Abstract
PURPOSE
Prospective data suggested a superiority of intraoperative MRI (iMRI) over 5-aminolevulinic acid (5-ALA) for achieving complete resections of contrast enhancement in glioblastoma surgery. We investigated this hypothesis in a prospective clinical trial and correlated residual disease volumes with clinical outcome in newly diagnosed glioblastoma.
METHODS
This is a prospective controlled multicenter parallel-group trial with two center-specific treatment arms (5-ALA and iMRI) and blinded evaluation. The primary end point was complete resection of contrast enhancement on early postoperative MRI. We assessed resectability and extent of resection by an independent blinded centralized review of preoperative and postoperative MRI with 1-mm slices. Secondary end points included progression-free survival (PFS) and overall survival (OS), patient-reported quality of life, and clinical parameters.
RESULTS
We recruited 314 patients with newly diagnosed glioblastomas at 11 German centers. A total of 127 patients in the 5-ALA and 150 in the iMRI arm were analyzed in the as-treated analysis. Complete resections, defined as a residual tumor ≤0.175 cm³, were achieved in 90 patients (78%) in the 5-ALA and 115 (81%) in the iMRI arm (P = .79). Incision-suture times (P < .001) were significantly longer in the iMRI arm (316 v 215 [5-ALA] minutes). Median PFS and OS were comparable in both arms. The lack of any residual contrast enhancing tumor (0 cm³) was a significant favorable prognostic factor for PFS (P < .001) and OS (P = .048), especially in methylguanine-DNA-methyltransferase unmethylated tumors (P = .006).
CONCLUSION
We could not confirm superiority of iMRI over 5-ALA for achieving complete resections. Neurosurgical interventions in newly diagnosed glioblastoma shall aim for safe complete resections with 0 cm³ contrast-enhancing residual disease, as any other residual tumor volume is a negative predictor for PFS and OS.
INTRODUCTION
A maximum safe resection of contrast-enhancing parts of glioblastomas is an established favorable prognostic factor.1-3 Intraoperative tools to support neurosurgeons in this regard include intraoperative MRI (iMRI) guidance and fluorescence-guided resections with 5-aminolevulinic acid (5-ALA; Gliolan; Medac, Wedel, Germany).4 The most recent prospective trials revealed an increase of complete contrast enhancement resections from 36%-68% with white light, to 65% with 5-ALA, and 96% with iMRI-guided surgery.5,6 The assumed superiority of iMRI was never proven in a direct comparison with 5-ALA; retrospective series show variable results.7-9 We performed this prospective controlled trial (PCT) to evaluate the assumed superiority and aimed to precisely analyze the role of residual tumor on postoperative MRI with 1-mm slice thickness.
CONTEXT
Key Objective
Prospective data suggested a superiority of intraoperative MRI (iMRI) over 5-aminolevulinic acid (5-ALA) for achieving complete resections (65% v 96%, respectively) of contrast enhancement in glioblastoma surgery. We aimed to investigate this hypothesis in a prospective clinical trial and correlated residual disease volumes with clinical outcome in newly diagnosed glioblastoma.
Knowledge Generated
We could not confirm superiority of iMRI over 5-ALA for achieving complete resections. Neurosurgical interventions in newly diagnosed glioblastoma shall aim for safe complete resections with 0 cm³ contrast-enhancing residual disease, as any other residual tumor volume is a negative predictor for progression-free survival and overall survival.
Relevance (I.K. Mellinghoff)
-
Maximum safe tumor resection represents an important first step in the treatment of glioblastoma and can be improved through the iMRI-guidance and fluorescence-guided resections with 5-ALA. The current study suggests that iMRI- and 5-ALA-guided resections result in similar rates of complete resections and postoperative residual tumor volumes.*
*Relevance section written by JCO Associate Editor Ingo K. Mellinghoff, MD.
METHODS
Study Design
The study Protocol (online only) was developed by the German Study Group for Intraoperative MRI (a collaborative working group of iMRI centers in Germany) in 2013. The aim was to assess the assumed superiority of iMRI over 5-ALA guidance for the resection of glioblastomas. We performed a nonrandomized PCT for the following reasons: (1) Ethics: It felt unjustifiable to withhold iMRI-guided resections to half of the patients in a randomized setting, given a possible increase of complete resections by almost 50% on the basis of the known data. Consequently, this might have led to a reduced number of patients giving their consent to participate. (2) Experience: iMRI centers had limited experience using 5-ALA, since it was a relatively new technology at that time, which might have been a bias. (3) Conviction: 5-ALA and iMRI centers were each highly experienced in and convinced of their respective techniques. The PCT design would allow each to contribute to the study with the best expertise in the field. Therefore, a prospective controlled parallel-group trial with two center-specific treatment arms (5-ALA and iMRI) was performed.
Patients
Patients were recruited at 11 centers in Germany. Inclusion and exclusion criteria were based on the protocol by Stummer et al6 with adaptions. Individuals age 18-80 years with suspected glioblastoma based on MRI, a planned complete resection of contrast enhancing tumor, and a Karnofsky performance score (KPS) of ≥60% were included. Main exclusion criteria were tumors of the midline, basal ganglia, cerebellum, brainstem, eloquent areas, multifocal glioblastoma, >50% noncontrast enhancing tumor, inability to provide informed consent, increased thrombosis risk, pregnancy or breastfeeding, hypersensibility to 5-ALA or porphyrins, renal or hepatic insufficiency, and inability to receive adjuvant therapy, judged by the treating surgeon. Patients with a final histologic result other than glioblastoma WHO grade 4 (according to the study protocol on the basis of the 200710 and formerly upcoming 201611 WHO classification) were excluded. All patients provided written informed consent. The protocol was approved by the lead ethics committee at the University of Tübingen (116/2014BO1) and each local ethics committee. This study was performed according to the Declaration of Helsinki and Good Clinical Practice standards.
Treatment
Patients at 5-ALA centers received 5-ALA (Gliolan) according to the manufacturer's recommendation (20 mg/kg bodyweight) dissolved in 50-mL drinking water 2-4 hours before induction of anesthesia. Patients at iMRI centers had a white-light resection with iMRI guidance (high-field MRI ≥ 1.5 T). The use of 5-ALA in the iMRI arm was not permitted. Other intraoperative tools (neuronavigation, ultrasound, and neuromonitoring) were permitted in both arms.
Patient Data
Patient data were pseudonymized centrally. Preoperative, postoperative, and follow-up MRI with 3D data (1-mm slices) were collected, perioperative and intraoperative data, including a surgeons' questionnaire, were analyzed, and KPS, National Institutes of Health Stroke Scale (NIHSS), and European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 (quality of life [QoL] questionnaire for patients with cancer) and QLQ-BN20 (additional module for patients with brain cancer) were completed.12,13 Patients were followed up at 3, 6, 9, and 12 months with MRI, clinical assessment, Response Assessment in Neuro-Oncology (RANO) criteria14 evaluation, KPS, NIHSS, and EORTC QLQ-C30 and QLQ-BN20. The individual study participation ended after 12 months. At trial closing, progression, last contact, and survival data were updated for all patients.
End Points
The primary end point was complete resections (yes/no), defined as a residual contrast-enhancing tumor ≤0.175 cm on postoperative MRI within 48 hours after surgery, evaluated by the central blinded neuroradiology reference (S.B.).5,6 The threshold to define complete resections was previously used in the landmark study by Stummer et al,6 aiming to prevent interpretation problems between tumor tissue and vessels in the available printed images with 5-mm slices. Secondary end points with central neuroradiology evaluation were preoperative and postoperative tumor volumes (cm³) and progression on the basis of MRI according to RANO criteria (imaging criteria only; clinical criteria were rated locally). Volumetric evaluation was performed with 3D Slicer.15 Secondary end points included progression-free survival (PFS), overall survival (OS), clinical data, histology, isocitrate dehydrogenase-1 (IDH-1) and methylguanine-DNA-methyltransferase (MGMT) promoter status, and QoL.
Comparability of Treatment Arms
A blinded centralized neurosurgical evaluation (O.G.) of preoperative tumors was performed as follows: (1) totally resectable, noneloquent, (2) totally resectable, eloquent, and (3) not totally resectable (exclusion criterion). Preoperative tumor volumes, clinical data, intraoperative tools used, and postoperative adjuvant therapy were compared between both treatment arms. Propensity scores were calculated to quantify and adjust for possible imbalances, including baseline tumor volume, anatomic localization, laterality, resectability and eloquence according to the reference, molecular characteristics, postoperatively assumed extent of resection (questionnaire), sex, and age.
Statistical Analysis
The sample size was calculated on the basis of a χ2 test. We expected to increase the number of patients with complete resections from 65% (Stummer et al6) in the control arm to 80% (on the basis of 96% described by Senft et al,5 but with an assumed overall lower number of expected complete resections according to additional available data of other studies9,16) in the interventional arm. Therefore, we needed 138 evaluable patients per arm (type 1 error 0.05, two-sided, type 2 error 80%; nQuery, v.7, Statsols, San Diego, CA). Because of the early primary end point, we expected only few dropouts and intended to allocate 154 patients to each arm. The primary analysis was a logistic regression taking propensity scores into account in obtaining the control or experimental intervention as covariates; the construction of the propensity scores also used a logistic regression analysis with study arm as outcome. All tests were defined in the statistics analysis plan before the analysis. The comparative statistical analysis for the primary outcome, PFS, and OS was performed using the above-mentioned propensity scores as covariate, accounting for potential imbalances in relevant cofactors in patients in different centers. The primary outcome (complete resection ≤0.175 cm³) was analyzed using logistic regression in the as-treated population, defined as all patients who did not violate major diagnostic inclusion criteria (Fig 1). Goodness of fit was assessed using the Hosmer-Lemeshow test. Multiple imputation was used to simulate missing values of the primary end point (predictor: propensity score, 500 imputation samples). The primary analysis population was the as-treated population, comprising all patients with singular and totally resectable glioblastoma. Since the sample size for the analysis of the primary end point without multiple imputation and the per-protocol analysis differed by <5% of patients, we did not perform a separate per-protocol analysis. In addition, analysis of covariance and the Cox regression model were used for continuous and censored outcomes. The proportional hazard assumption was checked by parallelism (visual inspection) of log minus log survival plots. Comparisons between categorical data were performed using the χ2-test or Fisher's exact test when expected frequencies were <5%. The Mann-Whitney test was used to compare non-normally distributed variables, and the independent-samples t test was used to compare normally distributed variables. Planned secondary analyses included the prognostic value of molecular markers and residual tumor volume with OS and PFS. The significance level was 0.05 (two-sided) for all analyses. However, only the primary analysis is confirmatory, and no adjustment for multiple testing was applied. Statistical analyses were performed using SPSS for Windows, v.26 (IBM, Armonk, NY).
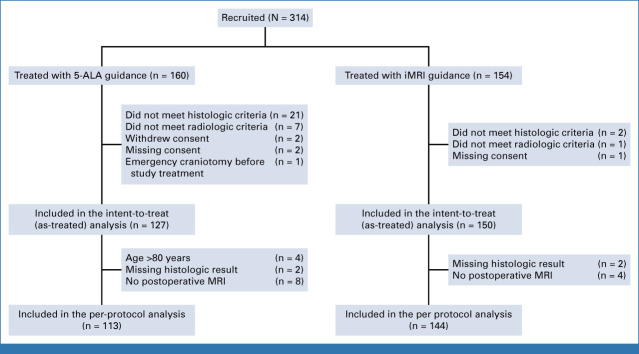
FIG 1.
Flow diagram. 5-ALA, 5-aminolevulinic acid; iMRI, intraoperative MRI.
RESULTS
We enrolled 314 patients at 11 German centers between July 1, 2015, and June 17, 2020; 160 were treated with 5-ALA and 154 with iMRI-guidance. For the final analysis, we excluded 23 patients with histologic results other than glioblastoma (12 metastasis, five gliosarcoma, two lymphoma, one abscess, and three other diagnoses), eight had major violations of radiologic inclusion criteria (preoperatively documented screening failures with inclusion of multifocal or eloquent tumors in three and five patients with planned partial resections), two patients withdrew their consent, three had a missing consent, and one had an emergency craniotomy. Therefore, the 5-ALA arm comprised 127 and the iMRI arm 150 patients in the as-treated analysis (Fig 1).
Baseline Characteristics
Both cohorts were comparable, except for lower percentages of patients without preoperative neurologic symptoms in the 5-ALA (32%) than in the iMRI (48%) arm (P = .01), a higher NIHSS in the 5-ALA (median, 1) than in the iMRI (median, 0) treatment arm (P = .048), and lower KPS in the 5-ALA (median, 90) than in the iMRI (median, 90) arm (P = .04; Table 1).
TABLE 1.
Preoperative and Postoperative Patient Characteristics

Intraoperative Characteristics
The mean incision-suture time (minutes) was significantly longer in the iMRI (316) than in the 5-ALA (215) arm (P < .001). Intraoperative neuromonitoring and neuronavigation were used comparably often; ultrasound was used more often with 5-ALA (54%) than with iMRI (34%; P = .001; Table 2).
TABLE 2.
Intraoperative Characteristics

Postoperative Characteristics
Significantly more patients had neurologic deficits postoperatively in the 5-ALA than in the iMRI treatment arm (56% 5-ALA v 43% iMRI; P = .042). However, the preoperative compared with postoperative improving (22% and 16%), stable (70% and 76%), or worsening (8% and 8%) deficits did not differ significantly between 5-ALA and iMRI, respectively (P = .43), with known baseline imbalance. More patients in the 5-ALA arm had neurologic deficits preoperatively compared with iMRI. Perioperative data were comparable between treatment arms (Table 1).
Primary End Point: Extent of Resection
Complete resection (defined as residual tumor ≤0.175 cm³) was achieved in 90 (78%) of patients in the 5-ALA and 115 (81%) in the iMRI treatment arm (odds ratio [OR], 1.09; 95% CI, 0.57 to 2.08; P = .79, adjusted for propensity scores; goodness of fit P = .74, after multiple imputation: 77.6% [5-ALA] and 80.9% [iMRI]; OR, 1.09; 95% CI, 0.57 to 2.07; P = .80). Analysis of complete resection defined as residual tumor = 0 cm³ was achieved in 83 (72%) in the 5-ALA and 92 (65%) in the iMRI treatment arm (P = .16, adjusted for propensity scores, goodness of fit P = .052). Median postoperative tumor volumes were comparably 0 cm³ in both arms (P = .36, adjusted for propensity scores; Table 3).
TABLE 3.
Primary End Point: Extent of Resection and Follow-Up Data
Follow-Up and Survival Data
Both treatment arms had similar PFS (hazard ratio [HR], 0.908; 95% CI, 0.686 to 1.202; P = .50, adjusted for propensity scores) and OS (HR, 0.997; 95% CI, 0.642 to 1.549; P = .99, adjusted for propensity scores; Figs 2A and 2B). MGMT promoter methylation conferred an OS advantage (median, 59.2 months; 95% CI, 19.5 to 99.1) over unmethylated tumors (median, 19.3 months; 95% CI, 16.7 to 22.0) in the full cohort (P = .002; HR, 0.483; 95% CI, 0.302 to 0.771; Fig 2C). The proportional hazard assumption was confirmed in each of the analyses. Follow-up parameters, QoL, and adjuvant treatment were comparable between treatment arms (Table 3; Appendix Table A1, online only).
FIG 2.

General survival data. Comparison of (A) PFS and (B) OS in the 5-ALA and iMRI treatment arms. (C) OS of the full cohort depending on the MGMT methylation status. 5-ALA, 5-aminolevulinic acid; iMRI, intraoperative MRI; MGMT, methylguanine-DNA-methyltransferase; OS, overall survival; PFS, progression-free survival.
Importance of Residual Tumor Volume
After categorization of the postoperative tumor volume into two groups (0- and >0-cm³ residual contrast enhancement), a longer median PFS (6.9 months; 95% CI, 5.0 to 8.9) and OS (27.9 months; 95% CI, 18.1 to 37.7) was seen in patients with 0 cm³ residual disease, compared with patients with >0 cm³ (3.8 months; 95% CI, 3.5 to 4.1 and 19.4 months; 95% CI, 15.6 to 23.2, respectively), and associated with significantly longer PFS (HR, 1.774; 95% CI, 1.325 to 2.375; P < .001) and OS (HR, 1.589; 95% CI, 1.0 to 2.526; P = .048; Figs 3A and 3B). Noteworthy, patients with 0 cm³ residual tumor volume had a significantly longer median PFS (6.9 months; 95% CI, 3.8 to 15.0) than patients with 0.001-0.175 cm³ (3.8 months; 95% CI, 3.1 to 6.2; HR, 1.717; 95% CI, 1.142 to 2.586; P = .009) or greater (3.8 months; 95% CI, 3.1 to 5.4; HR, 1.815; 95% CI, 1.276 to 2.582; P = .001; Fig 3C). Patients with 0 cm³ residual tumor had a longer median OS (27.9 months; 95% CI, 18.1 to 37.7) than patients with 0.001-0.175 cm³ (18.4 months; 95% CI, 5.7 to 31.1; HR, 1.814; 95% CI, 0.965 to 3.411; P = .044), or any greater residual volume (19.4 months; 95% CI, 16.7 to 22.1; HR, 1.454; 95% CI, 0.828 to 2.555; P = .41; Fig 3D).
FIG 3.
The role of residual tumor tissue. (A) PFS and (B) OS of patients with (>0 cm³) and without (0 cm³) contrast-enhanced residual tumor tissue. (C) PFS and (D) OS of patients with 0 cm³, 0-0.175 cm³, and >0.175 cm³ contrast-enhanced residual tumor volumes. OS, overall survival; PFS, progression-free survival.
MGMT Methylation and Residual Tumor Volume
The role of residual tumor volume differed significantly depending on MGMT methylation (Figs 4A and 4B): OS did not differ significantly between resections with 0- and >0-cm³ residual volumes in patients with methylated MGMT promoters (P = .62; HR, 0.806; 95% CI, 0.345 to 1.885). Patients with 0 cm³ residual tumor (median, 21.1 months; 95% CI, 14.7 to 27.5) had significantly longer OS compared with >0 cm³ (median, 16.1 months; 95% CI, 11.4 to 20.7) with unmethylated MGMT promoters (P = .006; HR, 2.347; 95% CI, 1.253 to 4.399).
FIG 4.

The role of methylation and residual tumor tissue. (A) Tumors with unmethylated MGMT promoters and complete (0 cm³) or incomplete (>0 cm³) resection of contrast enhancement, and (B) tumors with methylated MGMT promoters and complete (0 cm³) or incomplete (>0 cm³) resection of contrast enhancement. MGMT, methylguanine-DNA-methyltransferase.
White-Light Resections
As we did not include a white-light resection cohort, we performed an additional analysis with the raw data of the trial by Stummer et al6 (white-light cohort only, full data set analysis, n = 208), which had comparable inclusion criteria. Anatomic localization (P = .61), laterality (P = .88), and eloquence (P = .040) were similar in all three cohorts. Preoperative tumor volumes (cm³) of the white-light cohort (median, 30) were comparable with the 5-ALA (median, 29; P = .78), but smaller in the iMRI (median, 23; P = .002) arm. Postoperative tumor volumes (cm³) were significantly larger in the white-light (median, 0.50) than in the iMRI (median, 0; P < .001) or 5-ALA (median, 0; P < .001) cohort. A complete resection (≤0.175 cm³) was achieved significantly less often in the white-light (38.2%) than in the 5-ALA (78%; P < .001) or iMRI (81%; P < .001) cohort with a comparable KPS (median, 90; P = .341) 3 months postoperatively.
DISCUSSION
iMRI- and 5-ALA–guided resections led to similar rates of complete resections, postoperative residual tumor volumes, and survival data. The number of complete resections (≤0.175 cm³) in our 5-ALA arm (78%) is higher than that of Stummer et al6 (65%). As preoperative tumor volumes of both studies were comparable, we assume that our higher success rates might reflect increasing experience over time. The number of complete resections in our iMRI treatment arm (81%) is lower than that of Senft et al (96%),5 but consistent with the numbers used for our power calculation (80%) and with previously published monocentric (82%)16 and contemporary multicentric retrospective data (83.3%).17 A possible explanation for this might be seen in different preoperative median tumor volumes or selection bias on the basis of a monocentric study design in the study by Senft et al.5 Furthermore, preoperative neurologic performance (NIHSS, KPS, and neurologic deficits) of patients differed between both treatment arms. Additional adjusting analysis of the secondary end point PFS and OS revealed that this did not influence PFS and OS results (Appendix Tables A2 and A3).
The threshold to define complete resections in this study was residual contrast-enhanced tumor volume ≤0.175 cm³ on the basis of previous studies.6 However, the removal of all contrast-enhancing tumor tissue to 0 cm³ significantly improved PFS and OS (Figs 3A and 3B), independently of the residual volumes (Figs 3C and 3D). This finding is consistent with previous retrospective and cohort studies and has never been proven with such clarity in a prospective cohort.17-20
Postoperatively detected small residual tumors did not affect PFS and OS in methylated tumors, but in unmethylated ones which might be caused by a high efficacy of temozolomide in these patients (Figs 3A and 3B).
In our study, the median OS was 31.7 months in the 5-ALA and 22.9 months in the iMRI treatment arm (P = .99). Note that the higher median OS in the ALA treatment arm was a local statistical effect centered around 50% survival (Fig 2B). In the study by Stummer et al, completed before the establishment of the Stupp protocol involving radiochemotherapy with temozolomide, the median OS was 11.6 and 16.9 months, respectively, for incompletely and completely resected tumors.6,21,22 In recent studies, subgroup analyses of gross totally or maximal resected glioblastomas with radiochemotherapy showed a median OS of 18.5-20 months.20,23 Therefore, the results of our study are comparable or slightly better than contemporary survival data. This might be caused by the selection of fully resectable, nonmultifocal tumors in this study and the statistical effect described above.
The main limitation of this study is its nonrandomized design, which might have resulted in selection bias. Therefore, we have applied several safety measures and proofed excellent comparability of both treatment arms. No significant differences between centers were observed for the primary end point (P = .32, χ test), patient characteristics (preoperative tumor size P = .09, age P = .64, analysis of variance; sex P = .20, resectability P = .28, laterality P = .40, MGMT methylation P = .37 (χ2 test)), and PFS (P = .16, log-rank test). A potential center-specific bias was seen with significant differences in OS (P < .001), but this was well balanced between both treatment arms. Furthermore, surgeons finally judged the respective inclusion criteria of patients, which is a potential bias of this study despite comparable neurosurgical reference evaluation on resectability.
According to the newest WHO classification,24 only IDH-wild-type tumors can be considered glioblastomas. This study-protocol was designed on the basis of the 200710 and formerly upcoming 201611 WHO classification, and therefore also included IDH-1–mutated glioblastoma. Additional analysis excluding IDH-mutated tumors did not reveal meaningful changes (Table 3). This study was not powered and designed for long-term OS analysis, with a possible bias caused by the small numbers of patients at risk at later time points.
In conclusion, our study shows that 5-ALA and iMRI- guidance provide comparable surgical results with no superiority of iMRI. 5-ALA might be economically advantageous for glioblastoma resection, given high acquisition and running costs with significantly longer operating room times of iMRI. Our study showed that any contrast-enhancing residual tumor tissue is an adverse prognostic factor for OS and PFS. A safe resection with 0 cm³ residual tumor should always be pursued in patients with newly diagnosed glioblastoma. Subgroup analyses suggest this is especially relevant for patients with an unmethylated MGMT promoter.
ACKNOWLEDGMENT
The authors thank the patients and their relatives for their participation. The authors are grateful to all local investigators and study nurses. The authors thank all local neuroradiology and neuropathology departments and all our colleagues for supporting this study. The authors are also very grateful for the tremendous support and monitoring by the Center for Clinical Studies Tübingen (ZKS) and data management by the Department of Clinical Epidemiology and Applied Biostatistics Tübingen. The authors thank the University Hospital Tübingen and the Department of Neurosurgery for supporting this study with institutional monetary and staff support. The authors thank the photonamic GmbH & Co KG, Pinneberg, Germany, for providing the raw data of the trial by Stummer et al6 to perform a matched white-light resection cohort analysis.
APPENDIX 1.
We observed imbalances in preoperative Karnofsky performance score (KPS), National Institutes of Health Stroke Scale (NIHSS), and the frequency of neurologic symptoms between both study arms. Thus, we separately analyzed the prognostic value of these variables (univariate analysis) and two types of multivariate analysis: the first one including all prognostic factors and the second one including only multivariate significant predictors. As the three factors, KPS, NIHSS, and neurologic symptoms, are highly correlated with each other, only one of these (NIHSS for overall survival, KPS for progression-free survival) remained significant in the multivariate analysis.
It is important to note that the results for each study arm did not relevantly change after adjustment for these three prognostic factors.
TABLE A1.
Additional Therapies at Follow-Up in Patients

APPENDIX 2. ANALYSIS OF THE INFLUENCE OF PREOPERATIVE IMBALANCES BETWEEN BOTH TREATMENT ARMS ON PROGRESSION-FREE SURVIVAL AND OVERALL SURVIVAL
TABLE A2.
Analysis of the Influence of Preoperative Imbalances Between Both Treatment Arms on OS
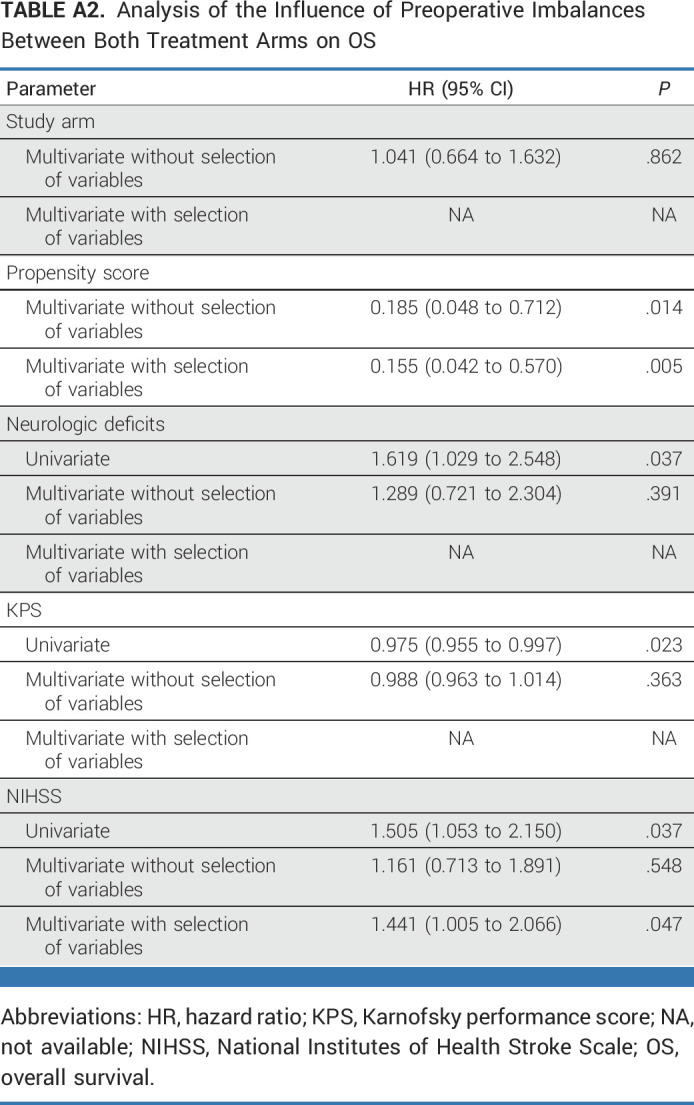
TABLE A3.
Analysis of the Influence of Preoperative Imbalances Between Both Treatment Arms on PFS

Walter Stummer
Consulting or Advisory Role: Alpheus Medical, SBI ALAPharma, NXDC
Speakers' Bureau: MSD Oncology
Research Funding: Zeiss (Inst), Photonamic (Inst)
Jan Coburger
Consulting or Advisory Role: BrainLAB
Stephanie Schipmann
Consulting or Advisory Role: NX Development Corp
Julian Rathert
Consulting or Advisory Role: Abbott
Roland Goldbrunner
Honoraria: Seagen
Consulting or Advisory Role: SeaGn
Michael Sabel
Honoraria: Integra LifeSciences
Speakers' Bureau: Novocure
Veit Rohde
Consulting or Advisory Role: Zeiss
Ghazaleh Tabatabai
Consulting or Advisory Role: Boehringer Ingelheim (Inst), CureVac (Inst), Bayer Germany (Inst), Advanced Accelerator Applications/Novartis (Inst)
Sotirios Bisdas
Consulting or Advisory Role: Image Analysis Group, Olea Medical
Andreas Unterberg
Stock and Other Ownership Interests: Bayer
Consulting or Advisory Role: Idorsia
Travel, Accommodations, Expenses: Idorsia
No other potential conflicts of interest were reported.
See accompanying Editorial, p. 5493
DISCLAIMER
This was an investigator-initiated trial, sponsored by the University Hospital of Tübingen, Germany. Independent monitoring was performed by the Center for Clinical Studies, Tübingen. The organizational costs of this trial were covered by private and institutional funding from the Department of Neurosurgery at the University of Tübingen. Participating centers did not receive financial compensation. Medac (Wedel, Germany) provided 5-ALA medication (Gliolan) for all patients included in the 5-ALA arm. Brainlab (Munich, Germany) provided the Quentry platform for sharing Digital Imaging and Communications in Medicine (DICOM) images without cost. The companies did not influence analysis or results. The corresponding author and the responsible biometrician have final accountability for the analysis and publication.
CLINICAL TRIAL INFORMATION
NCT02379572 (IMRI/ALA)
DATA SHARING STATEMENT
What data will be shared? Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendix).
What other documents will be available? Study protocol.
When will data be available? Beginning 12 months and ending 36 months after article publication.
With whom? Investigators whose written proposed use of the data has been approved by the working group of this article.
For what type of analysis? To achieve aims in the approved proposal.
By what mechanism will data be made available? By direct contact to the first author: constantin.roder@uni-tuebingen.de.
AUTHOR CONTRIBUTIONS
Conception and design: Constantin Roder, Walter Stummer, Jan Coburger, Moritz Scherer, Marco Skardelly, Paul Kremer, Ralf-Ingo Ernestus, Ghazaleh Tabatabai, Sotirios Bisdas, Andreas Unterberg, Christian Rainer Wirtz, Marcos Tatagiba
Provision of study materials or patients: Mario Löhr, Torben Scholz, Stephanie Schipmann, Andrej Pala, Ulrike Ernemann, Paul Kremer, Michael Sabel, Ghazaleh Tabatabai, Christian Rainer Wirtz, Marcos Tatagiba
Collection and assembly of data: Constantin Roder, Walter Stummer, Jan Coburger, Moritz Scherer, Patrick Haas, Christian von der Brelie, Marcel Alexander Kamp, Mario Löhr, Christina A. Hamisch, Torben Scholz, Stephanie Schipmann, Julian Rathert, Catrin Marlene Brand, Andrej Pala, Ulrike Ernemann, Florian Stockhammer, Paul Kremer, Roland Goldbrunner, Michael Sabel, Veit Rohde, Ghazaleh Tabatabai, Andreas Unterberg, Christian Rainer Wirtz, Marcos Tatagiba
Data analysis and interpretation: Constantin Roder, Walter Stummer, Moritz Scherer, Christian von der Brelie, Marco Skardelly, Andrej Pala, Ulrike Ernemann, Paul Kremer, Ralf-Ingo Ernestus, Veit Rohde, Ghazaleh Tabatabai, Peter Martus, Sotirios Bisdas, Oliver Ganslandt, Andreas Unterberg, Christian Rainer Wirtz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Intraoperative MRI-Guided Resection Is Not Superior to 5-Aminolevulinic Acid Guidance in Newly Diagnosed Glioblastoma: A Prospective Controlled Multicenter Clinical Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Walter Stummer
Consulting or Advisory Role: Alpheus Medical, SBI ALAPharma, NXDC
Speakers' Bureau: MSD Oncology
Research Funding: Zeiss (Inst), Photonamic (Inst)
Jan Coburger
Consulting or Advisory Role: BrainLAB
Stephanie Schipmann
Consulting or Advisory Role: NX Development Corp
Julian Rathert
Consulting or Advisory Role: Abbott
Roland Goldbrunner
Honoraria: Seagen
Consulting or Advisory Role: SeaGn
Michael Sabel
Honoraria: Integra LifeSciences
Speakers' Bureau: Novocure
Veit Rohde
Consulting or Advisory Role: Zeiss
Ghazaleh Tabatabai
Consulting or Advisory Role: Boehringer Ingelheim (Inst), CureVac (Inst), Bayer Germany (Inst), Advanced Accelerator Applications/Novartis (Inst)
Sotirios Bisdas
Consulting or Advisory Role: Image Analysis Group, Olea Medical
Andreas Unterberg
Stock and Other Ownership Interests: Bayer
Consulting or Advisory Role: Idorsia
Travel, Accommodations, Expenses: Idorsia
No other potential conflicts of interest were reported.
REFERENCES
- 1.Wen PY, Weller M, Lee EQ, et al. : Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol 22:1073-1113, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weller M, Le Rhun E, Preusser M, et al. : How we treat glioblastoma. ESMO Open 4:e000520, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wick W, Osswald M, Wick A, et al. : Treatment of glioblastoma in adults. Ther Adv Neurol Disord 11:1756286418790452, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fountain DM, Bryant A, Barone DG, et al. : Intraoperative imaging technology to maximise extent of resection for glioma: A network meta-analysis. Cochrane Database Syst Rev 1:CD013630, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senft C, Bink A, Franz K, et al. : Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol 12:997-1003, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Stummer W, Pichlmeier U, Meinel T, et al. : Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol 7:392-401, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Rogers CM, Jones PS, Weinberg JS: Intraoperative MRI for brain tumors. J Neurooncol 151:479-490, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Golub D, Hyde J, Dogra S, et al. : Intraoperative MRI versus 5-ALA in high-grade glioma resection: A network meta-analysis. J Neurosurg 134:484-498, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Roder C, Bisdas S, Ebner FH, et al. : Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: High-field iMRI versus conventional and 5-ALA-assisted surgery. Eur J Surg Oncol 40:297-304, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Louis DN, Ohgaki H, Wiestler OD, et al. : The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97-109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis DN, Perry A, Reifenberger G, et al. : The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathologica 131:803-820, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Aaronson NK, Ahmedzai S, Bergman B, et al. : The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365-376, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Taphoorn MJ, Claassens L, Aaronson NK, et al. : An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer 46:1033-1040, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Wen PY, Macdonald DR, Reardon DA, et al. : Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol 28:1963-1972, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. : 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30:1323-1341, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coburger J, Hagel V, Wirtz CR, et al. : Surgery for glioblastoma: Impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS One 10:e0131872, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah AS, Sylvester PT, Yahanda AT, et al. : Intraoperative MRI for newly diagnosed supratentorial glioblastoma: A multicenter-registry comparative study to conventional surgery. J Neurosurg 135:505-514, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Sanai N, Polley M-Y, McDermott MW, et al. : An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3-8, 2011 [DOI] [PubMed] [Google Scholar]
- 19.McGirt MJ, Chaichana KL, Gathinji M, et al. : Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110:156-162, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Karschnia P, Young JS, Dono A, et al. : Prognostic validation and refinement of a classification system for extent of resection in glioblastoma: A report of the RANO resect group. J Clin Oncol 40:2003, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stummer W, Reulen HJ, Meinel T, et al. : Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 62:564-576, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Stupp R, Mason WP, van den Bent MJ, et al. : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. Oncol Times 27:15-16, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Stupp R, Taillibert S, Kanner A, et al. : Effect of tumor-treating fields plus maintenance temozolomide vs. maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 318:2306-2316, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis DN, Perry A, Wesseling P, et al. : The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol 23:1231-1251, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
What data will be shared? Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendix).
What other documents will be available? Study protocol.
When will data be available? Beginning 12 months and ending 36 months after article publication.
With whom? Investigators whose written proposed use of the data has been approved by the working group of this article.
For what type of analysis? To achieve aims in the approved proposal.
By what mechanism will data be made available? By direct contact to the first author: constantin.roder@uni-tuebingen.de.