Summary
Background
The seasonal fluctuation in mortality and hospital admissions from respiratory diseases, with a winter peak and a summer trough, is widely recognized in extratropical countries. However, little is known about the seasonality of inpatient mortality and the role of ambient temperature remains uncertain. We aimed to analyse the association between ambient temperature and in-hospital mortality from respiratory diseases in the provinces of Madrid and Barcelona, Spain.
Methods
We used data on daily hospitalisations, weather (ie, temperature and relative humidity) and air pollutants (ie, PM2.5, PM10, NO2 and O3) for the Spanish provinces of Madrid and Barcelona during 2006–2019. We applied a daily time-series quasi-Poisson regression in combination with distributed lag non-linear models (DLNM) to assess, on the one hand, the seasonal variation in fatal hospitalisations and the contribution of ambient temperature, and on the other hand, the day-to-day association between temperature and fatal hospital admissions. The analyses were stratified by sex, age and primary diagnostic of hospitalisation.
Findings
The study analysed 1 710 012 emergency hospital admissions for respiratory diseases (mean [SD] age, 60.4 [31.0] years; 44.2% women), from which 103 845 resulted in in-hospital death (81.4 [12.3] years; 45.1%). We found a strong seasonal fluctuation in in-hospital mortality from respiratory diseases. While hospital admissions were higher during the cold season, the maximum incidence of inpatient mortality was during the summer and was strongly related to high temperatures. When analysing the day-to-day association between temperature and in-hospital mortality, we only found an effect for high temperatures. The relative risk (RR) of fatal hospitalisation at the 99th percentile of the distribution of daily temperatures vs the minimum mortality temperature (MMT) was 1.395 (95% eCI: 1.211–1.606) in Madrid and 1.612 (1.379–1.885) in Barcelona. In terms of attributable burden, summer temperatures (June–September) were responsible for 16.2% (8.8–23.3) and 22.3% (15.4–29.2) of overall fatal hospitalisations from respiratory diseases in Madrid and Barcelona, respectively. Women were more vulnerable to heat than men, whereas the results by diagnostic of admission showed heat effects for acute bronchitis and bronchiolitis, pneumonia and respiratory failure.
Interpretation
Unless effective adaptation measures are taken in hospital facilities, climate warming could exacerbate the burden of inpatient mortality from respiratory diseases during the warm season.
Funding
European Research Council Consolidator Grant EARLY-ADAPT, European Research Council Proof-of-Concept Grants HHS-EWS and FORECAST-AIR.
Keywords: Heat, Respiratory diseases, Hospital mortality
Research in context.
Evidence before this study
We searched PubMed from database inception until June 26, 2023, for articles published in English using the search terms “seasonality”, “temperature”, “heat”, “cold”, AND “hospitalisation”, “in-hospital mortality”, “respiratory diseases”. We found a reduced number of studies analysing the seasonal variation in inpatient mortality from respiratory diseases, and they were focusing solely on a subset of respiratory conditions accounting for a small fraction of all respiratory hospitalisations. Meanwhile, the contribution of ambient temperature to the seasonality of in-hospital mortality remained unexplored. On the other hand, although the day-to-day association between heat/cold and hospital admissions form a range of respiratory diseases (such as pneumonia, chronic obstructive pulmonary disease [COPD] and asthma) was extensively described, so far no study has focused on the fraction of hospitalisations resulting in death, and therefore, in the more severe cases of morbidity.
Added value of this study
We showed for the first time an inverse seasonal fluctuation between hospital admissions and inpatient mortality from respiratory diseases. Contrary to hospital admissions, higher during the cold season, the maximum incidence of inpatient mortality was during the summer. We also showed that the summer peak in in-hospital mortality from respiratory conditions was largely driven by high temperatures. Moreover, when analysing the day-to-day association between ambient temperature and hospital admissions resulting in death, we only found an effect for heat, thus confirming the results obtained in the seasonal analysis (ie, higher incidence of inpatient mortality in summer).
Implications of all the available evidence
Unless effective adaptation measures are taken in hospital facilities, climate warming could exacerbate the burden of inpatient mortality from respiratory diseases during the warm season.
Introduction
Ambient temperature is a major environmental contributor to adverse respiratory health.1,2 Short-term exposure to heat and cold, as well as temperature variability, increases morbidity and mortality from respiratory diseases, especially among vulnerable individuals with pre-existing conditions.3, 4, 5, 6, 7, 8, 9, 10, 11, 12 As a result of the projected increase in the exposure to extreme heat due to climate change,13 as well as its interaction with the rising prevalence of chronic respiratory diseases,14 population ageing15 and urbanisation,16 the heat-related respiratory adverse health outcomes are expected to worsen in the future, unless strong adaptation measures are put in place.1,17 By contrast, the negative effects of cold, which are nowadays far more common in extratropical countries,3,7 could be substantially reduced because of warming temperatures. These opposing trends in the impacts of heat and cold could in turn lead in the very long-term to a redefinition of the seasonality of respiratory morbidity and mortality.18,19 Moreover, climate change will also lead to more variability in temperature, which could translate into a substantial respiratory health burden.9,10,20
The seasonal fluctuation in mortality and hospital admissions from respiratory diseases, with a winter peak (mainly driven by respiratory infections) and a summer trough, is widely recognized in extratropical countries.21,22 However, to date, little is known about the seasonal variation in inpatient mortality, a surrogate for hospital performance in relation to severe respiratory events.23, 24, 25 Previous studies only focused on very specific respiratory diseases accounting for a very small fraction of all respiratory hospitalisations and found higher inpatient mortality in winter season.23, 24, 25 Meanwhile, the role of ambient temperature in the seasonal fluctuation of in-hospital mortality remains unexplored. On the other hand, although it has been extensively described that the daily exposure to heat and cold are associated with a greater risk of hospital admission from a range of respiratory diseases (such as pneumonia, chronic obstructive pulmonary disease [COPD] and asthma),6,8,26, 27, 28 so far no study has focused on the fraction of hospitalisations resulting in death, and therefore, in the more severe cases of morbidity.
In this study we assessed the seasonal variation in in-hospital mortality from respiratory diseases, the contribution of temperature to this seasonal variation, and the daily association between temperature and respiratory inpatient mortality in the provinces of Madrid and Barcelona, Spain. These research questions are of especial relevance because in-hospital deaths from respiratory conditions, which account for a large proportion of total respiratory mortality in Spain (64.4% during 2016–2018),29 could be more preventable than outpatient deaths, and therefore, being able to predict them could help to take preventive measures (eg, patient monitoring). Our results could inform climate change adaptation in healthcare facilities in a country that emerges as a major hotspot in terms of both the impact of global warming30 and human longevity rise.31
Methods
Data sources
This cross-sectional multicentre retrospective observational study used data on hospitalisations, weather (ie, temperature and relative humidity) and air pollution (ie, PM2.5, PM10, O3 and NO2) for the Spanish provinces of Madrid and Barcelona during 2006–2019, representing an average population of approximately 12 million people (26% of total population in Spain).
On the one side, the Spanish National Institute of Statistics (INE) provided individual-level records of hospital admissions both from public and private hospitals, which included the following variables for the patients: sex, age, date of admission and discharge, province of residence, type of admission (ie, ordinary or urgent), primary diagnostic of admission, type of discharge (ie, recovery, death, transfer, other) and length of hospital stay (in days). The dataset did not include unique identifier of patient, and therefore, readmissions or admissions belonging to the same subject could not be identified. The present study was restricted to emergency (ie, non-planned) hospital admissions due to respiratory disease (see ICD codes in the Appendix, p 3). Individual hospital records were aggregated by date of admission in order to conduct the statistical analyses described below.
Gridded (0.10° × 0.10°) observations of daily mean 2-m temperature (°C) and daily mean relative humidity (%) were derived from E-OBS (version 24.0e) of the European Climate Assessment and Dataset (ECA&D).32 Daily mean concentrations of PM2.5, PM10, NO2 and daily maximum 8-hour averages of O3 across Spain were estimated using a Quantile Machine Learning (QML) model framework at a spatial resolution of 10 km × 10 km. The model’s development involved integrating various data sources, including ground monitoring measurements,33 fine-mode and course-mode Aerosol Optical Depth (AOD),34 climate and air quality reanalysis data,35, 36, 37 and geographical features (eg, land-use, topography, road traffic).38,39 The model was trained using data from across Europe, covering the period from Jan 1, 2003, to Dec 31, 2020. To assess the accuracy of the model, we conducted a 10-fold validation. The results showed good performance, with correlation coefficients of 0.80, 0.79, 0.79, and 0.90 for PM2.5, PM10, NO2 and O3, respectively, when compared with site observations in Europe. The Normalized Root Mean Square Error (NRMSE) for PM2.5, PM10, NO2 and O3 predictions in Europe were found to be 1.84%, 2.07%, 8.99%, and 3.35%, respectively, in comparison to site observations. Both meteorological and air pollution data were transformed into provincial estimates by weighting the values with 1 km × 1 km gridded population counts for the year 2011 from INE.40
Statistical analysis
The statistical analyses were stratified by sex, age and diagnostic of hospitalisation and were done with R software (version 4.3.1) using the packages pbs (for cyclic splines), splines (for natural cubic splines) and dlnm (for distributed lag non-linear models).
Seasonality of in-hospital mortality
We first calculated the monthly case-fatality ratio (CFR) as the proportion of the number of hospital admissions resulting in death (regardless of the month when the death occurred) compared to the total number of hospital admissions in a given month:
| (1) |
and did a graphical representation of the CFR in order to have a first picture of the seasonal distribution of this variable (see Fig. 1). We then applied a daily time-series quasi-Poisson regression model41 to estimate the seasonality of hospital admissions resulting in death (μ):
| (2) |
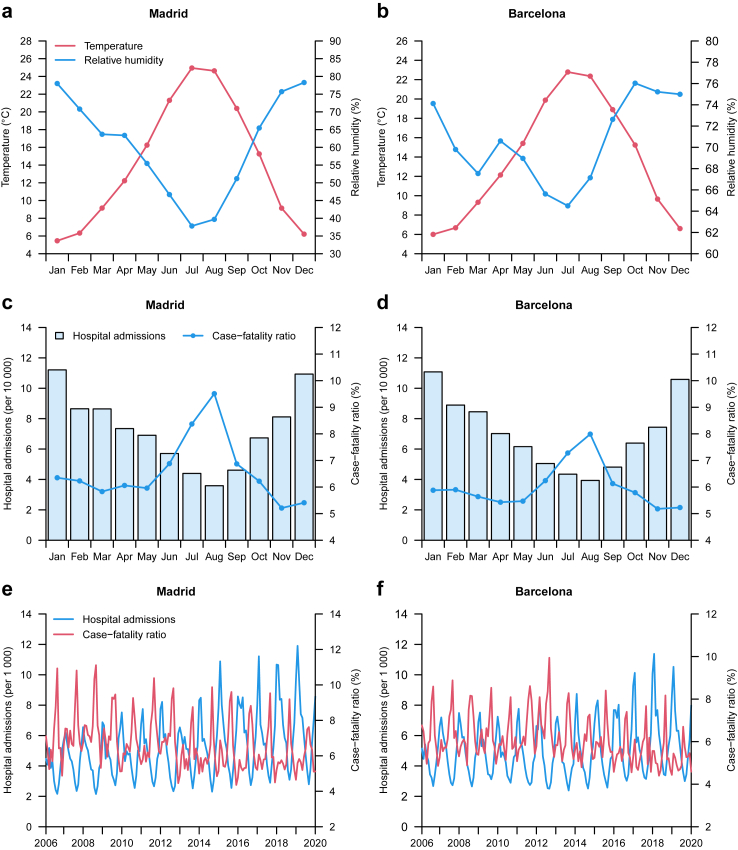
Fig. 1.
Monthly distribution of meteorological variables, hospital admissions and case-fatality ratio.
This model included the log-transformed total number of hospital admissions as an offset; an intercept (α); a natural cubic B-spline with 3 degrees of freedom (df) for the average age of the fatal admissions because it showed a seasonal fluctuation (see Appendix, p 4); a stratum defined by a two-way interaction term of year and day-of-the-week (dow) to control for the long-term trends and the weekly cycle of fatal hospital admissions; and a cyclic spline (cs) with four 4 df for day-of-the-year (doy, taking values from 1 to 366) to quantify the number of fatal hospitalisations on each calendar day. The seasonality, here referring to the association between the day-of-year and hospitalisations resulting in death, was reported as the relative risk (RR) of fatal hospital admission estimation at each calendar day with 95% empirical confidence interval (eCI) (see Fig. 2). Moreover, the ratio of the maximum fatal hospitalisation estimation at peak calendar day relative to the trough calendar day, ie, the peak-to-trough ratio (PTR), was used as a measure of seasonal amplitude.
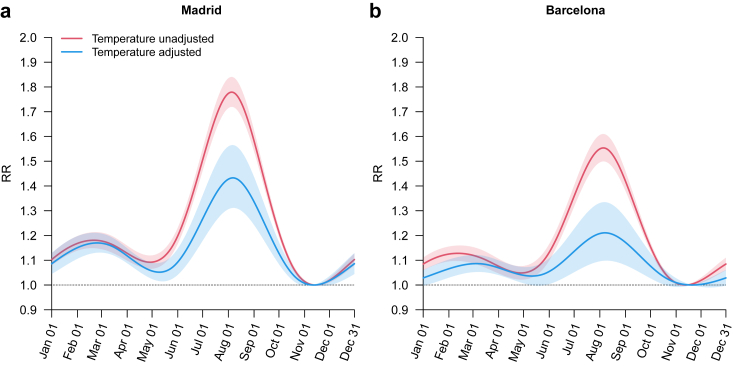
Fig. 2.
Seasonality of in-hospital mortality. RR = relative risk.
Role of temperature in the seasonality of in-hospital mortality
We introduced daily mean temperature into the model described above (equation (2)) in order to obtain the temperature-adjusted seasonality (see again Fig. 2):
| (3) |
Specifically, the short-term effects of temperature were modelled with a cross-basis function (cb) produced by DLNM,42 using a natural cubic B-spline both for the exposure-response and lag-response functions. The spline representing the exposure-response function in the cb was modelled with one internal knot placed at the 75th percentile of the daily mean temperature distribution. The lag period was extended up to 7 days, with an intercept and one internal knot placed at equally spaced values in the log scale to account for the delayed effects of temperature. Moreover, we also explored the potential confounding effect of relative humidity, air pollution (ie, PM2.5, PM10, O3 and NO2) and summer holidays on the role of temperature in the seasonality of in-hospital mortality by including separately the following terms in equation (3): a natural cubic B-spline with 2 df for relative humidity, a linear term for air pollutants, and a dummy or binary variable with value equal to 1 for July–August days.
Day-to-day association between temperature and in-hospital mortality
We performed a time-series quasi-Poisson regression in combination with DLNM to estimate the day-to-day association between temperature and in-hospital mortality counts. This model included the log-transformed total number of hospital admissions as an offset; an intercept; a natural cubic B-spline with 3 degrees of freedom (df) for the average age of fatal admissions; a categorical variable of day of the week to account for the weekly cycle in fatal hospital admissions; a natural cubic B-spline of time with 9 df per year to adjust for seasonal and long-term trends; a natural cubic B-spline with 2 df for relative humidity; and a cb of temperature with the same configuration as described above. Algebraically, the model can be written as:
| (4) |
where μ denotes the expected number of hospital admissions resulting in death; and ns the natural cubic spline. To test the potential confounding effect of air pollution on temperature-mortality association, we introduced separately in equation (4) a linear term for air pollutants (ie, PM2.5, PM10, NO2 and O3). We also analysed the effect modification of temperature by relative humidity and air pollutants by including in equation (4) an interaction between the cb function of temperature and dummy variables representing humidity and air pollution categories (low [below the median] and high [above the median]), and a linear term for the air pollutants in order to account for potential residual confounding. The temperature-hospitalisation associations captured by the cb were summarised as the RR of fatal hospital admission across the whole range of temperatures vs the minimum mortality temperature (MMT) with 95% eCI (see Fig. 3). We then used the RRs to compute monthly attributable fatal hospital admissions (ie, numbers and fractions of hospitalisations) following a methodology described elsewhere43 (see Fig. 4). In short, the RR corresponding to each day of the series was used to calculate the attributable fraction (AF) of fatal hospital admissions on that day and the next 7 days. Then, the daily attributable number (AN) of fatal hospital admissions was computed by multiplying the daily AF by the daily number of fatal hospitalisations. The number of AN in each month was separately aggregated from the daily series, and its ratio with the corresponding total number of hospital admissions provided the monthly AF. We calculated 95% eCI of attributable mortality using Monte Carlo simulations.
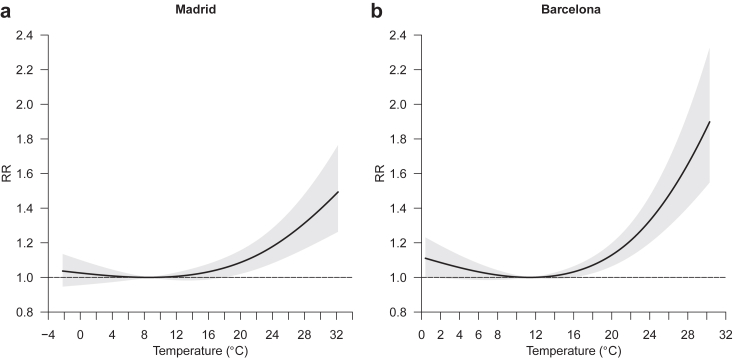
Fig. 3.
Day-to-day association between temperature and hospital admissions resulting in death. RR = relative risk.
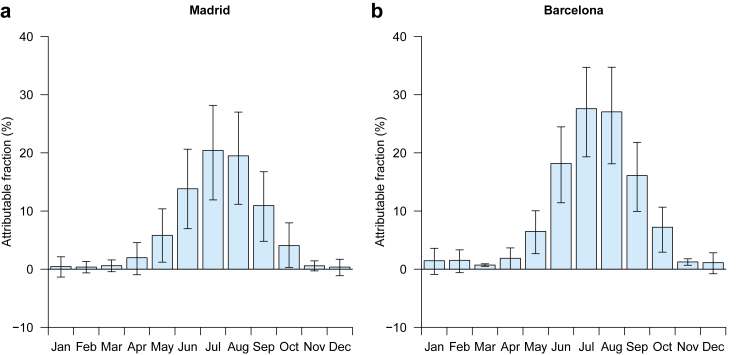
Fig. 4.
Temperature attributable fraction of hospital admissions resulting in death.
Sensitivity analyses
These abovementioned modelling choices were based on a quasi-likelihood version of the Akaike information criterion (Q-AIC) and thoroughly tested in sensitivity analyses. Specifically, we used different configuration for the cb function of temperature and different functions (linear and non-linear) for average age of the fatal admissions.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Multiple authors had full access to all of the data and the corresponding author had final responsibility to submit for publication.
Results
The study analysed 1 710 012 non-planned hospital admissions for respiratory diseases (mean [SD] age, 60.4 [31.0] years; 44.2% women), from which 103 845 resulted in death (81.4 [12.3] years; 45.1%). This number of hospitalisations accounted for 16.9% of all-cause non-planned admissions. Chronic lower respiratory diseases and pneumonia were the leading diagnostics of respiratory hospitalisations (48.8% of the total; see Appendix, p 5), although the highest case-fatality ratio was by far among lung diseases due to external agents (24.2%; see Appendix, p 5). The average value (interquartile range) of non-population-weighted daily mean temperature and relative humidity was, respectively, 14.3 °C (13.1) and 60.4% (31.9) in Madrid, and 13.8 °C (11.1) and 70.6% (14.7) in Barcelona. As expected, there was an inverse correlation between the daily mean temperature and daily mean relative humidity (Pearson correlation of −0.76 in Madrid and −0.24 in Barcelona).
Fig. 1 shows the monthly distribution of the number of hospital admissions and the CFR in the provinces of Madrid and Barcelona during 2006–2019. In both locations, the number of hospital admissions (including those resulting in death [see Appendix, p 6]) was higher in the cold season and lower in the warm season, with a peak in the month of January (the coldest one) and a minimum in the month of August (the hottest one after July). By contrast, the CFR drew the opposite seasonal pattern (see also age-standardised CFR in the Appendix, p 7), with maximum monthly incidence in summer (peak in August) and the minimum monthly incidence in autumn (minimum in November). When stratifying CFR by cause-specific respiratory disease (see Appendix, p 8), we saw that the higher CFR in the warm season was mainly driven by pneumonia, acute bronchitis and bronchiolitis, COPD and, especially, respiratory failure.
Fig. 2 depicts the estimation of the seasonality of hospital admissions resulting in death from the time-series regression model. Prior to the adjustment for ambient temperature (equation (2) in Methods section), a marked seasonal pattern with low fatal hospital admission in winter and a high fatal hospital admission in summer was observed, as already shown in Fig. 1. The shape of seasonality was similar between Madrid and Barcelona, but the amplitude of the seasonal variation was greater in Madrid. The temperature-unadjusted PTR was 1.554 (95% eCI: 1.499–1.610) and 1.779 (1.720–1.840) for Barcelona and Madrid, respectively. When accounting for temperature (equation (3) in Methods section), the shape of the seasonality was similar, but the amplitude showed a large decrease. The PTR was reduced to 1.433 (1.311–1.565) in Madrid and 1.211 (1.098–1.335) in Barcelona. It is important to note that the summer peak persisted after the adjustment for temperature, especially in Madrid, which underlines the presence of other factors contributing to higher fatal hospital admission during the summer, particularly in August. However, when accounting for the potential confounding effect of humidity, air pollution (ie, PM2.5, PM10, O3 and NO2) and summer holidays, the yielded estimates hardly changed (see Appendix, pp 9–10). Results by age group (ie, ≥60 years, ≥70 years and ≥80 years) were quite similar, while women displayed higher seasonal amplitude than men, both before and after adjustment for temperature (see Appendix, pp 11–12). All diagnostics of hospitalisation exhibited a seasonal peak during the summer season, except the lung diseases due to external agents, and high temperatures contributed importantly to this peak in the case of acute bronchitis and bronchiolitis, pneumonia and respiratory failure in Madrid and respiratory failure in Barcelona (see, Appendix, pp 13–14).
Fig. 3 reports the day-to-day association between daily mean temperature and hospital admissions resulting in death (equation (4) in Methods section). High temperatures contributed to an increase in the risk of fatal hospitalisation, especially in Barcelona, whereas low temperatures were not associated with this health variable (Fig. 3). Note that the effect of heat was immediate, as most of the impact took place in the first 3 days since the exposure (Appendix, p 15). The RR of fatal hospitalisation (accumulated across the lags) at the 99th (1st) percentile of the distribution of daily temperatures vs the MMT was 1.395 (95% eCI: 1.211–1.606) (1.017 [0.963–1.073]) in Madrid and 1.612 (1.379–1.885) (1.057 [0.990–1.129]) in Barcelona. Note that in Madrid (Barcelona) the 99th temperature percentile, 1st temperature percentile and the MMT corresponded to 30.0 °C (27.6 °C), 1.9 °C (4.0 °C) and 8.9 °C (11.4 °C), respectively. Risks by age group were similar, but women were more vulnerable to heat than men, especially in Madrid. Moreover, results by diagnostic of admission showed heat effects for acute bronchitis and bronchiolitis and pneumonia in Madrid and respiratory failure in Barcelona (see Appendix, p 16). Interestingly, neither relative humidity nor air pollutants (ie, PM2.5, PM10, NO2 and O3) played a statistically significant role in the association of heat with inpatient mortality (see Appendix, pp 17–18). Moreover, the reported estimates hardly changed after adjustment for air pollutants (see Appendix, p 19). Finally, in terms of mortality burden, summer temperatures (June–September) were responsible for 16.2% (8.8–23.3) and 22.3% (15.4–29.2) of overall fatal hospitalisations from respiratory diseases in Madrid and Barcelona, respectively (Fig. 4).
All sensitivity analyses suggested that the results reported were not dependent on modelling assumptions (see Appendix, pp 20–21).
Discussion
Our study showed for the first time a strong seasonal fluctuation in in-hospital mortality from respiratory diseases and demonstrated that temperature is an important driver of this phenomena. Contrary to hospital admissions, higher during the cold season, the maximum incidence of inpatient mortality was during the summer and was strongly related to high temperatures. Moreover, when analysing the day-to-day association between temperature and in-hospital mortality, we only found an effect for heat, thus confirming the results obtained in the seasonal analysis (ie, higher incidence of inpatient mortality in summer). These results have important implications for climate change health adaptation policies, and for the projections of climate change impacts on human health. Unless effective adaptation measures are taken in hospital facilities, climate warming might exacerbate the burden of inpatient mortality from respiratory diseases during the warm season.
In the present study, we described a maximum incidence of inpatient mortality in summer and a minimum in winter, which contrast with the seasonal pattern in hospital admissions. We also found that ambient temperature had an important contribution to the summer peak in in-hospital mortality, which might be related to increased severity of respiratory morbidity. However, although it is true that the adjustment for temperature reduced considerably the higher incidence of inpatient deaths during the summer, the August peak persisted, especially in Madrid, coinciding in time with the summer holidays and the eventual reorganisation of the healthcare services in many hospitals (eg, reduction in physicians), which could affect negatively the quality of medical care. Nonetheless, this hypothesis does not seem to be supported by the results of our study, which show no significant change in the seasonal pattern of in-hospital mortality after the adjustment for the July–August days in the time-series regression model (see Appendix, p 10), and therefore, further research is warranted to clarify this issue. On the other hand, when comparing the monthly CFR for respiratory diseases with the CFR for cardiovascular conditions (note that current research does not support that heat may contribute to cardiovascular hospitalisations5,44), we saw that cardiovascular CFR is at its lowest values during the summer months (see Appendix, p 22), though with a small peak in August, which reinforce the idea of a causal association between high temperatures and increased inpatient mortality from respiratory diseases.
It has been extensively described that the short-term (daily) exposure to heat and cold, as well as temperature variability, are associated with a greater risk of hospital admission from a range of respiratory diseases such as pneumonia, COPD and asthma.6,8,26, 27, 28 But so far no study has focused on the fraction of hospitalisations resulting in death, and therefore, in the more severe cases of morbidity. Here we assessed the day-to-day association between ambient temperature and hospital admissions resulting in death and we only found an effect for heat, thus confirming the results obtained in the seasonal analysis (ie, higher incidence of inpatient mortality in summer). In addition, it is also important to note that the impact of heat was immediate, in the first 3 days since the exposure, which suggest that the increase in acute respiratory outcomes during heat is more related to the aggravation of chronic and infectious respiratory diseases than the spread of new respiratory infections through indoor crowding to avoid heat, as respiratory infections usually take several days to cause symptoms.
In this study we found no association between low temperatures and the risk of fatal outcome in patients hospitalised for respiratory diseases. This might have to do with the fact that health services are increasingly prepared to deal with winter peaks in respiratory diseases, which might in turn help explain the results of a previous study4 in which we found a very large reduction in the effects of cold on overall respiratory mortality (hospital [about 65% in 2016–2018] and non-hospital deaths) in the most recent years in Spain, leading to a complete reversal of the seasonality of temperature-attributable mortality, with a shift of the maximum monthly incidence from winter to summer, and the minimum monthly incidence from early and late summer to winter. However, it is important to note that our findings for cold cannot be extrapolated to previous periods and other countries, which are characterised by different healthcare contexts.
The underlying physiological mechanisms by which heat trigger adverse health outcomes remain unclear, but they seem to be largely mediated by a thermoregulatory pathway.17,45 Under conditions of heat stress, the body activates heat loss responses of cutaneous vasodilation and sweat production (which subsequently evaporates and removes body heat) to limit elevations in core temperature, which can affect people differently based on, for example, age, pre-existing health conditions (eg, chronic cardiovascular-respiratory-kidney diseases, obesity, diabetes, etc) or even the use of certain medication.46 Vasodilation increases blood flow from the core to the skin and this allows more heat to be dissipated to the environment. Consequently, central blood volume is decreased and can be further reduced if sweating is not compensated by appropriate fluid intake (ie, dehydration). In response to this, heart rate and contractility increase, leading to a higher cardiac oxygen demand, which predisposes individuals with limited coronary flow reserve to ischaemia (ie, inadequate blood flow to other organs). Compounding factors of ischaemia and severe hyperthermia can cause cell damage (ie, necrosis), affecting critically the functioning of several vital organs, with the brain, heart, kidneys, intestines, liver, pancreas and lungs at greatest risk. Hyperthermia and ischaemia can also break down (i) cell membranes rendering the organs more permeable to pathogens and toxins, which induce a systemic inflammatory response that can activate a hypercoagulable state, potentially resulting in thrombosis; and (ii) skeletal muscle cells (ie, rhabdomyolysis), thereby releasing myoglobin that can cause acute renal failure by obstructing kidney tubules. Nevertheless, although adverse effects related to impaired thermoregulation may play an important role in heat-related respiratory diseases, a direct effect of breathing hot air is also plausible.6 For example, a study found that the inhalation of hot air triggered bronchoconstriction in patients with asthma.47
Consistently with the majority of previous studies on total mortality (ie, hospital and non-hospital mortality),48,49 we found that women have a much higher inpatient mortality from heat than men. This is very likely linked to sex-specific physiological differences in thermoregulation. Women have been reported to have a higher temperature threshold above which sweating mechanisms are activated, and a lower sweat output than men, which results in less evaporative heat loss, and therefore a larger susceptibility to the effects of heat.50 Another biological factor (perhaps less determinant) might have to do with the cardiovascular system; women are more likely to have high blood pressure after menopause.51
Last but not least, this study had strengths and limitations. On the one hand, we analysed high-quality morbidity and environmental data spanning 14 years, which allowed us to accurately characterise the seasonal variation in-patient mortality from respiratory diseases and its association with ambient temperature. Additionally, we used the most advanced modelling approaches, based on state-of-the-art methodologies in environmental epidemiology, which allowed us to obtain robust estimations while accounting for complex temporal patterns in the data. On the other hand, our findings might be affected by a selection bias if the characteristics of patients admitted to the hospital differ systematically by season, and therefore, they should be interpreted with caution. Although we controlled for age of the patients in the regression models, a variable showing a clear seasonal fluctuation (see Appendix, p 4), other patient characteristics (eg, comorbidities) were not taken into account because they were not available. Moreover, the study is limited to two Spanish provinces, and therefore, the generalizability our findings might be limited.
Contributors
HA designed the study, did the statistical analysis and drafted the manuscript. JGA, GR and JB contributed to the study design and edited the manuscript. ZC generated the air pollution data and edited the manuscript. RFMT processed the meteorological data. All authors revised the manuscript and approved the final version.
Data sharing statement
Health data can be obtained from the Spanish National Institute of Statistics (INE) under request (https://www.ine.es/infoine/?L=1). Meteorological data can be freely obtained from E-OBS gridded dataset (https://doi.org/10.24381/cds.151d3ec6). Air pollution data are available from the corresponding author upon reasonable request.
Declaration of interests
We declare no competing interests.
Acknowledgements
HA gratefully acknowledges funding from the European Union’s Horizon Europe research and innovation programme under grant agreement No 101065876 (MSCA Postdoctoral Fellowship TEMP-MOMO). HA, ZC, RFMT, and JB acknowledge funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 865564 (European Research Council Consolidator Grant EARLY-ADAPT, https://www.early-adapt.eu/). JB acknowledges funding from the European Union’s Horizon Europe Research and Innovation Programme under grant agreements No 101069213 (European Research Council Proof-of-Concept HHS-EWS) and 101123382 (European Research Council Proof-of-Concept FORECAST-AIR), and from the Spanish Ministry of Science and Innovation under grant agreement No RYC2018-025446-I (programme Ramón y Cajal). ISGlobal authors acknowledge support from the grant CEX2018-000806-S funded by MCIN/AEI/10.13039/501100011033, and support from the Generalitat de Catalunya through the CERCA Program. We acknowledge the E-OBS dataset from the EU-FP6 project UERRA (http://www.uerra.eu) and the Copernicus Climate Change Service, and the data providers in the ECA&D project (https://www.ecad.eu).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100757.
Appendix ASupplementary data
References
- 1.D’Amato G., Cecchi L., D’Amato M., Annesi-Maesano I. Climate change and respiratory diseases. Eur Respir Rev. 2014;23:161–169. doi: 10.1183/09059180.00001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaco J.M., Appel L.J., McGready J., Cutting G.R. The relationship of lung function with ambient temperature. PLoS One. 2018;13 doi: 10.1371/journal.pone.0191409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen R., Yin P., Wang L., et al. Association between ambient temperature and mortality risk and burden: time series study in 272 main Chinese cities. BMJ. 2018;363 doi: 10.1136/bmj.k4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achebak H., Devolder D., Ingole V., Ballester J. Reversal of the seasonality of temperature-attributable mortality from respiratory diseases in Spain. Nat Commun. 2020;11:2457. doi: 10.1038/s41467-020-16273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michelozzi P., Accetta G., De Sario M., et al. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 european cities. Am J Respir Crit Care Med. 2009;179:383–389. doi: 10.1164/rccm.200802-217OC. [DOI] [PubMed] [Google Scholar]
- 6.Anderson G.B., Dominici F., Wang Y., McCormack M.C., Bell M.L., Peng R.D. Heat-related emergency hospitalizations for respiratory diseases in the medicare population. Am J Respir Crit Care Med. 2013;187:1098–1103. doi: 10.1164/rccm.201211-1969OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Solanas È., Basagaña X. Temporal changes in the effects of ambient temperatures on hospital admissions in Spain. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu R., Zhao Q., Coelho M.S.Z.S., et al. Socioeconomic level and associations between heat exposure and all-cause and cause-specific hospitalization in 1,814 Brazilian cities: a nationwide case-crossover study. PLoS Med. 2020;17:1–18. doi: 10.1371/journal.pmed.1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim Y.-H., Hong Y.-C., Kim H. Effects of diurnal temperature range on cardiovascular and respiratory hospital admissions in Korea. Sci Total Environ. 2012;417–418:55–60. doi: 10.1016/j.scitotenv.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 10.Zhan Z., Zhao Y., Pang S., Zhong X., Wu C., Ding Z. Temperature change between neighboring days and mortality in United States: a nationwide study. Sci Total Environ. 2017;584–585:1152–1161. doi: 10.1016/j.scitotenv.2017.01.177. [DOI] [PubMed] [Google Scholar]
- 11.Xu R., Zhao Q., Coelho M.S.Z.S., et al. Socioeconomic inequality in vulnerability to all-cause and cause-specific hospitalisation associated with temperature variability: a time-series study in 1814 Brazilian cities. Lancet Planet Health. 2020;4:e566–e576. doi: 10.1016/S2542-5196(20)30251-5. [DOI] [PubMed] [Google Scholar]
- 12.Arsad F.S., Hod R., Ahmad N., et al. The impact of heatwaves on mortality and morbidity and the associated vulnerability factors: a systematic review. Int J Environ Res Public Health. 2022;19 doi: 10.3390/ijerph192316356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IPCC . Working group I contribution to the IPCC sixth assessment report. 2021. Climate change 2021: the physical science basis. [DOI] [Google Scholar]
- 14.Soriano J.B., Kendrick P.J., Paulson K.R., et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8:585–596. doi: 10.1016/S2213-2600(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutz W., Sanderson W., Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451:716–719. doi: 10.1038/nature06516. [DOI] [PubMed] [Google Scholar]
- 16.Huang K., Li X., Liu X., Seto K.C. Projecting global urban land expansion and heat island intensification through 2050. Environ Res Lett. 2019;14 [Google Scholar]
- 17.Ebi K.L., Capon A., Berry P., et al. Hot weather and heat extremes: health risks. Lancet. 2021;398:698–708. doi: 10.1016/S0140-6736(21)01208-3. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Solanas È., Quijal-Zamorano M., Achebak H., et al. Projections of temperature-attributable mortality in Europe: a time series analysis of 147 contiguous regions in 16 countries. Lancet Planet Health. 2021;5:e446–e454. doi: 10.1016/S2542-5196(21)00150-9. [DOI] [PubMed] [Google Scholar]
- 19.Quijal-Zamorano M., Martínez-Solanas È., Achebak H., et al. Seasonality reversal of temperature attributable mortality projections due to previously unobserved extreme heat in Europe. Lancet Planet Health. 2021;5:e573–e575. doi: 10.1016/S2542-5196(21)00211-4. [DOI] [PubMed] [Google Scholar]
- 20.Lee W., Kim Y., Sera F., et al. Projections of excess mortality related to diurnal temperature range under climate change scenarios: a multi-country modelling study. Lancet Planet Health. 2020;4:e512–e521. doi: 10.1016/S2542-5196(20)30222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madaniyazi L., Armstrong B., Chung Y., et al. Seasonal variation in mortality and the role of temperature: a multi-country multi-city study. Int J Epidemiol. 2022;51:122–133. doi: 10.1093/ije/dyab143. [DOI] [PubMed] [Google Scholar]
- 22.Upshur R.E., Moineddin R., Crighton E., Kiefer L., Mamdani M. Simplicity within complexity: seasonality and predictability of hospital admissions in the province of Ontario 1988–2001, a population-based analysis. BMC Health Serv Res. 2005;5:13. doi: 10.1186/1472-6963-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise R.A., Calverley P.M., Carter K., Clerisme-Beaty E., Metzdorf N., Anzueto A. Seasonal variations in exacerbations and deaths in patients with COPD during the TIOSPIR® trial. Int J Chron Obstruct Pulmon Dis. 2018;13:605–616. doi: 10.2147/COPD.S148393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller K., Hobohm L., Münzel T., Konstantinides S.V., Lankeit M. Sex-specific and age-related seasonal variations regarding incidence and in-hospital mortality of pulmonary embolism in Germany. ERJ Open Res. 2020;6 doi: 10.1183/23120541.00181-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho A.T.N., Shmelev A., Charbek E. Trends and seasonal variation of hospitalization and mortality of interstitial lung disease in the United States from 2006 to 2016. Respir Res. 2020;21:152. doi: 10.1186/s12931-020-01421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam H.C.Y., Li A.M., Chan E.Y.Y., Goggins W.B. The short-term association between asthma hospitalisations, ambient temperature, other meteorological factors and air pollutants in Hong Kong: a time-series study. Thorax. 2016;71:1097–1109. doi: 10.1136/thoraxjnl-2015-208054. [DOI] [PubMed] [Google Scholar]
- 27.Konstantinoudis G., Minelli C., Vicedo-Cabrera A.M., Ballester J., Gasparrini A., Blangiardo M. Ambient heat exposure and COPD hospitalisations in England: a nationwide case-crossover study during 2007–2018. Thorax. 2022;77:1098–1104. doi: 10.1136/thoraxjnl-2021-218374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu H., Sun S., Tang R., Chan K.-P., Tian L. Pneumonia hospitalization risk in the elderly attributable to cold and hot temperatures in Hong Kong, China. Am J Epidemiol. 2016;184:570–578. doi: 10.1093/aje/kww041. [DOI] [PubMed] [Google Scholar]
- 29.INE Death statistics according to cause of death. https://ine.es/dyngs/INEbase/en/operacion.htm?c=Estadistica_C&cid=1254736176780&menu=ultiDatos&idp=1254735573175
- 30.Giorgi F. Climate change hot-spots. Geophys Res Lett. 2006;33:1–4. [Google Scholar]
- 31.Foreman K.J., Marquez N., Dolgert A., et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392:2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornes R.C., van der Schrier G., van den Besselaar E.J.M., Jones P.D. An ensemble version of the E-OBS temperature and precipitation data sets. J Geophys Res Atmos. 2018;123:9391–9409. [Google Scholar]
- 33.European Environment Agency (EEA) European Environment Information and Observation Network (Eionet) https://www.eionet.europa.eu/
- 34.Aerosol NASA Robotic Network (AERONET) https://aeronet.gsfc.nasa.gov/
- 35.Copernicus Atmosphere Monitoring Service (CAMS) CAMS global reanalysis (EAC4) https://ads.atmosphere.copernicus.eu/cdsapp#!/dataset/cams-global-reanalysis-eac4?tab=overview
- 36.Gelaro R., McCarty W., Suárez M.J., et al. The modern-era retrospective analysis for research and applications, version 2 (MERRA-2) J Clim. 2017;30:5419–5454. doi: 10.1175/JCLI-D-16-0758.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muñoz-Sabater J. ERA5-land hourly data from 1981 to present. Copernicus Climate Change Service (C3S) Climate Data Store (CDS); 2019. [DOI] [Google Scholar]
- 38.Meijer J.R., Huijbregts M.A.J., Schotten K.C.G.J., Schipper A.M. Global patterns of current and future road infrastructure. Environ Res Lett. 2018;13 [Google Scholar]
- 39.Ching J., Mills G., Bechtel B., et al. WUDAPT: an urban weather, climate, and environmental modeling infrastructure for the Anthropocene. Bull Am Meteorol Soc. 2018;99:1907–1924. [Google Scholar]
- 40.INE Population and housing censuses. https://www.ine.es/dyngs/INEbase/en/operacion.htm?c=Estadistica_C&cid=1254736176992&menu=resultados&idp=1254735572981
- 41.Madaniyazi L., Tobias A., Kim Y., Chung Y., Armstrong B., Hashizume M. Assessing seasonality and the role of its potential drivers in environmental epidemiology: a tutorial. Int J Epidemiol. 2022;51:1677–1686. doi: 10.1093/ije/dyac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gasparrini A., Armstrong B., Kenward M.G. Distributed lag non-linear models. Stat Med. 2010;29:2224–2234. doi: 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasparrini A., Leone M. Attributable risk from distributed lag models. BMC Med Res Methodol. 2014;14:1–8. doi: 10.1186/1471-2288-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez-Solanas È., Basagaña X. Temporal changes in temperature-related mortality in Spain and effect of the implementation of a Heat Health Prevention Plan. Environ Res. 2019;169:102–113. doi: 10.1016/j.envres.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Mora C., Counsell C.W.W., Bielecki C.R., Louis L.V. Twenty-seven ways a heat wave can kill you. Circ Cardiovasc Qual Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.117.004233. [DOI] [PubMed] [Google Scholar]
- 46.Chen K., Dubrow R., Breitner S., et al. Triggering of myocardial infarction by heat exposure is modified by medication intake. Nat Cardiovasc Res. 2022;1:727–731. doi: 10.1038/s44161-022-00102-z. [DOI] [PubMed] [Google Scholar]
- 47.Hayes D., Collins P.B., Khosravi M., Lin R.-L., Lee L.-Y. Bronchoconstriction triggered by breathing hot humid air in patients with asthma. Am J Respir Crit Care Med. 2012;185:1190–1196. doi: 10.1164/rccm.201201-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hajat S., Kovats R.S., Lachowycz K. Heat-related and cold-related deaths in England and Wales: who is at risk? Occup Environ Med. 2007;64:93–100. doi: 10.1136/oem.2006.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Achebak H., Devolder D., Ballester J. Heat-related mortality trends under recent climate warming in Spain: a 36-year observational study. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanovich R., Ketko I., Charkoudian N. Sex differences in human thermoregulation: relevance for 2020 and beyond. Physiology. 2020;35:177–184. doi: 10.1152/physiol.00035.2019. [DOI] [PubMed] [Google Scholar]
- 51.Rosano G.M.C., Vitale C., Marazzi G., Volterrani M. Menopause and cardiovascular disease: the evidence. Climacteric. 2007;10:19–24. doi: 10.1080/13697130601114917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.