Abstract
PURPOSE
Tissue factor is highly expressed in cervical carcinoma and can be targeted by tisotumab vedotin (TV), an antibody-drug conjugate. This phase Ib/II study evaluated TV in combination with bevacizumab, pembrolizumab, or carboplatin for recurrent or metastatic cervical cancer (r/mCC).
METHODS
This open-label, multicenter study (ClinicalTrials.gov identifier: NCT03786081) included dose-escalation arms that assessed dose-limiting toxicities (DLTs) and identified the recommended phase II dose (RP2D) of TV in combination with bevacizumab (arm A), pembrolizumab (arm B), or carboplatin (arm C). The dose-expansion arms evaluated TV antitumor activity and safety at RP2D in combination with carboplatin as first-line (1L) treatment (arm D) or with pembrolizumab as 1L (arm E) or second-/third-line (2L/3L) treatment (arm F). The primary end point of dose expansion was objective response rate (ORR).
RESULTS
A total of 142 patients were enrolled. In dose escalation (n = 41), no DLTs were observed; the RP2D was TV 2 mg/kg plus bevacizumab 15 mg/kg on day 1 once every 3 weeks, pembrolizumab 200 mg on day 1 once every 3 weeks, or carboplatin AUC 5 on day 1 once every 3 weeks. In dose expansion (n = 101), the ORR was 54.5% (n/N, 18/33; 95% CI, 36.4 to 71.9) with 1L TV + carboplatin (arm D), 40.6% (n/N, 13/32; 95% CI, 23.7 to 59.4) with 1L TV + pembrolizumab (arm E), and 35.3% (12/34; 19.7 to 53.5) with 2L/3L TV + pembrolizumab (arm F). The median duration of response was 8.6 months, not reached, and 14.1 months, in arms D, E, and F, respectively. Grade ≥3 adverse events (≥15%) were anemia, diarrhea, nausea, and thrombocytopenia in arm D and anemia in arm F (none ≥15%, arm E).
CONCLUSION
TV in combination with bevacizumab, carboplatin, or pembrolizumab demonstrated manageable safety and encouraging antitumor activity in treatment-naive and previously treated r/mCC.
INTRODUCTION
Despite implementation of human papilloma virus vaccination and screening practices, some patients develop recurrent or metastatic cervical cancer (r/mCC), which is incurable.1-3 Chemotherapy doublets + bevacizumab were standard of care (SOC) for first-line (1L) treatment of eligible patients with r/mCC on the basis of results of the phase III GOG 240 trial.4,5 Current 1L SOC for patients with PD-L1–positive r/mCC is based on results of the KEYNOTE-826 study, which showed that the addition of pembrolizumab to a chemotherapy doublet with or without bevacizumab resulted in a superior overall survival (OS) benefit over the GOG240 regimen.4,6,7 However, additional 1L treatment options are needed for patients with r/mCC. Furthermore, limited OS after disease progression on 1L regimens8-10 marks the unmet need for new and effective treatment options in the second-line setting and beyond.
CONTEXT
Key Objective
Can treatment with tisotumab vedotin (TV) be safely and effectively combined with other anticancer therapies in cervical cancer, such as bevacizumab, carboplatin, or pembrolizumab, to improve treatment outcomes in patients with advanced cervical cancer?
Knowledge Generated
Combination treatment with TV + bevacizumab, or carboplatin, or pembrolizumab showed acceptable safety profiles and promising efficacy in pretreated patients during the dose escalation, supporting further evaluation of the selected recommended phase II dose in combination with current treatments (carboplatin or pembrolizumab) in earlier lines of treatment in the dose-expansion arms. First-line (1L) treatment with TV in combination with carboplatin and 1L or second-/third-line treatment with TV + pembrolizumab demonstrated encouraging and durable antitumor activity with tolerable and manageable safety profiles.
Relevance (G.F. Fleming)
-
While single-agent therapy with the antibody-drug conjugate TV is currently approved for pretreated recurrent or metastatic cervical cancer, these results lay the groundwork for future randomized explorations of combinations using this agent, potentially in an earlier line of therapy.*
*Relevance section written by JCO Associate Editor Gini F. Fleming, MD.
Tissue factor (TF) is expressed in many solid tumors, including cervical cancer.11 Tisotumab vedotin (TV) is a TF-directed antibody-drug conjugate with a proposed multimodal mechanism of action that includes direct cytotoxicity, bystander effects, antibody-dependent cellular cytotoxicity and phagocytosis, and induction of immunogenic cell death.12,13 TV monotherapy is approved in the United States for the treatment of adults with r/mCC who had disease progression on or after chemotherapy, on the basis of the clinically meaningful objective response rate (ORR) and duration of response (DOR) in the pivotal innovaTV 204/GOG-3023/ENGOT-cx6 study.14,15 Combining TV with chemotherapy, bevacizumab, and/or immunotherapies could allow for enhanced antitumor effects. Here, we present results from the proof-of-concept arms of the innovaTV 205/GOG-3024/ENGOT-cx8 study, evaluating the safety and antitumor activity of TV in combination with bevacizumab, pembrolizumab, or carboplatin in patients with r/mCC.
METHODS
Study Design
innovaTV 205/GOG-3024/ENGOT-cx8 (ClinicalTrials.gov identifier: NCT03786081) is an open-label, multicenter phase Ib/II study in patients with recurrent or stage IVB cervical cancer. The study was performed according to ENGOT-GOG model C.16 The objectives of the dose-escalation arms were to determine the maximum tolerated dose (MTD) and recommended phase II dose (RP2D) of TV when combined with bevacizumab (arm A), pembrolizumab (arm B), or carboplatin (arm C; Data Supplement, Fig S1, online only). The dose-expansion arms included TV-doublet combinations, with either carboplatin in the 1L setting (arm D) or pembrolizumab in the 1L (arm E) or second-line/third-line (2L/3L) settings (arm F; Data Supplement, Fig S1).
The study was approved by an independent ethics committee/institutional review boards at each site and was conducted in accordance with good clinical practice and the principles of the Declaration of Helsinki. All patients provided written informed consent.
Patients
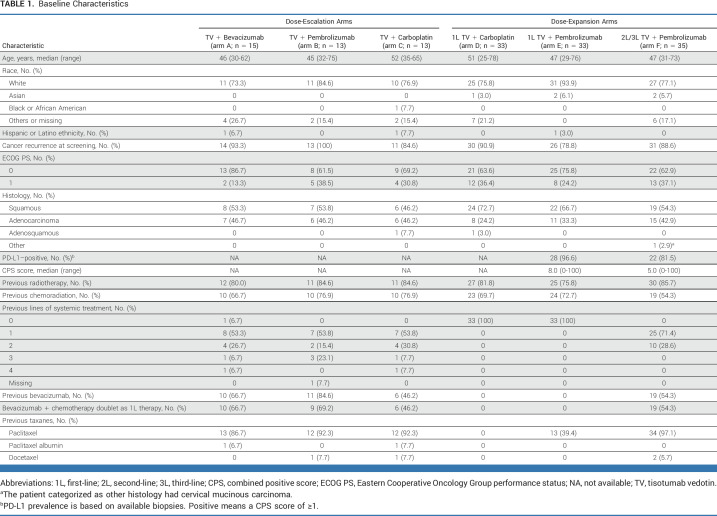
Adults (18 years and older) with recurrent or stage IVB squamous carcinoma, adenosquamous carcinoma, or adenocarcinoma of the cervix and measurable disease at baseline per RECIST v1.1 were enrolled.17 Patients eligible for dose-escalation arms A-C had disease progression on or after, or were ineligible or intolerant to, SOC treatments (ie, ≥2L). Patients enrolled in dose-expansion arms D (1L TV + carboplatin) and E (1L TV + pembrolizumab) had not received previous systemic therapy for r/mCC. Patients in arm F (2L/3L TV + pembrolizumab) had disease progression during or after one or two previous lines of systemic therapy in the r/mCC setting. Previous treatment with anti–PD-1 or anti–PD-L1 therapy was not permitted for patients enrolled in arms B, E, and F. Key exclusion criteria were risk of clinically significant bleeding issues and active ocular surface disease. Full eligibility criteria are listed in the Data Supplement (Table S1). Patients were enrolled regardless of PD-L1 status or TF expression.
Treatments
Patients received treatment intravenously once every 3 weeks until disease progression, unacceptable toxicity, or withdrawal of consent. Dose-escalation methods are given in detail in the Data Supplement. The MTD was defined as the dose below the lowest dose level that induced dose-limiting toxicity (DLT) in at least one third of patients. Each patient received a minimum of two 21-day treatment cycles before the RP2D was defined. Patients in the dose-expansion arms received treatment with the RP2D identified during dose escalation. To prevent ocular adverse events (AEs), all patients received prophylactic eye care (Data Supplement).
End Points
The dose-escalation primary end point was the incidence of DLTs and AEs. The dose-expansion primary end point was investigator-assessed ORR per RECIST v1.1.17 Secondary end points (dose escalation and dose expansion) included DOR, time to response (TTR), progression-free survival (PFS), OS, and AEs. Additional end points included immunogenicity and TF expression in tumor biopsies.
Assessments
AEs were monitored throughout treatment and at safety follow-up visits 30 days and 90 days after the last dose. Predefined AEs of special interest (AESIs) for TV included ocular, bleeding, and peripheral neuropathy AEs. AEs were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events v5.0.
Tumor imaging by computed tomography (or contrast-enhanced magnetic resonance imaging when appropriate or indicated) was performed at screening, every 6 weeks for the first 31 weeks of treatment in arms A-C and F, or for the first 37 weeks in arms D-E, and every 12 weeks thereafter in all arms. Objective responses (complete response [CR] or partial response [PR]) were confirmed by repeat imaging assessment, performed ≥4 weeks after first indication of response. Stable disease (SD) criteria are summarized in the Data Supplement. Disease control rate (DCR) was the combined rate of CR or PR lasting ≥4 weeks after first CR/PR and SD lasting ≥5 weeks after the first dose. Clinical benefit rate (CBR) was the combined rate of CR or PR lasting ≥4 weeks after first CR/PR and SD lasting ≥10 weeks, where minimum duration for SD was calculated as the time from the start of treatment to the last SD.
Assessments of TV-directed antidrug antibodies (ADAs) in plasma and TF expression are described in the Data Supplement.
Statistical Analyses
The safety analysis population included all patients who received at least one dose of study treatment. Efficacy analyses were conducted on the full analysis set, which was the same as the safety population but excluded patients with protocol violations (Fig 1). ORRs were estimated with exact Clopper-Pearson two-sided 95% CIs. Time-to-event outcomes (DOR, TTR, PFS, OS) were analyzed using Kaplan-Meier methods. Data were analyzed using SAS software v9.4 (SAS Institute, Inc, Cary, NC).
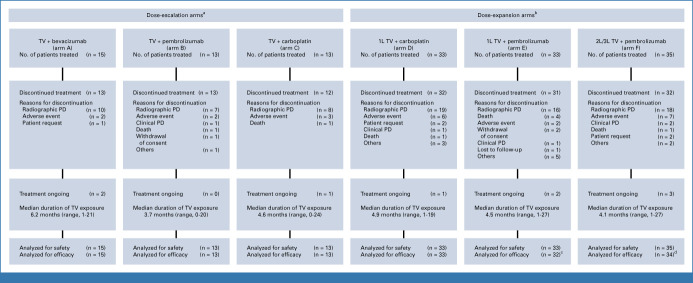
FIG 1.
CONSORT diagram. aData cutoff date for the dose-escalation arms was January 25, 2022. bData cutoff date for the dose-expansion arms was June 20, 2022. cOne patient received brentuximab and was not analyzed for efficacy. dOne patient did not have measurable disease at baseline and was not analyzed for efficacy. 1L, first-line; 2L, second-line; 3L, third-line; PD, progressive disease; TV, tisotumab vedotin.
RESULTS
Patients
A total of 142 patients were enrolled, including 41 patients in dose-escalation arms A-C between February 27, 2019, and October 6, 2020 (data cutoff January 25, 2022), and 101 patients in dose-expansion arms D-F from November 29, 2019, to December 15, 2020 (data cutoff June 20, 2022). In the dose-escalation arms, 61.0% of patients had previous 1L bevacizumab + platinum doublet chemotherapy, 73.2% had previous chemoradiotherapy, and 41.5% had ≥2 previous lines of treatment (Table 1). All 66 patients enrolled in dose-expansion arms D and E received study drug as 1L treatment for r/mCC; 71.2% had previous chemoradiotherapy. Of 35 patients in arm F (2L or 3L), 54.3% had previous chemoradiotherapy, 54.3% had received 1L bevacizumab + doublet chemotherapy, and 28.6% had received two previous lines of treatment.
TABLE 1.
Baseline Characteristics
Across dose-expansion and dose-escalation arms, nine patients (6.3%; two in arm A, one in arm C, one in arm D, two in arm E, and three in arm F) remained on study treatment and 133 (93.7%) had discontinued treatment, including 83 (58.5%) because of disease progression (radiographic or clinical) and 22 (15.5%) because of AEs (Fig 1). The median duration of TV exposure ranged from 3.7 to 6.2 months across arms (Fig 1).
Dose-Escalation Phase: Determination of RP2D
No DLTs were observed on the basis of Safety Data Monitoring Committee review. The MTD was not reached (NR) in any arm. AEs are summarized in the Data Supplement (Table S2). Grade 4 AEs considered related to any study treatment occurred in one patient in the TV + bevacizumab arm (arm A; rectal perforation), no patients in the TV + pembrolizumab arm (arm B), and four patients in the TV + carboplatin arm (arm C; thrombocytopenia [n = 2], hypomagnesemia [n = 1], and neutropenia, anemia, and thrombocytopenia combined [n = 1]). No fatal AEs related to any treatment were reported. Most AESIs were grade 1/2, and most events resolved or improved during the study (Data Supplement, Table S2). The most common bleeding AESI with TV + bevacizumab was epistaxis (66.7%; Data Supplement, Table S2). No clinically significant changes in clinical and coagulation laboratory results were observed. The RP2D was TV 2 mg/kg intravenously once every 3 weeks in combination with bevacizumab 15 mg/kg, pembrolizumab 200 mg, or carboplatin AUC 5 (each also administered once every 3 weeks).
Efficacy data and maximum change in target lesion size for the dose-escalation arms are provided in the Data Supplement (Table S3 and Fig S2, respectively).
Dose-Expansion Phase: Efficacy
1L TV + Carboplatin (arm D)
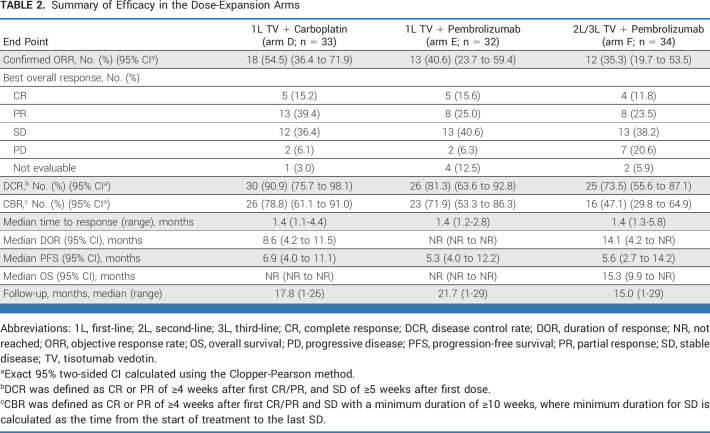
For patients treated with TV + carboplatin in 1L (n = 33), the ORR was 54.5%, the DCR was 90.9%, and the CBR was 78.8% (Table 2). In the response-evaluable population (patients with ≥1 postbaseline imaging assessment or who died before first postbaseline assessment; n = 32), the ORR was 56.3%, the DCR was 93.8%, and the CBR was 81.3% (Data Supplement, Table S4). Maximum change in target lesion size is shown in Figure 2A. Responses were ongoing in two of 18 responders at last assessment, one of whom remained on study treatment (Fig S3, Data Supplement). The median TTR was 1.4 months (range, 1.1-4.4). The median DOR was 8.6 months (95% CI, 4.2 to 11.5; Fig 2B). With a median follow-up of 17.8 months, the median PFS was 6.9 months (95% CI, 4.0 to 11.1; Data Supplement, Fig S4). The 1-year PFS rate was 28.1% (95% CI, 13.1 to 45.4). The 2-year PFS rate was 5.4% (95% CI, 0.4 to 20.8). Median OS was NR at data cutoff (14 patients [42.4%] had died; Fig 2C).
TABLE 2.
Summary of Efficacy in the Dose-Expansion Arms
FIG 2.
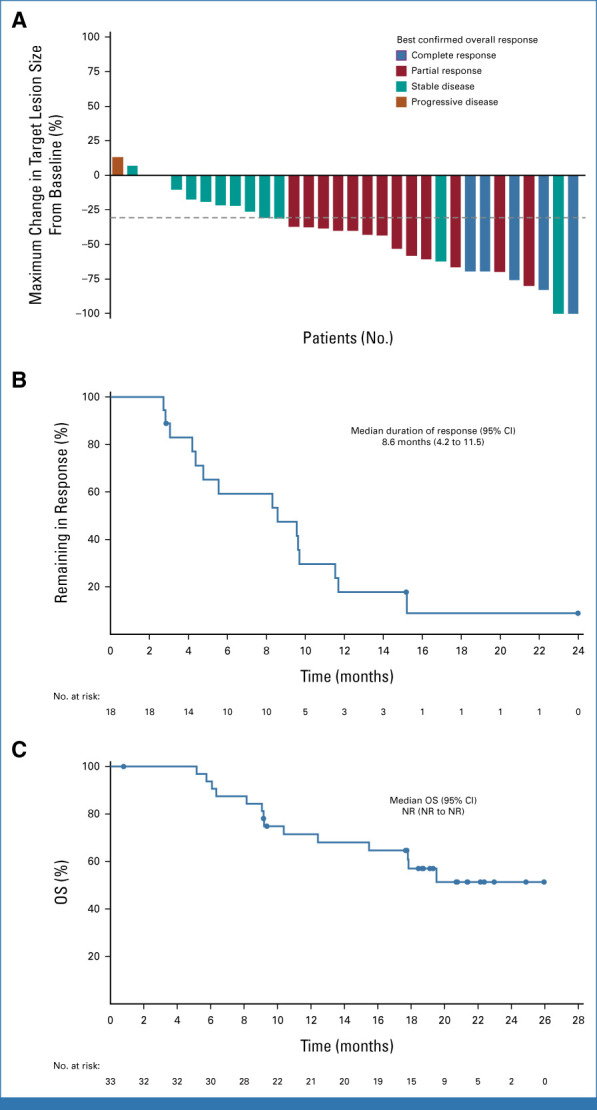
Efficacy outcomes in patients with r/mCC treated 1L with TV + carboplatin in the dose-expansion part (arm D). (A) Waterfall plot showing the maximum percentage change in target lesions. The dashed line indicates a 30% reduction from baseline. (B) Kaplan-Meier–estimated duration of response among the 18 patients with confirmed responses. (C) Kaplan-Meier–estimated overall survival. At data cutoff (June 20, 2022), the median follow-up was 17.8 months (range, 1-26). 1L, first-line; NR, not reached; OS, overall survival; r/mCC, recurrent or metastatic cervical cancer; TV, tisotumab vedotin.
1L TV + Pembrolizumab (arm E)
For treatment-naive patients who received TV + pembrolizumab (n = 32), the ORR was 40.6%, the DCR was 81.3%, and the CBR was 71.9% (Table 2; Fig 3A). In response-evaluable patients (n = 31), the ORR was 41.9%, the DCR was 83.9%, and the CBR was 74.2% (Data Supplement, Table S4). Seven of 13 responders had ongoing response at last assessment, two of whom remained on study treatment (Data Supplement, Fig S5). The median TTR was 1.4 months (range, 1.2-2.8); median DOR was NR (Fig 3B). With a median follow-up of 21.7 months, the median PFS was 5.3 months (95% CI, 4.0 to 12.2); the PFS rate was 37.1% (95% CI, 19.9 to 54.5) at 1 year and 28.9% (95% CI, 13.4 to 46.4) at 2 years (Data Supplement, Fig S6). Median OS was NR (13 deaths [40.6%]; Fig 3C).
FIG 3.
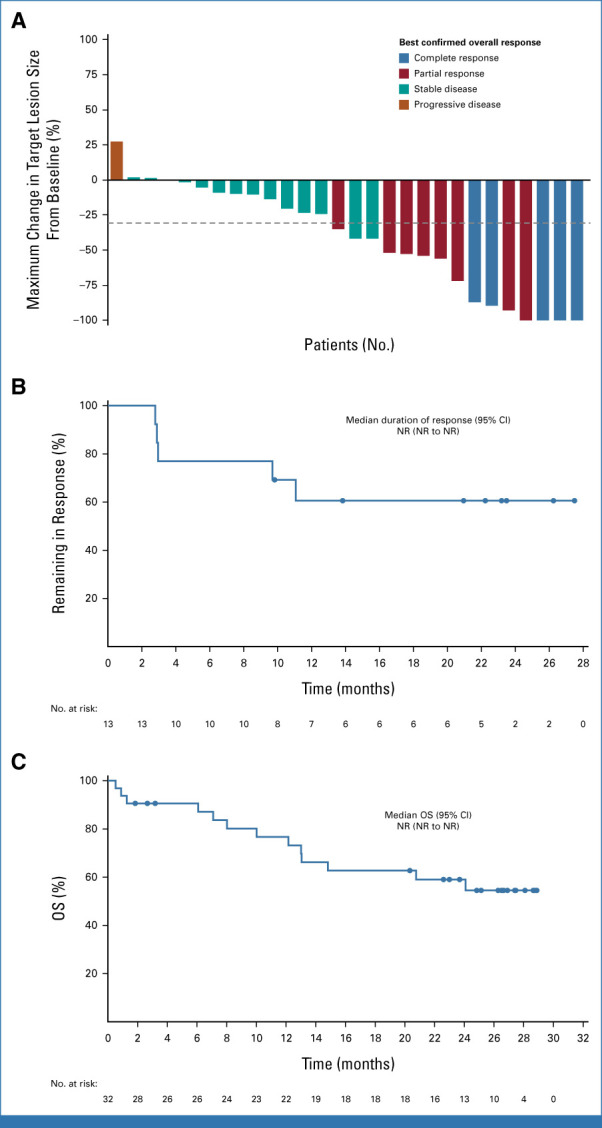
Efficacy outcomes in patients with r/mCC treated 1L with TV + pembrolizumab in the dose-expansion arm E. (A) Waterfall plot showing the maximum percentage change in target lesions. The dashed line indicates a 30% reduction from baseline. (B) Kaplan-Meier–estimated duration of response among the 13 patients with confirmed responses. (C) Kaplan-Meier–estimated overall survival. At data cutoff (June 20, 2022), the median follow-up was 21.7 months (range, 1-29). 1L, first-line; NR, not reached; OS, overall survival; r/mCC, recurrent or metastatic cervical cancer; TV, tisotumab vedotin.
2L or 3L TV + Pembrolizumab (arm F)
Among patients who received 2L or 3L treatment with TV + pembrolizumab (n = 34), the ORR was 35.3%, the DCR was 73.5%, and the CBR was 47.1% (Table 2; Fig 4A). In response-evaluable patients (n = 32), the ORR was 37.5%, the DCR was 78.1%, and the CBR was 50.0% (Data Supplement, Table S4). Responses were maintained in four of 12 responders at last assessment, three of whom remained on study treatment (Data Supplement, Fig S7). The median TTR was 1.4 months (range, 1.3-5.8). The median DOR was 14.1 months (95% CI, 4.2 to NR; Fig 4B). With a median follow-up of 15.0 months, the median PFS was 5.6 months (95% CI, 2.7 to 14.2; Data Supplement, Fig S8). The 1- and 2-year PFS rates were 34.5% (95% CI, 17.9 to 51.8) and 15.3% (95% CI, 4.9 to 31.2), respectively. The median OS was 15.3 months (95% CI, 9.9 to NR; 21 deaths [61.8%]; Fig 4C). The ORR was similar in patients with (33.3% [6 of 18]; 95% CI, 13.3 to 59.0) and without previous bevacizumab treatment (37.5% [6 of 16]; 95% CI, 15.2 to 64.6).
FIG 4.
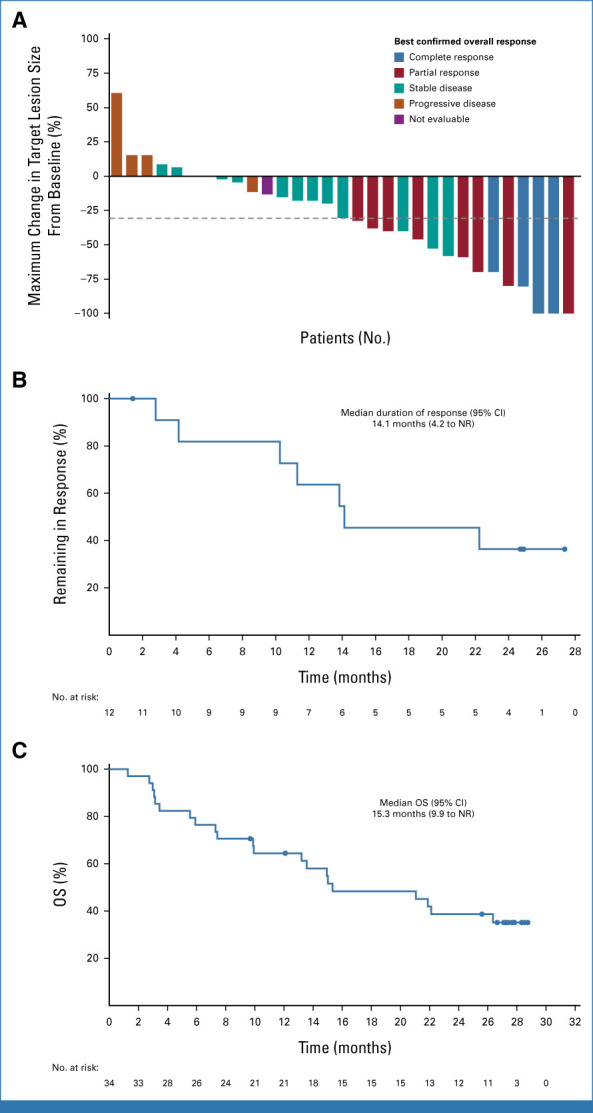
Efficacy outcomes in patients with r/mCC treated 2L or 3L with TV + pembrolizumab in the dose-expansion arm F. (A) Waterfall plot showing the maximum percentage change in target lesions. The dashed line indicates a 30% reduction from baseline. (B) Kaplan-Meier–estimated duration of response among the 12 patients with confirmed responses. (C) Kaplan-Meier–estimated overall survival. At data cutoff (June 20, 2022), the median follow-up was 15.0 months (range, 1-29). 2L, second line; 3L, third line; NR, not reached; OS, overall survival; r/mCC, recurrent or metastatic cervical cancer; TV, tisotumab vedotin.
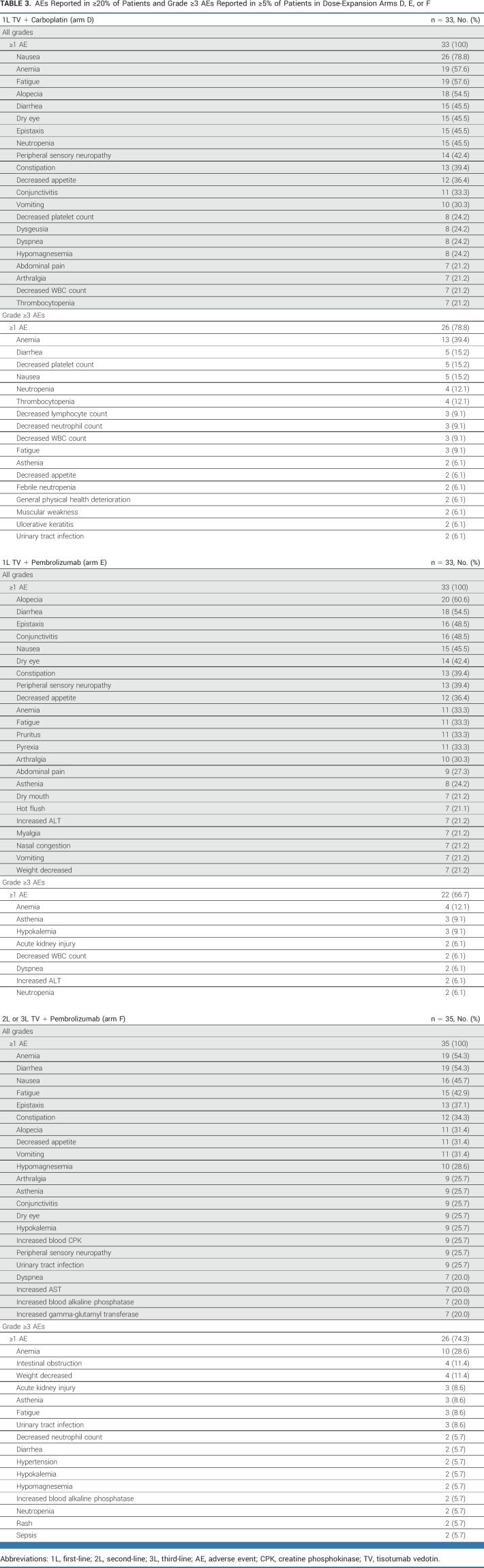
Dose-Expansion Phase: Safety
The most common AEs in the dose-expansion arms are listed in Table 3. Grade ≥3 AEs occurred in 78.8%, 66.7%, and 74.3% of patients in arms D, E, and F, respectively. AEs related to any study treatment are listed in the Data Supplement (Table S5). One treatment-related fatal AE (disseminated intravascular coagulation) occurred in arm E. The most common AEs leading to discontinuation of TV were ocular or peripheral neuropathy AEs (Data Supplement, Table S6).
TABLE 3.
AEs Reported in ≥20% of Patients and Grade ≥3 AEs Reported in ≥5% of Patients in Dose-Expansion Arms D, E, or F
Ocular AESIs were reported in 66.7%, 69.7%, and 54.3% of patients in arms D, E, and F, respectively (grade 3: 9.1%, 9.1%, and 2.9%, respectively; none grade ≥4; Data Supplement, Fig S9 and Table S7); the median time to onset was 33, 24, and 12 days, respectively. Ocular AEs resolved or improved in >85% of patients with events in each arm, with the median time to resolution of 21, 16, and 22 days in arms D, E, and F, respectively.
Peripheral neuropathy AESIs occurred in 60.6%, 51.5%, and 40.0% of patients in arms D, E, and F, respectively (grade 3: 12.1%, 3.0%, and 2.9%, respectively; none grade ≥4), with the median time to onset of 40, 85, and 105 days, respectively (Data Supplement, Fig S9 and Table S7). Peripheral neuropathy resolved or improved in >40% of patients with events in each arm, with the median time to resolution of 13, 43, and 49 days in arms D, E, and F, respectively.
Bleeding AESIs occurred in 57.6%, 66.7%, and 68.6% of patients in arms D, E, and F, respectively (grade ≥3: 6.1%, 6.1% [including 1 grade 5 disseminated intravascular coagulation], and 8.6%, respectively; Data Supplement, Fig S9); the median time to onset was 7-9 days. Bleeding AEs resolved or improved in >70% of patients with events in each arm, with the median time to resolution of 12, 34, and 7 days, respectively (Data Supplement, Table S7). Bleeding AEs related to TV treatment were reported in 42.4%, 54.5%, and 40.0% of patients in arms D, E, and F, respectively, most commonly grade 1/2 epistaxis (36.4%, 48.5%, and 25.7%, respectively). Thrombotic events are listed in the Data Supplement (Table S8); grade 3 thrombotic events included infusion site thrombosis (n = 1; 3.0%) in arm D, deep vein thrombosis (n = 1; 3.0%) in arm E, and pulmonary embolism (n = 1; 2.9%) in arm F.
Immune-related AEs in the pembrolizumab arms (arms E and F) are listed in the Data Supplement (Table S9). Immunogenicity results for TV ADAs are presented in the Data Supplement.
TF Expression
Membrane TF expression (≥1%) was confirmed for biopsy-evaluable patients in the dose-expansion arms (median [range] membrane H-score at baseline: arm D, 85.0 [2-290], n = 27; arm E, 120.0 [0-290], n = 30; arm F, 140.0 [14-285], n = 33). Tumor membrane H-scores for TF expression at baseline were not directly associated with response to treatment in arms D, E, or F (Data Supplement, Fig S10).
DISCUSSION
Doublet combination regimens of TV with bevacizumab, carboplatin, or pembrolizumab demonstrated acceptable safety and encouraging antitumor activity in patients with advanced r/mCC in the dose-escalation phase. Treatment history was similar to the real-world population,10,18,19 with the majority of patients having previous 1L bevacizumab + platinum doublet chemotherapy or previous chemoradiotherapy. Across arms A, B, and C, most AEs were grade 1/2 and no DLTs occurred with the TV-doublet combinations. Observed safety profiles were generally consistent with those known for each individual agent.4,15,20-23 The RP2D for TV in combination with bevacizumab, carboplatin, or pembrolizumab is the same as the approved dose for TV monotherapy.
Combining vascular endothelial growth factor angiogenesis inhibition (bevacizumab) with potential coagulation effects of TV via the TF target carries the potential for overlapping toxicities; however, data from this study suggest that there were no meaningful changes in coagulation parameters. For bevacizumab + TV, the bleeding AE rate was within the range of previous reports with TV monotherapy (57%-76%).15,20,21 Our results confirm the feasibility of adding TV to bevacizumab.
In the dose-expansion phase, 1L treatment with TV + carboplatin demonstrated a tolerable safety profile and encouraging antitumor activity, with numerically higher observed ORR (54.5%; CR, 15.2%) than that observed with cisplatin or topotecan + paclitaxel without bevacizumab (ORR, 36%; CR, 8%) in GOG 240.24 The CR rate observed with TV + carboplatin is comparable with that achieved with the current 1L SOC (12.9%-21.4%).6 TV + carboplatin appeared to have a more favorable tolerability profile than that previously noted with cisplatin + paclitaxel in previous studies.4,24,25 No grade 4 neutropenia was reported in patients receiving TV + carboplatin, whereas grade ≥4 neutropenia was reported in 26% of patients receiving cisplatin + paclitaxel in GOG 240.4,24,25 Peripheral neuropathy AEs, which are common with cisplatin/carboplatin + paclitaxel (any grade, 62%-77%; grade 3, 5%-9%),4,23 were reported in 60.6% of patients who received TV + carboplatin. The incidence of ocular AEs with TV + carboplatin (66.7%; grade 3, 9.1%) was comparable with that observed with TV monotherapy (53%15-65%20). The encouraging antitumor activity and tolerability of TV + carboplatin warrant further evaluation of this combination in the frontline setting.
Both pembrolizumab (in PD-L1+ tumors) and TV are approved in the United States as monotherapies for patients with r/mCC in the 2L setting.14,26 Pembrolizumab + chemotherapy showed improved OS versus chemotherapy alone in patients with PD-L1+ r/mCC in the 1L setting.6 There may be additive effects when combining TV and pembrolizumab. Clinical data suggest that the combination delivers a clinically meaningful response rate and prolonged DOR compared with each drug as monotherapy.15,22,27 The potential additive effects appear to be irrespective of previous therapy exposure. Additional research is needed to better understand the potential synergy. The immune-related AE profile with TV + pembrolizumab was comparable with that of pembrolizumab monotherapy in KEYNOTE-158 (n = 98).22 The observed additive clinical benefit and favorable safety profile support further evaluation of TV in combination regimens that may improve clinical outcomes for patients with treatment-naive and previously treated r/mCC.
A limitation of our study is small sample sizes of the combination arms, which limit our ability to make definitive conclusions regarding the efficacy of these combinations. Larger studies are needed to assess optimal treatment sequencing. The six arms of patients included in this phase Ib/II trial enabled systematic and careful evaluation of the feasibility of incorporating TV into established combination regimens. While these doublet data are encouraging, further investigation is ongoing to continue potential development of TV as part of evolving 1L SOC.
In conclusion, TV demonstrated acceptable safety and promising antitumor activity when administered in combination with bevacizumab, pembrolizumab, or carboplatin, supporting additional studies combining TV with individual backbone treatments for patients with r/mCC. Furthermore, TV in combination with carboplatin or pembrolizumab in the 1L setting showed durable antitumor activity and tolerable safety, supporting ongoing evaluation of triplet/quadruplet combinations of TV + carboplatin + pembrolizumab with or without bevacizumab as 1L treatment of r/mCC in an additional dose-expansion arm (arm H).
ACKNOWLEDGMENT
We thank the patients and their families and caregivers for participating in this study and all site personnel. This study is in collaboration with Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ. Medical writing and editorial support was provided by Lela Creutz, PhD, from Peloton Advantage, an OPEN Health company, and funded by Genmab.
Ignace Vergote
Consulting or Advisory Role: AstraZeneca, Elevar Therapeutics, Genmab, Immunogen, Jazz Pharmaceuticals, Mersana, MSD, Novocure, Sotio, Verastem, Zentalis, Roche, Agenus, Eisai, Novartis, Seagen, Akeso Biopharma, Bristol Myers Squibb, Deciphera, Exelixis, GlaxoSmithKline, Karyopharm Therapeutics, Oncoinvent, OncXerna Therapeutics, Regeneron, Sanofi
Research Funding: Roche (Inst), Amgen (Inst), Oncoinvent (Inst)
Travel, Accommodations, Expenses: Karyopharm Therapeutics, Genmab, Novocure
Els Van Nieuwenhuysen
Consulting or Advisory Role: Regeneron (Inst), Oncoinvent
Research Funding: AstraZeneca (Inst), Lilly (Inst), GlaxoSmithKline (Inst), Merck (Inst), Seagen (Inst)
Roisin E. O'Cearbhaill
Honoraria: GlaxoSmithKline, Curio Science, MJH Life Sciences, MJH/PER
Consulting or Advisory Role: Seagan, Aptitude Health, Fresenius Kabi, GlaxoSmithKline, Bayer, Regeneron, Carina Biotech, Immunogen, R-Pharm, GOG Foundation, Miltenyi Biotec, 2seventy bio
Research Funding: Juno Therapeutics (Inst), Sellas Life Sciences (Inst), Ludwig Institute for Cancer Research (Inst), TapImmune Inc (Inst), TCR2 Therapeutics (Inst), Regeneron (Inst), Genmab (Inst), Atara Biotherapeutics (Inst), GlaxoSmithKline (Inst), AstraZeneca/Merck (Inst), Syndax (Inst), Genentech (Inst), Kite/Gilead (Inst), GOG Foundation (Inst), Merck/Genentech (Inst), Acrivon Therapeutics (Inst), Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Hitech Health, Gathering Around Cancer, Society of Gynecologic Oncology
Other Relationship: JAMA Oncology
Uncompensated Relationships: Children's Medical Research Foundation (CMRF)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/785539
Domenica Lorusso
Consulting or Advisory Role: PharmaMar, AstraZeneca, Clovis Oncology, GlaxoSmithKline, MSD, Genmab, Seagen, Immunogen, Oncoinvest, Corcept Therapeutics, Sutro Biopharma, Novartis
Speakers' Bureau: AstraZeneca, Clovis Oncology, GlaxoSmithKline, MSD, PharmaMar, ImmunoGen, Seagen, Genmab
Research Funding: PharmaMar (Inst), Clovis Oncology (Inst), GlaxoSmithKline (Inst), MSD (Inst), AstraZeneca (Inst), Genmab (Inst), Seagen (Inst), Immunogen (Inst), Incyte (Inst), Novartis (Inst), Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, GlaxoSmithKline
Uncompensated Relationships: Gynecological Cancer InterGroup
Sharad Ghamande
Consulting or Advisory Role: Seagen
Speakers' Bureau: Tesaro/GSK, Eisai
Research Funding: Jounce Therapeutics (Inst), Astellas Pharma (Inst), Akeso Biopharma (Inst), Merck Serono (Inst), Incyte (Inst), Ellipses Pharma (Inst), Aravive (Inst), GlaxoSmithKline (Inst), Merck (Inst), Roche (Inst), Genentech (Inst), Takeda (Inst), Seagen (Inst), Advaxis (Inst), Bristol Myers Squibb (Inst), Clovis Oncology (Inst), AbbVie (Inst), Tesaro (Inst)
Dearbhaile C. Collins
Honoraria: Pfizer, Genmab, SeaGen, GlaxoSmithKline, Roche
Consulting or Advisory Role: Seagen, MSD
Research Funding: Pfizer (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Susana Banerjee
Stock and Other Ownership Interests: Perci Health
Honoraria: AstraZeneca, GlaxoSmithKline, Clovis Oncology, Pfizer, Immunogen, MSD Oncology, Mersana, Roche, Takeda, Amgen, Novocure
Consulting or Advisory Role: GlaxoSmithKline, MSD Oncology, Mersana, AstraZeneca, Seagen, OncXerna Therapeutics, Shattuck Labs, Immunogen, Regeneron, Novartis, Epsilogen
Research Funding: GlaxoSmithKline (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline, AstraZeneca, Verastem
Cara A. Mathews
Research Funding: AstraZeneca (Inst), Tesaro/GSK (Inst), Syros Pharmaceuticals (Inst), Astellas Pharma (Inst), Seagen (Inst), Deciphera (Inst), Moderna Therapeutics (Inst), Regeneron (Inst), Roche/Genentech (Inst), Pfizer (Inst), Laekna Therapeutics (Inst), EMD Serono (Inst), Merck (Inst), Genmab (Inst), Avenge Bio (Inst), Zentalis
Christine Gennigens
Honoraria: MSD Oncology, Ipsen, Pfizer, PharmaMar, AstraZeneca, GlaxoSmithKline, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: MSD Oncology, Bristol Myers Squibb/Celgene, Ipsen, AstraZeneca, GlaxoSmithKline, Eisai
Research Funding: AstraZeneca
Travel, Accommodations, Expenses: Ipsen, PharmaMar, Pfizer, MSD Oncology
David Cibula
Consulting or Advisory Role: AstraZeneca, Sotio, Roche, GlaxoSmithKline, Novocure, Akeso Biopharma, MSD, Mersana
Krishnansu S. Tewari
Honoraria: Tesaro, Clovis Oncology, Merck, Eisai, AstraZeneca, Genmab
Consulting or Advisory Role: Roche/Genentech, Tesaro, Clovis Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, Merck, Tesaro, Clovis Oncology, Eisai, Genmab
Research Funding: AbbVie (Inst), Genentech/Roche (Inst), Morphotek (Inst), Merck (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Kristine Madsen
Travel, Accommodations, Expenses: GlaxoSmithKline
Fatih Köse
Honoraria: Roche (Inst), AstraZeneca (Inst), MSD/Merck (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Pfizer (Inst), Takeda (Inst), Deva (Inst), Nobelpharma (Inst), Astellas Pharma (Inst), Janssen (Inst)
Consulting or Advisory Role: Roche, AstraZeneca, MSD/Merck, GlaxoSmithKline, Novartis, Pfizer, Takeda, Deva, Nobelpharma, Astellas Pharma, Janssen
Amanda L. Jackson
Consulting or Advisory Role: Ethicon, Seagen, Sutro Biopharma, AstraZeneca
Ingrid A. Boere
Consulting or Advisory Role: Tesaro/GSK (Inst), AstraZeneca (Inst)
Research Funding: GlaxoSmithKline (Inst)
Giovanni Scambia
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, PharmaMar, Roche, Tesaro
Speakers' Bureau: Clovis Oncology, MSD
Leslie M. Randall
Honoraria: BluPrint Oncology, PER, Curio Science
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, GOG Foundation, Merck, Mersana, Rubius Therapeutics, Myriad Genetics, Genentech/Roche, Seagen, Eisai, Novocure, AADi, On Target Laboratories, Immunogen
Speakers' Bureau: Genmab/Seagen
Research Funding: Genentech/Roche (Inst), On Target Laboratories (Inst), Pfizer (Inst), Aivita Biomedical (Inst), Tesaro (Inst), AstraZeneca (Inst), Merck (Inst), Akeso Biopharma (Inst), GEICO (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Seagen, Genmab, GOG Foundation
Jean-François Baurain
Consulting or Advisory Role: MSD Oncology (Inst), BMS (Inst), Novartis (Inst), Pierre Fabre (Inst), Sanofi/Regeneron (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), Sun Pharma (Inst)
Hannelore G. Denys
Consulting or Advisory Role: Pfizer (Inst), Roche (Inst), PharmaMar (Inst), AstraZeneca (Inst), Lilly (Inst), Novartis (Inst), Amgen (Inst), GlaxoSmithKline (Inst), MSD (Inst), Seagen (Inst), Gilead Sciences (Inst)
Research Funding: Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Pfizer (Inst), Roche (Inst), PharmaMar (Inst), Teva (Inst), AstraZeneca (Inst), Gilead Sciences (Inst)
Nelleke Ottevanger
Consulting or Advisory Role: AstraZeneca (Inst), GlaxoSmithKline (Inst), Nykode Therapeutics (Inst)
Frédéric Forget
Travel, Accommodations, Expenses: Ipsen, Teva
Camilla Mondrup Andreassen
Employment: Genmab
Stock and Other Ownership Interests: Genmab, Scandion Oncology, Novo Nordisk, Bavarian Nordic, 3M, AbbVie, Colgate Palmolive, Coloplast, Demant, Expres2ion Biotechnologies, Lundbeck, Johnson & Johnson/Janssen, Teva, Unilever
Michael J. Chisamore
Employment: Merck
Stock and Other Ownership Interests: Merck
Leonardo Viana Nicacio
Employment: Seagen
Stock and Other Ownership Interests: Florence Healthcare, Seagen
Honoraria: Seagen
Travel, Accommodations, Expenses: Seagen
Ibrahima Soumaoro
Employment: Genmab
Stock and Other Ownership Interests: Genmab, Bristol Myers Squibb
Travel, Accommodations, Expenses: Genmab, Bristol Myers Squibb
Bradley J. Monk
Leadership: US Oncology
Honoraria: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, Tesaro/GSK, Vascular Biogenics, GOG Foundation, Elevar Therapeutics, Novocure, Gradalis, Karyopharm Therapeutics, Bayer, EMD Serono/Merck, Sorrento Therapeutics, US Oncology, Myriad Pharmaceuticals, Novartis, OncoC4, Pieris Pharmaceuticals, Acrivon Therapeutics, Adaptimmune, HenRui, Laekna Health Care, Panavance Therapeutics, Verastem, Zentalis
Consulting or Advisory Role: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, GOG Foundation, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Myriad Pharmaceuticals, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, Tesaro/GSK, Vascular Biogenics, Gradalis, Karyopharm Therapeutics, Sorrento Therapeutics, Novocure, Bayer, Elevar Therapeutics, EMD Serono/Merck, Gradalis, US Oncology, Novartis, Pieris Pharmaceuticals, OncoC4, Adaptimmune, HenRui, Laekna Health Care, Panavance Therapeutics, Verastem, Zentalis
Speakers' Bureau: Roche/Genentech, AstraZeneca, Clovis Oncology, Eisai, Tesaro/GSK, Merck
Research Funding: Novartis (Inst), Amgen (Inst), Genentech (Inst), Lilly (Inst), Janssen (Inst), Array BioPharma (Inst), Tesaro (Inst), Morphotek (Inst), Pfizer (Inst), Advaxis (Inst), AstraZeneca (Inst), Immunogen (Inst), Regeneron (Inst), Nucana (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the International Gynecologic Cancer Society Annual Global Meeting, Rome, Italy, August 30-September 2, 2021; and at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by Genmab A/S and Seagen Inc.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Deidentified individual participant data collected during the trial will not be available upon request for further analyses by external independent researchers. Aggregated clinical trial data from the trial are provided via publicly accessible study registries/databases as required by law. For more information, please contact clinicaltrials@genmab.com.
AUTHOR CONTRIBUTIONS
Conception and design: Ignace Vergote, Dearbhaile C. Collins, David Cibula, Camilla Mondrup Andreassen, Lamar Eaton, Michael J. Chisamore, Leonardo Viana Nicacio, Ibrahima Soumaoro, Bradley J. Monk
Financial support: Anneke Westermann, Leonardo Viana Nicacio
Administrative support: Ingrid A. Boere, Leonardo Viana Nicacio
Provision of study materials or patients: Ignace Vergote, Els Van Nieuwenhuysen, Anneke Westermann, Sharad Ghamande, Dearbhaile C. Collins, Cara A. Mathews, Krishnansu S. Tewari, Ingrid A. Boere, Azmat Sadozye, Jean-François Baurain, Eelke Gort, Michal Zikán, Hannelore G. Denys, Nelleke Ottevanger, Michael J. Chisamore, Leonardo Viana Nicacio, Bradley J. Monk
Collection and assembly of data: Ignace Vergote, Els Van Nieuwenhuysen, Anneke Westermann, Domenica Lorusso, Susana Banerjee, Cara A. Mathews, Christine Gennigens, David Cibula, Krishnansu S. Tewari, Kristine Madsen, Fatih Köse, Ingrid A. Boere, Giovanni Scambia, Jean-François Baurain, Eelke Gort, Michal Zikán, Nelleke Ottevanger, Frédéric Forget, Camilla Mondrup Andreassen, Lamar Eaton, Leonardo Viana Nicacio, Ibrahima Soumaoro, Bradley J. Monk
Data analysis and interpretation: Ignace Vergote, Els Van Nieuwenhuysen, Anneke Westermann, Sharad Ghamande, Dearbhaile C. Collins, Susana Banerjee, Christine Gennigens, Krishnansu S. Tewari, Kristine Madsen, Amanda L. Jackson, Ingrid A. Boere, Leslie M. Randall, Azmat Sadozye, Jean-François Baurain, Hannelore G. Denys, Nelleke Ottevanger, Frédéric Forget, Camilla Mondrup Andreassen, Lamar Eaton, Michael J. Chisamore, Leonardo Viana Nicacio, Ibrahima Soumaoro, Bradley J. Monk
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Tisotumab Vedotin in Combination With Carboplatin, Pembrolizumab, or Bevacizumab in Recurrent or Metastatic Cervical Cancer: Results From the innovaTV 205/GOG-3024/ENGOT-cx8 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ignace Vergote
Consulting or Advisory Role: AstraZeneca, Elevar Therapeutics, Genmab, Immunogen, Jazz Pharmaceuticals, Mersana, MSD, Novocure, Sotio, Verastem, Zentalis, Roche, Agenus, Eisai, Novartis, Seagen, Akeso Biopharma, Bristol Myers Squibb, Deciphera, Exelixis, GlaxoSmithKline, Karyopharm Therapeutics, Oncoinvent, OncXerna Therapeutics, Regeneron, Sanofi
Research Funding: Roche (Inst), Amgen (Inst), Oncoinvent (Inst)
Travel, Accommodations, Expenses: Karyopharm Therapeutics, Genmab, Novocure
Els Van Nieuwenhuysen
Consulting or Advisory Role: Regeneron (Inst), Oncoinvent
Research Funding: AstraZeneca (Inst), Lilly (Inst), GlaxoSmithKline (Inst), Merck (Inst), Seagen (Inst)
Roisin E. O'Cearbhaill
Honoraria: GlaxoSmithKline, Curio Science, MJH Life Sciences, MJH/PER
Consulting or Advisory Role: Seagan, Aptitude Health, Fresenius Kabi, GlaxoSmithKline, Bayer, Regeneron, Carina Biotech, Immunogen, R-Pharm, GOG Foundation, Miltenyi Biotec, 2seventy bio
Research Funding: Juno Therapeutics (Inst), Sellas Life Sciences (Inst), Ludwig Institute for Cancer Research (Inst), TapImmune Inc (Inst), TCR2 Therapeutics (Inst), Regeneron (Inst), Genmab (Inst), Atara Biotherapeutics (Inst), GlaxoSmithKline (Inst), AstraZeneca/Merck (Inst), Syndax (Inst), Genentech (Inst), Kite/Gilead (Inst), GOG Foundation (Inst), Merck/Genentech (Inst), Acrivon Therapeutics (Inst), Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Hitech Health, Gathering Around Cancer, Society of Gynecologic Oncology
Other Relationship: JAMA Oncology
Uncompensated Relationships: Children's Medical Research Foundation (CMRF)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/785539
Domenica Lorusso
Consulting or Advisory Role: PharmaMar, AstraZeneca, Clovis Oncology, GlaxoSmithKline, MSD, Genmab, Seagen, Immunogen, Oncoinvest, Corcept Therapeutics, Sutro Biopharma, Novartis
Speakers' Bureau: AstraZeneca, Clovis Oncology, GlaxoSmithKline, MSD, PharmaMar, ImmunoGen, Seagen, Genmab
Research Funding: PharmaMar (Inst), Clovis Oncology (Inst), GlaxoSmithKline (Inst), MSD (Inst), AstraZeneca (Inst), Genmab (Inst), Seagen (Inst), Immunogen (Inst), Incyte (Inst), Novartis (Inst), Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, GlaxoSmithKline
Uncompensated Relationships: Gynecological Cancer InterGroup
Sharad Ghamande
Consulting or Advisory Role: Seagen
Speakers' Bureau: Tesaro/GSK, Eisai
Research Funding: Jounce Therapeutics (Inst), Astellas Pharma (Inst), Akeso Biopharma (Inst), Merck Serono (Inst), Incyte (Inst), Ellipses Pharma (Inst), Aravive (Inst), GlaxoSmithKline (Inst), Merck (Inst), Roche (Inst), Genentech (Inst), Takeda (Inst), Seagen (Inst), Advaxis (Inst), Bristol Myers Squibb (Inst), Clovis Oncology (Inst), AbbVie (Inst), Tesaro (Inst)
Dearbhaile C. Collins
Honoraria: Pfizer, Genmab, SeaGen, GlaxoSmithKline, Roche
Consulting or Advisory Role: Seagen, MSD
Research Funding: Pfizer (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Susana Banerjee
Stock and Other Ownership Interests: Perci Health
Honoraria: AstraZeneca, GlaxoSmithKline, Clovis Oncology, Pfizer, Immunogen, MSD Oncology, Mersana, Roche, Takeda, Amgen, Novocure
Consulting or Advisory Role: GlaxoSmithKline, MSD Oncology, Mersana, AstraZeneca, Seagen, OncXerna Therapeutics, Shattuck Labs, Immunogen, Regeneron, Novartis, Epsilogen
Research Funding: GlaxoSmithKline (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline, AstraZeneca, Verastem
Cara A. Mathews
Research Funding: AstraZeneca (Inst), Tesaro/GSK (Inst), Syros Pharmaceuticals (Inst), Astellas Pharma (Inst), Seagen (Inst), Deciphera (Inst), Moderna Therapeutics (Inst), Regeneron (Inst), Roche/Genentech (Inst), Pfizer (Inst), Laekna Therapeutics (Inst), EMD Serono (Inst), Merck (Inst), Genmab (Inst), Avenge Bio (Inst), Zentalis
Christine Gennigens
Honoraria: MSD Oncology, Ipsen, Pfizer, PharmaMar, AstraZeneca, GlaxoSmithKline, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: MSD Oncology, Bristol Myers Squibb/Celgene, Ipsen, AstraZeneca, GlaxoSmithKline, Eisai
Research Funding: AstraZeneca
Travel, Accommodations, Expenses: Ipsen, PharmaMar, Pfizer, MSD Oncology
David Cibula
Consulting or Advisory Role: AstraZeneca, Sotio, Roche, GlaxoSmithKline, Novocure, Akeso Biopharma, MSD, Mersana
Krishnansu S. Tewari
Honoraria: Tesaro, Clovis Oncology, Merck, Eisai, AstraZeneca, Genmab
Consulting or Advisory Role: Roche/Genentech, Tesaro, Clovis Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, Merck, Tesaro, Clovis Oncology, Eisai, Genmab
Research Funding: AbbVie (Inst), Genentech/Roche (Inst), Morphotek (Inst), Merck (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Kristine Madsen
Travel, Accommodations, Expenses: GlaxoSmithKline
Fatih Köse
Honoraria: Roche (Inst), AstraZeneca (Inst), MSD/Merck (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Pfizer (Inst), Takeda (Inst), Deva (Inst), Nobelpharma (Inst), Astellas Pharma (Inst), Janssen (Inst)
Consulting or Advisory Role: Roche, AstraZeneca, MSD/Merck, GlaxoSmithKline, Novartis, Pfizer, Takeda, Deva, Nobelpharma, Astellas Pharma, Janssen
Amanda L. Jackson
Consulting or Advisory Role: Ethicon, Seagen, Sutro Biopharma, AstraZeneca
Ingrid A. Boere
Consulting or Advisory Role: Tesaro/GSK (Inst), AstraZeneca (Inst)
Research Funding: GlaxoSmithKline (Inst)
Giovanni Scambia
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, PharmaMar, Roche, Tesaro
Speakers' Bureau: Clovis Oncology, MSD
Leslie M. Randall
Honoraria: BluPrint Oncology, PER, Curio Science
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, GOG Foundation, Merck, Mersana, Rubius Therapeutics, Myriad Genetics, Genentech/Roche, Seagen, Eisai, Novocure, AADi, On Target Laboratories, Immunogen
Speakers' Bureau: Genmab/Seagen
Research Funding: Genentech/Roche (Inst), On Target Laboratories (Inst), Pfizer (Inst), Aivita Biomedical (Inst), Tesaro (Inst), AstraZeneca (Inst), Merck (Inst), Akeso Biopharma (Inst), GEICO (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Seagen, Genmab, GOG Foundation
Jean-François Baurain
Consulting or Advisory Role: MSD Oncology (Inst), BMS (Inst), Novartis (Inst), Pierre Fabre (Inst), Sanofi/Regeneron (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), Sun Pharma (Inst)
Hannelore G. Denys
Consulting or Advisory Role: Pfizer (Inst), Roche (Inst), PharmaMar (Inst), AstraZeneca (Inst), Lilly (Inst), Novartis (Inst), Amgen (Inst), GlaxoSmithKline (Inst), MSD (Inst), Seagen (Inst), Gilead Sciences (Inst)
Research Funding: Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Pfizer (Inst), Roche (Inst), PharmaMar (Inst), Teva (Inst), AstraZeneca (Inst), Gilead Sciences (Inst)
Nelleke Ottevanger
Consulting or Advisory Role: AstraZeneca (Inst), GlaxoSmithKline (Inst), Nykode Therapeutics (Inst)
Frédéric Forget
Travel, Accommodations, Expenses: Ipsen, Teva
Camilla Mondrup Andreassen
Employment: Genmab
Stock and Other Ownership Interests: Genmab, Scandion Oncology, Novo Nordisk, Bavarian Nordic, 3M, AbbVie, Colgate Palmolive, Coloplast, Demant, Expres2ion Biotechnologies, Lundbeck, Johnson & Johnson/Janssen, Teva, Unilever
Michael J. Chisamore
Employment: Merck
Stock and Other Ownership Interests: Merck
Leonardo Viana Nicacio
Employment: Seagen
Stock and Other Ownership Interests: Florence Healthcare, Seagen
Honoraria: Seagen
Travel, Accommodations, Expenses: Seagen
Ibrahima Soumaoro
Employment: Genmab
Stock and Other Ownership Interests: Genmab, Bristol Myers Squibb
Travel, Accommodations, Expenses: Genmab, Bristol Myers Squibb
Bradley J. Monk
Leadership: US Oncology
Honoraria: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, Tesaro/GSK, Vascular Biogenics, GOG Foundation, Elevar Therapeutics, Novocure, Gradalis, Karyopharm Therapeutics, Bayer, EMD Serono/Merck, Sorrento Therapeutics, US Oncology, Myriad Pharmaceuticals, Novartis, OncoC4, Pieris Pharmaceuticals, Acrivon Therapeutics, Adaptimmune, HenRui, Laekna Health Care, Panavance Therapeutics, Verastem, Zentalis
Consulting or Advisory Role: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, GOG Foundation, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Myriad Pharmaceuticals, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, Tesaro/GSK, Vascular Biogenics, Gradalis, Karyopharm Therapeutics, Sorrento Therapeutics, Novocure, Bayer, Elevar Therapeutics, EMD Serono/Merck, Gradalis, US Oncology, Novartis, Pieris Pharmaceuticals, OncoC4, Adaptimmune, HenRui, Laekna Health Care, Panavance Therapeutics, Verastem, Zentalis
Speakers' Bureau: Roche/Genentech, AstraZeneca, Clovis Oncology, Eisai, Tesaro/GSK, Merck
Research Funding: Novartis (Inst), Amgen (Inst), Genentech (Inst), Lilly (Inst), Janssen (Inst), Array BioPharma (Inst), Tesaro (Inst), Morphotek (Inst), Pfizer (Inst), Advaxis (Inst), AstraZeneca (Inst), Immunogen (Inst), Regeneron (Inst), Nucana (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gargano JW, McClung N, Lewis RM, et al. : HPV type-specific trends in cervical precancers in the United States, 2008 to 2016. Int J Cancer 152:137-150, 2023 [DOI] [PubMed] [Google Scholar]
- 2.Pfaendler KS, Tewari KS: Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol 214:22-30, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Wagle NS, et al. : Cancer statistics, 2023. CA Cancer J Clin 73:17-48, 2023 [DOI] [PubMed] [Google Scholar]
- 4.Tewari KS, Sill MW, Long HJ, 3rd, et al. : Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 370:734-743, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang LT, Temin S, Camacho R, et al. : Management and care of women with invasive cervical cancer: American Society of Clinical Oncology resource-stratified clinical practice guideline. JCO Glob Oncol 2:311-340, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombo N, Dubot C, Lorusso D, et al. : Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med 385:1856-1867, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Chuang LT, Temin S, Berek JS: Management and care of patients with invasive cervical cancer: ASCO resource-stratified guideline rapid recommendation update. JCO Glob Oncol 8:e2200027, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alholm Z, Monk BJ, Ting J, et al. : Patient characteristics, treatment patterns and clinical outcomes among patients with previously treated recurrent or metastaticcervical cancer: a community oncology-based real-world analysis Annual Meeting of the Society of Gynecologic Oncology Women's Cancer, virtual, March 19-25, 2021 (poster) [DOI] [PubMed]
- 9.Musa FB, Brouwer E, Ting J, et al. : Trends in treatment patterns and costs of care among patients with advanced stage cervical cancer. Gynecol Oncol 164:645-650, 2022 [DOI] [PubMed] [Google Scholar]
- 10.Alholm Z, Monk BJ, Ting J, et al. : Patient characteristics, treatment patterns, and clinical outcomes among patients with previously treated recurrent or metastatic cervical cancer: A community oncology-based analysis. Gynecol Oncol 161:422-428, 2021 [DOI] [PubMed] [Google Scholar]
- 11.de Bono JS, Harris JR, Burm SM, et al. : Systematic study of tissue factor expression in solid tumors. Cancer Rep 6:e1699, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alley SC, Harris JR, Cao A, et al. : Abstract 221: Tisotumab vedotin induces anti-tumor activity through MMAE-mediated, Fc-mediated, and Fab-mediated effector functions in vitro. Cancer Res 79:221, 2019 [Google Scholar]
- 13.Breij EC, de Goeij BE, Verploegen S, et al. : An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res 74:1214-1226, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Tivdak [package insert]. Plainsboro, NJ, Seagen Inc, Genmab US Inc, 2022 [Google Scholar]
- 15.Coleman RL, Lorusso D, Gennigens C, et al. : Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 22:609-619, 2021 [DOI] [PubMed] [Google Scholar]
- 16.Vergote I, Coleman RL, Pignata S, et al. : Joint ENGOT and GOG Foundation requirements for trials with industry partners. Int J Gynecol Cancer 29:1094-1097, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Alholm Z, He D, Ting J, et al. : Real-world treatment drop-off among recurrent or metastatic cervical cancer patients: A US community oncology-based analysis. Gynecol Oncol 166:567-575, 2022 [DOI] [PubMed] [Google Scholar]
- 19.Sonawane K, Castellano T, Washington C, et al. : Factors associated with receipt of second-line recurrent or metastatic cervical cancer treatment in the United States: A retrospective administrative claims analysis. Gynecol Oncol Rep 44:101121, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong DS, Concin N, Vergote I, et al. : Tisotumab vedotin in previously treated recurrent or metastatic cervical cancer. Clin Cancer Res 26:1220-1228, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Yonemori K, Kuboki Y, Hasegawa K, et al. : Tisotumab vedotin in Japanese patients with recurrent/metastatic cervical cancer: Results from the innovaTV 206 study. Cancer Sci 113:2788-2797, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung HC, Ros W, Delord JP, et al. : Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 37:1470-1478, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Nishio S, Yonemori K, Usami T, et al. : Pembrolizumab plus chemotherapy in Japanese patients with persistent, recurrent or metastatic cervical cancer: Results from KEYNOTE-826. Cancer Sci 113:3877-3887, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tewari KS, Sill MW, Penson RT, et al. : Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 390:1654-1663, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiyama T, Mizuno M, Aoki Y, et al. : A single-arm study evaluating bevacizumab, cisplatin, and paclitaxel followed by single-agent bevacizumab in Japanese patients with advanced cervical cancer. Jpn J Clin Oncol 47:39-46, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keytruda [package insert]. Rahway, NJ, Merck & Co, Inc, 2023 [Google Scholar]
- 27.Chung H, Delord JP, Perets R, et al. : Pembrolizumab treatment of advanced cervical cancer: Updated results from the phase II KEYNOTE-158 study. Gynecol Oncol 162, 2021. (suppl 1; abstr S27) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified individual participant data collected during the trial will not be available upon request for further analyses by external independent researchers. Aggregated clinical trial data from the trial are provided via publicly accessible study registries/databases as required by law. For more information, please contact clinicaltrials@genmab.com.