Abstract
Inflammation and social behavior deficits are associated with a number of neuropsychiatric disorders. Chronic stress, a major risk factor for depression and other mental health conditions is known to increase inflammatory responses and social behavior impairments. Disturbances in mitochondria function have been found in chronic stress conditions, however the mechanisms that link mitochondrial dysfunction to stress-induced social behavior deficits are not well understood. In this study, we found that chronic restraint stress (RS) induces significant increases in serum cell-free mitochondrial DNA (cf-mtDNA) levels in mice, and systemic Deoxyribonuclease I (DNase I) treatment attenuated RS-induced social behavioral deficits. Our findings revealed potential roles of mitophagy and Mitochondrial antiviral-signaling protein (MAVS) in mediating chronic stress-induced changes in cf-mtDNA levels and social behavior. Furthermore, we showed that inhibition of Toll-like receptor 9 (TLR9) attenuates mtDNA-induced social behavior deficits. Together, these findings show that cf-mtDNA-TLR9 signaling is critical in mediating stress-induced social behavior deficits.
Subject terms: Neuroscience, Physiology
Introduction
Stressful experiences are part of day-to-day life, but chronic stress conditions have negative impact on our health and well-being. Chronic stress has been implicated in the pathophysiology of many neuropsychiatric disorders, especially in anxiety, mood disorders and post-traumatic stress disorder (PTSD) [1, 2]. Significant impairments in social behaviors and reductions in social cognition and social motivation have been reported in these disorders [3, 4]. In addition, rodent studies have shown impairments in social behavior such as reductions in social interaction following chronic stress conditions [5–11]. Furthermore, chronic stress-induced changes in social behavior are associated with reductions in molecules that play important roles in excitation/inhibition balance, and synaptic function [12–15]. However, the mechanism that links chronic stress to social behavior deficits is not well understood. It is known that systemic administration of antigens results in range of behavioral and mood changes in humans [16–20]. In addition, chronic stress conditions are associated with increased inflammatory responses in both humans and animal models [20–28]. In rodents, chronic unpredictable stress [20, 21], chronic adolescent stress [22], and repeated social defeat [23] have been shown to induce increases in proinflammatory markers in the central nervous system (CNS) and periphery. Also, repeated social defeat resulted in the infiltration of peripheral monocytes into the brain which were associated with anxiety-like behavior in mice [26, 29, 30].
Stressful conditions are also known to alter mitochondrial components, mitochondrial energy production capacity and mitochondrial morphology [31–33]. The mitochondrial DNA (mtDNA) is deficient in histones and effective repair mechanisms and therefore, mtDNA may be more prone to stress-induced damages [34–37]. Accordingly, stress conditions promote mtDNA release from the mitochondria to the cytosol or extracellular space [35]. The circulating cell-free mtDNA (cf-mtDNA) is known to activate Toll-like receptor 9 (TLR9) signaling which results in the activation of several proinflammatory cytokines [38]. Chronic stress has been shown to increase blood mtDNA levels in mice [37, 39]. In clinical studies, increased levels of blood mtDNA have been found in individuals who had endured either parental loss or childhood maltreatment and with psychopathology including major depression [40]. Higher blood mtDNA have also been found in subjects with clinical levels of depressive symptoms [41–44] and in suicide attempters [45]. Since mtDNA is associated with inflammation [46–48], it is important to understand the role of mtDNA in chronic stress-induced inflammation and behavioral effects.
Under physiological conditions, mitochondrial removal and replenishment are well balanced to maintain the mitochondrial function [49]. Mitophagy, selective autophagy of mitochondria, is a cellular mechanism that eliminates damaged mitochondria. It is known that impaired mitophagy process leads to the release of mtDNA into the cytoplasm and out into the extracellular space resulting in an elevated release of proinflammatory cytokines [50–53]. Among the various mitophagy-related molecules that protect mitochondria from pathogens and damage, mitochondrial antiviral-signaling protein (MAVS) is an integral part of the cellular stress response [54]. While MAVS plays a key role in maintaining mitochondrial homeostasis via autophagy [55] and is involved in propagating antiviral responses in the brain [56, 57], chronic activation of this pathway can result in inflammation-driven pathology [58–61].
In the present study, we investigated the role of mtDNA in mediating chronic stress-induced increases in neuroinflammation and social behavioral deficits in mice. Also, we examined whether inhibition of MAVS and TLR9 pathways could attenuate stress-induced changes in social behavior.
Materials and methods
Animals
Adult male CD1 mice, male C57BL/6 J mice, male TLR9-/- mice (TLR9 KO; strain #034449), male and female MAVS-/- mice (MAVS KO; strain #008634) of 8 weeks old and their age-matched wild type (WT) controls were purchased from The Jackson Laboratory. Animals were housed in the animal facility at The University of Texas Health Science Center at Houston or Augusta University. Mice were housed and maintained (5 mice per cage) in standard polypropylene cages in a 12-h light-dark cycle in compliance with the US National Institute of Health guidelines, which was approved by The University of Texas Health Science Center at Houston and Augusta University animal welfare guidelines. Mice were assigned to experimental groups based on their genotype. Mice were selected randomly in a blinded manner to perform the experiments.
Restraint stress (RS) procedure
In the RS paradigm, mice were restrained in well-ventilated 50 ml Falcon tubes for 2 h/day for 21 consecutive days. Control mice were housed under normal conditions in the usual cages. Mice were tested for behavior the day following the last restraint session.
Deoxyribonuclease I (DNase I) and rapamycin (RAPA) treatment
Mice were intraperitoneally (i.p.) injected with DNase I (100U; Sigma–Aldrich, MO, USA), RAPA (10 mg/kg; Sigma–Aldrich) or vehicle (PBS) twice a week during the RS paradigm.
Mitochondrial DNA (mtDNA) purification and treatment
Mitochondria were isolated from the spleen of mice exposed to RS using a mitochondria isolation kit (#89801; Thermo Fisher Scientific, MA, USA) following the manufacturer’s instructions. mtDNA were purified from mitochondrial pellets using DNeasy blood and tissue kits (Qiagen, Hilden, Germany). The DNA concentration and purity were examined by spectrophotometry. The endotoxin levels in the DNA samples were determined using the Limulus amoebocyte lysate assay (Thermo Scientific). For mtDNA treatment, WT and TLR9 KO mice were injected once with mtDNA (30 µg/mouse; i.p.) or vehicle (PBS) and behavior tests were performed at 4 h later. The above mtDNA concentration has been shown to induce systemic inflammation [62].
Cell-free mitochondrial DNA (cf-mtDNA) analysis by qRT-PCR
Total DNA was isolated from 250 μl serum using DNA isolation/purification kits (DNeasy blood and Tissue kit; Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Quantity of mtDNA was measured by qRT-PCR by analyzing mitochondrially encoded 12 S ribosomal RNA and cytochrome c oxidase I (Cox1) genes. Primers were synthesized by Integrated DNA Technologies. MasterCycler (Eppendorf, Westburg, NY, USA) was used to perform qRT-PCR using iTaqTM Universal SYBR® Green Supermix (Bio-Rad, CA, USA). Mitochondrial DNA levels were adjusted for nuclear DNA levels using 18 S rRNA expression and analyzed using the ΔCT method [62, 63].
Behavioral tests
Mice were tested for behavior in a room with ambient temperature, lighting, pressure and sound. Mice were habituated to the behavior rooms in their home cages 1 h prior to the testing. All behavioral tests were performed and scored blind to the treatment.
Three-chamber test
The three-chamber test was performed using an apparatus made of clear Plexiglas® with dimension of 19 cm × 45 cm × 22 cm. Two openings provided access to each compartment. Two identical wire containers were placed in the two side chambers. Test mouse was placed vertically in the apparatus’s middle chamber and allowed to move freely for initial 5 min to get habituated. Age, sex, and background-matched stranger mouse was then introduced in the one of the wire containers. The ventilated wire container allowed air exchange, but prevented direct physical contact. The time spent by test mouse in the different chambers (stranger mouse chamber, middle chamber and empty chamber) was video recorded for another 5 min.
Reciprocal social interaction test
In this test, the test mouse was allowed to move freely across the entire 3-chamber apparatus to interact physically with the age, sex, and background-matched stranger mouse for 5 min. The interaction between the mice was defined as close physical contact, nose-to-nose sniffing, ano-genital sniffing, and grooming. Time of interaction (initiated by the test mouse only) was video recorded for 5 min and scored blindly.
Flow cytometry
For the flow cytometry analysis, mouse whole blood was collected in heparin tube (#366667; BD, NJ, USA) after decapitation under anesthesia. RBC lysis buffer was added to lyse RBC from the whole blood according to manufacturer’s protocol (#420301; BioLegend, CA, USA). Following washing with PBS, cells were incubated with flow antibodies, CD11b (clone M1/70, 1:300), Ly6C (clone HK1.4, 1:300) from BioLegend, CA, USA and TLR9 (clone 26C593.2, 1/300) from Novus Biologicals, LLC, CO, USA. Cells were washed with PBS and fixed with fixation buffer (Affymetrix eBioscience). Samples were run in BD FACSAriaII instrument and were analyzed using BD FACSDiva software (BD Biosciences, San Jose, CA). Fluorescence Minus One (FMO) controls were used to accurately identify the positive cell populations for each marker. Specific markers on the cells were reported as the percentage of the number of gated events. Median fluorescence intensity derived from a fluorescence graph was used to study the level of cell surface expression.
Quantitative reverse transcriptase PCR (qRT-PCR)
For the qRT-PCR analyses, the prefrontal cortex (PFC) tissues from mice were collected immediately following decapitation under anesthesia, according to a mouse brain atlas [21]. Total RNA from the PFC samples was isolated by using a commercially available kit (SV RNA Isolation, Promega, Madison, WI, USA). MasterCycler (Eppendorf, Westburg, NY, USA) was used to perform qRT-PCR using iTaqTM Universal SYBR® Green Supermix (Bio-Rad, CA, USA). Primers specific to genes were synthesized by Integrated DNA Technologies. Housekeeping gene (beta2-microglobulin (B2m)) was used to normalize the gene of interest. A list of primers used is given in Table S1.
Statistical analysis
No statistical methods were used to predetermine sample size, but our sample sizes were similar to those reported in previous study [28]. Data were presented as mean ± SEM. Statistical analysis were done using two-tailed Student’s t-tests to compare two-group or Analysis of Variance (ANOVA) for the multiple-group comparisons. Grubbs’ test was performed to identify the significant outlier by using GraphPad online calculator [https://www.graphpad.com/quickcalcs/Grubbs1.cfm]. Bonferroni’s post hoc test was performed using GraphPad Prism 9.0.0 and p < 0.05 was considered significant.
Results
DNase I treatment attenuates chronic stress-induced social behavior deficits and increased inflammatory markers in the PFC
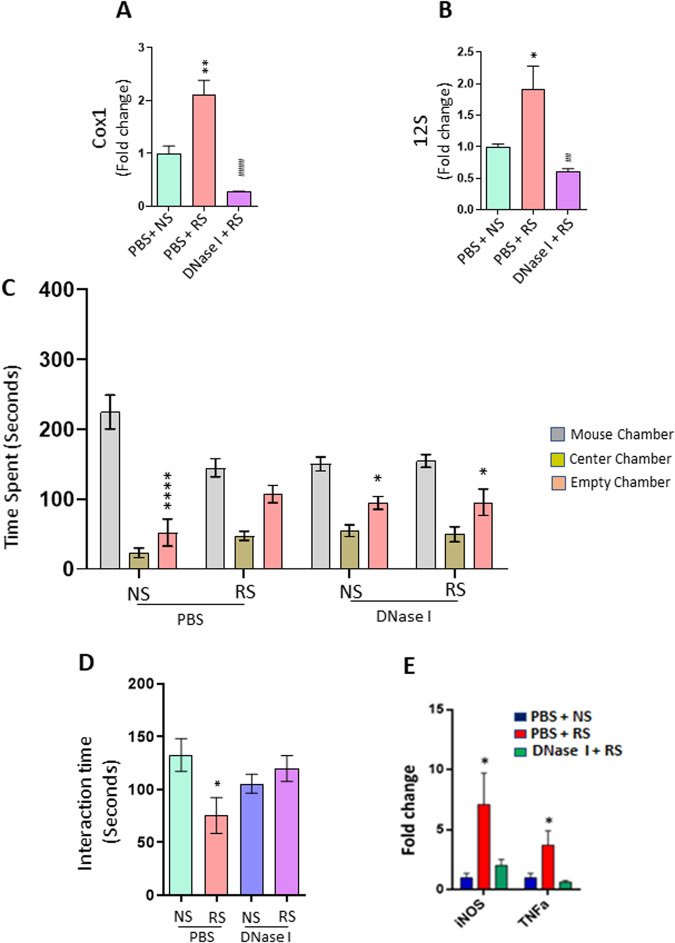
To determine the role of cf-mtDNA in mediating RS-induced social behavior deficits, we first measured the levels of cf-mtDNA in the serum of mice exposed to RS. The levels of two mtDNA genes, cytochrome c oxidase subunit I (Cox1) and mitochondrial 12 S rRNA gene (12 S) were examined. qPCR results showed significant increases in the expression of Cox1 and 12 S in the serum of mice exposed to RS (Fig. 1A, B). To examine the role of cf-mtDNA on RS-induced changes in social behavior, we performed three-chamber sociability and reciprocal social interaction tests in mice treated with DNase I during NS or RS exposure. DNase I is known to deplete cf-mtDNA [64, 65]. NS mice spent more time in the stranger mouse chamber than the empty cage chamber, whereas RS mice showed no preference for either chamber (Fig. 1C). However, RS mice injected with DNase I spent more time in the chamber housing stranger mouse than the empty cage chamber (Fig. 1C). In the reciprocal social interaction test, RS mice showed decreased interaction with a stranger mouse when compared with NS mice (Fig. 1D). Interestingly, treatment with DNase I significantly attenuated RS-induced decrease in interaction time in mice (Fig. 1D). Furthermore, we found that DNase I treatment significantly attenuated RS-induced increases in proinflammatory markers, iNOS and TNFα in the PFC, a key brain region implicated in social behavior (Fig. 1E). These results suggest that cf-mtDNA plays a critical role in RS-induced social behavior deficits and neuroinflammation.
Fig. 1. DNase I treatment attenuates chronic stress-induced social behavior deficits, and increased inflammatory markers in the PFC.
A, B Expression of serum mtDNA genes (Cox1 and 12 S) in no stress (NS) or restraint stress (RS) exposed mice treated with PBS or DNase I. Cox1; One-way ANOVA, **p < 0.01 (vs PBS + NS) and ####p < 0.0001 (vs PBS + RS), n = 4 per group. 12 S; One-way ANOVA, *p < 0.05 (vs PBS + NS) and ##p < 0.01 (vs PBS + RS), n = 4 per group. C, D DNase I treatment attenuated RS-induced deficits in social behavior. C Time in chamber in the three-chamber social interaction test. Two-way ANOVA, chamber (F (2, 81) = 87.81; p < 0.0001); interaction (chamber X treatment) (F (6, 81) = 6.174; p < 0.0001). *p < 0.05 and ****p < 0.0001 (mouse chamber vs empty chamber); n = 7–8 per group. D Reciprocal social interaction test; Two-way ANOVA, interaction (stress x treatment) (F (1, 23) = 6.575, p = 0.0173); *p < 0.05 vs PBS + NS; n = 6–7 per group. E mRNA expressions of proinflammatory cytokines in the PFC of RS mice treated with PBS or DNase I. iNOS; One-way ANOVA, *p < 0.05 (vs PBS + NS) n = 4–5 per group. TNFα; One-way ANOVA, *p < 0.05 (vs PBS + NS) n = 4–5 per group.
Rapamycin treatment attenuates chronic stress-induced increase in serum cf-mtDNA and social behavior deficits
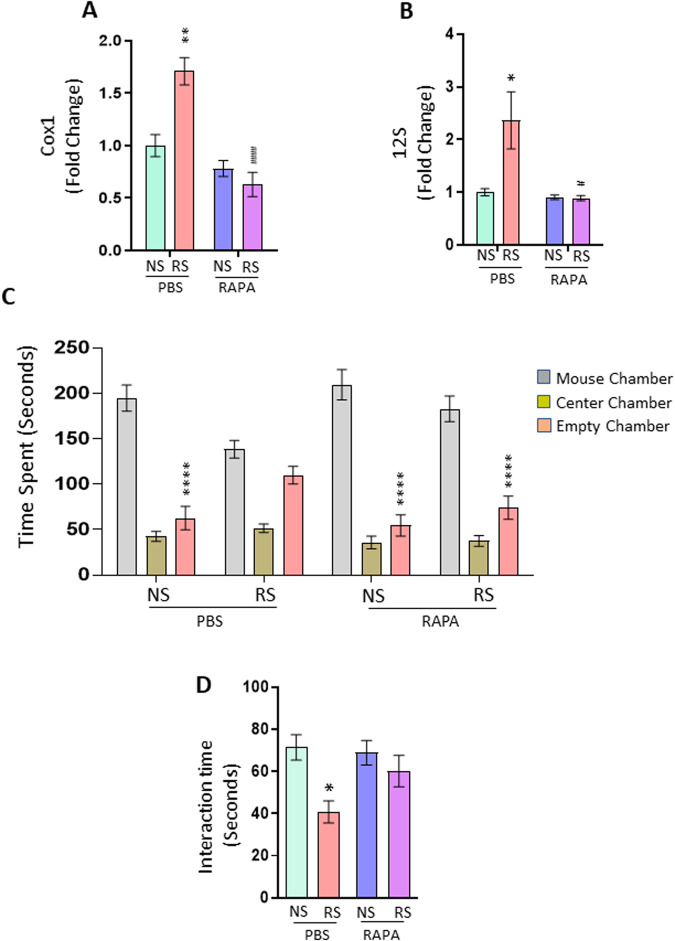
It is known that impaired mitophagy leads to the accumulation of dysfunctional mitochondria and mtDNA leakage to the cytosol whereas activation of mitophagy reduces inflammation by clearing damaged mitochondria [66]. Rapamycin (RAPA) is an activator of autophagy/mitophagy [67, 68]. Using the PBS-treated mice as control group, we examined whether RAPA treatment could attenuate RS-induced social behavior deficits and increases in cf-mtDNA levels. Gene expression data show that RAPA treatment significantly attenuated RS-induced increases in Cox1 and 12 S levels (Fig. 2A, B). To examine the effects of RAPA on chronic stress-induced changes in social behavior, we performed three-chamber sociability and reciprocal social interaction tests in mice treated with RAPA. RS mice injected with RAPA spent more time in the chamber housing stranger mouse than the empty cage chamber (Fig. 2C). Furthermore, RAPA treatment significantly attenuated RS-induced decrease in interaction time in mice (Fig. 2D).
Fig. 2. Rapamycin treatment attenuates chronic stress-induced increase in serum cf-mtDNA and behavior deficits.
A, B Expression of serum mtDNA genes (Cox1 and 12 S) in no stress (NS) or restraint stress (RS) exposed mice treated with PBS or Rapamycin (RAPA). Cox1; Two-way ANOVA, stress (F (1, 12) = 6.493, p = 0.0256); treatment (F (1, 12) = 35.32, p < 0.0001) and interaction (stress x treatment) (F (1, 12) = 15.55, P = 0.0019); **p < 0.01 (vs PBS + NS) and ####p < 0.0001 (vs PBS + RS), n = 4 per group. 12 S; Two-way ANOVA, stress (F (1, 12) = 5.953, p = 0.0312); treatment (F (1, 12) = 8.141, p = 0.0145) and interaction (stress x treatment) (F (1, 12) = 6.258, p = 0.0278); *p < 0.05 (vs PBS + NS) and #p < 0.05 (vs PBS + RS), n = 4 per group. C, D RAPA treatment attenuated RS-induced deficits in social behavior. C Time in chamber in the three-chamber social interaction test. Two-way ANOVA, chamber (F (2, 93) = 172.3, p < 0.0001); interaction (chamber X treatment) (F (6, 93) = 6.635, p < 0.0001). ****p < 0.0001 (mouse chamber vs empty chamber); n = 8–9 per group. D Reciprocal social interaction test; Two-way ANOVA, treatment (F (1, 31) = 8.976, p = 0.0053); *p < 0.05 vs PBS + NS; n = 8–9 per group.
Chronic stress-induced changes in cf-mtDNA levels and social behavior deficits are MAVS-dependent
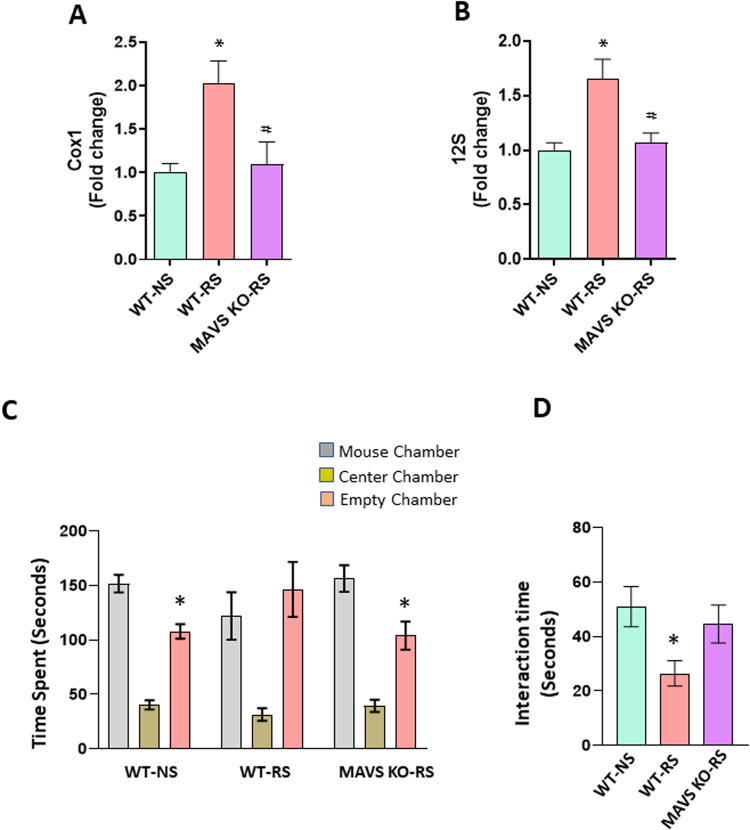
MAVS plays a key role in maintaining mitochondrial homeostasis via autophagy [55]. Activation of MAVS has been shown to recruit downstream signaling molecules and kinases, leading to the induction of type 1 interferon (IFN-I) proteins and other inflammatory cytokines [58–61, 69, 70]. Our earlier study has found significant increases in serum IFNβ levels in mice following RS, and systemic blockade of IFN-I signaling attenuated RS-induced social behavior deficits [28]. However, the role of MAVS in stress-induced changes in social behavior is not known. Here, we used MAVS KO mice to investigate MAVS function in RS-induced increases in cf-mtDNA levels and social behavior deficits. In male mice, MAVS deletion significantly attenuated RS-induced increases in serum Cox1 and 12 S levels (Fig. 3A, B). Furthermore, WT showed no preference for either chamber following RS, whereas MAVS KO mice exposed to RS showed a preference for the chamber housing stranger mouse than the empty cage chamber (Fig. 3C). Similarly, WT stressed mice showed decreased interaction with a stranger mouse compared to MAVS KO mice under RS exposure (Fig. 3D). Also, we found a significant attenuation in RS-induced social behavior deficits in female MAVS KO mice (Fig. S1). Overall, these results suggest that MAVS plays a key role in chronic stress-induced increases in cf-mtDNA levels and social behavior deficits.
Fig. 3. Chronic stress-induced changes in serum cf-mtDNA and social behavior deficits are MAVS-dependent.
A, B Expression of serum mtDNA genes (Cox1 and 12 S) in male wild type (WT) or MAVS KO mice exposed to no stress (NS) or restraint stress (RS). Cox1; One-way ANOVA, *p < 0.05 (vs WT-NS) and #p < 0.05 (vs WT-RS), n = 4 per group. 12 S; One-way ANOVA, *p < 0.05 (vs WT-NS) and #p < 0.05 (vs WT-RS), n = 4 per group. C, D MAVS deletion attenuated chronic stress-induced deficits in social behavior. C Time in chamber in the three-chamber social interaction test. Two-way ANOVA, chamber (F (2, 78) = 56.02, P < 0.0001); interaction (chamber X genotype) (F (4, 78) = 2.672, P = 0.0381). *p < 0.05 (mouse chamber vs empty chamber); n = 9–10 per group. D Reciprocal social interaction test; One-way ANOVA, *p < 0.05 vs WT-NS; n = 9–10 per group.
Chronic stress-stimulated mtDNA induces TLR9-dependent social behavior deficits
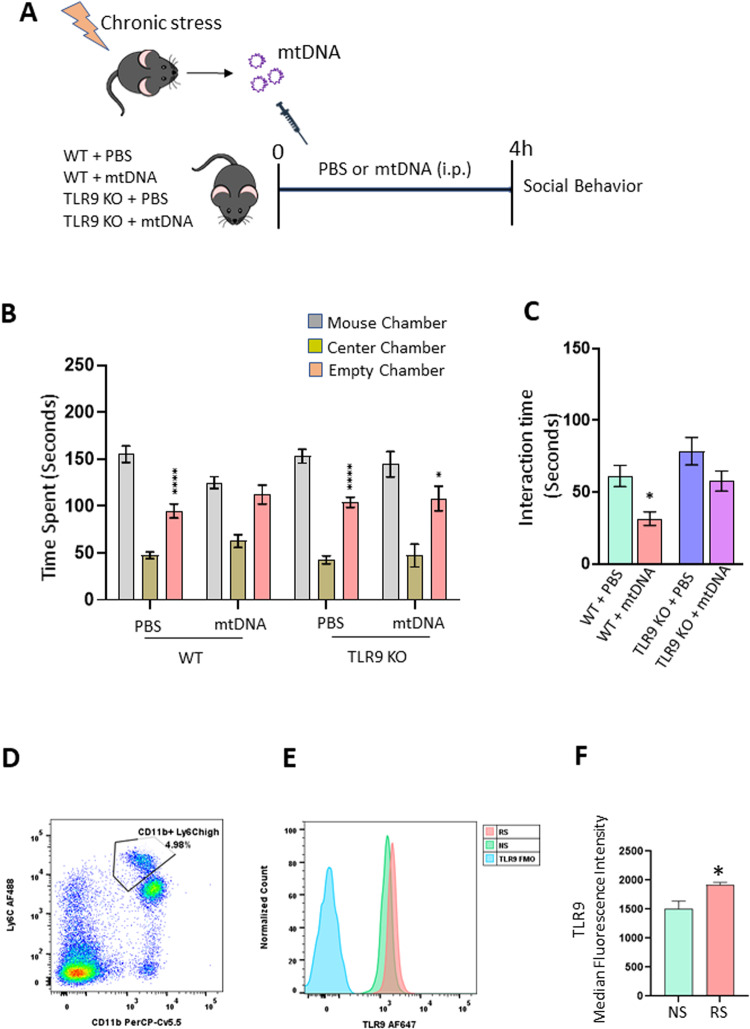
To determine if mtDNA is sufficient to cause social behavior deficits under chronic stress conditions, we administered chronic stress-stimulated mtDNA into control mice and social behavior was examined. For this, we have purified mtDNA from RS mice and injected it into WT control mice (Fig. 4A). We found that stress-exposed mtDNA was able to induce social behavior deficits in the three-chamber sociability test (Fig. 4B) and social interaction test (Fig. 4C) in control mice. mtDNA are recognized by TLR9 resulting in the activation of proinflammatory signaling pathways [38]. To determine the role of TLR9 in mediating mtDNA-induced social behavior deficits, mtDNA isolated from RS-exposed mice were administered to TLR9 KO mice (Fig. 4A). We found that the absence of TLR9 attenuated mtDNA-induced social behavior deficits (Fig. 4B, C). Using flow cytometry analysis, we found that TLR9 expression is increased in macrophages following RS (Fig. 4D–F).
Fig. 4. Chronic stress-stimulated mtDNA induces TLR9-dependent social behavior deficits.
A–C TLR9 deletion attenuated mtDNA-induced deficits in social behavior. A Experimental plan. B Time in chamber in the three-chamber social interaction test. Two-way ANOVA, chamber (F (2, 99) = 132.8, p < 0.0001); interaction (chamber X treatment) (F (6, 99) = 2.609, p = 0.0217). ****p < 0.0001 and *p < 0.05 (mouse chamber vs empty chamber); n = 7–10 per group. C Reciprocal social interaction test; Two-way ANOVA, treatment (F (1, 32) = 10.55, p = 0.0027), genotype (F (1, 32) = 7.809, p = 0.0087). *p < 0.05 vs WT-PBS; n = 6–10 per group. D, E Flow cytometry analysis showing increased TLR9 expression in blood macrophages of mice exposed to restraint stress (RS). D Gating strategy for flow cytometry analysis for CD11b+ Ly6Chigh cells. E TLR9 fluorescence intensity on gated CD11b+ Ly6Chigh cells in the blood of no stress (NS) and restraint stress (RS) mice. F Quantified TLR9 median fluorescence intensity represented in bar graph. Student’s t test; *p < 0.05 vs NS; n = 4 per group.
Discussion
Using restraint stress model, we found that chronic stress increases cf-mtDNA levels and inhibition of MAVS attenuates chronic stress-induced social behavior deficits in mice. Furthermore, we showed that TLR9 plays a critical role in mediating mtDNA-induced social behavior deficits.
Social behavioral impairments are a characteristic of a number of neurodevelopmental as well as neuropsychiatric disorders, including autism spectrum disorder (ASD), schizophrenia (SCZ), and major depressive disorder (MDD). Moreover, disturbances of mitochondrial function are known to cause social behavior deficits [71, 72]. Increased mitochondrial activity and the associated reduced GABAergic transmission have recently been shown to result in social behavioral deficits [73]. The high energy consumption by the brain requires mitochondria to mobilize the metabolites via the tricarboxylic acid cycle and ATP through oxidative phosphorylation [71, 72, 74]. Mitochondria play an important role in brain plasticity including neurogenesis and neurotransmission [74–79]. Therefore, any disturbance in mitochondrial homeostasis can influence the neuronal activity and brain function including social behavior.
Increased levels of plasma cf-mtDNA have been reported in suicide attempters [45] and major depressive disorder (MDD) subjects [41]. cf-mtDNA is a part of mitochondrial damage-associated molecular patterns (DAMPs) which are capable of eliciting inflammatory responses. A number of mechanisms of cf-DNA release from cells have been proposed which are categorized into two: cell death and active secretion. On the other hand, cf-mtDNA clearance is facilitated by either cf-mtDNA degradation by circulating DNases [80] or cf-mtDNA uptake by target cells [81]. mtDNA can elicit various proinflammatory signaling pathways by TLR9, cGAS-STING or inflammasome pathway depending on their localization. Cytoslic mtDNA is recognized by cGAS resulting in the activation of endoplasmic reticulum (ER)-localized STING and an interferon response. Similarly, cytosolic mtDNA stimulates inflammasome activity leading to increased levels of proinflammatory IL-1 and IL-8. On the other hand, mtDNA released into the blood is recognized by TLR9. We found that systemic reduction of cf-mtDNA using DNase I significantly blocked RS-induced neuroinflammation and social behavior deficits. Although we found an increase in cf-mtDNA following chronic stress exposure, the cellular source of the mtDNA is not known. Also, it is important to note that our findings on the effects of DNase I on social behavior were derived from experiments using one dose of DNase I. Future studies should examine the effects of additional doses of DNase I and other approaches to deplete mtDNA on social behavior.
In the present study, we have used the RS paradigm to investigate the effects of chronic stress on cf-mtDNA-mediated changes in inflammatory markers and social behavior. A number of previous studies have shown the effects of RS on anxiety and depression-like behaviors [82–85], brain connectivity [86, 87], hippocampal volume [88, 89], social behavior [28, 90] and cognitive functions [91–95] similar to those observed in depressed subjects [96]. In addition, RS exposure in rodents resulted in persistent low-grade inflammation, as shown by increases in peripheral levels of proinflammatory markers [28, 97, 98]. Although other chronic stress models such as chronic unpredictable stress and social defeat stress are known to induce neuroinflammation and behavioral abnormalities their effects on cf-mtDNA are not known.
Mitophagy is a key quality control mechanism that selectively eliminates dysfunctional mitochondria through autophagy [99]. Impairments in clearing defective mitochondria via mitophagy leads to the accumulation of mtDNA and subsequent proinflammatory response [100]. A number of studies have suggested a potential role of peripheral mtDNA in linking mitochondrial dysfunction to systemic inflammation [101]. Although the exact mechanism of mtDNA release into the circulation under chronic stress conditions is currently unknown, we believe that glucocorticoid signaling plays a causative role in stress-induced changes in mitochondrial homeostasis. In this regard, an in vitro study in primary human fibroblasts has found increase in mtDNA extrusion following dexamethasone treatment [102]. The impairments in the mitophagy process can lead to oxidative stress and reduced membrane potential [103]. The damaged mitochondria from defective mitophagy could result in the extrusion of components such as mtDNA into the cytosol and to the circulation leading to sustained inflammation. The effects of RAPA, a mammalian target of rapamycin (mTOR) inhibitor on social behavior has been tested in a number of studies using animal models of ASD. RAPA treatment has been shown to recover social deficits in Tsc mutant [104] and Cntnap2 −/− [105] mice. In addition, RAPA improved valproic acid-induced social deficits in mice [106]. Our data show a protective effect of RAPA against chronic stress-induced social behavior deficits. A number of studies have examined the effects of chronic stress on mTOR signaling. Chronic restraint stress (CRS) in rats for 6 h daily for 21 days has been shown to increase mTOR levels in the hippocampus [107]. However, another study in mice exposed to CRS for 4 h daily for 21 days found a decrease in the levels of phospho-mTOR/mTOR in the hippocampus [108]. Moreover, activation of the mTOR signaling pathway in the PFC plays an important role in the antidepressant action of ketamine [109]. On the other hand, inhibition of mTOR attenuated cognitive deficits and reduced amyloid-beta levels in a mouse model of Alzheimer’s disease [110]. These studies suggest that a balance between mTOR activation and inhibition is critical for neuroplasticity, and the timing and duration of stress play important role in the outcome.
MAVS is located on the outer membrane of mitochondria (OMM) and is known to promote proinflammatory signaling pathways following viral infections [60]. Specifically, MAVS activation results in mitochondrial dysfunction and subsequent release of ROS and mtDNA into the cytosol [111]. A number of mechanisms such as protein–protein interactions, mitochondrial dynamics and post-translational modifications have been implicated in the regulation of the expression and/or signaling of MAVS [112]. One such mitochondrial protein which has been identified as a negative regulator of MAVS is the nucleotide-binding domain and leucine-rich repeat containing family member, NLRX1 [113]. NLRX1 is present at the OMM and inhibits MAVS interactions with its signaling partners [114]. For example, NLRX1 negatively regulates infection-induced IFN-I signaling and IL-6 production in primary MEFs by inhibiting the interaction between MAVS and RIG-I [115]. It should be noted that molecules such as mitofusin 2 [116] and translocase of outer membrane 70 (Tom70) [117] have also been identified as MAVS interaction proteins. Although our study found an inhibition of stress-induced increases in cf-mtDNA levels and social behavior deficits in MAVS KO mice, additional studies are needed to elucidate the role of MAVS binding partners in mediating stress-induced behavioral changes.
TLRs play important role in regulating innate immunity and inflammation. Among the different TLRs, TLR9 is the only receptor for detecting DNA as well as oligodeoxynucleotides containing the CpG motifs and therefore, the cell-free DNA released from mitochondria which is rich in unmethylated CpGs can trigger inflammation via TLR9 [38]. mtDNA binds to TLR9 through a sequence specific binding to the N-terminal of the C-shaped leucine-rich repeat region of TLR9 [118]. Binding of DNA molecules to each monomer results in the dimerization of TLR9 [119] and subsequent interaction with myeloid differentiation primary response 88 (MYD88) adapter protein [47]. It is known that mitogen activated protein kinase (MAPK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathways participate in the TLR9-mediated transcription of proinflammatory cytokines [47]. Although most of the previous studies have investigated the role of TLR9 expressed in immune cells, it is also expressed in non-immune cells including neurons [120]. An earlier study has shown that TLR9 deficiency blocks chronic stress-induced changes of serum proinflammatory cytokines in mice [121]. Furthermore, a deficiency in TLR9 attenuated stress-enhanced corticosterone levels suggesting that TLR9 is necessary for the upregulation of the corticosterone in response to chronic stress [122].
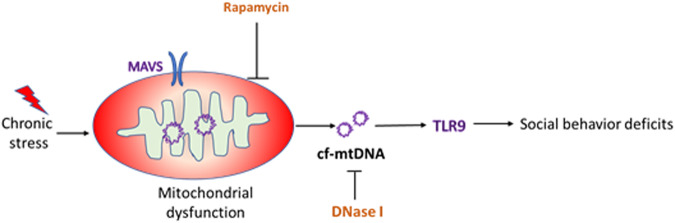
In summary, we uncovered a novel mechanism of chronic stress-induced social behavior deficits and our findings showed that cf-mtDNA-TLR9 signaling is critical in mediating stress-induced social behavior deficits (Fig. 5). A limitation of the present study is that we used global KO mice and therefore, the cell type-specific role of TLR9 or MAVS in mediating stress-induced behavior changes is not known. We are currently investigating the role of neuronal vs immune cell-specific function of TLR9 in neuroplasticity and behavior. Molecules such as MAVS and mTOR are the key mediators of stress-induced mitochondrial abnormalities and therefore, compounds that modulate either these pathways or TLR9 may represent potential therapeutic approaches to treat social behavior deficits seen in many neuropsychiatric conditions.
Fig. 5. cf-mtDNA-TLR9 signaling mediates chronic stress-induced social behavior deficits.
Mitochondrial antiviral-signaling protein (MAVS) plays a key role in maintaining mitochondrial homeostasis via autophagy. Rapamycin treatment and MAVS deletion attenuate chronic stress-induced increases in serum cf-mtDNA and social behavior deficits. In addition, Deoxyribonuclease I (DNase I) treatment attenuates chronic stress-induced social behavior deficits. mtDNA are recognized by Toll-like receptor 9 (TLR9) in macrophages resulting in the activation of proinflammatory signaling pathways and social behavior deficits.
Supplementary information
Acknowledgements
The authors acknowledge the funding support from US National Institute of Health/ National Institute of Mental Health (NIMH) grants (MH120876, MH121959 and MH128771), and the Merit Review Award (BX004758) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development to AP. The contents do not represent the views of the Department of Veterans Affairs or the United States Government. AP acknowledges the funding support from Louis A Faillace Endowed Chair in Psychiatry.
Author contributions
AP designed the research. AT, AB and CW performed the experiments and analyzed the data. AT prepared the initial manuscript draft. AP edited the manuscript. All authors had an opportunity to review and provide input on the final manuscript.
Competing interests
AP received pre-clinical research support from ACADIA Pharmaceuticals.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-023-02189-7.
References
- 1.Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–8. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66 Suppl 7:5–13. [PubMed] [Google Scholar]
- 4.Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16:559–72. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16:290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- 6.Beery AK, Kaufer D. Stress, social behavior, and resilience: insights from rodents. Neurobiol Stress. 2015;1:116–27.. doi: 10.1016/j.ynstr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Dawans B, Trueg A, Kirschbaum C, Fischbacher U, Heinrichs M. Acute social and physical stress interact to influence social behavior: the role of social anxiety. PLoS ONE. 2018;13:e0204665. doi: 10.1371/journal.pone.0204665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceccato S, Kettner SE, Kudielka BM, Schwieren C, Voss A. Social preferences under chronic stress. PLoS ONE. 2018;13:e0199528. doi: 10.1371/journal.pone.0199528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabanovic M, Liu H, Mlambo V, Aqel H, Chaudhury D. What it takes to be at the top: the interrelationship between chronic social stress and social dominance. Brain Behav. 2020;10:e01896. doi: 10.1002/brb3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchanan TW, Preston SD. Stress leads to prosocial action in immediate need situations. Front Behav Neurosci. 2014;8:5. doi: 10.3389/fnbeh.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrow AP, Bales NJ, Stover SA, Handa RJ. Chronic variable stress induces sex-specific alterations in social behavior and neuropeptide expression in the mouse. Endocrinology. 2018;159:2803–14.. doi: 10.1210/en.2018-00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo E, Sansing LH, Arnsten AFT, Datta D. Chronic stress weakens connectivity in the prefrontal cortex: architectural and molecular changes. Chronic Stress. 2021;5:24705470211029254. doi: 10.1177/24705470211029254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page CE, Coutellier L. Prefrontal excitatory/inhibitory balance in stress and emotional disorders: evidence for over-inhibition. Neurosci Biobehav Rev. 2019;105:39–51. doi: 10.1016/j.neubiorev.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Zheng Y, Yan J, Zhu C, Zeng X, Zheng S, et al. Early life stress induces different behaviors in adolescence and adulthood may related with abnormal medial prefrontal cortex excitation/inhibition balance. Front Neurosci. 2021;15:720286. doi: 10.3389/fnins.2021.720286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schedlowski M, Engler H, Grigoleit JS. Endotoxin-induced experimental systemic inflammation in humans: a model to disentangle immune-to-brain communication. Brain Behav Immun. 2014;35:1–8. doi: 10.1016/j.bbi.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24:558–63. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–9. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corona AW, Fenn AM, Godbout JP. Cognitive and behavioral consequences of impaired immunoregulation in aging. J Neuroimmune Pharmacol. 2012;7:7–23. doi: 10.1007/s11481-011-9313-4. [DOI] [PubMed] [Google Scholar]
- 20.Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, et al. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–20. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crider A, Feng T, Pandya CD, Davis T, Nair A, Ahmed AO, et al. Complement component 3a receptor deficiency attenuates chronic stress-induced monocyte infiltration and depressive-like behavior. Brain Behav Immun. 2018;70:246–56.. doi: 10.1016/j.bbi.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyter LM, Kelly SD, Harrell CS, Neigh GN. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain Behav Immun. 2013;30:88–94. doi: 10.1016/j.bbi.2013.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. beta-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–88. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- 25.McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP, et al. Sympathetic release of splenic monocytes promotes recurring anxiety following repeated social defeat. Biol Psychiatry. 2016;79:803–13.. doi: 10.1016/j.biopsych.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci. 2014;34:2583–91. doi: 10.1523/JNEUROSCI.3723-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schreiber G, Piehler J. The molecular basis for functional plasticity in type I interferon signaling. Trends Immunol. 2015;36:139–49. doi: 10.1016/j.it.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Tripathi A, Whitehead C, Surrao K, Pillai A, Madeshiya A, Li Y, et al. Type 1 interferon mediates chronic stress-induced neuroinflammation and behavioral deficits via complement component 3-dependent pathway. Mol Psychiatry. 2021;26:3043–59.. doi: 10.1038/s41380-021-01065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–33. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, et al. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry. 2014;75:970–81. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picard M, McEwen BS. Psychological stress and mitochondria: a conceptual framework. Psychosom Med. 2018;80:126–40.. doi: 10.1097/PSY.0000000000000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picard M, McEwen BS, Epel ES, Sandi C. An energetic view of stress: focus on mitochondria. Front Neuroendocrinol. 2018;49:72–85. doi: 10.1016/j.yfrne.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zemirli N, Morel E, Molino D. Mitochondrial dynamics in basal and stressful conditions. Int J Mol Sci. 2018;19:564. doi: 10.3390/ijms19020564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan SR, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci. 2004;359:109–21. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA. 1997;94:514–9. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Druzhyna NM, Wilson GL, LeDoux SP. Mitochondrial DNA repair in aging and disease. Mech Ageing Dev. 2008;129:383–90. doi: 10.1016/j.mad.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picard M, McEwen BS. Psychological stress and mitochondria: a systematic review. Psychosom Med. 2018;80:141–53.. doi: 10.1097/PSY.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimoto S, Fukuda D, Sata M. Emerging roles of Toll-like receptor 9 in cardiometabolic disorders. Inflamm Regen. 2020;40:18. doi: 10.1186/s41232-020-00118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai N, Chang S, Li Y, Li Q, Hu J, Liang J, et al. Molecular signatures of major depression. Curr Biol. 2015;25:1146–56. doi: 10.1016/j.cub.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS, et al. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiatry. 2016;79:78–86. doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindqvist D, Wolkowitz OM, Picard M, Ohlsson L, Bersani FS, Fernstrom J, et al. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology. 2018;43:1557–64.. doi: 10.1038/s41386-017-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung JK, Lee SY, Park M, Joo EJ, Kim SA. Investigation of mitochondrial DNA copy number in patients with major depressive disorder. Psychiatry Res. 2019;282:112616. doi: 10.1016/j.psychres.2019.112616. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Sundquist K, Rastkhani H, Palmer K, Memon AA, Sundquist J. Association of mitochondrial DNA in peripheral blood with depression, anxiety and stress- and adjustment disorders in primary health care patients. Eur Neuropsychopharmacol. 2017;27:751–8. doi: 10.1016/j.euroneuro.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Humphreys KL, Sisk LM, Manczak EM, Lin J, Gotlib IH. Depressive symptoms predict change in telomere length and mitochondrial DNA copy number across adolescence. J Am Acad Child Adolesc Psychiatry. 2020;59:1364–70 e2. doi: 10.1016/j.jaac.2019.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindqvist D, Fernstrom J, Grudet C, Ljunggren L, Traskman-Bendz L, Ohlsson L, et al. Increased plasma levels of circulating cell-free mitochondrial DNA in suicide attempters: associations with HPA-axis hyperactivity. Transl Psychiatry. 2016;6:e971. doi: 10.1038/tp.2016.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong F, Liang S, Zhong Z. Emerging Role of Mitochondrial DNA as a Major Driver of Inflammation and Disease Progression. Trends Immunol. 2019;40:1120–33.. doi: 10.1016/j.it.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Riley JS, Tait SW, Mitochondrial DNA. in inflammation and immunity. EMBO Rep. 2020;21:e49799. doi: 10.15252/embr.201949799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchi S, Guilbaud E, Tait SWG, Yamazaki T, Galluzzi L. Mitochondrial control of inflammation. Nat Rev Immunol. 2022;23,159–173. [DOI] [PMC free article] [PubMed]
- 49.Pickles S, Vigie P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 2018;28:R170–R85. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, et al. NF-kappaB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164:896–910. doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minton K. Inflammasome: anti-inflammatory effect of mitophagy. Nat Rev Immunol. 2016;16:206. doi: 10.1038/nri.2016.33. [DOI] [PubMed] [Google Scholar]
- 52.Zhong W, Rao Z, Xu J, Sun Y, Hu H, Wang P, et al. Defective mitophagy in aged macrophages promotes mitochondrial DNA cytosolic leakage to activate STING signaling during liver sterile inflammation. Aging Cell. 2022;21:e13622. doi: 10.1111/acel.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gkikas I, Palikaras K, Tavernarakis N. The role of mitophagy in innate immunity. Front Immunol. 2018;9:1283. doi: 10.3389/fimmu.2018.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ren Z, Ding T, Zuo Z, Xu Z, Deng J, Wei Z. Regulation of MAVS expression and signaling function in the antiviral innate immune response. Front Immunol. 2020;11:1030. doi: 10.3389/fimmu.2020.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun X, Sun L, Zhao Y, Li Y, Lin W, Chen D, et al. MAVS maintains mitochondrial homeostasis via autophagy. Cell Discov. 2016;2:16024. doi: 10.1038/celldisc.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hum NR, Bourguet FA, Sebastian A, Lam D, Phillips AM, Sanchez KR, et al. MAVS mediates a protective immune response in the brain to Rift Valley fever virus. PLoS Pathog. 2022;18:e1010231. doi: 10.1371/journal.ppat.1010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng J, Liao Y, Xiao L, Wu R, Zhao S, Chen H, et al. Autophagy regulates MAVS signaling activation in a phosphorylation-dependent manner in microglia. Cell Death Differ. 2017;24:276–87. doi: 10.1038/cdd.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–42. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Park S, Juliana C, Hong S, Datta P, Hwang I, Fernandes-Alnemri T, et al. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J Immunol. 2013;191:4358–66. doi: 10.4049/jimmunol.1301170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–61. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–82. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Deng S, Zhao S, Ai Y, Zhang L, Pan P, et al. Intra-peritoneal administration of mitochondrial DNA provokes acute lung injury and systemic inflammation via toll-like receptor 9. Int J Mol Sci. 2016;17:1425. doi: 10.3390/ijms17091425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiersma M, van Marion DMS, Bouman EJ, Li J, Zhang D, Ramos KS, et al. Cell-free circulating mitochondrial DNA: a potential blood-based marker for atrial fibrillation. Cells. 2020;9:1159. doi: 10.3390/cells9051159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weber C, Jenke A, Chobanova V, Yazdanyar M, Chekhoeva A, Eghbalzadeh K, et al. Targeting of cell-free DNA by DNase I diminishes endothelial dysfunction and inflammation in a rat model of cardiopulmonary bypass. Sci Rep. 2019;9:19249. doi: 10.1038/s41598-019-55863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ershova E, Sergeeva V, Klimenko M, Avetisova K, Klimenko P, Kostyuk E, et al. Circulating cell-free DNA concentration and DNase I activity of peripheral blood plasma change in case of pregnancy with intrauterine growth restriction compared to normal pregnancy. Biomed Rep. 2017;7:319–24. doi: 10.3892/br.2017.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez-Nuevo A, Zorzano A. The sensing of mitochondrial DAMPs by non-immune cells. Cell Stress. 2019;3:195–207. doi: 10.15698/cst2019.06.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villa-Cuesta E, Holmbeck MA, Rand DM. Rapamycin increases mitochondrial efficiency by mtDNA-dependent reprogramming of mitochondrial metabolism in Drosophila. J Cell Sci. 2014;127:2282–90. doi: 10.1242/jcs.142026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang J, Jiang J, Zuo Y, Gu Z. Rapamycin protects the mitochondria against oxidative stress and apoptosis in a rat model of Parkinson’s disease. Int J Mol Med. 2013;31:825–32. doi: 10.3892/ijmm.2013.1280. [DOI] [PubMed] [Google Scholar]
- 69.Fang R, Jiang Q, Zhou X, Wang C, Guan Y, Tao J, et al. MAVS activates TBK1 and IKKepsilon through TRAFs in NEMO dependent and independent manner. PLoS Pathog. 2017;13:e1006720. doi: 10.1371/journal.ppat.1006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–8. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 71.Hollis F, van der Kooij MA, Zanoletti O, Lozano L, Canto C, Sandi C. Mitochondrial function in the brain links anxiety with social subordination. Proc Natl Acad Sci USA. 2015;112:15486–91. doi: 10.1073/pnas.1512653112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hollis F, Kanellopoulos AK, Bagni C. Mitochondrial dysfunction in autism spectrum disorder: clinical features and perspectives. Curr Opin Neurobiol. 2017;45:178–87. doi: 10.1016/j.conb.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 73.Kanellopoulos AK, Mariano V, Spinazzi M, Woo YJ, McLean C, Pech U, et al. Aralar sequesters GABA into hyperactive mitochondria, causing social behavior deficits. Cell. 2020;180:1178–97 e20. doi: 10.1016/j.cell.2020.02.044. [DOI] [PubMed] [Google Scholar]
- 74.Vos M, Lauwers E, Verstreken P. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci. 2010;2:139. doi: 10.3389/fnsyn.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kann O, Kovacs R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–57. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 76.Khacho M, Slack RS. Mitochondrial dynamics in the regulation of neurogenesis: from development to the adult brain. Dev Dyn. 2018;247:47–53. doi: 10.1002/dvdy.24538. [DOI] [PubMed] [Google Scholar]
- 77.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Raefsky SM, Mattson MP. Adaptive responses of neuronal mitochondria to bioenergetic challenges: roles in neuroplasticity and disease resistance. Free Radic Biol Med. 2017;102:203–16. doi: 10.1016/j.freeradbiomed.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwarz TL. Mitochondrial trafficking in neurons. Cold Spring Harb Perspect Biol. 2013;5:a011304. doi: 10.1101/cshperspect.a011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–5. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zanini G, De Gaetano A, Selleri V, Savino G, Cossarizza A, Pinti M, et al. Mitochondrial DNA and exercise: implications for health and injuries in sports. Cells. 2021;10:2575. doi: 10.3390/cells10102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suvrathan A, Tomar A, Chattarji S. Effects of chronic and acute stress on rat behaviour in the forced-swim test. Stress. 2010;13:533–40. doi: 10.3109/10253890.2010.489978. [DOI] [PubMed] [Google Scholar]
- 83.Ulloa JL, Castaneda P, Berrios C, Diaz-Veliz G, Mora S, Bravo JA, et al. Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol Biochem Behav. 2010;97:213–21. doi: 10.1016/j.pbb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 84.Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:112–9. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 85.Bogdanova OV, Kanekar S, D’Anci KE, Renshaw PF. Factors influencing behavior in the forced swim test. Physiol Behav. 2013;118:227–39. doi: 10.1016/j.physbeh.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henckens MJ, van der Marel K, van der Toorn A, Pillai AG, Fernandez G, Dijkhuizen RM, et al. Stress-induced alterations in large-scale functional networks of the rodent brain. Neuroimage. 2015;105:312–22. doi: 10.1016/j.neuroimage.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 87.Seewoo BJ, Hennessy LA, Feindel KW, Etherington SJ, Croarkin PE, Rodger J. Validation of chronic restraint stress model in young adult rats for the study of depression using longitudinal multimodal mr imaging. eNeuro. 2020;7(4):ENEURO.0113-20.2020. [DOI] [PMC free article] [PubMed]
- 88.Alemu JL, Elberling F, Azam B, Pakkenberg B, Olesen MV. Electroconvulsive treatment prevents chronic restraint stress-induced atrophy of the hippocampal formation-A stereological study. Brain Behav. 2019;9:e01195. doi: 10.1002/brb3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee T, Jarome T, Li SJ, Kim JJ, Helmstetter FJ. Chronic stress selectively reduces hippocampal volume in rats: a longitudinal magnetic resonance imaging study. Neuroreport. 2009;20:1554–8. doi: 10.1097/WNR.0b013e328332bb09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park MJ, Seo BA, Lee B, Shin HS, Kang MG. Stress-induced changes in social dominance are scaled by AMPA-type glutamate receptor phosphorylation in the medial prefrontal cortex. Sci Rep. 2018;8:15008. doi: 10.1038/s41598-018-33410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y, Kan H, Yin Y, Wu W, Hu W, Wang M, et al. Protective effects of ginsenoside Rg1 on chronic restraint stress induced learning and memory impairments in male mice. Pharmacol Biochem Behav. 2014;120:73–81. doi: 10.1016/j.pbb.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 93.Huang P, Li C, Fu T, Zhao D, Yi Z, Lu Q, et al. Flupirtine attenuates chronic restraint stress-induced cognitive deficits and hippocampal apoptosis in male mice. Behav Brain Res. 2015;288:1–10. doi: 10.1016/j.bbr.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 94.Woo H, Hong CJ, Jung S, Choe S, Yu SW. Chronic restraint stress induces hippocampal memory deficits by impairing insulin signaling. Mol Brain. 2018;11:37. doi: 10.1186/s13041-018-0381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Y, Mao Y, Zhou D, Hu X, Wang J, Ma Y. Environmental enrichment and chronic restraint stress in ICR mice: effects on prepulse inhibition of startle and Y-maze spatial recognition memory. Behav Brain Res. 2010;212:49–55. doi: 10.1016/j.bbr.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 96.Wang Q, Timberlake MA, 2nd, Prall K, Dwivedi Y. The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:99–109. doi: 10.1016/j.pnpbp.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller ES, Apple CG, Kannan KB, Funk ZM, Plazas JM, Efron PA, et al. Chronic stress induces persistent low-grade inflammation. Am J Surg. 2019;218:677–83. doi: 10.1016/j.amjsurg.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu Y, Klomparens EA, Guo S, Geng X. Neuroinflammation caused by mental stress: the effect of chronic restraint stress and acute repeated social defeat stress in mice. Neurol Res. 2019;41:762–9. doi: 10.1080/01616412.2019.1615670. [DOI] [PubMed] [Google Scholar]
- 99.Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol. 2018;20:1013–22. doi: 10.1038/s41556-018-0176-2. [DOI] [PubMed] [Google Scholar]
- 100.Rai P, Roy JK. Rab11 regulates mitophagy signaling pathway of Parkin and Pink1 in the Drosophila model of Parkinson’s disease. Biochem Biophys Res Commun. 2022;626:175–86. doi: 10.1016/j.bbrc.2022.08.027. [DOI] [PubMed] [Google Scholar]
- 101.Newman LE, Shadel GS. Mitochondrial DNA release in innate immune signaling. Annu Rev Biochem. 2023;92,299–332. [DOI] [PMC free article] [PubMed]
- 102.Trumpff C, Marsland AL, Basualto-Alarcon C, Martin JL, Carroll JE, Sturm G, et al. Acute psychological stress increases serum circulating cell-free mitochondrial DNA. Psychoneuroendocrinology. 2019;106:268–76. doi: 10.1016/j.psyneuen.2019.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–30. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sato A, Kasai S, Kobayashi T, Takamatsu Y, Hino O, Ikeda K, et al. Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex. Nat Commun. 2012;3:1292. doi: 10.1038/ncomms2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xing X, Zhang J, Wu K, Cao B, Li X, Jiang F, et al. Suppression of Akt-mTOR pathway rescued the social behavior in Cntnap2-deficient mice. Sci Rep. 2019;9:3041. doi: 10.1038/s41598-019-39434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kotajima-Murakami H, Kobayashi T, Kashii H, Sato A, Hagino Y, Tanaka M, et al. Effects of rapamycin on social interaction deficits and gene expression in mice exposed to valproic acid in utero. Mol Brain. 2019;12:3. doi: 10.1186/s13041-018-0423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Polman JA, Hunter RG, Speksnijder N, van den Oever JM, Korobko OB, McEwen BS, et al. Glucocorticoids modulate the mTOR pathway in the hippocampus: differential effects depending on stress history. Endocrinology. 2012;153:4317–27. doi: 10.1210/en.2012-1255. [DOI] [PubMed] [Google Scholar]
- 108.Luo YF, Ye XX, Fang YZ, Li MD, Xia ZX, Liu JM, et al. mTORC1 signaling pathway mediates chronic stress-induced synapse loss in the hippocampus. Front Pharmacol. 2021;12:801234. doi: 10.3389/fphar.2021.801234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS ONE. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Valdes-Aguayo JJ, Garza-Veloz I, Badillo-Almaraz JI, Bernal-Silva S, Martinez-Vazquez MC, Juarez-Alcala V, et al. Mitochondria and mitochondrial DNA: key elements in the pathogenesis and exacerbation of the inflammatory state caused by COVID-19. Medicina. 2021;57:928. doi: 10.3390/medicina57090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jacobs JL, Coyne CB. Mechanisms of MAVS regulation at the mitochondrial membrane. J Mol Biol. 2013;425:5009–19. doi: 10.1016/j.jmb.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–7. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 114.Refolo G, Vescovo T, Piacentini M, Fimia GM, Ciccosanti F. Mitochondrial interactome: a focus on antiviral signaling pathways. Front Cell Dev Biol. 2020;8:8. doi: 10.3389/fcell.2020.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34:854–65. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yasukawa K, Oshiumi H, Takeda M, Ishihara N, Yanagi Y, Seya T, et al. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci Signal. 2009;2:ra47. doi: 10.1126/scisignal.2000287. [DOI] [PubMed] [Google Scholar]
- 117.Liu XY, Wei B, Shi HX, Shan YF, Wang C. Tom70 mediates activation of interferon regulatory factor 3 on mitochondria. Cell Res. 2010;20:994–1011. doi: 10.1038/cr.2010.103. [DOI] [PubMed] [Google Scholar]
- 118.Ohto U, Shibata T, Tanji H, Ishida H, Krayukhina E, Uchiyama S, et al. Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9. Nature. 2015;520:702–5. doi: 10.1038/nature14138. [DOI] [PubMed] [Google Scholar]
- 119.Ohto U, Ishida H, Shibata T, Sato R, Miyake K, Shimizu T. Toll-like receptor 9 contains two DNA binding sites that function cooperatively to promote receptor dimerization and activation. Immunity. 2018;48:649–58 e4. doi: 10.1016/j.immuni.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 120.Shintani Y, Drexler HC, Kioka H, Terracciano CM, Coppen SR, Imamura H, et al. Toll-like receptor 9 protects non-immune cells from stress by modulating mitochondrial ATP synthesis through the inhibition of SERCA2. EMBO Rep. 2014;15:438–45. doi: 10.1002/embr.201337945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xiang Y, Yan H, Zhou J, Zhang Q, Hanley G, Caudle Y, et al. The role of toll-like receptor 9 in chronic stress-induced apoptosis in macrophage. PLoS ONE. 2015;10:e0123447. doi: 10.1371/journal.pone.0123447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li H, Zhao J, Chen M, Tan Y, Yang X, Caudle Y, et al. Toll-like receptor 9 is required for chronic stress-induced immune suppression. Neuroimmunomodulation. 2014;21:1–7. doi: 10.1159/000354610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.