Abstract
Introduction
Eosinophils represent the most active cells in mammals that show protective and assistive activity in the host immune defence against helminth parasites. These cells are also responsible for the reduction of allergic and inflammatory reactions. The eosinophils play a key role in allergic reactions by secretion of different chemical molecules leading to swelling, lesions and granuloma onset.
Material and Methods
The study was carried out on 30 cats with inflammatory skin lesions. The cats ranged in age from seven months to 13 years, and had an average age of three years. The research methodology included information on the disease, dermatological conclusions, concomitant disorders, medical and laboratory data and the treatment strategy.
Results
In total, 30 cats were diagnosed with eosinophilic granuloma complex. The distribution of lesions was 87.1% in the skin and 12.9% at the skin–mucosal junction. The lesions increased and decreased with the seasons of spring and summer, and the onset of the disease usually coincided with exposure to fleas.
Conclusion
Eosinophilic granuloma complex in cats is a serious pathology and frequently requires lifelong treatment, so it is important to diagnose it quickly and accurately to ensure optimal treatment of affected animals.
Keywords: eosinophilic granuloma complex, eosinophilic dermatoses in cats, diagnosis, treatment
Introduction
Eosinophils are among the most misunderstood cells in mammalian biology, and the understanding of their multifaceted roles has undergone profound changes. Eosinophils were once believed to be helpful in the host’s natural defence against helminth parasites, as well as in reducing allergic inflammation by neutralising inflammatory mediators produced by mast cells. They were thought to be key factors in tissue damage in allergic inflammation because of their releasing of cytotoxic granular proteins that were previously thought to be useful for killing parasites. Furthermore, since they have been shown to release a wide range of pro-inflammatory substances, including granular proteins, lipid mediators, cytokines and chemokines, their role in inflammation was considered pro-inflammatory rather than anti-inflammatory (23).
From an aetiological point of view, eosinophilic granuloma complex (EGC) is currently considered a skin reaction caused by common allergic causes (18, 20, 25), including hypersensitivity reactions to fleas and environmental and food allergens (21). However, in many cases, no trigger factors were present. The pathogenesis of feline allergic skin diseases, in particular feline atopic syndrome, remains poorly understood compared to that of canine equivalents (4). Disorders with eosinophilic granuloma have immune-mediated or parasitic causes, although the specific functions and content of eosinophils in cats are currently unknown (5).
Some research has claimed that Mycobacterium thermoresistibile causes severe chronic skin infections in cats. Sick animals were treated with long-term specialised antibiotic medications, surgery, and skin restoration (22). It has been determined that systemic diseases in cats and dental illnesses are related. The main systemic diseases in cats, such as feline viral infections, complicated eosinophilic granulomas, immune system and gastrointestinal system disorders, liver and feeding disorders, diabetes mellitus and chronic kidney failure, can manifest with oral signs (6). Given the lack of information on the functions and content of eosinophils in cats, it is not surprising that our current understanding of EGC does not differ much from what was reported more than 35 years ago in one of the first descriptions of this disease (2).
The cat eosinophilic granuloma complex consists of a group of lesions defined as eosinophilic plaque, eosinophilic granuloma, and eosinophilic ulcers that affect the cat’s skin and mucous membranes (19). The condition, also known by the synonyms collagenolytic granuloma and linear granuloma, classically occurs in the form of various itchy, dense papules and plaques on the thigh, or in the form of single papular and nodular lesions located anywhere on the body, including the edge of the lips and in the oral cavity (1, 21). Oral diseases are extremely common among small companion animals, but most of them do not manifest clinical signs, so the diagnosis of EGC is usually established at a late stage (15, 16). In many cases, lesions are severe, can be chronic and recurrent, and are accompanied by varying and sometimes significant degrees of itching or pain. There is no proven feline age-related predisposition or well-documented breed predisposition to developing lesions. One study has reported a possible predisposition of female cats to EGC in a literature survey, but this has not been clearly documented (3).
Proliferative inflammatory eosinophilic granulomatosis is a common condition in cats causing lesions which can be located on the skin, the skin–mucosal membrane junctions, or in the oral cavity. Eosinophilic granuloma mainly affects the medial surface of the thigh, cheeks, tongue and palate. Pain is not common, the lesion may not cause itching if it is located on the skin, but nodular shapes in the oral cavity may make it difficult to swallow (12). Eosinophilic ulcers are usually recorded on the upper lip, eosinophilic plaque lesions most often occur on the abdomen and medial thigh, and eosinophilic granulomas usually appear on the tongue, hard palate and caudal surface of the thighs. It was found that the distribution of lesions was 40.0% in the oral cavity, 33.3% on the skin, and 26.6% at mucocutaneous junctions (7). Most cats with EGC were mixed breeds, on average three years old, and no predisposition to the disease according to sex was noted (8, 17). In contrast, according to other authors, oral signs were more frequent in males (n = 173; 58.4%), adults (aged 7 to 10 years (n = 88; 33.0%) and the European shorthair breed (n = 206; 73.6%). The gums were the site where oral lesions were most often detected (128 samples, 43.1%). Eosinophilic granuloma complex occurred in 11.1% (n = 33) of cases where cats suffered granulomas (19).
Previous research including a cytological analysis of smear samples found the number of eosinophils to be increasing and the cells to be organised into “islands.” Skin samples from cat eosinophilic granuloma complex underwent transmission electron microscopy, and the results showed “flame figures” with ultrastructurally normal collagen fibrils divided by oedema and encircled by a significant number of degranulating eosinophils (1). Histologically, the main lesion was characterised by a diffuse dermal/submucosal inflammatory infiltrate consisting of eosinophils. Often these foci were surrounded by macrophages and fewer multicellular giant histiocytic cells. Inflammatory infiltration of mast cells, lymphocytes, and plasma cells of mild to moderate severity was observed in the dermis and submucosal membrane. Ulcers of the epidermis and mucous membrane were observed in most lesions with inflammatory infiltrate from neutrophils. All cases were negative for feline herpesvirus 1 (FHV-1) on immunohistochemical assessment. Ulceration of the epidermis and mucosa was the most common sign of a complex of eosinophilic granulomas, especially in cases of oral lesions (7). Degranulation of eosinophils around collagen bundles represented the main pathogenetic event in these lesions, similar to “flame figures” in humans (10). The term “flame figures” can be more accurately used to refer to those foci of eosinophilic or partially basophilic debris commonly referred to as “collagen degeneration” (9).
Studies should include careful elimination of ectoparasites and the establishment of a strict diet before in vitro or in vivo allergy testing to detect environmental allergens. The most commonly used treatment options include antihistamines, glucocorticoids and cyclosporines (11, 18). Suspension of amoxicillin trihydrate-potassium clavulanate (Clavamox, Pfizer Animal Health) was effective for the treatment of eosinophilic plaques in cats, with a statistically significant reduction in the average lesion size by 96.2% (−7.60 cm), P = 0.0078 (24). A combination of oral prednisone and tacrolimus has been shown to be effective in reducing the recurrence of feline eosinophilic granuloma (13). The use of unibiol ointment for local treatment of ulcers in eosinophilic granuloma accelerated wound healing such that it had completed by as early as 10–14 days and had a good cosmetic result (14). Laser surgery was used for granuloma on the tongue, as the traditional surgical technique was not suitable because of the dense vasculature of the tongue and corresponding extensive bleeding which would be caused. After the operation, the animal quickly recovered on steroids, and its condition and quality of life improved significantly (12).
Despite common disorders associated with eosinophils in cats, including EGC, this pathology remains poorly understood. Therefore, the main objective of the work was to study feline EGC in a clinic setting. This was undertaken at the Zoovetcenter Veterinary Clinic in Shostka, Sumy Oblast (Ukraine). The features of the manifestation and clinical course of EGC in cats were investigated and the laboratory methods for such investigation were evaluated in animals with symptoms of skin damage, in order to differentiate the diagnosis of EGC and appraise the clinical effectiveness of the feline treatment regimen for the disease.
Material and Methods
Personnel at the Zoovetcenter clinic in Shostka in Ukraine’s Sumy Oblast drew blood from pet cats suspected of having EGC. Blood samples were collected from fasted cats of different breeds and ages and of both sexes from the lateral saphenous vein of the forelimb. The haematological indicator levels in blood serum were measured in the laboratory of the Department of Normal and Pathological Animal Anatomy at the Poltava State Agrarian University in Ukraine with an automatic veterinary haematological analyser (Element HT5; Heska, Barrie, ON, Canada), and an automatic biochemical express analyser (DRI-CHEM NX500; Fujifilm, Tokyo, Japan) was used for biochemical studies. The preparation of samples and determination of specific indicators were performed according to the instructions for the device and reagents.
LEUCODIF 200 reagents (Erba Lachema, Brno, Czech Republic) were used for rapid staining of blood smears. One tablet was dissolved in 250 mL of distilled water. Blood smears prepared on fat-free glass slides were left to dry in air. The solution was drained into a dye container. The smear was fixed by immersion five times for 1 s in reagent 1. After each immersion, the solution was allowed to drain, and its excess was removed on the edge of the container. The fixed smear was immersed three times for 1 s in reagent 2. After each immersion in this step, the solution was likewise allowed to drain and its excess was removed on the edge of the container. Completing these immersion steps, the smear was immersed six times for 1 s in reagent 3. Again, after each immersion, the solution was allowed to drain and its excess was removed on the edge of the container. The slide was treated with a washing solution and allowed to air dry. Blood smears were examined by optical microscopy and described in detail.
Nasal swabs which were collected at the Zoovetcenter clinic were tested in the laboratory of the Department of Normal and Pathological Animal Anatomy to study the presence of FHV-1 using a rapid immunochromatographic feline herpesvirus antigen (rhinotracheitis) test (FHV Ag; GenBody, Cheonan, South Korea). Thus, the pathology of feline herpesvirus infection was excluded in the differential diagnosis, because the clinical signs could be similar.
Biopsies were taken from the lesions of cats suspected of having EGC. Before a punch biopsy was taken at the Zoovetcenter clinic, a scarification test was performed to determine sensitivity to a local anaesthetic. The biopsy area was treated with an antiseptic, local anaesthesia was performed, a special perforating scalpel was inserted into the skin in spiral movements to the required depth (punch biopsy), and a column of biomaterial was removed. The material was immersed in a container with a fixing substance, and subsequently skin treatment was carried out. In some cases, granuloma removal was performed using a mono/bipolar radiofrequency electrosurgical unit (SURTRON 160; LED, Aprilia, Italy) and aluminium wound spray (Aluspray; Vetoquinol, Lure, France) to treat the wound surface. For treatment of EGC, a Royal Canin (Aimargues, France) hypoallergenic diet was recommended, and as topical therapy Stronghold solution (Zoetis, Parsippany, NJ, USA) once a month and Protopic ointment (LEO Pharma, Ballerup, Denmark) once a day for an extended period were prescribed. The medications administered in tablet form were Synulox (a combination of amoxycillin and clavulanic acid manufactured by Zoetis) at 50 mg twice a day for 14 days, prednisolone (Darnitsa, Kyiv, Ukraine) at 2 mg/kg for 14 days and the dose gradually reducing thereafter to the minimum effective dose, and Equoral (a cyclosporine preparation manufactured by Teva Pharmaceutical Industries, Opava, Czech Republic) at 7 mg/kg. After achieving a result, the dose of Equoral was reduced by half for 14–28 days, then to a maintenance dose once every two days for 14–28 days, and finally to once every three days for a duration of 2–4 months.
The diagnosis of eosinophilic granuloma was established comprehensively, taking into account anamnesis, clinical manifestations, and laboratory tests. Thirty cats confirmed to be EGC sufferers and having been treated by the Zoovetcenter clinic were the subject of a retrospective study over a 15-year period. The medical records were reviewed for the following information: general data (breed, age and sex), duration of the disease prior to examination, dermatological conclusions, laboratory and medical data, total duration of the disease, duration with and duration without treatment, and length of follow-up period.
Data for cats suffering from EGC were compared with data for the general cat population (n = 2,544) over the same time period by calculating relative risk (RR):
| 1 |
An RR of 2.0 or more was considered significant.
Results
Records from the 15-year timespan of January 2008 to December 2022 of cats diagnosed with EGC were selected. General data such as age, sex, breed, and spread of the lesion were collected and analysed. All cases were negative for FHV-1 in immunochromatographic assessment. In this study, 30 cats were diagnosed with the disease. Most of the patients were Sphinx cats (22/30). There was no sexual predisposition. The distribution of lesions of the eosinophilic granuloma complex was 87.1% in the skin and 12.9% at the skin–mucosal junction. The lesions increased and decreased with the seasons, being largest in spring and summer, and the onset of the disease usually coincided with the exposure (Fig. 1).
Fig. 1.
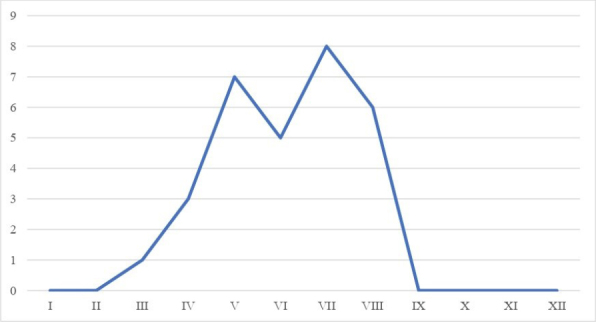
Outbreaks of eosinophilic granuloma complex depending on the season of the year (by month)
Eosinophilic granuloma complex was diagnosed in 1.18% (30/2,544 cats) of the cat dermatology cases and in 0.14% (30/20,048 cats) of all cats examined in the Zoovetcenter veterinary clinic over a 15-year period. Domestic hairless cats accounted for 73.3% (22/30) of cats with an eosinophilic granuloma complex and 80% of the cat population (RR = 1.1). The eosinophilic granuloma complex was also diagnosed in domestic long-haired (4 cases), Siamese (1 case), Manx (1 case), Himalayan (1 case) and Maine Coon (1 case) cats.
Neutered males, spayed females, intact males, and intact females were 37.2% (946), 24% (610), 23.6% (600), and 15.2% (386) of the cats with EGC, respectively. The same categories represented 37.9% (RR = 0.37), 37.9% (RR = 0.37), 9.99% (RR = 0.09) and 10.99% (RR = 0.10) of the total cat population, respectively.
The age of onset of the disease in cats ranged from 0.2 years to 13 years. Ninety-three percent (28 out of 30 cats) of the eosinophilic granuloma complex sufferers had an initial age <4 years; 53% (16 out of 30 cats) had an initial age <2 years and 40% (12 out of 30 cats) had an initial age <1 year. All cats were submitted to an initial lesion examination and had not received prior therapy. The most common lesions were on the lips (23 cases; 42%), caudal thighs (12 cases; 22%), and the chin (10 cases; 18%). Twenty-five out of the thirty (83%) cats had lesions in only one site (e.g. the chin, lips or thighs). Nine (30%) cats had linear lesions (Fig. 1) and twenty-one (70%) cats had papular or nodular lesions (Fig. 2). Only one cat had both morphological types. It was found that the lesions were symptomatic in only three cats, in which the lesions on the paws were painful or itchy. The distribution and morphological types of lesion were not associated with the age or sex of the cats. These data on lesions were mainly the results of anamnesis and physical examination. In 14 of the 30 (47%) cats, the lesion was described by the owners as “recently detected” before the examination in the Zoovetcenter veterinary clinic. In the remaining cats, the duration of the injury ranged from 2 weeks to 4 years.
Fig. 2.
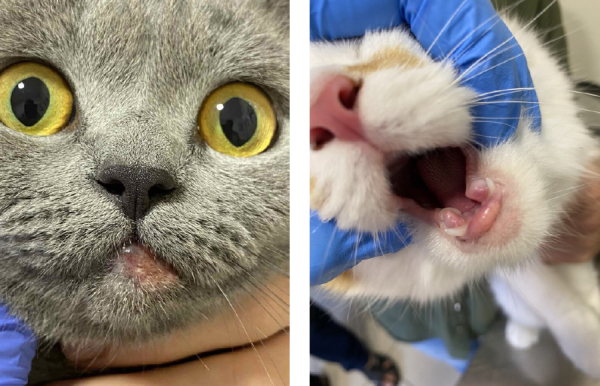
Raised granular type of lesion
In the EGC cases, raised, red, alopecial lesions were observed, which had a granular appearance (Fig. 2).
Skin lesions were single or in groups. They could be found anywhere on the body, but were usually found in a pattern as a band of hairless lesions along the back of the thighs. Lesions of this kind usually did not itch. Other areas where these granulomas could be detected were the chin, nose, muzzle and ears (Fig. 3).
Fig. 3.
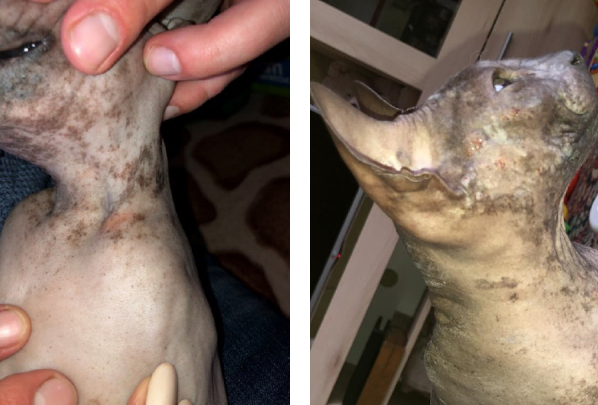
Idiopathic eosinophilic ulcer. Pink foci on the muzzle and neck
These lesions could appear anywhere on the body of the cat, but were most often seen on its hind legs (Fig. 4).
Fig. 4.
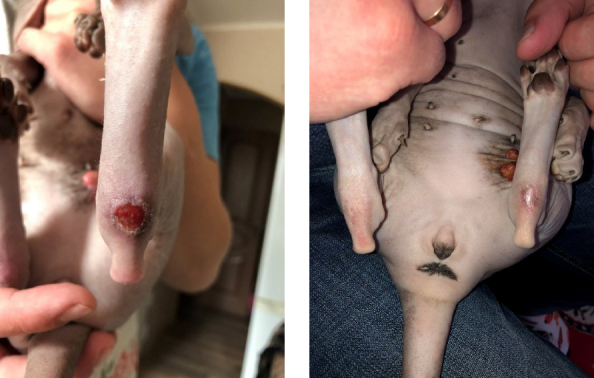
Eosinophilic ulcer on the hind limbs
Eosinophilic plaques were usually seen in the abdominal cavity or inner thigh. Typically they were raised orange-to-yellow erythematous plaques. When conducting biochemical blood tests, almost all indicators were observed to remain within the normal range, except for total bilirubin and creatine kinase, which were 1 ± 0.12 μmol/L and 163 ± 1.4 U/L, respectively (Table 1).
Table 1.
Average results of a biochemical blood test
| Indicators | Results | Normal range | Units |
|---|---|---|---|
| Blood urea nitrogen | 6.90 ± 0.03 | 6.28–11.71 | mmol/L |
| Creatinine | 71 ± 0.08 | 71–159 | umol/L |
| Blood urea nitrogen/creatinine | 24.1 ± 0.04 | ||
| Phosphorus | 1.73 ± 0.13 | 0.84–1.94 | mmol/L |
| Uric Acid | 26 ± 0.62 | 0–60 | umol/L |
| Total protein | 71 ± 0.71 | 57–78 | g/L |
| Albumin | 33 ± 0.03 | 23–35 | g/L |
| Globulin | 38 ± 0.06 | 27–52 | g/L |
| Albumin/globulin | 0.9 ± 0.15 | ||
| Glucose | 5.9 ± 1.37 | 3.9–8.2 | mmol/L |
| Alanine aminotransferase | 45 ± 2.6 | 22–84 | U/L |
| Aspartate aminotransferase | 30 ± 0.09 | 18–51 | U/L |
| Alkaline phosphatase | 24 ± 1.22 | 9–53 | U/L |
| Gamma-glutamyl transferase | 3 ± 0.04 | 1–10 | U/L |
| Total bilirubin | 1 ± 0.12 | 2–7 | umol/L |
| Amylase | 775 ± 2.7 | 200–1900 | U/L |
| Creatine kinase | 163 ± 1.4 | 17–150 | U/L |
When performing general blood test in cats eosinophilia was revealed (the average percentage of eosinophils being 14.8 ± 0.07%) and neutrophilia was noted (the average percentage of neutrophils being 17.35 ± 0.08%) (Table 2). In sick cats, a slight increase in white blood cells 22.80 ± 0.12 g/L was found in comparison with the upper limit of the normal range. It should be noted that the percentages of lymphocytes and monocytes were lower than normal (8.7 ± 0.21 and 0.4 ± 0.01, respectively).
Table 2.
Average results of complete blood count tests in cats
| Indicators | Results | Normal range | Units |
|---|---|---|---|
| Leukocytes | 22.80 ± 0.12 | 5.50–19.50 | 109/L |
| Neutrophils | 17.35 ± 0.08 | 3.12–12.58 | 109/L |
| Lymphocytes | 1.97 ± 0.05 | 0.73–7.86 | 109/L |
| Monocytes | 0.11 ± 0.03 | 0.07–1.36 | 109/L |
| Eosinophils | 3.36 ± 0,13 | 0.06–1.93 | 109/L |
| Basophils | 0.01 ± 0.34 | 0.00–0.12 | 109/L |
| Neutrophils | 76.1 ± 0.11 | 38.0–80.0 | % |
| Lymphocytes | 8.7 ± 0.21 | 12.0–45.0 | % |
| Monocytes | 0.4 ± 0.01 | 1.0–8.0 | % |
| Eosinophils | 14.8 ± 0.07 | 1.0–11.0 | % |
| Basophils | 0.0 ± 0.02 | 0.0–1.2 | % |
| Erythrocytes | 8.06 ± 0.15 | 4.60–10.20 | 1012/L |
| Haemoglobin | 120 ± 18.08 | 85–153 | 109/L |
| Haematocrit | 36.1 ± 0.34 | 26.0–47.0 | % |
| Average red blood cell volume | 44.7 ± 0.66 | 38.0–54.0 | fL |
| Average haemoglobin content in red blood cells | 14.9 ± 0.14 | 11.8–18.0 | Pg |
| Average haemoglobin concentration in red blood cells | 334 ± 3.12 | 290–360 | g/L |
| Red blood cell distribution index, % | 18.8 ± 0.17 | 16.0–23.0 | % |
| Platelets | 260 ± 5.58 | 100–518 | 109/L |
| Mean platelet volume | 13.9 ± 2.20 | 9.9–16.3 | fL |
A punch biopsy was performed in 19 of the 30 (63.3%) cats, and the biopsies revealed hyperplastic, superficial, deep perivascular dermatitis with eosinophilia and sometimes diffuse eosinophilic dermatitis. These histopathological signs are typical for eosinophilic granuloma in cats. In all cases, mixed inflammatory cells were detected, including neutrophils, macrophages and lymphocytes. No microbes (bacteria or fungi) were detected. Cats had diffuse, ulcerative, papular and nodular lesions in unusual places: the paw, the pulp of the paws and the plane and bridge of the nose. Eosinophilic microvesicles and microabscesses were observed in the epidermis. Follow-up information was available for 23 of the 30 (76.6%) cats and related to periods ranging from 2 months to 11 years. The follow-up periods were 1 year, 2 years and 4 years in 83.3% (25 out of 30), 43.3% (13 out of 30), and 20% (6 out of 30) of the patients, respectively. No relapses were recorded. Twelve (40%) cats received treatment. In some cases, surgical removal of the granulomas was performed. All of these cats went into remission during follow-up periods ranging from 2 months to 5 years, and no side effects of treatment were recorded. The remaining 18 (60%) cats did not receive treatment. Additional information was available for 20 of the 30 (66.6%) cats. These 20 had spontaneous regression of the lesion during follow-up periods ranging from 2 months to 11 years. The general duration of the disease was recorded for 16 out of 30 (53.3%) cats who recovered spontaneously without treatment and ranged from 1 to 9 months. The results of the treatment measures carried out indicate the effectiveness of using the treatment regimen.
Discussion
In this study, EGC was rare, accounting for 0.14% of cats visiting a veterinary clinic in 15 years. In parallel with other authors on the absence of any predisposition of one sex to EGC, we observed the highest percentage of eosinophilic granuloma complex occurrence among hairless cats (Sphinxes) (73.3%). The average age of cats with EGC was three years, and some authors reported a higher frequency in young animals and found this to be associated with a genetic predisposition (9, 10). The distribution of lesions of the eosinophilic granuloma complex was 87.1% in the skin and 12.9% at the skin–mucosal junction. Flame figures, which are flame-shaped eosinophilic structures in the dermis, form in feline EGC because eosinophils are recruited and degranulated and collagen fibres are partially disrupted but collagen fibrils are not damaged. These findings suggest that eosinophil accumulation and the release of granule contents represent the primary events in feline EGC (1). Histologically, inflammatory infiltrates in our research were most often detected in the submucosal and cutaneous layers, which consisted mainly of eosinophils associated with areas of flame figures. Around these sites, there was intense inflammatory infiltration consisting of frequently occurring macrophages and fewer multinucleated giant histiocytic cells, as described previously by other authors (3, 7). Ulceration of the epidermis and mucous membrane was a common sign of lesions of EGC, especially in cases where the oral cavity was affected. In this study, we were unable to assess the presence of FHV-1 in cases of the eosinophilic granuloma complex in cats. The area that was generally affected in cases of eosinophilic granuloma complex was the oral mucosa. This can be used to make a differential diagnosis and rule out other diseases that affect the same area.
Treatment of feline eosinophilic granuloma is most successful when based on the associated cause(s), and is therefore variously parasiticides, antimicrobials, novel diets, allergen-specific immunotherapy, and avoidance of triggers such as allergens, contactants, drugs or foreign bodies (5, 11, 17, 25). Various treatments for symptoms can be effective in particular cases: glucocorticoids, cyclosporine, immunomodulatory agents (levamisole, thiabendazole, mixed bacterial vaccines, chlorambucil and aurothioglucose), surgery, cryosurgery, laser surgery, radiation therapy and homeopathy (13, 14, 24). The results of the treatment measures carried out indicate the effectiveness of those regimens.
Eosinophilic granuloma complex in cats is a serious pathology and frequently requires lifelong treatment, so it is important to diagnose it quickly and accurately to ensure optimal treatment of affected animals.
Footnotes
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: The scientific activity of Prof. Grzegorz Woźniakowski is funded by the National Science Centre project: UMO-2020/39/B/NZ7/00493.
Animal Rights Statement: None required.
References
- 1.Bardagí M., Fondati A., Fondevila D., Ferrer L. Ultrastructural study of cutaneous lesions in feline eosinophilic granuloma complex Vet Dermatol. 2003;14:297. doi: 10.1111/j.1365-3164.2003.00357.x. . , , - , doi: . [DOI] [PubMed] [Google Scholar]
- 2.Bucci T.J. Intradermal granuloma associated with collagen degeneration in three cats J Am Vet Med Assoc. 1966;148:794. . , , –. . [PubMed] [Google Scholar]
- 3.Buckley L., Nuttall T. Feline eosinophilic granuloma complex(ities): some clinical clarification J Feline Med Surg. 2012;14:471. doi: 10.1177/1098612X12451549. . , , - , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diesel A. Cutaneous Hypersensitivity Dermatoses in the Feline Patient: A Review of Allergic Skin Disease in Cats Vet Sci. 2017;4:25. doi: 10.3390/vetsci4020025. . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diny N.L., Rose N.R., Čiháková D. Eosinophils in Autoimmune Diseases Front Immunol. 2017;8:484. doi: 10.3389/fimmu.2017.00484. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dokuzeylul B., Kayar A., Or M.E. Prevalence of systemic disorders in cats with oral lesions Vet Med (Praha) 2016;61:219. doi: 10.17221/8823-VETMED. . , , - , doi: . [DOI] [Google Scholar]
- 7.Ehlers L.P., Slaviero M., Piccolo Vargas T., Argenta F.F., Driemeier D., Amorim da Costa F.V., Pavarini S.P., Sonne L. Epidemiologic and Pathologic Aspects of Feline Eosinophilic Granuloma Complex Acta Sci Vet. 2019;47 doi: 10.22456/1679-9216.98316. . , , doi: . [DOI] [Google Scholar]
- 8.Falcão F., Faísca P., Viegas I., de Oliveira J.T., Requicha J.F. Feline oral cavity lesions diagnosed by histopathology: a 6-year retrospective study in Portugal J Feline Med Surg. 2020;22:977. doi: 10.1177/1098612X19900033. . , , - , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fondati A., Fondevila D., Ferrer L. Histopathological study of feline eosinophilic dermatoses Vet Dermatol. 2001;12:333. doi: 10.1046/j.0959-4493.2001.00253.x. . , , - , doi: . [DOI] [PubMed] [Google Scholar]
- 10.Fondati A., Fondevila D., Ferrer L. Piecemeal degranulation (PMD) morphology in feline circulating eosinophils Res Vet Sci. 2003;75:127. doi: 10.1016/s0034-5288(03)00040-7. . , , - , doi: . [DOI] [PubMed] [Google Scholar]
- 11.Hopke K.P., Sargent S.J. Novel presentation of eosinophilic granuloma complex in a cat J Feline Med Surg Open Reports. 2019;5:2055116919891548. doi: 10.1177/2055116919891548. . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovács K., Jakab C., Szász A. Laser-assisted removal of a feline eosinophilic granuloma from the back of the tongue Acta Vet Hung. 2009;57:417. doi: 10.1556/avet.57.2009.3.8. . , , - , doi: . [DOI] [PubMed] [Google Scholar]
- 13.Moon M., Suh G.H., Kwon Y.J., Kim H.J. Effective treatment of eosinophilic granuloma in a cat using tacrolimus with prednisolone J Biomed Transl Res. 2017;18:118. doi: 10.12729/jbtr.2017.18.3.118. . , , - , doi: . [DOI] [Google Scholar]
- 14.Mosiienko N.M., Kovalova L.O., Karpiuk V.V. Zastosuvannia mazi «Unibiol» dlia zahoiuvannia eozynofilnoi vyrazky u kotiv (Application of-«Unibiol»-ointment for the treatment of eosinophilic ulcers in cats-in Ukrainian); Aktualni problemy nezaraznoi patolohii tvaryn: materialy vseukrainskoi nauk.-prakt (Actual problems of non-contagious pathology of animals: All-Ukrainian scientific and practical internet conference – in Ukrainian); 2021. pp. 49–52. . In: . , pp. –. . [Google Scholar]
- 15.Niemiec B.A. Oral Pathology Top Companion Anim Med. 2008;23:59. doi: 10.1053/j.tcam.2008.02.002. . , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 16.Oliveira A., van den Broek A. The feline eosinophilic granuloma complex Comp Anim. 2006;11:48. doi: 10.1111/j.2044-3862.2006.tb00008.x. . , , –. , doi: [DOI] [Google Scholar]
- 17.Omelchenko H.O., Avramenko N.O. Kompleks eozynofilnoi hranulomy sered kotiv (Complex eosinophilic granuloma among cats – in Ukrainian); Dosiahnennia ta perspektyvy veterynarnoi nauky: materialy mizhnarodnoi naukovo-praktychnoi internet konferentsii molodykh vchenykh (Achievements and perspectives of veterinary science: international young scientists’ scientific and practical internet conference proceedings – in Ukrainian); 2022. pp. 74–75. . In: . , pp. –. . [Google Scholar]
- 18.Paterson S. Eosinophilic granuloma complex in the cat Comp Anim. 2016;21 doi: 10.12968/coan.2016.21.5.256. . , , doi: . [DOI] [Google Scholar]
- 19.Power H.T., Ihrke P.J. Selected feline eosinophilic skin diseases Vet Clin North Am Small Anim Pract. 1995;25:833. doi: 10.1016/s0195-5616(95)50130-5. . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 20.Pressanti C., Cadiergues M.C. Feline familial pedal eosinophilic dermatosis in two littermates J Feline Med Surg Open Rep. 2015;1:2055116915579683. doi: 10.1177/2055116915579683. . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott D.W., Miller W.H. Feline atopic dermatitis: A retrospective study of 194 cases (1988–2003) Jpn J Vet Dermatol. 2013;19:135. doi: 10.2736/jjvd.19.135. . , , - , doi: . [DOI] [Google Scholar]
- 22.Vishkautsan P., Reagan K.L., Keel M.K., Sykes J.E. Mycobacterial panniculitis caused by Mycobacterium thermoresistibile in a cat J Feline Med Surg Open Rep. 2016;2:2055116916672786. doi: 10.1177/2055116916672786. . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wechsler M.E., Munitz A., Ackerman S.J., Drake M.G., Jackson D.J., Wardlaw A.J., Dougan S.K., Berdnikovs S., Schleich F., Matucci A., Chanez P., Prazma C.M., Howarth P., Weller P.F., Merkel P.A. Eosinophils in Health and Disease: A State-of-the-Art Review Mayo Clin Proc. 2021;96:2694. doi: 10.1016/j.mayocp.2021.04.025. . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 24.Wildermuth B.E., Griffin C.E., Rosenkrantz W.S. Response of feline eosinophilic plaques and lip ulcers to amoxicillin trihydrate clavulanate potassium therapy: a randomised, double-blind placebo-controlled prospective study Vet Dermatol. 2012;23:110. doi: 10.1111/j.1365-3164.2011.01020.x. . , , - , doi: . [DOI] [PubMed] [Google Scholar]
- 25.Wisselink M.A., van Ree R., Willemse T. Evaluation of Felis domesticus allergen I as a possible autoallergen in cats with eosinophilic granuloma complex Am J Vet Res. 2002;63:338. doi: 10.2460/ajvr.2002.63.338. . , , –. , doi: . [DOI] [PubMed] [Google Scholar]


