Abstract
Introduction
Patients with EGFR-mutant NSCLC have a high incidence of brain metastases. The EGFR-directed tyrosine kinase inhibitor osimertinib has intracranial activity, making the role of local central nervous system (CNS)-directed therapies, such as radiation and surgery, less clear.
Methods
Patients with EGFR-mutant NSCLC and brain metastases who received osimertinib as initial therapy after brain metastasis diagnosis were included. Individual lesion responses were assessed using adapted RANO-BM criteria. CNS progression and local progression of brain metastasis from osimertinib start were analyzed using cumulative incidence treating death as a competing risk. Overall survival was estimated using Kaplan-Meier methodology.
Results
There were 36 patients who had a median interval from brain metastasis diagnosis to first-line osimertinib initiation of 25 days. In total, 136 previously untreated brain metastases were tracked from baseline. Overall, 105 lesions (77.2%) had complete response and 31 had partial response reflecting best objective response of 100%. Best response occurred at a median of 96 days (range: 28–1113 d) from baseline magnetic resonance imaging. This reflects a best objective response rate of 100%. Two-year overall survival was 80%. CNS progression rates at 1-, 2-, and 3-years post-osimertinib were 21%, 32%, and 41%, respectively. Lesion-level local failure was estimated to be 0.7% and 4.7% at 1- and 2-years post-osimertinib, respectively. No clinicodemographic factors including brain metastasis number were associated with post-osimertinib progression.
Conclusions
Intracranial response to osimertinib is excellent for patients with EGFR-mutant NSCLC with de novo, previously untreated brain metastases. Very low local failure rates support a strategy of upfront osimertinib alone in selected patients.
Keywords: Brain metastasis, Local recurrence, EGFR-mutant, Osimertinib, Non–small cell lung cancer
Introduction
The EGFR pathway activation is an oncologic driver of 15% to 40% of NSCLC.1 Incidence of brain metastases in patients with EGFR-mutant NSCLC (EGFRm) is estimated to be as high as 70%, considerably higher than that in non–EGFR-driven NSCLC.2, 3, 4, 5 Given that brain metastases have considerable risk of morbidity and mortality, optimal management remains an important challenge.
Surgery and radiotherapy are cornerstones of brain metastasis management and remain important and efficacious strategies irrespective of cancer type or molecular profile.6 Nevertheless, contemporary brain metastasis management now incorporates rational integration of central nervous system (CNS)-active systemic therapies when available. In the context of EGFRm, the third-generation EGFR-targeting tyrosine kinase inhibitor (TKI) osimertinib has been found to have both blood-brain tumor barrier penetrance and intracranial activity.7, 8, 9, 10, 11 These data have led to a recent ASCO-SNO-ASTRO guideline recommendation that osimertinib alone may be offered to patients with asymptomatic brain metastases with delay of local therapy until evidence of intracranial progression.12 This recommendation was made by informal consensus with the expert panel highlighting poor evidence quality to forego local therapy. This is likely because although most studies report global intracranial activity, durability and correlates of TKI responses, particularly with lesion-level dynamics, remain unclear.
The role of upfront local therapies, thus, remains controversial for patients with newly diagnosed brain metastases who are planning for osimertinib. We sought to analyze brain metastasis lesion-level characteristics, response dynamics, and CNS patterns of failure for systemic therapy-naive patients with previously untreated lesions treated with osimertinib.
Materials and Methods
Patient Selection
We performed an Institutional Review Board–approved retrospective cohort study, with waiver of informed consent given its retrospective nature. Patients were selected from an institutional database maintained by the Multidisciplinary Brain Metastasis Program at a National Cancer Institute–designated Comprehensive Cancer Center and were included if they had a diagnosis of EGFR-mutant NSCLC and treated with osimertinib between 2014 and 2022. Inclusion was further limited to patients who (1) received osimertinib alone as initial therapy after first brain metastasis diagnosis, (2) had at least one intact brain metastasis not previously treated with systemic or local therapy (stereotactic radiosurgery [SRS], whole brain radiotherapy, or surgery) at osimertinib initiation, and (3) had baseline brain magnetic resonance imaging (MRI) before, and at least one scan after, osimertinib initiation.
Response Evaluation
Lesions were tracked across subsequent scans until either discontinuation of osimertinib, first brain-directed radiotherapy or surgery, or death by a blinded neuroradiologist. Patients had the largest diameter of up to five of their largest eligible brain metastases measured in millimeters on sequential MRIs using thinnest available slice thicknesses (ranging from 1 to 5 mm), with no minimum lesion size requirement. Classification of intracranial response of individual lesions was determined using a modification of the RANO-BM criteria on a per-lesion basis as opposed to summing diameters.13 Complete response (CR) for a given lesion was defined as disappearance of the lesion, partial response (PR) as greater than or equal to 30% decrease, and progressive disease (PD) as greater than or equal to 20% increase in size relative to nadir or development of new brain metastasis. Stable disease was defined as any other scenario.
Study End Points
The primary end point of the study was CNS progression defined as either progression of existing brain metastasis or development of new parenchymal or leptomeningeal metastases. Local failure was defined as progression of an index lesion present before initiation of osimertinib. Pattern of first failure was noted to be within the CNS only, outside the CNS only, or simultaneous CNS/extra-CNS, defined as radiographic evidence of progression in both compartments within 1 month.
Statistical Analysis
Descriptive statistics were used to characterize the cohort and lesions studied. For overall survival (OS), follow-up was defined from osimertinib start to death (event) or last follow-up (censored). Kaplan-Meier methodology was used to describe survival. CNS progression rates after osimertinib were estimated with cumulative incidence considering death as a competing risk. Variables of interest were associated with CNS progression using the subdistribution hazard model where death was a competing event. Cumulative incidence rates for local failure at the lesion level were estimated in the competing risks setting with sandwich-based variance adjustment of 95% confidence intervals (CIs) owing to multiple lesions per patient. A generalized linear mixed model was attempted to associate lesion size with lesion progression where the correlation of repeated assessments within each lesion and the correlation of lesions within each patient were accounted for using a hierarchical model structure. Analyses and graphics were performed using SAS version 9.4 (The SAS Institute, Cary, NC) and R version 4.2.2 (The R Foundation for Statistical Computing).
Results
Patient Cohort
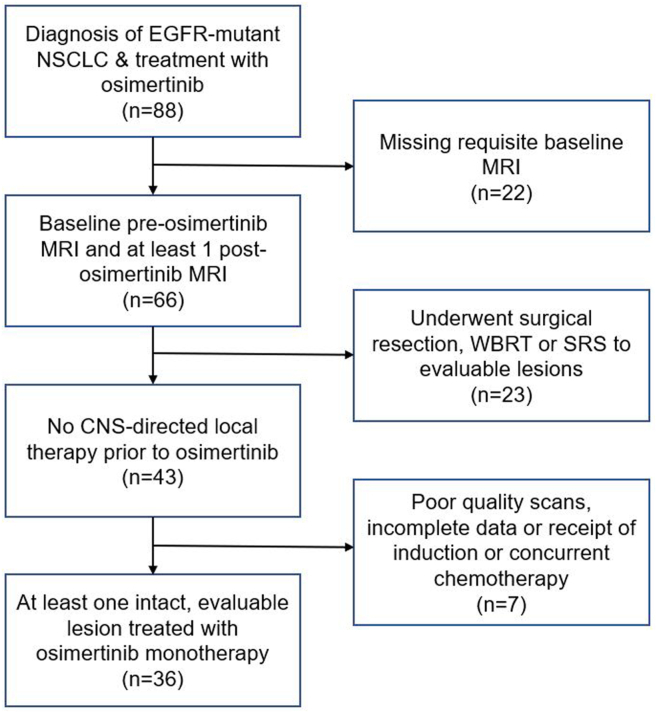
Of the 88 identified patients with EGFR-mutant NSCLC who received osimertinib for de novo brain metastases, 36 met the criteria for inclusion (Fig. 1). Overall, 69% were never-smokers and 72% female, and nearly all of whom (94%) were diagnosed with metastatic disease at presentation (Table 1). All but two patients had metastatic disease at NSCLC diagnosis, and 31 (86%) had extracranial metastases in addition to brain metastases. The remaining two patients had originally localized NSCLC treated without systemic therapy and later developed metachronous metastatic brain disease after initial treatment. Half of the patients had documented symptoms attributable to brain metastasis. No patient received any other cancer-directed systemic therapy before osimertinib initiation. The median duration from brain metastasis diagnosis to start of osimertinib was 25 days (interquartile range: 17.5–35.5 d). During the study period, four patients (11%) required osimertinib dose reductions owing to toxicity or intolerance.
Figure 1.
CONSORT diagram for patient inclusion. CNS, central nervous system; MRI, magnetic resonance imaging; SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy.
Table 1.
Cohort and Lesion Characteristics
| Characteristics | N = 36 |
|---|---|
| Median age at brain metastasis diagnosis (range) | 64 (47–83) |
| Female sex | 26 (72) |
| Never smoker | 25 (69) |
| Lung cancer stage at diagnosis | |
| I–III | 2 (5.6) |
| IV | 34 (94) |
| Initial ECOG score | |
| 0 | 11 (31) |
| 1 | 14 (39) |
| 2 | 4 (11) |
| Unknown | 7 (19) |
| Baseline brain metastasis burden | |
| 1 brain metastasis | 7 (19) |
| 2–5 brain metastases | 8 (22) |
| 6–10 brain metastases | 8 (22) |
| 11+ brain metastases | 13 (36) |
| Median lesion level baseline brain metastasis maximum diameter (cm) (range)a | 0.66 (0.15–5.00) |
| Median size of largest brain metastasis (cm) (range) | 0.99 (0.43–5.00) |
| Neurologic symptoms from brain metastases | 18 (50) |
| Extracranial status at the time of brain metastasis diagnosis | |
| Additional extracranial metastases | 31 (86) |
| No extracranial metastases | 5 (14) |
Note: All values are given in n (%).
N, number; ECOG, Eastern Cooperative Oncology Group.
N = 136.
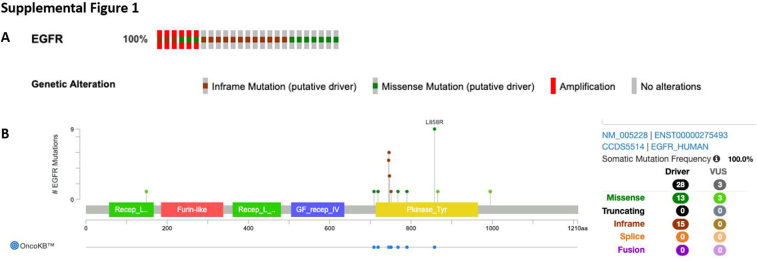
Before initiation of osimertinib, EGFR alteration was confirmed by the MSK-IMPACT or MSK-ACCESS next-generation sequencing platforms for 25 of 36 patients (69%) (Supplementary Fig. 1A).14 The remainder were diagnosed using a polymerase chain reaction–based molecular testing platform (n = 6) or next-generation sequencing panels (n = 5) performed elsewhere. Overall, the most common alteration was exon 19 deletion (n = 24, 67%) followed by L858R mutation (n = 8, 22%). Other alterations included two missense mutations (n = 2) and exon 19 insertion (n = 1). One patient was reported as “EGFR mutant” on an outside sequencing panel but the specific alteration was not available. Nearly all the identified mutations were driver mutations and found in the EGFR kinase domain; all alterations for patients sequenced using the MSK-IMPACT platform were deemed likely oncogenic by the OncoKB platform (Supplementary Fig. 1B).15
Lesion-Level Analysis
Just more than half of the patients (21 of 36, 58%) had six or more brain metastases at the time of osimertinib initiation (19% solitary brain metastasis, 22% two to five brain metastases). In total, 136 previously untreated brain metastases were present at baseline and subsequently tracked. Median baseline maximum diameter was 0.66 cm (range: 0.15–5.0 cm). In total, 42 lesions (30.9%) were more than 1 cm, 12 lesions (8.8%) more than 2 cm, and three lesions (2.2%) more than 3 cm at baseline. At the patient level, the largest brain metastasis had a median maximum diameter of 1.0 cm (range: 0.43–5.0). The decision to forego surgery or radiotherapy for larger lesions was made on a case-by-case basis after multidisciplinary discussion. Excluded patients had a similar distribution of lesion number, with significantly higher maximal tumor diameter (data not revealed).
Lesion-Level Response Rate
In total, 1129 baseline and post-treatment MRIs were reviewed. Of 136 tracked lesions, at the first reassessment, 64 lesions (47%) had CR, 66 (49%) had PR, and the remaining six (4.4%) were stable. Many brain metastases had continued response with further osimertinib exposure; 105 (77.2%) had CR as their best response during the recorded period. Of the 31 remaining lesions, all (31 of 31) had PR. Thus, in aggregate, the best overall response rate for osimertinib was 136 of 136 lesions (100%). The median time to best response from baseline MRI was 95.5 days (range: 28–1113 d). The median number of scans to best response (not including baseline MRI) was one (range: one to eight).
Patient-Level Outcomes
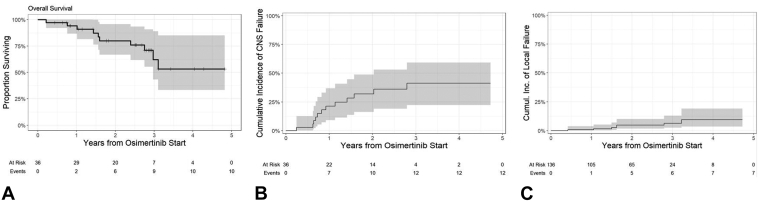
Median follow-up for survivors was 2.6 years from osimertinib initiation (range: 0.38–4.72 y). Median time between post-treatment surveillance MRIs was 86 days. Median OS for the cohort was not reached, and the 1-, 2-, and 3-year survival estimates post-osimertinib were 90.9% (95% CI: 81.1%–100%), 79.7% (95% CI: 65.0%–94.4%), and 62.4% (95% CI: 40.2%–84.6%), respectively (Fig. 2A).
Figure 2.
(A) Overall survival from the time of osimertinib initiation by Kaplan-Meier, and (B) cumulative incidence of CNS progression after start of osimertinib considering death as a competing risk. (C) Cumulative incidence of lesion-level local failure after the start of osimertinib considering death as a competing risk. CNS, central nervous system.
Overall, 21 patients had disease progression events at a median of 9.0 months post-osimertinib (range: 1.0–33.5 m). The most common site of first cancer progression was non-CNS only (n = 12, 33%), CNS only (n = 8, 22%), and mixed CNS/extra-CNS (n = 1, 3%). There were 12 CNS progression events (33%) which included local parenchymal only (n = 3), distant parenchymal only (n = 3), simultaneous local/distant parenchymal (n = 2), and leptomeningeal (n = 4). Figure 2B reveals the cumulative incidence of CNS progression post-osimertinib initiation. CNS progression rates at 1-, 2-, and 3-years post-initiation of therapy were 21.3% (95% CI: 9.2%–36.7%), 32.0% (95% CI: 16.3%–48.8%), and 41.2% (95% CI: 22.2%–59.3%), respectively. Of these relapses, eight were in the brain parenchyma, including progression of preexisting brain lesions alone (n = 3), development of new brain metastases (n = 3), and mixed progression of new/preexisting brain metastases (n = 2). Figure 2C reveals the cumulative incidence of local failure at the lesion level assuming death as a competing risk, which was estimated to be 0.7% (95% CI: 0.1%–4.6%) and 4.7% (95% CI: 2.0%–11.1%) at 1- and 2-years post-osimertinib, respectively. In addition, four patients relapsed with leptomeningeal disease, of which three had baseline leptomeningeal involvement which had stabilized on osimertinib for between 8 and 19 months. Of the 12 progressions, three (25%) were symptomatic, and all these patients had leptomeningeal disease. The remaining progression events were diagnosed radiographically.
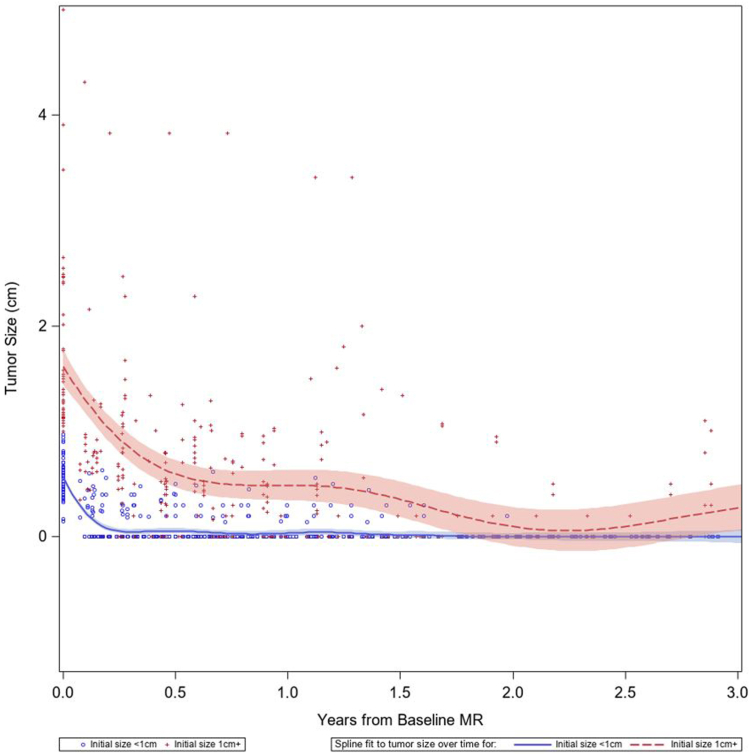
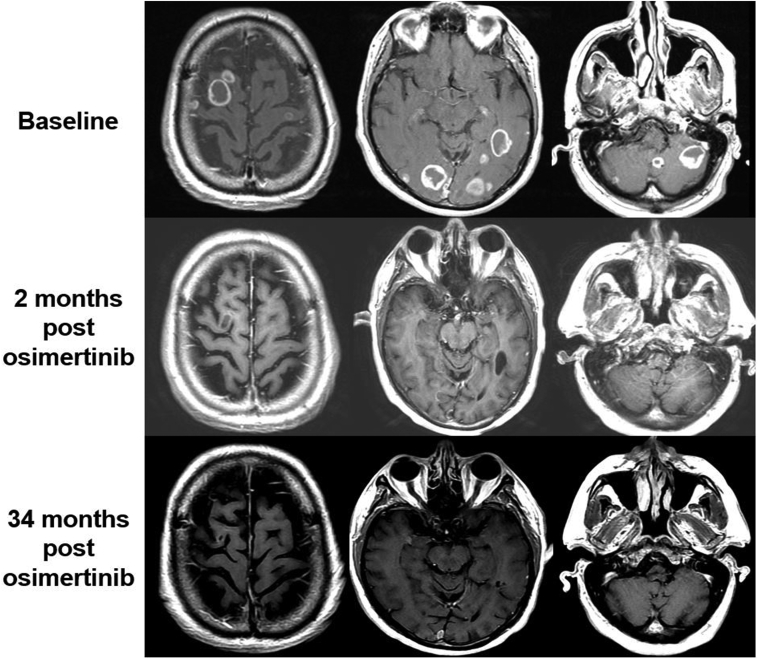
We did not identify any clinicodemographic factors significantly associated with risk of subsequent CNS progression post-osimertinib including baseline total number of brain metastases. Although the cohort predominantly consisted of patients with subcentimeter lesions, we could not formally test association of size with progression given few progression events. Figure 3 illustrates a spline-fit model of lesion-level sizes stratified by baseline brain metastasis size greater than or equal to 1 cm or less than 1 cm revealing a trend toward CR within the first year of therapy for smaller lesions and PR for larger lesions. Of note, six patients included in this series had at least one lesion above 2 cm and durable control was attained. Figure 4 illustrates an example of a patient with high burden of symptomatic brain metastases with almost three years of sustained response.
Figure 3.
Spline regression analysis of the brain metastasis maximum diameter after initiation of osimertinib. Lines are stratified by the baseline size of the brain metastasis and grouped as less than 1 cm (blue circle markers/blue line) or greater than or equal to 1 cm (red plus markers/red line).
Figure 4.
Case example of high burden of baseline brain metastasis with durable response to osimertinib. Top row illustrates representative index lesions from the baseline (pre-osimertinib) scan. Middle row illustrates the first MRI after initiation of osimertinib which already had significant regression of disease. The patient remained controlled intracranially until 34 months post-osimertinib, at which point he developed a subcentimeter new lesion which was subsequently treated with SRS. MRI, magnetic resonance imaging; SRS, stereotactic radiosurgery.
Salvage strategies to manage post-osimertinib CNS relapse were driven by the distribution of disease. Six patients were treated with radiotherapy (SRS, n = 5; whole brain radiotherapy, n = 1). One patient received multiagent chemotherapy (carboplatin, pemetrexed, bevacizumab) and one received increased osimertinib dose (80 mg, from 40 mg). Overall, there were five cases of subsequent leptomeningeal progression, four of which had not had any prior parenchymal progression events. Leptomeningeal disease was treated with craniospinal irradiation (n = 3) or involved field photon radiotherapy (n = 2).
Discussion
The CNS activity of osimertinib has been well established,11,16 with superior intracranial efficacy before generation EGFR TKI options.8 This has rapidly translated to decreased upfront utilization of surgery and radiotherapy for EGFRm brain metastases. Despite this evolution,12 the practice of relying solely on osimertinib and not also local therapy remains controversial and is not universally endorsed, given continued evidence gaps.11,17 At present, there are significant differences of opinion regarding optimal first-line strategy for asymptomatic brain metastases.18 This is likely due to challenges in data interpretation given significant heterogeneity in reported outcomes, which in many cases included patients with prior exposure to earlier-generation TKIs or chemotherapy, and relatively few longitudinal lesion-level assessments to guide longer-term success. At present, National Comprehensive Cancer Network guidelines for CNS malignancies state that it is reasonable to consider upfront CNS-active agents for asymptomatic patients. Nevertheless, they caution on universal endorsement given the lack of prospective clinical trials comparing the two strategies to assess what the impact of delayed radiation would be in terms of survival or in delay of neurologic deficit development.19
The approach herein including longitudinal imaging assessment of osimertinib-treated EGFRm brain metastases offers additional insight into the implications of foregoing local therapies in the upfront setting. The strength of this series is its homogeneity, only including patients with no prior systemic therapy exposure before osimertinib, enabling pure assessment of the intracranial control potential of the TKI alone. We found that most baseline lesions responded well to osimertinib and durably, with the first post-treatment MRI not necessarily indicative of best response, as many lesions continued to regress with further osimertinib treatment and with nearly 80% CNS control at 1 year.
Although other series have reported on CNS progression-free survival rates, there are relatively few published reports on the durability of lesion-level outcomes. This metric is more applicable for the decision of whether to prioritize upfront integration of focal therapies such as surgery and radiosurgery. The recent publication by Hui et al.20 investigated the local control potential of osimertinib in a cohort of 37 patients with 284 intact, nonirradiated lesions greater than or equal to 5 mm. They identified a 14% 1-year cumulative incidence of local brain metastasis failure and 4% in patients with first-line osimertinib, similar to the current series. Notably, their cohort included some pretreated patients, with 22% exposed to prior (non-osimertinib) TKIs, 22% to prior chemotherapy, and 8% to prior immunotherapy. They found that several factors were associated with increased local brain metastasis failure including uncontrolled primary tumor, poorer performance status, and increasing number of prior systemic therapies pre-osimertinib (which may predispose to C797S and other resistance mechanisms).21
Our study builds on this work with longer follow-up and a more homogenous population as all patients in our series were previously systemic therapy naive. We believe that this population is more clinically relevant in contemporary practice, where EGFR sequencing results return rapidly in the de novo brain metastatic setting and can inform upfront systemic therapy decision-making for such patients otherwise considered for local therapy. Our inability to confirm the factors they associated with local failure is likely due to the fact that their failure events were concentrated in their more heavily pretreated subgroup (which was not applicable in our more homogeneous cohort). Of note, excellent intracranial response rates for treatment-naive patients are comparable to those reported in the FLAURA study of upfront TKI utilization.8 Furthermore, Peled et al.16 reported a similarly strong intracranial objective response rate of 84% for 20 treatment-naive patients with asymptomatic brain metastases treated as part of a nonrandomized phase 2 study. They noted a median intracranial PFS of 11.8 months (95% CI: 7.7–NA) for this subgroup, and lesion-level analysis revealed a similar trend to those reported in Figure 3.
Taken together, there is growing evidence to support the approach of relying on osimertinib alone for de novo brain metastases, particularly for those with small lesions. Although osimertinib alone has good CNS activity, it remains unclear whether the combination of osimertinib plus upfront radiotherapy would improve outcomes even further. Such a paradigm of EGFR TKI plus upfront radiotherapy is supported in several analyses of first- and second-generation EGFR TKIs describing superior outcomes with combination therapy versus TKI alone.22, 23, 24 For example, in the multi-institutional Magnuson report, despite being enriched for more high-risk brain metastasis features, OS was significantly prolonged in patients who received upfront SRS followed by TKI versus TKI alone.22 The applicability of this finding to osimertinib, which has superior blood-brain tumor barrier penetration compared with earlier generation TKIs, is less clear. In a cohort of patients who received CNS-active TKI versus upfront SRS followed by TKI, Thomas et al.25 concluded that there was no significant difference in time to intracranial progression or treatment failure between the two strategies. Similar to the Magnuson analysis, brain metastases treated with upfront radiotherapy were larger and more symptomatic, so similar outcomes between the groups raise the real possibility that upfront radiation was able to help overcome some of these higher risk features.22,25
Going forward, we suspect that the management of these patients will become more individualized and nuanced. We were unable to identify lesion-level characteristics associated with local failure to provide recommendations for lesions where upfront local therapy should be strongly considered. Nevertheless, it is possible that despite more than 2 years of follow-up, we do not have sufficient follow-up to identify additional local failure events. Neither the Stanford series20 nor our work was able to reveal greater local failure risk for larger lesions, and the potential for dramatic, rapid responses is reported (Fig. 4).26 Nevertheless, we remain cautious and often advocate for upfront radiotherapy for larger lesions particularly in eloquent locations; this is driven by the uncertainty of the durability of response and the possibility that radionecrosis rates are significantly higher for patients with prior exposure to or concurrent utilization of EGFR-directed TKIs.27,28 Although Hui et al.20 suggested consideration of early integration of SRS for lesions which do not respond as rapidly to osimertinib given higher local failure risk, we did not observe this trend. Instead, we found that even large lesions continue to regress over time.
Numerous additional open questions remain around the CNS-directed management of EGFRm NSCLC. First, for resected brain metastases, which carry a progression rate of some 50% without adjuvant therapy, it remains unclear how adjuvant osimertinib compares to adjuvant SRS, which has excellent control rates and remains standard of care.29, 30, 31 A second question surrounds the optimal management of leptomeningeal disease, which is known to be enriched in EGFRm patients.32 In this series, utilization of proton craniospinal radiotherapy was used as a salvage strategy after osimertinib failure33 but the optimal sequencing of these interventions is uncertain. Thus, despite the potential for good local control of baseline brain metastases, we continue to advocate for close CNS surveillance and multidisciplinary management of this population given demonstrably lower overall CNS control.34,35
There are several limitations to this work. First, despite longitudinal tracking of more than 100 lesions, this is a single-institution cohort with inherent referral and selection biases given there were no consistent criteria to guide whether osimertinib alone was recommended. As expected, patients included in this study have smaller lesions than those excluded (in turn largely because of use of local therapy instead of osimertinib alone) given larger tumors often require resection, represent leptomeningeal metastasis plaques, or otherwise discouraged reliance on medical therapy alone given the established efficacy of radiation therapy. Further investigation is needed to determine the efficacy of medical strategies for large parenchymal tumors and leptomeningeal metastasis; however, in the real-world setting, these are less common and often require aggressive CNS-directed therapy given symptoms. We chose to include small lesions (<5 mm) to be as reflective of real-world practice as possible, though we acknowledge the difficulties and possibility of detection biases when tracking small lesions. Another consideration is that given this was retrospective, all post-treatment scans did not have uniform scan thickness. Despite these caveats, for our analysis, we feel that the general downward size trajectories are as informative as specific size changes. Finally, we adjusted variance estimates for local failure incidence to account for the interdependence of lesions within a patient; however, there are known biological cluster effects, beyond the characteristics of an individual brain metastasis alone, that could affect a patient's risk of brain metastasis progression (notably the emergence of resistance mechanisms and the potential for metastatic seeding).36
Conclusion
Intracranial response rates for osimertinib are excellent in patients with de novo, previously untreated brain metastases. Given very low rates of local failure, treatment with osimertinib alone without local therapies may be a reasonable first strategy for many patients, particularly those with subcentimeter lesions.
CRediT Authorship Contribution Statement
Brandon Imber: Conceptualization; Data curation; Funding acquisition; Methodology; Supervision; Roles/Writing—original draft; Writing—review and editing.
Ryka Sehgal: Data curation; Roles/Writing—original draft; Writing—review and editing.
Rachel Saganty: Data curation, Roles/Writing—original draft; Writing—review and editing.
Anne Reiner: Methodology; Visualization; Writing—review and editing.
A. Turan Illica: Data curation; Writing—review and editing.
Emily Miao: Writing—review and editing.
Bob T. Li: Writing—review and editing.
Gregory J. Riely: Writing—review and editing.
Helena Yu: Writing—review and editing.
Katherine Panageas: Methodology; Writing—review and editing.
Robert Young: Methodology; Writing—review and editing.
Luke Pike: Conceptualization; Data curation; Methodology; Supervision.
Nelson Moss: Conceptualization; Data curation; Funding acquisition; Methodology; Supervision; Roles/Writing—original draft; Writing—review and editing.
Acknowledgments
This study was supported in part by the National Institutes of Health and National Cancer Institute Cancer Center Support Grant P30 CA008748 to Selwyn M. Vickers.
Footnotes
Drs. Imber and Sehgal contributed equally as co-first authors.
Drs. Pike and Moss contributed equally as co-senior authors.
Disclosure: Dr. Imber reports receiving research funding and lecture honoraria from GT Medical Technologies; receiving research funding from AstraZeneca and Kazia Pharmaceuticals; and having DSMB/advisory board participation with Ono Pharmaceuticals and Telix Pharmaceuticals. Dr. Yu reports receiving grants from Cullinan, AstraZeneca, Daiichi, Pfizer, Black Diamond, Blueprint, Novartis, and Janssen, and consulting fees from AstraZeneca, Cullinan, Daiichi, Black Diamond, Blueprint, Amgen, Taiho, C4 Therapeutics, and Janssen. Dr. Li reports receiving grants from Amgen, Asia Society, AstraZeneca, BeiGene, Bolt Biotherapeutics, Daiichi Sankyo, and Roche, and royalties from Karger Publishers and Shanghai Jiao Tong University Press Co., Ltd. Dr. Riely reports receiving research funding from Pfizer, Roche, Mirati, Novartis, Eli Lilly, Rain Therapeutics, Merck, and Takeda; receiving meeting/travel support from Bayer; and having DSMB/advisory board participation with Novartis. Dr. Pike reports receiving consulting fees from Aviko Inc., Monograph Capital, Galera Therapeutics, Turnstone Biologics, and Monte Rosa Therapeutics and holding stock ownership in Schrodinger, Clovis Oncology, and Novavax. Dr. Young reports receiving consulting fees from ICON plc and Olea Medical and lecture fees from fMRI Consultants. Dr. Moss reports receiving research funding from GT Medical Technologies and research funding and consulting fees from AstraZeneca. The remaining authors declare no conflict of interest.
Cite this article as: Imber BS, Sehgal R, Saganty R, et al. Intracranial outcomes of de novo brain metastases treated with osimertinib alone in patients with newly diagnosed EGFR-mutant NSCLC. JTO Clin Res Rep. 2023;4:100607.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2023.100607.
Supplementary Data
Supplementary Figure 1.
References
- 1.Kelly W.J., Shah N.J., Subramaniam D.S. Management of brain metastases in epidermal growth factor receptor mutant non-small-cell lung cancer. Front Oncol. 2018;8:208. doi: 10.3389/fonc.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han G., Bi J., Tan W., et al. A retrospective analysis in patients with EGFR-mutant lung adenocarcinoma: is EGFR mutation associated with a higher incidence of brain metastasis? Oncotarget. 2016;7:56998–57010. doi: 10.18632/oncotarget.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu F., De Caluwe A., Anderson D., Nichol A., Toriumi T., Ho C. EGFR mutation status on brain metastases from non-small cell lung cancer. Lung Cancer. 2016;96:101–107. doi: 10.1016/j.lungcan.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Ge M., Zhuang Y., Zhou X., Huang R., Liang X., Zhan Q. High probability and frequency of EGFR mutations in non-small cell lung cancer with brain metastases. J Neuro Oncol. 2017;135:413–418. doi: 10.1007/s11060-017-2590-x. [DOI] [PubMed] [Google Scholar]
- 5.Zhao W., Zhou W., Rong L., et al. Epidermal growth factor receptor mutations and brain metastases in non-small cell lung cancer. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.912505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suh J.H., Kotecha R., Chao S.T., Ahluwalia M.S., Sahgal A., Chang E.L. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17:279–299. doi: 10.1038/s41571-019-0320-3. [DOI] [PubMed] [Google Scholar]
- 7.Ballard P., Yates J.W.T., Yang Z., et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–5140. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 8.Reungwetwattana T., Nakagawa K., Cho B.C., et al. CNS response to Osimertinib versus Standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non–small-cell lung cancer. J Clin Oncol. 2018;36:3290–3297. doi: 10.1200/JCO.2018.78.3118. [DOI] [PubMed] [Google Scholar]
- 9.Goss G., Tsai C.M., Shepherd F.A., et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol. 2018;29:687–693. doi: 10.1093/annonc/mdx820. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi H., Wakuda K., Fukuda M., et al. A Phase II study of osimertinib for radiotherapy-naive central nervous system metastasis from NSCLC: results for the T790M cohort of the OCEAN study (LOGIK1603/WJOG9116L) J Thorac Oncol. 2021;16:2121–2132. doi: 10.1016/j.jtho.2021.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Erickson A.W., Brastianos P.K., Das S. Assessment of effectiveness and safety of osimertinib for patients with intracranial metastatic disease: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogelbaum M.A., Brown P.D., Messersmith H., et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40:492–516. doi: 10.1200/JCO.21.02314. [DOI] [PubMed] [Google Scholar]
- 13.Lin N.U., Lee E.Q., Aoyama H., et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16:e270–e278. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 14.Cheng D.T., Mitchell T.N., Zehir A., et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-Impact): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn JMD. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakravarty D., Gao J., Phillips S., et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;1:1–16. doi: 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peled N., Kian W., Inbar E., et al. Osimertinib in advanced EGFR-mutant lung adenocarcinoma with asymptomatic brain metastases: an open-label, 3-arm, phase II pilot study. Neuro-Oncol Adv. 2022;4:vdab188. doi: 10.1093/noajnl/vdab188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elder J.B., Nahed B.V., Linskey M.E., Olson J.J. Review and evidence-based guidelines on the role of emerging and investigational Therapties for the treatment of adults with metastatic brain tumors. Neurosurgery. 2019;84:E201–E203. doi: 10.1093/neuros/nyy547. [DOI] [PubMed] [Google Scholar]
- 18.Fong C.H., Meti N., Kruser T., et al. Recommended first-line management of asymptomatic brain metastases from EGFR mutant and ALK positive non-small cell lung cancer varies significantly according to specialty: an international survey of clinical practice. J Thorac Dis. 2023;15:4367–4378. doi: 10.21037/jtd-22-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horbinski C., Nabors L.B., Portnow J., et al. [NCCN guidelines]® insights: central nervous system cancers, version 2.2022. J Natl Compr Cancer Netw. 2023;21:12–20. doi: 10.6004/jnccn.2023.0002. [DOI] [PubMed] [Google Scholar]
- 20.Hui C., Qu V., Wang J.Y., et al. Local control of brain metastases with osimertinib alone in patients with EGFR-mutant non-small cell lung cancer. J Neurooncol. 2022;160:233–240. doi: 10.1007/s11060-022-04145-x. [DOI] [PubMed] [Google Scholar]
- 21.Leonetti A., Sharma S., Minari R., Perego P., Giovannetti E., Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725–737. doi: 10.1038/s41416-019-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnuson W.J., Lester-Coll N.H., Wu A.J., et al. Management of brain metastases in tyrosine kinase inhibitor–naïve epidermal growth factor receptor–mutant non–small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35:1070–1077. doi: 10.1200/JCO.2016.69.7144. [DOI] [PubMed] [Google Scholar]
- 23.Du X.J., Pan S.M., Lai S.Z., et al. Upfront cranial radiotherapy vs. EGFR tyrosine kinase inhibitors alone for the treatment of brain metastases from non-small-cell lung cancer: a meta-analysis of 1465 patients. Front Oncol. 2018;8:603. doi: 10.3389/fonc.2018.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C., Lu X., Lyu Z., Bi N., Wang L. Comparison of up-front radiotherapy and TKI with TKI alone for NSCLC with brain metastases and EGFR mutation: a meta-analysis. Lung Cancer. 2018;122:94–99. doi: 10.1016/j.lungcan.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Thomas N.J., Myall N.J., Sun F., et al. Brain metastases in EGFR- and ALK-positive NSCLC: outcomes of central nervous system-penetrant tyrosine kinase inhibitors alone versus in combination with radiation. J Thorac Oncol. 2022;17:116–129. doi: 10.1016/j.jtho.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Zeeshan Ozair M., Giantini Larsen A.M., Eng J., Moss N.S. Exceptional response of a large and symptomatic EGFR-mutant brain metastasis to osimertinib: case report and review of the literature. JCO Precis Oncol. 2021;5 doi: 10.1200/PO.20.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J.M., Miller J.A., Kotecha R., et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neuro Oncol. 2017;133:357–368. doi: 10.1007/s11060-017-2442-8. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang H., Tao L., Wang X., et al. Tyrosine kinase inhibitor resistance increased the risk of cerebral radiation necrosis after stereotactic radiosurgery in brain metastases of non-small-cell lung cancer: a multi-institutional retrospective case-control study. Front Oncol. 2020;10:12. doi: 10.3389/fonc.2020.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown P.D., Ballman K.V., Cerhan J.H., et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bander E.D., Yuan M., Reiner A.S., et al. Durable 5-year local control for resected brain metastases with early adjuvant SRS: the effect of timing on intended-field control. Neuro-Oncol Pract. 2021;8:278–289. doi: 10.1093/nop/npab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bander E.D., El Ahmadieh T.Y., Chen J., et al. Outcomes following early postoperative adjuvant radiosurgery for brain metastases. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.40654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y.S., Jiang B.Y., Yang J.J., et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11:1962–1969. doi: 10.1016/j.jtho.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Yang J.T., Wijetunga N.A., Pentsova E., et al. Randomized Phase II trial of proton craniospinal irradiation versus photon involved-field radiotherapy for patients with solid tumor leptomeningeal metastasis. J Clin Oncol. 2022;40:3858–3867. doi: 10.1200/JCO.22.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss N.S., El Ahmadieh T.Y., Brown S., et al. Integrated multidisciplinary brain metastasis care reduces patient visits and shortens time to adjuvant irradiation. JCO Oncol Pract. 2022;18:e1732–e1738. doi: 10.1200/OP.22.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moss N.S., Beal K., Tabar V. Brain metastasis-a distinct oncologic disease best served by an integrated multidisciplinary team approach. JAMA Oncol. 2022;8:1252–1254. doi: 10.1001/jamaoncol.2022.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim M.Y., Oskarsson T., Acharyya S., et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]