Abstract
Background and Objectives
There is a lack of well-controlled US studies of intramuscular (IM) interferon beta (IFNβ)-1a use in pregnant women with multiple sclerosis; however, in the European Medicines Agency region, IFNβ formulations may be considered during pregnancy if clinically needed based on data from European Union cohort registries. The AVONEX Pregnancy Exposure Registry was established to prospectively study the effects of IM IFNβ-1a on the risk of birth defects and spontaneous pregnancy loss in a US population.
Methods
Pregnant women with multiple sclerosis exposed to IM IFNβ-1a within ~ 1 week of conception or during the first trimester were included. Participants were followed until there was a pregnancy outcome, live-born infants were followed until age 8–12 weeks. Data were collected on IM IFNβ-1a exposure, demographics, patient characteristics, medical history, and pregnancy outcomes, including live births (with or without birth defect), spontaneous abortions/miscarriages and fetal death/stillbirth, elective abortions (with and without birth defect), and ectopic pregnancies. A population-based birth defect surveillance program, the Metropolitan Atlanta Congenital Defects Program (MACDP), served as the primary external control group for evaluating the risk of birth defects.
Results
Three-hundred and two patients with a median (range) age of 31.0 (16–48) years and a median (range) gestational age at the time of enrollment of 10.1 (4–39) weeks were evaluable. Most patients (n = 278/302; 92%) reported IM IFNβ-1a exposure in the week before conception and most (n = 293/302; 97%) discontinued treatment before the end of the first trimester. Of 306 pregnancy outcomes, there were 272 live births, 28 spontaneous abortions of 266 pregnancies enrolled before 22 weeks’ gestation (rate 10.5%; 95% confidence interval 7.2–15.0), five elective abortions, and one stillbirth. There were 17 adjudicator-confirmed major birth defects of 272 live births (rate 6.3%; 95% confidence interval 3.8–10.0); the pattern of birth defects observed was not suggestive of a relationship to prenatal IM IFNβ-1a exposure.
Conclusions
This large US registry study suggests IM IFNβ-1a exposure during early pregnancy was not clinically associated with adverse pregnancy outcomes in women with multiple sclerosis. These findings help inform clinicians and patients in weighing the risks and benefits of IM IFNβ-1a use during pregnancy.
Clinical Trial Registration
ClinicalTrials.gov: NCT00168714, 15 September, 2005.
Key Points
| This national registry is the largest study to date to focus exclusively on the effects of intramuscular interferon beta-1a exposure in women with multiple sclerosis who are pregnant. |
| Results from this national registry suggest that intramuscular interferon beta-1a exposure within ~ 1 week of conception or during the first trimester of pregnancy is not clinically associated with adverse pregnancy outcomes. |
| Rates of spontaneous abortion were consistent with previous findings and no patterns of birth defects suggestive of an unusual distribution were observed. |
Introduction
The prevalence of multiple sclerosis (MS) is up to three times higher in women than in men [1–3], and the majority of women affected are of childbearing age [4]. Following the introduction of disease-modifying therapies (DMTs), the management of pregnancy in MS has changed over the last 2 decades in that women with MS are no longer discouraged from planning a family [4, 5]. Disease-modifying therapies are therefore likely to be widely used by women of childbearing potential. Intramuscular (IM) interferon beta-1a (IFNβ-1a) is indicated for the treatment of patients with relapsing forms of MS to slow the accumulation of physical disability and reduce the frequency of clinical exacerbations [6]. Having been commercially available for over 25 years as one of the first DMTs for MS, it has an established safety profile; however, there have been no adequate and well-controlled studies conducted in the USA with IM IFNβ-1a in pregnant women with MS.
In the US Food and Drug Administration (FDA) prescribing information, there is no clear recommendation for IM IFNβ-1a use during pregnancy. In 2020, the wording in the FDA prescribing information was also updated for IFNβ, indicating there is no increased risk with early pregnancy exposure in humans, “The majority of observational studies reporting on pregnancies exposed to interferon beta products did not identify an association between the use of interferon beta products during early pregnancy and an increased risk of major birth defects [6].” This recommendation is primarily based on two large epidemiological studies in Europe and Nordic regions of pregnancies exposed to IFNβ products [7–10], which led to the European Medicines Agency updated guidance allowing the use of IFNβ to be considered prior to conception, during pregnancy, and while breastfeeding if clinically needed [11, 12].
The AVONEX® Pregnancy Exposure Registry was established to study the effects of IM IFNβ-1a exposure during pregnancy in a US population, as mandated by the FDA. The aims of this prospective, longitudinal, observational, exposure-registration follow-up study were to detect any evidence of teratogenic effects in pregnancies inadvertently or intentionally exposed to IM IFNβ-1a, and to assess any potential increase in the risk of spontaneous loss.
Methods
The study population included pregnant women with MS exposed to IM IFNβ-1a within ~ 1 week of conception or during the first trimester of pregnancy. A participant was eligible if: the outcome of the pregnancy was unknown at the time of enrollment; she provided verbal consent to participate; and she provided contact information for herself, her healthcare provider (HCP), and the infant’s HCP (if applicable). Women were excluded if they were outside of the USA or defined as being reported retrospectively. The sample size calculations assumed 300 pregnancy exposures would result in 148 live births (assuming 20% were lost to follow-up and 62% of the clinically recognized pregnancies would result in live births), providing 80% power to detect an assumed relative risk (birth defect rate) detected of ≥ 2.75 (7.3%).
Participants were followed throughout pregnancy until there was an outcome; for pregnancies resulting in live births, the infants were followed until 8–12 weeks of age. Information on IM IFNβ-1a exposure, demographics, patient characteristics, medical history, and pregnancy outcomes was collected via telephone contact with participants at enrollment, mid-pregnancy (20–25 weeks’ gestation), and at 8–12 weeks after the estimated date of delivery [6]. A participant’s obstetric HCP was contacted after she provided information as to the pregnancy outcome and the infant’s pediatric HCP was contacted 8–12 weeks after the outcome. Evaluations of pregnancy outcomes included: live births (with or without birth defect); fetal loss (spontaneous abortions/miscarriages [loss occurring < 22 weeks gestation] and fetal death/stillbirth [loss occurring ≥ 22 weeks gestation; with and without birth defect]); elective abortions (with and without birth defects); and ectopic pregnancies. Measurements for live-born infants included: gestational age at outcome; birth weight; sex; head circumference at birth; length at birth; and Apgar scores at 1 and 5 min of age.
Methods used to facilitate enrollment included IM IFNβ-1a package inserts, informational letters, brochures, and digital and social outreach to raise awareness of the registry by participants and their HCPs. Data were monitored by an independent scientific advisory committee comprising four external experts in the specialties of teratology, epidemiology, maternal and fetal medicine, and neurology. The Metropolitan Atlanta Congenital Defects Program (MACDP), a population-based birth defect surveillance program administered by the Centers for Disease Control and Prevention [13, 14], was adopted as the primary external control group for evaluating the risk of birth defects. All birth defects were reviewed by a geneticist and coded according to the MACDP [15]. Each birth defect was assigned an organ system classification to facilitate identification of potential signals by grouping together similar defects [16], so that any potential causes for concern could be determined. For consistency with the MACDP [14, 17], the birth defect rate was calculated by dividing the number of outcomes with birth defects (i.e., live births and all fetal losses occurring at gestation ≥ 20 weeks with defects) by the total number of live births in the analysis population. The rate of spontaneous abortions was calculated by dividing the number of spontaneous abortions by the total number of pregnancies in the evaluable population that were enrolled prior to 22 weeks gestation. The rate of fetal deaths was calculated by dividing the number of fetal deaths by the sum of live births plus fetal deaths in the evaluable population. Calculations of 95% confidence intervals (CIs) were based on methods described previously [18].
The primary analysis population consisted of the evaluable population, also termed the evaluable population, consisted of patients with HCP-confirmed information on IM IFNβ-1a exposure and pregnancy outcomes who were prospectively enrolled in the registry between 3 March, 2004 and 8 September, 2011. A report of a pregnancy was considered closed when clear information on the exposure and the pregnancy outcome was obtained.
Standard Protocol Approvals, Registrations, and Patient Consent
The registry obtained central institutional review board approval for the study protocol and all amendments, which were performed in accordance with FDA guidance “Establishing Pregnancy Exposure Registries,” [19] the International Society for Pharmacoepidemiology’s Guidelines for Good Pharmacoepidemiology Practices [20], and the ethical principles outlined in the Declaration of Helsinki [21].
Verbal informed consent was obtained from participants in accordance with local practice and regulations. During the initial telephone encounter, participants were asked to provide verbal consent to enroll and to provide authorization for their HCPs, including their infant’s pediatrician, to release medical information to the registry.
Results
Participants
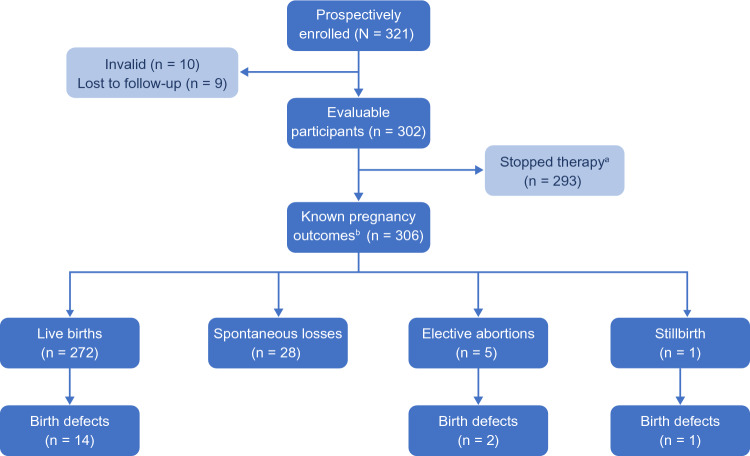
A total of 321 participants were prospectively enrolled in the registry between 3 March, 2004 and 8 September, 2011. Of these, 302 met the minimum criteria for enrollment with clear exposure and outcome information confirmed by their HCPs, and therefore comprised the evaluable population; nine (2.8%) participants were lost to follow-up, and ten (3.1%) were deemed invalid (Fig. 1). The median age of the evaluable population was 31.0 years (range 16–48 years), and 81 (26.8%) participants were aged > 35 years. The median gestational age at the time of enrollment was 10.1 weeks (range 4–39 weeks) (Table 1). Most participants (n = 278; 92.1%) reported exposure to IM IFNβ-1a in the week prior to conception; 24 (7.9%) reported that the earliest pregnancy exposure began in the first trimester. The majority of women (n = 293; 97%) discontinued IM IFNβ-1a before the end of their first trimester; five (1.7%) continued IM IFNβ-1a into the second trimester, and four (1.3%) continued into the third trimester (Table 1). The median maternal age at the time of stopping IM IFNβ-1a exposure was 31.0 years (range 16─48 years). The mean [standard deviation (SD)] duration of IM IFNβ-1a exposure during pregnancy was 5.1 (5.1) weeks (range: the week prior to conception to 40 weeks).
Fig. 1.
Registry enrollment and outcomes. aThe Stopped therapy population included all participants in the evaluable population who discontinued intramuscular (IM) interferon beta-1a (IFNβ-1a) therapy within the first trimester of pregnancy, defined as prior to gestational week 14. b306 pregnancy outcomes were reported in the evaluable population, which included four sets of twins (eight infants) and 298 singleton infants
Table 1.
Participant characteristics at the time of enrollment and duration of IM IFNβ-1a exposure
| Characteristic | Evaluable patients (N = 302) |
|---|---|
| Duration of MSa, years | |
| Mean (SD) | 4.6 (4.25) |
| Median (range) | 3.6 (0–24) |
| Age at enrollment, years | |
| Mean (SD) | 31.7 (5.57) |
| Median (range) | 31.0 (16–48) |
| Age category at enrollment, years, n (%) | |
| < 25 | 39 (12.9) |
| 25 to ≤ 35 | 182 (60.3) |
| > 35 | 81 (26.8) |
| Race/ethnicity, n (%) | |
| White | 196 (64.9) |
| Black | 70 (23.2) |
| Hispanic | 24 (7.9) |
| Asian | 2 (0.7) |
| Other | 10 (3.3) |
| Gestational week at enrollment | |
| Mean (SD) | 12.6 (7.4) |
| Median (range) | 10.1 (4–39) |
| Duration of IM IFNβ-1a exposure during pregnancy,b weeks | |
| Mean (SD) | 5.1 (5.1) |
| Median (range) | 4.1 (0–40) |
| Earliest exposure of IM IFNβ-1a during pregnancy, n (%) | |
| ≤ 1 week prior to conception | 278 (92.1) |
| First trimester | 24 (7.9) |
| Latest exposure of IM IFNβ-1a during pregnancy, n (%) | |
| ≤ 1 week prior to conception | 6 (2.0) |
| First trimester | 287 (95) |
| Second trimester | 5 (1.7) |
| Third trimester | 4 (1.3) |
IFNβ-1a interferon beta-1a, IM intramuscular, MS multiple sclerosis, SD standard deviation
aOne patient was diagnosed with clinically isolated syndrome, for which she was being treated with IM IFNβ-1a (301/302 patients diagnosed with MS)
bPatients with duration of exposure = zero ended therapy ≤ 1 week prior to conception
Most participants (n = 204; 67.5%) had experienced a prior pregnancy (Table 2). Three (1.5%) reported a previous pregnancy ending in fetal death/stillbirth; 64 (31.4%) reported a prior history of one or more spontaneous abortions. Only two (1.0%) participants reported a history of offspring with birth defects (one spina bifida; one infant death at 6 weeks of age related to gangrenous bowel).
Table 2.
Maternal history of participants
| Maternal history, n (%) | Patients |
|---|---|
| Number of previous pregnancies | (N = 302) |
| 0 | 98 (32.5) |
| 1 | 86 (28.5) |
| 2 | 53 (17.5) |
| 3 | 31 (10.3) |
| 4 | 19 (6.3) |
| ≥ 5 | 15 (5.0) |
| Number of previous live infants of those with a prior pregnancy | (N = 204) |
| 0 | 34 (16.7) |
| 1 | 91 (44.6) |
| 2 | 46 (22.6) |
| 3 | 18 (8.8) |
| 4 | 9 (4.4) |
| ≥ 5 | 6 (2.9) |
| Number of previous spontaneous abortions of those with a prior pregnancy | (N = 204) |
| 0 | 140 (68.6) |
| 1 | 46 (22.5) |
| 2 | 12 (5.9) |
| 3 | 6 (2.9) |
| 4 | 0 |
| ≥ 5 | 0 |
| Number of previous fetal deaths of those with a prior pregnancy | (N = 204) |
| 0 | 201 (98.5) |
| 1 | 3 (1.5) |
| 2 | 0 |
| 3 | 0 |
| 4 | 0 |
| ≥ 5 | 0 |
Pregnancy Outcomes
A total of 306 pregnancy outcomes were reported, including four sets of twins and 298 singleton infants: 272 live births, 28 spontaneous abortions, five elective abortions, and one stillbirth (Fig. 1). There were 266 pregnancies enrolled before 22 weeks’ gestation, giving a spontaneous abortion rate of 10.5% (95% CI 7.2–15.0). The one fetal death/stillbirth reported in this registry occurred at 32.4 weeks gestation; the fetal death rate was 0.4% (95% CI 0.0–2.3), calculated based on the sum of all evaluable live births and fetal deaths (n = 273).
Birth Defects
There were 16 prenatal tests suggesting a possible malformation, 23 birth defects of the live births, three elective abortions (two at ≥ 20 weeks gestation), and one fetal death/stillbirth reported by the HCP. Of those, advisory committee-confirmed major birth defects were reported in 17 outcomes: 14 in live-born infants, two in elective abortions ≥ 20 weeks gestation, and one in a fetal death/stillbirth (Fig. 1; Table 3). In the unconfirmed cases, the geneticist did not have sufficient information for confirmation of the defect. Clusters were reported for hypospadias (three live-born male infants), Down syndrome (one live-born infant and one stillborn infant), and Marcus Gunn syndrome (two live-born infants). None of these were judged as temporally related to IM IFNβ-1a exposure by the geneticist or were of a known cause and therefore temporality was irrelevant; both Down syndrome cases listed advanced maternal age as a confounding factor. The remaining birth defects were diverse in organ system classification; several of these defects were not temporally related to prenatal IM IFNβ-1a exposure as judged by the geneticist based on the intersection of the timing of IM IFNβ-1a exposure and the development of the structure impacted (Table 3). Of the nine pregnancies with second-trimester or third-trimester exposure, one had a reported birth defect (diaphragmatic hernia). The MACDP reports a birth defect prevalence rate of 2.67% among live-born infants in the MACDP population [14, 17]; the prevalence of geneticist-confirmed birth defects in the evaluable population in this registry was 17/272 (6.3% [95% CI 3.8–10.0]).
Table 3.
Advisory committee-confirmed clinically major birth defectsa
| Birth defect/s | Maternal age, years | Pregnancy outcome | Gestational age,b weeks | Duration of IM IFNβ-1a exposure during pregnancy (weeks) | Confounding factors | Temporally attributed to IM IFNβ-1a exposure? |
|---|---|---|---|---|---|---|
| Hypospadias | 35 | Live birth | 34.1 | 4.1 | – | No |
| Hypospadias | 27 | Live birth | 40.1 | 3.4 | – | No |
| Hypospadias | 36 | Live birth | 36.6 | 6.0 | – | No |
| Down syndrome | 35 | Live birth | 38.4 | 4.1 | Advanced maternal age | No known causec |
| Down syndrome | 39 | Stillbirth | 32.4 | 6.1 | Advanced maternal age | No known causec |
| Marcus Gunn syndrome | 32 | Live birth (twin) | 31.4 | 1.3 | – | No |
| Marcus Gunn syndrome | 30 | Live birth | 39.4 | 2.6 | – | No known causec |
| Amniotic band syndrome | 29 | Live birth | 27.9 | 5.6 | – | Unknown |
| Bilateral hearing loss | 33 | Live birth | 39.1 | 2.0 | – | Unknown |
| Sensory hearing loss | 34 | Live birth | 38.1 | 1.0 | – | No |
| Club feet | 36 | Live birth | 37.1 | 4.1 | – | Unknown |
| Diaphragmatic hernia | 26 | Live birth | 41.0 | 18.3 | – | Unknown |
| Hydronephrosis | 22 | Live birth | 33.1 | 0.6 | – | No |
| Interrupted aortic arch and esophagus | 32 | Live birth | 39.9 | 3.9 | – | Unknown |
| Pyloric stenosis | 36 | Live birth | 39.3 | 4.3 | – | Unknown |
| Spina bifida | 28 | Elective abortion | 23.4 | 5.0 | – | Unknown |
| Trisomy 8 mosaicism | 30 | Elective abortion | 22.1 | 4.0 | – | Unknown |
IFNβ-1a interferon beta-1a, IM intramuscular
aConfirmed clinically major birth defects in live births and fetal losses > 20 weeks’ gestation
bGestational age at identification of the birth defect
cGeneticist coded the defect as a defect with a known cause; therefore, temporality may be irrelevant. Shaded rows represent birth defects clustered in cases of ≥ 2
Characteristics of Live Births
There were 272 live births in the evaluable population; mean (SD) gestational age of mothers at birth was 38.3 (3.0) years (Table 4). Mean (SD) birth weight was 3169 (689) g; 92% of babies were of normal birth weight, 5% low birth weight (< 2500 g), and 3% very low birth weight (< 1500 g). Mean (SD) Apgar scores were 8.0 (1.6) at 1 minute and 8.8 (1.0) at 5 minutes. In singletons born without geneticist-confirmed birth defects, the majority (92%) of births were full term (≥ 36 weeks gestation), 4.5% were preterm (< 36 weeks gestation), and 3.7% were very preterm (< 32 weeks gestation). Median (range) head circumference at birth was 34.0 cm. For reference, median head circumference at birth is 35.8 cm for US male newborns and 34.7 cm for US female newborns [22].
Table 4.
Characteristics of live births
| Characteristic, n (%) | Live births (N = 272)a |
|---|---|
| Sexb | |
| Female | 137 (50.9) |
| Male | 132 (49.1) |
| Gestational age at birth, yearsb | |
| Mean (SD) | 38.3 (3.0) |
| Median (range) | 38.9 (23–47) |
| Birth weight, gc | |
| Mean (SD) | 3169 (689) |
| Median (range) | 3260 (530–4848) |
| Birth length, cmd | |
| Mean (SD) | 49.2 (4.4) |
| Median (range) | 50.1 (17–57) |
| Head circumference, cme | |
| Mean (SD) | 33.7 (2.8) |
| Median (range) | 34.0 (13–40) |
| Apgar score, 1 minf | |
| Mean (SD) | 8.0 (1.6) |
| Median (range) | 8.0 (1–10) |
| Apgar score, 5 ming | |
| Mean (SD) | 8.8 (1.0) |
| Median (range) | 9.0 (2–10) |
| Characteristics in singleton births without geneticist-confirmed birth defects (n = 246) | |
| Gestational age, n (%)h | |
| Full term (≥ 36 weeks gestation) | 223 (91.8) |
| Preterm (< 36 weeks gestation) | 11 (4.5) |
| Very preterm (< 32 weeks gestation) | 9 (3.7) |
| Birth weight, n (%)i | |
| Normal | 205 (91.9) |
| Low (< 2500 g) | 11 (4.9) |
| Very low (< 1500 g) | 7 (3.1) |
SD standard deviation
aIncludes four sets of twins
bn = 269
cn = 249
dn = 224
en = 196
fn = 206
gn = 205
hn = 243
in = 223
Discussion
Results from this large national registry suggest that IM IFNβ-1a exposure within ~1 week of conception or during the first trimester of pregnancy was not clinically significantly associated with adverse pregnancy outcomes in women with MS. The majority of singleton births were full term and of normal birth weight. The spontaneous abortion rate of 10.5% (95% CI 7.2–15.0) reported in the current registry is consistent with findings from the general population in a Danish register-based study (10.9%) [23], a Finnish and Swedish cohort pregnancy registry (12%) [9], a US study (15.9%) [24], a large epidemiological study in Europe (10.7%) [8], and, importantly, those from a longitudinal study including untreated pregnant women with MS (9.8%) [9, 10, 25]. Although the rate of birth defects in the current registry, 6.3% (95% CI 3.8–10.0), was statistically significantly higher than that in the selected MACDP comparison group (2.67%), we did not observe patterns of defects that were suggestive of an unusual distribution. Specifically, there were three cases of hypospadias in 132 male infants in this registry compared with one case in 250 male infants born in the USA [26]. In the Finnish and Swedish cohort pregnancy registry, the prevalence of major congenital anomalies in live births was 1.8% (95% CI 0.9–3.1) and 3.3% (95% CI 2.2–4.4) in IFNβ-exposed and MS DMT-unexposed pregnancies, respectively [9], 2.8% in a large European epidemiological study [8], and ranged from 0% to 7.7% in a longitudinal study of DMT-treated women with MS, untreated pregnant women with MS, and non-MS pregnant controls[25].
The study design and choice of reference group from population-based surveillance data can influence the interpretation of our results given this registry did not include a comparator group of pregnant women with MS who were not treated with IM IFNβ-1a. European Surveillance of Congenital Anomalies (EUROCAT) is a European network of population-based congenital anomaly registries [27] and has strict criteria for major congenital anomalies. The MACDP is a regionally specific surveillance system in a well-defined geographic area with data collected through medical record abstraction. The differences in both the populations and terminology used in EUROCAT and MACDP may lead to different classifications of birth defects and therefore make the birth defect rate appear higher or lower when in fact this difference is because of the classification instead of a true difference in the risk of a birth defect. The MACDP classification was used in this study, i.e., live births and all fetal losses occurring at gestation ≥ 20 weeks with defects. This registry includes a population of potentially high risk, chronically ill, systematically monitored women with MS who were receiving IM IFNβ-1a at approximately the time of conception or during the first trimester of pregnancy. Women with MS, and their HCPs, actively and voluntarily participated in this registry throughout pregnancy and early infancy; therefore, there is potential for bias given that there was considerable opportunity to detect adverse outcomes through multiple encounters between the registry, the participant, and her HCPs.
Several publications have discussed the results of exposure to IFNβ formulations during pregnancy [7–9, 25, 28–34], though the current study is the largest to date that focuses exclusively on IM IFNβ-1a in patients with MS. Higher rates of spontaneous abortion have been shown in pregnant patients with MS exposed to IFNβ formulations, including one small study of IFNβ-1b (n = 21; 28%), versus unexposed patients [25, 28, 29]. Other small studies [30, 31], and also larger cohort studies [7–9, 32–34], support findings of the current registry and showed no evidence that exposure to IFNβ formulations before conception or during pregnancy increases the risk of spontaneous abortion. Furthermore, none of the studies [7–9, 25, 28–34] suggested any increased risk of congenital abnormalities in babies born to women with MS who received treatment with IFNβ formulations prior to, or during, pregnancy. Some of the more recent safety data from large cohort studies investigating exposure to IFNβ during pregnancy—the European IFNβ Pregnancy Registry, which evaluated 948 pregnancies across 26 countries [8], and the Nordic cohort study including outcomes from 797 pregnancies [9]—led to the European Medicines Agency releasing an update to guidance allowing the use of IFNβ to be considered prior to conception, during pregnancy, and while breastfeeding [12].
Pregnancy exposure registries are valuable tools for assessing the safety of biopharmaceutical products in pregnancy. This registry represents the first and largest prospective study to date of the safety of IM IFNβ-1a in human pregnancy. Limitations of the study include the lack of a comparison group of pregnant patients with MS who were not exposed to IM IFNβ-1a, and the short follow-up of up to 12 weeks for live-born infants. It would be beneficial to evaluate the longer term effects of exposure to IM IFNβ-1a during pregnancy to understand whether there is any effect on developmental outcomes. Recently published preliminary data from the German Multiple Sclerosis and Pregnancy Registry (median 2-year follow-up), and retrospective data from a post-authorization safety study (PRIMA) including physical growth curves to 4 years, show no indication that IFNβ-1a exposure during pregnancy (or lactation) adversely affected children’s development [35, 36]. However, because of the low number of patients with exposure in the second and third trimesters in previous studies and in our registry, additional studies are needed to assess longer term exposure during pregnancy. The European Medicines Agency has requested a cohort study to assess the risk of IFNβ exposure in later-stage pregnancies. As a result, the noninterventional drug utilization study, INFORM (EUPAS38736) [37] is being conducted to determine if the number of exposed pregnancies is adequate for a cohort study.
The prospective nature of the registry is a major strength as it reduces the bias of missing data and meets the criteria based on FDA guidelines. Early enrollment (mean [SD] gestational age at enrollment was 12.6 [7.4] weeks) minimizes bias associated with prenatal testing and the knowledge of pregnancy outcomes prior to enrollment; however, enrollment even earlier would have increased the likelihood of capturing spontaneous abortions occurring nearer the start of the first trimester. The rate of previous spontaneous abortions in those patients with a prior pregnancy was 31% (64/204), suggesting the true rate is likely higher, as others have also suggested [38]. Despite its limitations, the findings of the registry are generally consistent with previous findings and are informative to clinicians and their patients for weighing the risks and benefits of IM IFNβ-1a exposure during pregnancy.
Conclusions
Findings from this large national registry study suggest IM IFNβ-1a exposure during early pregnancy was not clinically significantly associated with adverse pregnancy outcomes in women with MS. Thus, these findings will help inform clinicians and patients when weighing the risks and benefits of IM IFNβ-1a use during pregnancy.
Acknowledgments
Lindsey O’Mahony, PhD (on behalf of Excel Scientific Communications, Southport, CT, USA) wrote the first draft of the manuscript based on input from the authors, and Allison Terry (Excel Scientific Solutions) copyedited and styled the manuscript as per journal requirements; both were funded by Biogen (Cambridge, MA, USA).
Declarations
Funding
Biogen (Cambridge, MA, USA) sponsored this study and provided funding for medical writing support in the development of this paper.
Conflicts of Interest/Competing Interests
Bianca Weinstock-Guttman has participated in speaker’s bureaus and/or served as a consultant for, and/or received grant/research support from Biogen, EMD Serono, Novartis, Genentech, Celgene/Bristol Myers Squibb, Sanofi Genzyme, Bayer, Janssen, Labcorp, and Horizon. She serves on the editorial board of BMJ Neurology, Children, CNS Drugs, MS International, and Frontiers Epidemiology. Amy Perrin Ross has acted as a consultant for Biogen, EMD Serono, Novartis, Celgene/BMS, Roche, Sanofi Genzyme, Genentech, Janssen, Horizon, and Alexion. Jonathan Planton, Kurt White, Avni Pandhi, Andres Greco, Achint Kumar, Nicholas Everage, and Megan Vignos are employees of and may hold stock/stock options in Biogen.
Ethics Approval
The registry obtained central institutional review board approval for the study protocol and all amendments, which were performed in accordance with FDA guidance “Establishing Pregnancy Exposure Registries,” the International Society for Pharmacoepidemiology’s Guidelines for Good Pharmacoepidemiology Practices, and the ethical principles outlined in the Declaration of Helsinki.
Consent to Participate
Verbal informed consent was obtained from participants in accordance with local practice and regulations. During the initial telephone encounter, participants were asked to provide verbal consent to enroll and to provide authorization for their healthcare providers, including their infant’s pediatrician, to release medical information to the registry.
Consent for Publication
Not applicable.
Availability of Data and Material
The trial is registered on ClinicalTrials.gov (NCT00168714). Request for the data supporting this manuscript should be submitted to https://vivli.org/.
Code Availability
Not applicable.
Authors’ Contributions
In addition to the contributions listed below, all authors read and approved the final version. BW-G, APR, JP, NE: analysis and interpretation of the data and drafting/critically revising the manuscript; KW, AP, AG, AK: collection of the data, analysis and interpretation of the data, and drafting/critically revising the manuscript; MV: post-hoc plan of statistical analyses, analysis and interpretation of the data, and drafting/critically revising the manuscript.
References
- 1.Leray E, Moreau T, Fromont A, Edan G. Epidemiology of multiple sclerosis. Rev Neurol (Paris). 2016;172(1):3–13. doi: 10.1016/j.neurol.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Gilmour H, Ramage-Morin PL, Wong SL. Multiple sclerosis: prevalence and impact. Health Rep. 2018;29(1):3–8. [PubMed] [Google Scholar]
- 3.Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029–e1040. doi: 10.1212/WNL.0000000000007035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simone IL, Tortorella C, Ghirelli A. Influence of pregnancy in multiple sclerosis and impact of disease-modifying therapies. Front Neurol. 2021;12:697974. doi: 10.3389/fneur.2021.697974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villaverde-González R. Updated perspectives on the challenges of managing multiple sclerosis during pregnancy. Degener Neurol Neuromuscul Dis. 2022;12:1–21. doi: 10.2147/DNND.S203406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biogen Inc. Avonex US prescribing information. 2021. https://hcp.avonex.com/content/dam/commercial/avonex/hcp/en_us/pdf/Avonex_US_Prescribing_Information.pdf. Accessed 22 Apr 2023.
- 7.Thiel S, Langer-Gould A, Rockhoff M, Haghikia A, Queisser-Wahrendorf A, Gold R, et al. Interferon-beta exposure during first trimester is safe in women with multiple sclerosis: a prospective cohort study from the German Multiple Sclerosis and Pregnancy Registry. Mult Scler. 2016;22(6):801–809. doi: 10.1177/1352458516634872. [DOI] [PubMed] [Google Scholar]
- 8.Hellwig K, Geissbuehler Y, Sabidó M, Popescu C, Adamo A, Klinger J, et al. Pregnancy outcomes in interferon-beta-exposed patients with multiple sclerosis: results from the European Interferon-beta Pregnancy Registry. J Neurol. 2020;267(6):1715–1723. doi: 10.1007/s00415-020-09762-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakkarainen KM, Juuti R, Burkill S, Geissbuhler Y, Sabido M, Popescu C, et al. Pregnancy outcomes after exposure to interferon beta: a register-based cohort study among women with MS in Finland and Sweden. Ther Adv Neurol Disord. 2020;13:1756286420951072. doi: 10.1177/1756286420951072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korjagina M, Hakkarainen KM, Burkill S, Geissbuhler Y, Sabido M, Everage N, et al. Prevalence of adverse pregnancy outcomes after exposure to interferon beta prior to or during pregnancy in women with MS: stratification by maternal and newborn characteristics in a register-based cohort study in Finland and Sweden. Mult Scler Relat Disord. 2021;48:102694. doi: 10.1016/j.msard.2020.102694. [DOI] [PubMed] [Google Scholar]
- 11.Krysko KM, Bove R, Dobson R, Jokubaitis V, Hellwig K. Treatment of women with multiple sclerosis planning pregnancy. Curr Treat Options Neurol. 2021;23(4):11. doi: 10.1007/s11940-021-00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varyte G, Zakareviciene J, Ramasauskaite D, Lauzikiene D, Arlauskiene A. Pregnancy and multiple sclerosis: an update on the disease modifying treatment strategy and a review of pregnancy's impact on disease activity. Medicina (Kaunas). 2020;56(2):49. [DOI] [PMC free article] [PubMed]
- 13.Correa-Villasenor A, Cragan J, Kucik J, O'Leary L, Siffel C, Williams L. The Metropolitan Atlanta Congenital Defects Program: 35 years of birth defects surveillance at the Centers for Disease Control and Prevention. Birth Defects Res A Clin Mol Teratol. 2003;67(9):617–624. doi: 10.1002/bdra.10111. [DOI] [PubMed] [Google Scholar]
- 14.Correa A, Cragan JD, Kucik JE, Alverson CJ, Gilboa SM, Balakrishnan R, et al. Reporting birth defects surveillance data 1968–2003. Birth Defects Res A Clin Mol Teratol. 2007;79(2):65–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC). Metropolitan Atlanta Congenital Defect Program (MACDP) six-digit code defect list, version 08/07. 2007.
- 16.Scheuerle A, Tilson H. Birth defect classification by organ system: a novel approach to heighten teratogenic signalling in a pregnancy registry. Pharmacoepidemiol Drug Saf. 2002;11(6):465–475. doi: 10.1002/pds.726. [DOI] [PubMed] [Google Scholar]
- 17.Correa A, Cragan JD, Kucik JE, Alverson CJ, Gilboa SM, Balakrishnan R, et al. Erratum. Birth Defects Res A Clin Mol Teratol. 2008;82(1):41–62. doi: 10.1002/bdra.20434. [DOI] [Google Scholar]
- 18.Fleiss J, Levin B, Paik M. Statistical methods for rates and proportions. 3. Hoboken: Wiley; 2003. [Google Scholar]
- 19.US Food and Drug Administration. Guidance for industry: establishing pregnancy exposure registries. In: US Department of Health and Human Services, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, editors. Rockville: US Food and Drug Administration; 2002.
- 20.International Society for Pharmacoepidemiology (ISPE) Guidelines for good pharmacoepidemiology practices. Pharmacoepidemiol Drug Saf. 2008;17(2):200–208. doi: 10.1002/pds.1471. [DOI] [PubMed] [Google Scholar]
- 21.World Medical A World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Data table of infant head circumference-for-age charts. 2001 August 23, 2001. https://www.cdc.gov/growthcharts/html_charts/hcageinf.htm. Accessed 15 Jun 2023.
- 23.Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320(7251):1708–1712. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anokute CC. Epidemiology of spontaneous abortions: the effect of previous abortions. J R Soc Health. 1987;107(1):31–33. doi: 10.1177/146642408710700114. [DOI] [PubMed] [Google Scholar]
- 25.Weber-Schoendorfer C, Schaefer C. Multiple sclerosis, immunomodulators, and pregnancy outcome: a prospective observational study. Mult Scler. 2009;15(9):1037–1042. doi: 10.1177/1352458509106543. [DOI] [PubMed] [Google Scholar]
- 26.Donaire AE, Mendez MD. Hypospadias. Treasure Island: StatPearls; 2023. [Google Scholar]
- 27.Boyd PA, Haeusler M, Barisic I, Loane M, Garne E, Dolk H. Paper 1: the EUROCAT network: organization and processes. Birth Defects Res A Clin Mol Teratol. 2011;91:S2–15. doi: 10.1002/bdra.20780. [DOI] [PubMed] [Google Scholar]
- 28.Sandberg-Wollheim M, Frank D, Goodwin TM, Giesser B, Lopez-Bresnahan M, Stam-Moraga M, et al. Pregnancy outcomes during treatment with interferon beta-1a in patients with multiple sclerosis. Neurology. 2005;65(6):802–806. doi: 10.1212/01.wnl.0000168905.97207.d0. [DOI] [PubMed] [Google Scholar]
- 29.Boskovic R, Wide R, Wolpin J, Bauer DJ, Koren G. The reproductive effects of beta interferon therapy in pregnancy: a longitudinal cohort. Neurology. 2005;65(6):807–811. doi: 10.1212/01.wnl.0000180575.77021.c4. [DOI] [PubMed] [Google Scholar]
- 30.Patti F, Cavallaro T, Lo Fermo S, Nicoletti A, Cimino V, Vecchio R, et al. Is in utero early-exposure to interferon beta a risk factor for pregnancy outcomes in multiple sclerosis? J Neurol. 2008;255(8):1250–1253. doi: 10.1007/s00415-008-0909-4. [DOI] [PubMed] [Google Scholar]
- 31.Hellwig K, Gold R. Glatiramer acetate and interferon-beta throughout gestation and postpartum in women with multiple sclerosis. J Neurol. 2011;258(3):502–503. doi: 10.1007/s00415-010-5758-2. [DOI] [PubMed] [Google Scholar]
- 32.Amato MP, Portaccio E, Ghezzi A, Hakiki B, Zipoli V, Martinelli V, et al. Pregnancy and fetal outcomes after interferon-beta exposure in multiple sclerosis. Neurology. 2010;75(20):1794–1802. doi: 10.1212/WNL.0b013e3181fd62bb. [DOI] [PubMed] [Google Scholar]
- 33.Coyle PK, Sinclair SM, Scheuerle AE, Thorp JM, Jr, Albano JD, Rametta MJ. Final results from the Betaseron (interferon β-1b) Pregnancy Registry: a prospective observational study of birth defects and pregnancy-related adverse events. BMJ Open. 2014;4(5):e004536. doi: 10.1136/bmjopen-2013-004536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler. 2011;17(4):423–430. doi: 10.1177/1352458510394610. [DOI] [PubMed] [Google Scholar]
- 35.Friedmann N, Ciplea AI, Thiel S, Gold R, Hellwig K. Interferon- or peginterferon-beta 1a exposure during pregnancy in women with multiple sclerosis: outcomes on child development. Mult Scler. 2022;28:182. [Google Scholar]
- 36.Begus-Nahrmann Y, Taipale K, Niemczyk G, Rehberg-Weber K, Klehmet J. Interferon beta exposure during pregnancy and breastfeeding: impact on birth outcome and child development: results from the post-authorisation safety study PRIMA. Mult Scler. 2022;28:463. doi: 10.1016/j.msard.2023.104844. [DOI] [PubMed] [Google Scholar]
- 37.Sabidó MS-WK, Grimes N, Prach LM, Zhao L, Hakkarainen KM. Interferon beta exposure in the 2nd and 3rd trimester of pregnancy a register based drug utilisation study in Finland and Sweden. Mult Scler. 2021;27(2S):656. [Google Scholar]
- 38.Crowe HM, Wesselink AK, Wise LA, Jick SS, Rothman KJ, Mikkelsen EM, et al. Pre-pregnancy migraine diagnosis, medication use, and spontaneous abortion: a prospective cohort study. J Headache Pain. 2022;23(1):162. doi: 10.1186/s10194-022-01533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]