Abstract
Objective
Basilar artery occlusion (BAO) secondary to traumatic vertebral artery (VA) dissection caused by vertebral fracture is a rare cause of acute ischemic stroke, and optimal management, such as antithrombotic agents, surgical fixation, and parent artery occlusion (PAO), has been controversial. We report a case in which mechanical thrombectomy and PAO were performed for a BAO due to right VA dissection caused by a transverse foramen fracture of the axis vertebra.
Case Presentation
A patient in her 80s suffered from a backward fall, and a neck CT revealed a fracture and dislocation of the right lateral mass of the axis and a compressed transverse foramen. The patient was instructed to admit and to remain in bed rest; however, she suddenly lost consciousness the following day. The CTA revealed right VA occlusion and BAO; therefore, the patient underwent mechanical thrombectomy and the BAO was successfully reperfused but the VA stenotic dissection remained. PAO of the right VA was performed on the fifth day after the accident to prevent BAO recurrence.
Conclusion
Mechanical thrombectomy is an effective treatment for BAO caused by VA dissection, and PAO may contribute to the prevention of stroke recurrence.
Keywords: spine fracture, cerebrovascular injury, vertebral artery injury, mechanical thrombectomy, parent artery occlusion
Introduction
Cervical spine fractures and dislocations are associated with vertebral artery injury (VAI) in 0.53–39% of cases,1–3) and VAI is related to cerebral infarction in about 20% of patients.4–7) Although the efficacies of antithrombotic therapy,7,8) surgical fixation,6,9,10) and endovascular treatment7,11,12) for vertebral artery (VA) dissection have been previously reported, there are few case reports on the use of mechanical thrombectomy for basilar artery embolism due to traumatic VA dissection.13–16) In addition, indications for prophylactic parent artery occlusion (PAO) have not been established yet.7,9,12,17) Here, we report a case in which mechanical thrombectomy was performed for basilar artery occlusion (BAO) secondary to right VA dissection caused by a transverse foramen fracture of the axis vertebra. PAO of the dissected VA was performed after discussion with a spinal surgeon.
Case Presentation
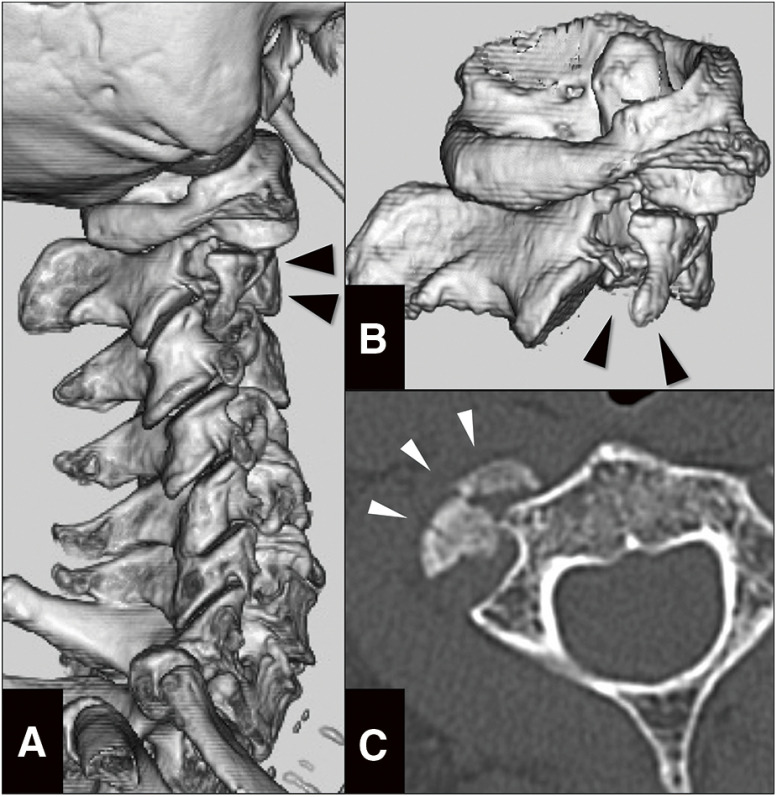
A patient in her 80s with hypertension slipped and suffered from a backward fall at home and was brought to our hospital by an ambulance. The patient had a right forearm laceration, an occipital blow, and right cervical pain, with no neurological disorders. The head CT showed no abnormal results. The neck CT revealed a fractured and dislocated right lateral mass of the axis and a compressed transverse foramen (Fig. 1A–1C) without prevertebral soft-tissue swelling or hematoma. Hence, the patient was admitted and underwent conservative management in the orthopedic ward, including neck collar fixation, bed rest, and gatch-up restriction of up to 30 degrees.
Fig. 1. CT at arrival. (A) 3D-CT showing spinal fracture at axis vertebra. (B) 3D-CT in the lateral view showing the fracture line and dislocation (black arrowheads) of the lateral mass of the second cervical spine. (C) Axial CT showing dislocation of the lateral mass of the second cervical vertebra (white arrowheads).
On the second day of hospitalization, the patient suddenly lost consciousness, evolving to a Glasgow Coma Scale (GCS) score of 3. The patient was intubated and placed on a ventilator, and the head CT demonstrated no intracranial hemorrhage, while the CTA showed right VA occlusion and BAO (Fig. 2A) without paravertebral extravasation. Considering the contraindication of intravenous infusion of recombinant tissue-type plasminogen activator in trauma cases, an emergency percutaneous mechanical thrombectomy was performed.
Fig. 2. Angiography images on the second day of hospitalization. (A) 3D-CTA performed upon worsening showing no blood flow on the basilar top and right proximal VA. (B) Preoperative left subclavian angiography indicating a vending VA origin. (C) Preoperative brachiocephalic angiography depicting a slow flow in the right VA. (D–E) Right vertebral angiography on anteroposterior (D) and lateral views (E) demonstrating VA dissection at the V3 segment. (F) Selective angiography distal to the dissection demonstrating BAO. (G) Microcatheter basilar angiography showing complete reperfusion. (H) Microcatheter right vertebral angiography at the lower V2 segment showing stagnating flow after balloon percutaneous transluminal angioplasty. (I) Left vertebral angiography depicting normal basilar perfusion.
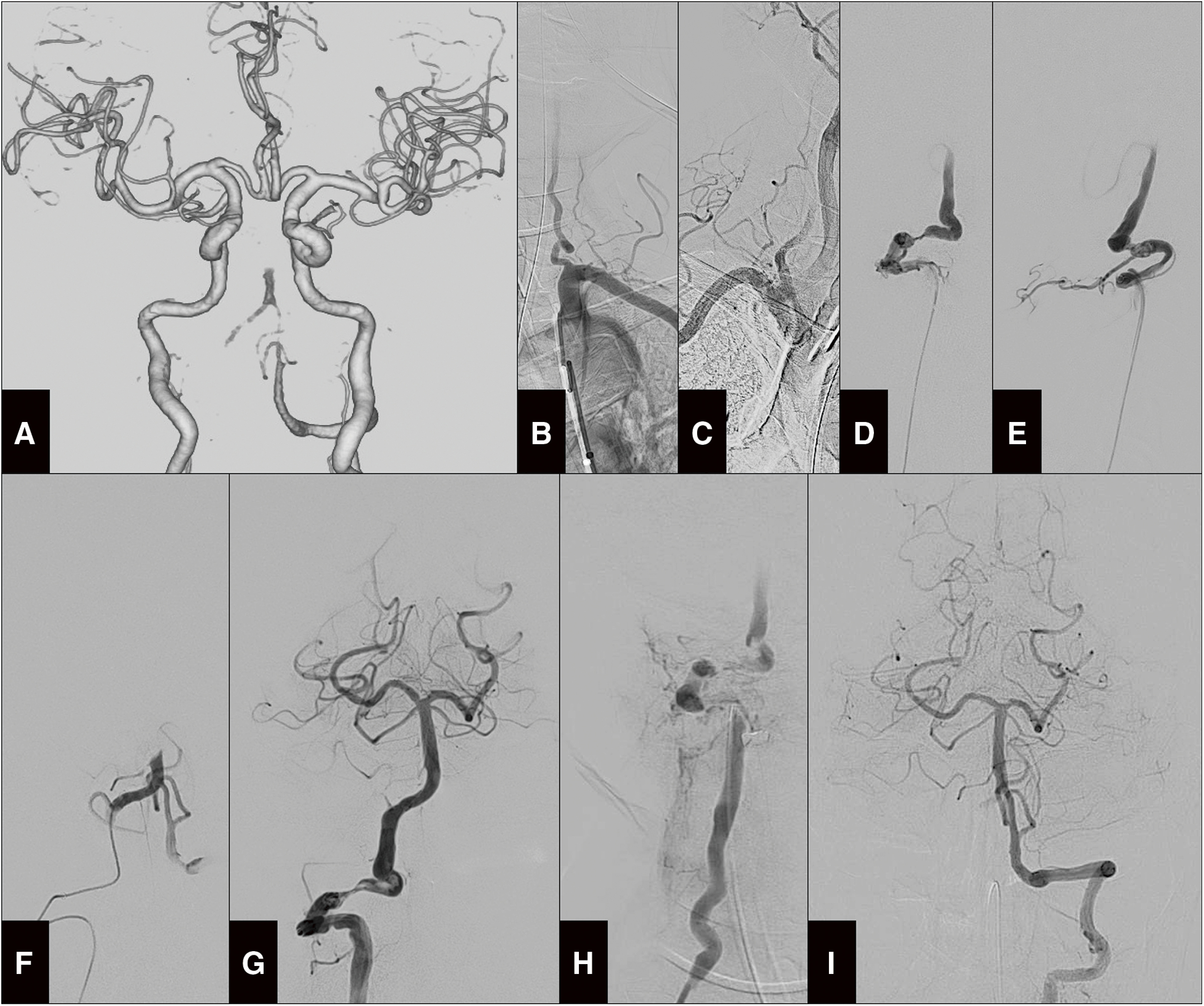
A 6F Flexor Shuttle Guiding Sheath (Cook Medical Inc., Bloomington, IN, USA) was deployed to the left subclavian artery via the right femoral artery. Left subclavian angiography was performed to approach the left VA depicted on CTA; however, the severe tortuosity observed at the origin of the left VA hindered the advancement of the guiding sheath (Fig. 2B). Next, the guiding sheath was placed in the right subclavian artery to approach the right stagnant VA (Fig. 2C). The right subclavian artery was also highly tortuous, and therefore, the guiding sheath could not advance further. Consequently, the right VA was approached from the guiding sheath placed in the subclavian artery using a Chikai-14 microguidewire (Asahi Intecc, Aichi, Japan), Marksman microcatheter (Medtronic, Irvine, CA, USA), and Penumbra 5MAX ACE reperfusion catheter (Penumbra, Alameda, CA, USA). Angiography of the microcatheter at the V2 segment revealed right VA blood flow stagnation and severe stenotic dissection of the V3 segment (Fig. 2D–2E).
The reperfusion catheter could not reach beyond the dissection site; it was reluctantly placed proximally. The microcatheter was advanced intracranially through the stenotic lesion and a BAO was diagnosed (Fig. 2F). Due to the standby position of the reperfusion catheter, it was necessary to pass the stent retriever through the dissection for thrombectomy. The damage to the dissected site was worry; therefore, a smaller stent retriever was selected among those in regular use. Two passes with a Trevo XP 3×20 mm stent retriever (Stryker, Kalamazoo, MI, USA) were performed and the basilar complex was recanalized (Fig. 2G). Finally, balloon dilatation of the right VA with Gateway Over-The-Wire (Stryker) was gently performed twice. The VA did not dilate and blood flow remained stagnant (Fig. 2H); hence, we considered stent implantation. However, it had to require strong antithrombotic therapy, and it could cause hemorrhagic complications to traumatic injured sites. Moreover, the patient had suffered from traumatic vertebral fracture the day before and needed to be considered for cervical fixation surgery. We assessed stent implantation to be invalid. A left vertebral angiogram confirmed favorable blood flow (Fig. 2I). Therefore, the procedure was terminated, with the right VA showing a Denver grade 2 stenosis.4) The anterior spinal artery originated from the distal right VA, and the right posterior inferior cerebellar artery (PICA) derived from the V4 segment (Fig. 2F); therefore, PAO for the right VA (V3 segment) was considered to prevent the recurrence of embolism. However, we could not decide on the PAO; therefore, as an alternative, 200 mg of acetylsalicylic acid was administered through a nasogastric tube, and an intravenous infusion of ozagrel sodium 80 mg was started immediately. The two antiplatelet drugs with short half-lives were selected to diminish the adverse effect of future traumatic changes and surgical treatment.
The patient was extubated on the day after the endovascular surgery and presented slight disorientation, facial paralysis, dysarthria, and ataxia. GCS score improved to 13 (E3V4M6) compared to before mechanical thrombectomy. Diffusion-weighted images (DWIs) showed multiple infarctions in the posterior circulatory territory (Fig. 3A). Intracranial and cervical MRA (Fig. 3B and 3C) demonstrated a reperfused basilar artery and a right VA with stagnant blood flow. As a maintenance dose, 100 mg of acetylsalicylic acid was only maintained.
Fig. 3. Post-thrombectomy images. (A) DWIs showing multiple infarctions in the posterior circulation territory. (B and C) Intracranial (B) and cervical (C) MRAs on the third hospitalization day showing the reperfused basilar and stagnant right vertebral arteries. (D) 3D-CTA on the fifth admission day demonstrating persistent severe stenotic dissection of the right VA without new dissection of the left VA. DWIs: diffusion-weighted images; VA: vertebral artery.
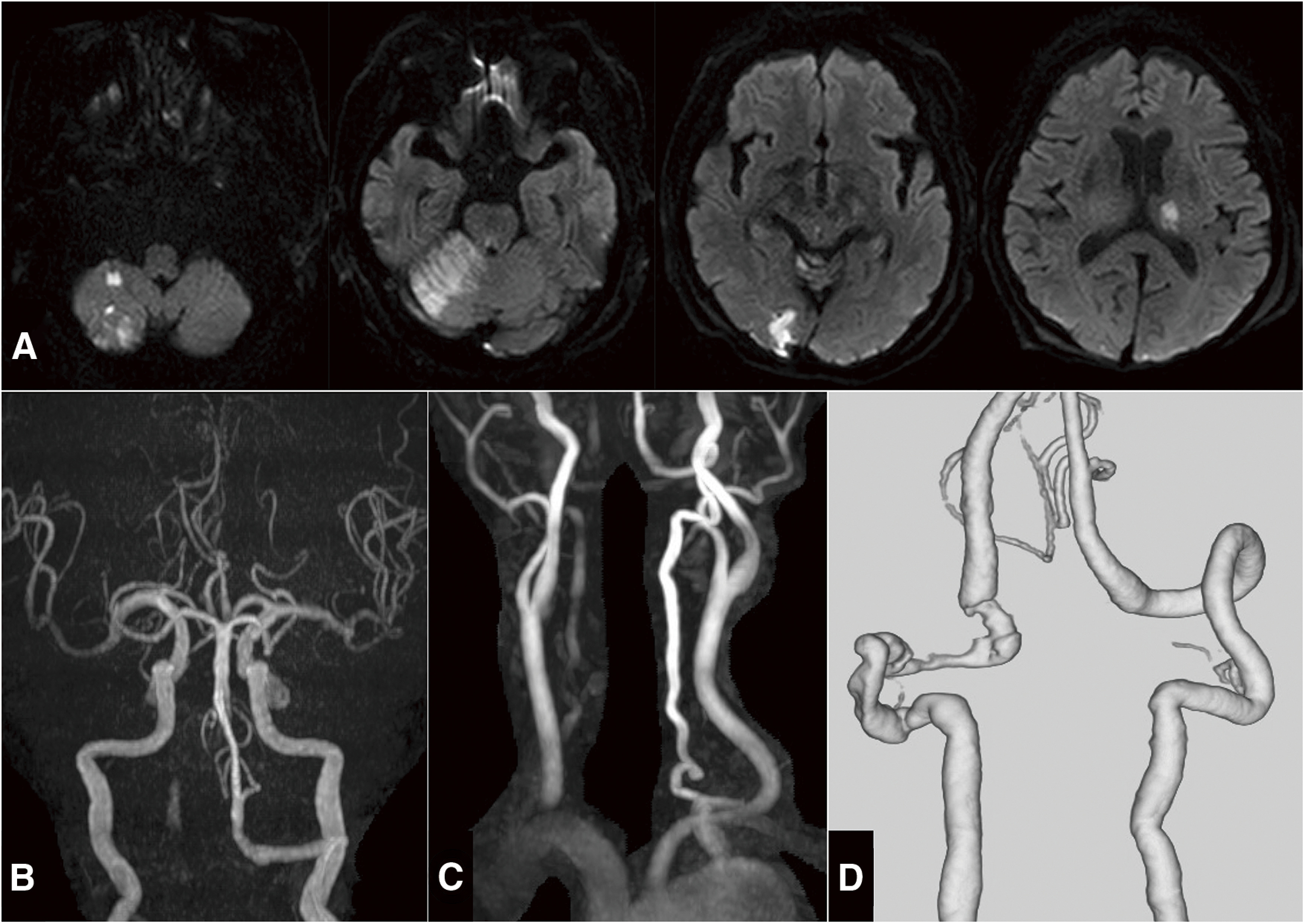
After several days of radiographic consideration and neurological examination, the spine surgeon indicated that the cervical spine was sufficiently stable and supportive, requiring no further fusion surgery and behavioral restrictions. This patient had a distal embolization even on bed rest and under gatch-up restriction on the second day of hospitalization; hence, there was a possibility of BAO recurrence. Moreover, the CTA on the fifth day of hospitalization showed persistent severe stenosis of the right VA without left VA new dissection (Fig. 3D). The possibility of fatal distal embolization from the remaining dissection could not be ruled out if rehabilitation is performed without limitation. The indication of PAO was controversial; hence, we discussed it with the spine surgeon and carefully obtained informed consent from her family. The PAO of the right VA was selected to prevent recurrence of the distal embolism.
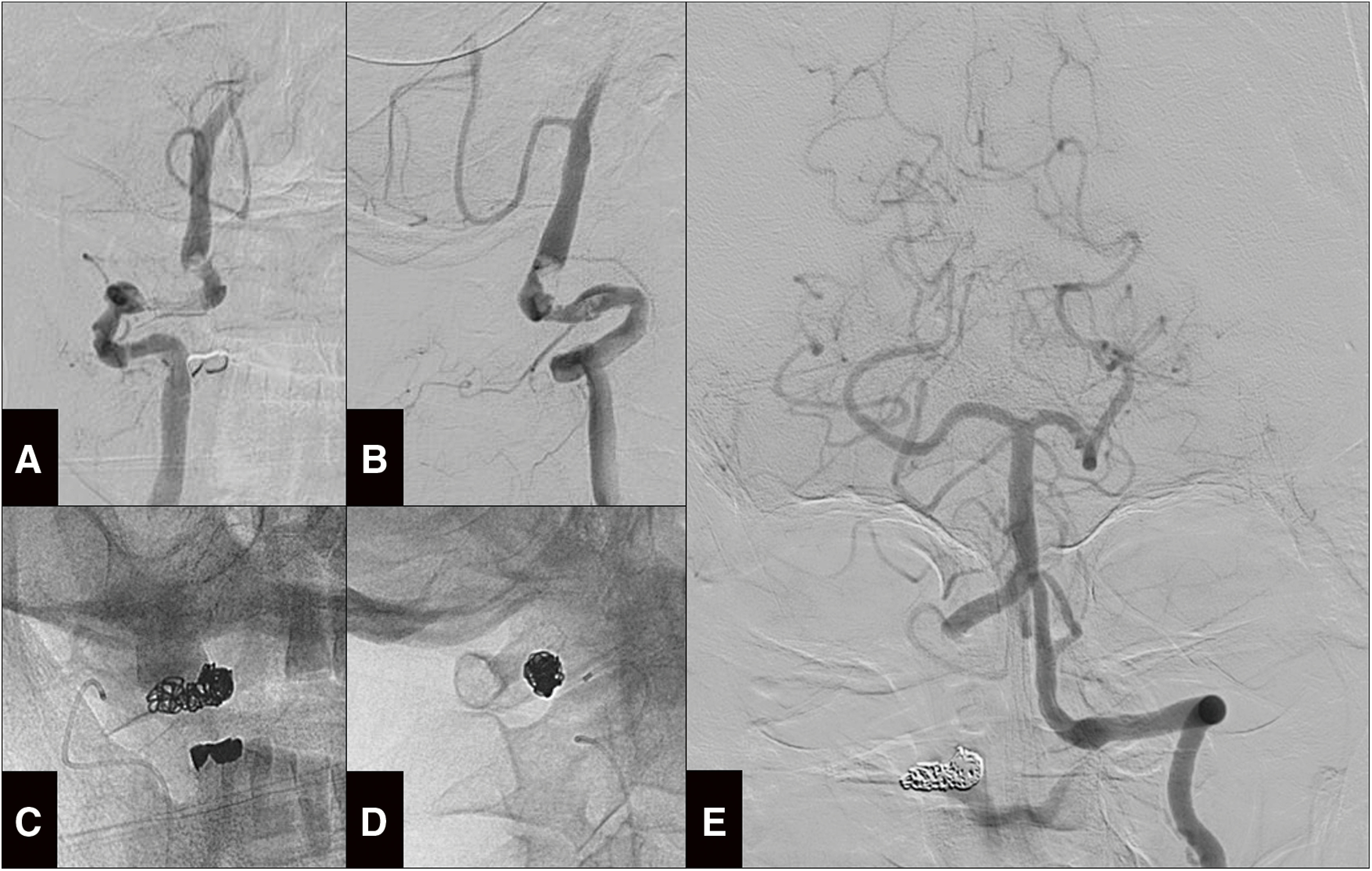
Preoperative right vertebral angiography revealed severe stenotic dissection and stagnant blood flow (Fig. 4A and 4B). The only V3 segment of the right VA was locally occluded using Plowler Select Plus microcatheter (Cerenovus, Raynham, MA, USA) and GDC Fibered VortX coils (Boston Scientific, Marlborough, MA, USA) (Fig. 4C and 4D) without occlusion of the V4 perforator. Postoperative left vertebral angiography revealed antegrade flow to the basilar artery and retrograde flow to the right PICA (Fig. 4E). The origin of anterior spinal artery was depicted in the previous angiogram between the union and the right PICA (Fig. 2F). The PAO was safely achieved. The patient did not show new neurological deficits or DWI abnormalities after PAO. Right vertebral retrograde flow was confirmed using intracranial MRA. The antiplatelet therapy was abandoned. She was transferred to a rehabilitation hospital on the 35th day of hospitalization with slight ataxia, gait disturbance, and a modified Rankin Scale score of 4.
Fig. 4. PAO for the right dissected VA on the fifth day of hospitalization. (A and B) Preoperative right vertebral angiography from the anteroposterior (A) and lateral (B) views showing the stagnating flow and persistent V3 dissection. (C and D) The coil mass placed on the V3 segment on the anteroposterior (C) and lateral (D) views. (E) Postoperative left vertebral angiography demonstrating normal flow to the right posterior inferior cerebellar artery.
Discussion
Approximately 70%–78% of VAI cases are associated with cervical spine fractures.1) The most frequent causes of this disease are motor vehicle accidents and falls,1,2,5,6,18) and VAI is also closely associated with head trauma.9) Torina et al. reviewed the MRI and MRA results of spinal cord injuries and found that VA thrombosis was more common in the lower cervical spine because the VA passes through the transverse foramen of the C6, C5, or even C4 vertebrae.19) In contrast, VAI occurs even at the C2 level, as in our case.3) Ding et al. focused on the correlation between C2 fractures and VAI, reporting that bony fragments within the foramen transversarium were associated with a greater risk of VAI.3) Similarly, our patient had a fractured axis vertebra, a dislocated lateral mass, and a compressed transverse foramen that caused VAI. VAI is classified based on the Denver grading system,4) and in combination with the respective stroke risks, its grade should be used to decide the therapeutic options.1,4) The present case had Denver grade 2 VAI; its high rate of stroke,4–7) low rate of natural healing,4) and high rate of pseudoaneurysm transformation4,9) have been reported in previous studies.
VAI is a devastating condition with a high subsequent stroke rate of about 20%.4–7) Biffl et al. mentioned that the time window from trauma to stroke is more than 10–72 hours.4,18) Early diagnosis and prophylactic treatment are essential in acute clinical settings. Medical therapy, including anticoagulation or antiplatelet agents, is a well-established treatment course that decreases stroke rates by 1%–3.9%.7,8) Furthermore, cervical spine surgery may reduce the risk of distal embolism and hemodynamic compromise by restoring cervical spine alignment and decompressing the blood vessels.6,9,10) Early posterior fusion surgery performed within 24 hours after injury without endovascular embolization or antithrombotic therapy has been reported to be a safe and effective treatment option for asymptomatic VA occlusion associated with cervical spine trauma.10) In contrast, cervical manipulation during surgery can further damage blood vessels, accelerate thrombus growth, and result in embolism, as evidenced by several reports of cerebral infarction in the posterior circulation after cervical spine surgery for traumatic cervical spine injury.17) Although controversial, the efficacy of endovascular treatment has been reported.7,9,11,12) In a retrospective study of 23 patients with cervical fractures and VA occlusion who required acute cervical spine surgery, preoperative PAO for VA occlusion was associated with a lower incidence of radiological thromboembolic stroke than surgery without preoperative PAO.12) PAO for VAI before cervical spine surgery for unstable cervical spine injuries is a treatment option for stroke prevention.12,17) As in this case, the necessity of a stable cervical spine injury is more controversial; however, several severe BAO cases from minor neck trauma were reported.13–15) VAI due to cervical spine fracture extends to V4 in only 2% of patients,6) and simple proximal occlusion is usually sufficient to prevent distal embolization.12) Therefore, prophylactic PAO would be safe without extradural origin of the PICA, contralateral hypoplasia, or contralateral injury. In this case, PAO indication, which was performed for secondary prevention after BAO, may have been appropriate. However, when considering secondary prevention of embolic stroke, it should be decided earlier by matching the favorable timing of the stroke onset.4,7,18)
Since thrombectomy was recently established, the usefulness of endovascular treatment for BAO caused by trauma has been reported.13–16) However, and unlike cardiogenic cerebral embolism, many cases present with dissection or severe stenosis in the proximal artery, and the approach to it has to be carefully considered. In this case, the basilar artery was assessed through the normal (clean-road) and occluded abnormal (dirty-road) VA, and mechanical thrombectomy was performed using antegrade or reverse revascularization variants.20) Various endovascular approaches may also be feasible, with the choice depending on the anatomy, accessibility, and cause, such as dissection or stenosis due to cervical spinal deviation. We considered that the approach from the dissected blood vessel had risks, such as bleeding; therefore, the procedure was attempted from the clean road, the left VA. However, its severe proximal tortuosity prevented access to the basilar artery. Switching to a left brachial artery puncture might have allowed safer thrombectomy from the left VA clean-road approach (Fig. 2B)20); however, left VA dissection might have occurred. We selected to guide the microcatheter distally beyond the right VA dissection and pass the dissection twice with a stent retriever to remove the basilar artery thrombus. Fortunately, complete recanalization of the BAO was obtained without complications. Finally, the right VA did not dilate, and blood flow remained stagnant; hence, we attempted to dilate the VA with balloon angioplasty or stent implantation. However, left vertebral angiography confirmed favorable blood flow. Therefore, the procedure was terminated, resulting in severe stenosis of the right VA. PAO at the initial surgery could have been more effective in preventing BAO by matching the favorable timing stated previously.4,7,18) We decided to perform PAO after confirming the CTA findings without left VA new dissection and spontaneous right VA healing. This patient initially had distal embolization even on bed rest and under gatch-up restriction; therefore, we decided to perform PAO for secondary prevention of BAO after obtaining informed consent. The PAO would also have prevented pseudoaneurysm transformation. The good recovery from BAO shows the reasonability of this decision, as it not only addressed the possibility of recurrent thromboembolic events but also mitigated the risk of subarachnoid hemorrhaging.
Conclusion
In this report, we present the case of a patient who experienced BAO following VA dissection resulting from a cervical spine fracture. Mechanical thrombectomy is an effective treatment for BAO caused by VAI, and PAO may contribute to stroke recurrence prevention and reduction in the risk of subarachnoid hemorrhage. Although individualized treatment strategies for VAI should be considered, this case highlights the potential efficacy of endovascular therapy. Further research and case reports are necessary to better understand the pathophysiology and the optimal management of this condition.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1).Shafafy R, Suresh S, Afolayan JO, et al. Blunt vertebral vascular injury in trauma patients: ATLS(®) recommendations and review of current evidence. J Spine Surg 2017; 3: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).R S, Gem K, A N, et al. Vertebral artery injury in cervical spine fractures: a cohort study and review of the literature. Ulster Med J 2020; 89: 89–94. [PMC free article] [PubMed] [Google Scholar]
- 3).Ding T, Maltenfort M, Yang H, et al. Correlation of C2 fractures and vertebral artery injury. Spine 2010; 35: E520–E524. [DOI] [PubMed] [Google Scholar]
- 4).Biffl WL, Ray CE, Jr., Moore EE, et al. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg 2002; 235: 699–706; discussion, 706–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Esnault P, Cardinale M, Boret H, et al. Blunt cerebrovascular injuries in severe traumatic brain injury: incidence, risk factors, and evolution. J Neurosurg 2017; 127: 16–22. [DOI] [PubMed] [Google Scholar]
- 6).Foreman PM, Griessenauer CJ, Chua M, et al. Corrective spinal surgery may be protective against stroke in patients with blunt traumatic vertebral artery occlusion. J Neurosurg Spine 2015; 23: 665–670. [DOI] [PubMed] [Google Scholar]
- 7).Stein DM, Boswell S, Sliker CW, et al. Blunt cerebrovascular injuries: does treatment always matter? J Trauma 2009; 66: 132–143; discussion, 143–144. [DOI] [PubMed] [Google Scholar]
- 8).Burlew CC, Biffl WL, Moore EE, et al. Blunt cerebrovascular injuries: redefining screening criteria in the era of noninvasive diagnosis. J Trauma Acute Care Surg 2012; 72: 330–335; discussion 336–337, quiz 539. [DOI] [PubMed] [Google Scholar]
- 9).Merrill S, Clifton W, Valero-Moreno F, et al. Vertebral artery injury with coinciding unstable cervical spine trauma: mechanisms, evidence-based management, and treatment options. Cureus 2020; 12: e7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Asukai M, Ushirozako H, Suda K, et al. Safety of early posterior fusion surgery without endovascular embolization for asymptomatic vertebral artery occlusion associated with cervical spine trauma. Eur Spine J 2022; 31: 3392–3401. [DOI] [PubMed] [Google Scholar]
- 11).Pham MH, Rahme RJ, Arnaout O, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery 2011; 68: 856–866; discussion, 866. [DOI] [PubMed] [Google Scholar]
- 12).Indo M, Oya S, Shojima M, et al. Prevention of thromboembolic infarction after surgery for traumatic cervical fracture with vertebral artery occlusion by preoperative endovascular coil embolization. World Neurosurg 2019; 129: e838–e844. [DOI] [PubMed] [Google Scholar]
- 13).Ali N, Al-Chalabi M, Salahuddin H. Successful mechanical thrombectomy for basilar artery occlusion in a seven-year-old male. Cureus 2021; 13: e13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Mikkelsen R, Dalby RB, Hjort N, et al. Endovascular treatment of basilar artery thrombosis secondary to bilateral vertebral artery dissection with symptom onset following cervical spine manipulation therapy. Am J Case Rep 2015; 16: 868–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Ladner TR, He L, Jordan LC, et al. Mechanical thrombectomy for acute stroke in childhood: how much does restricted diffusion matter? BMJ Case Rep 2014; 2014(nov12 1): bcr2014011465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Ratnasabapathy U, Purcell M, Bhattacharya JJ. Endovascular rescue of vertebro-basilar thrombosis in cervical spine injury. Spinal Cord Ser Cases 2018; 4: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Tumialán LM, Theodore N. Basilar artery thrombosis after reduction of cervical spondyloptosis: a cautionary report. J Neurosurg Spine 2012; 16: 492–496. [DOI] [PubMed] [Google Scholar]
- 18).Biffl WL, Moore EE, Offner PJ, et al. Optimizing screening for blunt cerebrovascular injuries. Am J Surg 1999; 178: 517–521. [DOI] [PubMed] [Google Scholar]
- 19).Torina PJ, Flanders AE, Carrino JA, et al. Incidence of vertebral artery thrombosis in cervical spine trauma: correlation with severity of spinal cord injury. AJNR Am J Neuroradiol 2005; 26: 2645–2651. [PMC free article] [PubMed] [Google Scholar]
- 20).Cohen JE, Leker RR, Gomori JM, et al. Emergent revascularization of acute tandem vertebrobasilar occlusions: endovascular approaches and technical considerations – confirming the role of vertebral artery ostium stenosis as a cause of vertebrobasilar stroke. J Clin Neurosci 2016; 34: 70–76. [DOI] [PubMed] [Google Scholar]