Abstract
Traumatic brain injury (TBI) caused by acoustic blast overpressure (ABO) is frequently associated with chronic visual deficits in military personnel and civilians. In this study, we characterized retinal gliotic response in adult male rats following a single ABO exposure directed to one side of the head. Expression of gliosis markers and intermediate filaments was assessed at 48 h and 1 wk post-ABO exposure, in comparison to age-matched non-exposed control retina. In response to a single ABO exposure, type III IF, glial fibrillary acidic protein (GFAP) was variably induced in a subpopulation of retinal Müller glia in ipsilateral eyes. ABO-exposed eyes exhibited radial Müller glial GFAP filament extension through the inner plexiform layer (IPL) and the inner nuclear layer (INL) through the retina in both the nasal quadrant and juxta-optic nerve head (jONH) eye regions at 1 wk post-ABO. We observed an ~6-fold increase (p ≤ 0.05) in radial glial GFAP immunolabeling in the IPL in both eye regions, in comparison to regionally matched controls. Similarly, GFAP extension through the INL into the outer retina was elevated ~3-fold, p ≤ 0.05 in the nasal retina, but exhibited wider variability in the jONH retina. In contrast, constitutive type III IF vimentin exhibited greater remodeling in retinal Müller glia through the jONH retina compared to the nasal retina in response to ABO. We observed areas of lateral vimentin remodeling through the Müller glial end-feet, and greater mid-outer retinal radial vimentin IF extension in a subpopulation of glia at 1 wk post-ABO. We also observed a significant increase in total retinal levels of the type III IF desmin in ABO-exposed retina vs. controls (~1.6-fold, p ≤ 0.01). In addition, ABO-exposure elicited varied glial induction of developmentally regulated type VI family IFs (nestin and synemin) in subpopulations of Müller cells at 48 h and 1 wk post-ABO. We demonstrate that multiple glial phenotypes emerge in the rat retina following a single ABO exposure, rather than a global homogeneous retinal glial response, involving less well characterized IF protein forms which warrant further investigation in the context of ABO-induced retinal gliosis.
1. Introduction
Exposure of military service personnel and civilians to powerful acoustic blast overpressure (ABO) shock waves arising from improvised explosive devices and ever-advancing weaponry has increased dramatically in modern conflicts (Bochicchio et al., 2008; Owens et al., 2008; Ritenour and Baskin, 2008). Consequently, blast-associated traumatic brain injury (TBI), auditory damage and visual system dysfunction have become highly prevalent war injuries in recent years (Dougherty et al., 2011; Fausti et al., 2009; Rosenfeld et al., 2013). Long-term visual impairment is common in returning war veterans exposed to blasts, presenting with visual disturbances including photophobia, diplopia, visual acuity deficits, reading difficulties, convergence (binocular) and accommodation (focusing) insufficiency (Cockerham et al., 2011; Lemke et al., 2013; Magone et al., 2014; Saunders and Echt, 2012), and visual field deficits (Goodrich et al., 2013; Lemke et al., 2016). While penetrating eye injuries are generally detected on-site, the interplay between TBI-and visual system damage following ABO confounds immediate vision assessment, and closed-globe eye injuries are hard to detect, manifesting gradually, with visual impairment often reported only several weeks, months or even years following blast exposure (Goodrich et al., 2013; Lemke et al., 2016; Magone et al., 2014). With the greater use of explosives in both combat and domestic settings there is a growing need to find prophylactic and post-exposure treatments to limit visual impairment arising from ABO. Unfortunately, the modes and mechanisms of retinal and wider ocular damage are a complex combination of primary (i.e., attributable directly to the blast wave), secondary, (penetrating, from blast-propelled shrapnel), and tertiary (rapid acceleration and sudden impact of the body) effects, which interplay during and following ABO exposure (Greer et al., 2016; Kalayci et al., 2020; Sherwood et al., 2014). In particular, the effects of single or multiple ABO exposure(s) on the integrity and functionality of the neural retina remain poorly understood. Exposure to an ABO shock wave inflicts biological damage via extreme pressurization and expansion as the wave-front moves through tissues, with reflection of compression waves at density boundaries, and inertial effects from differential acceleration of structures, causing asynchronous movement of tissues (Ravin et al., 2012; Ritenour and Baskin, 2008). Furthermore, pressure wave reflection and amplification in in silico models of ABO exposure to the human skull predict resonation and amplification of a blast wave off the eye socket (orbital bone) and within the orbit (Hua et al., 2017; Rossi et al., 2012).
A variety of animal models of ocular ABO exposure have been developed in recent years, most employing modified compressed air-driven shock tube devices, which allow specific parameters to be set, including peak sound pressure and distance from the source to point of contact, and the ability to perform single or multiple blasts (Choi et al., 2015; Mammadova et al., 2017; Thomas et al., 2020; Wang et al., 2014). While animal models vary in configuration, blast intensity and single to multiple exposure paradigms, the emerging findings generally indicate direct damage to the retina arising from ocular ABO exposure—including retinal ganglion cell (RGC) death, retinal inflammation, increases in oxidative stress markers, oxidative protein modification, optic nerve damage, and acute reactive gliosis (Allen et al., 2018; Bernardo-Colon et al., 2019; Mammadova et al., 2017). Reactive gliosis is a universal response to CNS injury, whereby glia undergo hypertrophy with rapid induction of type III family intermediate filaments, typically GFAP and vimentin, and coordinate the release of trophic factors and cytokines providing structural and regenerative support (de Hoz et al., 2016; Lewis and Fisher, 2003). Importantly, glial reactivity is context-specific and heterogeneous—however, prolonged proliferative gliosis, structural hypertrophy and occupation of vacated injury sites by hypertrophying glia often culminates in scarring, inflammation, and further loss of neurons (de Hoz et al., 2016; Pekny and Pekna, 2014, 2016). Beyond GFAP and vimentin, the IF superfamily constitutes six distinct classified groups sharing sequence and structural motifs; an N-terminal head-, central α-helical rod-, and C-terminal tail-domain, represented by over 70 separate gene products in humans, each with unique developmental structural/biophysical roles and properties (Sanghvi-Shah and Weber, 2017), diversity further amplified by splice-forms and combinatorial assembly (Potokar et al., 2020). IF assembly initiates via central rod-domain coiled-coil homo- or heterodimerization followed by lateral, antiparallel tetramerization, assembly into octamers-of-tetramers (unit-length filaments), end-to-end annealing and compaction, their lateral apolar structure conferring varied elastic and tensile properties (for a detailed review, see (Herrmann and Aebi, 2016)). During reactive gliosis, the composition of upregulated IF sub-types in the polymerizing network may be injury-specific, with unique IF cytoskeletal re-modeling and filament bundling properties (Charrier and Janmey, 2016; Lewis and Fisher, 2003).
Retinal gliosis overtly manifests in the large, radial Müller cells, which project processes bidirectionally from INL cell bodies inwards to endfeet at the basal lamina vitreal border at the inner limiting membrane (ILM), and outwards, to apical microvilli at the external limiting membrane (ELM) (Bringmann et al., 2006; Vecino et al., 2016). In quiescent retina, GFAP is largely restricted to the Müller endfeet and astroglia along the ILM; upon retinal injury and with ageing, upregulation and extension of GFAP through the radially extending Müller glial processes produces striking filamentous arrays in gliotic retina (Bignami and Dahl, 1979; Bringmann et al., 2006; Eisenfeld et al., 1984; Reichenbach and Bringmann, 2020; Vecino et al., 2016). Constitutively expressed vimentin is often coordinately regulated alongside GFAP in activated glia undergoing structural hypertrophy (Pekny et al., 1999); in reactive Müller cells, vimentin-driven IF extension into the sub-retinal space can lead to retinal scarring (Fernandez-Sanchez et al., 2015; Lewis and Fisher, 2003). Another type III IF, desmin, is ubiquitous in cardiac, skeletal, and smooth muscle cells, where it serves to maintain the mechanical integrity and tensile strength of muscle fibers (Agnetti et al., 2022; Li et al., 1997; Paulin and Li, 2004) and could impart similar mechanical properties in other cell types and tissues. Desmin has not been characterized extensively in the neural retina, per se, but is known to be constitutively expressed normally through the inner Müller glia and vascular pericytes (Dahl and Bignami, 1982), as well as in retinal pathologies involving vascular elements (e.g., pericyte ensheathment, retinopathy of prematurity) (Chan-Ling et al., 2004; Hughes and Chan-Ling, 2004; Hughes et al., 2006). With relevance to the present study, it has been shown that repetitive primary blast injury induced in rats in a manner similar to that used here, causes disruption of the blood-brain barrier and increase of desmin-positive pericytes (Uzunalli et al., 2021).
Beyond the type III subfamily, the unusually large, developmentally-regulated type VI IF proteins nestin and synemin are recognized glial activation markers in the CNS and retina (Luna et al., 2010; Xue et al., 2010). Characterized by extended C-terminal tails with multi-protein docking sites, and non-consensus head domains that preclude homopolymer assembly (Eliasson et al., 1999; Titeux et al., 2001), synemin and nestin must integrate into IFs via obligatory heterodimer formation with type III vimentin/desmin or with GFAP or as IF-associated proteins (Jing et al., 2007; Khanamiryan et al., 2008; Steinert et al., 1999). Synemin serves as a platform/A-kinase anchoring protein (AKAP) regulating signaling during muscle hypertrophy (Li et al., 2014), bridges IF to myofibrillar z-lines, and connects peripheral myofibrils to costameres at the sarcolemma (Bellin et al., 2001). In cardiomyocytes, specific synemin isoforms stabilize cell-cell junctions (α-synemin) or bridge desmin filaments to the z-lines (β-synemin (Lund et al., 2012)), forming a large cytoskeletal interactome including dystrophin, α-actinin, and vinculin (Bhosle et al., 2006; Mizuno et al., 2001; Sandoval et al., 1983). Nestin and synemin both exhibit developmentally-regulated expression patterns— β-synemin in pluripotent embryonic stem cells, and α-synemin with nestin in neural-progenitor cells (de Souza Martins et al., 2011). Early synemin expression in developing human retina occurs in the nerve fiber layer/ganglion cell layers (NFL/GCL), rises transiently in Müller glial end-feet (colocalizing with vimentin), and later predominates in NFL/GCL (Tawk et al., 2003). Nestin was initially identified as a transient marker in radial glial cells of the embryonic rat CNS (Hockfield and McKay, 1985), and is extensively expressed throughout dividing radial neural processes in developing rat retina, and later restricted to glial end-feet and retinal endothelial/pericyte progenitors (Lee et al., 2012).
In this study, we assessed retinal expression patterns of multiple IF protein subtypes following a single ABO exposure to understand macroglial IF cytoskeletal remodeling in the eye post exposure. We find that, rather than a homogeneous retinal macroglial response, a single ABO exposure elicited multiple cytoskeletal IF induction and remodeling patterns among retinal macroglia. Remarkably, type III IF protein induction and remodeling exhibited high cell-to-cell variability in GFAP induction among Müller cells, and regional variation in glial GFAP assembly through the retina post-ABO. Vimentin cytoskeletal IF hypertrophy occurred in Müller glia close to the ONH-emergence, and the less-well characterized type III IF desmin exhibited elevated total retinal expression post ABO without overt glial cytoskeletal remodeling. We further found that subsets of retinal Müller glia and astrocytes exhibited elevated expression of type VI IFs nestin and synemin, indicating another layer of pleiomorphic retinal glial cytoskeletal IF responses to ABO. Our study reveals remarkably heterogeneous retinal glial responses to a single ABO exposure in a rat TBI model, and demonstrates the participation of less-well characterized IF protein subtypes, sometimes in the absence of archetypal glial GFAP induction, in cytoskeletal remodeling during glial activation and reactivity arising from a single ABO exposure.
2. Materials and methods
2.1. Animals and experimental design
Outbred adult male Long-Evans rats (Blue Spruce, HsdBlu:LE; Envigo or Charles River Laboratories) were employed in this study. Blast exposure was performed on rats of approximately 3.5 mo of age (~400–450 g) at the Buffalo VA Medical Center. Age-matched animals from each cohort were reserved to serve as non-exposed controls. All procedures involving animal experimentation were approved by the VA Western New York Healthcare System (VAWNYHS) Institutional Animal Care and Use Committee (IACUC) and conformed to the standards of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications, 8th Ed. 2011). Housing consisted of shoe-box style transparent plastic cages; water and standard rodent chow (Envigo) were provided ad libitum on a 12:12 light:dark cycle (light onset at 6:00 a.m., off at 6:00 p.m.). For biochemical analysis, tissues were harvested from 48 h to 1 wk post-ABO cohorts. Blast exposed rats were reserved either for whole-eye fixation and embedment (48 h post-ABO (n = 5); 1 wk post-ABO (n = 6)), or for harvesting whole retina lysates (48 h post-ABO (n = 5); 1 wk post-ABO (n = 6)). Non-exposed control rats were likewise reserved (whole-eye fixation, cohort-matched (n = 7), and (n = 6) for 48 h and 1 wk post-ABO, respectively) or for whole retina lysate analysis (total (n = 6), 3 rats each per matched cohort used for Western blotting analyses). Tissue harvesting and fixation was performed as described below (Section 2.3: Indirect Immunofluorescence and Confocal Microscopy), and whole retina lysates were flash-frozen in liquid nitrogen and processed as described below (Section 2.6: SDS-PAGE, Western blotting and densitometric analyses of whole retina lysates).
2.2. Acoustic blast overpressure (ABO) exposure
The procedures employed for ABO exposure were as described in detail previously (see (Allen et al., 2018; Allen et al., 2021)). A schematic of the shock-tube apparatus and animal placement is shown in Supplemental Fig. S1. In brief, closed-globe, single ABO exposure to the ipsilateral eye of anesthetized rats was performed using a modified shock tube device (Allen et al., 2021) and an “open”-configuration holder with the head and neck exposed (holder side view, Supplemental Fig. S1A; housing custom designed and manufactured by Custom Collaborators, Clarence Center, Buffalo, NY). The open-holder allowed largely unconstrained head movement upon blast, albeit with cushioning of the contralateral side of the head, to reduce impact and recoil. The holder was aligned so that the direction of the blast was perpendicular to the body axis, with the head positioned so that the ipsilateral blast-facing (right) eye was 2.5 in. (6.35 cm) from the muzzle of the shock tube, Supplemental Fig. S1B). Acoustic blasts were caused by instantaneous release of compressed nitrogen back-pressure within the shock tube by rupture (with a computer-controlled solenoid-driven arrow), of a pressurized (at 80-psi criterion backpressure) brass foil diaphragm (Precision Brand, brass shim, #17135, 0.001”, Daemar Inc. Lawrenceville, GA), generating sound amplitudes of ~195 dB SPL (~63 kPa [kPa]). ABO’s generated from lower than criterion backpressures occasionally arose as a result of diaphragm slippage, and these individual instances are denoted with stars in Western blotting analyses, with their respective psi-values given (see Section 3.1.).
2.3. Eye orientation and embedding, sectioning and antigen retrieval
Eyes were harvested at 48 h or 1 wk post-ABO exposure alongside age-matched, non-exposed controls. Enucleated eyes were immersion fixed overnight at 4 °C in phosphate buffered saline (PBS) containing a final concentration of 4% formaldehyde (prepared from 16% aqueous formaldehyde solution, #15710, Electron Microscopy Sciences [EMS] Hatfield, PA). Fixed eyes were then cryoprotected in 30% (w:v) sucrose (#57501, Thermo Fisher Scientific) in PBS at 4 °C, and then equilibrated overnight at 4 °C in 2:1 (v:v) mixture of O.C.T. (Optimal Cutting Temperature Compound, Tissue-Tek®, #62550, Electron Microscopy Sciences) and 30% sucrose in PBS. Eyes were oriented along the vertical axis in a cryomold, using the superior pole as a reference (marked at harvesting) and embedded in O.C.T. over dry ice-chilled undenatured ethanol. Cryosections (10 μm thick) were cut along the naso-temporal axis through the nasal hemisphere toward the equatorial meridian, and through the ONH.
Antigen retrieval:
For detection of synemin, vimentin and desmin immunolabeling in PFA-fixed rat retina cryosections, we performed heat-mediated antigen retrieval as follows. In brief, eye cryosections were equilibrated in tris buffered saline (TBS), pH 7.6, for at least 10 min before immersing in low pH IHC antigen retrieval solution (#00-4955-58, Thermo Fisher Scientific) preheated to 80 °C, and subjected to sustained heating for 3 × 5 min microwave cycles on low power, to reach and maintain a temperature range of 85–90 °C for a total of 15 min. The sections were allowed to cool to room temperature before reequilibrating in TBS and commencing with indirect immunofluorescence analysis.
2.4. Indirect immunofluorescence and confocal microscopy
For indirect immunofluorescence (IHC), retinal cryosections were processed and probed as described previously (Ramachandra Rao et al., 2018); primary antibodies were used at the dilutions described in Table 1. The use of mouse-host primary antibodies was avoided where possible to circumvent cross-reactivity with endogenous rat-IgG. For all IHC protocols, incubation in primary antibodies was performed at 4 °C overnight in a humidified chamber.
Table 1.
Antibodies, sources, hosts/clonality, and working dilutions of stocks.
| Antibody/reagent | Clone/Cat. No. | Source | Host/clonality | Dilution used |
|---|---|---|---|---|
| GAPDH | D16H11/5174 | Cell Signaling Technology® | Mouse monoclonal | 1:2000 WBa |
| GFAP | GA5/3670 | Cell Signaling Technology® | Mouse monoclonal | 1:1000 WB 1:200 IHCb |
| GFAP | Z0334 | DAKO | Rabbit polyclonal | 1:500 IHC |
| Desmin | GTX103557 | Genetex | Rabbit polyclonal | 1:200 IHC 1:1000 WB |
| Nestin | AF2736 | R&D | Systems Goat polyclonal | 1:250 IHC |
| Nestin | 401/556309 | BD Pharmingen | Mouse monoclonal | 1:100 IHC 1:1000 WB |
| Synemin | SAB4200138 | Millipore | Sigma Rabbit polyclonal | 1:200 IHC 1:1000 WB |
| Synemin | B-8/sc-376944 | Santa Cruz Biotechnology, Inc. | Mouse monoclonal | 1:200 IHC 1:1000 WB |
| Synemin | A-8/sc-374484 | Santa Cruz Biotechnology, Inc. | Mouse monoclonal | 1:100 IHC 1:1000 WB |
| Vimentin | EPR3776 Ab92547 |
Abcam | Rabbit monoclonal | 1:1000 WB |
| Vimentin | PA5–27231 | Invitrogen | Rabbit polyclonal | 1:200 IHC |
| Glutamine Synthetase | TA301447 | OriGene | Rabbit polyclonal | 1:250 IHC |
WB, Western blotting.
IHC, immunohistochemistry.
In preabsorption experiments, synemin-pSAB primary antibody was incubated in the presence of a 10 M excess of the peptide immunogen (>95% pure, Custom Peptide Synthesis Services, Thermo Fisher Scientific) during incubation. Following standard washes to remove unbound primary, detection was performed by 1 h incubation in the species-appropriate secondary Alexa-Fluor conjugates (anti-whole IgG F(ab’)2 fragments coupled to Alexa-Fluor ® Plus dyes, Thermo Fisher Scientific, 1:500 dilution), and nuclei counterstained with 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/ml). Sections were examined with a Leica TCS SPEII DMI4000 scanning laser confocal fluorescence microscope. Images were captured at the same laser and gain settings within each set of analyses, using the 40X oil immersion (RI-1.518) objective under nominal laser intensity (10–35% of maximum intensity), arbitrary gain and offset values, optimized for signal-to-noise. As previously described, intense radial GFAP expression was typically observed in Müller glia at the far peripheries of the retina in both control and blast exposed eyes, and therefore avoided in all imaging experiments. Notably, a small proportion of cohort-matched control retinas, (~17%), exhibited higher basal GFAP expression through the inner-Müller glial IPL processes (see Supplemental Fig. S2), and therefore we used cohort-matched controls in regional quantification analyses (see below, Section 2.5.).
2.5. Image analysis
Confocal z-stacks were split into their constituent channels and converted into 8-bit greyscale images in ImageJ® (FIJI) (Schindelin et al., 2012). GFAP-channel greyscale images were equally thresholded (70–255), and specific retinal layers were selected across the entire length of each image (~275.3 μm, at 3.75 pixel/micron resolution), using the multi-point tool to trace each region of interest (ROI). Successively outer retinal ROIs were traced contiguously from the inner to outer retinal layers. For the very outer neural retina, ROIs were traced to include the ONL and the photoreceptor inner segments (IS)— to account for hypertrophied gliotic processes exceeding the ELM. The mean % area of each ROI occupied by GFAP signal was calculated by running the [Analyze Particles] macro, with particle size parameters set to 0-infinity μm2 and limited to threshold. The equivalent regional analyses from matched cohort controls were used to normalize each data set and calculate fold-differences in GFAP content between non-exposed and ABO-exposed retinas.
2.6. Immunoprecipitation of synemin from retina lysates
Non-exposed control retina lysates with a total protein content of 500 μg per immunoprecipitation (IP) were pre-cleared with 30 μl (packed volume) of Protein A/G-agarose PLUS (#sc-2003, Santa Cruz Biotechnology, Santa Cruz, CA) pre-equilibrated in RIPA buffer (#9806, Cell Signaling Technology, Danvers, MA) for 30 min, rotating at 4 °C. A/G-agarose was collected by low-speed centrifugation at 100×g for 3 min 4 °C, and the supernatants incubated with freshly equilibrated 30 μl packed volume A/G agarose and 2.5 μg of either normal mouse IgG, synemin A8 monoclonal, or pSAB synemin polyclonal overnight, rotating, at 4 °C. A/G-agarose-IP complexes were collected at 100×g for 3 min 4 °C, and post-IP supernatants were removed and retained for analysis. The A/G-agarose-IP complexes were washed three times with ice-cold 1 ml RIPA buffer before eluting in 50 μl 200 mM glycine, pH 2.2, and neutralizing in Tris-HCl pH 8.8. Eluted proteins were collected using gel-loading tips to exclude A/G agarose and diluted in 2x Laemmli Buffer (LC2676 Novex™, Thermo Fisher) for SDS-PAGE analysis.
2.7. In vitro Calpain-1 digestion of rat retina and desmin-overexpression lysates
Retinal lysates (10 μg total protein per retina) or 200 ng of Desmin-OE lysate in RIPA buffer, omitting protease inhibitor cocktail, were diluted 1:5 (v/v) in calpain digest buffer: Tris-HCl pH 7.5, 150 mM NaCl, 2 mM CaCl2, 2.5 mM β-mercaptoethanol, 0.2% (v/v) Triton X-100 and 0.3% (v/v). Parallel reactions were set up with or without protease inhibitor EGTA (at 2 mM) combined with 2 U of active Calpain-1 (Abcam, #ab91019), and subjected to rapid digest for 1 min or 5 min at 30 °C before quenching in 1:1 v/v 2x Laemmli sample buffer before and boiling for 5 min prior to analysis by SDS-PAGE.
2.8. SDS-PAGE, Western blotting, densitometric analyses of whole retina lysates
Whole, flash-frozen retinas were lysed in RIPA buffer and processed as described previously (Ramachandra Rao et al., 2018). A 1:80 ratio of mammalian protease inhibitor cocktail (P8340, Millipore Sigma, St. Louis, MO) throughout post-lysis processing; we noted that several IF proteins, including GFAP, were highly sensitive to degradation post-lysis. Equal Briefly, protein content was assessed by micro-BCA assay (#23227, Pierce, Thermo Fisher) and equal quantities of retinal lysate protein were resolved by electrophoresis on 10% Mini--PROTEAN® TGX™ Precast Protein Gels, (#4561036, Bio-Rad, Hercules, CA) before Western transfer to LF-PVDF (#1620264, Bio Rad). To test the specificity of B8 mAb for synemin, 2 μg of T-antigen driven full-length human synemin overexpression lysate (HEK293T cells) versus empty vector HEK293 T control lysate (#LS-G79757, LifeSpan Biosciences, Seattle, WA), were used as positive and negative controls, respectively. Desmin pAb was preabsorbed against full-length recombinant human vimentin (NBP2–35139, Novus Biologicals, Centennial, CO) and recombinant GFAP at 10 M and 5 M excess, respectively, for 1 h room temperature prior to probing. Otherwise, primary antibodies were diluted and applied as stated in Table 1. HRP-conjugated goat--anti-rabbit or anti-mouse secondary antibodies were applied as appropriate (#7074P2 and #7076P2, respectively, Cell Signaling Technology, 1:10,000), using luminol-peroxide containing Clarity Max ECL reagent (#1705062, Bio-Rad) for chemiluminescence detection, imaged on a ChemiDoc™ MP System (Bio-Rad). Densitometric analysis and quantification was performed using Image Lab software (Bio-Rad). GAPDH was used as a housekeeping protein for loading normalization.
2.9. Antibody cross-reactivity assessment and protein standards employed
The class III IF subgroup share high sequence homology and we assessed cross-reactivity for the major type III-IF targeting antibodies used to a panel of recombinant type III IF family proteins. Western blot analysis was performed, probing parallel blots of 10 ng human GFAP-GST (N-terminally tagged) fusion protein (H00002670-P01, Novus Biologicals), 10 ng recombinant human vimentin (NBP2–35139, Novus Biologicals) or 10 μg (total protein) of desmin overexpression (OE) lysate (HEK293 T-antigen driven full-length human desmin, NBL1–09840, Novus Biologicals), using both 10 μg matched empty-vector HEK293T lysate and 10 ng protease-free BSA (A3059, Millipore-Sigma, St Louis, MO) as negative controls.
Recombinant proteins were resolved by TGX-PAGE and transferred to PVDF in parallel before probing with type III IF raised antibodies to assess cross-reactivity (Supplemental Fig. S3A). GFAP mAb GA5 was the most monospecific antibody, recognizing exclusively recombinant GST-tagged GFAP ~75 kDa, and smeared degradation products. Anti-vimentin rabbit mAb EPR 3776 cross-reacted lightly with desmin overexpression lysate which migrated as a range of products between ~30 and ~55 kDa—while anti-desmin rabbit pAb GTX103557 (Genetex, Irvine, CA, USA, see antibody Table 1.) also cross-reacted weakly with recombinantly expressed GST-GFAP and vimentin. The widely used GFAP polyclonal, pAb z0334 (DAKO, Agilent Boulder, CO, USA, see antibody Table 1.) exhibited cross-reactivity with overexpressed desmin (Fig. S3A), but notably, was the most sensitive probe for its target antigen, with detection pAb z0334 ≫ GFAP mAb GA5 (middle panels, rGST- GFAP detection, Supplemental Fig. S3A). Importantly, despite minimal desmin cross-reactivity, GFAP pAB z0334 produced both basal- and induced-retinal immunolabeling patterns consistent with the predominant targeting of GFAP by IHC (Section 3.1., Fig. 1).
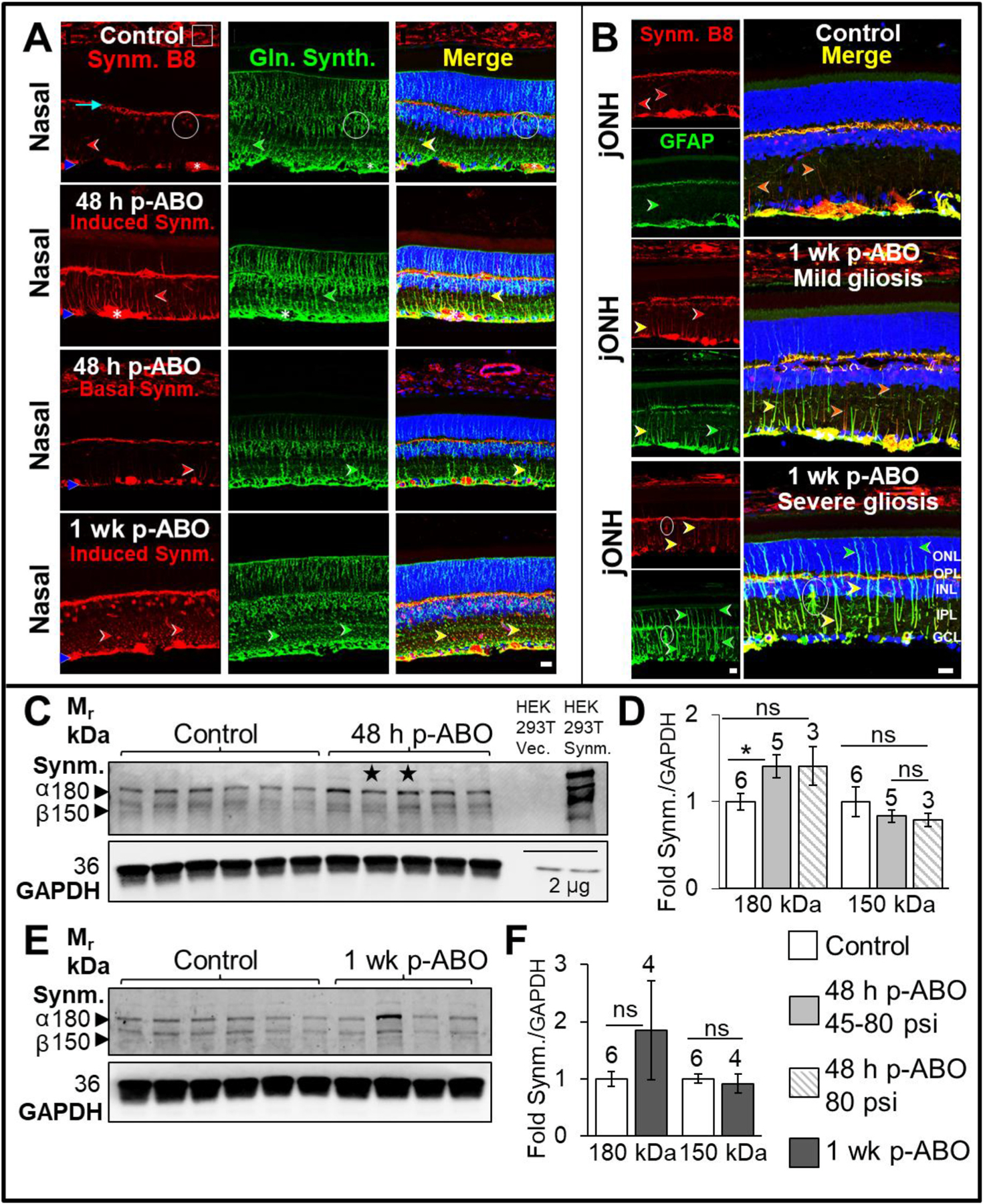
Fig. 1. Müller glial GFAP induction and assembly in response to a single ABO exposure.
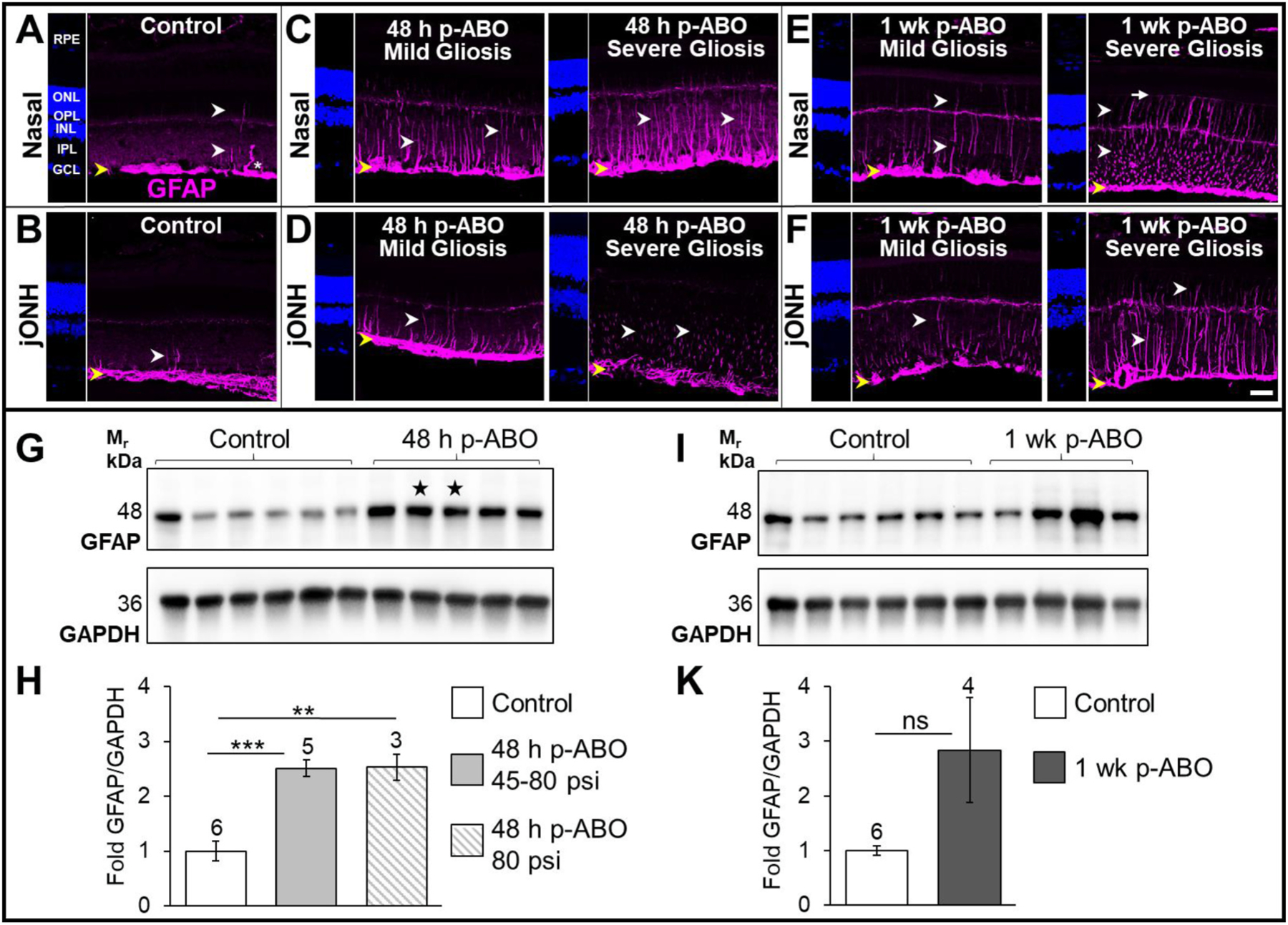
Top: Representative confocal images of GFAP-immunolabeling (magenta) in nasal- and juxta-optic nerve head (jONH) retinal cross-sections from control and ABO-exposed eyes. A, B. Control retinal GFAP localization, sampled in the nasal eye quadrant and jONH, respectively. In control retina GFAP expression was restricted predominantly to the inner-retinal Müller glial endfeet and astrocytic processes (yellow arrows), with occasional radially extending GFAP-IF I glial processes (white arrows); GFAP was expressed in occasional IPL-protruding astrocytic processes (asterisk). C. Nasal quadrant retinal GFAP expression at 48 h post-ABO. GFAP was variably induced in retinal Müller glia post-ABO, exhibiting a classic radial glial IF cytoskeletal extension, indicative of reactive gliosis, ranging from sporadic (mild) to widespread (severe) Müller glial GFAP induction (white arrows). D. jONH retinal GFAP at 48 h post-ABO. Müller glial GFAP-induction exhibited greater variation through the jONH post-ABO, again with ranging density of Müller glial GFAP induction (mild-severe, white arrows). E. Nasal retina at 1 wk post-ABO; varied gliosis was observed with occasionally extended GFAP filaments in mild gliotic retina contrasted with profound GFAP induction and assembly through the outer retina in severely gliotic retina post-ABO with GFAP often extending to the apical Müller extents at the ELM (white arrows and long white arrow, respectively). F. jONH retina at 1 wk post-ABO; GFAP extension post-ABO in the jONH retina mirrored that in the nasal quadrant, exhibiting sparse to dense glial GFAP upregulation but with even greater cell-to-cell variability in induction, albeit with outer retinal filament extension in severely gliotic jONH-retina (white arrows). Scale bar 20 μm. Nuclei counterstained with DAPI (blue). Bottom: G, I; Western blot analyses of GFAP expression in rat retina lysates, and H, K; densitometric quantification. Elevated retinal GFAP was observed by 48 h post-ABO exposure, with varied GFAP upregulation among individual retina samples from 1 wk post-ABO cohort eyes. Open bars: non-exposed controls; light gray bars: 48 h ABO; hatched bars: ABO generated only from criterion 80 psi backpressure; and dark gray bars: 1 wk ABO. Sample sizes shown above bars. Stars denote ABO generated using shock tube backpressures of 56 psi and 45 psi, left to right, respectively. **p ≤ 0.01, ***p ≤ 0.001. Abbreviations: ELM, external limiting membrane; GCL, ganglion cell layer; GFAP, glial fibrillary acidic protein; ONL, outer nuclear layer; OPL, outer plexiform layer; RPE, retinal pigmented epithelium. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Preabsorption of desmin pAb with 10 M excess vimentin eliminated minimal cross reactivity (Supplemental Fig. S3B), however, desmin pAb most sensitively detected desmin in all tests. Furthermore, 10 μg desmin-overexpression lysate or matched empty vector (HEK293T) lysate was used to preabsorb 0.3 μg desmin pAb, revealing efficient competition for binding desmin protein (Supplemental Fig. S3C). We found that in each instance, the major immunoreactive species for each was the target immunogen for each antibody, and that endogenous levels of each IF would be well below the 10 ng tested recombinant forms. We did, nevertheless perform preabsorption of the desmin pAB against vimentin and GFAP before probing for retinal desmin in Western blots as described above in Section 2.8.
2.10. Statistical analysis
Numerical values for all measures are expressed as mean ± standard error of the mean (SEM). Statistical significance was assessed using Student’s t-test, with significance threshold set at a p-value <0.05. Increasingly stringent statistical significance levels are designated as follows: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
3. Results
3.1. Varied Müller glial GFAP induction and assembly in response to a single ABO exposure
Retinal gliosis was monitored by GFAP-immunolabeling and confocal immunofluorescence microscopy of retina cross sections prepared from the nasal and jONH eye regions of ABO-exposed and non-exposed control eyes (see Section 2.4). In control retinas, GFAP was largely restricted to the macroglial end-feet along the ILM (yellow arrows, Fig. 1A; nasal, B; jONH), with occasional radial filamentous GFAP extension through Müller cell processes, and protruding astrocytes (white arrows and asterisk, respectively, Fig. 1A and B). In response to a single ABO-exposure, ipsilateral eyes exhibited varied Müller glial GFAP induction and radial filamentous extension, with mild to severe retinal gliosis at 48 h post-ABO in both eye regions (white arrows, Fig. 1C–D). Similarly, varied glial GFAP induction was observed at 1 wk post-ABO (white arrows, Fig. 1E–F), however, greater outer-retinal GFAP-IF extension was observed, with GFAP occasionally reaching the apical Müller glial limits at the ELM in severely gliotic retina (white arrows, and long arrow, respectively, Fig. 1E–F).
Noting the range of retinal gliosis observed following a single ABO exposure, we next assessed total retinal GFAP levels in individual whole-retina lysates. In agreement with IHC analyses, total retinal GFAP protein expression at 48 h post-exposure was elevated compared to control retinas (Fig. 1G–H). As described in Methods, Section 2.2., two of the ABO exposures performed for 48 h end-point analyses were generated from ~2/3 criterion backpressures of 56 psi and 45 psi, respectively. Interestingly, retinas harvested from these ABO-exposures exhibited similarly elevated total GFAP expression levels (stars, Fig. 1G). Average retinal GFAP expression levels increased by ~2.5-fold over controls in 48 h post-ABO samples, (p ≤ 0.001, ±0.15, Fig. 1H); similar average fold-increase in retinal GFAP was observed within the criterion 80 psi sample subset (hashed gray bar, ~2.5-fold, ±0.24, p ≤ 0.01). In 1 wk post-ABO harvested retinas, GFAP expression varied widely (Fig. 1I–K); while average GFAP expression levels were ~2.8-fold higher than non-exposed controls, the change was not statistically different (Fig. 1K). These results are consistent with varied glial GFAP-induction, remodeling and IF-extension following ABO-exposure observed above (Fig. 1A–F) and the range of gliotic responses observed post-ABO.
3.2. Quantification of GFAP deposition in specific retinal layers and eye regions following single ABO exposure
Inner retina and ILM:
Quantification of GFAP immunolabeling in selected retinal layers was performed in equally thresholded binary images using ImageJ software (see Section 2.5, Image Analysis). Compared to cohort-matched non exposed control retina (open bars, Fig. 2), GFAP labeling in the nasal eye quadrant at 48 h post-ABO retina (solid light gray bars, Fig. 2.) was modestly elevated through the ILM, corresponding to GFAP expressed through the glial endfeet and astrocytes (by ~1.3-fold, ±0.09, p ≤ 0.05, Fig. 2A). In nasal retinas harvested at 1 wk post-ABO (nasal retina—solid dark gray bars, Fig. 2.), a robust elevation in GFAP content through the ILM was observed (~1.6-fold increase over non-exposed controls, ±0.14, p ≤ 0.05, Fig. 2B). In retina imaged adjacent to the ONH at 48 h ABO (dotted light gray bars, Fig. 2) GFAP immunolabeling through the ILM was not significantly different in ABO retina compared to controls at either 48 h, or 1 wk post exposure (Fig. 2A–B). Thus, in response to a single ABO, GFAP was elevated through the inner-retinal glial processes in the ILM in the nasal-eye region, but was comparable to controls in the jONH retina.
Fig. 2. Regional quantification of GFAP immunolabeling through retinal layers in nasal eye quadrant and jONH retina at 48 h- and 1 wk-post-ABO vs cohort matched controls.
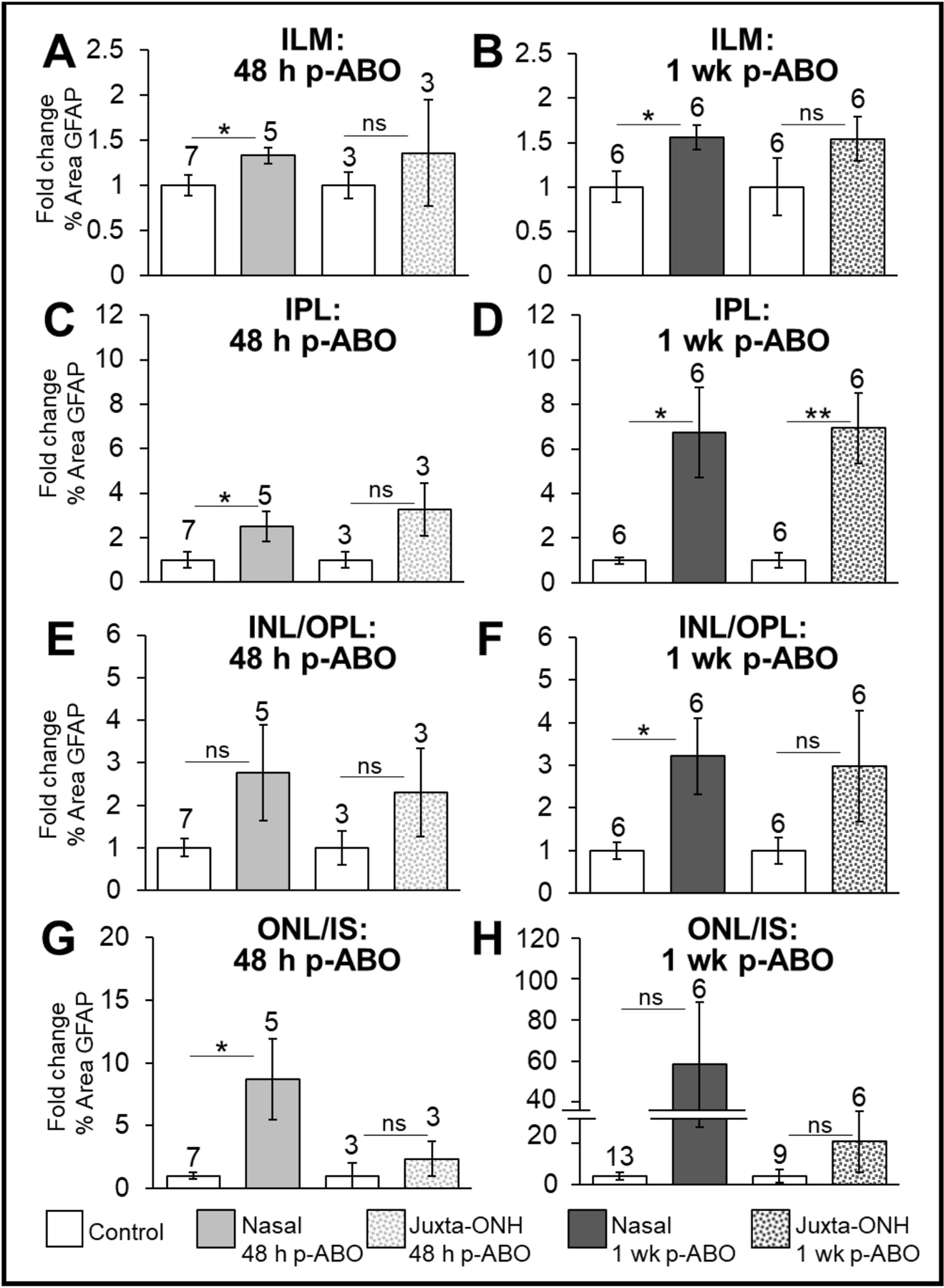
Particle analysis (FIJI, ImageJ) was used to measure mean % area GFAP immunolabeling in defined retinal regions of equally-thresholded confocal z-stack images and expressed as fold-change relative to matched cohort controls. A, C, E, G: Fold-change in % Area GFAP immunolabeling in each retinal region at 48 h post-ABO. B, D, F, H: Fold-change in % Area GFAP immunolabeling in each retinal region at 1 wk post-ABO. Open bars: cohort-matched control retinas; solid light gray bars: nasal eye quadrant 48 h post-ABO; dotted light gray bars: jONH 48 h post-ABO; solid dark gray bars: nasal eye, 1 wk ABO; dotted dark gray bars: jONH 1 wk post-ABO. Greater GFAP content in the outer retina was observed at 1 wk post-ABO compared to 48 h post-ABO and greater overall GFAP extension was seen in the nasal quadrant vs the jONH retina. *p ≤ 0.05, **p ≤ 0.01. Abbreviations: as in legend, Fig. 1.
Inner plexiform layer:
GFAP extending through the glial processes in the IPL of the nasal retina was increased on average by ~2.5-fold over controls at 48 h post single ABO, ±0.65, p ≤ 0.05, Fig. 2C, while a greater overall increase was observed at 1 wk ABO, ~6.75-fold, ±2.05, p ≤ 0.05, Fig. 2D. In the jONH retina, this pattern was echoed, GFAP extending through the IPL was variable at 48h post exposure, and not significantly different from controls, but substantially elevated, with an average ~6.9-fold increase at 1 wk post-ABO, ±1.57, p ≤ 0.01; Fig. 2D.
Mid-retina, INL and OPL:
GFAP extending through the INL/OPL was not significantly elevated over control retinas in nasal or jONH eye regions at 48 h post-ABO, (Fig. 2E). However, by 1 wk post-ABO, overall GFAP content through the INL/ONL retina layers in the nasal retina was significantly increased over non-exposed control eyes (by ~3.2-fold, ±0.89, p ≤ 0.05, Fig. 2F); in contrast to the jONH, where filamentous GFAP extending through the retinal ONL/INL was not significantly different from controls, (Fig. 2F).
Outer retina:
Filamentous GFAP extending into the ONL, and as far as the ELM, was significantly increased over control retina in the nasal eye region at 48 h ABO, (by ~8.7-fold, ±3.22, p ≤ 0.05, Fig. 2G); however, in the jONH, outer retinal GFAP was comparable to controls (Fig. 2G). At 1 wk ABO, outer retinal GFAP exhibited a higher range but with large variation in both nasal and jONH eye regions, with no overall significant differences over controls. (Fig. 2H).
To summarize, GFAP was significantly increased through the ILM and IPL in response to a single ABO by 48 h post exposure in the nasal-but not the jONH-eye regions and further elevated at 1 wk ABO, with mirroring fold-increases. Overall, GFAP extending through the INL/OPL was more robustly elevated in nasal eye region than in the jONH by 1 wk post-ABO and GFAP deposition varied widely through the outer retina in both eye regions in.
3.3. Glial vimentin re-modelling in the retinal ONH-emergence and jONH post-ABO without gross changes in total retinal protein levels
We next assessed retinal vimentin expression and distribution in relation to its type III IF co-polymerizing partner, GFAP, in control and ABO-exposed eyes. In GFAP/vimentin co-immunolabelled control retina, vimentin was constitutively expressed through the inner Müller glial cell processes, with tapered intensity through the outer retina (Vim.+ green arrows, Control, Fig. 3A–B), in contrast to occasional radial GFAP positive glia, (GFAP + red arrows, Control Fig. 3A–B). Interestingly GFAP predominated through the ILM astrocytic processes (upward blue arrows, Control, Merge, Fig. 3A–B). In the nasal quadrant, at both 48 h and 1 wk post-ABO, GFAP-induction varied among the population of Müller glia, with both Vim+ IF/GFAP− or Vim+/GFAP+ glial processes apparent (green and red arrows, respectively, Fig. 3A–B). Notably, through many Vim+/GFAP+ glial processes, gradated type III filament subtype expression was observed, with cytoskeletal vimentin decreasing toward the outer Müller glial processes, while GFAP usually extended further towards the outer extent of the processes at the ELM (yellow arrows GFAP+/Vim+ and Vim−/GFAP+, red arrows, Fig. 3A–B). Interestingly, astrocytes protruding into the IPL were predominantly GFAP+ in control and ABO-exposed eyes, even when the processes appeared grossly hypertrophic (asterisk, 48 h post-ABO, Fig. 3A).
Fig. 3. Retinal Glial vimentin IF-remodeling and total protein in response to single ABO exposure.
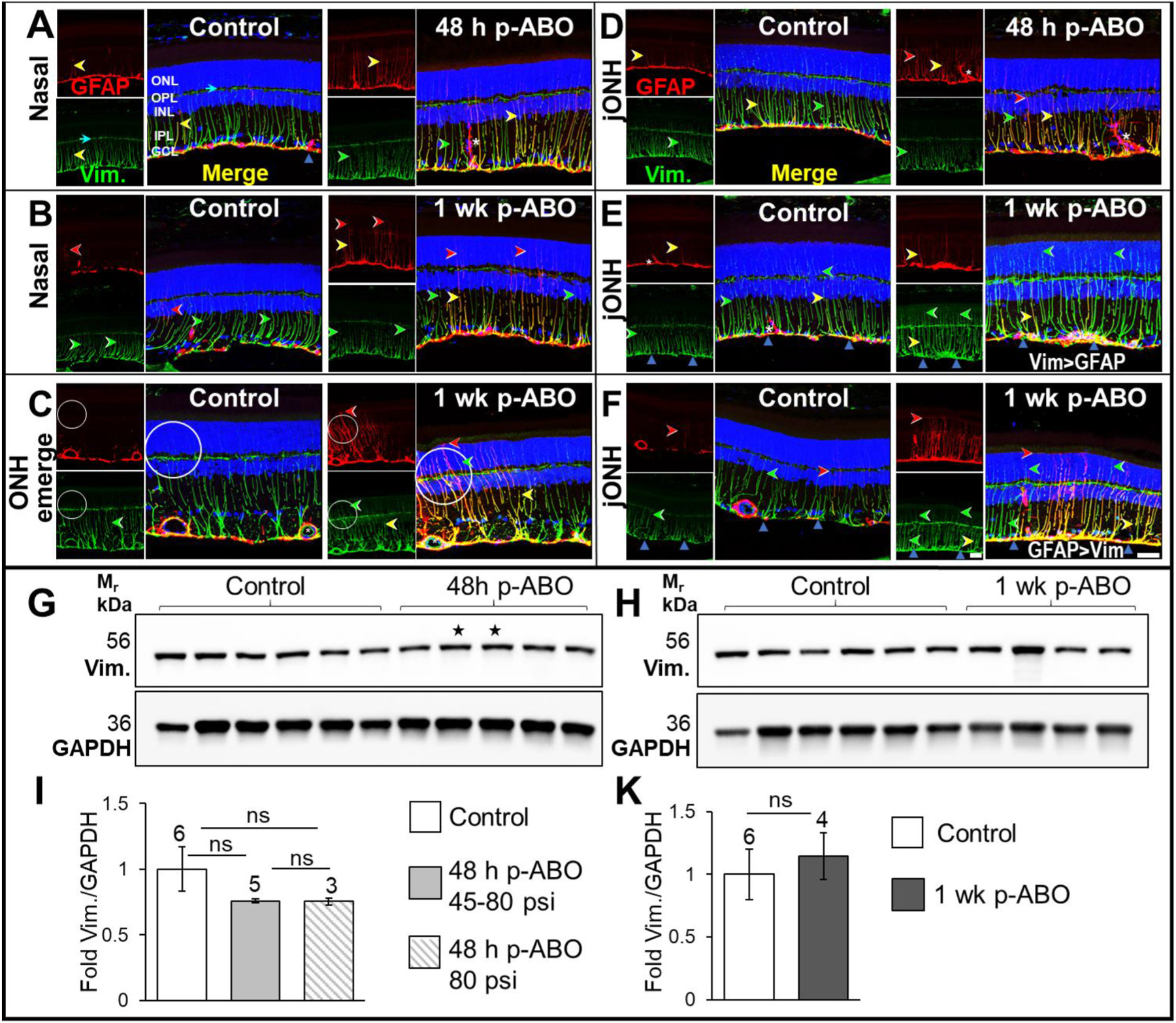
Top: A. GFAP (red) and vimentin (green) co-immunolabeling in retina cross sections from the nasal eye quadrant in control and 48 h post-ABO exposed eyes. Left; Non-exposed control retinas exhibited a typical constitutive expression pattern extending through the Müller cell processes with tapered intensity through the mid- and outer retina. Right; At 48 h post-ABO, no obvious changes in retinal vimentin were observed, while GFAP was induced in a sub-population of glia, (GFAP+/Vim+, yellow arrows) with a remaining varied proportion of Vim+ glia present (green arrows). Occasional GFAP + hypertrophic IPL astrocytes were observed (asterisk). B, Similarly, compared to regionally matched controls (left), no overt changes in retinal vimentin expression was observed at 1 wk post-ABO (right) in the nasal eye quadrant. GFAP often extended further into the outer retina without vimentin co-label in post-ABO retina(red arrows). C. Through the ONH emergence, vimentin was robustly expressed through inner retinal macroglia in non-exposed control eyes (left), exhibiting partial overlap with GFAP. At 1 wk post-ABO, regional vimentin and GFAP remodeling was apparent through some glial processes (right) along the ONH emergence, with regional cytoskeletal hypertrophy (circled). D. Compared to region-matched jONH control retina (left), 48 h post-ABO jONH retina (right) exhibited varied GFAP+/Vim−, Vim+/GFAP−, and Vim+/GFAP+ glia, without gross changes in vimentin expression at this earlier time point post-ABO (red, green, and yellow arrows, respectively). Interestingly, hypertrophied astrocytes protruding into the retinal IPL were observed at 48 h post-ABO which expressed GFAP but little to no vimentin (asterisk). E. At 1 wk post ABO, regional vimentin hypertrophy was apparent in a subpopulation of Müller glia in the jONH retina (right), with non-tapered glial cytoskeletal vimentin through the mid-to-outer retinal processes, often without GFAP co-labeling (Vim > GFAP, green arrows). F. Another regional comparison within the jONH eye region, with control retina (left) vs 1 wk post-ABO retina, with glia exhibiting extensive GFAP induction and assembly, and accompanying vimentin cytoskeletal remodeling apparent 1 wk post-ABO, with robust glial cytoskeletal Vim+ and GFAP+ co-expression, the latter extending further through to the outer retinal glial processes at the ELM in severely gliotic regions (GFAP > Vim, red arrows). Scale bar, 20 μm. Bottom: G, H. Western blot analyses in whole individual retina lysates at 48 h- and 1 wk post-exposure, respectively, and I, K. densitometric quantification. No overall changes in total retinal vimentin expression levels were observed following ABO. Open bars, non-exposed controls; light gray bar, 48 h post-ABO; hatched bar, 48 h post-ABO generated only from criterion 80 psi backpressure; and dark gray bar, 1 wk post-ABO. Sample sizes shown above bars. Stars denote ABO generated using shock tube backpressures of 56 psi and 45 psi, left to right, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
At the optic nerve head emergence, Vimentin and GFAP were robustly expressed through the inner retinal macroglial processes; at 1 wk post-ABO, regional glial cytoskeletal hypertrophy was observed in gliotic Müller processes, (circled, Fig. 3C); retinal vascular vimentin expression appeared comparable in control and ABO-exposed eyes (asterisks, Fig. 3C). Notably, at 48 h post-ABO, retinal vimentin appeared unchanged through the jONH retina, despite radial GFAP induction (Vim.+/GFAP+ processes, yellow arrows, Fig. 3D), with a proportion of constitutive Vim.+/GFAP− processes (green arrows, Fig. 3D). Remarkably, hypertrophied astrocytes were observed in the IPL at 48 h post-ABO, exhibiting enlarged stellate processes, with a predominantly GFAP+/Vim− cytoskeleton (asterisk, Fig. 3D). By contrast, at the later time point, 1 wk post-ABO, hypertrophied cytoskeletal vimentin was observed through clusters of Müller glia, notably, without tapered labeling compared to controls, and greater label through to the outer retina (green arrows, 1 wk post-ABO jONH, Fig. 3E); even in sparsely GFAP+ induced Müller glial processes, with little GFAP assembly post-ABO, hypertrophied radial vimentin-IF was still observed through a subpopulation of jONH-glial processes at 1 wk post ABO, (GFAP+/Vim + yellow, and Vim+/GFAP−, green arrows, Vim > GFAP, Fig. 3E). Vimentin also exhibited regional remodeling through the jONH in another glial subpopulation where GFAP-induction was robust 1 wk post-ABO, but again with filamentous GFAP assembly generally extending further through to the very outer extents of the Müller glial processes (GFAP+/Vim+, yellow and GFAP+/Vim−, red arrows, GFAP > Vim, Fig. 3F). Western blot analysis showed comparable and robust vimentin expression levels among individual non-exposed control and ABO-exposed retina lysates (Fig. 3G–K) and there were no changes in total retinal vimentin at either 48 h- or 1 wk post-ABO (Fig. 3I–K). While vimentin underwent regional re-modeling in Müller glia post-ABO exposure, total retinal vimentin levels were comparable between ABO-exposed and control rat retinas.
3.4. Altered total retinal desmin protein levels without overt radial glial desmin extension following a single ABO exposure
Of the major type III IF sub-family species, desmin is the least well-characterized in retinal glia. Desmin exhibits unique tensile properties among type III family IFs, providing sustained stress resistance in striated muscle (Charrier and Janmey, 2016; Kreplak et al., 2008). To examine if desmin participated in cytoskeletal remodeling in rat retina post-ABO, we first characterized its expression in non-exposed control retina. Co-labeling for GFAP and desmin (Fig. 4A–B) revealed constitutive retinal Müller glial desmin expression in control eyes, predominantly through the early glial processes, and robust expression in presumptive horizontal cell processes in the OPL (cyan arrows, control, Fig. 4A), consistent with previous findings (Dahl and Bignami, 1982). Desmin pAb also labeled retinal vasculature, likely pericytes, (asterisk, control, Fig. 4A), where desmin ensheathment provides vascular stability (Chan-Ling et al., 2004). Notably, GFAP was more extensive through the ILM macroglia, while more delicate desmin was observed in control retina (upward blue arrows, Control, Fig. 4A). Occasional radial glial GFAP-desmin co-expression was also observed (yellow arrowhead, Control, Fig. 4A).
Fig. 4. Altered expression of retinal Type III IF desmin following single ABO exposure.
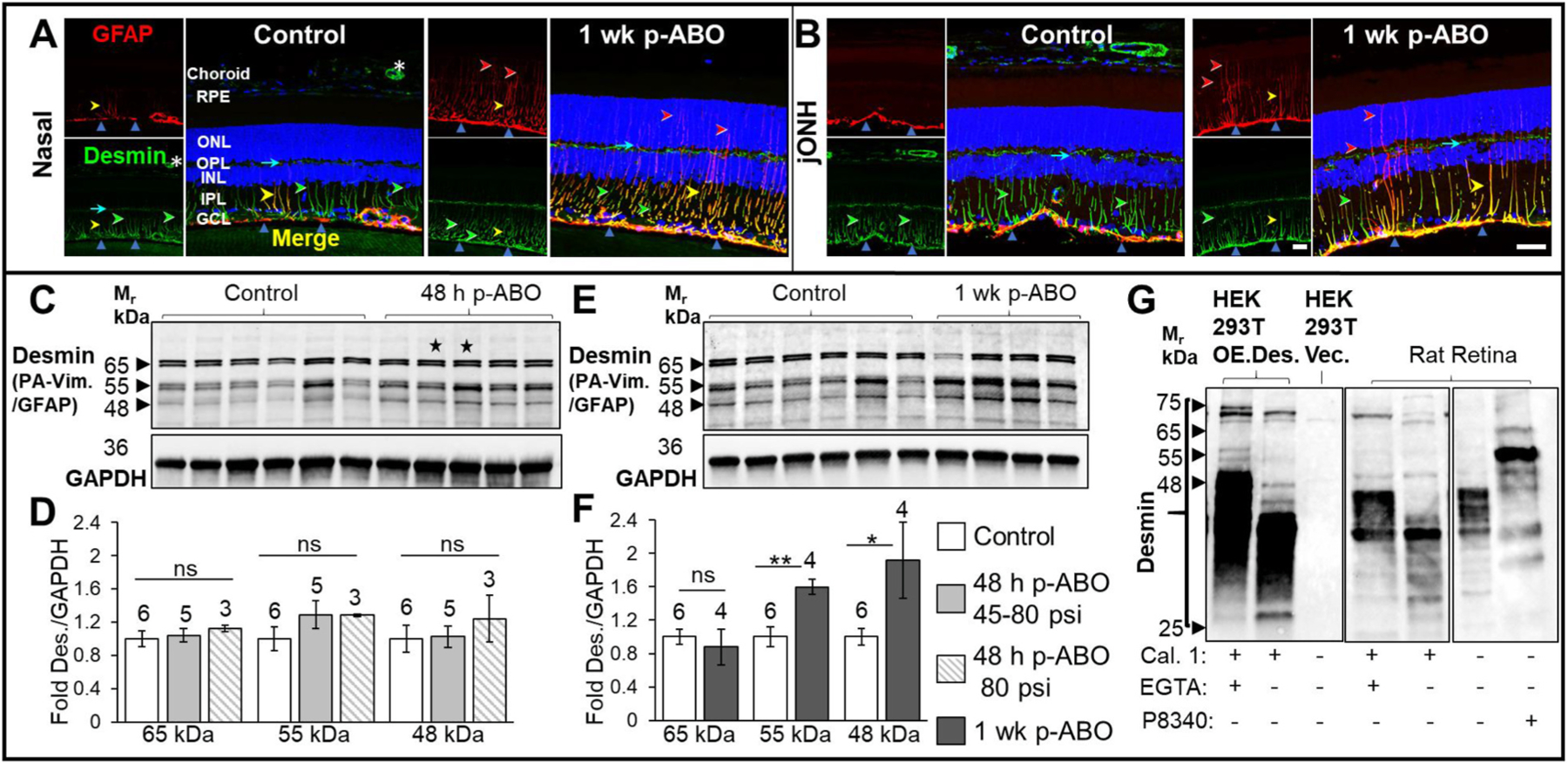
Upper panels: A. Representative images of non-exposed control retinal desmin immunolabeling from nasal control and 1 wk post-ABO retinas. Left, Control, Nasal retina; GFAP-IF (red) was restricted largely to the astroglia/ILM, (upward blue arrows), while desmin (green) was constitutively expressed through Müller glial processes (green arrows). Desmin expression through OPL processes (cyan arrows) and vascular pericytes (asterisk) was observed. Right,1 wk post-ABO Nasal retina; Radial GFAP upregulation at 1 wk ABO without overt constitutive radial desmin remodeling was observed, with graded Müller cell co-expression of both IFs (yellow arrows); GFAP often extended into the outer retina without accompanying desmin IF (red arrows). While no overt changes in radial glial desmin were observed post-ABO, IF-remodeling through the ILM was observed, with greater lateral GFAP+/desmin+ overlap through the inner retinal cytoskeletal structures (upward blue arrows). B. jONH Control retina (left) and 1 wk post-ABO (right) exhibited a similar GFAP/desmin co-immunolabeling pattern, with discrete lateral spreading of inner retinal IF post-exposure compared to controls. Lower panels: C,E. Western blot analyses of whole retina lysates probed using desmin-pAb following preabsorption against full-length vimentin and GFAP proteins. Sample sizes shown above bars. Immunoreactive doublets at 64/65 kDa, 54/55 kDa, and 47/48 kDa were observed (black arrows). 55 KDa is consistent with the predicted unmodified molecular weight of desmin. A lower-migrating species at Mr ~48 kDa could correspond to calpain-protease degradation product from desmin processing which migrates ~10 kDa lower than full-length desmin (Aweida et al., 2018). While no overall changes in desmin levels were seen at 48 h post-ABO, an increase in the levels of 54/55 kDa and 47/48 kDa desmin forms was observed at 1 wk post-ABO (E). D,F. Quantification of the major immunoreactive desmin forms confirmed no overall changes in retinal desmin protein levels at 48 h post-ABO. Quantification of 1 wk ABO lysates revealed elevated levels of 54/55 kDa, and 47/48 kDa desmin forms, consistent with the expected non-modified and lightly degraded desmin forms, respectively, and no significant change in 64/65 kDa species. G. Western blot analyses of Calpain 1 (Cal. 1) digested overexpressed-desmin and rat retina lysates probed with desmin pAb. 200 ng desmin overexpression lysate or 10 μg rat retina lysate was digested with 2 U calpain 1 with or without the presence of 2 mM protease inhibitor EGTA. Desmin pAb detected multiple MW protein forms ranging from 25 to 75 kDa in HEK293T desmin OE-lysate, absent in vector-control HEK293T lysates, and similar endogenous desmin expression patterns in rat retina lysates, with Cal. 1, resulting in collapse of the immunoreactive desmin forms between 25 and 35 kDa in both overexpression and rat retina lysates. Fully protected retina lysates showed even higher range of desmin species, showing that digest conditions (30 °C, without protease inhibitor cocktail, P8340), also led to minimal desmin degradation. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
At 48 h post-ABO, retinal desmin appeared similar to region matched controls, albeit with varied GFAP induction (not shown). At 1 wk post-ABO, radial glial processes exhibited a mixture of GFAP+/desmin+-IF, GFAP+/desmin−-IF, and GFAP−/desmin+-IF, as observed at 48 h ABO; notably, desmin filaments tapered through the retinal IPL, with no obvious radial extension post-ABO, while GFAP frequently extended into the outer retina (yellow, red, and green arrows, respectively, nasal/jONH, 1 wk post-ABO, Fig. 4A–B). Interestingly, while radial glial desmin appeared largely unchanged, there was greater co-labeling of laterally extending GFAP+/desmin IF+ through the macroglial processes along the ILM, consistent with re-modelled cytoskeletal IF structure in the inner retinal glia (upward blue arrows, nasal/jONH, 1wk post-ABO, Fig. 4A–B).
We next assessed total retinal desmin expression in age/cohort matched non-exposed and post-ABO harvested retinas. We extensively characterized desmin pAb (above) and found minimal cross-reactivity with overexpressed, purified type-III IFs, which migrate similarly by SDS-PAGE (Fig. S3). Therefore, we performed preabsorption (PA) of desmin pAb against both GFAP and vimentin recombinant proteins prior to probing Western blots of ABO- and non-exposed control retina lysates (see cross reactivity and preabsorption, Supplemental Figs. S3A–C). As shown in Fig. 4C, E, Vim/GFAP PA-desmin pAb detected multiple immunoreactive desmin forms in individual control rat retina lysates, and similarly in 48 h or 1 wk post-ABO lysates, migrating as major doublets at ~65 kDa, ~55 kDa and ~48 kDa. Quantification revealed no significant changes in the retinal levels of either desmin form at 48 h ABO compared to control (Fig. 4D). However, at 1 wk post-ABO, both ~55- and ~48 kDa desmin species were significantly elevated compared to controls (Fig. 4E), with quantification showing a ~1.6-fold, ±0.09, (p ≤ 0.01) fold-increase in ~55 kDa desmin and a 1.9-fold, ±0.46, p ≤ 0.05 fold-increase in ~48 kDa desmin over control levels by 1 wk post-ABO, Fig. 4F.
Desmin is subject to a series of ubiquitination and phosphorylation events which increase its MW in muscle, but which then increase susceptibility of desmin to cleavage by calpain proteases during muscle wasting and clearance (Aweida et al., 2018; Vermeer et al., 2021). As shown in Fig. 4G, Western blot analysis of desmin overexpression lysate (HEK293T) alongside rat retina lysates subjected to calpain-1 (Cal. 1) digest, with or without protease inhibitor EGTA (see Section 2.7), revealed a complex and similar degradation pattern in both retina and HEK293T-OE lysates. Furthermore, we found that digest conditions (absence of protease inhibitor cocktail P8340), still led to residual proteolytic degradation of desmin, since lysates fully protected with P8340 (as for all control and ABO-treated lysates) exhibited the range of 48–65 kDa species (Fig. 4G). Since desmin has a predicted MW of ~55 kDa, the most significantly elevated doublet post 1 wk ABO corresponds to full-length desmin without extensive post-translational modification, with modest degradation to ~48 kDa, consistent with elevated total retinal desmin but with low-level degradation, the latter may be attributable to pre- or post-lysis degradation.
3.5. Altered synemin expression patterns in retinal Müller cells and astrocytes in response to single ABO exposure
Retinal Müller cells are known to respond to mechanical injury, specifically retinal detachment, by upregulating type VI-family IF protein synemin (Luna et al., 2010). Like other type VI IFs, synemin is unable to self-polymerize, and must incorporate into the IF-network as an obligate IF-heterodimer, or as an IF-associated protein (Jing et al., 2007); alternative splicing produces two major high MW tail-domain isoforms (Titeux et al., 2001). We first examined basal retinal synemin expression, (using a rabbit pAb to avoid mAb-on rat IgG/Fc cross reactivity, predicted to detect both major synemin isoforms, denoted pSAB, see Table 1).
As shown in Fig. S4A, pSAB detected synemin expression in a variety of cell types and structures in control rat retina, including RGCs (asterisk), OPL processes (cyan arrow), retinal and choroidal vasculature (boxed), ILM astrocytic processes (yellow arrows) and occasional delicate synemin traversing the Müller glia processes (white arrowhead), with diffuse label in some INL cell bodies (circled), closely matching previously described immunolocalization (Luna et al., 2010). At 1 wk post-ABO, synemin was upregulated in discrete clusters of retinal Müller glia predominantly through the IPL (white arrowheads, Fig. S4B), and regionally through macroglial processes along the ILM (white and yellow arrowhead, respectively, Fig. S4B). Furthermore, retinal pSAB immunolabeling was completely inhibited by preabsorption with ~10 M excess of the immunogenic peptide (proprietary sequence, Millipore Sigma, Fig. S4C). pSAB, however, did not sensitively detect endogenous synemin by Western analysis, and only detected retinal protein levels following enrichment via immunoprecipitation (IP) from rat retinal lysates via pSAB or via synemin mAb clone A8, and detected HEK293T-overexpressed Human synemin (Fig. S4D). Synemin mAb clone B8 (see Antibodies Table 1.), however, exhibited greater sensitivity than pSAB of mAb A8 for endogenous retinal synemin by IHC and by Western blot analyses. For detailed analyses we therefore used mAb B8 for IHC and total protein analyses of our experimental groups, including 48 h post-ABO. As shown in Fig. 5A, synemin mAb B8 immunolabeling of non-exposed control retina (synemin: red channel, Müller cells: anti-Gln Synthetase pAb, green channel, Fig. 5E) revealed similar retinal synemin localization to pSAB (Fig. S4); through OPL processes, and RGC cytosol (top panel, Fig. 5A, cyan arrow, asterisk, respectively), with basal Müller glial synemin expression in rare radial processes and INL cell bodies (top panel; Fig. 5A, red and yellow arrows, and Müller cell processes: green arrows and bodies: circled). At 48 h post-ABO robust radially-extending synemin was occasionally observed in a subpopulation of glia, but with basal expression evident through much of the nasal retina (48 h post-ABO Fig. 5A, middle panels, red and yellow arrows). However, at 1 wk post-ABO we observed profound synemin induction, in discrete clusters of glial processes through the nasal quadrant retina (1 wk post-ABO, Fig. 5A, bottom panel yellow arrows) in agreement with our above findings with pSAB (Fig. S4).
Fig. 5. Synemin upregulation in a subpopulation of retinal Müller glia following single ABO exposure.
Upper panels: A. Representative rat retina cross sections through the nasal eye quadrant, immunolabeled for Synemin (mAb clone B8, red), and Glutamine Synthetase (green). Control; Nasal Retina, (Top Left): mAb B8 immunolabeling revealed basal synemin expression in a variety of cell-types in rat retina and choroid, including RGCs (asterisk), choroidal vasculature (box), presumptive horizontal processes (through the OPL, cyan arrowhead), astrocytes, Müller glial end feet (ILM, blue arrowhead), and faint radial expression through cell processes (white arrowhead). Expression in a subset of INL cell bodies was observed (circled). 48 h post-ABO; Nasal Retina, (Middle-Left panels): Occasional filamentous radial synemin labeling was observed through the IPL in rare Müller glial processes at 48h post blast, with most of the glia exhibiting basal to no radial synemin expression. 1 wk post-ABO, Nasal Retina (bottom left): By 1 wk post-ABO, clusters of Müller glial processes exhibited robust synemin expression. Moreover, contiguous lateral synemin labeling through the ILM end-feet and astrocytic processes was often observed, indicating macroglial synemin cytoskeletal re-modeling (blue arrows, ILM). B. Control, jONH Retina (Top Right); comparable basal retinal synemin expression was observed in jONH retina to that observed in the nasal eye quadrant. 1 wk post-ABO, (mildly gliotic retina, Middle panel, Right); variable Müller glial synemin induction was observed in mildly gliotic retina at 1 wk post-ABO, often without robust GFAP co-induction (orange arrows); graded filamentous synemin colocalization with GFAP in co-expressing Müller glial processes was observed (orange-yellow arrows). Synemin may interact directly with GFAP as an associated binding protein or indirectly in IF heterodimers via vimentin/desmin. 1 wk post-ABO (severely gliotic retina, bottom panel, Right); elevated radial glial synemin expression, with extended, thickened hypertrophic cytoskeletal appearance was observed through a sub-population of Müller cell processes in severely gliotic jONH retina 1 wk post-ABO, glia often exhibiting profound GFAP co-induction (yellow arrows). Hypertrophic GFAP+/Synemin+ astrocytes in the IPL were occasionally observed (circled). Notably, radial filamentous GFAP generally extended further into the outer Müller glial processes than synemin post-ABO (green arrows). Scale bar, 20 μm. Lower panels: C-F. Western blot analyses of whole retinal synemin levels probed using mAb B8. Both alpha (180 kDa)- and beta (150 kDa)- synemin isoforms were detected by B8. C-D. In 48 h post-ABO harvested rat retinas, modest elevation in retinal alpha-synemin levels were observed. Sample sizes shown above bars. Stars denote ABO generated using shock tube backpressures of 56 psi and 45 psi, left to right, respectively. mAb B8 also detected overexpressed synemin (HEK293T system), with little to no signal in matched vector-only control lysates, demonstrating high target specificity of mAb B8 to synemin. E,F. In 1 wk post-ABO retinas there was no differences in either synemin isoform protein levels compared to controls. *p ≤ 0.05, **p ≤ 0.01. Abbreviations: as in legend, Fig. 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
GFAP/synemin co-immunolabeling of the jONH retina revealed a similar basal synemin expression pattern in jONH control retina to that observed in nasal retina controls (top panels, Fig. 5A–B). At 1 wk post-ABO, elevated radial synemin was observed in mildly gliotic retina, with varied co-expression of GFAP+ (middle panel, Fig. 5B, yellow and orange arrows); interestingly, some Synemin+ glia exhibited minimal GFAP, and vimentin and/or GFAP heteropolymers may enable co-polymerization in radially extending filaments (in lieu of a robust GFAP+ scaffold, orange arrowheads, middle panel; Fig. 5B). In severely gliotic retina, clusters of Müller glia exhibited robust, thickened radial synemin-IF extending further across the IPL and into the INL, and GFAP further into the outer retina, despite hypertrophic appearance of both IFs (red, yellow and green arrows, bottom panel, Fig. 5B). Hypertrophied astrocytes with GFAP+/Synemin+ cytoskeletal-IF were also observed post-ABO within the IPL (bottom panel, Fig. 5B, circled).
In Western blotting analyses of total retinal synemin (Fig. 5C–F) mAb B8 detected both major synemin MW isoforms in single retina lysates (arising from differential retention of a 936-base pair intronic region, extending the c-terminus in the larger form by 312 amino acids, giving α-synemin, Mr ~180 kDa and β-synemin, Mr ~150 kDa (Titeux et al., 2001)). No substantial changes in either synemin isoform were detected in response to ABO, with only a minor increase in the levels of α-synemin observed at 48 h-post-ABO (~1.4-fold over controls, ±0.13, p ≤ 0.05, Fig. 5C–D); no significant changes in retinal synemin levels were observed at 1 wk post-ABO (Fig. 5E–F). Indeed, synemin up-regulation was observed in a relatively small pool of Müller glia in response to ABO, and protein upregulation may precede IF-integration or binding to the cytoskeletal scaffold, moreover, wider, constitutive synemin expression in a range of other retinal cell types and compartments would further mask the cell-specific changes occurring in activated Müller glia subsets.
3.6. Upregulation of neural progenitor marker and type VI-IF nestin in rat retinal glia in response to ABO-exposure
Nestin re-expression in adult glia may presage reversion to a progenitor-like state— while full trans-differentiation of Müller glia has not been demonstrated in higher mammals (Dvoriantchikova et al., 2019)— nestin expressing glia may at least represent a reparative state in various retinal injury models in mammals (Luna et al., 2010; Xue et al., 2010; Zou et al., 2013). As shown in (Fig. 6A), nestin (goat-host pAb)/GFAP GA5 mAb co-immunolabeling (see Table 1) revealed nestin expression in a variety of retinal and choroidal cell types in control rat eyes; interestingly, some vasculature labeling was observed indicating some endothelial expression beyond multipotent vascular progenitors (asterisks, Fig. 6A), as recently proposed in adult Human vascular endothelium (Dusart et al., 2018). Variable basal nestin through occasional Müller glial processes, either with or without GFAP co-expression was observed in control eyes (yellow and red arrowheads, respectively IPL, Fig. 6A). The latter suggests co-polymerization with a non-GFAP partner such as vimentin or desmin. Punctate nestin clusters through the IPL, reminiscent of interdigitating Müller glial branches was also observed (square, control, Fig. 6A). Retinal Müller endfeet and astrocytic processes exhibited delicate nestin in controls (blue arrowheads ILM, and emerging astrocyte, circled, Control, Fig. 6A). In 48 h post-ABO exposed eyes, retinal nestin was upregulated through subpopulations of Müller glia, with profound radial nestin extension, (red, yellow arrows, 48 h p-ABO, Fig. 6A), and with more extensive nestin observed through the Müller glial end feet (blue arrowheads, 48 h p-ABO, Fig. 6A). A mixture of predominantly GFAP+, nestin+ and graded co-expression GFAP+/nestin+ Müller glia were observed post-ABO (green, red, and yellow arrows 48h p-ABO, Fig. 6A). At 1 wk post-ABO, variable nestin induction persisted in subpopulations of Müller glia in both nasal and jONH eye regions, (red, yellow arrows, 1 wk post-ABO, Fig. 6B). Retinal immunolabeling using nestin mAb clone-401, Supplemental Fig. S5), further verified the induction pattern we observed, with low basal nestin expression observed in occasional Müller glia processes in control retina (white arrows, Supplemental Fig. S5A), and varied glial induction at 48 h- and 1 wk post-ABO (white arrows, Supplemental Fig. S5 B–C).
Fig. 6. Variable nestin induction in retinal Müller glia following ABO exposure.
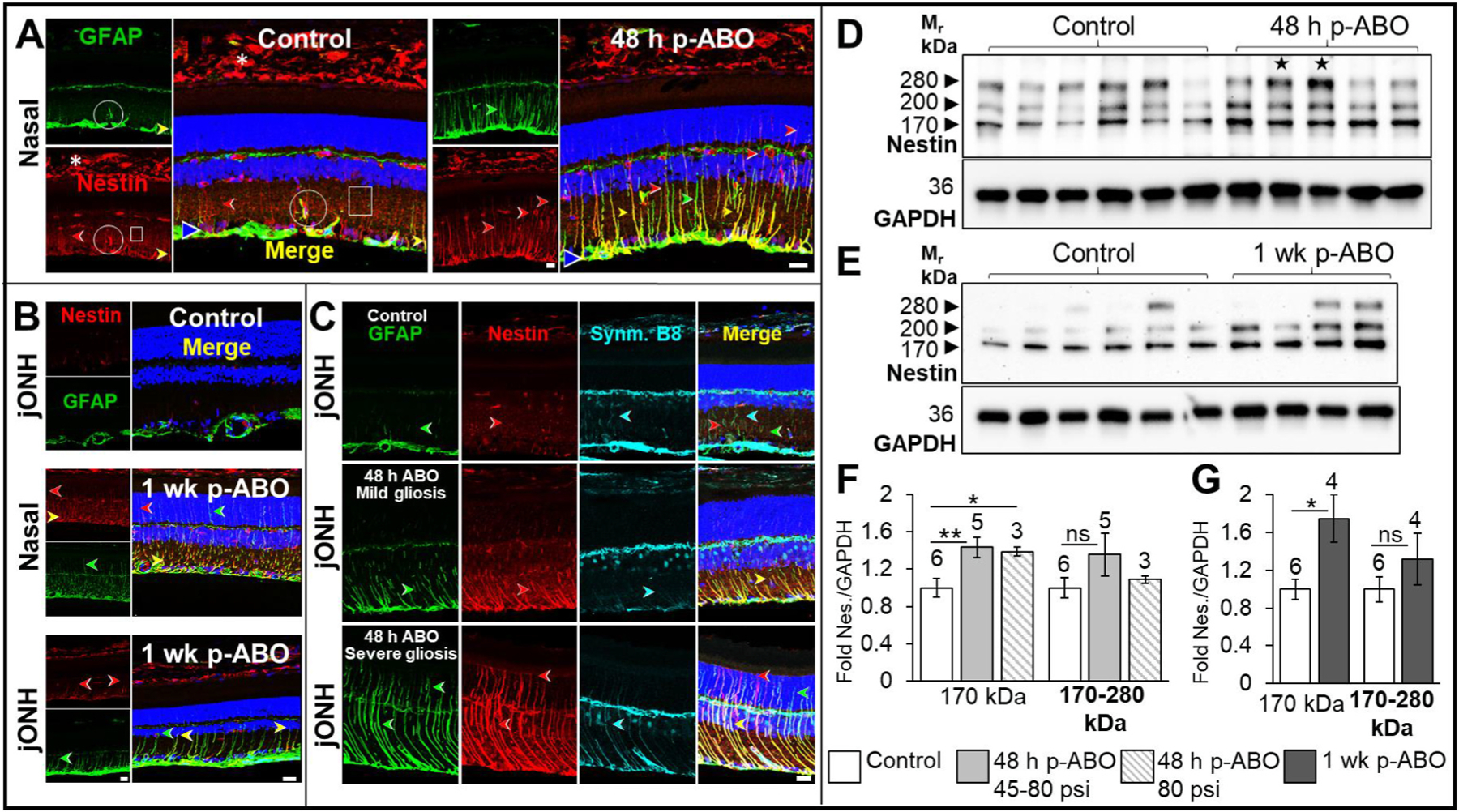
A. GFAP (green) and Nestin (red) co-immunolabeling in nasal-quadrant retina cross sections. Left; Control nasal retina exhibited typical GFAP localization through the inner-retinal macroglia (blue arrow) with occasional radial IF extension, and rare Müller glial GFAP+/nestin+ coexpression, or nestin+ filamentous labeling alone (yellow and red arrows, respectively). Endothelia (asterisks), also exhibited nestin expression, and diffuse nestin labeling through the IPL may indicate lateral glial soma or possibly neuronal expression (boxed). Nestin co-labeling with GFAP through protruding astrocytic processes associated with blood vessels is indicated (circled). Right; at 48 h post-ABO, varied Müller glial induction of GFAP and nestin through radial glial processes was observed, with multiple IF-subtypes exhibiting either predominant cytoskeletal nestin (potentially co-polymerized with vimentin, red arrows), predominant GFAP (green arrows) or graded GFAP/nestin (yellow arrows). Robust cytoskeletal nestin labeling extended into the outer retinal glial processes in some Müller cells (red arrows, outer retina). B. 1 wk post ABO GFAP/nestin immunolabeling, in nasal and jONH retina, compared to controls (top panel and left panel A), nestin was variably induced through Müller glia, with varied GFAP co-induction. C. Triple immunolabeling for synemin (cyan) GFAP (green) and nestin (red) in jONH retina.,Top; in non-exposed control retinas, faint intermittent GFAP, nestin and synemin IF structures were rarely observed through glial processes in the IPL, but interestingly, GFAP+ processes generally co-labeled for synemin, suggesting heteropolymerization within the IF cytoskeletal network, or synemin association with GFAP as an IFAP. Middle: jONH retina, with mild gliosis, at 48 h post-ABO exhibited variable GFAP and Nestin upregulation in some Müller glia, often without accompanying synemin induction. Bottom: jONH retina, with severe gliosis, at 48 h post-ABO with profound induction and extension of GFAP, nestin and synemin through radial Müller glial processes (green, red and cyan arrows, respectively). Interestingly, nestin often exhibited the most extended IF-assembly in some gliotic regions post-ABO, nestin assembly reaching the outer retinal space and ELM, often without GFAP co-labeling (red arrow, outer retina). Scale bars 20 μm. Bottom; D-G. Western blotting analyses of whole individual retina lysates using anti-nestin mAb-401. D,E. Expression of multiple high molecular weight nestin forms was observed in both control and post-ABO rat retina lysates, consistent with known post-translational phosphorylation events (Sahlgren et al., 2001) and multiple translated nestin splice-forms expressed in Rattus norvegicus (splice forms CRA_a-c Genbank EDM00748.1 to 750.1). We observed that the ~170 kDa nestin species in Western blots (the major predicted un-modified form) was modestly upregulated post-ABO. F,G. Densitometric quantification summarizing the protein level changes observed for 170 kDa nestin, and summed 170–280 kDa migrating nestin forms, normalized to housekeeping protein GAPDH for 48 h and 1 wk post-ABO, respectively. Open bars, non-exposed controls; light gray bar, 48 h post-ABO; hatched bar, 48 h post-ABO generated only from use of criterion 80 psi backpressure; and dark gray bar, 1 wk post-ABO. Sample sizes shown above bars. Stars (F) denote ABO generated using shock tube backpressures of 56 psi and 45 psi, left to right, respectively. *p ≤ 0.05, **p ≤ 0.01. Abbreviations: as in legend, Fig. 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Triple immunolabeling for nestin (red), synemin (cyan), and GFAP (green), of jONH retina in 48 h post-ABO eyes (Fig. 6C) showed nestin- and synemin -IF extension commensurate with the severity of gliosis; interestingly, hypertrophic nestin was sometimes observed through the radial processes and ELM, often without GFAP-co-label, among graded GFAP+/synemin+/nestin+ Müller cells in severely gliotic retina (red arrows, severe gliosis, Fig. 6C).
Western blot analysis of total retinal nestin protein expression (Fig. 6D–G) revealed multiple high molecular weight immunoreactive species migrating between Mr ~170 kDa and Mr ~280 kDa by SDS-PAGE (Fig. 6D–E). Nestin possesses an extended C-terminal tail containing charged peptide repeats (Steinert et al., 1999) which may affect migration by SDS-PAGE. Moreover, nestin is modified by phosphorylation (Sahlgren et al., 2001). There are at least three alternatively spliced nestin protein products described in rat (isoforms: CRA_a, GenBank EDM00748.1, Mr ~160–170 kDa), CRA_b, Genbank EDM00749.1 (Mr ~210–220 kDa), and CRA_c; and Genbank EDM00750.1 (Mr ~190–200 kDa). Overall, Mr ~170 kDa nestin exhibited modest increases in total retinal expression levels in ABO-exposed eyes (48 h post-ABO: ~1.4-fold increase, ±0.11, p ≤ 0.01, and 1 wk post-ABO: ~1.7-fold increase, ±0.25, compared to non-exposed control retinas, p ≤ 0.05, Fig. 6F–G). While 170 kDa retinal nestin was elevated in response to ABO, total HMW nestin was not statistically different in ABO-exposed versus control retinas (Fig. 6F–G). Thus, the Mr ~170 kDa nestin species appeared to be the predominant upregulated form in rat retina following ABO exposure in our model— reflecting specific isoform upregulation— and/or altered post-translational modifiers.
4. Discussion
The increasing use of high-order explosives in recent military conflicts has led to an alarming increase in the incidence and severity of ABO-exposures with TBI (Scott et al., 2017). In particular, chronic vision-problems are frequently reported long after an ABO-exposure in returning military service personnel (Cockerham et al., 2011; Lemke et al., 2013), which has galvanized efforts to characterize the mechanisms ABO-related visual dysfunction. In this study, we sought to characterize retinal gliosis in a rodent ABO-model, to ascertain the severity and nature of Müller glial reactive phenotypes. A variety of rodent ABO models have been developed in recent years, ranging from single- or multiple- ABO exposure paradigms, (lapsed or consecutive), low-intensity (Heyburn et al., 2019), or varied-intensity ABO waves (Harper et al., 2019), and either focused ABO directly over the eye globe, with the body constrained, as in many primary blast studies (Bernardo-Colon et al., 2019) or with free head or body movement. Importantly, here we employed an “open” holder configuration, with blast delivered laterally over the right eye and head, allowing unconstrained head movement and ABO-associated coup-contrecoup brain injury—indeed, we found that y-maze performance was compromised revealing long-term cognitive deficits using this open configuration (Allen et al., 2021). While we cannot isolate the primary blast wave effects in this study, ABO with associated head- and coupled eye-movements are usually encountered together in a real-life scenario, and retinal responses to ABO are likely to interact with wider CNS and visual systems effects post exposure. TBI and ocular ABO models are intended to identify key characteristics and molecular mechanisms of ocular pathology related to ABO, but not to recapitulate the exact glial- and related injury processes which would occur in humans; by maintaining free head motion with ABO, we aim to provide insight into ABO with TBI. Interestingly, we find subject-to-subject differences in the degree of retinal GFAP induction in our ABO model, which are likely related to the extent of head and neck movements, severity of eye motion. Key molecular factors include basal retinal GFAP levels before ABO, and underlying biological differences among blast subjects, which, while limited in out-bred rodents, are unavoidable variables in whole animal studies. In the current study, male Long-Evans rats were exclusively employed, representing the larger proportion of front-line combat military personnel, and the most affected military demographic of casualties with TBI-related injuries (Tovar et al., 2021)—however, representation and comparison of gliotic responses in female TBI models are important considerations for future work. Importantly, while we report ABO in relatively small sample sizes in this study, we observe crucial commonalities in the glial responses to ABO between subjects in protein-level and IHC analyses. In order to capture the early stages of Müller glial IF induction, we performed analyses at 48 h and 1 wk post-ABO. Regional GFAP-immunolabel quantification provided further insight into the overall patterns of GFAP induction, which varied both between blast subjects and regionally through the eye post-ABO (section 3.2, Fig. 2). Interestingly, quantification showed that nasal- and jONH-GFAP induction echoed one another at each time point post-ABO, but the extent of GFAP assembly through the outer retina varied widely, with greater overall deposition in the nasal eye quadrant than in the jONH. Mechanical stresses and pressurization forces and wave reflection/amplification will vary at various positions through the eye. In our rat model blast orientation was lateral (perpendicular) to the ABO with the head~1 inch from the muzzle— since rats possess laterally facing eyes (Wallace et al., 2013), ABO exposure was essentially applied face-on. The outer retinal surface may therefore be more vulnerable to the shockwave front than the deeper eye structures. Indeed, with the eye anchored by the optic nerve head and collagen-reinforced pia mater surrounding the exiting axonal fibers (Schwaner et al., 2020), there may be greater mechanical stability in this eye region— but the retina may also encounter inertial forces as the eye rotates around the optic nerve during ABO. However, in silico modeling of the head-motion and blast wave forces across the eye would be required to fully address these questions.
While vimentin has been reported to be co-induced alongside GFAP in a variety of retinal disease states (Gonzalez-Riquelme et al., 2021; Luna et al., 2010; Fernandez-Sanchez et al., 2015), we found that jONH retina exhibited clusters of glia with apparent vimentin hypertrophy without concomitant GFAP induction following ABO exposure (Section 3.3, Fig. 3). While vimentin did not undergo gross uncontrolled hypertrophy through to the sub-retinal space, IF-remodeling and bundle thickening through the radial glial processes may help to stabilize retinal structure following ABO. While GFAP and vimentin type III IFs have been extensively characterized in retina, the role of desmin in retinal macroglia is not well known. Desmin is better characterized in muscle, owing to its dysregulation and aggregation in desminopathies (Clemen et al., 2013). The desmin pAb we characterized for use in this study effectively detected constitutive desmin through inner-retinal Müller glial processes and pericytes, consistent with its known retinal distribution with limited overlap with GFAP, indicating separate glial IF networks in the resting state (Dahl and Bignami, 1982). We observe that desmin localization remains largely unchanged with ABO –with modest upregulation through end-feet—despite dynamic changes in total retinal protein levels. Thus, we do not propose that desmin undergoes vast re-modelling or localization changes with ABO exposure—but that rather it may be reinforced and incorporated through glial end feet in response to mechanical perturbation following ABO. Desmin protein abundance is tightly regulated by a cycle of phosphorylation, ubiquitination and proteolysis via calpain family proteases in muscle tissue (Aweida et al., 2018; Vermeer et al., 2021), but we did not observe overt degradation of desmin in rat retina following ABO exposure—indeed full-length desmin (~55 kDa) was elevated, and modest increase in ~48 kDa-desmin was observed indicating modest processing, consistent with retinal desmin upregulation and minimal degradation in our rodent ABO model. At least one proteomic study has shown concomitant up-regulation of GFAP and desmin IFs in gliotic human retina arising from proliferative vitreoretinopathy (Eastlake et al., 2018), however, it is not yet clear how desmin participates in reactive gliosis pathways, and what function it may serve through the Müller glial end feet and inner retinal processes.
Beyond type-III family IFs, a single ABO exposure elicited varied induction of developmentally regulated type VI-family IFs nestin and synemin in retinal Müller cells in our rodent ABO model. Nestin exhibited acute extension through the length of Müller glia, prominent by 48 h post-ABO, while synemin upregulation appeared most overtly at 1 wk post-ABO, in pockets of inner retina Müller glia. Synemin is known to associate with GFAP as an IF-associated protein (Jing et al., 2007), but may heterodimerize with vimentin, which we found largely unaltered in our ABO model, to achieve the extended-filamentous synemin we observed in rat retina post-ABO exposure. Synemin expression is tightly regulated in the developing human retina, early expression occurring through the nerve-fiber layer and GCL, followed by transient expression in the Müller glial endfeet (Tawk et al., 2003). Interestingly, synemin protein is likely stabilized by the presence of other IF-proteins in the cytoskeletal network (Jing et al., 2007). Intriguingly, the well-known synemin binding partner, muscle-associated dystrophin, is also functionally important in retina, and expressed in Müller glia, where it is thought to mediate cell-adhesion and signaling, anchoring the glia to the actin cytoskeleton (Claudepierre et al., 2000). While structural stabilization is certainly a strong possibility for the function of upregulated glial synemin, there may also be a reparative role for sub-populations of activated Müller cells during the retinal response to ABO. The acute retinal glial responses we observe in our rodent TBI model are heterogeneous, with potential further divergence of established subtypes; these may adapt to local neurotoxic stress and damage. In age-related macular degeneration and injury models, Müller glia also diverge into varied adaptive subsets, expressing new transcriptomes—with either maladaptive, or compensatory profiles (Liu et al., 2022; Tomita et al., 2021). While beyond the scope of the current study, single cell transcriptomics has emerged as important tool for analyzing Müller cell heterogeneity—we now view these principal glia as complex populations of cells capable of varied responses to disease states (Lamas and Martinez-Colin, 2022).
Re-expression of the neural progenitor marker nestin at early stages of the gliotic response to ABO could represent Müller glia undergoing partial de-differentiation to regenerative states. Indeed, nestin re-expression is described in other retinal injury models (Luna et al., 2010; Xue et al., 2010). Vast upregulation of nestin through the cerebral cortex was previously observed in cortical impact TBI, with activation of caspase-3 and apoptosis (Kaneko et al., 2012). However, we do not observe overt retinal degeneration in our rodent ABO model (Allen et al., 2018, 2021), more consistent with a protective and stabilizing component among the acute gliotic sub-populations we observe post-blast exposure. We cannot rule out an interaction between gliosis and altered retinal function, since optomotor response tracking, to assess visual deficits, measured bi-monthly between 2 and 8 mo post single ABO exposure, revealed significant decreases in spatial frequency and contrast sensitivity in ipsilateral eyes of blast exposed rats compared to matched non-exposed controls in our longitudinal studies, while ERG a-wave and b-waves were not significantly altered post-ABO exposure using the open holder conformation (Allen et al., 2018, 2021).
Specific nestin isoform expression patterns influence viability and differentiation; for example, nestin short-isoform, expressed in dorsal-root ganglia, is cytoprotective during oxidative stress responses (Huang et al., 2009; Su et al., 2013). While Nestin+/GFAP− astroglia traditionally thought to assume a proliferative state (Sergent-Tanguy et al., 2006), Nestin+ Müller glia post-ABO may represent different proliferative sub-sets (if differentially positive for cell division markers), as shown in retinal detachment and laser injury models in the rat eye (Kohno et al., 2006; Luna et al., 2010). In future work it would be informative for nestin+/GFAP− and nestin+/GFAP+ Müller cells emerging post-ABO to be similarly phenotyped. Nestin has a complex phosphoproteome: 11 phospho-serine sites have been formally identified and are associated with neural stem cell progenitors (Namiki et al., 2012; Sahlgren et al., 2001). In retinal lysates we observe a complex mixture of high molecular weight nestin forms, the lowest-migrating band subject to upregulation following ABO exposure (Section 3.6, Fig. 6.), which could indicate de-phosphorylation, or altered transcription. In future work, beyond the scope of the present acute study, both intermediate and longitudinal time points within matched cohorts would help determine the relative persistence of GFAP, nestin and synemin intermediate filaments, and whether the proportion of Müller glia expressing developmentally-regulated IFs diminish in frequency, remain stable, or undergo further remodeling. It is yet unknown whether any scarring would result as a consequence of continued IF-extension, and glial process invasion of the sub-retinal space (e.g. rare hypertrophied nestin+/GFAP− Müller glia, or Vimentin+/GFAP− glia, some which exhibited pronounced outer-retinal IF-cytoskeletal incorporation). We acknowledge that the observed retinal glial phenotypes are static snapshots of likely dynamic and potentially asynchronous emergent glial populations following ABO exposure. While an unavoidable limitation, the study compensates on several fronts—being the first to provide an in-depth analyses of macroglial expression patterns of the less well-characterized type III desmin and type VI synemin IF proteins among current models of ABO-associated retinal gliosis, and furthermore, performing regional analyses post-ABO. Together our results indicate that the Müller cells are able to tailor their IF expression profiles, potentially to provide appropriate structural and regenerative environment to the surrounding neurons, indicative of coordinated reactivity between neighboring cells. Our work reveals that ABO exposure can impact the neural retina differentially through the eye globe, eliciting varied temporal and spatial glial responses.
5. Conclusions
In our rodent model of single lateral ABO exposure to the rat eye, we find acute retinal gliosis emergent by 48 h post-ABO, characterized by profound GFAP induction through the inner retinal Müller endfeet and extension through to the outer retinal processes. Moreover, gliosis following ABO may be accompanied by altered retinal expression of class III-IF desmin, a classic myocyte-marker and muscle-associated IF. Finally, ABO induced varied Müller glial re-expression of developmentally regulated type VI-IFs nestin and synemin, exhibiting varied reactive phenotypes. The non-uniform nature of glial reactivity across the rodent eye in our ABO model indicates complex remodeling of the IF network, with regional sensitivity across the eye. By understanding the retinal glial responses to ABO in greater detail, we gain insight into the protective as well as the pathological aspects of Müller glial activation in the retina, and by applying greater focus to the range of retinal glial responses will allow investigators to assess gliosis more accurately in other ocular injury models. Eventually, this knowledge can be applied to determine the relative efficacy of therapeutics in ABO models, designed to mitigate or prevent deterioration of retinal function and structure following ABO exposure.
Supplementary Material
Acknowledgments
We thank Rebecca Benz, Kristie Kilby, Alexis Shivers (Buffalo VAMC VMU staff) for technical assistance in the course of this study. This work was supported in part by: a VA MERIT Award from the Department of Veterans Affairs/BLR&D Service (BX002439) to SJF and MTP; a VA BLR&D Research Career Scientist Award (15F-RCS-001) to SJF; a VA RR&D Research Career Scientist Award (RX003134) to MTP; a VA Career Development Award (8RX00292) to RSA; the Georgia Research Alliance (MTP, CTM, RSA); NEI Core Grant P30 EY006360 (MTP and RSA); and by facilities and resources provided by the VA Western New York Healthcare System (LAS, SRR, SJF) and the Atlanta VAMC (MTP, CTM, RSA). Portions of this work were presented in preliminary form at the 2020 Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO 2020). The opinions expressed herein do not reflect those of the Department of Veteran Affairs, the National Institutes of Health, or the U.S. Government.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.exer.2023.109585.
Data availability
Data will be made available on request.
References
- Agnetti G, Herrmann H, Cohen S, 2022. New roles for desmin in the maintenance of muscle homeostasis. FEBS J 289, 2755–2770. [DOI] [PubMed] [Google Scholar]
- Allen RS, Motz CT, Feola A, Chesler KC, Haider R, Ramachandra Rao S, Skelton LA, Fliesler SJ, Pardue MT, 2018. Long-term functional and structural consequences of primary blast overpressure to the eye. J. Neurotrauma 35, 2104–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RS, Motz CT, Singh A, Feola A, Hutson L, Douglass A, Ramachandra Rao S, Skelton LA, Cardelle L, Bales KL, Chesler K, Gudapati K, Ethier CR, Harper MM, Fliesler SJ, Pardue MT, 2021. Dependence of visual and cognitive outcomes on animal holder configuration in a rodent model of blast overpressure exposure. Vis. Res 188, 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aweida D, Rudesky I, Volodin A, Shimko E, Cohen S, 2018. GSK3-beta promotes calpain-1-mediated desmin filament depolymerization and myofibril loss in atrophy. J. Cell Biol 217, 3698–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellin RM, Huiatt TW, Critchley DR, Robson RM, 2001. Synemin may function to directly link muscle cell intermediate filaments to both myofibrillar Z-lines and costameres. J. Biol. Chem 276, 32330–32337. [DOI] [PubMed] [Google Scholar]
- Bernardo-Colon A, Vest V, Cooper ML, Naguib SA, Calkins DJ, Rex TS, 2019. Progression and pathology of traumatic optic neuropathy from repeated primary blast exposure. Front. Neurosci 13, 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosle RC, Michele DE, Campbell KP, Li Z, Robson RM, 2006. Interactions of intermediate filament protein synemin with dystrophin and utrophin. Biochem. Biophys. Res. Commun 346, 768–777. [DOI] [PubMed] [Google Scholar]
- Bignami A, Dahl D, 1979. The radial glia of Muller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp. Eye Res 28, 63–69. [DOI] [PubMed] [Google Scholar]
- Bochicchio GV, Lumpkins K, O’Connor J, Simard M, Schaub S, Conway A, Bochicchio K, Scalea TM, 2008. Blast injury in a civilian trauma setting is associated with a delay in diagnosis of traumatic brain injury. Am. Surg 74, 267–270. [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A, 2006. Muller cells in the healthy and diseased retina. Prog. Retin. Eye Res 25, 397–424. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Page MP, Gardiner T, Baxter L, Rosinova E, Hughes S, 2004. Desmin ensheathment ratio as an indicator of vessel stability: evidence in normal development and in retinopathy of prematurity. Am. J. Pathol 165, 1301–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier EE, Janmey PA, 2016. Mechanical properties of intermediate filament proteins. Methods Enzymol 568, 35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Greene WA, Johnson AJ, Chavko M, Cleland JM, McCarron RM, Wang HC, 2015. Pathophysiology of blast-induced ocular trauma in rats after repeated exposure to low-level blast overpressure. Clin. Exp. Ophthalmol 43, 239–246. [DOI] [PubMed] [Google Scholar]
- Claudepierre T, Dalloz C, Mornet D, Matsumura K, Sahel J, Rendon A, 2000. Characterization of the intermolecular associations of the dystrophin-associated glycoprotein complex in retinal Muller glial cells. J. Cell Sci 113 Pt 19, 3409–3417. [DOI] [PubMed] [Google Scholar]
- Clemen CS, Herrmann H, Strelkov SV, Schroder R, 2013. Desminopathies: pathology and mechanisms. Acta Neuropathol 125, 47–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerham GC, Rice TA, Hewes EH, Cockerham KP, Lemke S, Wang G, Lin RC, Glynn-Milley C, Zumhagen L, 2011. Closed-eye ocular injuries in the Iraq and Afghanistan wars. N. Engl. J. Med 364, 2172–2173. [DOI] [PubMed] [Google Scholar]
- Dahl D, Bignami A, 1982. Immunohistological localization of desmin, the muscle-type 100 A filament protein, in rat astrocytes and Muller glia. J. Histochem. Cytochem 30, 207–213. [DOI] [PubMed] [Google Scholar]
- de Hoz R, Rojas B, Ramirez AI, Salazar JJ, Gallego BI, Trivino A, Ramirez JM, 2016. Retinal macroglial responses in Health and disease. BioMed Res. Int 2016, 2954721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Martins SC, Agbulut O, Diguet N, Larcher JC, Paulsen BS, Rehen SK, Moura-Neto V, Paulin D, Li Z, Xue Z, 2011. Dynamic expression of synemin isoforms in mouse embryonic stem cells and neural derivatives. BMC Cell Biol 12, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty AL, MacGregor AJ, Han PP, Heltemes KJ, Galarneau MR, 2011. Visual dysfunction following blast-related traumatic brain injury from the battlefield. Brain Inj 25, 8–13. [DOI] [PubMed] [Google Scholar]
- Dusart P, Fagerberg L, Perisic L, Civelek M, Struck E, Hedin U, Uhlen M, Tregouet DA, Renne T, Odeberg J, Butler LM, 2018. A systems-approach reveals human nestin is an endothelial-enriched, angiogenesis-independent intermediate filament protein. Sci. Rep 8, 14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoriantchikova G, Seemungal RJ, Ivanov D, 2019. Development and epigenetic plasticity of murine Muller glia. Biochim. Biophys. Acta Mol. Cell Res 1866, 1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastlake K, Heywood WE, Banerjee P, Bliss E, Mills K, Khaw PT, Charteris D, Limb GA, 2018. Comparative proteomic analysis of normal and gliotic PVR retina and contribution of Muller glia to this profile. Exp. Eye Res 177, 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenfeld AJ, Bunt-Milam AH, Sarthy PV, 1984. Muller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest. Ophthalmol. Vis. Sci 25, 1321–1328. [PubMed] [Google Scholar]
- Eliasson C, Sahlgren C, Berthold CH, Stakeberg J, Celis JE, Betsholtz C, Eriksson JE, Pekny M, 1999. Intermediate filament protein partnership in astrocytes. J. Biol. Chem 274, 23996–24006. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Wilmington DJ, Gallun FJ, Myers PJ, Henry JA, 2009. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. J. Rehabil. Res. Dev 46, 797–810. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sanchez L, Lax P, Campello L, Pinilla I, Cuenca N, 2015. Astrocytes and muller cell alterations during retinal degeneration in a transgenic rat model of retinitis pigmentosa. Front. Cell. Neurosci 9, 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Riquelme MJ, Galindo-Romero C, Lucas-Ruiz F, Martinez-Carmona M, Rodriguez-Ramirez KT, Cabrera-Maqueda JM, Norte-Munoz M, Vidal-Sanz M, Agudo-Barriuso M, 2021. Axonal injuries cast long shadows: long term glial activation in injured and contralateral retinas after unilateral axotomy. Int. J. Mol. Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich GL, Flyg HM, Kirby JE, Chang CY, Martinsen GL, 2013. Mechanisms of TBI and visual consequences in military and veteran populations. Optom. Vis. Sci 90, 105–112. [DOI] [PubMed] [Google Scholar]
- Greer N, Sayer N, Kramer M, Koeller E, Velasquez T, 2016. Prevalence and Epidemiology of Combat Blast Injuries from the Military Cohort 2001–2014, Prevalence and Epidemiology of Combat Blast Injuries from the Military Cohort 2001–2014. Washington (DC). [PubMed] [Google Scholar]
- Harper MM, Woll AW, Evans LP, Delcau M, Akurathi A, Hedberg-Buenz A, Soukup DA, Boehme N, Hefti MM, Dutca LM, Anderson MG, Bassuk AG, 2019. Blast preconditioning protects retinal ganglion cells and reveals targets for prevention of neurodegeneration following blast-mediated traumatic brian injury. Invest. Ophthalmol. Vis. Sci 60, 4159–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H, Aebi U, 2016. Intermediate Filaments: Structure and Assembly. Cold Spring Harb Perspect Biol 8 (11), a018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyburn L, Abutarboush R, Goodrich S, Urioste R, Batuure A, Statz J, Wilder D, Ahlers ST, Long JB, Sajja V, 2019. Repeated low-level blast overpressure leads to endovascular disruption and alterations in TDP-43 and Piezo2 in a rat model of blast TBI. Front. Neurol 10, 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockfield S, McKay RD, 1985. Identification of major cell classes in the developing mammalian nervous system. J. Neurosci 5, 3310–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Wang Y, Gu L, 2017. Primary blast waves induced brain dynamics influenced by head orientations. Biomed Eng Lett 7, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Wu CM, Shi GY, Wu GC, Lee H, Jiang MJ, Wu HL, Yang HY, 2009. Nestin serves as a prosurvival determinant that is linked to the cytoprotective effect of epidermal growth factor in rat vascular smooth muscle cells. J. Biochem 146, 307–315. [DOI] [PubMed] [Google Scholar]
- Hughes S, Chan-Ling T, 2004. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest. Ophthalmol. Vis. Sci 45, 2795–2806. [DOI] [PubMed] [Google Scholar]
- Hughes S, Gardiner T, Hu P, Baxter L, Rosinova E, Chan-Ling T, 2006. Altered pericyte-endothelial relations in the rat retina during aging: implications for vessel stability. Neurobiol. Aging 27, 1838–1847. [DOI] [PubMed] [Google Scholar]
- Jing R, Wilhelmsson U, Goodwill W, Li L, Pan Y, Pekny M, Skalli O, 2007. Synemin is expressed in reactive astrocytes in neurotrauma and interacts differentially with vimentin and GFAP intermediate filament networks. J. Cell Sci 120, 1267–1277. [DOI] [PubMed] [Google Scholar]
- Kalayci M, Er S, Tahtabasi M, 2020. Bomb explosion: ocular effects of primary, secondary and tertiary mechanisms. Clin. Ophthalmol 14, 1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Tajiri N, Yu S, Hayashi T, Stahl CE, Bae E, Mestre H, Franzese N, Rodrigues A Jr., Rodrigues MC, Ishikawa H, Shinozuka K, Hethorn W, Weinbren N, Glover LE, Tan J, Achyuta AH, van Loveren H, Sanberg PR, Shivsankar S, Borlongan CV, 2012. Nestin overexpression precedes caspase-3 upregulation in rats exposed to controlled cortical impact traumatic brain injury. Cell Med 4, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanamiryan L, Li Z, Paulin D, Xue Z, 2008. Self-assembly incompetence of synemin is related to the property of its head and rod domains. Biochemistry 47, 9531–9539. [DOI] [PubMed] [Google Scholar]
- Kohno H, Sakai T, Kitahara K, 2006. Induction of nestin, Ki-67, and cyclin D1 expression in Muller cells after laser injury in adult rat retina. Graefes Arch. Clin. Exp. Ophthalmol 244, 90–95. [DOI] [PubMed] [Google Scholar]
- Kreplak L, Herrmann H, Aebi U, 2008. Tensile properties of single desmin intermediate filaments. Biophys. J 94, 2790–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas M, Martinez-Colin EJ, 2022. Muller cell molecular heterogeneity: facts and predictions. ASN Neuro 14, 17590914221106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Park HS, Shin JM, Chun MH, Oh SJ, 2012. Nestin expressing progenitor cells during establishment of the neural retina and its vasculature. Anat Cell Biol 45, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke S, Cockerham GC, Glynn-Milley C, Cockerham KP, 2013. Visual quality of life in veterans with blast-induced traumatic brain injury. JAMA Ophthalmol 131, 1602–1609. [DOI] [PubMed] [Google Scholar]
- Lemke S, Cockerham GC, Glynn-Milley C, Lin R, Cockerham KP, 2016. Automated perimetry and visual dysfunction in blast-related traumatic brain injury. Ophthalmology 123, 415–424. [DOI] [PubMed] [Google Scholar]
- Lewis GP, Fisher SK, 2003. Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int. Rev. Cytol 230, 263–290. [DOI] [PubMed] [Google Scholar]
- Li Z, Mericskay M, Agbulut O, Butler-Browne G, Carlsson L, Thornell LE, Babinet C, Paulin D, 1997. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J. Cell Biol 139, 129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Parlakian A, Coletti D, Alonso-Martin S, Hourde C, Joanne P, Gao-Li J, Blanc J, Ferry A, Paulin D, Xue Z, Agbulut O, 2014. Synemin acts as a regulator of signalling molecules during skeletal muscle hypertrophy. J. Cell Sci 127, 4589–4601. [DOI] [PubMed] [Google Scholar]
- Liu B, He J, Zhong L, Huang L, Gong B, Hu J, Qian H, Yang Z, 2022. Single-cell transcriptome reveals diversity of Muller cells with different metabolic- mitochondrial signatures in normal and degenerated macula. Front. Neurosci 16, 1079498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna G, Lewis GP, Banna CD, Skalli O, Fisher SK, 2010. Expression profiles of nestin and synemin in reactive astrocytes and Muller cells following retinal injury: a comparison with glial fibrillar acidic protein and vimentin. Mol. Vis 16, 2511–2523. [PMC free article] [PubMed] [Google Scholar]
- Lund LM, Kerr JP, Lupinetti J, Zhang Y, Russell MA, Bloch RJ, Bond M, 2012. Synemin isoforms differentially organize cell junctions and desmin filaments in neonatal cardiomyocytes. Faseb. J 26, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magone MT, Kwon E, Shin SY, 2014. Chronic visual dysfunction after blast-induced mild traumatic brain injury. J. Rehabil. Res. Dev 51, 71–80. [DOI] [PubMed] [Google Scholar]
- Mammadova N, Ghaisas S, Zenitsky G, Sakaguchi DS, Kanthasamy AG, Greenlee JJ, West Greenlee MH, 2017. Lasting retinal injury in a mouse model of blast-induced trauma. Am. J. Pathol 187, 1459–1472. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Thompson TG, Guyon JR, Lidov HG, Brosius M, Imamura M, Ozawa E, Watkins SC, Kunkel LM, 2001. Desmuslin, an intermediate filament protein that interacts with alpha -dystrobrevin and desmin. Proc. Natl. Acad. Sci. U. S. A 98, 6156–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki J, Suzuki S, Masuda T, Ishihama Y, Okano H, 2012. Nestin protein is phosphorylated in adult neural stem/progenitor cells and not endothelial progenitor cells. Stem Cell. Int 2012, 430138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens BD, Kragh JF Jr., Wenke JC, Macaitis J, Wade CE, Holcomb JB, 2008. Combat wounds in operation Iraqi freedom and operation enduring freedom. J. Trauma 64, 295–299. [DOI] [PubMed] [Google Scholar]
- Paulin D, Li Z, 2004. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp. Cell Res 301, 1–7. [DOI] [PubMed] [Google Scholar]
- Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallen A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisen J, 1999. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J. Cell Biol 145, 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M, 2014. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol. Rev 94, 1077–1098. [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M, 2016. Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Biophys. Acta 1862, 483–491. [DOI] [PubMed] [Google Scholar]
- Potokar M, Morita M, Wiche G, Jorgacevski J, 2020. The diversity of intermediate filaments in astrocytes. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra Rao S, Pfeffer BA, Mas Gomez N, Skelton LA, Keiko U, Sparrow JR, Rowsam AM, Mitchell CH, Fliesler SJ, 2018. Compromised phagosome maturation underlies RPE pathology in cell culture and whole animal models of Smith-Lemli-Opitz Syndrome. Autophagy 1–22. [DOI] [PMC free article] [PubMed]
- Ravin R, Blank PS, Steinkamp A, Rappaport SM, Ravin N, Bezrukov L, Guerrero-Cazares H, Quinones-Hinojosa A, Bezrukov SM, Zimmerberg J, 2012. Shear forces during blast, not abrupt changes in pressure alone, generate calcium activity in human brain cells. PLoS One 7, e39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A, 2020. Glia of the human retina. Glia 68, 768–796. [DOI] [PubMed] [Google Scholar]
- Ritenour AE, Baskin TW, 2008. Primary blast injury: update on diagnosis and treatment. Crit. Care Med 36, S311–S317. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JV, McFarlane AC, Bragge P, Armonda RA, Grimes JB, Ling GS, 2013. Blast-related traumatic brain injury. Lancet Neurol 12, 882–893. [DOI] [PubMed] [Google Scholar]
- Rossi T, Boccassini B, Esposito L, Clemente C, Iossa M, Placentino L, Bonora N, 2012. Primary blast injury to the eye and orbit: finite element modeling. Invest. Ophthalmol. Vis. Sci 53, 8057–8066. [DOI] [PubMed] [Google Scholar]
- Sahlgren CM, Mikhailov A, Hellman J, Chou YH, Lendahl U, Goldman RD, Eriksson JE, 2001. Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J. Biol. Chem 276, 16456–16463. [DOI] [PubMed] [Google Scholar]
- Sandoval IV, Colaco CA, Lazarides E, 1983. Purification of the intermediate filament-associated protein, synemin, from chicken smooth muscle. Studies on its physicochemical properties, interaction with desmin, and phosphorylation. J. Biol. Chem 258, 2568–2576. [PubMed] [Google Scholar]
- Sanghvi-Shah R, Weber GF, 2017. Intermediate filaments at the junction of mechanotransduction, migration, and development. Front. Cell Dev. Biol 5, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders GH, Echt KV, 2012. Blast exposure and dual sensory impairment: an evidence review and integrated rehabilitation approach. J. Rehabil. Res. Dev 49, 1043–1058. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9 (7), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaner SA, Feola AJ, Ethier CR, 2020. Factors affecting optic nerve head biomechanics in a rat model of glaucoma. J. R. Soc., Interface 17, 20190695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TE, Kirkman E, Haque M, Gibb IE, Mahoney P, Hardman JG, 2017. Primary blast lung injury - a review. Br. J. Anaesth 118, 311–316. [DOI] [PubMed] [Google Scholar]
- Sergent-Tanguy S, Michel DC, Neveu I, Naveilhan P, 2006. Long-lasting coexpression of nestin and glial fibrillary acidic protein in primary cultures of astroglial cells with a major participation of nestin(+)/GFAP(−) cells in cell proliferation. J. Neurosci. Res 83, 1515–1524. [DOI] [PubMed] [Google Scholar]
- Sherwood D, Sponsel WE, Lund BJ, Gray W, Watson R, Groth SL, Thoe K, Glickman RD, Reilly MA, 2014. Anatomical manifestations of primary blast ocular trauma observed in a postmortem porcine model. Invest. Ophthalmol. Vis. Sci 55, 1124–1132. [DOI] [PubMed] [Google Scholar]
- Steinert PM, Chou YH, Prahlad V, Parry DA, Marekov LN, Wu KC, Jang SI, Goldman RD, 1999. A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co- assembly in vitro to form heteropolymers with type III vimentin and type IV alpha- internexin. J. Biol. Chem 274, 9881–9890. [DOI] [PubMed] [Google Scholar]
- Su PH, Chen CC, Chang YF, Wong ZR, Chang KW, Huang BM, Yang HY, 2013. Identification and cytoprotective function of a novel nestin isoform, Nes-S, in dorsal root ganglia neurons. J. Biol. Chem 288, 8391–8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawk M, Titeux M, Fallet C, Li Z, Daumas-Duport C, Cavalcante LA, Paulin D, Moura-Neto V, 2003. Synemin expression in developing normal and pathological human retina and lens. Exp. Neurol 183, 499–507. [DOI] [PubMed] [Google Scholar]
- Thomas CN, Courtie E, Bernardo-Colon A, Essex G, Rex TS, Ahmed Z, Blanch RJ, 2020. Assessment of necroptosis in the retina in a repeated primary ocular blast injury mouse model. Exp. Eye Res 197, 108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titeux M, Brocheriou V, Xue Z, Gao J, Pellissier JF, Guicheney P, Paulin D, Li Z, 2001. Human synemin gene generates splice variants encoding two distinct intermediate filament proteins. Eur. J. Biochem 268, 6435–6449. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Qiu C, Bull E, Allen W, Kotoda Y, Talukdar S, Smith LEH, Fu Z, 2021. Muller glial responses compensate for degenerating photoreceptors in retinitis pigmentosa. Exp. Mol. Med 53, 1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar MA, Bell RS, Neal CJ, 2021. Epidemiology of blast neurotrauma: a meta- analysis of blast injury patterns in the military and civilian populations. World Neurosurg 146, 308–314 e303. [DOI] [PubMed] [Google Scholar]
- Uzunalli G, Herr S, Dieterly AM, Shi R, Lyle LT, 2021. Structural disruption of the blood-brain barrier in repetitive primary blast injury. Fluids Barriers CNS 18, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC, 2016. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res 51, 1–40. [DOI] [PubMed] [Google Scholar]
- Vermeer MC, Bolling MC, Bliley JM, Arevalo Gomez KF, Pavez-Giani MG, Kramer D, Romero-Herrera PH, Westenbrink BD, Diercks GF, van den Berg MP, Feinberg AW, Sillje HHW, van der Meer P, 2021. Gain-of-function mutation in ubiquitin-ligase KLHL24 causes desmin degradation and dilatation in hiPSC-derived engineered heart tissues. J. Clin. Invest 131 (17), e140615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DJ, Greenberg DS, Sawinski J, Rulla S, Notaro G, Kerr JN, 2013. Rats maintain an overhead binocular field at the expense of constant fusion. Nature 498, 65–69. [DOI] [PubMed] [Google Scholar]
- Wang HC, Choi JH, Greene WA, Plamper ML, Cortez HE, Chavko M, Li Y, Dalle Lucca JJ, Johnson AJ, 2014. Pathophysiology of blast-induced ocular trauma with apoptosis in the retina and optic nerve. Mil. Med 179, 34–40. [DOI] [PubMed] [Google Scholar]
- Xue L, Ding P, Xiao L, Hu M, Hu Z, 2010. Nestin, a new marker, expressed in Muller cells following retinal injury. Can. J. Neurol. Sci 37, 643–649. [DOI] [PubMed] [Google Scholar]
- Zou YY, Kan EM, Lu J, Ng KC, Tan MH, Yao L, Ling EA, 2013. Primary blast injury-induced lesions in the retina of adult rats. J. Neuroinflammation 10, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


