Summary
Background
Immunocompromised individuals are not optimally protected by COVID-19 vaccines and potentially require additional preventive interventions to mitigate the risk of severe COVID-19. We aimed to characterise and describe the risk of severe COVID-19 across immunocompromised groups as the pandemic began to transition to an endemic phase.
Methods
COVID-19-related hospitalisations, intensive care unit (ICU) admissions, and deaths (01/01/2022-31/12/2022) were compared among different groups of immunocompromised individuals vs the general population, using a retrospective cohort design and electronic health data from a random 25% sample of the English population aged ≥12 years (Registration number: ISRCTN53375662).
Findings
Overall, immunocompromised individuals accounted for 3.9% of the study population, but 22% (4585/20,910) of COVID-19 hospitalisations, 28% (125/440) of COVID-19 ICU admissions, and 24% (1145/4810) of COVID-19 deaths in 2022. Restricting to those vaccinated with ≥3 doses of COVID-19 vaccine (∼84% of immunocompromised and 51% of the general population), all immunocompromised groups remained at increased risk of severe COVID-19 outcomes, with adjusted incidence rate ratios (aIRR) for hospitalisation ranging from 1.3 to 13.1. At highest risk for COVID-19 hospitalisation were individuals with: solid organ transplant (aIRR 13.1, 95% confidence interval [95% CI] 11.2–15.3), moderate to severe primary immunodeficiency (aIRR 9.7, 95% CI 6.3–14.9), stem cell transplant (aIRR 11.0, 95% CI 6.8–17.6), and recent treatment for haematological malignancy (aIRR 10.6, 95% CI 9.5–11.9). Results were similar for COVID-19 ICU admissions and deaths.
Interpretation
Immunocompromised individuals continue to be impacted disproportionately by COVID-19 and have an urgent need for additional preventive measures beyond current vaccination programmes. These data can help determine the immunocompromised groups for which targeted prevention strategies may have the highest impact.
Funding
This study was funded by AstraZeneca UK.
Keywords: COVID-19, Retrospective studies, Immunocompromised, Hospitalisation, ICU admission, Mortality, England, Epidemiology
Research in context.
Evidence before this study
We searched Medline from 1 March 2020 to 31 March 2023, using search terms (“SARS-CoV-2” OR “COVID-19”) AND (“immunocompromised patient” [tiab] OR “immunocompromized patient” [tiab] OR “compromised host” [tiab] OR “compromized host” [tiab] OR “immune compromise” [tiab] OR “immune compromised host” [tiab] OR “immune compromised patient” [tiab] OR “immunocompromise” [tiab] OR “immunocompromised host” [tiab] OR “immunocompromized host” [tiab]) AND (“hospitalization” OR “hospitalisation” OR “ICU” OR “mortality”), to identify studies that reported on increased risk of severe COVID-19 events in immunocompromised individuals, compared with the general population. Seven citations of interest were retrieved, of which three were retrospective cohort studies, but none of these studies covered the period after March 2022.
Added value of this study
The INFORM study aims to assess and characterise the COVID-19 risk and burden among immunocompromised groups using routinely collected electronic healthcare record data from a random sample representative of the total English population. These initial results provide critical, and urgently needed, granular-level data for describing immunocompromised populations that remained at increased risk of severe COVID-19 throughout 2022, despite vaccination programmes being well established. Results show that immunocompromised individuals accounted for 3.9% of the population of England, but 22% of COVID-19 hospitalisations, 28% of COVID-19 ICU admissions, and 24% of COVID-19 deaths, despite >80% having received ≥3 doses of a COVID-19 vaccine. In a fully vaccinated population adjusted for sex, age, and number of non-immunocompromising comorbidities, COVID-19 risk remained elevated across immunocompromised groups by 1.3 to 13.1-fold for COVID-19 hospitalisation and 1.3 to 19.9-fold for COVID-19 deaths, compared with the general population, with solid organ transplant recipients comprising the group at highest risk for both hospitalisation and death.
Implications of all the available evidence
While vaccines may be sufficient to safeguard most immunocompetent individuals against severe COVID-19, the highly disproportionate risk for the immunocompromised population found in this study underscores the urgent need for additional preventive measures against COVID-19 in this vulnerable population. The results of our study can be used to determine the immunocompromised groups at highest risk and support an evidence-based approach to ensure that preventive interventions combined with effective vaccination programmes are targeted towards individuals where interventions are likely to have the greatest impact, thus enabling policymakers to make informed decisions and ensure equitable access policies.
Introduction
In late 2021, a worldwide resurgence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) occurred with the rapid spread of the Omicron variant. In 2022, the considerable reduction in coronavirus disease (COVID-19) hospitalisation and mortality compared with the early phase of the pandemic was attributable to broad access to COVID-19 vaccines, SARS-CoV-2 infection and reinfection acquired immunity,1, 2, 3, 4, 5, 6 attenuated viral pathogenicity of Omicron,7,8 increased availability of effective treatments such as neutralising monoclonal antibodies and antivirals,9,10 and the advent of bivalent COVID-19 vaccine boosters.11 Yet in the UK, COVID-19 accounted for 3.9% of all deaths in 2022 with a total of 12,017 deaths from January to May 2023.12 In the United States, hospitalisation rates were about three times higher for COVID-19 than influenza and respiratory syncytial virus between October 2022 and April 2023.13, 14, 15, 16 Thus, although the World Health Organization declared that COVID-19 no longer constitutes a public health emergency of international concern on 5 May 2023, SARS-CoV-2 continues to cause substantial disease.17
In particular, COVID-19 remains a threat for immunocompromised individuals, owing to their suboptimal response to vaccines18, 19, 20 and the lifting of non-pharmaceutical preventive public health measures.6 In immunocompromised individuals, COVID-19 vaccines elicit shorter duration of protection,21 lower seroconversion rates post-vaccination,21, 22, 23 and reduced neutralisation activity.22 Immunocompromised individuals may consequently be at increased risk of hospitalisation and death even after vaccination.20 For instance, during the early Omicron era in British Columbia, Canada, severely immunocompromised individuals were 16 times more likely to be hospitalised for COVID-19 than immunocompetent individuals.24 Additionally, in a study conducted in the United States using a large health claims database (the Healthcare Integrated Research Database) from April 2020 to March 2022, 23.5% of the immunocompromised individuals had a COVID-19-related hospitalisation.25 To reduce their risk of SARS-CoV-2 infection and severe outcomes, immunocompromised individuals are recommended to continue to self-isolate,26,27 heavily impacting their mental health and health-related quality of life.28,29
However, immunocompromised individuals comprise a heterogeneous population with varying degrees of immunosuppression that can be attributable to either the underlying disease itself, treatment with immunosuppressive medications or therapies, and/or the duration of the condition or treatment.21,30, 31, 32 The wide range of conditions considered immunocompromising has led to differing categorisations (e.g., who should be considered moderately vs severely immunocompromised), resulting in important inconsistencies in determining who should be targeted and prioritised for COVID-19 preventive measures, such as COVID-19 booster dose vaccination or pre-exposure prophylaxis (PrEP) with long-acting monoclonal antibodies.
Using routinely collected, national primary and secondary care data in England, the overarching aim of the INFORM study (INvestigation oF cOvid-19 Risk among iMmunocompromised populations) was to provide a comprehensive assessment of COVID-19 impact, risk, and cost among immunocompromised populations and other high-risk populations compared with the general population since the start of the pandemic. Here we present the first results from INFORM focused on understanding which immunocompromised groups have remained at high-risk of severe COVID-19. The first aim, reported here, was to define which immunocompromised groups remain at the highest risk of severe COVID-19 outcomes, as the pandemic has transitioned to an endemic phase.
Methods
Study design and data sources
This is a retrospective cohort study in England that used de-identified routinely collected electronic healthcare record data from primary and secondary care, linked to national data on COVID-19 surveillance, vaccination status, primary care medication dispensations, and mortality, accessed via the National Health Services (NHS) Digital. Datasets used included: General Practice Extraction Service Data for Pandemic Planning and Research (GDPPR), COVID-19 Second Generation Surveillance System (SGSS) from Pillar 1 and Pillar 2, COVID-19 vaccination status data, Hospital Episode Statistics (HES), NHS Business Service Authority (BSA) dispensing data, Office of National Statistics (ONS) data, and Personal Demographics Service (PDS) data (see Supplement S1 for further information). This study was registered on the International Traditional Medicine Clinical Trial Registry (ISRCTN, ISRCTN53375662).
Cohort and sample selection
The study population consisted of a random sample of 25% of all individuals aged ≥12 years, registered and active (i.e., legal residents) in the NHS-linked national datasets on 1 January 2022. This age cutoff aligned with cutoffs used for COVID-19 vaccination programmes in the UK and globally (based on the non-paediatric label for the mRNA vaccines) and target age groups for COVID-19 PrEP programmes.
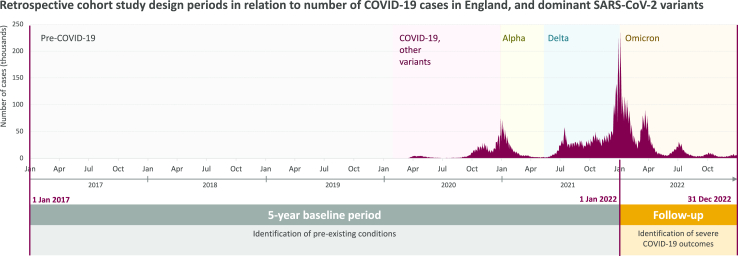
The overall study period was between 1 January 2017 and 31 December 2022 (Fig. 1). A period of 60 months, from 1 January 2017 to 31 December 2021, was used to assess patient characteristics and identify pre-existing chronic conditions; the period from 1 January 2022 (index date) to 31 December 2022 was used to assess occurrence of COVID-19 outcomes. Data were available from all datasets from the start to end of the study period (Note: the GDPPR dataset includes all patients registered to a GP practice and alive on 31 October 2019. Baseline data from GDPPR prior to October 2019 were also available from NHS Digital).
Fig. 1.
Study Period.
Chart sources: GOV. UK dashboard https://coronavirus.data.gov.uk/ and Our World in Data https://ourworldindata.org/coronavirus#explore-the-global-situation.
Outcomes
Main outcomes were: COVID-19-related hospitalisation, defined as ≥1 in-patient overnight stay with COVID-19 recorded as the primary diagnosis (discharge and rehospitalisation within 7 days were considered to be the same event); COVID-19-related intensive care unit (ICU) admission, defined as ICU admission within a hospitalisation episode with COVID-19 as the primary diagnosis; and COVID-19-related mortality, defined as a death in ONS mortality data with COVID-19 recorded as a cause of death, or an in-hospital death during a COVID-19 hospitalisation.
Population characteristics
Baseline demographic and clinical characteristics, including age, sex, ethnicity, body mass index (BMI), Index of Multiple Deprivation (IMD),33 and number of non-immunocompromising comorbidities (0, 1, 2, or ≥3) associated with an increased risk of severe COVID-19 outcomes were described for the study population using the GDPPR dataset. Comorbidities included diabetes, current or former smoking, Down's syndrome, sickle cell disease, thalassemia, multiple sclerosis, motor neurone disease, myasthenia gravis, Huntington's disease, chronic heart disease, cerebrovascular disease, chronic liver disease, chronic lung disease, pulmonary hypertension, and cystic fibrosis (see Supplement S2 for further details). Continuous measures were summarised using mean, standard deviation, median, interquartile range, and minimum and maximum values; categorical measures were summarised using numbers and percentages.
Definitions of immunocompromised individuals
Immunocompromised individuals were grouped according to predefined immunocompromising conditions and/or treatment strategies defined during the baseline period using the GDPPR, HES, and BSA datasets. Individuals were labelled as having ≥1 immunocompromising condition according to: 1) a broad definition that encompassed all conditions/treatments generally considered immunocompromising; and 2) a stringent definition based on criteria aligned with UK pandemic guidelines.34,35
“Broadly-defined immunocompromised” included any individual with ≥1 of the following:
-
•
Primary immunodeficiency: primary immunodeficiency ≤5 years prior to 1 January 2022.
-
•
Secondary immunodeficiency: immunosuppressive therapy [ies] ≤12 months prior to 1 January 2022.
-
•
High-dose or long-term moderate dose corticosteroids: chronic immune-mediated inflammatory diseases who received high-dose corticosteroids (≥20 mg prednisolone per day) for >10 days in the month prior to 1 January 2022; or chronic immune-mediated inflammatory disease who received long-term moderate dose corticosteroids (10 mg prednisolone per day for >4 weeks) ≤3 months prior to 1 January 2022; or high-dose steroids (>40 mg prednisolone per day for >1 week) for any reason in the month prior to 1 January 2022.
-
•
End-stage kidney disease (ESKD): ESKD ≤5 years prior to 1 January 2022.
-
•
Solid organ transplant: solid organ or islet transplant, not including corneal transplants ≤5 years prior to 1 January 2022.
-
•
Stem cell transplants: haematopoietic stem cell transplants 2 years prior to 1 January 2022.
-
•
Solid tumour: solid tumour [s] ≤5 years prior to 1 January 2022.
-
•
Haematological malignancy: haematological malignancy [ies] ≤5 years prior to 1 January 2022.
-
•
Advanced or untreated human immunodeficiency virus (HIV): clinical manifestations of symptomatic HIV (i.e., diagnostic code for HIV/acquired immunodeficiency syndrome (AIDS) or AIDS-defining conditions) ≤12 months prior to 1 January 2022. As CD4 counts were not available in the data, only patients with AIDS-defining illness were captured.
“Stringently-defined immunocompromised” comprised a subset of the broadly-defined and included any individual with ≥1 of the following:
-
•
Moderate to severe primary immunodeficiency: common variable immunodeficiency disease, severe combined immunodeficiency, DiGeorge syndrome, or Wiskott-Aldrich syndrome ≤5 years prior to 1 January 2022.
-
•
Active treatment with non-corticosteroid immunosuppressive or immunomodulatory therapy: treatment with alkylating agents, antimetabolites, transplant-related immunosuppressive drugs, cancer chemotherapeutic agents classified as severely immunosuppressive, TNF blockers, and other biologic agents that are immunosuppressive or immunomodulatory (e.g., B-cell depleting agents) ≤12 months prior to 1 January 2022.
-
•
High-dose corticosteroids: treatment with high-dose corticosteroids steroids (i.e., ≥20 mg prednisone or equivalent per day when administered ≥2 weeks) in the month prior to 1 January 2022.
-
•
Solid organ transplant: solid organ transplant (excluding corneal transplants) or islet transplant ≤2 years prior to 1 January 2022.
-
•
Stem cell transplant: haematopoietic stem cell transplant ≤2 years prior to 1 January 2022.
-
•
Solid or haematologic malignancy on active treatment: solid tumour [s] or haematologic malignancies on treatment ≤6 months prior to 1 January 2022.
-
•
Chronic lymphocytic leukaemia, non-Hodgkin's lymphoma, multiple myeloma, acute leukaemia: chronic lymphocytic leukaemia, non-Hodgkin's lymphoma, multiple myeloma, or acute leukaemia ≤2 years prior to 1 January 2022.
-
•
Advanced or untreated HIV: same definition as above for broadly-defined.
Immunocompromised groups were not mutually exclusive, i.e., individuals can be immunocompromised due to ≥1 condition/treatment. To more thoroughly assess COVID-19 burden and risk associated with specific immunocompromised groups, additional subgroups were included. For solid tumours and haematological malignancies, this included subgroups based on recency of treatment (≤6 months, 6–12 months, and >12 months); for solid organ transplant, this included individuals receiving their transplant ≤1 year and >1–2 years prior and on anti-rejection therapy. Further details of all definitions are provided in Supplement S3. The comparison group for individuals with a particular immunocompromising condition corresponds to all individuals who do not have that specific condition, including those with other immunocompromising conditions.
Statistical analysis
Crude incidence rates (IRs) per 1000 person-years were estimated for COVID-19 hospitalisations, ICU admissions and mortality by dividing the total number of COVID-19 outcome events by the sum of person time at-risk across all individuals in the analysis set multiplied by 1000. Time at risk was calculated as time from 1 January 2022 to the earlier of 31 December 2022 or date of all-cause death. Time at-risk for COVID-19 hospitalisations did not consider the duration of the hospitalisation events or any time between hospital admissions ≤7 days apart. Similarly, time in ICU, or between ICU admissions ≤7 days apart, was not considered as time at risk for COVID-19 ICU admissions.
Adjustment for age, sex, and number of non-immunocompromising comorbidities (0, 1, 2, or ≥3, see list above) was used to enable comparison of severe COVID-19 outcomes among immunocompromised groups with the general population. Adjusted incidence rate ratios (aIRRs) were estimated using a Poisson model with offset for person time at risk. For hospitalisations, the model predicted total hospitalisations per person over the study period, allowing for multiple hospitalisations for an individual. Individuals were censored with the same approach as crude IRs. Censoring was based on the earliest of all-cause death or end of study period. Results are presented for broadly- and stringently-defined immunocompromised individuals, and by specific immunocompromising conditions/treatments, comparing individuals in each group/subgroup (numerator: exposed to the immunocompromising condition/treatment) to those not in the group/subgroup (denominator: unexposed). Analysis was repeated for fully vaccinated individuals, defined as individuals who had received ≥3 doses of a COVID-19 vaccine on or before 1 January 2022.
The number of hospitalisations per 1000 individuals, relative to the size of the immunocompromised group/subgroup and absolute number of hospitalisations was used to quantify the potential impact of additional preventive interventions (such as social distancing, lockdown, prophylactic monoclonal treatment, etc.), alongside effective vaccination programmes, if successfully targeted towards specific populations within the broad or stringent definitions. The 25% random sample was scaled (multiplied by 4) to estimate the total size of groups/subgroups and number of hospitalisations within the whole population of England (Supplement S4).
Ethics
Access to anonymised data was facilitated through the NHS-Digital Secure Data Environment (SDE). The SDE provides secure access to the health and care data held by NHS-Digital, eliminating the need for it to leave NHS-Digital. All analysis was conducted in the secure environment without any personal data transfer. The study protocol was exempted from Ethics Application by the Health Research Authority under the Integrated Research Application System application number 304305.
Reporting
The Strengthening the Reporting of Observational studies in Epidemiology (STROBE, https://www.strobe-statement.org/download/strobe-checklist-cohort-case-control-and-cross-sectional-studies-combined) checklist was used to guide transparent reporting of our work.36 Due to disclosure rules, cells with counts <10 were suppressed, while other counts were rounded to the nearest 5 or 0.
Patient and public involvement
Patients were involved in the review of the study protocol and analysis plan.
Results
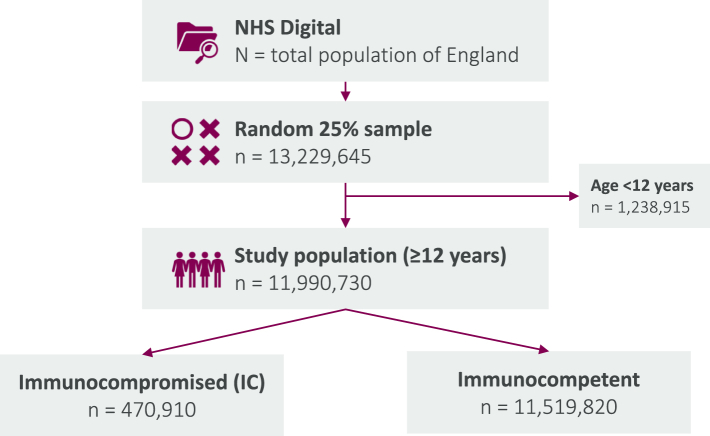
Study population and prevalence of immunocompromised groups
The study population consisted of 11,990,730 individuals aged ≥12 years (Fig. 2). Compared with the total study population, immunocompromised individuals were older (mean age 66.1 years for broadly-defined and 64.7 years for stringently-defined vs 45.2, respectively), had more comorbidities (20.4% and 21.3% with ≥3 vs 6.5%, respectively), and were more likely to be vaccinated with ≥3 COVID-19 vaccine doses (84.5% and 83.6% vs 50.5%, respectively), whereas the proportions of female and highly deprived individuals and mean BMI were similar (Table 1).
Fig. 2.
INFORM study flowchart. a NHS digital includes 98% of England's population; b Active treatment with non-corticosteroid immunosuppressive or immunomodulatory therapy(ies); c Chronic lymphocytic leukaemia, non-Hodgkin's lymphoma, multiple myeloma, acute leukaemia. Numbers in cells below 10 are suppressed, otherwise numbers are rounded to 0 or 5, in compliance with NHS Digital suppression rules.
Table 1.
Baseline characteristics of the study population ≥12 years-old, England, NHS-Digital, 2022.
| Characteristic | Total population |
Broadly-defined immunocompromised |
Stringently-defined immuncompromised |
|---|---|---|---|
| N = 11,990,730 | N = 470,910 | N = 83,185 | |
| Age, mean (SD) | 45.2 (20.7) | 66.1 (16.4) | 64.7 (17.0) |
| Sex (n, %) | |||
| Male | 5,953,930 (49.7) | 224,860 (47.8) | 41,475 (49.9) |
| Female | 6,036,080 (50.3) | 246,040 (52.2) | 41,710 (50.1) |
| Ethnicity (n, %)a | |||
| White | 6,450,440 (53.8) | 388,380 (82.5) | 69,015 (83.0) |
| Black | 398,490 (3.3) | 13,165 (2.8) | 2730 (3.3) |
| South Asian | 641,405 (5.3) | 18,735 (4.0) | 3765 (4.5) |
| Other | 621,110 (5.2) | 14,645 (3.1) | 2955 (3.6) |
| Unknown | 3,879,283 (32.4) | 35,984 (7.6) | 4721 (5.7) |
| Index of Multiple Deprivation± (n, %) | |||
| Most deprived 20% | 2,975,810 (24.8) | 97,085 (20.6) | 17,380 (20.9) |
| Second most deprived 20% | 2,539,420 (21.2) | 91,975 (19.5) | 16,355 (19.7) |
| Third most deprived 20% | 2,154345 (18.0) | 89,250 (19.0) | 15,905 (19.1) |
| Second least deprived 20% | 2,100,470 (17.5) | 96,765 (20.5) | 17,045 (20.5) |
| Least deprived 20% | 1,946,735 (16.2) | 94,925 (20.2) | 16,400 (19.7) |
| Body Mass Index, for individuals aged 18+ years | |||
| Mean (SD)b | 27.1 (6.6) | 27.9 (6.2) | 27.6 (6.1) |
| Median (IQR)c | 26.2 (7.8) | 27.1 (7.3) | 26.8 (7.3) |
| Minimum, Maximum | 10.0, 90.0 | 10.0, 90.0 | 10.0, 89.9 |
| Number of non-immunocompromising comorbidities (n,%)d | |||
| 0 | 5,776,290 (48.2) | 116,385 (24.7) | 19,670 (23.6) |
| 1 | 3,839,335 (32.0) | 148,525 (31.5) | 25,785 (31.0) |
| 2 | 1,597,535 (13.3) | 109,950 (23.3) | 20,010 (24.1) |
| ≥3 | 777,565 (6.5) | 96,045 (20.4) | 17,725 (21.3) |
| Number of doses of a COVID-19 vaccine (n, %) | |||
| 0 | 2,642,845 (22.0) | 23,705 (5.0) | 4095 (4.9) |
| 1 | 830,965 (6.9) | 6675 (1.4) | 1520 (1.8) |
| 2 | 2,456,280 (20.5) | 42,690 (9.1) | 8015 (9.6) |
| ≥3 | 6,060,635 (50.5) | 397,835 (84.5) | 69,555 (83.6) |
| Death during follow-up (n, %) | 122,155 (1.0) | 33,985 (7.2) | 10,025 (12.1) |
IQR – interquartile range, SD – standard deviation.
Ethnicity distribution in the total population is greatly biased by the large proportion of “Unknown”. 2021 Census data for England and Wales point to an ethnicity distribution very similar to that observed in the IC populations: 82% White and 18% Black, Asian, mixed or other subgroup. Source: https://www.ethnicity-facts-figures.service.gov.uk.
Patients with missing values excluded from denominator in calculations of summary statistics.
IQR between the 25% and 75% percentile of the associated distribution.
Comorbidities included diabetes, current or former smoking, Down's syndrome, sickle cell disease, thalassemia, multiple sclerosis, motor neurone disease, myasthenia gravis, Huntington's disease, chronic heart disease, cerebrovascular disease, chronic liver disease, chronic lung disease, pulmonary hypertension, and cystic fibrosis.
The prevalence of immunocompromised individuals was 3.9% when broadly-defined (n = 470,910) and 0.7% when stringently-defined (n = 83,185) (Table 2), with stringently-defined representing a 17.7% subset of the broadly-defined. Differences in the absolute number of broadly-vs stringently-defined immunocompromised individuals were primarily driven by stringently-defined only including solid tumours with active treatment in the last 6 months (n = 27,700 of 309,395 individuals with solid tumours in the last 5 years) (Table 2). Stringently-defined also only included: active treatment of haematological malignancies in the last 6 months (n = 8420 of 60 270 individuals with haematological malignancies in the last 5 years); active treatment with non-corticosteroid immunosuppressive or immunomodulatory therapy (n = 11,365 of 89,885 individuals with secondary immunodeficiency); moderate to severe primary immunodeficiency (n = 1270 of 9320 individuals with any primary immunodeficiency); solid organ transplant in the last 2 years or on anti-rejection therapies (n = 4245 or 2640, respectively, of 7375 with solid organ transplant in the last 5 years); and high-dose corticosteroids treatment (n = 3365 of 4130 with high-dose or long-term moderate-dose corticosteroids). Immunocompromised groups/subgroups were not mutually exclusive, with most overlap occurring with solid organ transplant (20% of ESKD and 3% of secondary immunodeficiency patients).
Table 2.
COVID-19-related hospitalisations (COVID-19 cases, number of patients, proportion, crude IR per 1000 person-years, and IRR) in the population aged >12 years, overall and by immunocompromising condition, England, NHS-Digital, 2022.
| Overall Population |
COVID-19 Hospitalisationsd |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N total study populatione | % of total populationb | Patients hospitalisede | N hospitalisationse | % of hospitalisationsb | Total person-time (years) | Crude IR (per 1000 patient years) | IR 95% CI | Adjusted IRRc | IRR 95% CI | |
| Overall study population | ||||||||||
| All individuals | 11,990,730 | 100.00 | 20,600 | 20,910 | 100.0 | 11,987,570 | 1.7 | 1.6–1.8 | ||
| Total broadly defined immunocompromised | 470,910 | 3.90 | 4465 | 4585 | 21.9 | 470,055 | 9.7 | 9.4–10.0 | 2.16 | 2.09–2.24 |
| Total stringently defined immunocompromised | 83,185 | 0.70 | 1595 | 1675 | 8.0 | 82,860 | 20.2 | 19.5–20.9 | 4.61 | 4.38–4.85 |
| Primary immunodeficiency | 9320 | 0.08 | 185 | 195 | 0.9 | 9285 | 21.0 | 19.0–23.0 | 5.54 | 4.79–6.39 |
| Moderate to severe primary immunodeficiencya | 1270 | 0.01 | 25 | 30 | 0.1 | 1265 | 22.9 | 17.4–28.4 | 9.40 | 6.48–13.63 |
| Secondary immunodeficiency | 89,885 | 0.70 | 830 | 845 | 4.1 | 89,760 | 9.4 | 8.7–10.1 | 2.86 | 2.66–3.07 |
| Active treatment with non-corticosteroid immunosuppressive or immunomodulatory therapya | 11,365 | 0.09 | 250 | 255 | 1.2 | 11,315 | 22.7 | 20.9–24.5 | 5.50 | 4.85–6.23 |
| High-dose, long-term moderate-dose corticosteroids | 4130 | 0.03 | 40 | 40 | 0.2 | 4120 | 9.7 | 6.6–12.8 | 1.90 | 1.39–2.61 |
| High-dose corticosteroidsa | 3365 | 0.03 | 20 | 20 | 0.1 | 3365 | 6.5 | 3.1–9.9 | 1.41 | 0.92–2.17 |
| End-stage kidney disease | 23,315 | 0.20 | 580 | 590 | 2.8 | 23,210 | 25.5 | 24.2–26.8 | 3.83 | 3.52–4.16 |
| Solid organ transplant ≤5 years prior | 7375 | 0.06 | 215 | 215 | 1.0 | 7345 | 29.1 | 26.8–31.4 | 11.60 | 10.10–13.31 |
| ≤1 year priora | 2705 | 0.02 | 75 | 75 | 0.4 | 2695 | 28.2 | 24.4–32.0 | 11.37 | 9.03–14.31 |
| 1–2 years priora | 1540 | 0.01 | 45 | 45 | 0.2 | 1530 | 28.1 | 23.1–33.1 | 11.62 | 8.56–15.77 |
| On anti-rejection therapiesa | 2640 | 0.02 | 85 | 85 | 0.4 | 2630 | 32.0 | 28.2–35.8 | 12.05 | 9.68–15.00 |
| Stem cell transplant ≤2 years priora | 1435 | 0.01 | 35 | 35 | 0.2 | 1430 | 24.5 | 19.3–29.7 | 14.24 | 10.15–19.97 |
| Solid tumour ≤5 years prior | 309,395 | 2.60 | 2380 | 2415 | 11.5 | 308,890 | 7.8 | 7.4–8.2 | 1.41 | 1.35–1.47 |
| With active treatment (≤6 months prior)a | 27,700 | 0.20 | 420 | 430 | 2.0 | 27,610 | 15.5 | 14.3–16.7 | 3.66 | 3.31–4.03 |
| With recent treatment (6–12 months prior) | 6985 | 0.06 | 75 | 75 | 0.4 | 6965 | 11.1 | 8.7–13.5 | 2.57 | 2.04–3.23 |
| With distant treatment (>12 months prior) | 36,480 | 0.30 | 200 | 205 | 1.0 | 36,445 | 5.6 | 4.6–6.6 | 1.26 | 1.10–1.45 |
| Haematological malignancy ≤5 years prior | 60 270 | 0.50 | 1075 | 1145 | 5.5 | 60,035 | 19.1 | 18.3–19.9 | 3.58 | 3.36–3.80 |
| With active treatment (≤6 months prior)a | 8420 | 0.07 | 380 | 420 | 2.0 | 8330 | 50.3 | 48.2–52.4 | 10.69 | 9.68–11.80 |
| With recent treatment (6–12 months prior) | 2250 | 0.02 | 60 | 70 | 0.3 | 2240 | 31.2 | 27.1–35.3 | 6.60 | 5.20–8.39 |
| With distant treatment (>12 months prior) | 11,990 | 0.10 | 160 | 165 | 0.8 | 11,955 | 14.0 | 12.2–15.8 | 2.87 | 2.45–3.35 |
| Chronic lymphocytic leukaemia, non-Hodgkin's lymphoma, multiple myeloma, acute leukaemia (≤2 years prior)a | 35,720 | 0.30 | 855 | 925 | 4.4 | 35,525 | 26.1 | 25.1–27.1 | 4.84 | 4.52–5.18 |
| Advanced or untreated HIVa | 580 | 0.01 | <10 | <10 | <0.05 | 575 | 13.9 | 5.7–22.1 | 2.26 | 1.11–4.58 |
| Vaccinated population (≥3 doses of a COVID-19 vaccine) | ||||||||||
| All individuals | 6,060,635 | 100.00 | 14,365 | 14,600 | 100.0 | 6,058,430 | 2.4 | 2.3–2.5 | ||
| Total broadly defined immunocompromised | 397,835 | 6.60 | 3525 | 3625 | 24.8 | 397,190 | 9.1 | 8.8–9.4 | 2.17 | 2.09–2.26 |
| Total stringently defined immunocompromised | 69,560 | 1.10 | 1265 | 1335 | 9.2 | 69,295 | 19.3 | 18.6–20.0 | 4.71 | 4.44–4.99 |
| Primary immunodeficiency | 6605 | 0.10 | 145 | 155 | 1.1 | 6580 | 23.6 | 21.2–26.0 | 5.81 | 4.94–6.83 |
| Moderate to severe primary immunodeficiencya | 870 | 0.01 | 20 | 20 | 0.2 | 870 | 25.3 | 18.7–31.9 | 9.70 | 6.33–14.87 |
| Secondary immunodeficiency | 75,465 | 1.20 | 670 | 685 | 4.7 | 75,365 | 9.1 | 8.4–9.8 | 3.02 | 2.79–3.27 |
| Active treatment with non-corticosteroid immunosuppressive or immunomodulatory therapya | 9370 | 0.20 | 190 | 200 | 1.4 | 9335 | 21.4 | 19.4–23.4 | 5.54 | 4.81–6.39 |
| High-dose, long-term moderate dose corticosteroids | 3510 | 0.06 | 35 | 35 | 0.2 | 3505 | 10.0 | 6.7–13.3 | 2.16 | 1.54–3.02 |
| High-dose corticosteroidsa | 2770 | 0.05 | 20 | 20 | 0.1 | 2770 | 7.6 | 3.9–11.3 | 1.75 | 1.13–2.71 |
| End-stage kidney disease | 18,055 | 0.30 | 420 | 430 | 2.9 | 17,980 | 23.9 | 22.4–25.4 | 3.74 | 3.39–4.13 |
| Solid organ transplant ≤5 years prior | 5765 | 0.10 | 170 | 170 | 1.2 | 5740 | 29.8 | 27.2–32.4 | 13.12 | 11.23–15.31 |
| ≤1 year priora | 2065 | 0.03 | 55 | 55 | 0.4 | 2055 | 26.3 | 22.0–30.6 | 11.77 | 8.96–15.47 |
| 1–2 years priora | 1200 | 0.02 | 35 | 35 | 0.2 | 1195 | 28.4 | 22.7–34.1 | 12.95 | 9.18–18.26 |
| On anti-rejection therapiesa | 2075 | 0.03 | 60 | 60 | 0.4 | 2065 | 28.1 | 23.8–32.4 | 11.72 | 9.01–15.26 |
| Stem cell transplant ≤2 years priora | 995 | 0.02 | 20 | 20 | 0.1 | 995 | 18.1 | 11.9–24.3 | 10.96 | 6.83–17.56 |
| Solid tumour ≤5 years prior | 265,960 | 4.40 | 1890 | 1915 | 13.1 | 265,600 | 7.2 | 6.8–7.6 | 1.40 | 1.33–1.47 |
| With active treatment (≤6 months prior)a | 23,475 | 0.40 | 325 | 335 | 2.3 | 23,410 | 14.3 | 13.0–15.6 | 3.65 | 3.26–4.08 |
| With recent treatment (6–12 months prior) | 5960 | 0.10 | 50 | 50 | 0.4 | 5945 | 8.7 | 6.2–11.2 | 2.23 | 1.69–2.95 |
| With distant treatment (>12 months prior) | 31,305 | 0.50 | 165 | 170 | 1.2 | 31,275 | 5.5 | 4.4–6.6 | 1.33 | 1.14–1.55 |
| Haematological malignancy ≤5 years prior | 50,665 | 0.80 | 865 | 925 | 6.3 | 50,470 | 18.4 | 17.5–19.3 | 3.62 | 3.38–3.87 |
| With active treatment (≤6 months prior)a | 6960 | 0.10 | 290 | 325 | 2.2 | 6895 | 47.1 | 44.7–49.5 | 10.63 | 9.50–11.89 |
| With recent treatment (6–12 months prior) | 1875 | 0.03 | 45 | 50 | 0.3 | 1865 | 25.7 | 21.2–30.2 | 5.80 | 4.34–7.74 |
| With distant treatment (>12 months prior) | 10,165 | 0.20 | 135 | 140 | 1.0 | 10,135 | 13.9 | 12.0–15.8 | 3.03 | 2.55–3.59 |
| Chronic lymphocytic leukaemia, non-Hodgkin's lymphoma, multiple myeloma, acute leukaemia (≤2 years prior)a | 30,295 | 0.50 | 695 | 755 | 5.2 | 30,135 | 25.1 | 24.0–26.2 | 4.96 | 4.60–5.35 |
| Advanced or untreated HIVa | 455 | 0.007 | <10 | <10 | <0.07 | 450 | 15.5 | 6.3–24.7 | 2.55 | 1.20–5.43 |
CI – confidence interval. IR – incidence rate per 1000 person-years. IRR – incidence rate ratio.
Stringent immunocompromised subgroups.
Percentages are based on the total number individuals or COVID-19 events in the overall 12+ population.
Incidence rate ratios (IRR) adjusted for age, sex, and number of non-immunocompromising comorbidities, comparing the incidence rate of individuals with the condition specified in each row to the incidence rate in individuals who do not have the condition specified in that row.
Any overnight hospital stay with COVID-19 recorded as the primary diagnosis; for IRR calculations, hospital readmission within one week of discharge is not considered a new hospitalisation.
Counts are rounded to the nearest 5 in compliance with the NHS Privacy Policy.
Incidence of severe COVID-19 outcomes and relative rates
A total of 20 910 COVID-19 hospitalisations, 440 COVID-19 ICU admissions, and 4810 COVID-19 deaths were recorded for the overall study population, corresponding to IR of 1.7 [95% confidence interval (95% CI) 1.6–1.8], 0.04 (95% CI 0–0.09), and 0.4 (95% CI 0.3–0.5) per 1000 person-years, respectively (Tables 2 and 3). Among immunocompromised individuals, COVID-19 hospitalisation, ICU admission, and mortality rates were about 6-fold higher for broadly-defined (9.7, 95% CI 9.4–10.0; 0.3, 95% CI 0–0.6; and 2.5, 95% CI 2.2–2.8 per 1000 person-years, respectively) and about 11-fold higher for stringently-defined (20.2, 95% CI 19.5–20.9; 0.9, 95% CI 0.2–1.6; and 4.6, 95% CI 3.9–5.3 per 1000 person-years, respectively) than for the unexposed population. After adjusting for age, sex, and the number of non-immunocompromising comorbidities, aIRRs remained significantly increased for both the broadly- and stringently-defined when compared to unexposed: 2.16 (95% CI 2.09–2.24) and 4.61 (95% CI 4.38–4.85), respectively, for COVID-19 hospitalisations; 4.13 (95% CI 3.30–5.18) and 12.48 (95% CI 9.60–16.22), respectively, for COVID-19 ICU admissions; 1.97 (95% CI 1.84–2.11) and 3.99 (95% CI 3.58–4.46), respectively, for COVID-19 deaths (Tables 2 and 3; Supplement S5).
Table 3.
COVID-19-related ICU admissions and mortality (COVID-19 cases, number of patients, proportion, crude IR per 1000 person-years, and IRR) in the population aged >12 years, overall and by immunocompromising condition, England, NHS-Digital, 2022.
| COVID-19 ICU |
COVID-related mortality |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N ICUa, d | % of ICUb | Total person-time (years) | Crude IR | IR 95% CI | Adjusted IRRc | IRR 95% CI | N deathsa | % of deathsb | Total person-time (years) | Crude IR | IR 95% CI | Adjusted IRRc | IRR 95% CI | |
| Overall study population | ||||||||||||||
| All individuals | 440 | 100.0 | 11,990,553 | 0.04 | 0.0–0.09 | 4810 | 100.0 | 11,930,335 | 0.4 | 0.3–0.5 | ||||
| Total broadly defined immunocompromised | 125 | 28.1 | 470,844 | 0.3 | 0.0–0.6 | 4.13 | 3.30–5.18 | 1145 | 23.8 | 452,710 | 2.5 | 2.2–2.8 | 1.97 | 1.84–2.11 |
| Total stringently defined immunocompromised | 75 | 16.5 | 83,144 | 0.9 | 0.2–1.6 | 12.48 | 9.60–16.22 | 355 | 7.4 | 77,810 | 4.6 | 3.9–5.3 | 3.99 | 3.58–4.46 |
| Primary immunodeficiency | <10 | <2.2 | 9315 | 1.0 | 0.0–3.0 | 13.29 | 6.76–26.14 | 45 | 1.0 | 9070 | 5.1 | 3.0–7.2 | 5.66 | 4.20–7.61 |
| Moderate to severe primary immunodeficiencye | 0 | 0.0 | 1270 | NA | NA | NA | NA | <10 | <0.2 | 1245 | 4.0 | 0.0–9.5 | 8.30 | 3.39–20.32 |
| Secondary immunodeficiency | 25 | 5.7 | 8969 | 0.3 | 0.0–0.9 | 4.12 | 2.72–6.24 | 195 | 4.0 | 88,535 | 2.2 | 1.5–2.9 | 2.99 | 2.58–3.47 |
| Active treatment with non-corticosteroid immunosuppressive or immunomodulatory therapye | 15 | 3.2 | 11,353 | 1.2 | 0.0–3.1 | 15.03 | 8.71–25.95 | 60 | 1.2 | 11,020 | 5.4 | 3.5–7.3 | 5.53 | 4.26–7.19 |
| High-dose, long-term moderate dose corticosteroids | 0 | 0.0 | 4130 | NA | NA | NA | NA | <10 | <0.2 | 3930 | 2.0 | 0.–5.1 | 1.65 | 0.81–3.34 |
| High-dose corticosteroidse | 0 | 0.0 | 3366 | NA | NA | NA | NA | <10 | <0.2 | 3160 | 1.3 | 0.0–4.8 | 1.14 | 0.42–3.09 |
| End-stage kidney disease | 35 | 8.1 | 23,297 | 1.5 | 0.3–2.8 | 13.95 | 9.74–19.97 | 145 | 3.1 | 21,820 | 6.7 | 5.4–8.0 | 3.81 | 3.22–4.50 |
| Solid organ transplant ≤5 years prior | 15 | 3.2 | 7364 | 1.9 | 0.0–4.2 | 24.74 | 14.28–42.85 | 35 | 0.7 | 7220 | 4.7 | 2.4–7.0 | 13.03 | 9.22–18.41 |
| ≤1 year priore | <10 | <2.2 | 2702 | 2.6 | 0.0–6.4 | 33.25 | 15.44–71.57 | 10 | 0.2 | 2645 | 4.2 | 0.4–8.0 | 12.25 | 6.69–22.42 |
| 1–2 years priore | <10 | <2.2 | 1538 | 0.7 | 0.0–5.6 | 8.48 | 1.14–62.90 | 10 | 0.2 | 1505 | 6.6 | 1.6–11.6 | 18.74 | 9.95–35.33 |
| On anti-rejection therapiese | <10 | <2.2 | 2635 | 2.3 | 0.0–6.1 | 27.59 | 12.07–63.05 | 10 | 0.2 | 2595 | 4.6 | 0.8–8.4 | 12.09 | 6.78–21.58 |
| Stem cell transplant ≤2 years priore | <10 | <2.2 | 1434 | 1.4 | 0.0–6.6 | 24.92 | 6.03–102.98 | <10 | <0.2 | 1390 | 1.4 | 0.0–6.6 | 6.36 | 1.55–26.18 |
| Solid tumour ≤5 years prior | 35 | 7.9 | 309,379 | 0.1 | 0.0–0.5 | 1.22 | 0.85–1.74 | 625 | 13.0 | 295,030 | 2.1 | 1.7–2.5 | 1.30 | 1.19–1.42 |
| With active treatment (≤6 months prior)e | <10 | <2.2 | 27,698 | 0.2 | 0.0–1.4 | 2.42 | 1.06–5.52 | 60 | 1.3 | 24,405 | 2.5 | 1.3–3.7 | 2.58 | 2.00–3.33 |
| With recent treatment (6–12 months prior) | <10 | <2.2 | 6985 | 0.3 | 0.0–2.6 | 3.20 | 0.78–13.23 | 10 | 0.2 | 6400 | 1.7 | 0.0–4.1 | 1.72 | 0.94–3.14 |
| With distant treatment (>12 months prior) | <10 | <2.2 | 36,479 | 0.08 | 0.0–1.1 | 0.93 | 0.29–2.98 | 60 | 1.2 | 35,355 | 1.7 | 0.6–2.8 | 1.52 | 1.17–1.98 |
| Haematological malignancy ≤5 years prior | 55 | 12.7 | 60,240 | 0.9 | 0.1–1.7 | 12.37 | 9.23–16.57 | 300 | 6.2 | 57,730 | 5.2 | 4.4–6.0 | 3.32 | 2.95–3.74 |
| With active treatment (≤6 months prior)e | 25 | 5.9 | 8401 | 3.2 | 1.1–5.4 | 37.94 | 25.39–56.69 | 90 | 1.9 | 7745 | 11.9 | 9.7–14.1 | 10.12 | 8.20–12.50 |
| With recent treatment (6–12 months prior) | <10 | <2.2 | 2248 | 2.2 | 0.0–6.4 | 24.92 | 10.12–61.37 | 10 | 0.2 | 2140 | 5.6 | 1.4–9.8 | 4.64 | 2.60–8.26 |
| With distant treatment (>12 months prior) | <10 | <2.2 | 11,989 | 0.5 | 0.0–2.3 | 5.70 | 2.50–12.99 | 45 | 0.9 | 11,615 | 3.9 | 2.1–5.7 | 2.99 | 2.22–4.04 |
| Chronic lymphocytic leukaemia, non-Hodgkin's lymphoma, multiple myeloma, acute leukaemia (≤2 years prior)e | 50 | 11.8 | 35,691 | 1.5 | 0.4–2.5 | 18.92 | 14.00–25.58 | 235 | 4.9 | 34,080 | 6.9 | 5.8–8.0 | 4.49 | 3.93–5.13 |
| Advanced or untreated HIVe | 0 | 0.0 | 578 | NA | NA | NA | NA | <10 | <0.2 | 515 | 5.8 | 0.0–14.4 | 3.12 | 1.13–11.39 |
| Vaccinated population (≥3 doses of a COVID-19 vaccine) | ||||||||||||||
| All individuals | 255 | 100 | 6,060,529 | 0.04 | 0.0–0.1 | 3425 | 100.0 | 6,013,350 | 0.6 | 0.5–0.7 | ||||
| Total broadly defined immunocompromised | 90 | 36.1 | 397,784 | 00.2 | 0–0.5 | 4.66 | 3.56–6.11 | 855 | 24.9 | 383,315 | 2.2 | 1.9–2.5 | 1.98 | 1.83–2.14 |
| Total stringently defined immunocompromised | 55 | 22 | 69,524 | 0.08 | 0.08–1.6 | 14.34 | 10.56–19.48 | 295 | 8.7 | 65,260 | 4.6 | 3.8–5.4 | 4.55 | 4.03–5.14 |
| Primary immunodeficiency | <10 | <3.9 | 6601 | 1.4 | 0–3.8 | 20.43 | 10.33–40.41 | 35 | 1.1 | 6410 | 5.6 | 3.2–8.0 | 6.09 | 4.35–8.51 |
| Moderate to severe primary immunodeficiencye | 0 | 0 | 872 | 0 | NA | NA | <10 | <0.3 | 855 | 4.7 | 0–11.4 | 9.43 | 3.47–25.65 | |
| Secondary immunodeficiency | 20 | 7.8 | 75,450 | 0.3 | 0–1.0 | 4.84 | 3.03–7.72 | 130 | 3.9 | 74,400 | 1.8 | 1.1–2.5 | 2.81 | 2.35–3.35 |
| Active treatment with non-corticosteroid immunosuppressive or immunomodulatory therapye | 10 | 3.9 | 9363 | 1.2 | 0–3.2 | 17.49 | 9.42–32.47 | 45 | 1.3 | 9100 | 4.9 | 2.9–6.9 | 5.72 | 4.24–7.73 |
| High-dose, long-term moderate dose corticosteroids | 0 | 0 | 3511 | 0 | NA | NA | <10 | <0.3 | 3365 | 1.8 | 0–5.2 | 1.65 | 0.73–3.74 | |
| High-dose corticosteroidse | 0 | 0 | 2771 | 0 | NA | NA | <10 | <0.3 | 2635 | 0.8 | 0–4.7 | 0.76 | 0.18–3.12 | |
| End-stage kidney disease | 25 | 10.2 | 18,040 | 1.4 | 0–2.9 | 16.15 | 10.55–24.74 | 100 | 2.9 | 16,935 | 6.0 | 4.5–7.5 | 3.68 | 3.00–4.50 |
| Solid organ transplant ≥5 years prior | 10 | 3.9 | 5759 | 1.9 | 0–4.5 | 20.93 | 2.82–155.56 | 30 | 0.9 | 5650 | 5.3 | 2.7–7.9 | 17.91 | 12.38–25.90 |
| ≤1 year priore | <10 | <3.9 | 2062 | 2.9 | 0–7.2 | 46.31 | 20.12–106.68 | 10 | 0.3 | 2020 | 5.4 | 1.1–9.7 | 19.60 | 10.70–35.92 |
| 1–2 years priore | 0 | 0 | 1200 | 0 | NA | NA | <10 | <0.3 | 1180 | 5.9 | 0.2–11.6 | 19.94 | 9.35–42.53 | |
| On anti-rejection therapiese | <10 | <3.9 | 2071 | 2.4 | 0–6.7 | 35.93 | 14.47–89.25 | 10 | 0.3 | 2045 | 5.4 | 1.1–9.7 | 13.32 | 4.89–36.27 |
| Stem cell transplant ≤2 years priore | 25 | 9.8 | 996 | 0 | NA | NA | <10 | <0.3 | 970 | 1.0 | 0–7.2 | 5.16 | 0.70–38.17 | |
| Solid tumour ≤5 years prior | <10 | <3.9 | 265,950 | 0.09 | 0–0.5 | 1.22 | 0.79–1.86 | 465 | 13.6 | 254,460 | 1.8 | 1.4–2.2 | 1.29 | 1.17–1.43 |
| With active treatment (≤6 months prior)e | <10 | <3.9 | 23,475 | 0.2 | 0–1.5 | 2.85 | 1.15–7.05 | 50 | 1.5 | 20,840 | 2.4 | 1.1–3.7 | 2.91 | 2.19–3.86 |
| With recent treatment (6–12 months prior) | <10 | <3.9 | 5958 | 0.2 | 0–2.7 | 2.27 | 0.31–16.86 | <10 | <0.3 | 5505 | 1.1 | 0–3.8 | 1.29 | 0.57–2.91 |
| With distant treatment (>12 months prior) | 40 | 16.5 | 31,302 | 0.03 | 0–1.1 | 0.43 | 0.06–3.20 | 40 | 1.2 | 30,390 | 1.3 | 0.2–2.4 | 1.43 | 1.04–1.96 |
| Haematological malignancy ≤5 years prior | 20 | 7.1 | 50,638 | 0.8 | 0–1.7 | 13.58 | 9.65–19.11 | 250 | 7.3 | 48,570 | 5.1 | 4.2–6.0 | 3.72 | 3.26–4.25 |
| With active treatment (≤6 months prior)e | <10 | <3.9 | 6950 | 2.7 | 0.4–5.1 | 38.33 | 23.71–61.97 | 75 | 2.2 | 6430 | 11.5 | 9.1–13.9 | 11.26 | 8.90–14.26 |
| With recent treatment (6–12 months prior) | <10 | <3.9 | 1870 | 2.1 | 0–6.7 | 28.43 | 10.36–78.02 | 10 | 0.3 | 1780 | 5.6 | 1.0–10.2 | 5.31 | 2.82–10.00 |
| With distant treatment (>12 months prior) | 40 | 15.3 | 10,164 | 0.6 | 0–2.5 | 8.00 | 3.50–18.32 | 40 | 1.2 | 9850 | 4.1 | 2.1–6.1 | 3.59 | 2.61–4.94 |
| Chronic lymphocytic leukaemia, non-Hodgkin's lymphoma, multiple myeloma, acute leukaemia (≤2 years prior)e | 0 | 0 | 30,269 | 1.3 | 0.2–2.4 | 20.55 | 14.46–29.20 | 200 | 5.9 | 28,955 | 6.9 | 5.8–8.0 | 5.18 | 4.48–5.99 |
| Advanced or untreated HIVe | 25 | 9.8 | 454 | 0 | NA | NA | <10 | <0.3 | 405 | 4.9 | 0–14.6 | 2.85 | 0.69–11.75 | |
CI – confidence interval. IR – incidence rate per 1000 person-years. IRR – incidence rate ratio. NA – not applicable.
Counts are rounded to the nearest 5 in compliance with the NHS Privacy Policy.
Percentages are based on the total number of events.
Incidence rate ratios (IRR) adjusted for age, sex, and number of non-immunocompromising comorbidities, comparing the incidence rate of individuals with the condition specified in each row to the incidence rate in individuals who do not have the condition specified in that row.
Number of patients with ≥1 COVID-19 ICU admission.
Stringent immunocompromised subgroups.
Among specific immunocompromised subgroups, aIRRs for COVID-19 hospitalisation were highest for stem cell transplants in the last 2 years (14.24, 95% CI 10.15–19.97) followed by solid organ transplant (11.6, 95% CI 10.10–13.31, Table 2, Supplement S5). Among solid organ transplant, aIRRs for those receiving a transplant in the last 2 years or on anti-rejection therapies remained similar to the aIRRs for those receiving solid organ transplant in the last 5 years. In contrast, for solid tumours or haematological malignancies in the last 5 years, aIRRs increased with recency of active treatment: for haematological malignancies treated within ≤6 months and 6–12 months, aIRRs were 10.69 (95% CI 9.68–11.8) and 6.6 (95% CI 5.2–8.39), respectively, compared with 2.87 (95% CI 2.45–3.35) for those treated >12 months prior; for solid tumours treated ≤6 months and 6–12 months prior, aIRRs were 3.66 (95% CI 3.31–4.03) and 2.57 (95% CI 2.04–3.23), respectively, compared with 1.26 (95% CI 1.1–1.45) for those treated >12 months prior. Other subgroups showing a greater than 5-fold increased risk included primary immunodeficiency and secondary immunodeficiency due to active treatment with non-corticosteroid immunosuppressive or immunomodulatory therapy. Results were similar for COVID-19 ICU admissions and deaths, although small sample sizes for some subgroups (e.g., stem cell transplants and high-dose corticosteroid treatment) resulted in wide CIs and limited interpretability of results (Table 3; Supplement S5).
Results were also similar for the 6,060,635 individuals vaccinated with ≥3 COVID-19 vaccine doses, with aIRRs among the broadly- and stringently-defined of: 2.17 (95% CI 2.09–2.26) and 4.71 (95% CI 4.44–4.99), respectively, for COVID-19 hospitalisations, 4.66 (95% CI 3.56–6.11) and 14.34 (95% CI 10.56–19.48), respectively, for COVID-19 ICU admissions, and 1.98 (95% CI 1.83–2.14) and 4.55 (95% CI 4.03–5.14), respectively, for COVID-19 deaths. Solid organ transplant patients remained the highest-risk group (aIRR 13.12 [95% CI 11.23–15.31] for COVID-19 hospitalisations) (Tables 2 and 3; Supplement S6).
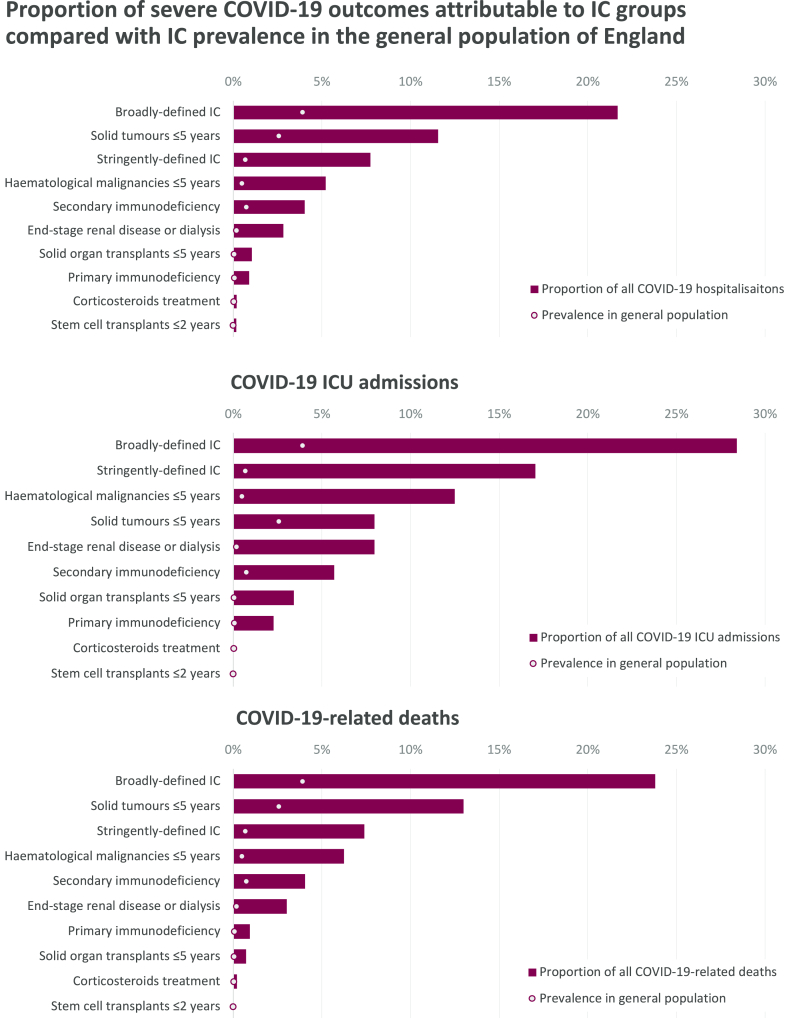
Proportion of severe COVID-19 outcomes attributable to immunocompromising conditions
Immunocompromised individuals accounted for a disproportionately high percentage of severe COVID-19 outcomes (Tables 2 and 3; Fig. 3). The broadly-defined comprised 3.9% of the overall population vs 21.9% of COVID-19 hospitalisations, 28.1% of ICU admissions, and 23.8% of deaths; stringently-defined 0.7% of the overall population vs 8.0% of COVID-19 hospitalisations, 16.5% of ICU admissions, and 7.4% of deaths. Similarly, in those with ≥3 vaccine doses, the broadly-defined comprised 6.6% of the total population vs 24.8% of COVID-19 hospitalisations, 36.1% of ICU admissions, and 24.9% of deaths; stringently-defined 1.1% of the total population vs 9.2% of COVID-19 hospitalisations, 22.0% of ICU admissions, and 8.7% of deaths (Tables 2 and 3). Across immunocompromised groups, this disproportionality ranged from approximately 3- to 25-fold in the overall population and approximately 2- to 22-fold in the fully-vaccinated populations.
Fig. 3.
The incidence rate ratio of COVID-19 hospitalisation, ICU admission and mortality in immunocompromised groups compared to the total study population. † Stringently-defined immunocompromised groups, HIV – human immunodeficiency virus, ICU – intensive care unit.
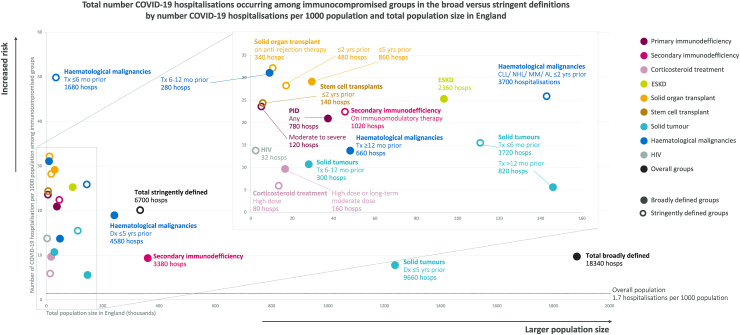
Potential population-level impact of preventive interventions
The study sample corresponded to ∼48 million individuals and ∼83,640 total COVID-19 hospitalisations in England's general population aged ≥12 years. For each immunocompromised group/subgroup, Fig. 4 shows the total population size in England (x axis), the total number of hospitalisations that could potentially be prevented if all of those individuals were successfully targeted by a preventive intervention in addition to and alongside current vaccination programmes, and the total number of potentially preventable hospitalisations per 1000 population (y axis). Thus, successful targeting of the ∼1.9 million broadly-vs ∼0.3 million stringently-defined with a 100% effective intervention (in addition to vaccination), in theory, could have prevented up to 18,340 vs 6700 of the total COVID-19 hospitalisations, corresponding to up to 9.7 vs 20.1 COVID-19 hospitalisations per 1000 persons in each group, respectively, due to the higher number of individuals broadly defined as immunocompromised. With 80% effectiveness of an additional preventive intervention alongside vaccination, up to 14,672 broadly-defined vs 5360 stringently-defined hospitalisations could have been prevented, equating to 7.8 vs 16.1 hospitalisations per 1000 persons in each group, respectively. However, there was substantial overlap between groups/subgroups included in the broad (solid circles) and stringent (open circles) definitions, meaning several of those groups/subgroups corresponded to similar levels of potential impact. In particular, targeting of all solid organ transplant in the last 5 years (broad definition) instead of last 2 years (stringent definition) increased the total number of potentially preventable COVID-19 hospitalisations from 480 to 860 as well as the number per 1000 from 28.3 to 29.2; haematological malignancies treated in the last 6–12 months, excluded from the stringent definition, was similarly associated with 31.1 potentially preventable COVID-19 hospitalisations per 1000 persons and a relatively small total population size (n = 9000). Results were similar when limited to the fully vaccinated population (Supplement S7).
Fig. 4.
Total number COVID-19 hospitalisations occurring among immunocompromised groups in 2022 by number of COVID-19 hospitalisations per 1000 population and total population size in England. AL – acute leukaemia, CLL – chronic lymphocytic leukaemia, Dx – diagnosis, ESKD – end-stage kidney disease, HIV – human immunodeficiency virus, hosps – hospitalisations, MM – multiple myeloma, mo – months, NHL – non-Hodgkin's lymphoma, PID – primary immunodeficiency, Tx – treatment, yrs – years.
Discussion
Using data collected throughout 2022, this large population-based study found that immunocompromised individuals remain disproportionally affected by COVID-19, accounting for 3.9% of the population of England, but >20% of COVID-19 hospitalisations, ICU admissions, and deaths, despite >80% of these immunocompromised individuals having received ≥3 COVID-19 vaccine doses. Among fully vaccinated individuals, COVID-19 risk remained elevated across immunocompromised groups, with aIRRs ranging from 1.3 to 13.1 for COVID-19 hospitalisation, 1.2 to 46.3 for ICU admissions, and 1.3 to 19.9 for COVID-19 mortality. The risk was particularly high in several immunocompromised groups/subgroups captured in the stringent definition aligned with UK pandemic guidelines, such as individuals with recent organ transplant (≤2 years prior), active treatment (≤6 months prior) for haematological malignancies, and moderate to severe primary immunodeficiency. However, similar levels of risk and potential impact for preventive interventions was also indicated for groups/subgroups not covered in this stringent definition, such as any solid organ transplant within the last 5 years, any primary immunodeficiency, and haematological cancer treated 6–12 months prior.
While vaccines may be sufficient to safeguard most immunocompetent individuals against severe COVID-19, the risk for the immunocompromised individuals described in this study underscores the urgent need for additional preventive measures against COVID-19 in this vulnerable population. The impact of COVID-19 on immunocompromised individuals is not surprising, as they are known to have impaired immune responses to vaccines, including lower antibodies levels, reduced neutralisation activity,37 and shorter duration of protection,22 often remaining insufficiently protected even after booster doses.22,23 Previous retrospective cohort studies in Canada24 and the United Kingdom,38,39 which included the early phase of the Omicron period, showed that fully vaccinated immunocompromised individuals were at a higher risk of breakthrough hospitalisation, ICU admission, and death. Once hospitalised, immunocompromised patients with COVID-19 had an increased risk of ICU admission or in-hospital death, irrespective of vaccination status, compared with non-immunocompromised patients.32,40,41 Our study extends these observations to after the emergence of Omicron, as the pandemic began to transition into the endemic era. As immunocompromised individuals form a large (∼1.9 million in England), heterogeneous population, employing broad pharmaceutical interventions may prove challenging. Conversely, limiting the focus to the stringently-defined immunocompromised group as per UK pandemic guidelines (∼360,000 in England) may exclude important high-risk groups. Results from this study can assist in identifying those immunocompromised individuals at highest risk and support an evidence-based approach to ensuring that preventive interventions are targeted towards the groups where interventions are likely to have the most impact, enabling policymakers to make informed and equitable decisions. They are also important for raising awareness among immunocompromised individuals, healthcare providers, and especially the general population, as some may argue that the pandemic is now over, and vaccine boosters are adequate to protect this population.
The strength of the INFORM study resides in the use of linked, high-quality routine health data from multiple sources (e.g., primary care records and prescription data, hospital records, national immunisation records and surveillance data), allowing comprehensive and granular assessment of immunocompromised groups/subgroups compared to unexposed populations in a large sample, representative of England's general population. We postulate these findings may be extrapolated to similar countries/regions where local data do not exist. Data source limitations included the fact that the GDPPR dataset does not capture general practitioner registration date, meaning patients with <12 months of baseline data could not be excluded, and prevalence of pre-existing chronic conditions may be underestimated, likely resulting in IRR underestimation as the unexposed population may include some immunocompromised individuals. Lack of CD4 count data meant only those with AIDS-defining illness could be captured. We have analysed only COVID-19-related outcomes, not all-cause outcomes, and therefore, a comparison with the pre-COVID-19 period is not feasible.
Other limitations include IRRs that were only adjusted for age, sex, and non-immunocompromising comorbidity count. We did not adjust for chronic kidney disease to avoid overlap and collinearity with ESKD. We also did not adjust for vaccination status. Instead, we stratified our analysis by vaccination status, showing aIRRs for the overall population (12+ years) and aIRRs restricted to fully vaccinated individuals (≥3 doses). When comparing the aIRRs for the overall population and the fully vaccinated very minor changes only in magnitude (with the same direction) are observed. This can be explained by the high proportion (>80%) of fully vaccinated individuals among the immunocompromised. A full examination of the effect of time since last vaccination, number of doses, type of vaccination, and prior infection was not carried out as the methodology required is beyond the scope of this analysis. In addition, vaccine effectiveness and waning in immunocompromised individuals has been examined elsewhere.42,43
These initial results focused on COVID-19 hospitalisation, ICU admissions, and deaths and therefore do not capture the full extent of the impact COVID has on immunocompromised patients, which can include negative consequences related to underlying disease progression, interrupted treatment pathways, and a decline in health-related quality of life. Furthermore, the high complexity of the group stratification resulted in a significant reduction in the number of patients per group. To address this issue, in a future study we aim to integrate machine learning techniques, to overcome the limitations of traditional statistical methods and capture complex relationships and hidden patterns that might be missed by conventional approaches. This could potentially improve variable selection and feature extraction but also enhance the predictive power of our analyses. Finally, there was not any assessment of other high-risk groups that are also likely to be disproportionally impacted by COVID-19, including the elderly and other highly co-morbid populations that may have suboptimal response to vaccination due to immunosenescense.44, 45, 46
In conclusion, these first results of the INFORM study demonstrate that, as the threat of COVID-19 continues, and despite high rates of vaccination, immunocompromised individuals remain highly disproportionately impacted by COVID-19, accounting for more than 20% of COVID-19 hospitalisations, ICU admissions, and deaths in England. Furthermore, results suggest that while UK pandemic guidelines have successfully targeted several of the key high-risk immunocompromised groups, there are potential opportunities to improve such guidelines and ensure more equitable policies and recommendations, especially as we look towards new strategies to help protect this important population.
Contributors
ST, SD, SA, RAE, JKQ, NJ, YL, and SG designed the study. YL, MY, KE, SG, and NJ collected the data. SV, LC, KE, MY, YL, and NJ performed statistical analysis. All authors interpreted the data. RM, ST, CF, MY, RY, and LC performed data visualisation. ST, SD, RM, LC, NJ, RY, MY, KE, RAE, and JKQ wrote the initial draft of the manuscript. All authors provided critical conceptual input, and critically revised the manuscript. All authors had full access to all study data and approved the decision to submit for publication.
Data sharing statement
Data for these analyses were accessed within the NHS secure data environment. Only aggregated, rounded and small-number suppressed outputs from the environment are permitted by the NHS. Because of this, the authors are unable to share the underlying patient level data. For enquiries about data access, please visit https://digital.nhs.uk/services/secure-data-environment-service/secure-data-environment#accessing-data.
Declaration of interests
ST, SD, SA, SV, LC, CF, RM are employees of and may own stocks or shares in AstraZeneca.
RY is an employee of P95 Epidemiology & Pharmacovigilance, which received a consultancy fee for the conduct of the current study from AstraZeneca.
YL, MY, KE, SG, NJ are employees of Evidera, which received funding for the conduct of the current study from AstraZeneca.
EB reports receiving research grant from Vaccitech, consulting fee from AstraZeneca and Vaccitech, owning shares in Perspectum Diagnostics, and holding patents in HBV and HCV Viral Vectored Vaccine work.
SB reports receiving consulting fees from AstraZeneca.
RAE reports receiving funding from Evidera for providing advice throughout the current study, research grants from NIHR, UKRI, Wolfson Foundation, Genetec/Roche to her institution for works not related to current manuscript, speaker fee from Boehringer Ingelheim and Moderna, support from Chiesi for attending British Thoracic Society (BTS), and serving non paid as European Respiratory Society (ERS) Group 01.02 Pulmonary Rehabilitation and Chronic Care secretary and American Thoracic Society (ATS) Pulmonary Rehabilitation Assembly chair.
PM reports receiving consulting fee from AstraZeneca and The Binding Site, speaker fee from AstraZeneca, Moderna, AbbVie, and support for attending meetings from AstraZeneca.
JKQ reports receiving funding from Evidera for providing advice throughout the current study, research grants from Medical Research Council (MRC), Health Data Research UK (HDR UK), GSK, Boehringer Ingelheim, Asthma + Lung UK and AstraZeneca to her research institution, and consulting fee from GSK, Evidera, AstraZeneca, Insmed.
Acknowledgements
The authors would like to thank Sean H Lim, MBChB, PhD (University of Southampton), Lance Turtle, MBBS, MRCP, DTMH, PhD (University of Liverpool), Lennard Lee, MRCP, BmBCh, MA, DPhil (University of Oxford), and Sundeep Kaul, PhD (National Health Service) for critical review of the manuscript; Valerie Olson, PhD (Evidera) for data analysis support as the statistical programmer; Ana Goios, PhD, Marc Baay, PhD, and Alejandra Gonzalez Diaz, BA (all at P95 Epidemiology & Pharmacovigilance, Leuven, Belgium) for visualisation, medical writing, and editorial support; and Carla Bezjian, PhD, Susana Rodríguez Santiago, PhD (Virgo, New York, USA), and Lisa J White, PhD (Director, Model Health Ltd) for visualisation support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100747.
Appendix A. Supplementary data
References
- 1.COVID-19 Forcasting Team Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet. 2023;401(10379):833–842. doi: 10.1016/S0140-6736(22)02465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wrenn J.O., Pakala S.B., Vestal G., et al. COVID-19 severity from Omicron and Delta SARS-CoV-2 variants. Influenza Other Respir Viruses. 2022;16(5):832–836. doi: 10.1111/irv.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolter N., Jassat W., Walaza S., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward I.L., Bermingham C., Ayoubkhani D., et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study. BMJ. 2022;378 doi: 10.1136/bmj-2022-070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu F.H., Jia Y.J., Zhao D.Y., et al. Clinical outcomes of the severe acute respiratory syndrome coronavirus 2 Omicron and Delta variant: systematic review and meta-analysis of 33 studies covering 6 037 144 coronavirus disease 2019-positive patients. Clin Microbiol Infect. 2023;29(7):835–844. doi: 10.1016/j.cmi.2023.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flahault A., Calmy A., Costagliola D., et al. No time for complacency on COVID-19 in Europe. Lancet. 2023 doi: 10.1016/s0140-6736(23)01012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigal A. Milder disease with Omicron: is it the virus or the pre-existing immunity? Nature Rev Immunol. 2022;22(2):69–71. doi: 10.1038/s41577-022-00678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki R., Yamasoba D., Kimura I., et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature. 2022;603(7902):700–705. doi: 10.1038/s41586-022-04462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan D.J., Focosi D., Hanley D., et al. Outpatient regimens to reduce COVID-19 hospitalizations: a systematic review and meta-analysis of randomized controlled trials. medRxiv. 2023 doi: 10.1002/jmv.29310. 2022.05.24.22275478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tibble H., Mueller T., Proud E., et al. Uptake of monoclonal antibodies and antiviral therapies for COVID-19 in Scotland. Lancet. 2023;401(10371):101–102. doi: 10.1016/S0140-6736(22)02398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbel R., Peretz A., Sergienko R., et al. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: a retrospective cohort study. Lancet Infect Dis. 2023;23(8):914–921. doi: 10.1016/S1473-3099(23)00122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Office for National Statistics . 2023. Deaths registered weekly in England and Wales, provisional.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/weeklyprovisionalfiguresondeathsregisteredinenglandandwales [Google Scholar]
- 13.Centers for Disease Control and Prevention COVID-NET laboratory-confirmed COVID-19 hospitalizations. https://covid.cdc.gov/covid-data-tracker/#covidnet-hospitalization-network
- 14.Centers for Disease Control and Prevention Laboratory-confirmed influenza hospitalizations. https://gis.cdc.gov/GRASP/Fluview/FluHospRates.html
- 15.Centers for Disease Control and Prevention RSV-associated hospitalization surveillance network. https://www.cdc.gov/rsv/research/rsv-net/dashboard.html21 Accessed March 2023.
- 16.Xie Y., Choi T., Al-Aly Z. Risk of death in patients hospitalized for COVID-19 vs seasonal influenza in fall-winter 2022-2023. JAMA. 2023;329(19):1697–1699. doi: 10.1001/jama.2023.5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . 2023. Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic.https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic [Google Scholar]
- 18.Duly K., Farraye F.A., Bhat S. COVID-19 vaccine use in immunocompromised patients: a commentary on evidence and recommendations. Am J Health Syst Pharm. 2022;79(2):63–71. doi: 10.1093/ajhp/zxab344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prendecki M., Clarke C., Edwards H., et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis. 2021;80(10):1322–1329. doi: 10.1136/annrheumdis-2021-220626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoham S., Batista C., Ben Amor Y., et al. Vaccines and therapeutics for immunocompromised patients with COVID-19. eClinicalMedicine. 2023;59 doi: 10.1016/j.eclinm.2023.101965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee A., Wong S.Y., Chai L.Y.A., et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tartof S.Y., Slezak J.M., Puzniak L., et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet Reg Health Am. 2022;9 doi: 10.1016/j.lana.2022.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tartof S.Y., Slezak J.M., Puzniak L., et al. Immunocompromise and durability of BNT162b2 vaccine against severe outcomes due to omicron and delta variants. Lancet Respir Med. 2022;10(7):e61–e62. doi: 10.1016/S2213-2600(22)00170-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahremand T., Yao J.A., Mill C., Piszczek J., Grant J.M., Smolina K. COVID-19 hospitalisations in immunocompromised individuals in the Omicron era: a population-based observational study using surveillance data in British Columbia, Canada. Lancet Reg Health Am. 2023;20 doi: 10.1016/j.lana.2023.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ketkar A., Willey V., Pollack M., et al. Assessing the risk and costs of COVID-19 in immunocompromised populations in a large United States commercial insurance health plan: the EPOCH-US Study. Curr Med Res Opin. 2023:1–16. doi: 10.1080/03007995.2023.2233819. [DOI] [PubMed] [Google Scholar]
- 26.UK Health Security Agency . 2023. COVID-19: guidance for people whose immune system means they are at higher risk.https://www.gov.uk/government/publications/covid-19-guidance-for-people-whose-immune-system-means-they-are-at-higher-risk/covid-19-guidance-for-people-whose-immune-system-means-they-are-at-higher-risk [Google Scholar]
- 27.Patel P., Twentyman E., Koumans E., et al. Information for persons who are immunocompromised regarding prevention and treatment of SARS-CoV-2 infection in the context of currently circulating Omicron sublineages–United States, January 2023. Morb Mortal Wkly Rep. 2023;72(5):128–131. doi: 10.15585/mmwr.mm7205e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Gessa G., Price D. The impact of shielding during the COVID-19 pandemic on mental health: evidence from the English Longitudinal Study of Ageing. Br J Psychiatry. 2022;221(4):637–643. doi: 10.1192/bjp.2022.44. [DOI] [PubMed] [Google Scholar]
- 29.Snooks H., Watkins A., Lyons J., et al. Did the UK's public health shielding policy protect the clinically extremely vulnerable during the COVID-19 pandemic in Wales? Results of EVITE Immunity, a linked data retrospective study. Pub Health. 2023;218:12–20. doi: 10.1016/j.puhe.2023.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel M., Chen J., Kim S., et al. Analysis of MarketScan data for immunosuppressive conditions and hospitalizations for acute respiratory illness, United States. Emerg Infect Dis. 2020;26(8):1720–1730. doi: 10.3201/eid2608.191493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belsky J.A., Tullius B.P., Lamb M.G., Sayegh R., Stanek J.R., Auletta J.J. COVID-19 in immunocompromised patients: a systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect. 2021;82(3):329–338. doi: 10.1016/j.jinf.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turtle L., Thorpe M., Drake T.M., et al. Outcome of COVID-19 in hospitalised immunocompromised patients: an analysis of the WHO ISARIC CCP-UK prospective cohort study. PLoS Med. 2023;20(1) doi: 10.1371/journal.pmed.1004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noble M., Wright G., Smith G., Dibben C. Measuring multiple deprivation at the small-area level. Environ Plan A. 2006;38(1):169–185. [Google Scholar]
- 34.UK Department of Health & Social Care . 2023. Defining the highest-risk clinical subgroups upon community infection with SARS-CoV-2 when considering the use of neutralising monoclonal antibodies (nMABs) and antiviral drugs: independent advisory group report.https://www.gov.uk/government/publications/higher-risk-patients-eligible-for-covid-19-treatments-independent-advisory-group-report-march-2023/defining-the-highest-risk-clinical-subgroups-upon-community-infection-with-sars-cov-2-when-considering-the-use-of-neutralising-monoclonal-antibodies [Google Scholar]
- 35.Department of Health & Social Care . 2021. Joint Committee on Vaccination and Immunisation (JCVI) advice on third primary dose vaccination.https://www.gov.uk/government/publications/third-primary-covid-19-vaccine-dose-for-people-who-are-immunosuppressed-jcvi-advice/joint-committee-on-vaccination-and-immunisation-jcvi-advice-on-third-primary-dose-vaccination [Google Scholar]
- 36.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung M.W., Dayam R.M., Shapiro J.R., et al. Third and fourth vaccine doses broaden and prolong immunity to SARS-CoV-2 in immunocompromised adult patients. medRxiv. 2023 doi: 10.4049/jimmunol.2300190. 2023.03.01.23286513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agrawal U., Bedston S., McCowan C., et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. Lancet. 2022;400(10360):1305–1320. doi: 10.1016/S0140-6736(22)01656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nafilyan V., Ward I.L., Robertson C., Sheikh A. Evaluation of risk factors for postbooster omicron COVID-19 deaths in England. JAMA Netw Open. 2022;5(9) doi: 10.1001/jamanetworkopen.2022.33446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singson J.R.C., Kirley P.D., Pham H., et al. Factors associated with severe outcomes among immunocompromised adults hospitalized for COVID-19–COVID-NET, 10 States, March 2020-February 2022. Morb Mortal Wkly Rep. 2022;71(27):878–884. doi: 10.15585/mmwr.mm7127a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nevejan L., Ombelet S., Laenen L., et al. Severity of COVID-19 among hospitalized patients: Omicron remains a severe threat for immunocompromised hosts. Viruses. 2022;14(12):2736. doi: 10.3390/v14122736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Britton A., Embi P.J., Levy M.E., et al. Effectiveness of COVID-19 mRNA vaccines against COVID-19-associated hospitalizations among immunocompromised adults during SARS-CoV-2 Omicron Predominance–VISION Network, 10 states, December 2021-August 2022. Morb Mortal Wkly Rep. 2022;71(42):1335–1342. doi: 10.15585/mmwr.mm7142a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferdinands J.M., Rao S., Dixon B.E., et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. BMJ. 2022;379 doi: 10.1136/bmj-2022-072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aw D., Silva A.B., Palmer D.B. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu W., Wong G., Hwang Y.Y., Larbi A. The untwining of immunosenescence and aging. Semin Immunopathol. 2020;42(5):559–572. doi: 10.1007/s00281-020-00824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hägg S., Religa D. COVID vaccination in older adults. Nat Microbiol. 2022;7(8):1106–1107. doi: 10.1038/s41564-022-01166-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.