosed after study enrollment, other than nonmelanoma skin cancers, were considered, but an analysis was also performed treating progression as a competing risk, that is, excluding SM reported after progression of HL. Subgroup analyses and adjusted analyses comparing PET2+ and PET– patients used prespecified known risk factors, with the latter not reduced using any model building techniques. All analyses were performed using STATA version 16.1 (STATACORP, TX). P values are two-sided with P < .05 considered significant.
RESULTS
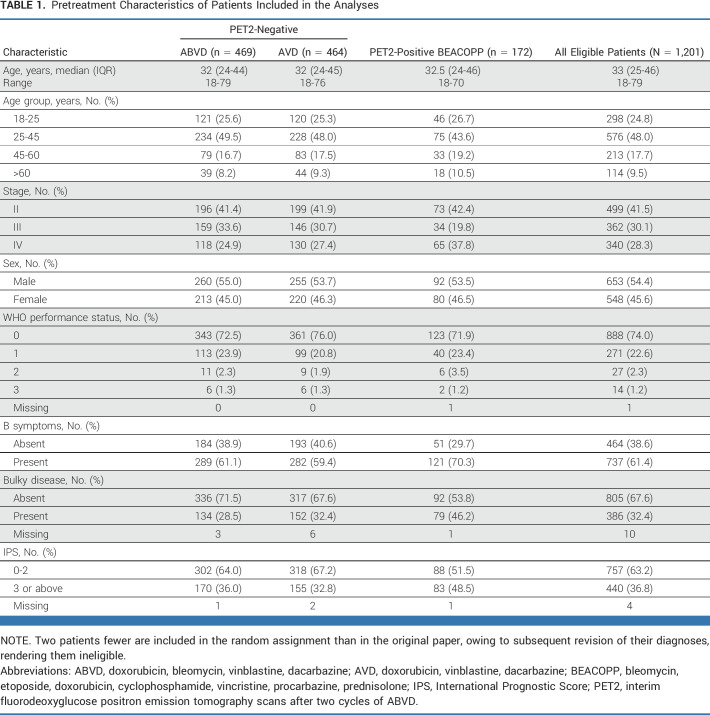
Between 2008 and 2012, 1,201 eligible patients were registered at 138 participating centers (Table 1). Their median age was 33 years (IQR, 25-46; range, 18-79), 54.5% of patients were male, 41.5% had stage II disease, 30.1% had stage III, and 28.3% had stage IV. Systemic B symptoms were present in 61.4%, and 32.4% had bulky disease defined as a thorax to mass transverse diameter ratio of >33% for mediastinal masses or an extramediastinal mass of >10 cm. Overall consolidation radiotherapy was administered in 79 patients (6.6%): 33 randomly assigned after a negative iPET and 43 treated with BEACOPP after positive iPET.
TABLE 1.
Pretreatment Characteristics of Patients Included in the Analyses
After a median follow-up of 7.3 years (IQR, 5.3-8.7), the overall PFS at 7 years is 78.2% (95% CI, 75.6 to 80.5) and the OS is 91.6% (95% CI, 89.7 to 93.2; Appendix Fig A1, online only). The 7-year PFS and OS were 84.7% (95% CI, 81.0 to 87.7) and 95.6% (95% CI, 93.2 to 97.2) for stage II patients and 73.4% (95% CI, 69.7 to 76.8) and 88.7% (95% CI, 85.7- 91.0) for stage III-IV patients, respectively (Appendix Fig A2). In 52.2% of patients 60 years and younger and with stage III-IV disease (a comparable cohort with SWOG S0816), the 7-year PFS was 75.5% (95% CI, 71.7 to 78.8) and the OS was 91.4% (95% CI, 88.6 to 93.5). Late events were more likely to be deaths in remission than relapses, with 23 late events beyond 5 years: eight recurrences and 15 deaths in remission and just three relapses occurring beyond year 7 (Appendix Fig A3).
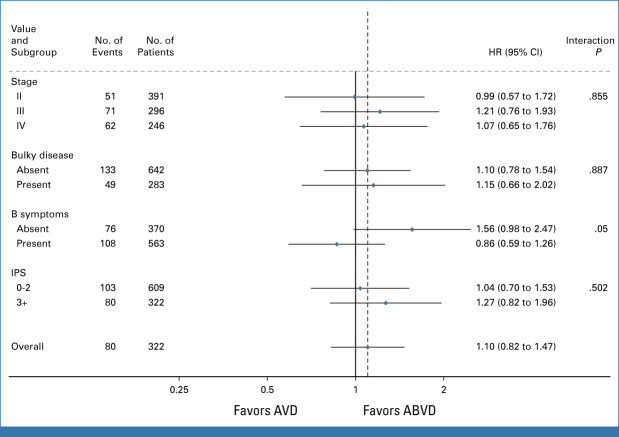
After a negative per-protocol PET2, 933 patients were randomly assigned to continue ABVD or AVD. The 3-year PFS was 85.5% (95% CI, 81.9 to 88.4) in the ABVD arm and 84.3% (95% CI, 80.6 to 87.3) in the AVD arm (HR, 1.10; 95% CI, 0.82 to 1.47). With this long-term analysis, the lower CI for the 1.3% difference in 3-year PFS (95% CI, –3.0 to 4.7) now falls within the predefined noninferiority margin of 5%. Per-protocol analysis, excluding those who did not start their randomized allocation, was similar, 1.0% (95% CI, –3.4 to 4.5). At 7 years, the PFS for ABVD was 81% (95% CI, 76.9 to 84.4), and for AVD, it was 79.2% (95% CI, 75.1 to 82.8; Fig 1A). Subgroup analyses of PFS by stage, International Prognostic Score, the presence of bulky disease, and B-symptoms did not indicate significant interactions with the treatment arm (Appendix Fig A4). In the iPET-negative group, the OS was 93.3% at 7 years (95% CI, 91.3 to 94.9) with no significant difference between arms, ABVD: 93.2% (95% CI, 90.2 to 95.3), AVD: 93.5% (95% CI, 90.5 to 95.5), HR, 0.84 (95% CI, 0.51 to 1.37; Fig 2A).
FIG 1.
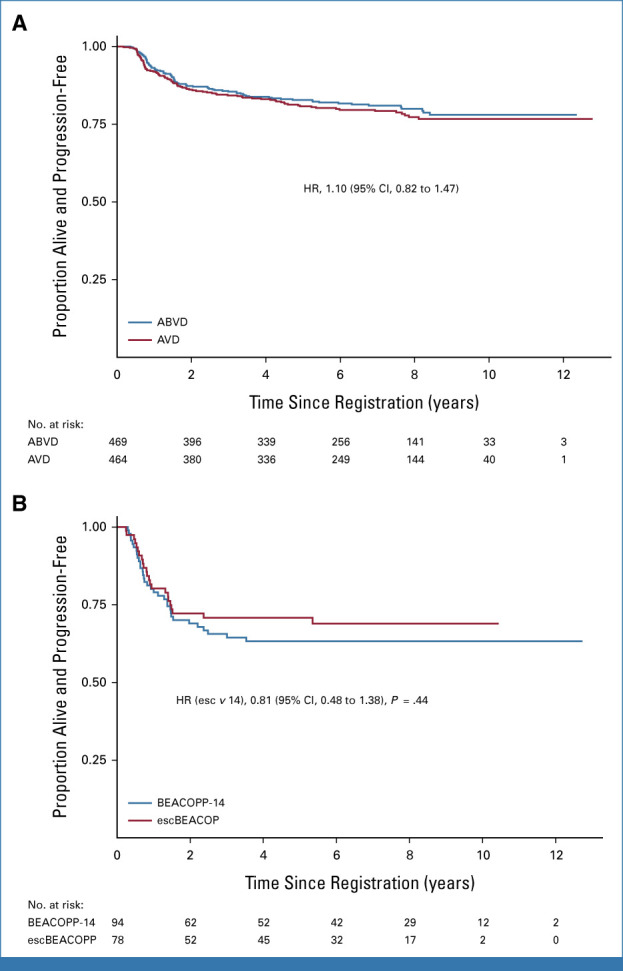
(A) PFS of PET2-negative patients by randomized arm. (B) PFS for PET2-positive patients who received intensified therapy with either escBEACOPP or BEACOPP14. ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; AVD, doxorubicin, vinblastine, dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone; escBEACOPP, escalated BEACOPP; PET2, interim fluorodeoxyglucose positron emission tomography scans after two cycles of ABVD; PFS, progression-free survival.
FIG 2.
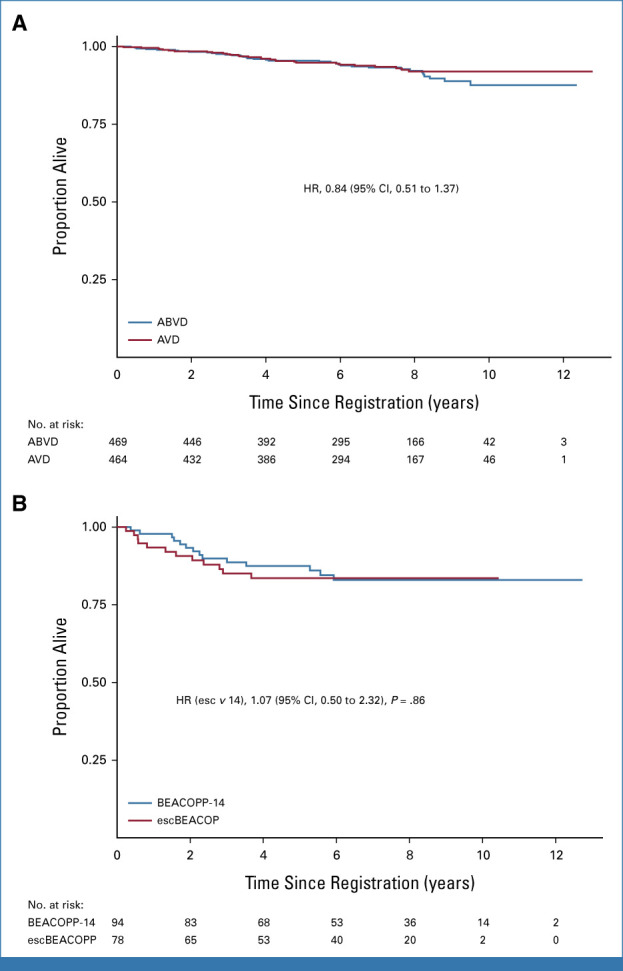
(A) OS of PET2-negative patients by randomized arm. (B) OS for PET2-positive patients who received intensified therapy with either escBEACOPP or BEACOPP14. ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; AVD, doxorubicin, vinblastine, dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone; escBEACOPP, escalated BEACOPP; OS, overall survival; PET2, interim fluorodeoxyglucose positron emission tomography scans after two cycles of ABVD.
Failure to achieve PET2 negativity was significantly associated with inferior PFS and OS, even when adjusted for baseline prognostic factors (age, sex, stage, WHO Performance status, B symptoms, baseline bulky disease): HRs 2.03 (95% CI, 1.50 to 2.75) and 2.90 (95% CI, 1.82 to 4.64), both P < .001. Among 172 patients with a positive PET2, the 7-year PFS was 65.9% (95% CI, 58.1 to 72.6; Fig 1B) and the 7-year OS was 83.2% (95% CI, 76.2 to 88.3; Fig 2B). No difference in PFS and OS rates has been observed comparing patients allocated to treatment with escBEACOPP or BEACOPP-14.
Analysis of SMs
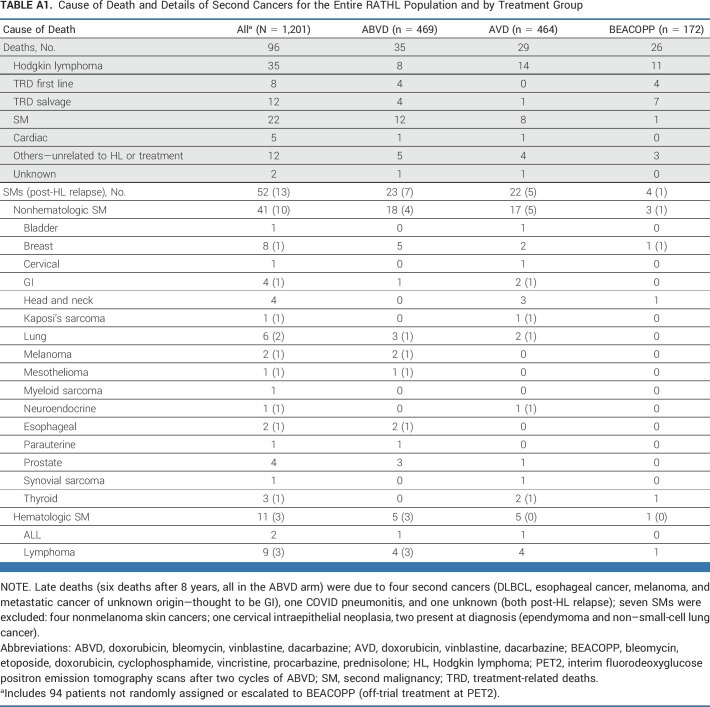
Overall, 52 SMs were reported, including three SMs in patients taken off study at PET2 (Appendix Table A1). Of the remaining 49 SMs, 39 occurred in patients without HL progression, not related to second-line therapy. Thirty-eight solid SMs were diagnosed during follow-up: 18, 17, and 3 in the ABVD, AVD, and BEACOPP groups, respectively. Hematologic SMs were diagnosed in five, five, and one patient treated with ABVD, AVD, and BEACOPP, respectively. The cumulative incidence of SMs at 7 years was 5.1% (95% CI, 3.2 to 8.1) and 5.8% (95% CI, 3.8 to 9.0) for those receiving ABVD/AVD and 2.5% (95% CI, 0.8 to 7.7) for those escalated to BEACOPP (Appendix Fig A5). Risk of SM increased with age but not with the use of radiotherapy. When considering only SMs reported preprogression, the rates were 3.7% (95% CI, 2.2 to 6.2), 4.0% (95% CI, 2.4 to 6.6), and 1.3% (95% CI, 0.3 to 5.1).
Analysis of Causes of Death
In total, 96 patients have died: 35 because of HL progression; 20 treatment-related, including 8 deaths during first-line therapy and 12 during subsequent lines of therapy; 22 because of a second malignancy; five because of cardiac events; 12 because of other causes; and two unknown. The rates of HL-related deaths at 7 years were similar: 3.7% (95% CI, 2.2 to 6.1) ABVD and 2.8% (95% CI, 2.3 to 6.2) AVD (Appendix Table A1).
DISCUSSION
This analysis with extended follow-up strengthens the previous finding that patients with a negative PET2 scan after two cycles of ABVD can be de-escalated to AVD chemotherapy without inferior outcomes. The omission of bleomycin in later cycles of treatment has not resulted in a greater likelihood of disease recurrence, but conversely, it does not appear to have significantly improved survival in the longer term, probably because respiratory failure caused by bleomycin-related lung injury is a rare cause of death.2 However, there was only one death ascribed to treatment-related toxicity (and this was after salvage treatment) among patients randomly assigned to AVD, compared with eight among those continuing ABVD, four during initial therapy and four after salvage.
Starting with ABVD and intensifying with BEACOPP for patients with a positive PET2 remains a controversial subject.3-5 It is reassuring that the responses seen to this treatment have proved to be durable, with only one PFS event beyond 5 years. Similarly, the absence of excess numbers of SMs stands in contrast to the SWOG S8016 trial, in which the cumulative incidence among 48 patients who received six cycles of escalated BEACOPP was 14%.6 It is possible that the more limited use of BEACOPP in the RATHL trial (four cycles of escalated BEACOPP or six BEACOPP-14) may account for this difference.
Taken overall, the results of this study, with a 7-year survival of 92% for the whole population, support the use of an interim PET response–adapted approach for patients with advanced-stage HL when ABVD is chosen as initial therapy. Recent results from the ECHELON1 study support the use of brentuximab vedotin with AVD (BV-AVD) as a new standard of care for patients with stage III-IV diease.7 Furthermore, the SWOG1826 trial showed improved PFS after nivolumab-AVD compared with BV-AVD in a similar group of patients,8 albeit with only a follow-up of 12 months. Neither of these trials used a response-adapted ABVD comparator arm, and longer-term follow-up will help clarify the true benefit of adding new agents to the AVD backbone. These studies together provide a basis for future strategies to further improve the results, by testing regimens that might increase the initial response rate, identify novel biomarkers to better predict response, or provide other means to escalate therapy among patients with a poor initial response measured by fluorodeoxyglucose-PET.
APPENDIX
FIG A1.
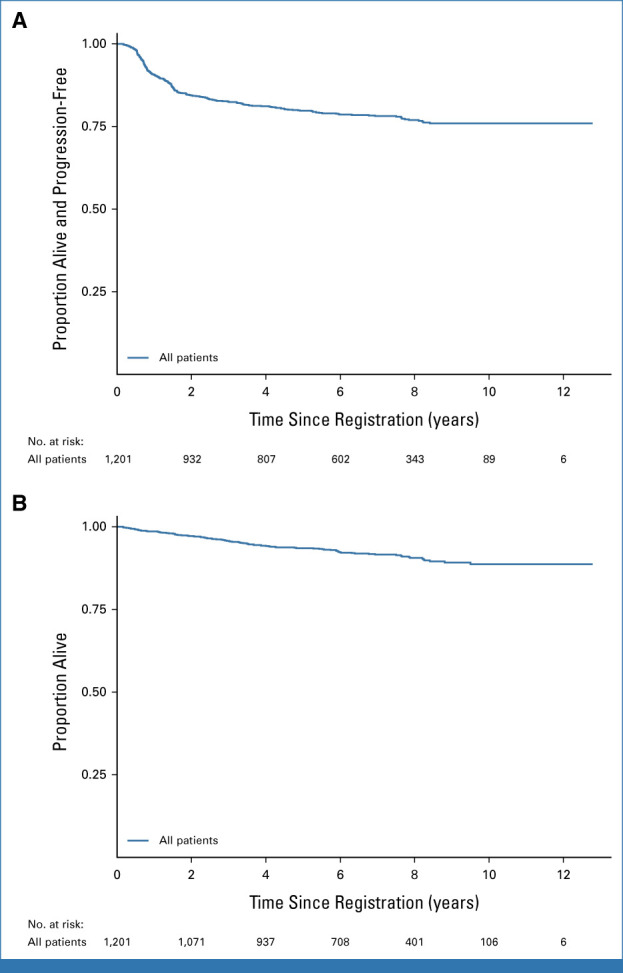
PFS and OS (whole RATHL population). PFS: 205 progressions and 44 deaths without progression: 5-year rate 79.8% (95% CI, 77.33 to 82.0); 7-year rate 78.2% (95% CI, 75.6 to 80.5). OS: 96 deaths: 5-year rate 93.5% (95% CI, 91.9 to 94.9); 7-year rate 91.6% (95% CI, 89.7 to 93.2). OS, overall survival; PFS, progression-free survival.
FIG A2.
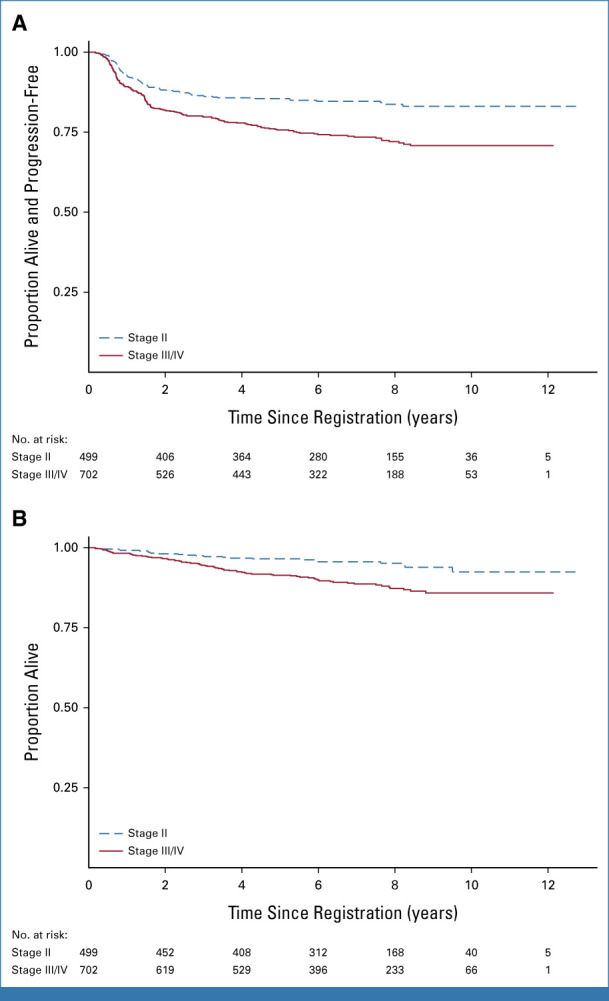
PFS and OS for stage II and for patients with stage III-IV disease. Seven-year PFS for stage II 84.7% (95% CI, 81.0 to 87.7) and III/IV 73.4% (95% CI, 69.7 to 76.8). Seven-year OS for stage II 95.6% (95% CI, 93.2 to 97.2) and III/IV 88.7% (95% CI, 85.7 to 91.0). OS, overall survival; PFS, progression-free survival.
FIG A3.
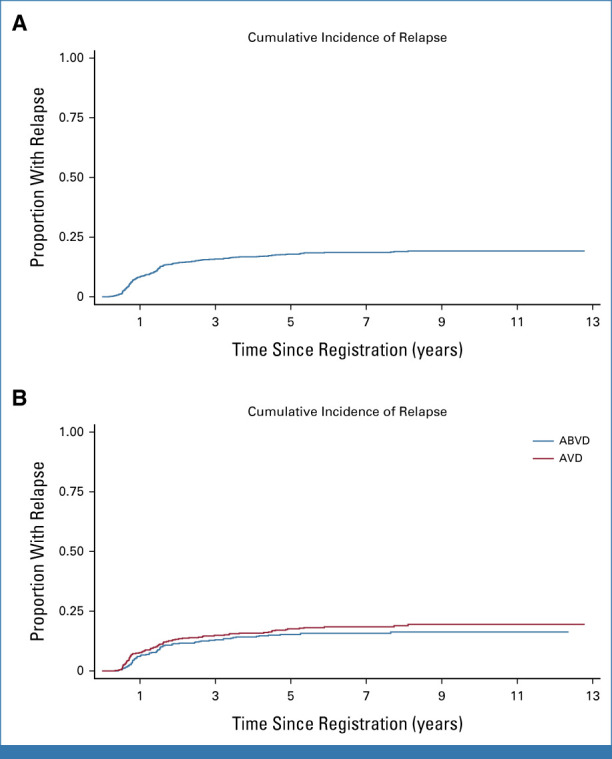
(A) Cumulative incidence of relapse in the whole RATHL cohort. Seven-year rate: 18.6% (95% CI, 16.4 to 21.0). (B) Cumulative incidence of relapse in randomly assigned PET patients: 7-year rates: 15.8% (95% CI, 12.7 to 19.5) ABVD and 18.5% (95% CI, 12.7 to 19.5) AVD; HR 1.20 (95% CI, 0.87 to 1.64), P = .25. ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; AVD, doxorubicin, vinblastine, dacarbazine; HR, hazard ratio; PET, positron emission tomography.
FIG A4.
Subgroup analysis for PFS in randomly assigned patients. HR, hazard ratio; IPS, international prognostic score; PFS, progression-free survival.
FIG A5.
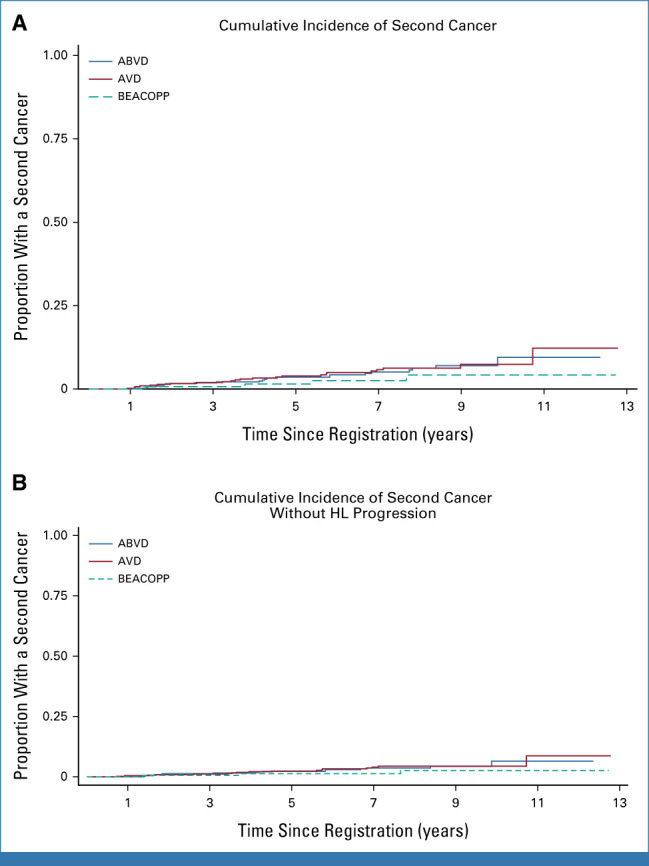
(A) Cumulative incidence of SM by treatment group. Seven-year rates: ABVD 5.1% (95% CI, 3.2 to 8.1), AVD 5.8% (95% CI, 3.8 to 9.0), and BEACOPP 2.5% (95% CI, 0.8 to 7.7). (B) Cumulative incidence of SM by treatment group excluding SM after HL progression: ABVD 3.7% (95% CI, 2.2 to 6.2), AVD 4.0% (95% CI, 2.4 to 6.6), and BEACOPP 1.3% (95% CI, 3.2 to 5.1). ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; AVD, doxorubicin, vinblastine, dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone; HL, Hodgkin lymphoma; SM, secondary malignancy.
TABLE A1.
Cause of Death and Details of Second Cancers for the Entire RATHL Population and by Treatment Group
Stefano Luminari
Consulting or Advisory Role: Roche, Gilead Sciences, Celgene, Genmab, Regeneron, Incyte, BeiGene
Travel, Accommodations, Expenses: Celgene, BeiGene
Alexander Fossa
Honoraria: BMS Norway, Gilead Sciences, AbbVie, Takeda
Judith Trotman
Research Funding: Beigene (Inst), Roche/Genentech (Inst), Pharmacyclics (Inst), Janssen-Cilag (Inst), Takeda (Inst), BMS (Inst), Cellectar (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Daniel Molin
Honoraria: Roche, Merck, Bristol Myers Squibb, Takeda
Gunilla Enblad
Consulting or Advisory Role: Gilead Sciences (Inst), Pierre Fabre
Speakers’ Bureau: MSD Oncology
Leanne Berkahn
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Honoraria: Janssen
Consulting or Advisory Role: Janssen Oncology
Research Funding: AbbVie
Uncompensated Relationships: BeiGene
Sally F. Barrington
Research Funding: Bristol Myers Squibb (Inst), Amgen (Inst), Pfizer (Inst), Novartis Pharmaceuticals UK Ltd (Inst), AstraZeneca (Inst), Takeda (Inst)
John Radford
Stock and Other Ownership Interests: AstraZeneca, GlaxoSmithKline, Smith & Nephew
Honoraria: Takeda, ADC Therapeutics
Consulting or Advisory Role: Takeda, Seagen, Novartis, SOBI
Speakers’ Bureau: Takeda, Seagen, Novartis
Research Funding: Takeda
Travel, Accommodations, Expenses: Takeda, ADC Therapeutics
Amy A. Kirkwood
Honoraria: Kite/Gilead
Consulting or Advisory Role: Janssen
Research Funding: Takeda (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), Roche (Inst), BeiGene (Inst)
Peter W.M. Johnson
Honoraria: Epizyme, InCyte
Consulting or Advisory Role: Epizyme
Patents, Royalties, Other Intellectual Property: Combined use of Fc gamma RIIb (CD32b) and CD20-specific antibodies. WO Patent, PCT/GB2011/051572; EU11760819.0
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the Annual Meeting of the American Society of Hematology, New Orleans, LA, December 10-13, 2022.
SUPPORT
P.W.M.J.: Cancer Research UK CRUK/07/033. L.B.: Leukemia and Blood Cancer New Zealand. J.T.: Cancer Australia. S.F.B.: National Institute for Health and Care Research (NIHR) RP-2016-07-001, Wellcome/EPSRC Centre For Medical Engineering, King’s College London WT203148/Z/16/Z.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Individual participant data will be made available, including data dictionaries, for approved data sharing requests. Individual participant data will be shared, after deidentification and normalization of information (text, tables, figures, and appendices). Anonymized data will be available for researchers who provide a completed Data Sharing Agreement that describes a methodologically sound proposal for the purpose of the approved proposal. Data will be shared once all relevant parties approve and sign the Data Sharing Agreement. Requests should be addressed to the clinical trials unit: ctc.rathl@ucl.ac.uk
AUTHOR CONTRIBUTIONS
Conception and design: Stefano Luminari, Alexander Fossa, Judith Trotman, Gunilla Enblad, Leanne Berkahn, Sally F. Barrington, John Radford, Peter W.M. Johnson
Provision of study materials or patients: Stefano Luminari, Alexander Fossa, Daniel Molin, Gunilla Enblad, Leanne Berkahn, Sally F. Barrington, John Radford, Massimo Federico, Peter W.M. Johnson
Collection and assembly of data: Stefano Luminari, Alexander Fossa, Judith Trotman, Daniel Molin, Francesco d’Amore, Gunilla Enblad, Leanne Berkahn, John Radford, Massimo Federico, Amy A. Kirkwood, Peter W.M. Johnson
Data analysis and interpretation: Stefano Luminari, Alexander Fossa, Gunilla Enblad, Sally F. Barrington, John Radford, Massimo Federico, Amy A. Kirkwood, Peter W.M. Johnson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Follow-Up of the Response-Adjusted Therapy for Advanced Hodgkin Lymphoma Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Stefano Luminari
Consulting or Advisory Role: Roche, Gilead Sciences, Celgene, Genmab, Regeneron, Incyte, BeiGene
Travel, Accommodations, Expenses: Celgene, BeiGene
Alexander Fossa
Honoraria: BMS Norway, Gilead Sciences, AbbVie, Takeda
Judith Trotman
Research Funding: Beigene (Inst), Roche/Genentech (Inst), Pharmacyclics (Inst), Janssen-Cilag (Inst), Takeda (Inst), BMS (Inst), Cellectar (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Daniel Molin
Honoraria: Roche, Merck, Bristol Myers Squibb, Takeda
Gunilla Enblad
Consulting or Advisory Role: Gilead Sciences (Inst), Pierre Fabre
Speakers’ Bureau: MSD Oncology
Leanne Berkahn
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Honoraria: Janssen
Consulting or Advisory Role: Janssen Oncology
Research Funding: AbbVie
Uncompensated Relationships: BeiGene
Sally F. Barrington
Research Funding: Bristol Myers Squibb (Inst), Amgen (Inst), Pfizer (Inst), Novartis Pharmaceuticals UK Ltd (Inst), AstraZeneca (Inst), Takeda (Inst)
John Radford
Stock and Other Ownership Interests: AstraZeneca, GlaxoSmithKline, Smith & Nephew
Honoraria: Takeda, ADC Therapeutics
Consulting or Advisory Role: Takeda, Seagen, Novartis, SOBI
Speakers’ Bureau: Takeda, Seagen, Novartis
Research Funding: Takeda
Travel, Accommodations, Expenses: Takeda, ADC Therapeutics
Amy A. Kirkwood
Honoraria: Kite/Gilead
Consulting or Advisory Role: Janssen
Research Funding: Takeda (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), Roche (Inst), BeiGene (Inst)
Peter W.M. Johnson
Honoraria: Epizyme, InCyte
Consulting or Advisory Role: Epizyme
Patents, Royalties, Other Intellectual Property: Combined use of Fc gamma RIIb (CD32b) and CD20-specific antibodies. WO Patent, PCT/GB2011/051572; EU11760819.0
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hokland P, Shah M, David K, et al. : How I treat advanced Hodgkin lymphoma—A global view. Br J Haematol 190:837-850, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson P, Federico M, Kirkwood A, et al. : Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med 374:2419-2429, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Press OW, Li H, Schöder H, et al. : US intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: Southwest Oncology Group S0816. J Clin Oncol 34:2020-2027, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallamini A, Tarella C, Viviani S, et al. : Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: Long-term results of the GITIL/FIL HD 0607 trial. J Clin Oncol 36:454-462, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Zinzani PL, Broccoli A, Gioia DM, et al. : Interim positron emission tomography response-adapted therapy in advanced-stage Hodgkin lymphoma: Final results of the phase II part of the HD0801 study. J Clin Oncol 34:1376-1385, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Stephens DM, Li H, Schöder H, et al. : Five-year follow-up of SWOG S0816: Limitations and values of a PET-adapted approach with stage III/IV Hodgkin lymphoma. Blood 134:1238-1246, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansell SM, Radford J, Connors JM, et al. : Overall survival with brentuximab vedotin in stage III or IV Hodgkin’s lymphoma. N Engl J Med 387:310-320, 2022 [DOI] [PubMed] [Google Scholar]
- 8.Herrera AF, LeBlanc ML, Castellino SM, et al. : SWOG S1826, a randomized study of nivolumab(N)-AVD versus brentuximab vedotin(BV)-AVD in advanced stage (AS) classic Hodgkin lymphoma (HL). J Clin Oncol 41, 2023. (suppl 17; abstr LBA4) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data will be made available, including data dictionaries, for approved data sharing requests. Individual participant data will be shared, after deidentification and normalization of information (text, tables, figures, and appendices). Anonymized data will be available for researchers who provide a completed Data Sharing Agreement that describes a methodologically sound proposal for the purpose of the approved proposal. Data will be shared once all relevant parties approve and sign the Data Sharing Agreement. Requests should be addressed to the clinical trials unit: ctc.rathl@ucl.ac.uk