Abstract
Systemic combination chemotherapy and intrathecal chemotherapy markedly increased the survival rate of children with ALL. In the past two decades, the use of minimal (measurable) residual disease (MRD) measurements early in therapy improved risk group stratification with subsequent treatment intensifications for patients at high risk of relapse, and enabled a reduction of treatment for low-risk patients. The recent development of more sensitive MRD technologies may further affect risk stratification. Molecular genetic profiling has led to the discovery of many new subtypes and their driver genetic alterations. This increased our understanding of the biological basis of ALL, improved risk classification, and enabled implementation of precision medicine. In the past decade, immunotherapies, including bispecific antibodies, antibody-drug conjugates, and cellular therapies directed against surface proteins, led to more effective and less toxic therapies, replacing intensive chemotherapy courses and allogeneic stem-cell transplantation in patients with relapsed and refractory ALL, and are now being tested in newly diagnosed patients. It has taken 50-60 years to increase the cure rate in childhood ALL from 0% to 90% by stepwise improvements in chemotherapy. This review provides an overview of how the developments over the past 10-15 years mentioned above have significantly changed the diagnostic and treatment approach in ALL, and discusses how the integrated use of molecular and immunotherapeutic insights will very likely direct efforts to cure those children with ALL who are not cured today, and improve the quality of life for survivors who should have decades of life ahead. Future efforts must focus on making effective, yet very expensive, new technologies and therapies available to children with ALL worldwide.
INTRODUCTION
Approximately one of 1,500 newborns develop ALL before their 18th birthday. Long-term survival rates increased from zero in the 1950s to over 90% in the past decade in high-income countries (Fig 1).1-3 This is attributable to stepwise refinement and intensification of chemotherapy regimens to improve both systemic and CNS control, by more sophisticated use of allogenic hematopoietic stem-cell transplantation (HSCT), improved supportive care, and more recently, improved risk stratification incorporating genomic features and early treatment response quantified by minimal (measurable) residual disease (MRD). In the past decade, genomic analyses have identified many new genetic subclasses of ALL, refining classification of ALL and increased understanding of disease biology.4 For some subtypes, precision medicine strategies have been or will soon be introduced. The very recent introduction of immunotherapies, including bispecific antibodies, antibody-drug conjugates (ADC), and cellular therapies, has changed treatment for refractory/relapsed (r/r) ALL and is now entering frontline trials.
FIG 1.
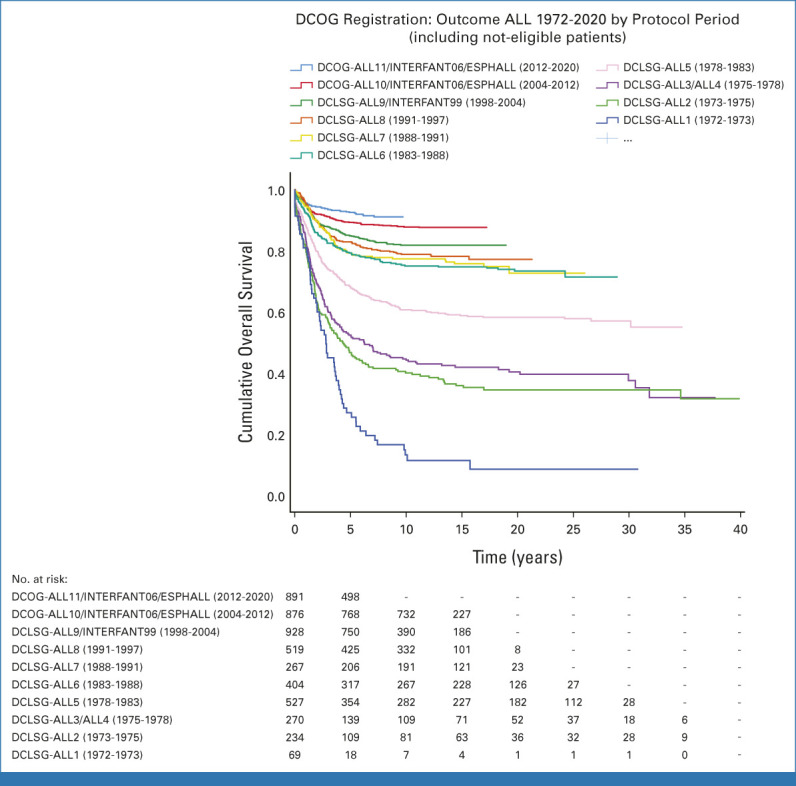
Outcome of Dutch children with ALL from 1972 to 2020. DCLSG, Dutch Childhood Leukemia Study Group; DCOG, Dutch Childhood Oncology Group.
We review how these developments have and will continue to lead to significant changes in treatment of childhood ALL. We anticipate that this will change ALL from an incurable disease in 1960 to a curable disease for (nearly) all patients and will also significantly reduce the incidence of major toxicities and thereby improve quality of life for survivors.
CURRENT THERAPY
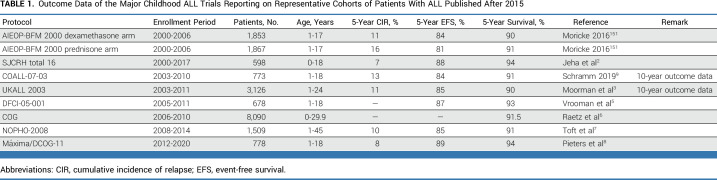
Treatment of childhood ALL involves multiagent chemotherapy, administered in rotating combinations combined with CNS prophylaxis, administered over approximately 2-2.5 years. Regimens typically include induction, followed by one or two consolidation courses, (delayed) intensification and maintenance. Five drugs form the backbone: glucocorticoids (prednisone and dexamethasone), vincristine, asparaginase, methotrexate, and 6-mercaptopurine. Additional drugs include anthracyclines (daunorubicin and doxorubicin), cytosine arabinoside (araC), and cyclophosphamide. Table 1 summarizes the most recent outcome data of the major study groups worldwide.2,3,5-9
TABLE 1.
Outcome Data of the Major Childhood ALL Trials Reporting on Representative Cohorts of Patients With ALL Published After 2015
Dexamethasone has greater antileukemic efficacy, but more side effects, than prednisone.10 Regular 5- to 7-day pulses of corticosteroids and vincristine are widely used during maintenance, but their value is debatable and likely depends on the other components of therapy and the patient subset.11 The benefit of high-dose versus intermediate-dose methotrexate also depends on the type of ALL.12,13 Truncation of the asparaginase schedule because of intolerance or silent inactivation of the drug increases the relapse risk.14-16 Therapeutic drug monitoring is used to detect silent inactivation of asparaginase and facilitates the timely switch to alternative asparaginases.17,18 How much asparaginase is needed for each subtype defined by MRD or genetics remains to be elucidated.19 The optimal length of ALL therapy is about 2-2.5 years for both boys and girls.20 Although 1-year total therapy worsens the overall outcome, half of the patients are long-term survivors with this shorter therapy and limited maintenance duration,21 but it is unclear how to recognize who will be cured with shorter therapy in advance.21 Genotyping of the host to adapt chemotherapy is currently limited to the detection of TPMT and NUDT15 polymorphisms that influence 6-mercaptopurine metabolism, with clinical consequences.22
Intrathecal therapy is essential to prevent CNS relapses.23 The benefit of triple intrathecal therapy (methotrexate, hydrocortisone, and cytarabine) versus intrathecal methotrexate is debatable; the intensity of intrathecal versus systemic therapy may influence the site of relapse.24,25 Cranial radiotherapy reduces the CNS relapse rate only in children with CNS3 disease, but does not influence survival even in this group.26 A recent report from the Children's Oncology Group shows that outcome in T-cell (T)-ALL is worse with CNS3 status, even with cranial irradiation.27 Flow cytometric detection of CNS leukemia is more reliable than morphology and positivity is associated with inferior outcome,28 which is being tested in the ongoing ALLTogether01 protocol. HSCT indications have been reduced by improvements in chemotherapy and are currently T-ALL with induction failure,29 BCR::ABL1-positive ALL with a poor MRD response, and subgroups of KMT2A-rearranged (KMT2A-R) infant ALL.30 For high-risk ALL subtypes, including KMT2A-R,31 hypodiploidy <44 chromosomes,32,33 and TCF3::HLF, its value is unproven.
MRD
Monitoring early responses to chemotherapy by MRD significantly improved the prediction of relapse and thereby risk group stratification. MRD is measured by flow cytometry using the leukemic immunophenotype or by PCR of clonotypic Ig/TCR rearrangements or fusion genes and genomic deletions.34 Next-generation sequencing–based MRD35,36 is more sensitive and more specific. It has not yet been widely implemented in clinical trials but may replace other technologies in the future. MRD-based stratification allows for a reduction of treatment intensity, thereby improving the quality of life for low-risk patients, while not jeopardizing their high survival rate. Persistent MRD identifies a small high-risk group who should be allocated to intensive chemotherapy and HSCT37 or new precision medicine or immunotherapeutic options to increase survival. The latter may have dual advantages since they also cause fewer side effects.38
Successful examples of therapy reduction exist. In some trials, MRD-based low-risk patients do not receive corticosteroid/vincristine pulses, whereas medium-risk patients do. MRD-guided reductions are possible such as reduction of the intensification course and reduction of cumulative anthracycline dose.8,39-42 The possibility of therapy reductions confronts clinicians and patients/parents with the dilemma of treating all low-risk patients with intensive therapy leading to the lowest relapse risk but increased risk of side effects, versus deintensified therapy for the majority of low-risk patients with better quality of life but possibly a slightly higher relapse rate for a few patients highly likely to be salvaged with rescue therapy. Therapy reductions are mainly applied in good-risk genetic subtypes, for example, ETV6::RUNX1, high hyperdiploidy (51-67 chromosomes), and/or those with negative end-of-induction MRD.43
SIDE EFFECTS
The most important acute side effects of chemotherapy influencing quality of life are bacterial and fungal infections caused by myelosuppression and the immunosuppressive effect of glucocorticoids, vincristine-induced neuropathy, methotrexate encephalopathy, pancreatitis,44 and intracerebral venous thrombosis caused by asparaginase, and osteonecrosis, myopathy, and behavioral problems caused by glucocorticoids. Many side effects are reversible, but a small percentage of patients with pancreatitis44 and about 60% of patients with symptomatic osteonecrosis45 experience long-term consequences. In patients receiving HSCT, infertility and chronic graft-versus-host disease cause additional serious toxicity. With current survival rates of >90%, quality of life is receiving more attention, leading to outcome indicators cocreated by professionals and survivors focused on toxicity-free survival.46,47
MOLECULAR GENETIC PROFILING
Genomic analyses have identified multiple subtypes of B-progenitor (Fig 2) and T-lineage ALL, their drivers, and cooperating genomic alterations, and showed that many children have germline genomic variations that influence leukemia susceptibility and treatment response. Constitutional syndromes such as Down syndrome and ataxia telangiectasia are associated with an increased risk of ALL.48 Genome-wide association studies identified multiple noncoding polymorphisms influencing the risk of developing ALL, commonly at loci encoding tumor suppressors or hematopoietic transcription factors (ARID5B, BAK1, CDKN2A/CDKN2B, BMI1-PIP4K2A, CEBPE, ELK3, ERG, GATA3, IGF2BP1, IKZF1 IKZF3, USP7, and LHPP). The risk associated with each is subtle but combinatorial.49 Several may influence acquisition of somatic driver alterations, such as germline GATA3 alterations and CRLF2 rearrangement in Ph-like ALL.50 Pathogenic germline coding variants have been described in multiple genes in ALL, including TP53, PAX5, IKZF1, and ETV6.51-53 These variants have been identified in both familial ALL, which is rare, and sporadic cases with no known family history, at a relatively high frequency (1%-2% of cases). Several germline pathogenic variants show associations with ALL subtypes, such as TP53 and low hypodiploidy (30-39 chromosomes), and ETV6 with high-hyperdiploid ALL. Germline IKZF1 variants also influence drug responsiveness in ALL.54
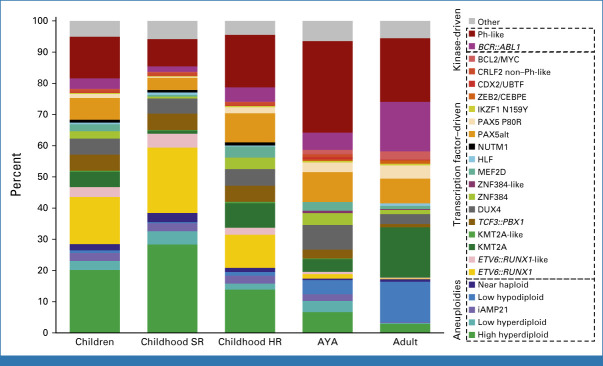
FIG 2.
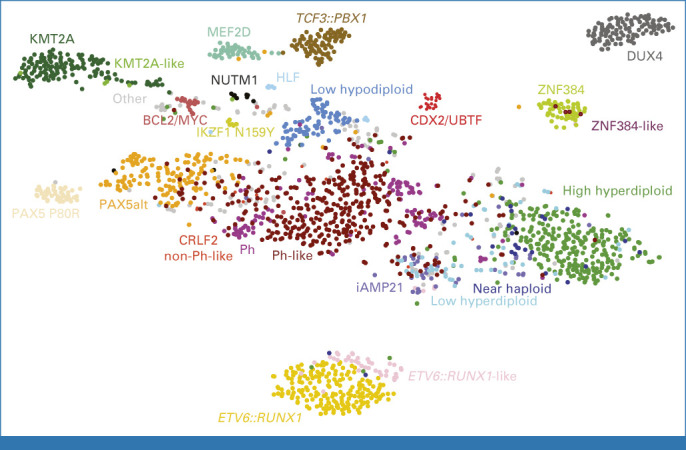
Subtyping of B-ALL using whole-transcriptome sequencing. t-SNE depiction of childhood and adult B-ALL adapted from Kimura et al.70 Subtypes are color-coded and labeled accordingly. t-SNE, t-weighted stochastic neighbor embedding.
Over 20 subtypes of B-ALL are now recognized, most of which were unknown before genomic profiling as they are not detectable by conventional cytogenetics55 (Figs 2 and 3). Aneuploid B-ALL subtypes are high-hyperdiploid ALL characterized by gains of at least five chromosomes, most commonly 4, 10, 14, 17, 18, 21, and X, and favorable outcome.56 Hypodiploid ALL, with multiple chromosomal losses, has two subtypes associated with poor outcome: near-haploid ALL with 25-29 chromosomes, and low-hypodiploid ALL with 30-39 chromosomes.32,57 Low-hypodiploid ALL is almost always associated with biallelic TP53 alteration, including a germline variant in about half of the pediatric cases; near-haploid ALL has a similar gene expression profile and mutational spectrum (Ras pathway, CREBBP) to hyperdiploid ALL, suggesting a common origin.4,51,55 B-ALL with intrachromosomal amplification of chromosome 21 (iAMP21) arises from breakage-fusion-bridge cycles and chromothripsis of chromosome 21, and rarely, constitutional alterations of chromosomes 15 or 21.58 This subtype has been associated with poor prognosis, ameliorated with more intensive treatment regimens.59
FIG 3.
Frequency of B-ALL subtypes. Histogram of prevalence of B-ALL subtypes according to age. Childhood <15 years; AYA 15-39 years; adult >39 years. AYA, adolescent young adult; HR, high risk; SR, standard risk.
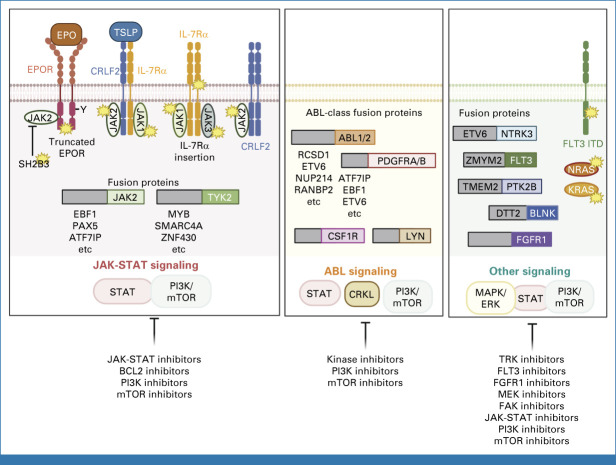
Chimeric fusion oncoproteins are a hallmark of B-ALL, and commonly arise in utero, years before clinically manifest leukemia, consistent with the requirement for additional genomic alterations to promote leukemogenesis. Several subtypes are defined by a single rearrangement, such as ETV6::RUNX1, TCF3::PBX1, BCR::ABL1, and translocations involving KMT2A.60 By contrast, several recently described subtypes have multiple fusion partners converging on a single gene: MEF2D-rearranged,61,62 NUTM1-rearranged,63 and ZNF384-rearranged ALL.64 NUTM1-rearranged ALL is observed in infants without KMT2A-R.63 ZNF384 rearrangement is characterized by the expression of myeloid antigens diagnosed as ALL or mixed-phenotype acute leukemia that are otherwise biologically indistinguishable, and prone to shift in immunophenotype during disease progression64,65 or exposure to CD19-directed immunotherapy. Additional subtypes are defined by characteristic alterations in specific genes (PAX5 P80R, PAX5alt with heterogeneous PAX5 alterations, and IKZF1 N159Y).55,66 Rearrangement of DUX4 to immunoglobulin or other enhancers defines a subtype of favorable-risk ALL,67-69 albeit with elevated levels of MRD early in therapy. Rare, but high-risk cases have dual alterations that deregulate CDX2 and encode UBTF::ATXN7L370. Ph-like (BCR::ABL1-like) ALL71,72 exhibits a transcriptomic signature similar to BCR::ABL1 ALL, and is the most genomically diverse form of ALL, with rearrangements and mutations of at least 16 cytokine receptors and tyrosine kinases, most commonly deregulating JAK-STAT and ABL1-class signaling pathways73 (Fig 4); this subtype increases in incidence with age and is associated with poor outcome.
FIG 4.
Pathways and therapeutic targets in BCR::ABL-like B-ALL. Schematic of genomic subtypes of BCR::ABL-like B-ALL, and potential therapeutic targeting opportunities, grouped according to kinase driver (JAK-STAT pathway, ABL1-class, and other). JAK-STAT, Janus kinase–signal transducers and activators of transcription; mTOR, mammalian target of rapamycin;PI3K, phosphatidylinositol 3-kinase.
Genomic subtypes are associated with relapse risk and chemotherapy response,74 providing a rationale for comprehensive genomic analysis at diagnosis. Although integrated whole-genome sequencing and transcriptome sequencing provide the most detailed molecular portrait of ALL, transcriptome sequencing provides an analysis of gene expression, mutations, and chromosomal rearrangements, and enables the identification of most risk-stratifying genomic alterations in ALL. Transcriptome sequencing also identifies cases that phenocopy canonical subtypes with similar gene expression but alternative genomic drivers (eg, ETV6::RUNX1-like ALL).67,69,75 Notably, the time for patients to be considered cured is 6 years after diagnosis, irrespective of the prognosis of each genetic subtype.3 In combination with MRD and cytogenetic abnormalities, copy-number alterations (eg, the UKALL-CNA profile in the ALLTogether01 protocol)38 are being incorporated into risk-stratification algorithms.
Genomic alterations are less established in the management of T-ALL, as this entity is less common, and more frequently driven by noncoding, enhancer alterations that deregulate T-lineage transcription factors and oncogenes that require genome sequencing for identification.76 However, this landscape is changing, with recent studies comprehensively defining the taxonomy of T-ALL and showing associations between subtype, outcome, and treatment failure.77 Similarly, acute leukemias of ambiguous lineage that exhibit either minimal differentiation or immunophenotypic features of multiple hematopoietic lineages (most commonly B and myeloid, or T and myeloid) have been poorly characterized from a genomic perspective. In addition to known associations with KMT2A and BCR::ABL1, several new entities have recently been identified that transcend immunophenotypic criteria, including ZNF384-rearranged (either B-ALL or B/myeloid) leukemia78 and BCL11B-rearranged (early T-cell precursor [ETP] or T/myeloid) leukemia.79,80
PRECISION MEDICINE IN B-ALL
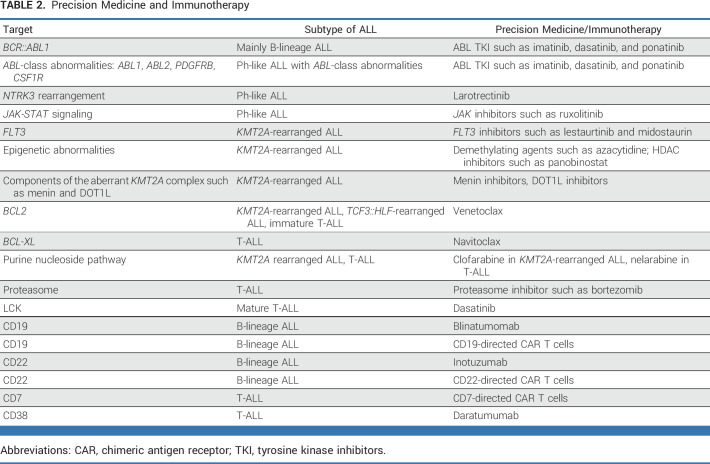
BCR::ABL1-rearranged (Philadelphia chromosome–positive, Ph+) ALL was the first genetic subtype for which targeted therapy became available. Several large collaborative studies have shown that the addition of ABL tyrosine kinase inhibitors (TKI) to chemotherapy has improved outcome.81-83 Also, the need for HSCT in these patients has significantly been reduced.83-85 Before the advent of TKI therapy, most children with BCR::ABL1-positive ALL received HSCT; currently, only those with high MRD after two courses of chemotherapy and TKI (5%-10%) are transplanted in first remission. Imatinib and dasatinib are used because of more safety data in pediatrics,86,87 whereas third-/fourth-generation TKIs such as ponatinib are commonly used in adults because of the much higher prevalence of BCR::ABL1 and ABL1-class Ph-like ALL in adults, and more data on TKI safety. A randomized trial88 showed that dasatinib led to better outcome than imatinib in children with BCR::ABL1 ALL, but because of limited follow-up and the poor outcome of imatinib-treated patients, this has not yet led to the routine implementation of dasatinib.88 Very recently, highly promising results were obtained with chemotherapy-free treatment regimens in adult BCR::ABL1-rearranged ALL. These protocols, consisting of a TKI (usually ponatinib) plus several courses of blinatumomab and inotuzumab,89 showed low toxicity and excellent early relapse-free survival rates, raising the potential that adult BCR::ABL1-rearranged ALL could be a favorable subtype, and that similarly favorable results may be observed in childhood BCR::ABL1-ALL (Table 2).
TABLE 2.
Precision Medicine and Immunotherapy
Ph-like ALL is more common than BCR::ABL1 ALL in children (approximately 10%-15% v approximately 2%-3%) but has a similarly poor outcome without the use of TKIs.90 Both have a very high incidence of IKZF1 deletions, which further worsens their poor outcome.84,91 Rexinoids92 and FAK inhibitors93 ameliorate the biologic effects of IKZF1 alterations, but have not been tested clinically. The Máxima/DCOG group observed improved outcomes by prolonging therapy with a third year.8 Only one of eight BCR::ABL1-like cases carry an ABL-class fusion (ABL1, ABL2, PDGFRB, CSF1R, and very rare others) that can be targeted by the ABL TKIs.94 This led to the addition of TKIs to chemotherapy in small cohorts with encouraging results,95 as well as for larotrectinib for NTRK3-rearranged Ph-like ALL96 (Fig 3). The efficacy of TKIs in ABL1-class Ph-like ALL is being tested in the COG/EsPhALL Ph+ ALL, ALLtogether1, and St Jude Total Therapy 17 protocols.
In BCR::ABL1-like cases with mutations activating JAK-STAT signaling, approved JAK inhibitors such as ruxolitinib are less consistently effective than ABL1 inhibitors in ABL1-class cases.97 Ruxolitinib is being evaluated by the COG (ClinicalTrials.gov identifier: NCT02723994), but outcome data are not yet available.
Specific therapy protocols have been developed for KMT2A-R ALL.98-100 Infants with low MRD at the end of induction have a better outcome when treated with ALL-like consolidation (course 1B), and those with high MRD benefit from AML-like consolidation therapy (ADE-MAE courses).101 KMT2A-R ALL carries a specific gene expression profile including overexpression of wild-type FLT3. Limited efficacy was observed of a FLT3 inhibitor (FLT3i) as a single agent in infant KMT2A-R ALL.102 A randomized study adding FLT3i to chemotherapy showed no overall benefit, but suggested a benefit in a small subset identified by inhibition of phosphorylated FLT3 or by ex vivo sensitivity to the compound.103 KMT2A-R ALL is mainly characterized by epigenetic abnormalities caused by the KMT2A fusion protein104 and shows high sensitivity to demethylating agents and HDAC inhibitors ex vivo.105 However, a clinical trial with azacytidine in infant KMT2A-R ALL did not show promising results.106 Recently, precision medicine therapies have focused on components of the KMT2A protein complex such as DOT1L and menin. The clinical effect of DOT1L inhibitors was disappointing, but recent phase I/II trials with menin inhibitors showed promising early results,107 leading to development of multiple upcoming trials. Because of high BCL2 expression, venetoclax has also been studied in KMT2A-R ALL.108 Venetoclax also showed promising preclinical activity in TCF3::HLF rearranged ALL108,109 and T-ALL (see below). Finally, because of the association of low-hypodiploid ALL and TP53 mutations, TP53 is an attractive therapeutic target, but this has not yet led to clinical trials.
PRECISION MEDICINE IN T-ALL
Precision medicine is more difficult in T-ALL.110 Nelarabine is a DNA-terminating nucleoside prodrug that is metabolized into arabinosylguanine nucleotide triphosphate and preferentially accumulates in T lymphoblasts.111 It was highly active in early-phase trials for r/r T-ALL but was also associated with significant and often severe neurotoxicity.112 COG ALL0434 demonstrated that it could be added safely in newly diagnosed T-ALL/lymphoma (T-LLy) and improved outcome modestly, with a notable impact on CNS relapses.13,113 The COG also tested the proteasome inhibitor bortezomib in T-ALL/T-LLy with complicated results but a suggestion that bortezomib improved outcome in T-lymphoblastic lymphoma.114 Preclinical data have shown alterations in BCL2 signaling pathways in T-ALL, with the ETP subset showing BCL2 dependence and remaining non-ETP cases dependent on BCL-XL.115 These observations prompted efforts to target these pathways with navitoclax (BCL-XL) and/or venetoclax (BCL2). Promising results of an early-phase clinical trial resulted in a trial of low-dose navitoclax plus venetoclax in r/r pediatric B- and T-ALL (ClinicalTrials.gov identifier: NCT05192889).116 Ex vivo drug sensitivity studies showed that subsets of T-ALLs are very sensitive to dasatinib, with LCK activation driving dasatinib sensitivity associated with high BCL-XL and low BCL2 expression.108,117 These results raise the possibility of testing subtype-specific targeted therapies in T-ALL (Table 2).
IMMUNOTHERAPY
Immunotherapy has revolutionized the landscape of B-ALL therapy, showing very high remission rates in highly refractory patients118 and significantly less acute toxicity than intensive chemotherapy. Three approaches have become mainstays of treatment for r/r ALL and are now being tested in newly diagnosed patients (Table 2).
Blinatumomab is a bispecific T-cell engager genetically engineered monoclonal antibody recognizing CD19, expressed on the surface of essentially all B-ALLs, and CD3, expressed on the surface of essentially all T cells. Dual binding by blinatumomab brings cytotoxic T cells to CD19+ B cells, enabling cell killing. Because blinatumomab has a short half-life, it is administered by continuous infusion, typically for 28 days. The side-effect profile of blinatumomab is very different from that of chemotherapy with little neutropenia or mucositis, but some unique CNS toxicities, including seizures and hallucinations, and occasional development of cytokine release syndrome (CRS), usually in patients with high disease burden. Blinatumomab was demonstrated to be highly active in r/r adult and then pediatric B-ALL,119 leading to testing in randomized trials for the first relapse of ALL in North America and Europe. These showed that replacing one to two cycles of intensive chemotherapy with blinatumomab in children with high- and intermediate-risk relapsed ALL, followed by HSCT, significantly improved outcomes with this approach now considered to be standard of care.120,121 The COG also showed that replacement of one cycle of intensive chemotherapy with blinatumomab and addition of two cycles later in therapy improved outcome for children with lower-risk first relapse of B-ALL, but this benefit was limited to those with bone marrow relapse, whereas those with isolated CNS relapse fared poorly.122 A very recent pilot study in KMT2A-R infant ALL showed that addition of blinatumomab to the Interfant-06 backbone significantly improved outcome.123 Multiple cooperative groups are now testing blinatumomab in newly diagnosed ALL, with some adding the agent to backbone therapy and others using it to replace components of chemotherapy.
Inotuzumab is an ADC composed of a monoclonal antibody recognizing CD22, expressed on most B-ALLs, and the chemotherapy agent calicheamicin. After binding to surface CD22, inotuzumab is internalized and then calicheamicin is released into the cell by the lysozyme. The landmark INO-VATE trial showed that inotuzumab was superior to intensive chemotherapy for adult r/r ALL.124 Phase I and II trials conducted by the COG and European groups showed excellent activity in pediatric r/r ALL, with remission rates over 50%, allowing many patients to proceed to HSCT.125-127 Inotuzumab is administered with once a week intravenous 1-hour infusions for 3 weeks, and is well tolerated in heavily pretreated patients. One challenge is the potential for hepatic toxicity if given relatively close to previous HSCT or proximate to subsequent HSCT. Most pediatric trials have used inotuzumab as a single agent, whereas current trials are combining it with chemotherapy generally to replace other components such as anthracyclines. Several groups (COG and ALLtogether) are now testing inotuzumab in patients with newly diagnosed ALL.
The progress with antibody-based therapy is less in T-ALL. The anti-CD38 monoclonal antibody daratumumab has shown good efficacy in preclinical models of T-ALL and in small clinical series of relapsed T-ALL.128,129 Except for infusion reactions, no significant side effects have been reported. Studies on newly diagnosed T-ALL are planned.
Chimeric antigen receptor (CAR) T-cell therapy has recently entered the mainstream. Development of highly active (third-generation) CAR T cells required genetically engineered constructs with an extracellular ScFv antibody fragment that recognizes a cellular target such as CD19 with intracellular CD3zeta and costimulatory domains (41BB or CD137) or CD28 that mediate cell expansion, persistence and engagement, and cytotoxicity.130 CD19-directed CAR T cells were shown to be highly active in pediatric r/r ALL, with complete remission (CR) rates over 90% in heavily pretreated patients, most of whom failed three or more lines of therapy and had relapsed after HSCT.131-134 When treated with high disease burden, there is a high risk of CRS, including severe fluid overload, hypoxemia, and hypotension. Patients with severe CRS had very high interleukin-6 levels, and tocilizumab, a monoclonal antibody that inhibits binding of IL-6 to the IL-6 receptor, was highly effective in treating CRS,135 which was critical to the clinical development of CAR T cells.131 The CD19 CAR T-cell agent tisagenlecleucel was US Food and Drug Administration approved in 2017 for children and young adults with r/r ALL on the basis of the worldwide ELIANA trial.132 Subsequent real-world experience with tisagenlecleucel in r/r pediatric ALL showed very similar results to this pivotal trial with CR rates about 85% (almost all MRD-negative), event-free survival of 50%-55% at 12 months, and overall survival of about 75% at 12 months, all dramatically better than any other therapy.136 CAR T-cell therapy is also effective in CNS leukemia137,138 and other extramedullary sites,139 and its efficacy was shown in high-risk leukemias including infant KMT2A-R ALL,140,141 although perhaps less in ALL with TP53 aberrations. CAR T-cell therapy appears curative by itself in some cases, with the longest-responding patient now 11 years postinfusion without subsequent therapy and dozens of patients in CR 5+ years. However, about 50% of patients relapse with either CD19+ disease (loss of CAR T-cell persistence or activity) or CD19-negative disease (antigen escape).134 Several factors predict the lack of response and/or relapse142-144: high disease burden, loss of MRD response, early (within approximately 6 months) loss of B-cell depletion, and low plasma levels of fludarabine as part of the preinfusion lymphodepletion schedule.142-145 Loss of B-cell aplasia is a surrogate marker of CAR activity since CD19 CAR T cells also kill normal B cells. A critical question is whether CAR T cells should be used as a definitive therapy or as a bridge to HSCT.146 The nature of the CAR costimulatory domain (41BB v CD28) influences persistence, and this may influence the decision for definitive versus bridge therapy.
CAR T cells directed against CD22 have also been tested,147 generally showing high activity but less persistence than CD19-CARs, and there is great interest in testing dual targeting of CD19 and CD22, either with bicistronic CAR constructs allowing CAR T cells to recognize both proteins, or with mixtures of individual CD19- and CD22-targeted CARs.148
As there are no cell surface markers distinguishing normal and malignant T cells, the development of CAR T cells against T-ALL has the risk of CAR T-cell fratricide. However, several strategies to overcome this risk have been developed, and the first successful small studies with CAR T cells directed against CD7 have been reported.149,150
Given the impressive activity in r/r ALL, there is great interest in testing CAR T-cell therapy in earlier phases of disease such as high-risk first relapse or very high-risk newly diagnosed ALL.
In conclusion, in the past two decades, the use of MRD significantly improved stratification with subsequent treatment reductions or intensifications that improved outcome. More recently, molecular genetic profiling led to the discovery of many new genetic subclasses, which has increased our understanding of the biological basis of ALL, improved risk classification, and enabled refinement of precision medicine regimens. Very recently, immunotherapeutic approaches, including bispecific antibodies, ADC, and cellular therapies, led to more effective and less-toxic therapies replacing intensive chemotherapy courses and HSCT in relapsed ALL and are now being tested in newly diagnosed patients.
It has taken 50-60 years to increase the cure rate in childhood ALL from 0% to 90% by stepwise improvements in chemotherapy. The developments described herein have been developed over the past 10-15 years and will very likely direct efforts to cure those children with ALL who are not cured today, and improve the quality of life for survivors who should have decades of life ahead. The results discussed here are limited to countries that have next-generation sequencing assays and targeted treatment options at their disposal. Achieving cure that is truly universal will require development of cost-effective mechanisms to make these new technologies and therapies available to children with ALL worldwide.
ACKNOWLEDGMENT
C.G.M. is the recipient of an NCI Outstanding Investigator Award (R35 CA297695) and is the William E. Evans Endowed Chair. S.P.H. is the Jeffrey E. Perelman Distinguished Chair in Pediatrics at the Children's Hospital of Philadelphia.
Rob Pieters
Honoraria: Jazz Pharmaceuticals, Kite, a Gilead company, Novartis, Servier/Pfizer
Consulting or Advisory Role: Kite, a Gilead company, Jazz Pharmaceuticals, Servier
Travel, Accommodations, Expenses: Kite, a Gilead company, Jazz Pharmaceuticals, Servier
Charles G. Mullighan
Stock and Other Ownership Interests: Amgen
Honoraria: Amgen, Illumina
Consulting or Advisory Role: Illumina, Faze Medicines, Beam Therapeutics
Speakers' Bureau: Amgen, Pfizer
Research Funding: Loxo, Pfizer, AbbVie
Patents, Royalties, Other Intellectual Property: Inventor on a pending patent application related to gene-expression signatures for detection of underlying Philadelphia chromosome-like events and therapeutic targeting in leukemia (PCT/US2012/069228), WO 2021/022076 A1. This patent highlight shows representative PROTAC compounds bound to JAK2, where ruxolitinib and baricitinib bind to the human JAK2 JH1. Furthermore, representative data illustrate protein degradation, cytotoxicity, and effect of the JAKSTAT signaling pathway of the PROTAC compounds in MHHCALL-4 cells, Marcus Fisher, Fatemeh Keramatnia, Kevin Mcgowan, Jaeki Min, Gisele A. Nishiguchi, Jeanine Price, Zoran Rankovic, Das Sourav, Charles G. Mullighan, Yunchao Chang 2021 Substituted N-(2-(2,6-DIOXOPIPERIDIN-3-YL)-1,3-DIOXOISOINDOLIN-5-YL)Arylsulfonamide Analogs as Modulators of Cereblon Protein, Application No: PCT/US2021/051648 Filed: September 23, 2021. Patent pending (Inst)
Travel, Accommodations, Expenses: Amgen, Illumina
Stephen P. Hunger
Stock and Other Ownership Interests: Amgen, Merck
Honoraria: Jazz Pharmaceuticals, Servier/Pfizer
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Charles G. Mullighan
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Advancing Diagnostics and Therapy to Reach Universal Cure in Childhood ALL
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Rob Pieters
Honoraria: Jazz Pharmaceuticals, Kite, a Gilead company, Novartis, Servier/Pfizer
Consulting or Advisory Role: Kite, a Gilead company, Jazz Pharmaceuticals, Servier
Travel, Accommodations, Expenses: Kite, a Gilead company, Jazz Pharmaceuticals, Servier
Charles G. Mullighan
Stock and Other Ownership Interests: Amgen
Honoraria: Amgen, Illumina
Consulting or Advisory Role: Illumina, Faze Medicines, Beam Therapeutics
Speakers' Bureau: Amgen, Pfizer
Research Funding: Loxo, Pfizer, AbbVie
Patents, Royalties, Other Intellectual Property: Inventor on a pending patent application related to gene-expression signatures for detection of underlying Philadelphia chromosome-like events and therapeutic targeting in leukemia (PCT/US2012/069228), WO 2021/022076 A1. This patent highlight shows representative PROTAC compounds bound to JAK2, where ruxolitinib and baricitinib bind to the human JAK2 JH1. Furthermore, representative data illustrate protein degradation, cytotoxicity, and effect of the JAKSTAT signaling pathway of the PROTAC compounds in MHHCALL-4 cells, Marcus Fisher, Fatemeh Keramatnia, Kevin Mcgowan, Jaeki Min, Gisele A. Nishiguchi, Jeanine Price, Zoran Rankovic, Das Sourav, Charles G. Mullighan, Yunchao Chang 2021 Substituted N-(2-(2,6-DIOXOPIPERIDIN-3-YL)-1,3-DIOXOISOINDOLIN-5-YL)Arylsulfonamide Analogs as Modulators of Cereblon Protein, Application No: PCT/US2021/051648 Filed: September 23, 2021. Patent pending (Inst)
Travel, Accommodations, Expenses: Amgen, Illumina
Stephen P. Hunger
Stock and Other Ownership Interests: Amgen, Merck
Honoraria: Jazz Pharmaceuticals, Servier/Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hunger SP, Lu X, Devidas M, et al. : Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the Children's Oncology Group. J Clin Oncol 30:1663-1669, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeha S, Pei D, Choi J, et al. : Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol 37:3377-3391, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moorman AV, Antony G, Wade R, et al. : Time to cure for childhood and young adult acute lymphoblastic leukemia is independent of early risk factors: Long-term follow-up of the UKALL2003 trial. J Clin Oncol 40:4228-4239, 2022 [DOI] [PubMed] [Google Scholar]
- 4.Brady SW, Roberts KG, Gu Z, et al. : The genomic landscape of pediatric acute lymphoblastic leukemia. Nat Genet 54:1376-1389, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vrooman LM, Blonquist TM, Harris MH, et al. : Refining risk classification in childhood B acute lymphoblastic leukemia: Results of DFCI ALL Consortium Protocol 05-001. Blood Adv 2:1449-1458, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raetz E, Lu X, Devidas M, et al. : Continued improvements in overall survival (OS) in children with newly diagnosed acute lymphoblastic leukemia (ALL): A Children's Oncology Group (COG) report. Pediatr Blood Cancer 65:e27057, 2018. 29603868 [Google Scholar]
- 7.Toft N, Birgens H, Abrahamsson J, et al. : Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia 32:606-615, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Pieters R, de Groot-Kruseman H, Fiocco M, et al. : Improved outcome for ALL by prolonging therapy for IKZF1 deletion and decreasing therapy for other risk groups. J Clin Oncol 41:4130-4142, 2023 [DOI] [PubMed] [Google Scholar]
- 9.Schramm F, Zur Stadt U, Zimmermann M, et al. : Results of CoALL 07-03 study childhood ALL based on combined risk assessment by in vivo and in vitro pharmacosensitivity. Blood Adv 3:3688-3699, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teuffel O, Kuster SP, Hunger SP, et al. : Dexamethasone versus prednisone for induction therapy in childhood acute lymphoblastic leukemia: A systematic review and meta-analysis. Leukemia 25:1232-1238, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Guolla L, Breitbart S, Foroutan F, et al. : Impact of vincristine-steroid pulses during maintenance for B-cell pediatric ALL: A systematic review and meta-analysis. Blood 141:2944-2954, 2023 [DOI] [PubMed] [Google Scholar]
- 12.Larsen EC, Devidas M, Chen S, et al. : Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: A report from Children's Oncology Group Study AALL0232. J Clin Oncol 34:2380-2388, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunsmore KP, Winter SS, Devidas M, et al. : Children's Oncology Group AALL0434: A phase III randomized clinical trial testing nelarabine in newly diagnosed T-cell acute lymphoblastic leukemia. J Clin Oncol 38:3282-3293, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brigitha LJ, Fiocco M, Pieters R, et al. : Hypersensitivity to Pegylated E.coli asparaginase as first-line treatment in contemporary paediatric acute lymphoblastic leukaemia protocols: A meta-analysis of the Ponte di Legno Toxicity working group. Eur J Cancer 162:65-75, 2022 [DOI] [PubMed] [Google Scholar]
- 15.Gottschalk Hojfeldt S, Grell K, Abrahamsson J, et al. : Relapse risk following truncation of pegylated asparaginase in childhood acute lymphoblastic leukemia. Blood 137:2373-2382, 2021 [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Wang C, Raetz EA, et al. : Impact of asparaginase discontinuation on outcome in childhood acute lymphoblastic leukemia: A report from the Children's Oncology Group. J Clin Oncol 38:1897-1905, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloos RQH, Pieters R, Jumelet FMV, et al. : Individualized asparaginase dosing in childhood acute lymphoblastic leukemia. J Clin Oncol 38:715-724, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Tong WH, Pieters R, Kaspers GJ, et al. : A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood 123:2026-2033, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brigitha LJ, Pieters R, van der Sluis IM: How much asparaginase is needed for optimal outcome in childhood acute lymphoblastic leukaemia? A systematic review. Eur J Cancer 157:238-249, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Teachey DT, Hunger SP, Loh ML: Optimizing therapy in the modern age: Differences in length of maintenance therapy in acute lymphoblastic leukemia. Blood 137:168-177, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato M, Ishimaru S, Seki M, et al. : Long-term outcome of 6-month maintenance chemotherapy for acute lymphoblastic leukemia in children. Leukemia 31:580-584, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Moriyama T, Yang W, Smith C, et al. : Comprehensive characterization of pharmacogenetic variants in TPMT and NUDT15 in children with acute lymphoblastic leukemia. Pharmacogenet Genomics 32:60-66, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards S, Pui CH, Gayon P, et al. : Systematic review and meta-analysis of randomized trials of central nervous system directed therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 60:185-195, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salzer WL, Burke MJ, Devidas M, et al. : Impact of intrathecal triple therapy versus intrathecal methotrexate on disease-free survival for high-risk B-lymphoblastic leukemia: Children's Oncology Group Study AALL1131. J Clin Oncol 38:2628-2638, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matloub Y, Lindemulder S, Gaynon PS, et al. : Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with intrathecal methotrexate: Results of the Children's Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children's Oncology Group. Blood 108:1165-1173, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vora A, Andreano A, Pui CH, et al. : Influence of cranial radiotherapy on outcome in children with acute lymphoblastic leukemia treated with contemporary therapy. J Clin Oncol 34:919-926, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gossai N, Devidas M, Chen Z, et al. : Central nervous system status is prognostic in T-cell acute lymphoblastic leukemia: A Children's Oncology Group report. Blood 141:1802-1811, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Haas V, Pieters R, van der Sluijs-Gelling AJ, et al. : Flowcytometric evaluation of cerebrospinal fluid in childhood ALL identifies CNS involvement better then conventional cytomorphology. Leukemia 35:1773-1776, 2021 [DOI] [PubMed] [Google Scholar]
- 29.Schrappe M, Hunger SP, Pui CH, et al. : Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med 366:1371-1381, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mann G, Attarbaschi A, Schrappe M, et al. : Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukaemia: Results from the Interfant-99 Study. Blood 116:2644-2650, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Attarbaschi A, Moricke A, Harrison CJ, et al. : Outcomes of childhood noninfant acute lymphoblastic leukemia with 11q23/KMT2A rearrangements in a modern therapy era: A retrospective international study. J Clin Oncol 41:1404-1422, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNeer JL, Devidas M, Dai Y, et al. : Hematopoietic stem-cell transplantation does not improve the poor outcome of children with hypodiploid acute lymphoblastic leukemia: A report from Children's Oncology Group. J Clin Oncol 37:780-789, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pui CH, Rebora P, Schrappe M, et al. : Outcome of children with hypodiploid acute lymphoblastic leukemia: A retrospective multinational study. J Clin Oncol 37:770-779, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrappe M: Detection and management of minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program 2014:244-249, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Svaton M, Skotnicova A, Reznickova L, et al. : NGS better discriminates true MRD positivity for the risk stratification of childhood ALL treated on an MRD-based protocol. Blood 141:529-533, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood B, Wu D, Crossley B, et al. : Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood 131:1350-1359, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borowitz MJ, Wood BL, Devidas M, et al. : Prognostic significance of minimal residual disease in high risk B-ALL: A report from Children's Oncology Group study AALL0232. Blood 126:964-971, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enshaei A, O'Connor D, Bartram J, et al. : A validated novel continuous prognostic index to deliver stratified medicine in pediatric acute lymphoblastic leukemia. Blood 135:1438-1446, 2020 [DOI] [PubMed] [Google Scholar]
- 39.Pieters R, de Groot-Kruseman H, Van der Velden V, et al. : Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: Study ALL10 from the Dutch Childhood Oncology Group. J Clin Oncol 34:2591-2601, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Vora A, Goulden N, Wade R, et al. : Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): A randomised controlled trial. Lancet Oncol 14:199-209, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Ariffin H, Chiew EKH, Oh BLZ, et al. : Anthracycline-free protocol for favorable-risk childhood ALL: A noninferiority comparison between Malaysia-Singapore ALL 2003 and ALL 2010 studies. J Clin Oncol 41:3642-3651, 2023 [DOI] [PubMed] [Google Scholar]
- 42.Schrappe M, Bleckmann K, Zimmermann M, et al. : Reduced-intensity delayed intensification in standard-risk pediatric acute lymphoblastic leukemia defined by undetectable minimal residual disease: Results of an international randomized trial (AIEOP-BFM ALL 2000). J Clin Oncol 36:244-253, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Schore R, Angiolillo A, Kairalla JA, et al. : Outstanding outcomes with two low intensity regimens in children wth low-risk B-ALL: A report from COG AALL0932. Leukemia 37:1375-1378, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolthers BO, Frandsen TL, Baruchel A, et al. : Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: An observational Ponte di Legno Toxicity Working Group study. Lancet Oncol 18:1238-1248, 2017 [DOI] [PubMed] [Google Scholar]
- 45.te Winkel ML, Pieters R, Hop WC, et al. : Prospective study on incidence, risk factors, and long-term outcome of osteonecrosis in pediatric acute lymphoblastic leukemia. J Clin Oncol 29:4143-4150, 2011 [DOI] [PubMed] [Google Scholar]
- 46.van Kalsbeek RJ, Hudson MM, Mulder RL, et al. : A core outcome set to measure quality of survival for childhood cancer—A joint international consensus statement from the International Childhood Cancer Outcome Project. Nat Med 29:1340-1348, 2023 [DOI] [PubMed] [Google Scholar]
- 47.Andres-Jensen L, Attarbaschi A, Bardi E, et al. : Severe toxicity free survival: Physician-derived definitions of unacceptable long-term toxicities following acute lymphocytic leukaemia. Lancet Haematol 8:e513-e523, 2021 [DOI] [PubMed] [Google Scholar]
- 48.Brown AL, de Smith AJ, Gant VU, et al. : Inherited genetic susceptibility to acute lymphoblastic leukemia in Down syndrome. Blood 134:1227-1237, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gocho Y, Yang JJ: Genetic defects in hematopoietic transcription factors and predisposition to acute lymphoblastic leukemia. Blood 134:793-797, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang H, Zhang H, Luan Y, et al. : Noncoding genetic variation in GATA3 increases acute lymphoblastic leukemia risk through local and global changes in chromatin conformation. Nat Genet 54:170-179, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holmfeldt L, Wei L, Diaz-Flores E, et al. : The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet 45:242-252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moriyama T, Metzger ML, Wu G, et al. : Germline genetic variation in ETV6 and risk of childhood acute lymphoblastic leukaemia: A systematic genetic study. Lancet Oncol 16:1659-1666, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah S, Schrader KA, Waanders E, et al. : A recurrent germline PAX5 mutation confers susceptibility to pre-B cell acute lymphoblastic leukemia. Nat Genet 45:1226-1231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Churchman ML, Qian M, Te Kronnie G, et al. : Germline genetic IKZF1 variation and predisposition to childhood acute lymphoblastic leukemia. Cancer Cell 33:937-948.e8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gu Z, Churchman ML, Roberts KG, et al. : PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat Genet 51:296-307, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paulsson K, Johansson B: High hyperdiploid childhood acute lymphoblastic leukemia. Genes Chromosomes Cancer 48:637-660, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Harrison CJ, Moorman AV, Broadfield ZJ, et al. : Three distinct subgroups of hypodiploidy in acute lymphoblastic leukaemia. Br J Haematol 125:552-559, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Schwab C, Ryan S, et al. : Constitutional and somatic rearrangement of chromosome 21 in acute lymphoblastic leukaemia. Nature 508:98-102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moorman AV, Robinson H, Schwab C, et al. : Risk-directed treatment intensification significantly reduces the risk of relapse among children and adolescents with acute lymphoblastic leukemia and intrachromosomal amplification of chromosome 21: A comparison of the MRC ALL97/99 and UKALL2003 trials. J Clin Oncol 31:3389-3396, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Iacobucci I, Kimura S, Mullighan CG: Biologic and therapeutic implications of genomic alterations in acute lymphoblastic leukemia. J Clin Med 10:31-54, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu Z, Churchman M, Roberts K, et al. : Genomic analyses identify recurrent MEF2D fusions in acute lymphoblastic leukaemia. Nat Commun 7:13331, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohki K, Butler ER, Kiyokawa N, et al. : Clinical characteristics and outcomes of B-cell precursor ALL with MEF2D rearrangements: A retrospective study by the Ponte di Legno Childhood ALL Working Group. Leukemia 37:212-216, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boer JM, Valsecchi MG, Hormann FM, et al. : Favorable outcome of NUTM1-rearranged infant and pediatric B cell precursor acute lymphoblastic leukemia in a collaborative international study. Leukemia 35:2978-2982, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirabayashi S, Ohki K, Nakabayashi K, et al. : ZNF384-related fusion genes define a subgroup of childhood B-cell precursor acute lymphoblastic leukemia with a characteristic immunotype. Haematologica 102:118-129, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dickerson KM, Qu C, Gao Q, et al. : ZNF384 fusion oncoproteins drive lineage aberrancy in acute leukemia. Blood Cancer Discov 3:240-263, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li JF, Dai YT, Lilljebjorn H, et al. : Transcriptional landscape of B cell precursor acute lymphoblastic leukemia based on an international study of 1,223 cases. Proc Natl Acad Sci U S A 115:E11711-E11720, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lilljebjorn H, Henningsson R, Hyrenius-Wittsten A, et al. : Identification of ETV6-RUNX1-like and DUX4-rearranged subtypes in paediatric B-cell precursor acute lymphoblastic leukaemia. Nat Commun 7:11790, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang J, McCastlain K, Yoshihara H, et al. : Deregulation of DUX4 and ERG in acute lymphoblastic leukemia. Nat Genet 48:1481-1489, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwab C, Cranston RE, Ryan SL, et al. : Integrative genomic analysis of childhood acute lymphoblastic leukaemia lacking a genetic biomarker in the UKALL2003 clinical trial. Leukemia 37:529-538, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimura S, Montefiori L, Iacobucci I, et al. : Enhancer retargeting of CDX2 and UBTF::ATXN7L3 define a subtype of high-risk B-progenitor acute lymphoblastic leukemia. Blood 139:3519-3531, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. : A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: A genome-wide classification study. Lancet Oncol 10:125-134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mullighan CG, Su X, Zhang J, et al. : Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med 360:470-480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roberts KG, Li Y, Payne-Turner D, et al. : Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med 371:1005-1015, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee SHR, Yang W, Gocho Y, et al. : Pharmacotypes across the genomic landscape of pediatric acute lymphoblastic leukemia and impact on treatment response. Nat Med 29:170-179, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeha S, Choi J, Roberts KG, et al. : Clinical significance of novel subtypes of acute lymphoblastic leukemia in the context of minimal residual disease-directed therapy. Blood Cancer Discov 2:326-337, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Easton J, Shao Y, et al. : The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet 49:1211-1218, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pölönen P, Elsayed A, Di Giacomo D, et al. : Comprehensive genome characterization of childhood T-ALL links oncogene activation mechanism and subtypes to prognosis. Blood 140:1727-1729, 2022 [Google Scholar]
- 78.Alexander TB, Gu Z, Iacobucci I, et al. : The genetic basis and cell of origin of mixed phenotype acute leukaemia. Nature 562:373-379, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Montefiori LE, Bendig S, Gu Z, et al. : Enhancer hijacking drives oncogenic BCL11B expression in lineage-ambiguous stem cell leukemia. Cancer Discov 11:2846-2867, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Giacomo D, La Starza R, Gorello P, et al. : 14q32 rearrangements deregulating BCL11B mark a distinct subgroup of T-lymphoid and myeloid immature acute leukemia. Blood 138:773-784, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Biondi A, Schrappe M, De Lorenzo P, et al. : Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): A randomised, open-label, intergroup study. Lancet Oncol 13:936-945, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schultz KR, Carroll A, Heerema NA, et al. : Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children's Oncology Group study AALL0031. Leukemia 28:1467-1471, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biondi A, Gandemer V, De Lorenzo P, et al. : Imatinib treatment of paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (EsPhALL2010): A prospective, intergroup, open-label, single-arm clinical trial. Lancet Haematol 5:e641-e652, 2018 [DOI] [PubMed] [Google Scholar]
- 84.Slayton WB, Schultz KR, Kairalla JA, et al. : Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with Philadelphia chromosome-positive acute lymphoblastic leukemia: Results of Children's Oncology Group trial AALL0622. J Clin Oncol 36:2306-2314, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hunger S, Tran TH, saha V, et al. : Dasatinib with intensive chemotherapy in de novo pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: A report from the single-arm, open-label, phase 2 CA180-372/AALL1122 study. Lancet Haematol 10:e510-e520, 2023 [DOI] [PubMed] [Google Scholar]
- 86.Zwaan CM, Rizzari C, Mechinaud F, et al. : Dasatinib in children and adolescents with relapsed or refractory leukemia: Results of the CA180-018 phase I dose-escalation study of the Innovative Therapies for Children with Cancer Consortium. J Clin Oncol 31:2460-2468, 2013 [DOI] [PubMed] [Google Scholar]
- 87.Aplenc R, Blaney SM, Strauss LC, et al. : Pediatric phase I trial and pharmacokinetic study of dasatinib: A report from the Children's Oncology Group phase I consortium. J Clin Oncol 29:839-844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen S, Chen X, Cai J, et al. : Effect of dasatinib vs imatinib in the treatment of pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: A randomized clinical trial. JAMA Oncol 6:358-366, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jabbour E, Short NJ, Jain N, et al. : Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: A US, single-centre, single-arm, phase 2 trial. Lancet Haematol 10:e24-e34, 2023 [DOI] [PubMed] [Google Scholar]
- 90.den Boer ML, Cario G, Moorman AV, et al. : Outcomes of paediatric patients with B-cell acute lymphocytic leukaemia with ABL-class fusion in the pre-tyrosine-kinase inhibitor era: A multicentre, retrospective, cohort study. Lancet Haematol 8:e55-e66, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mullighan CG, Miller CB, Radtke I, et al. : BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature 453:110-114, 2008 [DOI] [PubMed] [Google Scholar]
- 92.Churchman ML, Low J, Qu C, et al. : Efficacy of retinoids in IKZF1-mutated BCR-ABL1 acute lymphoblastic leukemia. Cancer Cell 28:343-356, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Churchman ML, Jones L, Evans K, et al. : Efficacy of focal adhesion kinase inhibition in combination with dasatinib in BCR-ABL1 acute lymphoblastic leukemia. Blood 126:3766, 2015 [Google Scholar]
- 94.Boer JM, Steeghs EM, Marchante JR, et al. : Tyrosine kinase fusion genes in pediatric BCR-ABL1-like acute lymphoblastic leukemia. Oncotarget 8:4618-4628, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moorman AV, Schwab C, Winterman E, et al. : Adjuvant tyrosine kinase inhibitor therapy improves outcome for children and adolescents with acute lymphoblastic leukaemia who have an ABL-class fusion. Br J Haematol 191:844-851, 2020 [DOI] [PubMed] [Google Scholar]
- 96.Nardi V, Ku N, Frigault MJ, et al. : Clinical response to larotrectinib in adult Philadelphia chromosome-like ALL with cryptic ETV6-NTRK3 rearrangement. Blood Adv 4:106-111, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tasian SK, Doral MY, Borowitz MJ, et al. : Aberrant STAT5 and PI3K/mTOR pathway signaling occurs in human CRLF2-rearranged B-precursor acute lymphoblastic leukemia. Blood 120:833-842, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pieters R, De Lorenzo P, Ancliffe P, et al. : Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the Interfant-06 protocol: Results from an international phase III randomized study. J Clin Oncol 37:2246-2256, 2019 [DOI] [PubMed] [Google Scholar]
- 99.Tomizawa D, Miyamura T, Imamura T, et al. : A risk-stratified therapy for infants with acute lymphoblastic leukemia: A report from the JPLSG MLL-10 trial. Blood 136:1813-1823, 2020 [DOI] [PubMed] [Google Scholar]
- 100.Dreyer ZE, Dinndorf PA, Camitta B, et al. : Analysis of the role of hematopoietic stem-cell transplantation in infants with acute lymphoblastic leukemia in first remission and MLL gene rearrangements: A report from the Children's Oncology Group. J Clin Oncol 29:214-222, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stutterheim J, van der Sluis IM, de Lorenzo P, et al. : Clinical implications of minimal residual disease detection in infants with KMT2A-rearranged acute lymphoblastic leukemia treated on the Interfant-06 protocol. J Clin Oncol 39:652-662, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zwaan CM, Soderhall S, Brethon B, et al. : A phase 1/2, open-label, dose-escalation study of midostaurin in children with relapsed or refractory acute leukaemia. Br J Haematol 185:623-627, 2019 [DOI] [PubMed] [Google Scholar]
- 103.Brown PA, Kairalla JA, Hilden JM, et al. : FLT3 inhibitor lestaurtinib plus chemotherapy for newly diagnosed KMT2A-rearranged infant acute lymphoblastic leukemia: Children's Oncology Group trial AALL0631. Leukemia 35:1279-1290, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stumpel DJ, Schneider P, van Roon EH, et al. : Specific promoter methylation identifies different subgroups of MLL-rearranged infant acute lymphoblastic leukemia, influences clinical outcome, and provides therapeutic options. Blood 114:5490-5498, 2009 [DOI] [PubMed] [Google Scholar]
- 105.Garrido Castro P, van Roon EHJ, Pinhancos SS, et al. : The HDAC inhibitor panobinostat (LBH589) exerts in vivo anti-leukaemic activity against MLL-rearranged acute lymphoblastic leukaemia and involves the RNF20/RNF40/WAC-H2B ubiquitination axis. Leukemia 32:323-331, 2018 [DOI] [PubMed] [Google Scholar]
- 106.Guest E, Kairalla J, Devidas M, et al. : A pilot study of azatidine as epigenetic priming for chemotherapy in infants less than 1 year of age with KMT2A-rearranged acute lymphoblastic leukemia (ALL): Results from the Children's Oncology Group (COG) trial AALL15P1. Blood 140:3256-3257, 2022 [Google Scholar]
- 107.Issa GC, Aldoss I, DiPersio J, et al. : The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature 615:920-924, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frismantas V, Dobay MP, Rinaldi A, et al. : Ex vivo drug response profiling detects recurrent sensitivity patterns in drug-resistant acute lymphoblastic leukemia. Blood 129:e26-e37, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fischer U, Forster M, Rinaldi A, et al. : Genomics and drug profiling of fatal TCF3-HLF-positive acute lymphoblastic leukemia identifies recurrent mutation patterns and therapeutic options. Nat Genet 47:1020-1029, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cordo V, van der Zwet JCG, Cante-Barrett K, et al. : T-cell acute lymphoblastic leukemia: A roadmap to targeted therapies. Blood Cancer Discov 2:19-31, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kadia TM, Gandhi V: Nelarabine in the treatment of pediatric and adult patients with T-cell acute lymphoblastic leukemia and lymphoma. Expert Rev Hematol 10:1-8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zwaan CM, Kowalczyk J, Schmitt C, et al. : Safety and efficacy of nelarabine in children and young adults with relapsed or refractory T-lineage acute lymphoblastic leukaemia or T-lineage lymphoblastic lymphoma: Results of a phase 4 study. Br J Haematol 179:284-293, 2017 [DOI] [PubMed] [Google Scholar]
- 113.Hayashi RJ, Winter SS, Dunsmore KP, et al. : Successful outcomes of newly diagnosed T lymphoblastic lymphoma: Results from Children's Oncology Group AALL0434. J Clin Oncol 38:3062-3070, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Teachey DT, Devidas M, Wood BL, et al. : Children's Oncology Group trial AALL1231: A phase III clinical trial testing bortezomib in newly diagnosed T-cell acute lymphoblastic leukemia and lymphoma. J Clin Oncol 40:2106-2118, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chonghaile TN, Roderick JE, Glenfield C, et al. : Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov 4:1074-1087, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pullarkat VA, Lacayo NJ, Jabbour E, et al. : Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov 11:1440-1453, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gocho Y, Liu J, Hu J, et al. : Network-based systems pharmacology reveals heterogeneity in LCK and BCL2 signaling and therapeutic sensitivity of T-cell acute lymphoblastic leukemia. Nat Cancer 2:284-299, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brivio E, Baruchel A, Beishuizen A, et al. : Targeted inhibitors and antibody immunotherapies: Novel therapies for paediatric leukaemia and lymphoma. Eur J Cancer 164:1-17, 2022 [DOI] [PubMed] [Google Scholar]
- 119.von Stackelberg A, Locatelli F, Zugmaier G, et al. : Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 34:4381-4389, 2016 [DOI] [PubMed] [Google Scholar]
- 120.Locatelli F, Zugmaier G, Rizzari C, et al. : Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA 325:843-854, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brown PA, Ji L, Xu X, et al. : Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA 325:833-842, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hogan LE, Brown PA, Ji L, et al. : Children's Oncology Group trial AALL1331: Phase 3 trial of blinatumomab in children, adolescents and young adults with low -risk B-cell ALL in first relapse. J Clin Oncol 41:4118-4129, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van der Sluis IM, de Lorenzo P, Kotecha RS: Blinatumomab added to upfront chemotherapy in infant lymphoblastic leukemia. N Engl J Med 388:1572-1581, 2023 [DOI] [PubMed] [Google Scholar]
- 124.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. : Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med 375:740-753, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brivio E, Locatelli F, Lopez-Yurda M, et al. : A phase 1 study of inotuzumab ozogamicin in pediatric relapsed/refractory acute lymphoblastic leukemia (ITCC-059 study). Blood 137:1582-1590, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pennesi E, Michels N, Brivio E, et al. : Inotuzumab ozogamicin as single agent in pediatric patients with relapsed and refractory acute lymphoblastic leukemia: Results from a phase II trial. Leukemia 36:1516-1524, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.O'Brien MM, Ji L, Shah NN, et al. : Phase II trial of inotuzumab ozogamicin in children and adolescents with relapsed or refractory B-cell acute lymphoblastic leukemia: Children's Oncology Group Protocol AALL1621. J Clin Oncol 40:956-967, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bride KL, Vincent TL, Im SY, et al. : Preclinical efficacy of daratumumab in T-cell acute lymphoblastic leukemia. Blood 131:995-999, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cerrano M, Bonifacio M, Olivi M, et al. : Daratumumab with or without chemotherapy in relapsed and refractory acute lymphoblastic leukemia. A retrospective observational Campus ALL study. Haematologica 107:996-999, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.June CH, Sadelain M: Chimeric antigen receptor therapy. N Engl J Med 379:64-73, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grupp SA, Kalos M, Barrett D, et al. : Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368:1509-1518, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maude SL, Laetsch TW, Buechner J, et al. : Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439-448, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maude SL, Frey N, Shaw PA, et al. : Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507-1517, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Laetsch TW, Maude SL, Rives S, et al. : Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA trial. J Clin Oncol 41:1664-1669, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kadauke S, Myers RM, Li Y, et al. : Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: A prospective clinical trial. J Clin Oncol 39:920-930, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pasquini MC, Hu ZH, Curran K, et al. : Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv 4:5414-5424, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jacoby E, Ghorashian S, Vormoor B, et al. : CD19 CAR T-cells for pediatric relapsed acute lymphoblastic leukemia with active CNS involvement: A retrospective international study. Leukemia 36:1525-1532, 2022 [DOI] [PubMed] [Google Scholar]
- 138.Leahy AB, Newman H, Li Y, et al. : CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: A post-hoc analysis of pooled data from five clinical trials. Lancet Haematol 8:e711-e722, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holland EM, Yates B, Ling A, et al. : Characterization of extramedullary disease in B-ALL and response to CAR T-cell therapy. Blood Adv 6:2167-2182, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moskop A, Pommert L, Baggott C, et al. : Real-world use of tisagenlecleucel in infant acute lymphoblastic leukemia. Blood Adv 6:4251-4255, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ghorashian S, Jacoby E, De Moerloose B, et al. : Tisagenlecleucel therapy for relapsed or refractory B-cell acute lymphoblastic leukaemia in infants and children younger than 3 years of age at screening: An international, multicentre, retrospective cohort study. Lancet Haematol 9:e766-e775, 2022 [DOI] [PubMed] [Google Scholar]
- 142.Lamble AJ, Myers RM, Taraseviciute A, et al. : Preinfusion factors impacting relapse immunophenotype following CD19 CAR T cells. Blood Adv 7:575-585, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dekker L, Calkoen FG, Jiang Y, et al. : Fludarabine exposure predicts outcome after CD19 CAR T-cell therapy in children and young adults with acute leukemia. Blood Adv 6:1969-1976, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schultz LM, Baggott C, Prabhu S, et al. : Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: A pediatric real-world chimeric antigen receptor consortium report. J Clin Oncol 40:945-955, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Myers RM, Shah NN, Pulsipher MA: How I use risk factors for success or failure of CD19 CAR T cells to guide management of children and AYA with B-cell ALL. Blood 141:1251-1264, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Buechner J, Caruana I, Kunkele A, et al. : Chimeric antigen receptor T-cell therapy in paediatric B-cell precursor acute lymphoblastic leukaemia: Curative treatment option or bridge to transplant? Front Pediatr 9:784024, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fry TJ, Shah NN, Orentas RJ, et al. : CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 24:20-28, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cordoba S, Onuoha S, Thomas S, et al. : CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: A phase 1 trial. Nat Med 27:1797-1805, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pan J, Tan Y, Wang G, et al. : Donor-derived CD7 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia: First-in-human, phase I trial. J Clin Oncol 39:3340-3351, 2021 [DOI] [PubMed] [Google Scholar]
- 150.Zhang M, Chen D, Fu X, et al. : Autologous nanobody-derived fratricide-resistant CD7-CAR T-cell therapy for patients with relapsed and refractory T-cell acute lymphoblastic leukemia/lymphoma. Clin Cancer Res 28:2830-2843, 2022 [DOI] [PubMed] [Google Scholar]
- 151.Möricke A, Zimmermann M, Valsecchi MG, et al. : Dexamethasone vs prednisone in induction treatment of pediatric ALL: Results of the randomized trial AIEOP-BFM ALL 2000. Blood 127:2101-2112, 2016 [DOI] [PubMed] [Google Scholar]