Abstract
Three additional ATPase genes, clustered in the order ahaH, ahaI, and ahaK, were found upstream of the previously characterized genes ahaECFABDG coding for the archaeal A1Ao ATPase from Methanosarcina mazei. ahaH, the first gene in the cluster, is preceded by a conserved promoter sequence. Northern blot analysis revealed that the clusters ahaHIK and ahaECFABDG are transcribed as one message. AhaH is a hydrophilic polypeptide and is similar to peptides of previously unassigned function encoded by genes preceding postulated ATPase genes in Methanobacterium thermoautotrophicum and Methanococcus jannaschii. AhaI has a two-domain structure with a hydrophilic domain of 39 kDa and a hydrophobic domain with seven predicted transmembrane α helices. It is similar to the 100-kDa polypeptide of V1Vo ATPases and is therefore suggested to participate in proton transport. AhaK is a hydrophobic polypeptide with two predicted transmembrane α helices and, on the basis of sequence comparisons and immunological studies, is identified as the proteolipid, a polypeptide which is essential for proton translocation. However, it is only one-half and one-third the size of the proteolipids from M. thermoautotrophicum and M. jannaschii, respectively. ahaK is expressed in Escherichia coli, and it is incorporated into the cytoplasmic membrane despite the different chemical natures of lipids from archaea and bacteria. This is the first report on the expression and incorporation into E. coli lipids of a membrane integral enzyme from a methanogens, which will facilitate analysis of the structure and function of the membrane domain of the methanoarchaeal ATPase.
A major goal in understanding the physiology of methanogens has been to delineate the mechanism by which they produce ATP. In recent years it has become evident that methanogenic archaea do not synthesize ATP by substrate level phosphorylation but couple the conversion of all substrates known to the generation of ion gradients, H+ and Na+, across the cytoplasmic membrane (5, 19). All methanogens investigated so far contain a H+-translocating A1Ao ATPase. These enzymes have been purified from a number of organisms, but depending on the isolation procedure, two to six nonidentical subunits have been found (3, 11–14, 26, 35). Recently, the genome sequencing projects revealed eight putative ATPase genes on the chromosomes of Methanobacterium thermoautotrophicum and Methanococcus jannaschii, as deduced from sequence comparisons (2, 28). However, the exact subunit composition of the ATPase remains to be established.
In an ongoing project dealing with the elucidation of the mechanism of ATP synthesis in methanogens, we have purified the ATP synthase from Methanosarcina mazei and concluded from biochemical and molecular data that the hydrophilic domain contains at least seven subunits and that the corresponding genes (ahaECFABDG) are clustered on the chromosome (35). Furthermore, a 36- and a 7-kDa polypeptide were found in the purified enzyme and were assigned to the membrane domain; the 7-kDa polypeptide was identified as the proteolipid. Unfortunately, neither the genes encoding the 36- and 7-kDa polypeptides nor any genes encoding any other potential membrane-spanning subunits were found in that study (35). In order to determine unequivocally the subunit composition of the A1Ao ATPase from the methylotrophic M. mazei, we sequenced the region upstream of ahaE and found three more ATPase genes. Two encode subunits of the membrane domain, the proteolipid and the homolog of the 100-kDa polypeptide from V1Vo ATPases. The third encodes a hydrophilic peptide similar to deduced gene products of previously unassigned function in M. thermoautotrophicum and M. jannaschii.
We will show here that the proteolipid of M. mazei is expressed in Escherichia coli and is targeted to the cytoplasmic membrane, which will facilitate future work on the analysis of the structure and function of the proton conductance pathway in methanoarchaeal ATPases.
MATERIALS AND METHODS
Organisms and plasmids.
M. mazei Gö1 (DSM 3647) was obtained from the Deutsche Sammlung für Mikroorganismen und Zellkulturen, Braunschweig, Federal Republic of Germany. M. mazei was grown under strictly anaerobic conditions as described by Hippe et al. (9). Wild-type Saccharomyces cerevisiae (SF838-1Då MATα ade6 leu2-3 leu2-112 ura3-52 his4-519 pep4-3 gal2) and vma3Δ (SF838-1Då vma3-Δ1, isogenic with SF838-1Då except for vma3Δ::ura), vma11Δ (RHA107, isogenic with SF838-1Då except for vma11Δ::leu), and vma16Δ (LGY10, isogenic with SF838-1Då except for vma16Δ::leu) mutants were obtained from T. H. Stevens, University of Oregon, Eugene, and were grown on synthetic dropout medium on yeast extract-peptone-dextrose (YEPD) at pH 5.0 or 7.0 as described elsewhere (27, 36). E. coli DH5α (supE44 ΔlacU169 Φ80 lacZΔM15 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 [8]), DK8 (1100Δ[uncB-uncC] ilv::Tn10 [16]), MJM413 (F+ asnA+ asnB31 thi-1 recA56 srl-1300::Tn10 atpE1003 [leu-4→amber] [7]), and LE392 (supE44 supF58 hsdR514 galK2 galT22 metB trpR55 lacY1 [22]) were grown at 37°C on Luria-Bertani medium or on minimal medium supplemented with methionine and tryptophan (each at 50 μg/ml). Where indicated, either 1% glucose or 1% succinate was used as a carbon and energy source (31). Plasmids used were pJLA603 (25), pHSG398, pHSG399 (30), pVT100-U, pVT102-U (32), and pRT1001, which is an XbaI subclone of pEW1 (35).
For the expression studies, ahaK was amplified by PCR by introducing an NdeI restriction site at the 5′ end and a BamHI restriction site at the 3′ end. The 252-bp fragment containing ahaK of M. mazei (primer ahaK/U [5′-GAATAAACATATGGTAGACGCAGCA-3′] and primer ahaK/R [5′-TAGGATCCTAAATTTAAGTAAAATC-3′]) was cloned into pJLA603, giving rise to pRT200. To complement the proteolipid mutant of E. coli, MJM413, plasmids pRT201 (pHSG398::ahaK) and pRT202 (pHSG399::ahaK) were constructed by cloning the XhoI/BamHI fragment of pRT200 into pHSG399 and pHSG398; in pRT202 and pRT201, ahaK is oriented colinear and with opposite polarity to the lac promoter, respectively. For complementation studies of the Δvma11::leu and Δvma16::leu proteolipid mutants of S. cerevisiae, the ahaK gene was cloned into the yeast expression vectors pVT100-U and pVT102-U under the control of the adh promoter. To complement the S. cerevisiae, Δvma3::ura mutant, a leucine cassette was inserted into the uracil cassette in pVT100-U and pVT102-U, giving rise to pVM1001 and pVM1002, respectively. The ahaK gene was cloned into the four above-mentioned plasmids after digestion of pRT201 with PstI and BamHI. The resulting plasmids were named pRT203 (pVT100-U::ahaK), pRT204 (pVT102-U::ahaK), pVM1003 (pVM1001::ahaK), and pVM1004 (pVM1002::ahaK). In pRT203 and pVM1003, the gene is oriented 5′→3′ with respect to the adh promoter; in pRT204 and pVM1004, it is oriented with the opposite polarity.
Northern blotting.
RNA was prepared from M. mazei grown on methanol (200 mM). Cells were harvested anaerobically (4,000 rpm; 10 min; 4°C) at an optical density at 600 nm (OD600) of 0.6, and RNA isolation and blotting were carried out as described elsewhere (22), with the following modifications. The pellet was resuspended in 30 mM sodium acetate (pH 5.5)–1.5% sodium dodecyl sulfate) (SDS), and RNA was isolated by extraction with phenol (equilibrated with 20 mM sodium acetate–1 mM EDTA–0.1% [wt/vol] SDS [pH 5.5]) and chloroform-isoamyl alcohol (24:1) at 65°C.
Molecular procedures.
All procedures were performed according to standard techniques (22). DNA sequences were determined from nested deletion clones by the chain termination method of Sanger et al. and were analyzed on a UNIX computer by using the Genetics Computer Group package (6, 23).
Expression studies.
For complementation assays, E. coli MJM413 was transformed with the plasmids indicated and plated on Tanaka plates containing glucose and chloramphenicol. Transformants were streaked in parallel on Tanaka plates containing glucose and chloramphenicol and on Tanaka plates containing succinate and chloramphenicol in the absence or presence of isopropyl-β-d-thiogalactopyranoside (IPTG) and were incubated at different temperatures. Yeast competent cells were transformed with the plasmids indicated and plated on synthetic dropout medium. Transformants were streaked in parallel on YEPD plates at pH 5.0 or 7.0 and were incubated at 30°C. For heterologous expression of AhaK in E. coli DK8, competent cells were transformed with the plasmids indicated, and expression was achieved by thermal induction (42°C) after the cultures reached an OD600 of 0.1. The cultures remained at 42°C for another 3 to 4 h. SDS-polyacrylamide gel electrophoresis (PAGE) was performed on 12% polyacrylamide gels according to the procedure given in reference 24.
Immunological studies.
Western blotting with SDS-polyacrylamide gels was performed as described elsewhere (35). Polyclonal antibodies against AhaK (34) were obtained from F. Mayer, Göttingen, Federal Republic of Germany.
Nucleotide sequence accession number.
The nucleotide sequences reported in this paper have been submitted to GenBank under accession no. U47274.
RESULTS
Nucleotide sequence of the 5′ end of the aha operon from M. mazei and its transcriptional analysis.
In a previous study, the genes ahaECFABDG were found to encode hydrophilic subunits of the A1Ao ATPase of M. mazei (35). The DNA sequence upstream of ahaE of M. mazei was determined. Directly upstream of ahaE is an apparently noncoding, AT-rich region of 211 bp. Upstream of the intergenic region, three ATPase genes, designated ahaH, ahaI and ahaK, were identified; they are 330, 1,950, and 243 bp long, respectively. ahaH overlaps with ahaI by 8 bp, and ahaI and ahaK are separated by only 4 bp. Each of the genes is preceded by a well-placed and well-conserved Shine-Dalgarno sequence, and translation is initiated by an ATG codon in all cases. Upstream of ahaH is an AT-rich region which contains two potential archaeal promoter sequences (Fig. 1). The DNA sequence (1,200 bp) upstream of this potential promoter sequence does not contain any ATPase genes.
FIG. 1.
DNA and deduced amino acid sequences of the 5′ terminus of the aha operon. The start of ahaH is indicated. Conserved residues of putative promoter sequences (boxes A and B) are marked by asterisks. The putative ribosome binding site of ahaH is underlined.
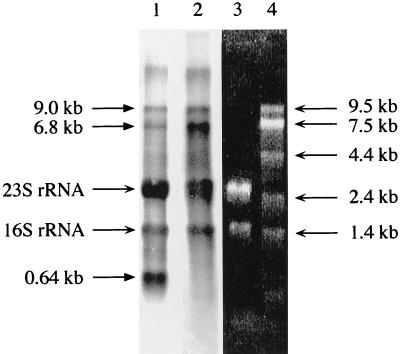
The gene cluster ahaECFABDG clearly codes for subunits of the A1Ao ATPase (35). However, sequence comparisons alone do not allow the assignment of AhaHIK to subunits of the A1Ao ATPase, since the ahaHIK cluster is separated from the larger cluster by 211 bp and since M. mazei has been shown to possess two structurally related ATPases (1). When total RNA was isolated from cells grown on methanol and was hybridized against ahaE or ahaK, the same pattern was observed, indicating that both clusters belong to the same transcriptional unit (Fig. 2). The 9.0-kb transcript corresponds well to a mRNA covering ahaH through ahaG. Whether the 6.8-kb transcript is derived from a second transcriptional start point or by a modification of the 9.0-kb transcript remains to be elucidated. The 0.64-kb transcript hybridizing to the proteolipid-encoding gene, ahaK, indicates an additional definite transcription of ahaK only. This is of particular importance because the proteolipid is present in 9 to 12 copies per ATPase molecule. In bacteria, enhanced synthesis of the proteolipid is achieved by translational but not by transcriptional regulation (18). Putative, but not well-conserved, promoter sequences are found approximately 0.6 kb upstream of a weak transcriptional terminator downstream of ahaK.
FIG. 2.
Northern blot of total RNA of methanol-grown cells of M. mazei. Lane 1, autoradiograph of a sample probed with ahaK; lane 2, autoradiograph of a sample probed with ahaE; lane 3, total RNA in formaldehyde agarose gel; lane 4, RNA standard in formaldehyde agarose gel.
AhaH.
AhaH has a molecular weight of 12,200, consists of 109 residues, and has a deduced pI of 4.74. The peptide is highly charged; 21 and 16% of the residues are acidic and basic, respectively. Database searches identified the following deduced gene products with previously unassigned function as homologs of AhaH: MJ0223 of M. jannaschii (25% identity) (2), MT0961 of M. thermoautotrophicum (25% identity) (28), and AF1158 of Archaeoglobus fulgidus (29% identity) (15). Twenty-three and 44% of the amino acids of AhaH are identical and similar, respectively, to those of NtpF of Enterococcus hirae (29). Therefore, there is compelling reason to designate MJ0223, MT0961, and AF1158 atpHMj, atpHMt, and atpHAf. The fact that ahaH, atpHMj, atpHMt, and atpHAf are the first genes in their operons suggests that they could correspond to uncI of bacterial F1Fo ATPases (33), but only 12% of the amino acids of UncI of E. coli and AhaH were found to be identical.
AhaI.
ahaI encodes a peptide of 649 residues with a molecular weight of 72,048 and a calculated pI of 5.80. Twenty-seven, 30, and 35% of its residues are conserved in AtpIMt, AtpIMj, and AtpIAf, respectively, and 20 (Vph1p of S. cerevisiae [17]) to 24% (Vph1p of Bos taurus [21]) of its residues are conserved in the 100-kDa subunits of the V1Vo ATPases of eucarya. Twenty-seven percent of the residues of NtpI from E. hirae are identical to those of AhaI. Hydrophobicity plots of AhaI propose a highly hydrophilic N-terminal domain of 39 kDa and a hydrophobic C-terminal domain of 33 kDa. Garnier analysis of the hydrophilic domain of AhaI predicts a highly α-helical structure, as is the case with subunit b of the F1Fo ATPases. Interestingly, the similarities of the hydrophilic domain of subunit b of the F1Fo ATPases to the hydrophilic domains of AhaI and AtpIMj are 22 and 26%, respectively. The hydrophobic C termini of AhaI and AtpI are predicted to have six to seven putative transmembrane helices. The similarity to subunit a of the F1Fo ATPases is below 20%.
AhaK.
ahaK codes for a protein with 80 residues and a molecular mass of 7.9 kDa. The pI was determined to be 4.04. Hydrophobicity plots predict one hairpin with two transmembrane α helices. It is very similar to proteolipids from other archaea such as M. thermoautotrophicum (35% identity) (28), M. jannaschii (45% identity) (2), A. fulgidus (52% identity) (15), Sulfolobus acidocaldarius (31% identity) (4), and Halobacterium salinarum (52% identity) (10). Thirty-three percent of the residues are conserved in AhaK and NtpK from E. hirae (29). On the basis of its molecular mass, AhaK is more similar to the proteolipids of bacteria, but on the basis of sequence analysis, it is more closely related to the proteolipids of V1Vo ATPases from eucarya; the degrees of identity range from 26.7 to 31.4%. The active carboxylate of helix 2, which is conserved in all proteolipids known so far, is present at position 65 (Fig. 3). Since the proteolipid is essential for proton translocation, it was analyzed in more detail.
FIG. 3.
Alignment of the proteolipids from M. mazei (Mma), M. thermoautotrophicum (Mth) (28), M. jannaschii (Mja) (2), A. fulgidus (Afu) (15), H. salinarum (Hsa) (10), and S. acidocaldarius (Sac) (4). Conserved residues are shaded, and putative transmembrane segments are boxed. The boundaries of the transmembrane segments are hypothetical. Leader sequences of the proteolipids from H. salinarum and S. acidocaldarius are not shown.
Expression of the proteolipid in E. coli.
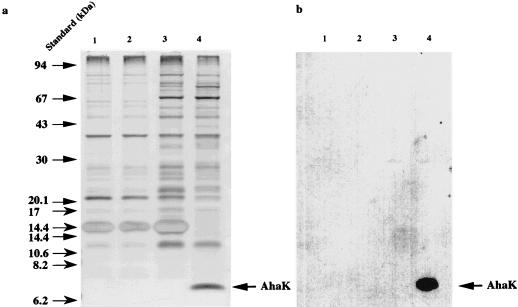
To facilitate a biochemical and biophysical analysis of the proteolipid from methanogens, we cloned ahaK from M. mazei into the expression vector pJLA603, and the resulting plasmid, pRT200, was transformed into the ATPase-negative E. coli strain DK8. Upon induction of expression, the growth of the host cells ceased (data not shown). Cells were harvested and separated into cytoplasmic and membrane fractions and were subjected to SDS-PAGE. Only the membranes of E. coli DK8(pRT200) contained a polypeptide of 7 kDa, which cross-reacted with an antibody directed against the proteolipid of M. mazei (Fig. 4). Interestingly, the proteolipid was inserted into the E. coli cytoplasmic membrane despite the different chemical natures of lipids from bacteria and archaea. This offers a new opportunity to study the proteolipid from methanogens in a heterologous system.
FIG. 4.
SDS-PAGE and Western blot of the proteolipid from M. mazei expressed in E. coli. ahaK was expressed by raising the temperature to 42°C at an OD600 of 0.1. Cells were harvested at an OD600 of 1. Protoplasts were prepared, lysed by osmotic shock, and separated into cytoplasm and membrane fractions, and the membrane fractions were subjected to SDS-PAGE (a) and Western blotting (b). Lanes 1, DK8(pJLA603), not induced; lanes 2, DK8(pJLA603), induced; lanes 3, DK8(pRT200), not induced; lanes 4, DK8(pRT200), induced.
DISCUSSION
The A1Ao ATPase operon from M. mazei and its implications for the subunit composition of methanoarchaeal ATPases.
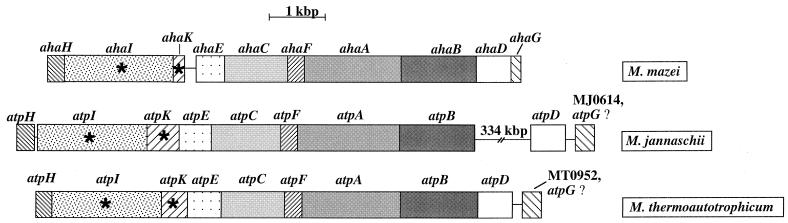
The molecular data revealed that the A1Ao ATPase of M. mazei is encoded by 10 genes, ahaH through ahaG (Fig. 5). Apparently, the gene structure at the 5′ end is identical in all archaeal A1Ao and bacterial V1Vo ATPase operons known so far. It is noteworthy that AhaH and AhaI have homologs not in F1Fo ATPases but in V1Vo ATPases. According to its molecular mass, AhaK, the proteolipid, is similar to F1Fo ATPases, but its primary structure is more similar to that of the proteolipids of V1Vo ATPases than to those of F1Fo ATPases. Attempts to genetically complement proteolipid mutants of E. coli and S. cerevisiae failed (see Materials and Methods), although ahaK was expressed in both, as shown by Western blots (data for expression in S. cerevisiae are not shown). Apparently, it was not assembled functionally into F1Fo or V1Vo ATPases. Taken together, the subunit composition and any given polypeptide of the methanoarchaeal A1Ao ATPase are more similar to V1Vo than to F1Fo ATPase subunits. This demonstrates a close evolutionary linkage of A1Ao and V1Vo ATPases.
FIG. 5.
Organization of genes in methanoarchaeal A1Ao ATPase operons. Genes encoding hydrophobic subunits are marked by asterisks. Homologous genes are depicted by the same pattern. Data are from references 2 and 28.
Structure of the membrane domain.
From the data presented in this study, it is obvious that the Ao domain consists of only two different subunits, the homolog of the 100-kDa subunit of V1Vo ATPases and the proteolipid. This is in contrast to previous results in which a 36-kDa protein, but not AhaI, was found in the membrane fraction of the purified enzyme (35). However, it is conceivable that AhaI is sensitive to proteolysis, and a loss of the hydrophilic domain during the purification procedure would leave only a ∼36-kDa domain in the membrane, as reported for the 100-kDa subunits of V1Vo ATPases.
One of the major differences between A1Ao and V1Vo ATPases known hitherto was the sizes of their proteolipids; this obvious difference was believed to be the reason for the apparent inability of V1Vo ATPases to synthesize ATP (20). An experimental approach verifying or questioning this assumption was hindered by the fact that the 7-kDa proteolipid from M. mazei was not assembled functionally into the F1Fo ATPase from E. coli or the V1Vo ATPase from S. cerevisiae (see above). Fortunately, a solution to this interesting question comes with the genomic sequences from M. jannaschii and M. thermoautotrophicum (2, 28). The proteolipid of M. thermoautotrophicum is predicted to be 15.6 kDa with four transmembrane helices, and that of M. jannaschii is predicted to be 21.3 kDa with six transmembrane helices. From the genomic sequences it is evident that the A1Ao ATPases are the only ATPases present in M. jannaschii and M. thermoautotrophicum. Since these organisms gain energy only by ion gradient-driven phosphorylation, there is no doubt that they function as ATP synthases, and direct experimental proof that the A1Ao ATPases from methanogens function as ATP synthases is available (19).
Two important conclusions have to be drawn from these observations: first, duplication of the proteolipid genes is already observed in archaea, and second, it not accompanied by a failure of the ATPase to synthesize ATP. Therefore, the apparent inability of V1Vo ATPases to synthesize ATP can no longer be attributed to the size of the proteolipid respective to its number of transmembrane α helices. More likely to be important is the number of active carboxylates per ATPase. Assuming a constant number of 12 hairpins per enzyme molecule, the F1Fo ATPase contains 12 glutamates, the A1Ao ATPases of M. thermoautotrophicum and M. mazei also contain 12, and the A1Ao ATPase of M. jannaschii contains 8, but the V1Vo ATPases contain only 6.
ACKNOWLEDGMENTS
This work was supported by a grant from the Deutsche Forschungsgemeinschaft.
We are grateful to F. Mayer, University of Göttingen, for the generous gift of the AhaK-specific antibody. V.M. appreciated the warm welcome, stimulating discussions, and introduction to yeast genetics by T. H. Stevens, L. A. Graham, and colleagues during a stay in Eugene, Oreg., in which the expression studies of ahaK in yeast were initiated.
REFERENCES
- 1.Becher B, Müller V. ΔμNa+ drives the synthesis of ATP via an ΔμNa+− translocating F1F0-ATP synthase in membrane vesicles of the archaeon Methanosarcina mazei Gö1. J Bacteriol. 1994;176:2543–2550. doi: 10.1128/jb.176.9.2543-2550.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bult C J, White O, Olsen G J, Zhou L X, Fleischmann R D, Sutton G G, Blake J A, Fitzgerald L M, Clayton R A, Gocaynee J D, Kerlavage A R, Dougherty B A, Tomb J F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Weidman J F, Fuhrmann J L, Nguyen D, Utterback T R, Kelley J M, Peterson J D, Sadow P W, Hanna M C, Cotton M D, Roberts K M, Hurst M A, Kaine B P, Borodovsky M, Klenk H-P, Fraser C M, Smith H O, Woese C R, Venter J G. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Konisky J. Characterization of a membrane-associated ATPase from Methanococcus voltae, a methanogenic member of the Archaea. J Bacteriol. 1993;175:5677–5682. doi: 10.1128/jb.175.17.5677-5682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denda K, Konishi J, Oshima T, Date T, Yoshida M. A gene encoding the proteolipid subunit of Sulfolobus acidocaldarius ATPase complex. J Biol Chem. 1989;264:7119–7121. [PubMed] [Google Scholar]
- 5.Deppenmeier U, Müller V, Gottschalk G. Pathways of energy conservation in methanogenic Archaea. Arch Microbiol. 1996;165:149–163. [Google Scholar]
- 6.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis program for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraga D, Fillingame R H. Essential residues in the polar loop region of subunit c of Escherichia coli F1F0 ATP synthase definded by random oligonucleotide-primed mutagenesis. J Bacteriol. 1991;173:2639–2643. doi: 10.1128/jb.173.8.2639-2643.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 9.Hippe H, Caspari D, Fiebig K, Gottschalk G. Utilization of trimethylamine and other methyl compounds and methane formation by Methanosarcina barkeri. Proc Natl Acad Sci USA. 1979;76:494–498. doi: 10.1073/pnas.76.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ihara K, Watanabe S, Sugimura K, Mukohata Y. Identification of proteolipid from an extremely halophilic archaeon, Halobacterium salinarum, as an N,N′-dicyclohexyl-carbodiimide binding subunit of ATP synthase. Arch Biochem Biophys. 1997;341:267–272. doi: 10.1006/abbi.1997.9972. [DOI] [PubMed] [Google Scholar]
- 11.Inatomi K. ATP-dependent H+-pump activity in inverted vesicles of Methanosarcina mazei Gö1 and characterization of membrane ATPase. J Bacteriol. 1996;178:2424–2426. doi: 10.1128/jb.178.8.2424-2426.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inatomi K I. Characterization and purification of the membrane-bound ATPase of the archaebacterium Methanosarcina barkeri. J Bacteriol. 1986;167:837–841. doi: 10.1128/jb.167.3.837-841.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inatomi K I, Kamagata Y, Nakamura K. Membrane ATPase from the aceticlastic methanogen Methanothrix thermophila. J Bacteriol. 1993;175:80–84. doi: 10.1128/jb.175.1.80-84.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inatomi K I, Maeda M, Futai M. Dicyclohexylcarbodiimide-binding protein is a subunit of the Methanosarcina barkeri ATPase complex. Biochem Biophys Res Commun. 1989;162:1585–1590. doi: 10.1016/0006-291x(89)90856-5. [DOI] [PubMed] [Google Scholar]
- 15.Klenk H-P, Clayton R A, Tomb J-F, White O, Nelson K E, Ketchum K A, Dodsen R J, Gwinn M, Hickey E K, Peterson J D, Richardson D L, Kerlavage A R, Graham D E, Kyrpides N C, Fleischmann R D, Quackenbush J, Lee N H, Sutton G G, Gill S, Kirkness E F, Dougherty B A, McKenny K, Adams M D, Loftus B, Peterson S, Reich C I, McNeil L K, Badger J H, Glodek A, Zhou L, Overbeek R, Gocayne J D, Weidman J F, McDonald L, Utterback T, Cotton M D, Spriggs T, Artiach P, Kaine B P, Sykes S M, Sadow P W, D’Andrea K P, Bowman C, Fujii C, Garland S A, Mason T M, Olsen G J, Fraser C M, Smith H O, Woese C R, Venter J C. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeglobus fulgidus. Nature. 1997;390:364–370. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 16.Klionsky D J, Brusilow W S A, Simoni R D. In vivo evidence for the role of the ɛ subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J Bacteriol. 1984;160:1055–1060. doi: 10.1128/jb.160.3.1055-1060.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manolson M F, Proteau D, Preston R A, Stenbit A, Roberts B T, Hoyt M A, Preuss D, Mulholland J, Botstein D, Jones E W. The VPH1 gene encodes a 95-kDa integral membrane polypeptide required for in vivo assembly and activity of the yeast vacuolar H+-ATPase. J Biol Chem. 1992;267:14294–14303. [PubMed] [Google Scholar]
- 18.McCarthy J E G, Schairer H U, Sebald W. Translational initiation frequency of atp genes from Escherichia coli: identification of an intercistronic sequence that enhances translation. EMBO J. 1985;4:519–526. doi: 10.1002/j.1460-2075.1985.tb03659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller V, Blaut M, Gottschalk G. Bioenergetics of methanogenesis. In: Ferry J G, editor. Methanogenesis. New York, N.Y: Chapman & Hall; 1993. pp. 360–406. [Google Scholar]
- 20.Nelson N. Evolution of organellar proton-ATPases. Biochim Biophys Acta. 1992;1100:109–124. doi: 10.1016/0005-2728(92)90072-a. [DOI] [PubMed] [Google Scholar]
- 21.Peng S B, Crider B P, Xie X-S, Stone D K. Alternative mRNA splicing generates tissue-specific isoforms of 116-kDa polypeptide of vacuolar proton pump. J Biol Chem. 1994;269:17262–17266. [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:369–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 25.Schauder B, Blöcker H, Frank R, McCarthy J E G. Inducible expression vectors incorporating the Escherichia coli atpE translational initiation region. Gene. 1987;52:279–283. doi: 10.1016/0378-1119(87)90054-0. [DOI] [PubMed] [Google Scholar]
- 26.Scheel E, Schäfer G. Chemiosmotic energy conservation and the membrane ATPase of Methanolobus tindarius. Eur J Biochem. 1990;187:727–735. doi: 10.1111/j.1432-1033.1990.tb15360.x. [DOI] [PubMed] [Google Scholar]
- 27.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 28.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, Harrison D, Hoang L, Keagle P, Lumm W, Pothier B, Qiu D, Spadafora R, Vicaire R, Wang Y, Wierzbowski J, Gibson R, Jiwani N, Caruso A, Bush D, Safer H, Patwell D, Prabhakar S, McDougall S, Shimer G, Goyal A, Pietrokovski S, Church G M, Daniels C J, Mao J-I, Rice P, Nölling J, Reeve J N. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takase K, Kakinuma S, Yamato I, Konishi K, Igarashi K, Kakinuma Y. Sequencing and characterization of the ntp gene cluster for vacuolar-type Na+-translocating ATPase of Enterococcus hirae. J Biol Chem. 1994;269:11037–11044. [PubMed] [Google Scholar]
- 30.Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZα-complementation and chloramphenicol-resistance selection. Gene. 1987;61:63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka S, Lerner S A, Lin E C C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967;93:642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vernet T, Dignard D, Thomas D Y. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52:225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- 33.Walker J E, Saraste M, Gay N J. The unc operon. Nucleotide sequence, regulation and structure of ATP-synthase. Biochim Biophys Acta. 1984;768:164–200. doi: 10.1016/0304-4173(84)90003-x. [DOI] [PubMed] [Google Scholar]
- 34.Wilms R. Ph.D. thesis. GöttingenGöttingen, Germany: University of ; 1992. [Google Scholar]
- 35.Wilms R, Freiberg C, Wegerle E, Meier I, Mayer F, Müller V. Subunit structure and organization of the genes of the A1Ao ATPase from the archaeon Methanosarcina mazei Gö1. J Biol Chem. 1996;271:18843–18852. doi: 10.1074/jbc.271.31.18843. [DOI] [PubMed] [Google Scholar]
- 36.Yamashiro C T, Kane P M, Wolczyk D F, Preston R A, Stevens T H. Role of vacuolar acidification in protein sorting and zymogen activation: a genetic analysis of the yeast vacuolar proton-translocating ATPase. Mol Cell Biol. 1990;10:3737–3749. doi: 10.1128/mcb.10.7.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]