ABSTRACT
As the proportion of older people in the world’s population steadily increases, there is an urgent need to identify ways to support healthy aging. The gut microbiome has been proposed to be involved in aging-related diseases and has become an attractive target for improving health in older people. Herein, we cover the relationship between the gut microbiome and chronological age in adults, and then, we discuss the gut microbiome features associated with frailty, as a hallmark of unhealthy aging in older people. Furthermore, we describe the effects of microbiome-targeted interventions, such as dietary patterns and consumption of probiotics, prebiotics, and synbiotics, on modulating the gut microbiome composition and further promoting healthy aging. Further studies are needed to explore the underlying mechanisms of gut microbiome-induced aging complications and to develop personalized microbiome-based strategies for reducing the severity of frailty or preventing the onset of frailty in older adults.
KEYWORDS: Gut microbiome, healthy aging, frailty, dysbiosis, intervention
Introduction
Globally, the number of older people and their proportion in the population are increasing. According to the World Population Prospects 2022, the proportion of the global population aged 65 years or above is expected to increase from 10% in 2022 to 16% in 2050, owing to declining fertility and increasing longevity.1 Nevertheless, an extension of lifespan does not necessarily mean an extension of healthspan, the functional and disease-free period of life; there is a gap of 9 years between lifespan and healthspan.2 It is therefore important to understand the mechanism of healthy aging and to identify appropriate interventions that can delay disease onset and reduce severity of diseases in advanced ages.
Aging is a complex biological process that is induced by the accumulation of cellular and molecular damage over time, such as stem cell exhaustion, genetic instability, telomere attrition, cellular senescence, and deregulated nutrient sensing.3 These result in chronic inflammation, which in turn increases the risk of various chronic diseases including type 2 diabetes, cardiovascular diseases, osteoarthritis, and Alzheimer’s disease.4 These deficits also lead to increased frailty, which is a state of increased vulnerability to poor resolution of homeostasis when exposed to stressors.5 Aging processes are influenced by genetic and non-genetic (including lifestyle and environmental) factors, and thus, modulating non-genetic factors is a feasible strategy for promoting healthy aging.
Recent reports indicate that the gut microbiome has tremendous potential in affecting host health by fermenting indigestible food components into absorbable metabolites, maintaining the intestinal integrity, regulating the immune system, and protecting from pathogens.6 In addition, there is increasing evidence that dysbiosis of the gut microbiome is associated with the aforementioned age-related chronic diseases,7 which means that the gut microbiome may have the potential to act as a major regulator of the aging process. Therefore, in recent years, the gut microbiome has become an attractive target for interventions to promote healthy aging.
In this review, we summarize how the gut microbiome changes with age, whether there are differences in the gut microbiome composition between healthy and unhealthy aging, and whether gut microbiome-targeted interventions can improve older people’s health.
The aging-related gut microbiome
The gut microbiome composition of adults is relatively stable throughout life. In healthy adults, the gut microbiome is dominated by Firmicutes and Bacteroidetes, representing over 90% of gut microbes, and smaller proportions of Actinobacteria, Proteobacteria, and Verrucomicrobia.8 Despite the stability of the adult gut microbiome, chronological age is one of the factors that have large effects on inter-individual variations of gut microbiome composition in the Dutch,9,10 Belgian,11 American,12,13 and Chinese populations.14
Studies of the gut microbiome of adults aged ≤90 years have shown associations of individual gut microbial taxa with age (Table 1). For example, in a study performed by our group on 890 South Korean adults, the abundance of short chain fatty acid (SCFA)-producing bacteria, such as Dorea, Blautia, and Coprococcus, decreased with aging, while that of pathobionts, such as Streptococcus, Klebsiella, and Haemophilus, increased with aging. In addition, the abundance of Bacteroides decreased with aging, while Prevotella showed the opposite relationship in this population.17 A study of 1,596 Japanese adults showed that the abundance of Blautia decreased with aging, consistent with the results of a study of South Koreans, while that of another SCFA-producer, Roseburia, increased with aging.18 In a Chinese population, the enrichments of Bacteroides, Bifidobacterium, and Coprococcus, as well as various pathobionts, were observed in older people aged ≥50 years, in contrast to younger people aged <50 years. Additionally, the gut microbiome of older Chinese individuals was enriched by LPS biosynthesis- and SCFA degradation-related metabolic pathways, which were also positively correlated with several species enriched in older adults, such as Escherichia coli, Klebsiella pneumoniae, and Bacteroides fragilis.19 The differences in aging-related gut microbial changes among populations may be related to differences in diets, lifestyles, or health conditions of populations, even among people belonging to the same ethnic groups. Nevertheless, it was also reported that there are common age-related gut microbial species in different ethnic populations, including Chinese, Israeli, and Dutch adults. Across all three populations, B. bifidum and B. breve showed negative associations with age, while multiple species from the genera Klebsiella, Campylobacter, and Streptococcus showed positive associations with age. This study also showed the significant enrichment of genes related to toxicity, bacterial communication, and adhesion in the gut microbiome of older adults.20 Although particular gut microbes are associated with chronological age, it is not known whether they are related to healthy or unhealthy aging.
Table 1.
Studies investigating associations between the gut microbiome and age.
| Country | N | Age | Method of gut microbiome evaluation | Gut microbes associated with increased age | References |
|---|---|---|---|---|---|
| Italy | 69 | 22–109 years | 16S (V3–V4) |
Coprococcus, Roseburia, Faecalibacterium ↓ Oscillospira, Odoribacter, Butyricimonas, Eggerthella, Akkermansia, Anaerotruncus, Synergistaceae, Bilophila, Christensenellaceae ↑ |
Biagi, 201615 |
| China | 168 | 24–83 years 90–102 years |
16S (V4–V5) | Clostridium cluster XIVa, Ruminococcaceae, Akkermansia, Christensenellaceae ↑ | Kong, 201616 |
| South Korea | 890 | 20–90 years | 16S (V3–V4) |
Bacteroides, Oscillospira, Dorea, Blautia, Coprococcus ↓ Streptococcus, Veillonella, Haemophilus, Klebsiella, Prevotella ↑ |
Lim, 202117 |
| Japan | 1596 | 20–83 years | 16S (V3–V4) |
Blautia, Parabacteroides ↓ Roseburia ↑ |
Park, 202118 |
| China | 614 | 19–49 years 50–87 years |
Shotgun |
Alistipes putredinis, Barnesiella intestinihominis, Megamonas funiformis, Parabacteroides merdae, Subdoligranulum unclassified ↓ Bacteroides (B. cellulosilyticus, B. fragilis, B. intestinalis, B. ovatus, Bacteroides sp 4 3 47FAA, B. thetaiotaomicron), Bifidobacterium (B. longum, B. pseudocatenulatum), Clostridium bolteae, Escherichia (E. coli, Escherichia unclassified), Parabacteroides (P. distasonis, Parabacteroides unclassified), Ruminococcus gnavus, Klebsiella pneumoniae, Dialister invisus, Veillonella unclassified, Mitsuokella multacidawere, Coprococcus eutactus ↑ |
Yan, 202219 |
| China Netherlands Israel |
4346 (China: 2,338 Netherlands: 1,133 Israel: 875) |
18–81 years (China: 26–76 years Netherlands: 18–81 years Israel: 18–70 years) |
Shotgun | Common features in three cohorts Bifidobacterium bifidum, Bifidobacterium breve ↓ Campylobacter concisus, Citrobacter koseri, Klebsiella pneumoniae/Klebsiella variicola group, Klebsiella oxytoca, Klebsiella pneumoniae, Veillonella atypica, Streptococcus gordonii, Lactobacillus salivarius, Pseudoflavonifractor capillosus, Clostridium saccharolyticum, Coprococcus catus, Ruminococcus lactaris, Klebsiella variicola/pneumoniae, Butyrivibrio crossotus ↑ |
Zhang, 202120 |
Centenarians (individuals aged 100 years and older) are considered as a model of healthy aging, as they have reached the extreme limits of human lifespan by surviving, escaping, or delaying age-associated diseases.21 To identify the longevity-specific gut microbiome features, Biagi et al. investigated the gut microbiome in Italian adults of a wide range of ages, including centenarians (99–104 years old) and semi-supercentenarians (105–109 years old). They found that the abundance of Coprococcus, Roseburia, and Faecalibacterium was negatively associated with age, while the abundance of Oscillospira, Odoribacter, and Butyricimonas was positively associated with age. In particular, the gut microbiome of semi-supercentenarians was enriched by Akkermansia and Christensenellaceae.15 Similarly, the enrichment of Akkermansia, Clostridium cluster XIVa, Ruminococcaceae, and Christensenellaceae was observed in the gut microbiome of long-living Chinese people (≥90 years old),16 suggesting that these taxa may contribute to longevity. However, it is important to note that long-living people are not uniformly healthy.
The gut microbiome and frailty
Although aging has several common features in health conditions, as described above, there are differences in the health status of each individual, even at the same age. Therefore, it is necessary to distinguish between the characteristics of the gut microbiome related to healthy and unhealthy aging for identifying the microbial signatures with potential for use in microbiome-based interventions targeted to healthy aging in older people. In this review, we focus on the association of the gut microbiome with frailty as a hallmark of unhealthy aging in older people.
Frailty
Frailty is a common biologic syndrome in older adults and is characterized by reduced physiological reserve and resistance to stressors, accompanied by increased vulnerability to negative health outcomes, such as falls, disability, and hospitalization.22 Frailty is also characterized by its high level of heterogeneity among people of a similar age.23 As the impairment of multiple systems is related to the progression of frailty, a frailty assessment is conducted through complex tests. Multiple frailty assessment instruments have been developed for application in various populations and forms of clinical practice. The two most commonly used frailty measurements are Fried’s Frailty Phenotype and Rockwood’s Frailty Index.24 In the Fried’s Frailty Phenotype model, people with three or more of the five phenotypes, including weak grip strength, low energy expenditure, slow gait speed, self-reported exhaustion, and unintentional weight loss, are considered frail.22 In Rockwood’s Frailty Index of accumulative deficits, frailty is defined as the sum of health deficits, such as signs, symptoms, disabilities, and diseases, divided by the total number of deficits measured.25 However, there is no gold-standard instrument for frailty. Despite the complexity of the frailty assessment, it is considered to be a better indicator of health status in older adults than chronological age, as frailty is a significant predictor of mortality in older people.5
Risk factors for frailty include sociodemographic (e.g., advanced age, female sex, and living alone), lifestyle (e.g., physical inactivity and low protein intake), clinical (e.g., chronic diseases, multimorbidity, and polypharmacy), and biological (e.g., inflammation and micronutrient deficits) factors.26 Among these, modifiable risk factors, such as lifestyle, can be potential targets for prevention of frailty onset or progression. Recently, several studies have reported a link between the gut microbiome and frailty,27–32 suggesting that certain gut microbiome profiles may be another risk factor for frailty. At the same time, because the gut microbiome is potentially modifiable, it may be altered to prevent and treat frailty.
Gut microbiome signatures in frail and non-frail older people
Differences in the gut microbiome composition between frail and non-frail older people have been described in multiple populations (Table 2). For instance, in the gut microbiome of Chinese community dwellers whose frailty was quantified using Fried’s Frailty Phenotype, the abundance of Prevotella, Faecalibacterium, Roseburia, and Blautia was significantly lower in frail older adults, while that of some beneficial bacteria, such as Akkermansia, Bifidobacterium, and Lactobacillus, as well as Klebsiella was higher than those in non-frail older adults.27 A lower abundance of Prevotella copri in frailer older adults was also observed in our study of Korean community dwellers,28 where the frailty assessment was performed using the Korean Frailty Index.33 In addition, the Korean community dwellers’ samples were clustered into two enterotypes based on their gut microbiome composition, represented by Prevotella and Bacteroides, and none of the frail older adults’ samples were assigned to the Prevotella enterotype. The negative association of Coprococcus eutactus and positive association of Bacteroides fragilis and Clostridium hathewayi with the frailty index were also observed.28 Another study investigating frailty association with the gut microbiome in community-dwelling females, whose frailty was quantified using Rockwood’s Frailty Index, from the TwinsUK cohort showed that Faecalibacterium prausnitzii was less abundant in frailer individuals, while Eubacterium dolichum and Eggerthella lenta were more abundant in frailer individuals.29
Table 2.
Studies investigating associations between the gut microbiome and frailty.
| Country | Participants (n, age range) | Frailty instruments | Method of gut microbiome evaluation | Gut microbes associated with increased frailty | References |
|---|---|---|---|---|---|
| China | Community dwellers (94, 70–92) | Fried’s Frailty Phenotype | 16S (V3–V4) |
Parabacteroides, Akkermansia, Klebsiella, Bifidobacterium, Lactobacillus, Pyramidobacter, Alistipes, Dysgonomonas ↑ Faecalibacterium, Roseburia, Prevotella, Megamonas, Blautia, Phascolarctobacterium, Megasphaera, Haemophilus ↓ |
Xu, 202127 |
| South Korea | Community dwellers (176, 70–90) | Korean Frailty Index | 16S (V3–V4) |
Bacteroides fragilis, Clostridium hathewayi ↑ Prevotella copri, Coprococcus eutactus ↓ |
Lim, 202128 |
| UK | Younger community dwelling female twins (728, 42–86) | Rockwood’s Frailty Index | 16S (V4) |
Eubacterium dolichum, Eggerthella lenta ↑ Faecalibacterium prausnitzii ↓ |
Jackson, 201629 |
| US | Nursing home older adults (166, 65–?) | Clinical Frailty Scale | Shotgun |
Bacteroides dorei, Flavonifractor plautii ↑ Bacteroides vulgatus, Anaerostipes hadrus, Faecalibacterium prausnitzii ↓ |
Haran, 202130 |
| US | Skilled nursing facility dwellers (SNFD) (22, 65–97) Community dwellers (CD) (25, 65–91) Young adult (YA) (95, 18–55) |
Rockwood’s Frailty Index Fried’s Frailty Phenotype Physical Activity Scale for the Elderly |
Shotgun |
Clostridium species ↑ in SNFD no Prevotella-rich enterotype in SNFD |
Larson, 202231 |
| Multiple (meta-analysis) |
Community dwellers, nursing homes, hospitalized (340, 63–83) | Fried’s Frailty Phenotype Clinical Frailty Scale Groningen Frailty Indicator etc. |
Shotgun, 16S |
Eggerthella lenta, Eubacterium cylindroides, Eubacterium dolichum ↑ Alistipes shahii, Faecalibacterium prausnitzii, Roseburia inulinivorans ↓ |
Almeida, 202232 |
Older adults in nursing homes tend to be more frail34 and have a higher prevalence of polypharmacy than do community-dwelling older adults.35 Nevertheless, a decrease in F. prausnitzii abundance was consistently observed in the frailer older adults in the nursing home population, similar to what was observed in community-dwelling populations. In addition, more abundant Flavonifractor plautii were observed in the frailer individuals. The frailty of the nursing home population was measured using the Clinical Frailty Scale.30 Recently, a study comparing the microbiome of skilled nursing facility-dwelling older adults (SNFDs), community-dwelling older adults (CDs), and younger adults was reported.31 In the study, the frailty was assessed using Rockwood’s Frailty Index, Fried’s Frailty Phenotype, and the Physical Activity Scale for the Elderly. The gut microbiome of the SNFDs had a significantly increased abundance of Clostridium species. In addition, the study participants were clustered into three enterotypes that were dominated by Ruminococcus, Bacteroides, and Prevotella, based on their gut microbiome composition, and SNFD samples were classified only as the Ruminococcus or Bacteroides enterotype. Authors of the above study also investigated the abundance of virulence genes in the gut microbiome of older adults and showed that more virulence genes were enriched in SNFDs than in CDs, indicating that frailer older adults tend to have more virulence genes in their gut microbiome.31
A recent meta-analysis of previous studies on gut microbiome composition in frail and non-frail older adults, including community dwellers, hospitalized individuals/nursing home residents, and chronic kidney patients, showed a lower relative abundance of F. prausnitzii, Alistipes shahii, and Roseburia inulinivorans species and a higher relative abundance of E. lenta, Eubacterium cylindroides, and E. dolichum in frail older adults than in non-frail older adults.32
Collectively, these studies used different kinds of frailty instruments based on different theoretical concepts; this may limit the comparability of research results, considering that there is high heterogeneity in the strength of associations between different frailty instruments and total mortality.36 Nevertheless, there are some consistent frailty-related features of the gut microbiome. A decrease in the abundance of butyrate-producing bacteria, especially Faecalibacterium, Roseburia, and Coprococcus, has consistently been observed. Butyrate plays an important role in inflammation regulation by inhibiting the production of pro-inflammatory cytokines, such as IFN-γ, TNF-α, IL-1β, IL-6, and IL-8, and stimulating the induction of IL-10 and TGF-β. In addition, butyrate plays a crucial role in enhancing the barrier function of intestinal epithelial cells via upregulating the expression of mucin 2 (MUC2) and tight junction proteins.37 Thus, the reduction of the abundance of butyrate-producing bacteria in frail older adults may lead to a decrease in butyrate levels, and in turn, an increase in gut permeability, resulting in the entrance of bacteria and their products into the circulatory system and a chronic low-grade inflammatory status known as inflammageing. Inflammageing is a strong risk factor for multiple aging-related diseases and physical and cognitive disability, all of which are typical elements in frailty.38 Therefore, butyrate-producing bacteria may have the propensity to improve frailty in older people.
The reduced abundance of Prevotella was also consistently observed in frailer older adults. Prevotella is one of the most dominant genera in the human gut microbiome, but the role of its members is not completely understood and has remained controversial. Previous studies have shown that Prevotella is more abundantly found in populations with a high-fiber diet,39,40 and this genus is involved in improving glucose metabolism induced by dietary fiber.41 In contrast, other studies have shown that increased Prevotella spp. abundances are associated with new-onset rheumatoid arthritis, insulin resistance, and persistent gut inflammation.42–44 These discrepancies may be due to the species-level and strain-level diversity of Prevotella. In the human gut, Prevotella spp. mainly comprise P. copri and Prevotella stercorea, but 22 additional Prevotella species-level genome bins were recently identified using metagenomic approaches.45 In addition, P. copri comprises four genetically and functionally distinct clades.46 Therefore, further studies on the function of Prevotella in healthy aging are needed in consideration of the species- and strain-level variability.
Another frailty-related gut microbiome feature is an increase in the abundance of pathobionts such as Eggerthella, B. fragilis, C. hathewayi, and Enterobacteriaceae. Eggerthella is considered an opportunistic pathogen, and it is associated with several chronic diseases, such as rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease.47–49 A recent study showed that E. lenta induced Th17 cell activation via a strain-specific enzyme, cardiac glycoside reductase 2 (Cgr2), and then increased IL-17A production in the gut, resulting in intestinal inflammation. C. hathewayi is associated with type 2 diabetes and coronavirus disease-19 disease severity,50,51 and it is involved in the production of trimethylamine, the precursor of the proatherogenic compound trimethylamine N-oxide (TMAO), from choline.52 Although additional pathophysiological mechanisms need to be further elucidated, these results suggest that changes in the gut microbiome in frail older adults may be closely related to the control of host metabolism and inflammation and, thus, hold potential as a therapeutic target to ameliorate frailty (Figure 1).
Figure 1.
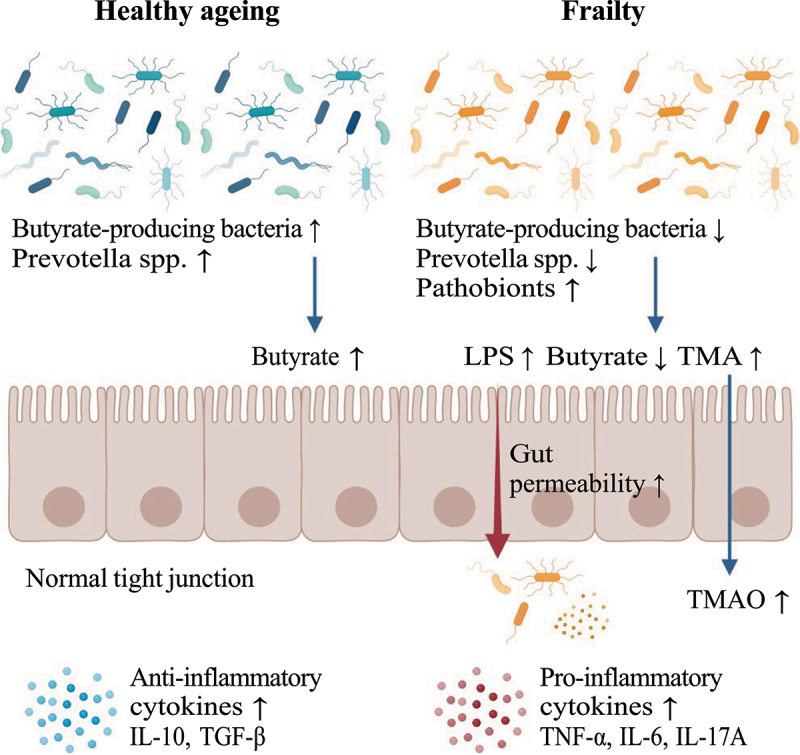
Gut microbiome signatures in healthy and frail older adults. Created with BioRender.com.
Microbiome-targeted interventions to improve health status in older people
The fact that the gut microbiome is associated with health status in older people means that the modulation of the gut microbiome has the potential to promote healthy aging. Microbiome-targeted interventions include probiotics, prebiotics, synbiotics, and diet. Although many microbiome intervention studies have been conducted in animals, such as mice, rats, and drosophila, this review focused on intervention studies in humans. We performed a PubMed search using the following search terms “((prebiotics or probiotics or synbiotics or diet) AND (gut microbiome)) AND (elderly[Title/Abstract] OR older[Title/Abstract])” with the filter “Full text, Randomized Controlled Trial, English, Aged: 65+ years”. After screening, we identified 23 studies relevant to our interests (Table 3). Most intervention studies on older people did not assess improvements in frailty or specific diseases but instead examined other health effects such as changes in gut microbiome composition, metabolic and inflammatory biomarkers, or cognitive functions.
Table 3.
Summary of randomized controlled trials on the gut microbiome of older adults.
| Subjects | Location | Study design | Type of intervention | Intervention period | Age inclusion criteria | Age (mean±SD) | No. of subjects, F/M ratio | Alteration in the gut microbiome | Measured outcome | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy | United States | A randomized, doubled-blind, placebo-controlled crossover study | Probiotics (Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9–1, and Bifidobacterium longum MM2) | 3 weeks per intervention | 65–80 | 69.8 ± 0.7 (mean±SEM) |
32 (22/10) |
Bifidobacteria and lactic acid bacteria ↑ Escherichia coli ↓ Faecalibacterium prausnitzii prevalence ↑ |
Increase in anti-inflammatory IL-10 | Spaiser, 201553 |
| Older women with low bone mineral density | Sweden | A randomized, placebo-controlled, double-blind, clinical trial | Lactobacillus reuteri ATCCPTA 6475 | 12 months | 75–80 | Probiotics: 76.4 ± 1.0 Placebo: 76.3 ± 1.1 |
Probiotics: 45 (45/0) Placebo: 45 (45/0) |
– | Reduction in loss of total volumetric bone mineral density | Nilsson, 201854 |
| Healthy | Italy | A randomized, double-blind, placebo-controlled trial | Bifidobacterium longum Bar33 and Lactobacillus helveticus Bar13 mixture | 30 days | ≥75 | 84.6 ± 7.8 | 98 (29/69) | – | Improvements in immunity (naive, activated memory, regulatory T cells, B cells, and natural killer cell activity ↑, memory T cells ↓) | Finamore, 201955 |
| Healthy | South Korea | A randomized, double-blind, placebo-controlled, multicenter clinical trial | Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI mixture | 12 weeks | ≥65 | Probiotic: 71.11 ± 5.02 Placebo: 72.00 ± 3.36 |
Probiotic: 27 (17/10) Placebo: 26 (10/16) |
Probiotic: Eubacterium, Allisonella, Clostridiales, and Prevotellaceae ↓ | Improvement in mental flexibility test and stress scores Increase in the serum BDNF levels | Kim, 202156 |
| Healthy | United States | A randomized, placebo-controlled, double-blinded trial | Lactobacillus rhamnosus GG (LGG) | 12 weeks | middle-aged (52–59) and older adults (60–75) | Probiotic: 64.4 ± 5.5 Placebo: 64.2 ± 5.4 |
Probiotic: 86 (48/38) Placebo: 83 (55/28) |
Probiotic: LGG ↑ | Improvement in cognitive function in persons meeting the criteria for mild cognitive impairment57 | Aljumaah, 202258 |
| Older adults with declining memory | Japan | A randomized, double-blind, placebo-controlled trial | Lactiplantibacillus plantarum OLL2712 | 12 weeks | ≥65 | Probiotic: 76.8 ± 4.6 Placebo: 76.9 ± 4.9 |
Probiotic: 39 (21/18) Placebo: 39 (21/18) |
Probiotic: Lachnoclostridium, Monoglobus, Oscillibacter ↓ | Improvements in composite memory and visual memory scores | Sakurai, 202259 |
| Flu-vaccinated healthy older individuals | Italy | A placebo-controlled, randomized, double-blind, clinical trial | Probiotic mixture | 28 days | 60–80 | Probiotic: 62.56 ± 4.93 Placebo: 64.92 ± 6.04 |
Probiotic: 25 (17/8) Placebo: 25 (19/6) |
Probiotic: Dialister, Lachnospira, Bifidobacterium, and Lachnoclostridium ↑ Alistipes, Dorea, Parabacteroides, and Clostridium ↓(after intervention) |
Common infectious disease symptom ↓ Improvement of total antioxidant capacity and βdefensin2 levels |
Sandionigi, 202260 |
| Older people with malnutrition in long-term care | China | A randomized, single-blind clinical trial | Clostridium butyricum | 12 weeks | ≥65 | Probiotic: 81.64 ± 5.01 Control: 85.38 ± 4.92 |
Probiotics: 11 (8/3) Placebo: 8 (5/3) |
Coprobacillus species, Carnobacterium divergens, Corynebacterium_massiliense↑ the beneficial organisms Akkermansia muciniphila and Alistipes putredinis ↑ |
Improvements in immunity and levels of nutrition biomarkers such as IFN-γ, occludin, and prealbumin | Liu, 202261 |
| Older individuals with mild cognitive impairment (MCI) | China | A randomized, placebo-controlled study | Probiotic mixture | 12 weeks | >60 | Probiotic: 76.40 ± 9.61 Placebo: 75.30 ± 9.75 |
Probiotic: 21 (11/10) Placebo: 21 (10/11) |
Probiotic: Blautia, Lachnospiraceae, Muribaculaceae, Haemophilus, Coprococcus, Ruminococcus, Anaerostipes, Erysipelotrichaceae, Prevotellaceae, Pantoea↑ | Improvement in cognitive function and sleep quality | Fei, 202362 |
| Residents of a long-term care facility or community dwellers | Canada | A prospective, placebo-controlled, randomized, double-blinded study | MSPrebiotic (resistant starch) | 12 weeks | elderly (ELD): ≥ 70 mid-age (MID): 30–50 |
ELD: 78.4 ± 7.7 MID: 41.6 ± 5.61 |
ELD: 42 (25/17) MID: 42 (24/18) |
MSPrebiotic: Bifidobacteria↑(in both ELD and MID) | MSPrebiotic: Butyrate↑(in ELD) | Alfa, 201863 |
| Healthy | UK | A randomized, cross-over, double-blinded study | Wheat bran arabinoxylan oligosaccharides (AXOS) | 10 days per intervention | 60 | 67.67 ± 1 (mean±SEM) |
21 (13/8) | AXOS: Bifidobacterium↑ | No significant effects on glucose, HDL, LDL, triglycerides, and fecal calprotectin | Chung, 202064 |
| Community-dwelling individuals | Sweden | A randomized, placebo-controlled, parallel clinical trial | Arabinoxyland Oat β–glucan |
6 weeks | ≥65 | Arabinoxylan: 69.0 (66.0–71.5) Oat β–glucan: 69.0 (66.0–72.0) Placebo: 70.5 (67.0–78.3) (Median [IQR]) |
Arabinoxylan: 17 (8/9) Oat β–glucan: 15 (6/9) Placebo: 17 (8/9) |
No significant differences in the gut microbiota composition before and after intervention | No significant effects on acute indomethacin-induced intestinal hyperpermeability | Ganda, 202065 |
| Healthy | Japan | A randomized, single-blind, parallel design |
Helianthus tuberosus (dietary fiber) morning intake group (MG)/evening intake group (EG) |
1 week | ≥65 | MG: 74.3 ± 0.9 EG: 74.6 ± 1.7 (mean±SEM) |
MG: 18 (9/9) EG: 17 (8/9) |
MG: Ruminococcus ↓ | MG: favorable effect on the postprandial glucose level | Kim, 202066 |
| Healthy | Netherlands | A double-blind, placebo-controlled trial | Chicory long-chain inulin | 2 months | 55–80 | Inulin: 62.2 ± 6.9 Placebo: 63.7 ± 8.1 (mean±SD) |
Inulin: 13 (4/9) Placebo: 13 (4/9) |
Bifidobacterium angulatum and Bifidobacterium ruminantium ↑ Alistipes shahii and Anaerostipes hadrus↑ |
No significant effects on immune response and SCFAs | Kiewiet, 202167 |
| Healthy | Brazil | A randomized, double-blinded, placebo-controlled trial | Synbiotic: soy and yacon extracts containing Bifidobacterium animalis ssp. lactis BB-12 Placebo: soy and yacon extracts |
4 weeks | ≥65 | Synbiotic: 67 Placebo: 71 (mean) |
Synbiotic: 14 Placebo: 15 |
No significant differences in the number of Bifidobacteria, Clostridia, or Enterobacteria | Increase in polyamines levels in both groups No significant effect on the production of proinflammatory cytokines |
Manzoni, 201768 |
| Older patients with MetS | Italy | A double-blind, randomized, placebo-controlled, parallel-group clinical trial | A synbiotic formula of Lactobacillus plantarum PBS067, Lactobacillus acidophilus PBS066 and Lactobacillus reuteri PBS072 with active prebiotics | 60 days | 65–80 | Synbiotic: 72 ± 3 Placebo: 71 ± 3 |
Synbiotic: 30 (16/14) Placebo: 30 (17/13) |
– | Decrease in MetS syndrome prevalence, levels of several cardiovascular risk factors, and markers of insulin resistance | Cicero, 202169 |
| Older individuals with increased intestinal permeability (IP) | Italy | A randomized, controlled, crossover trial | Polyphenol-rich diet | 8 weeks per intervention | ≥60 | 77 (70–87) (Median [IQR]) |
51 (29/22) | Fiber-fermenting and butyrate-producing bacteria↑(the family Ruminococcaceae and members of the genus Faecalibacterium)70 Positive correlation between polyphenol metabolites and Butyricicoccus | A significant reduction of the IP marker, zonulin, in older individuals affected by “leaky gut”70 | Peron, 202171 |
| Healthy | Italy, France, Germany | An open label, randomized, controlled trial | A: RISTOMED diet B: RISTOMED diet + VSL#3 probiotic blend |
8 weeks | 65–85 | A: 70.5 ± 4 B: 69.7 ± 3.9 |
A: 31 (17/14) B: 31 (16/15) |
B: Bifidobacteria↑ | B: erythrocyte sedimentation rate ↓ serum folate and serum vitamin B12 ↑ Plasma homocysteine ↓ |
Valentini, 201572 |
| Healthy | United States | A randomized, double-blind, placebo-controlled, crossover study | Probiotic: HPD+ B. bifidum HA-132, B. breve HA-129, B. longum HA-135, L. acidophilus HA-122, L. plantarum HA-119Prebiotic: HPD + inulinSynbiotic: HPD + probiotic plus inulinPlacebo: HPD alone | 2 weeks per high-protein diet (HPD) intervention | ≥65 | 73.7 ± 5.6 | 26 (26/0) | HPD periods (pooled): Lactobacillus, Lactococcus, Streptococcus ↑ butyrate producers (Roseburia and Anaerostipes) ↓ |
No significant effects on indicators of wellness Fat-free mass ↑ |
Ford, 202073 |
| Non-frail or pre-frail individuals | UK, France, Netherlands, Italy, and Poland | A randomized, multicenter, single-blind, controlled trial | Mediterranean diet | 12 months | 65–79 | MedDiet: 71 (65–79) Control: 71 (65–79) (median [min-max]) |
MedDiet: 324 (182/141) Control: 289 (144/145) |
MedDiet adherence ↑: Faecalibacterium prausnitzii, Roseburia (R. hominis), Eubacterium (E. rectale, E. eligens, E. xylanophilum), Bacteroides thetaiotaomicron, Prevotella copri, and Anaerostipes hadrus ↑ (DietPositive)/ Ruminococcus torques, Collinsella aerofaciens, Coprococcus comes, Dorea formicigenerans, Clostridium ramosum, Veillonella dispar, Flavonifractor plautii, and Actinomyces lingnae ↓ (DietNegative) |
No direct association between dietary adherence scores and frailty DietPositive taxa: positive association with several markers of lower frailty and improved cognitive function, and negative association with inflammatory markers including C-reactive protein and interleukin-17 |
Ghosh, 202074 |
| Healthy | Australia | A randomized, crossover clinical trial | Low-anthocyanin plum nectar | 8 weeks per intervention | ≥55 | 70 ± 10 | 31 (17/14) | No significant effects on gut microbiome | No significant effects on cognition, blood pressure, or anti-inflammatory biomarkers | Igwe, 202075 |
| Healthy | New Zealand | A randomized, parallel-group design | A diet containing the recommended dietary intake (RDA) of protein/a diet containing twice the RDA (2RDA) | 10 weeks | ≥70 | RDA: 74.7 ± 3.9 2RDA: 73.7 ± 3.3 |
RDA: 15 (0/15) 2RDA: 13 (0/13) |
No significant effects on the gut microbiome | No significant effects on the fecal VOC 2RDA: circulatory TMAO↑ LDL cholesterol↑ No differences in other biomarkers of CVD risk and insulin sensitivity76 |
Mitchell, 202077 |
| Community-dwelling older adults | Finland/Netherlands | A multicenter randomized controlled trial | Dietary advice aimed at increasing protein intake | 6 months | ≥65 | Diet: 74.6 ± 4.8 Control: 74.1 ± 4.7 |
Diet: 47 (19/28) Control: 43 (24/19) |
No significant effects on the gut microbiome | No significant effects on appetite or brain activity | Fluitman, 202378 |
Probiotics, prebiotics, and synbiotics
According to the definition by the International Scientific Association for Probiotics and Prebiotics (ISAPP), probiotics include any “live microorganism that, when administered in adequate amounts, confers a health benefit on the host”79 and prebiotics include any “substrate that is selectively utilized by a host microorganism, conferring a health benefit”.80 More recently, the ISAPP defined synbiotics as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host”.81 Probiotics, prebiotics, and synbiotics are considered to be a cost-effective strategy for improving gut microbiome homeostasis and health status.
Lactobacillus and bifidobacterium are the most commonly used probiotics. Several clinical trials have demonstrated that probiotics have beneficial potential for decreasing inflammatory levels and improving cognitive function in older people. For example, the intake of a probiotic mixture (B. bifidum G9–1, B. longum MM2, and L. gasseri KS-13) for 3 weeks in healthy older adults resulted in a significant increase in IL-10 levels and an increase in the prevalence of an anti-inflammatory commensal bacterium, F. prausnitzii,53 which was associated with less frail phenotypes.29,30,32 Intervention studies of another probiotic mixture (B. longum Bar33 and Lactobacillus helveticus Bar13) and Clostridium butyricum for 30 days and 12 weeks, respectively, also showed significant improvements in immunity.55,61 In the latter study, increases in the abundance of beneficial bacteria, such as Akkermansia muciniphila and Alistipes putredinis, were observed after the intervention.61 Additionally, the consumption of probiotics containing B. bifidum BGN4 and B. longum BORI for 12 weeks by healthy older adults significantly increased levels of serum blood brain-derived neurotrophic factor (BDNF), which is known to be essential for learning and memory, while significantly reducing the abundance of inflammation-causing gut bacteria, including Eubacterium, Allisonella, and Prevotellaceae.56 In older adults with declining memory, L. plantarum OLL2712 consumption for 12 weeks significantly improved composite memory and visual memory and decreased the relative abundances of Lachnoclostridium, Monoglobus, and Oscillibacter, which are related to the inflammatory response.59 Similarly, the intake of probiotics is reported to be effective in improving cognitive function.58,62 In addition, 1-year daily supplementation with L. reuteri 6475 reduced bone loss in older women with low bone mineral density,54 and the abundance of L. reuteri was increased by the intervention compared to the baseline.82 Collectively, these studies demonstrate that the consumption of probiotics may inhibit the growth of inflammation-related gut microbes and/or promote the growth of beneficial gut microbes, which in turn may have effects such as improving immune function, cognitive function, and even bone health in older people.
Prebiotics include polyols (e.g. xylitol, sorbitol, and mannitol), oligosaccharides (e.g. inulin, fructooligosaccharides [FOS], and galactooligosaccharides), and fibers (e.g. cellulose, pectins, and β-glucans). Intervention studies with wheat bran arabinoxylan oligosaccharides, chicory long-chain inulin, and resistant starch for 10 days, 2 months, and 12 weeks, respectively, in older participants showed a significant increase in the abundance of Bifidobacteria.63,64,67 Resistant starch consumption resulted in a significant increase in the relative proportion of fecal butyrate in older adults.63 However, chicory long-chain inulin consumption did not change fecal SCFA concentrations or affect immunity.67 Furthermore, wheat-bran arabinoxylan oligosaccharide supplementation did not result in changes in metabolic biomarkers and fecal calprotectin levels.64 These intervention studies showed that these dietary supplements are bifidogenic but failed to induce changes in measured outcomes in older people.
Synbiotics are combinations of probiotics and prebiotics, which may synergistically act in the gut to confer beneficial effects on host health. For example, consumption of a synbiotic formula of three probiotics (L. plantarum PBS067, L. acidophilus PBS066, and L. reuteri PBS072) with active prebiotics (inulin and FOS) in older patients with metabolic syndrome (MetS) for 2 months significantly improved metabolic parameters, such as waist circumference, total cholesterol levels, and triglyceride levels, and reduced serum hsCRP and TNF-alpha levels.69
The number of probiotics, prebiotics, and synbiotics intervention studies in older adults remains limited. Although several studies, including the above examples, have shown that the consumption of probiotics, prebiotics, and synbiotics may improve health in older people, their efficacies may vary depending on dosage and treatment duration.83,84 It is also still unclear how long these effects will last after cessation of supplementation. For example, the probiotic loads in feces dropped to baseline within 1 month after 28 days of 11-strain probiotic consumption in healthy young adults,85 but in the gut of 30% of healthy young individuals taking B. longum AH1206 for 2 weeks, B. longum AH1206 remained detectable for at least 6 months after consumption cessation.86 These studies indicate that the persistence of probiotics in the gut after consumption cessation can be influenced by the probiotic strain and/or the host. As most studies discussed in this section did not conduct follow-up assessments, or conducted follow-up only for 1–2 weeks after probiotics treatment, longer follow-ups are needed to determine the long-term effects of probiotics in older people. In addition, the efficacy of probiotic gut mucosal colonization during consumption varies among different persons, depending on baseline host transcriptional and microbiome features.85 Therefore, more intervention studies are needed involving probiotics, prebiotics, and synbiotics in a larger number of older adults to assess their effects on improving older adult health, to determine the optimal formula, dosage, and administration duration and to develop methods for predicting individual responses to treatment.
Diet
Recent studies have shown that specific dietary factors may influence older adult health through the modulation of gut microbiome. Examples of such studies are presented below.
The MedDiet is characterized by a high intake of vegetables, legumes, fruits, nuts, and olive oil, a low intake of red meats and refined grains, and a low-to-moderate intake of wine.87 Adherence to the MedDiet has a protective effect on the development of frailty88,89 and beneficial effects on the improvement of inflammation90 and cognitive function in older people.91 Ghosh et al. performed a 1-year MedDiet intervention in older people across five European countries to investigate whether it could induce alterations of the gut microbiome and contribute to the reduction of frailty as assessed by Fried’s Frailty Phenotype. A higher level of adherence to the MedDiet was associated with increased abundance of P. copri and butyrate-producing bacteria, such as F. prausnitzii, Roseburia hominis, Eubacterium rectale, and Eubacterium xylanophilum; these taxa have been associated with reduced frailty and inflammatory status and improved cognitive function. This study indicated that the MedDiet can beneficially modulate the composition of the gut microbiome, which has the potential to improve frailty in older adults.74
Polyphenols, which have antioxidant and anti-inflammatory activity, are degraded into active phenolic metabolites by gut microbes; consequently, polyphenols and/or their metabolites may affect gut microbial composition.92 A polyphenol-rich diet intervention for 8 weeks significantly reduced blood pressure and levels of serum zonulin, which is an intestinal permeability marker involved in tight junction modulation, in older adults, and induced significant increases in the abundance of butyrate-producing bacteria Butyricicocci and F. prausnitzii in the gut microbiome.70 Additionally, these butyrate-producing bacteria were positively correlated with serum theobromine and methylxanthines derived from cocoa and/or green tea, and these metabolites were inversely correlated with serum zonulin.71 These studies indicated that a polyphenol-rich diet may contribute to reinforcement of the intestinal barrier through increased butyrate and/or specific metabolites (owing to the resulting increase in the abundance of butyrate-producing bacteria).93 Although the intervention failed to show improvement in inflammatory markers, a polyphenol-rich diet has the potential to attenuate the risk of inflammation, by improving intestinal barrier function, which is closely associated with the development and progression of frailty.94
In older adults, an inadequate protein intake is associated with an increased risk of developing sarcopenia and frailty95,96. The current recommended dietary allowance (RDA) for protein is 0.8 g/kg/day, but higher-protein intake than the RDA has been proposed to prevent or postpone frailty in advanced age.96 There are several interventions to determine the effects of high protein intake on the gut microbiome in older adults. A high-protein diet (HPD, 1.5 to 2.2 g/kg/day protein) consumption with and without a probiotic and/or prebiotic in healthy older women did not significantly change their general wellness but increased fat-free mass. The HPD intervention induced increases in the abundance of Lactobacillus, Lactococcus, and Streptococcus and decreases in the abundance of butyrate-producing bacteria, Roseburia, and Anaerostipes in the gut microbiome.73 Because reduced abundances of butyrate producers have been consistently observed to be associated with frailer phenotypes, as discussed earlier, HPD-associated changes in the gut microbiome may be unfavorable for older adults’ gut microbiome health. However, in other two HPD intervention studies on older adults (1.6 g/kg/day protein for 10 weeks and 1.2 g/kg/day protein for 6 months), no significant changes were observed in the gut microbiome after the interventions.77,78 Alternatively, intakes of 1.6 g/kg/day protein for 10 weeks increased circulatory concentrations of TMAO,76 a bacterial metabolite derived from dietary choline and carnitine that is associated with an increased risk of cardiovascular diseases.97 Therefore, it is necessary to comprehensively evaluate whether an HPD is beneficial to the health of older adults in various aspects.
The effects of changes to dietary habits, including the examples presented above, may vary depending on an individual’s baseline gut microbiome composition. Recently, Karakan et al. reported that in patients with irritable bowel syndrome (IBS), specific personalized diets designed using machine-learning algorithms considering individual gut microbiome profiles improved IBS symptom severity and significantly increased the abundance of Faecalibacterium in the gut microbiome, compared with a standard IBS diet.98 To improve frailty more efficiently in older people, it is necessary to apply personalized dietary interventions based on an individual’s gut microbiome composition, as demonstrated in the IBS study.
Conclusions and future perspectives
Recent studies have highlighted that the gut microbiome is associated not only with age but also with frailty in older people. These findings indicate that the gut microbiome may play an important role in the aging process. However, most studies reporting frailty-related gut microbiome features have been conducted with a cross-sectional study design, which cannot distinguish whether frailty-related gut microbiome features are the cause or effect of frailty. Long-term longitudinal studies are needed to identify causal relationships between the gut microbiome and frailty or unhealthy aging. In addition, considering the high variability of specific species and strains, metagenomic analyses with a strain-level resolution are warranted.
Various microbiome-targeted interventions to improve health in older adults have been performed, and the effects of interventions varied across studies. In the case of probiotics, prebiotics, and synbiotics, the response to treatment can be affected by dosage, duration, and their components. Furthermore, each individual’s age, sex, lifestyle, health condition, and baseline gut microbiome composition can influence the response to treatment. Therefore, it is necessary to develop algorithms that can maximize the personal effect of a given intervention. Most intervention studies for modulating the gut microbiome composition and improving health status have been conducted in conjunction with a diet that was already in practice, including specific dietary habits or the use of probiotic species. As there is mounting evidence for several microbial and metabolite candidates associated with healthy aging, such as F. prausnitzii and butyrate, clinical trials for these candidates are needed to test their safety and efficacy in reducing the severity of frailty or delaying the onset of frailty in older adults. It is also important to gain a better understanding of the mechanism of action of the gut microbiome in age-related diseases and frailty in older people.
Funding Statement
This work was supported by the Main Research Program [E0170600-07] of the Korea Food Research Institute (KFRI), funded by the Ministry of Science and ICT.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
No new data were created or analyzed in this study.
References
- 1.United Nations . 2022. Department of economic and social affairs, population division. World population prospects 2022: summary of results. Report No.: UN DESA/POP/2022/TR/NO.3.
- 2.Garmany A, Yamada S, Terzic A.. Longevity leap: mind the healthspan gap. NPJ Regen Med. 2021;6:57. doi: 10.1038/s41536-021-00169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–18. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571(7764):183–192. doi: 10.1038/s41586-019-1365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015;31(1):69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buford TW. (Dis)trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5(1):80. doi: 10.1186/s40168-017-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gacesa R, Kurilshikov A, Vich Vila A, Sinha T, Klaassen MAY, Bolte LA, Andreu-Sanchez S, Chen L, Collij V, Hu S, et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature. 2022;604(7907):732–739. doi: 10.1038/s41586-022-04567-7. [DOI] [PubMed] [Google Scholar]
- 10.Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Sci. 2016;352(6285):565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, et al. Population-level analysis of gut microbiome variation. Sci. 2016;352(6285):560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 12.McDonald D, Hyde E, Debelius JW, Morton JT, Gonzalez A, Ackermann G, Aksenov AA, Behsaz B, Brennan C, Chen Y, et al. American gut: An open platform for citizen science microbiome research. mSystems. 2018;3(3):e00031–18. doi: 10.1128/mSystems.00031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilmanski T, Diener C, Rappaport N, Patwardhan S, Wiedrick J, Lapidus J, Earls JC, Zimmer A, Glusman G, Robinson M, et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. 2021;3(2):274–286. doi: 10.1038/s42255-021-00348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Wu W, Zheng HM, Li P, McDonald D, Sheng HF, Chen MX, Chen ZH, Ji GY, Zheng ZD, et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med. 2018;24(10):1532–1535. doi: 10.1038/s41591-018-0164-x. [DOI] [PubMed] [Google Scholar]
- 15.Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, et al. Gut microbiota and extreme longevity. Curr Biol. 2016;26(11):1480–1485. doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Kong F, Hua Y, Zeng B, Ning R, Li Y, Zhao J. Gut microbiota signatures of longevity. Curr Biol. 2016;26(18):R832–R833. doi: 10.1016/j.cub.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Lim MY, Hong S, Bang SJ, Chung WH, Shin JH, Kim JH, Nam YD, David LA. Gut microbiome structure and association with host factors in a Korean population. mSystems. 2021;6(4):e0017921. doi: 10.1128/mSystems.00179-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park J, Kato K, Murakami H, Hosomi K, Tanisawa K, Nakagata T, Ohno H, Konishi K, Kawashima H, Chen YA, et al. Comprehensive analysis of gut microbiota of a healthy population and covariates affecting microbial variation in two large Japanese cohorts. BMC Microbiol. 2021;21(1):151. doi: 10.1186/s12866-021-02215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan H, Qin Q, Yan S, Chen J, Yang Y, Li T, Gao X, Ding S. Comparison of the gut microbiota in different age groups in China. Front Cell Infect Microbiol. 2022;12:877914. doi: 10.3389/fcimb.2022.877914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Zhong H, Li Y, Shi Z, Ren H, Zhang Z, Zhou X, Tang S, Han X, Lin Y, et al. Sex- and age-related trajectories of the adult human gut microbiota shared across populations of different ethnicities. Nat Aging. 2021;1(1):87–100. doi: 10.1038/s43587-020-00014-2. [DOI] [PubMed] [Google Scholar]
- 21.Evert J, Lawler E, Bogan H, Perls T. Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci. 2003;58:232–237. doi: 10.1093/gerona/58.3.M232. [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 23.Mitnitski A, Howlett SE, Rockwood K. Heterogeneity of human aging and its assessment. J Gerontol A Biol Sci Med Sci. 2017;72(7):877–884. doi: 10.1093/gerona/glw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: Implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Wang Y, Li H, Dai Y, Chen D, Wang M, Jiang X, Huang Z, Yu H, Huang J, et al. Altered fecal microbiota composition in older adults with frailty. Front Cell Infect Microbiol. 2021;11:696186. doi: 10.3389/fcimb.2021.696186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim MY, Hong S, Kim JH, Nam YD, Le Couteur D. Association between gut microbiome and frailty in the older adult population in Korea. J Gerontol A Biol Sci Med Sci. 2021;76:1362–1368. doi: 10.1093/gerona/glaa319. [DOI] [PubMed] [Google Scholar]
- 29.Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE, O’Toole PW, Spector TD, Steves CJ. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8(1):8. doi: 10.1186/s13073-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haran JP, Zeamer A, Ward DV, Dutta P, Bucci V, McCormick BA, Le Couteur D. The nursing home older adult gut microbiome composition shows time-dependent dysbiosis and is influenced by medication exposures, age, environment, and frailty. J Gerontol A Biol Sci Med Sci. 2021;76:1930–1938. doi: 10.1093/gerona/glab167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson PJ, Zhou W, Santiago A, Driscoll S, Fleming E, Voigt AY, Chun OK, Grady JJ, Kuchel GA, Robison JT, et al. Associations of the skin, oral and gut microbiome with aging, frailty and infection risk reservoirs in older adults. Nat Aging. 2022;2(10):941–955. doi: 10.1038/s43587-022-00287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almeida HM, Sardeli AV, Conway J, Duggal NA, Cavaglieri CR. Comparison between frail and non-frail older adults’ gut microbiota: A systematic review and meta-analysis. Ageing Res Rev. 2022;82:101773. doi: 10.1016/j.arr.2022.101773. [DOI] [PubMed] [Google Scholar]
- 33.Hwang HS, Kwon IS, Park BJ, Cho B, Yoon JL, Won CW. The validity and reliability of Korean frailty index. J Korean Geriatr Soc. 2010;14(4):191–202. doi: 10.4235/jkgs.2010.14.4.191. [DOI] [Google Scholar]
- 34.Kojima G. Prevalence of frailty in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(11):940–945. doi: 10.1016/j.jamda.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 35.Burato S, Leonardi L, Antonazzo IC, Raschi E, Ajolfi C, Baraghini M, Chiarello A, Delmonte V, Di Castri L, Donati M, et al. Comparing the prevalence of polypharmacy and potential drug-drug interactions in nursing homes and in the community dwelling elderly of Emilia Romagna region. Front Pharmacol. 2020;11:624888. doi: 10.3389/fphar.2020.624888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aguayo GA, Vaillant MT, Donneau AF, Schritz A, Stranges S, Malisoux L, Chioti A, Guillaume M, Muller M, Witte DR, et al. Comparative analysis of the association between 35 frailty scores and cardiovascular events, cancer, and total mortality in an elderly general population in England: an observational study. PLoS Med. 2018;15(3):e1002543. doi: 10.1371/journal.pmed.1002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Wang J, He T, Becker S, Zhang GL, Li DF, Ma X. Butyrate: A double-edged sword for health? Adv Nutr. 2018;9:21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrucci L, Fabbri E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Sci. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Bjorck I, Backhed F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015;22(6):971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 44.Leite AZ, Rodrigues NC, Gonzaga MI, Paiolo JCC, de Souza CA, Stefanutto NAV, Omori WP, Pinheiro DG, Brisotti JL, Matheucci Junior E, et al. Detection of increased plasma interleukin-6 levels and prevalence of Prevotella copri and Bacteroides vulgatus in the feces of type 2 diabetes patients. Front Immunol. 2017;8:1107. doi: 10.3389/fimmu.2017.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tett A, Pasolli E, Masetti G, Ercolini D, Segata N. Prevotella diversity, niches and interactions with the human host. Nat Rev Microbiol. 2021;19(9):585–599. doi: 10.1038/s41579-021-00559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tett A, Huang KD, Asnicar F, Fehlner-Peach H, Pasolli E, Karcher N, Armanini F, Manghi P, Bonham K, Zolfo M, et al. The Prevotella copri complex comprises four distinct clades underrepresented in westernized populations. Cell Host & Microbe. 2019;26(5):666–679.e7. doi: 10.1016/j.chom.2019.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA. 2017;114(40):10713–10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Zhang DY, Jia HJ, Feng Q, Wang DH, Liang D, Wu XN, Li JH, Tang LQ, Li Y, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21(8):895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 49.Alexander M, Ang QY, Nayak RR, Bustion AE, Sandy M, Zhang B, Upadhyay V, Pollard KS, Lynch SV, Turnbaugh PJ. Human gut bacterial metabolism drives Th17 activation and colitis. Cell Host & Microbe. 2022;30(1):17–30.e9. doi: 10.1016/j.chom.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuo T, Zhang F, Lui GCY, Yeoh YK, AYL L, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 52.Romano KA, Vivas EI, Amador-Noguez D, Rey FE, Blaser MJ. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio. 2015;6(2):e02481. doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spaiser SJ, Culpepper T, Nieves C Jr., Ukhanova M, Mai V, Percival SS, Christman MC, Langkamp-Henken B. Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2 ingestion induces a less inflammatory cytokine profile and a potentially beneficial shift in gut microbiota in older adults: a randomized, double-blind, placebo-controlled, crossover study. J Am Coll Nutr. 2015;34:459–469. doi: 10.1080/07315724.2014.983249. [DOI] [PubMed] [Google Scholar]
- 54.Nilsson AG, Sundh D, Backhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018;284(3):307–317. doi: 10.1111/joim.12805. [DOI] [PubMed] [Google Scholar]
- 55.Finamore A, Roselli M, Donini L, Brasili DE, Rami R, Carnevali P, Mistura L, Pinto A, Giusti A, Mengheri E. Supplementation with Bifidobacterium longum Bar33 and Lactobacillus helveticus Bar13 mixture improves immunity in elderly humans (over 75 years) and aged mice. Nutrition. 2019;63-64:184–192. doi: 10.1016/j.nut.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Kim CS, Cha L, Sim M, Jung S, Chun WY, Baik HW, Shin DM, Le Couteur D. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci. 2021;76:32–40. doi: 10.1093/gerona/glaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanborn V, Azcarate-Peril MA, Updegraff J, Manderino L, Gunstad J. Randomized clinical trial examining the impact of lactobacillus rhamnosus gg probiotic supplementation on cognitive functioning in middle-aged and older adults. Neuropsychiatr Dis Treat. 2020;16:2765–2777. doi: 10.2147/NDT.S270035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aljumaah MR, Bhatia U, Roach J, Gunstad J, Azcarate Peril MA. The gut microbiome, mild cognitive impairment, and probiotics: A randomized clinical trial in middle-aged and older adults. Clin Nutr. 2022;41:2565–2576. doi: 10.1016/j.clnu.2022.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Sakurai K, Toshimitsu T, Okada E, Anzai S, Shiraishi I, Inamura N, Kobayashi S, Sashihara T, Hisatsune T. Effects of Lactiplantibacillus plantarum OLL2712 on memory function in older adults with declining memory: a randomized placebo-controlled trial. Nutrients. 2022;14(20):4300. doi: 10.3390/nu14204300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandionigi A, De Giani A, Tursi F, Michelotti A, Cestone E, Giardina S, Zampolli J, Di Gennaro P, Abbassi MS. Effectiveness of multistrain probiotic formulation on common infectious disease symptoms and gut microbiota modulation in flu-vaccinated healthy elderly subjects. Biomed Res Int. 2022;2022:1–16. doi: 10.1155/2022/3860896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu L, Chen X, Liu L, Qin H. Clostridium butyricum potentially improves immunity and nutrition through alteration of the microbiota and metabolism of elderly people with malnutrition in long-term care. Nutrients. 2022;14(17):3546. doi: 10.3390/nu14173546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fei Y, Wang R, Lu J, Peng S, Yang S, Wang Y, Zheng K, Li R, Lin L, Li M. Probiotic intervention benefits multiple neural behaviors in older adults with mild cognitive impairment. Geriatr Nurs. 2023;51:167–175. doi: 10.1016/j.gerinurse.2023.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Alfa MJ, Strang D, Tappia PS, Graham M, Van Domselaar G, Forbes JD, Laminman V, Olson N, DeGagne P, Bray D, et al. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin Nutr. 2018;37:797–807. doi: 10.1016/j.clnu.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 64.Chung WSF, Walker AW, Bosscher D, Garcia-Campayo V, Wagner J, Parkhill J, Duncan SH, Flint HJ. Relative abundance of the Prevotella genus within the human gut microbiota of elderly volunteers determines the inter-individual responses to dietary supplementation with wheat bran arabinoxylan-oligosaccharides. BMC Microbiol. 2020;20(1):283. doi: 10.1186/s12866-020-01968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ganda Mall JP, Fart F, Sabet JA, Lindqvist CM, Nestestog R, Hegge FT, Keita AV, Brummer RJ, Schoultz I. Effects of dietary fibres on acute indomethacin-induced intestinal hyperpermeability in the elderly: a randomised placebo controlled parallel clinical trial. Nutrients. 2020;12:1954. doi: 10.3390/nu12071954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim HK, Chijiki H, Nanba T, Ozaki M, Sasaki H, Takahashi M, Shibata S. Ingestion of Helianthus tuberosus at breakfast rather than at dinner is more effective for suppressing glucose levels and improving the intestinal microbiota in older adults. Nutrients. 2020;12(10):3035. doi: 10.3390/nu12103035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiewiet MBG, Elderman ME, El Aidy S, Burgerhof JGM, Visser H, Vaughan EE, Faas MM, de Vos P. Flexibility of gut microbiota in ageing individuals during dietary fiber long-chain inulin intake. Mol Nutr Food Res. 2021;65(4):e2000390. doi: 10.1002/mnfr.202000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manzoni MSJ, Rossi EA, Pauly-Silveira ND, Pinto RA, Roselino MN, Carlos IZ, Quilles MB, de Abreu Gloria MB, Cavallini DCU. Consumption effect of a synbiotic beverage made from soy and yacon extracts containing Bifidobacterium animalis ssp. lactis BB-12 on the intestinal polyamine concentrations in elderly individuals. Food Res Int. 2017;99:495–500. doi: 10.1016/j.foodres.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 69.Cicero AFG, Fogacci F, Bove M, Giovannini M, Borghi C. Impact of a short-term synbiotic supplementation on metabolic syndrome and systemic inflammation in elderly patients: a randomized placebo-controlled clinical trial. Eur J Nutr. 2021;60(2):655–663. doi: 10.1007/s00394-020-02271-8. [DOI] [PubMed] [Google Scholar]
- 70.Bo C D, Bernardi S, Cherubini A, Porrini M, Gargari G, Hidalgo-Liberona N, Gonzalez-Dominiguez R, Zamora-Ros R, Peron G, Marino M, et al. A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: the maple randomised controlled trial. Clin Nutr. 2021;40:3006–3018. doi: 10.1016/j.clnu.2020.12.014. [DOI] [PubMed] [Google Scholar]
- 71.Peron G, Gargari G, Merono T, Minarro A, Lozano EV, Escuder PC, Gonzalez-Dominguez R, Hidalgo-Liberona N, Del Bo C, Bernardi S, et al. Crosstalk among intestinal barrier, gut microbiota and serum metabolome after a polyphenol-rich diet in older subjects with “leaky gut”: the MaPLE trial. Clin Nutr. 2021;40:5288–5297. doi: 10.1016/j.clnu.2021.08.027. [DOI] [PubMed] [Google Scholar]
- 72.Valentini L, Pinto A, Bourdel-Marchasson I, Ostan R, Brigidi P, Turroni S, Hrelia S, Hrelia P, Bereswill S, Fischer A, et al. Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota - the “RISTOMED project”: randomized controlled trial in healthy older people. Clin Nutr. 2015;34:593–602. doi: 10.1016/j.clnu.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 73.Ford AL, Nagulesapillai V, Piano A, Auger J, Girard SA, Christman M, Tompkins TA, Dahl WJ. Microbiota stability and gastrointestinal tolerance in response to a high-protein diet with and without a prebiotic, probiotic, and synbiotic: a randomized, double-blind, placebo-controlled trial in older women. J Acad Nutr Diet. 2020;120(4):500–516.e10. doi: 10.1016/j.jand.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 74.Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, Giampieri E, Jennings A, Candela M, Turroni S, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69(7):1218–1228. doi: 10.1136/gutjnl-2019-319654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Igwe EO, Roodenrys S, Probst YC, Do Rosario V, Netzel ME, Hong HT, Netzel G, Phan ADT, Charlton KE. Low anthocyanin plum nectar does not impact cognition, blood pressure and gut microbiota in healthy older adults: a randomized crossover trial. Nutr Res. 2020;82:74–87. doi: 10.1016/j.nutres.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell SM, Milan AM, Mitchell CJ, Gillies NA, D’Souza RF, Zeng N, Ramzan F, Sharma P, Knowles SO, Roy NC, et al. Protein intake at twice the RDA in older men increases circulatory concentrations of the microbiome metabolite trimethylamine-N-oxide (TMAO). Nutrients. 2019;11:2207. doi: 10.3390/nu11092207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitchell SM, McKenzie EJ, Mitchell CJ, Milan AM, Zeng N, D’Souza RF, Ramzan F, Sharma P, Rettedal E, Knowles SO, et al. A period of 10 weeks of increased protein consumption does not alter faecal microbiota or volatile metabolites in healthy older men: a randomised controlled trial. J Nutr Sci. 2020;9:e25. doi: 10.1017/jns.2020.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fluitman KS, Wijdeveld M, Davids M, van Ruiten CC, Reinders I, Wijnhoven HAH, Keijser BJF, Visser M, Nieuwdorp M, IJ RG, et al. Personalized dietary advice to increase protein intake in older adults does not affect the gut microbiota, appetite or central processing of food stimuli in community-dwelling older adults: a six-month randomized controlled trial. Nutrients. 2023;15:332. doi: 10.3390/nu15020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The International Scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 80.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 81.Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, et al. The International Scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17(11):687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li P, Ji B, Luo H, Sundh D, Lorentzon M, Nielsen J. One-year supplementation with Lactobacillus reuteri ATCC PTA 6475 counteracts a degradation of gut microbiota in older women with low bone mineral density. NPJ Biofilms Microbio. 2022;8(1):84. doi: 10.1038/s41522-022-00348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McLoughlin RF, Berthon BS, Jensen ME, Baines KJ, Wood LG. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106(3):930–945. doi: 10.3945/ajcn.117.156265. [DOI] [PubMed] [Google Scholar]
- 84.Kim YA, Keogh JB, Clifton PM. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr Res Rev. 2018;31(1):35–51. doi: 10.1017/S095442241700018X. [DOI] [PubMed] [Google Scholar]
- 85.Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RB, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–1405.e21. doi: 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 86.Maldonado-Gomez MX, Martinez I, Bottacini F, O’Callaghan A, Ventura M, van Sinderen D, Hillmann B, Vangay P, Knights D, Hutkins RW, et al. Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome. Cell Host & Microbe. 2016;20(4):515–526. doi: 10.1016/j.chom.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 87.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6):1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 88.Talegawkar SA, Bandinelli S, Bandeen-Roche K, Chen P, Milaneschi Y, Tanaka T, Semba RD, Guralnik JM, Ferrucci L. A higher adherence to a Mediterranean-style diet is inversely associated with the development of frailty in community-dwelling elderly men and women. J Nutr. 2012;142(12):2161–2166. doi: 10.3945/jn.112.165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leon-Munoz LM, Guallar-Castillon P, Lopez-Garcia E, Rodriguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc. 2014;15(12):899–903. doi: 10.1016/j.jamda.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 90.Wu PY, Chen KM, Tsai WC. The Mediterranean dietary pattern and inflammation in older adults: a systematic review and meta-analysis. Adv Nutr. 2021;12:363–373. doi: 10.1093/advances/nmaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loughrey DG, Lavecchia S, Brennan S, Lawlor BA, Kelly ME. The impact of the Mediterranean diet on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Adv Nutr. 2017;8:571–586. doi: 10.3945/an.117.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu M, Luo Q, Nie R, Yang X, Tang Z, Chen H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit Rev Food Sci Nutr. 2021;61(13):2175–2193. doi: 10.1080/10408398.2020.1773390. [DOI] [PubMed] [Google Scholar]
- 93.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pothier K, Gana W, Bailly N, Fougere B. Associations between frailty and inflammation, physical, and psycho-social health in older adults: a systematic review. Front Psychol. 2022;13:805501. doi: 10.3389/fpsyg.2022.805501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coelho-Junior HJ, Calvani R, Azzolino D, Picca A, Tosato M, Landi F, Cesari M, Marzetti E. Protein intake and sarcopenia in older adults: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19:8718. doi: 10.3390/ijerph19148718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coelho-Junior HJ, Marzetti E, Picca A, Cesari M, Uchida MC, Calvani R. Protein intake and frailty: a matter of quantity, quality, and timing. Nutrients. 2020;12(10):2915. doi: 10.3390/nu12102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karakan T, Gundogdu A, Alagozlu H, Ekmen N, Ozgul S, Tunali V, Hora M, Beyazgul D, Nalbantoglu OU. Artificial intelligence-based personalized diet: a pilot clinical study for irritable bowel syndrome. Gut Microbes. 2022;14(1):2138672. doi: 10.1080/19490976.2022.2138672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study.


