Abstract
We present a case of a 67-year-old male with heart failure with reduced ejection fraction due to a previous myocardial infarction, slow-conducting atrial fibrillation with an indication of pacemaker implantation, and sustained ventricular tachycardia, requiring defibrillation support in prevention of sudden cardiac death. Current guidelines recommend biventricular over conventional right ventricular pacing for patients with ejection fraction <40 % and atrioventricular conduction disorders in either sinus rhythm or atrial fibrillation, but in patients with a narrow baseline QRS complex, biventricular pacing still induces a form of electrical dyssynchrony. In our case we combined the advantages of conduction system pacing in form of left bundle branch area pacing, in order to prevent further left ventricular deterioration due to newly induced dyssyncrony, and also defibrillation support, using a single-chamber defibrillator with a DF-1/IS-1 connection.
Keywords: Conduction system pacing, Left bundle branch area pacing, Heart failure, Single-chamber defibrillator
Learning objective
In patients with a pacing indication for slow-conducting atrial fibrillation and a concomitant implantable defibrillator indication, using a single-chamber defibrillator with a DF-1/IS-1 connection is the most cost-efficient option, while providing the combined advantage of physiological pacing and defibrillation support.
Introduction
Left bundle branch area pacing (LBBAP) has been established over the past few years as a feasible conduction system pacing technique and has been increasingly used in patients with pacing indications for bradyarrhythmias or cardiac resynchronization therapy [1]. The combination of physiological pacing and defibrillation support in the same patient could be challenging in terms of device selection. In most centers, the usual choice would be cardiac resynchronization therapy with a defibrillator (CRT—D) device, with the LBBAP lead connected to the left ventricular port.
We present a case of a patient with permanent slow-conducting atrial fibrillation and ventricular tachycardia caused by structural heart disease, who received both conduction system pacing and defibrillation support using a single-chamber defibrillator.
Case report
We present the case of a 67-year-old Caucasian male referred to our clinic for pacemaker implantation. The patient had a history of previous anterior myocardial infarction complicated with an apical aneurysm, with subsequent surgical revascularization of the anterior descending artery using the left internal mammary artery, diabetes mellitus, dyslipidemia, and permanent atrial fibrillation.
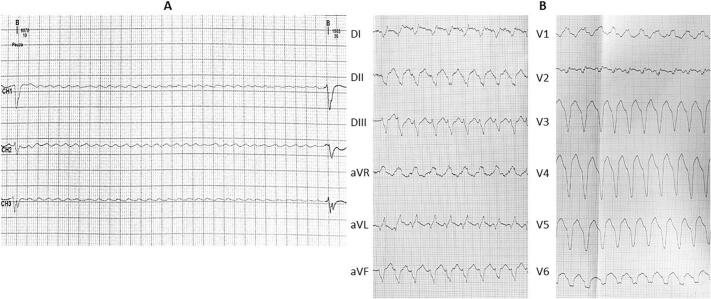
Ambulatory 24-h electrocardiogram (ECG) monitoring performed at another institution detected atrial fibrillation with the mean heart rate of 52 bpm (67 bpm during daytime and 42 bpm at night), with multiple pauses exceeding 6 s at night and 4 s during daytime, associated with symptoms such as light-headedness and shortness of breath (Fig. 1A).
Fig. 1.
(A) 24-Hour ambulatory ECG tracing depicting a period of ventricular asystole of over 6 s. (B) 12‑lead ECG tracing showing ventricular tachycardia originating from the left ventricular apical region. ECG, electrocardiography.
During the initial hospital stay, the patient also presented an auto-limited, sustained ventricular tachycardia, originating from the apical region (Fig. 1B).
The echocardiography showed an enlarged left ventricle (left ventricular telediastolic diameter of 72 mm, with telediastolic volume of 214 mL) with an apical aneurysm and akinetic apical segments of the left ventricle walls, with severely reduced global left ventricular function (left ventricular ejection fraction 19 % measured using Simpson's biplane method), atrial enlargement, and moderate mitral regurgitation. The N-terminal pro-B-type natriuretic peptide (NT-proBNP) value was 635 pg/mL, other laboratory results were unremarkable.
Taking into account the clinical and paraclinical findings, we considered that the patient had a class I indication for both permanent cardiac pacing and defibrillation support. In this context, we opted for the use of a single-chamber implantable cardiac defibrillator (ICD) with a DF-1/IS-1 connector, along with the implantation of an additional lead for LBBAP.
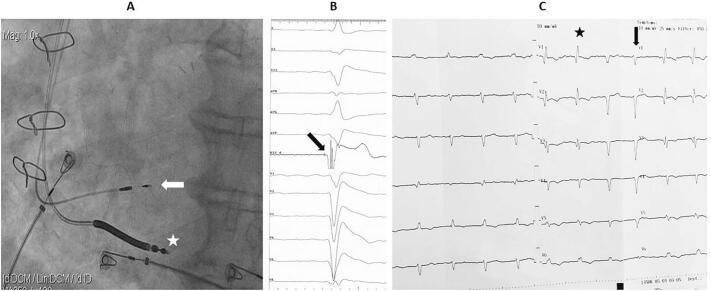
First, a standard single-coil defibrillation lead was placed in the right ventricular apex (Fig. 2A). Secondly, a 3830 SelectSecure lead (Medtronic, Minneapolis, MN, USA) was placed through a C315 His catheter (Medtronic) at the basal interventricular septum, where pacing revealed a positive complex in lead DII, a negative complex in lead DIII, and a “W” pattern in lead V1 (QS complex in V1 with a notch at the nadir of the QRS). With repeated clockwise rotations, the lead penetrated deep into the septum until ectopic beats of a right bundle branch block morphology were seen (Fig. 2A). Intracardiac electrograms recorded at the lead tip revealed a left bundle branch potential (Fig. 2B), and pacing at this site generated a narrow QRS complex with a right bundle branch block morphology similar in duration to the baseline complex (Fig. 2C).
Fig. 2.
(A) Fluoroscopic image in LAO projection showing the final position of the leads: the defibrillator lead in the right ventricular apex (white asterisk) and the LBBAP lead penetrating the interventricular septum (white arrow). (B) Intraprocedural electrogram revealing the left bundle branch potential recorded at the LBBAP lead tip (black arrow). (C) Final 12‑lead ECG showing paced QRS complexes (black asterisk) of right bundle branch block morphology with a similar duration as the alternating intrinsic QRS complexes (black arrow).
LAO, left anterior oblique; LBBAP, left bundle branch area pacing; ECG, electrocardiogram.
The LBBAP lead was connected to the IS-1 port of the defibrillator and the high-voltage connector to the corresponding port, while the IS-1 connector of the defibrillation lead was capped.
The final interrogation of the ICD recorded a pacing threshold of 0.5 V at 0.4 ms pulse duration and an excellent sensing threshold of 14 mV. The pacing component of the defibrillator was programmed in VVIR mode 70 bpm.
The patient was discharged the next day with an optimized treatment for heart failure (sacubitril/valsartan 49/51 mg twice a day, bisoprolol 10 mg once a day, eplerenone 25 mg once a day, and empagliflozin 10 mg once a day), anticoagulation with apixaban 5 mg twice a day and antiarrhythmic therapy (amiodarone 200 mg once a day).
At the six-month follow-up, the patient was clinically stable, without signs of congestion, and with improved exercise capacity. The interrogation of the device showed stable pacing threshold and sensing (0.5 V at 0.4 ms pulse duration and, respectively >12 mV), with 88 % ventricular pacing and no significant ventricular arrhythmias under beta-blocker in high doses (bisoprolol 10 mg per day). At the echocardiographic evaluation, left ventricular function was slightly improved (left ventricular ejection fraction 24 % measured using Simpson's biplane method) without any marked dyssynchrony. The NT-proBNP value dropped from 635 pg/mL to 402 pg/mL.
Discussion
Right ventricular pacing (RVP) is associated with a decrease in left ventricular function in the long term due to the asynchronous electrical activation and the resulting differential workload of opposing ventricular wall segments. Common predictors of pacing-induced cardiomyopathy are a baseline reduced ejection fraction and wide, paced QRS complexes [2,3].
Therefore, current guidelines recommend biventricular over conventional RVP for patients with heart failure (ejection fraction <40 %) and atrioventricular conduction disorders in either sinus rhythm or atrial fibrillation [4].
Based on this argument, the common option for patients with a reduced ejection fraction, an indication of permanent cardiac pacing, and a concomitant indication for defibrillation support would be to implant a biventricular CRT-D device. This is also available for patients with permanent atrial fibrillation, as was the case of our patient, with the difference that, since the atrial lead is not necessary, the atrial port could be plugged.
Biventricular pacing was shown to reduce the risk of heart failure progression and mortality compared to RVP in patients with reduced ejection fraction and a pacing indication. Unfortunately, in patients with a narrow baseline QRS complex, biventricular pacing still induces a form of electrical dyssynchrony, due to the merging of two non-physiological depolarization wavefronts: one arising from the left ventricular epicardium and another from the right ventricular endocardium. In this regard, studies have reported adverse outcomes with biventricular pacing in narrow QRS patients, and, as a consequence, this procedure is not recommended with the sole intention of cardiac resynchronization therapy in these patients [5].
Furthermore, biventricular pacing is highly dependent on the patient's coronary sinus anatomy, and it requires careful interventricular interval programming to ensure adequate ventricular electrical synchronization.
On the other hand, LBBAP is the latest physiological cardiac pacing technique implemented in clinical practice. The goal of the procedure is to capture the ramifications of the left bundle branch and thus, to use the intrinsic conduction system for very fast and synchronous activation of the left ventricle. Compared to His bundle pacing, LBBAP has the advantage of a significantly lower pacing threshold, higher sensing thresholds, a much wider target area, and an overall higher success rate [6]. Compared to RVP, studies have proven the superiority of LBBAP in terms of clinical status and left ventricular ejection fraction preservation [7]. Also, in patients with cardiac resynchronization indication, LBBAP was superior to biventricular pacing, leading to higher ejection fraction and smaller left ventricular volumes [8].
In our patient, we needed the combined advantages of conduction system pacing, to prevent further left ventricular deterioration during pacing in the form of pacing-induced cardiomyopathy, and also defibrillation support. To achieve this goal, we opted for the most cost-efficient option, to use a single-chamber defibrillator with a DF-1/IS-1 connection, since the atrial lead was redundant. We placed the LBBAP lead in the IS-1 port and the high-voltage shock connector in the dedicated port and capped the pace/sense connector of the defibrillator lead.
The obvious disadvantage of using a biventricular CRT-D device for these cases is the significantly increased cost of the procedure and sometimes the difficulty of placing the coronary sinus lead. By using our method, several advantages can be easily identified: the procedure is a lot cheaper, the LBBAP procedure has a high success rate in experienced hands, there is no need for complex programming since there are no interventricular intervals to manage, and there is no reason to worry about avoiding a high pacing burden. Also, the sensing threshold is usually very good since the sensing vector in bipolar mode spans the entire width of the septum, so there is no risk of ventricular fibrillation undersensing.
The only major limitation to this approach is the presence of significant scarring in the interventricular septum, which makes lead penetration very difficult, and also, once it reaches the left side of the septum, could be associated with ineffective pacing and low sensing thresholds. Fortunately, in our case, the myocardial scar was limited to the apical septum, so there were no concerns with lead placement in the basal part of the septum.
Also, there is the risk of lead-to‑lead interaction between the LBBAP and the defibrillator lead, which may lead to inappropriate sensing of non-cardiac signals or lead erosion. This can be easily avoided by placing the leads as far apart as possible and observing them fluoroscopically in different projections for contact and interaction.
To date, two prospective studies have addressed the possibility of using DF-1/IS-1 defibrillators for LBBAP, both of them in patients with an indication for cardiac resynchronization therapy. Ten patients were included in the CROSS-LEFT pilot study, and the authors showed that in this configuration there was adequate detection and treatment of the induced ventricular fibrillation and a significant improvement in left ventricular function [9]. In the second study, Ponnusamy et al. confirmed these results in 11 patients with successful LBBAP with stable pacing and sensing thresholds and no complications over the follow-up period, including inappropriate discharges [10].
The key message of this report is that an individualized approach to each device implantation could provide the patient with an effective therapy while reducing both the risks of the procedure and the associated costs.
Author contributions
All authors have read and agreed to the published version of the.
manuscript.
Funding
No external funding.
Informed consent statement
written informed consent has been obtained from the patient to publish this paper.
Declaration of competing interest
the authors of this work have nothing to disclose.
Acknowledgments
None.
References
- 1.Jastrzębski M., Kiełbasa G., Cano O., Curila K., Heckman L., De Pooter J., et al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J. 2022;43:4161–4173. doi: 10.1093/eurheartj/ehac445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khurshid S., Liang J.J., Owens A., Lin D., Schaller R., Epstein A.E., et al. Longer paced QRS duration is associated with increased prevalence of right ventricular pacing-induced cardiomyopathy. J Cardiovasc Electrophysiol. 2016;27:1174–1179. doi: 10.1111/jce.13045. [DOI] [PubMed] [Google Scholar]
- 3.Curtis A.B., Worley S.J., Adamson P.B., Chung E.S., Niazi I., Sherfesee L., et al. Biventricular versus right ventricular pacing in heart failure patients with atrioventricular BLOCK (BLOCK HF) trial investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–1593. doi: 10.1056/NEJMoa1210356. [DOI] [PubMed] [Google Scholar]
- 4.Glikson M., Nielsen J.C., Kronborg M.B., Michowitz Y., Auricchio A., Barbash I.M., et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–3520. doi: 10.1093/eurheartj/ehab699. [DOI] [PubMed] [Google Scholar]
- 5.Ruschitzka F., Abraham W.T., Singh J.P., Bax J.J., Borer J.S., Brugada J., et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369:1395–1405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 6.Zhuo W., Zhong X., Liu H., Yu J., Chen Q., Hu J., et al. Pacing characteristics of his bundle pacing vs. left bundle branch pacing: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.849143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S., Guo J., Tao A., Zhang B., Bao Z., Zhang G. Clinical outcomes of left bundle branch pacing compared to right ventricular apical pacing in patients with atrioventricular block. Clin Cardiol. 2021;44:481–487. doi: 10.1002/clc.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Zhu H., Hou X., Wang Z., Zou F., Qian Z., et al. Randomized trial of left bundle branch vs biventricular pacing for cardiac resynchronization therapy. J Am Coll Cardiol. 2022;80:1205–1216. doi: 10.1016/j.jacc.2022.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Clementy N., Bodin A., Ah-Fat V., Babuty D., Bisson A. Dual-chamber ICD for left bundle branch area pacing: the cardiac resynchronization and arrhythmia sensing via the left bundle (cross-left) pilot study. J Interv Card Electrophysiol. 2023;66:905–912. doi: 10.1007/s10840-022-01342-6. [DOI] [PubMed] [Google Scholar]
- 10.Ponnusamy S.S., Ramalingam V., Ganesan V., Syed T., Kumar M., Mariappan S., et al. Left bundle branch pacing-optimized implantable cardioverter-defibrillator (LOT-ICD) for cardiac resynchronization therapy: a pilot study. Heart Rhythm O2. 2022;3(6Part B):723-7 doi: 10.1016/j.hroo.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]