Abstract
Background
Limited data is available on awareness and clinical management of the airway pressure release ventilation (APRV) mode of ventilation for acute respiratory distress syndrome (ARDS) patients among physicians who work at in adult critical areas. This study aimed to assess the knowledge and current practice of using APRV mode with ARDS patients and identify barriers to not using this mode of ventilation among physicians who work in adult critical areas in Saudi Arabia.
Methods
Between November 2022 and April 2023, a cross-sectional online survey was disseminated to physicians who work in adult critical areas in Saudi Arabia. The characteristics of the respondents were analyzed using descriptive statistics. Percentages and frequencies were used to report categorical variables.
Results
Overall, 498 physicians responded to the online survey. All responders (498, 100 %) reported that APRV is indicated in patients with ARDS, but 260 (52.2 %) did not know if there was an institutionally approved APRV protocol. Prone positioning was the highest recommended intervention by 164 (33.0 %) when a conventional MV failed to improve oxygenation in patients with ARDS. 136 (27.3 %) responders stated that the P-high should be set equal to the plateau pressure on a conventional ventilator while 198 (39.8 %) said that P-low should be 0 cmH2O. Almost half of (229, 46.0 %) responders stated that the T-high should be set between 4 and 6 s, while 286 (57.4 %) said that the T-low should be set at 0.4–0.8 s. The maximum allowed tidal volume during the release phase should be 4–6 ml/kg. Moreover, just over half (257, 51.6 %) believed that the maximum allowed P-high setting should be 35 cmH2O. One third of the responders (171, 34.3 %) stated that when weaning patients with ARDS while in APRV mode, the P-high should be reduced gradually to reach a target of 10 cmH2O. However, 284 (36.9 %) thought that the T-high should be gradually increased to reach a target of 10 s. Most responders (331, 66.5 %) felt that the criteria to switch the patient to CPAP would be to have an FiO2 ≤ 0.4, P-high ≤10 cm H2O, and T-high ≥10 s. Lack of training has been the most common barrier to not using APRV by 388 (77.9 %).
Conclusion
There is a lack of consensus on the use of APRV mode, probably due to several barriers. While there were some agreements on the management of ventilation and oxygenation, there were variations in the selection of the initial setting of APRV. Education, training, and the presence of standardized protocols may help to provide better management.
Keywords: ARDS, APRV, Physicians, Saudi Arabia, Mechanical ventilation
1. Introduction
Airway pressure release ventilation (APRV) is a safe and effective mode of ventilation classified as intermittent mandatory ventilation [1,2]. The APRV mode applies two levels of continuous positive airway pressure (CPAP) and allow unrestricted spontaneous breathing regardless of the ventilator cycle, so patients can be more comfortable [3]. This mode of ventilation is designed to improve oxygenation by increasing mean airway pressure (MAP) and achieve lung recruitment while maintaining convenient peak airway pressure (PIP) in acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) patients [4]. The findings of previous studies demonstrated significant improvements in gas exchange and arterial oxygenation as measured by the arterial oxygen pressure/the fraction of inspired oxygen (PaO2/FIO2) ratio when using APRV mode compared with other modes of ventilation for ARDS patients [[5], [6], [7]]. More importantly, the APRV mode has been shown to improve oxygenation and a subsequent decrease in length of stay and ventilator days in adult patients with coronavirus disease (COVID-19) requiring mechanical ventilation [8].
Despite the wide use of APRV mode as a rescue therapy for ARDS patients in many intensive care units (ICU) around the world, the settings of this mode are slightly different in terminology, but the concepts are similar to other conventional modes [2,9]. APRV settings include P-high, T-high, P-low, and T-low, and the users of this mode should be aware of the rationales and differences of these setting variables on the mechanical ventilators. P-high reflects the high level of CPAP pressure that applies for a prolonged period (T-high) to promote adequate lung volume and achieve alveolar recruitment. P-low reflects the low level of (CPAP pressure) that is typically applied for a short period of time (T-low); most carbon dioxide removal occurs at this level. Conceptually, spontaneous breathing in APRV mode is allowed at any point during the ventilatory cycle, and the main goal of this mode is to maintain adequate oxygenation and achieve recruitment without overdistention of the lung during P-high and provide adequate ventilation during P-low level to avoid air trapping or intrinsic positive end-expiratory pressure (PEEP) [2,9,10].
To date, there are limited studies investigating the knowledge and clinical management of using the APRV mode for ARDS patients among clinical practitioners [11,12]. Therefore, our aim in this study was to assess the knowledge and current practice of using APRV mode with ARDS patients and identify barriers to not using this ventilatory mode among physicians who work at critical care units in Saudi Arabia.
2. Methods
2.1. Study design and instrument
This cross-sectional study used a questionnaire that was developed by experts (respiratory therapists, ICU physicians, and pulmonary physicians) who have experience with the APRV mode based on the available literature [10,11,13]. The questionnaire consisted of 25 items in three sections, including collecting data about demographics, knowledge and clinical practice of APRV mode, and barriers to not using APRV mode. Choices in Section 2 of the questionnaire (knowledge and practice of APRV mode) were based on available references and strategies for using and operating the APRV mode [10,11,13]. The questionnaire was piloted and evaluated by ten (ICU physicians and pulmonary physicians) to ensure the comprehensibility and clarity of the questions as well as the answers.
2.2. Data collection and sampling
The questionnaire was available and distributed via the SurveyMonkey platform from November 2022 to April 2023. ICU physicians and pulmonary physicians were invited to participate in this survey via professional groups on social media platforms. Additionally, the survey was sent to members of the Saudi Critical Care Society and the Saudi Thoracic Society. Moreover, five data collectors from different regions of Saudi Arabia were responsible for distributing the survey at their regional hospitals. A convenience sampling strategy was used, and ICU physicians and pulmonary physicians who work at adult critical areas in Saudi Arabia were the main target in this survey. The consent of participants and information about the study as well as point of contact were introduced at the beginning of the questionnaire. Before starting the survey, we stated the consent as follows “By answering the first question, you voluntarily agree to participate in this study and give your consent to your anonymous data being used for research purposes.” Additionally, all potential participants were required to consent before participation. To avoid duplication and repetitive responses, participants were allowed to fill out the survey link once only. The expected time to complete the questionnaire was 10 min.
2.3. Data analysis
Data collection was automatically collected and exported to an Excel file. Data verification was initially performed by the main author, and a second author was available or cross-verification to reduce the likelihood of errors during data entry. The data were analyzed using descriptive analysis. Frequency and percentages were used to summarize the results. The R studio software was used to calculate the responses and data visualization.
2.4. Ethical approval
Ethical approval for this study was obtained from the Bioethics and Research Committee at Jazan University, Saudi Arabia (REC-44/04/364).
3. Results
Demographics and characteristics: Overall, 498 physicians responded to the survey. Half of respondents 284 (56.6 %) had 1–5 years of experience, followed by 113 (22.7 %) respondents had less than a year of clinical experience, 70 (14.1 %) respondents had from six to ten years of clinical experience, and 33 (6.6 %) had more than ten years of clinical experience. (see Table 1).
Table 1.
The demographics and characteristics of responders.
| Characteristic | N = 498 |
|---|---|
| Sex, n (%) | |
| Female | 189 (38.0) |
| Male | 309 (62.0) |
| Geographical location, n (%) | |
| Western Region | 203 (40.8) |
| Central Region | 188 (37.8) |
| Southern Region | 67 (13.5) |
| Eastern Region | 38 (7.6) |
| Northern Region | 2 (0.4) |
| Workplace, n (%) | |
| Ministry of Health Hospitals | 251 (50.4) |
| Ministry of Defense Hospitals | 113 (22.7) |
| Ministry of National Guard Health Affairs Hospitals | 60 (12.0) |
| University Hospitals | 30 (6.0) |
| King Faisal Specialist Hospitals & Research Centre (Riyadh, Jeddah, Medina) | 19 (3.8) |
| Ministry of Interior Hospitals | 16 (3.2) |
| Private Hospitals | 7 (1.4) |
| Royal Commission Hospitals | 2 (0.4) |
| Experience, n (%) | |
| Less than 1 year | 113 (22.7) |
| 1–5 years | 282 (56.6) |
| 6–10 years | 70 (14.1) |
| More than 10 years | 33 (6.6) |
Two-third of responders 329 (66.1 %) reported usually caring for one or two patients with ARDS per shift. A considerable majority 356 (71.5 %) reported usually working in critical care units. Most responders reported that they had not received adequate training on APRV mode 385 (77.3 %) and only 155 (31.1 %) ever used APRV for patients with ARDS. Half of the responders 260 (52.2 %) did not know whether APRV was used within their working hospital. Moreover, 324 (65.1 %) did not know whether there was an institutionally approved APRV protocol. (Supplementary Table 1).
3.1. Awareness about APRV mode
All responders 498 (100 %) reported that APRV is indicated in patients with ARDS whereas 390 (78.3 %) reported that APRV is recommended in patients with COVID-19. One-third of responders 164 (33.0 %) stated that the highest recommended intervention when conventional MV fails to improve oxygenation in patients with ARDS would be prone positioning, followed by pulmonary vasodilators 132 (26.5 %), whereas the use of APRV was only recommended by 88 (17.7 %) (Supplementary Table 1)
3.2. Clinical management of APRV mode in ARDS patients
3.2.1. APRV initial settings
When initiating APRV mode in patients with ARDS, over one-quarter of responders 136 (27.3 %) believed that the P-high should be set equal to the plateau pressure on a conventional ventilator, while nearly as many 135 (27.1 %) reported setting the P-high 2–5 cmH2O above the mean airway pressure on conventional ventilator. When setting the P-low, over one-third of responders 198 (39.8 %) stated that a 0 cmH2O P-low should be used, followed by 110 (22.1 %) responders who thought that the P-low should match the PEEP level of the conventional ventilation. Nearly half of the responders 229 (46.0 %) stated that the T-high should be set at between 4 and 6 s, whereas over one quarter 138 (27.7 %) thought this should be 2–3 s. Majority of responders 286 (57.4 %) reported that the T-low should be set at 0.4–0.8 s, followed by 101 (20.3 %) responders who recommended the T-low to be set per the desired inspiratory to expiratory (I:E) ratio seconds (Supplementary Table 1).
3.2.2. APRV management
Most responders 337 (67.7 %) reported that when using APRV, the maximum allowed tidal volume during the release phase should be 4–6 ml/kg. Moreover, 257 (51.6 %) reported that the maximum allowed P-high setting should be 35 cmH2O followed by 132 (26.5 %) who reported 40 cmH2O. Just over half 258 (51.8 %) reported that pressure support can be used during spontaneous breaths in the APRV mode in patients with ARDS (Supplementary Table 1).
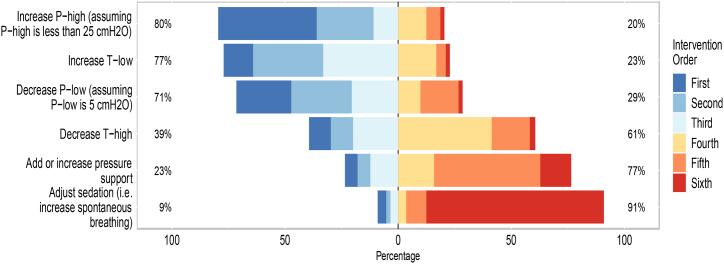
When utilizing APRV in managing patients with ARDS, 217 (44 %) reported increasing P-high (assuming P-high is less than 25 cmH2O), followed by 121 (24 %) reported decreasing P-low (assuming P-low is 5 cmH2O) would be their first and second prefatory choices to manage hypoxia. Fig. 1 shows the preferred order of adjusting the APRV parameters when PaCO2 is elevated, and pH is lowered in patients with ARDS.
Fig. 1.
The order of interventions when levels of pH are unacceptably low and the PaCO2 is elevated in patients with ARDS.Legend: The X-axis represents the percentage of intervention order. The first to third intervention are on the left side of the figure, whereas the fourth to sixth interventions are on the right side. The Y axis shows the interventions. Withing the figure box, the Y axis shows the percentages of being a first to third intervention (left) or being a fourth to sixth intervention (right). The complete percentages of the intervention orders are reported in Supplementary Table 2.
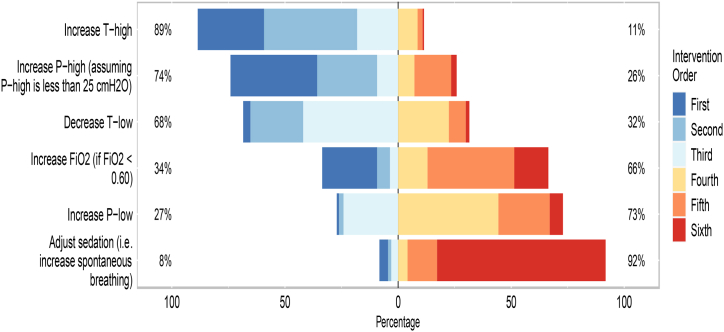
When utilizing APRV in managing patients with ARDS, 191 (38 %) responders reported increasing the T-high followed by 191 (38 %) increasing the P-high (assuming P-high is less than 25 cmH2O) would be their first and second prefatory choices to manage respiratory acidosis. Fig. 2 shows the order of adjusting the APRV parameters when PaO2 is low in patients with ARDS.
Fig. 2.
The order of interventions when levels of oxygen are unacceptably low in patients with ARDS.Legend: The X-axis represents the percentage of intervention order. The first to third intervention are on the left side of the figure, whereas the fourth to sixth interventions are on the right side. The Y axis shows the interventions. Withing the figure box, the Y axis shows the percentages of being a first to third intervention (left) or being a fourth to sixth intervention (right). The complete percentages of the intervention orders are reported in Supplementary Table 3.
3.2.3. APRV weaning and discontinuation
One-third 171 (34.3 %) of responders, stated that when weaning patients with ARDS while in APRV mode, the P-high should be reduced gradually in an attempt to reach a target of 10 cmH2O, followed by 132 (26.5 %) responders, who reported a target of 15 cmH2O. Moreover, 284 (36.9 %) reported that the T-high should be gradually increased in an attempt to reach a target of 10 s, followed by 128 (25.7 %) who reported the target should be 7 s. When oxygenation goals are achieved and the patient with ARDS is clinically stable, most responders 331 (66.5 %) reported that the criteria to switch the patient to CPAP would be to have an FiO2 ≤ 0.4, P-high ≤10 cm H2O, and T-high ≥10 s (Supplementary Table 1).
3.2.4. Barriers to using APRV mode in ARDS patients
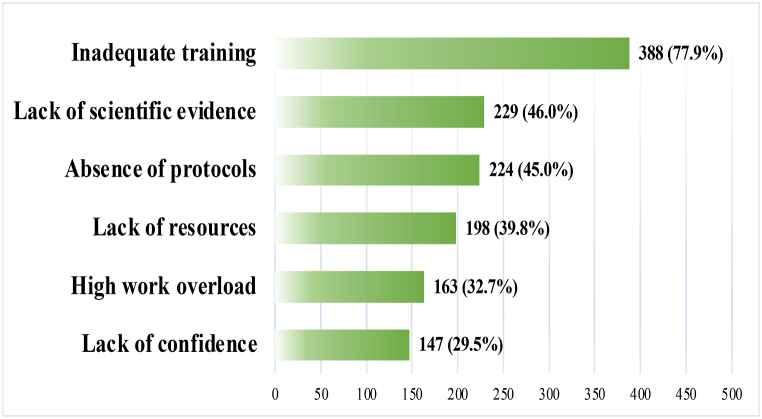
Several barriers were reported by responders. The majority 388 (77.9 %) mentioned the lack of training, followed by a lack of evidence 229 (46.0 %) and the absence of standardized protocols 224 (45.0 %). All reported barriers are depicted in Fig. 3.
Fig. 3.
The most common barriers to not using APRV mode.
4. Discussion
To the best of our knowledge, this is the first national study to assess the knowledge and current practice of using the APRV mode in ARDS patients among ICU and pulmonary physicians in Saudi Arabia, as well as the barriers to not using the APRV mode in ARDS patients. In general, there was no consensus on which intervention should be considered when conventional MV fails to improve the oxygenation status in ARDS patients. Moreover, variations were observed in the initial APRV settings, but there was an overall modest agreement on how to manage ventilation and oxygenation as well as how to wean off and discontinue APRV use.
In treating ARDS patients with worsening oxygenation, several strategies can be considered, including APRV mode, high frequency oscillations, and prone positioning [14]. Among these, APRV mode has shown physiological benefit by promoting alveolar recruitment and gas exchange [[5], [6], [7]]. However, it is not straightforward mode, as it requires selecting and adjusting four important parameters (P-high, T-high, P-low, and T-low) in order to provide such a benefit. Consequently, guidelines have been developed to assist clinicians in initiating and manipulating APRV parameters [10,13]. The current guidelines recommendation for these parameters are that P-high should be set at a level sufficient for lung recruitment (typically between 15 and 25 cm H2O), P-low should be set at 0 cm H2O to facilitate ventilation, T-high should be set at 4–6 s to ensure adequate oxygenation and alveoli recruitment, T-low should be set based on expiratory flow pattern analysis (set at around 0.4–0.8 s) to prevent lung decruitment during expiration [10,13]. However, the current guidelines for APRV mode have limitations. First, they lack standardized recommendations regarding specific parameters settings, leading to variability in clinical practice. Second, the use of arbitrary numbers suggesting initial setting, the guideline may not account for the individual patient variability and specific clinical conditions, leading to potential suboptimal setting for patients. Last, the evidence supporting the effectiveness of APRV mode is still evolving, and there is a need for more robust clinical trials to validate and refine the guidelines for its use.
At 44 % of institutions, the APRV mode was the third choice (17.7 %) for ARDS patients who had not improved with conventional MV. This suggests a lower preference among physicians in Saudi Arabia. This could be due to several factors, such as limited familiarity or experience with APRV mode, lack of strong evidence supporting its superiority over other strategies, and institutional factors (i.e. available resources, equipment and protocol). Our findings differ from previous work by Miller et al. [11], where APRV was the first choice. The variations may be due to the larger sample size (498 participants) compared to Miller et al.’s small sample size (60 participants). Moreover, Miller et al.’s survey included only AARConnect members, potentially causing sampling bias, whereas our study targeted all critical care physicians using convenience sampling.
In our study, we observed variations in APRV parameters settings, with some deviating from established guidelines [10,13]. Particularly, the T-low, which guidelines suggest setting based on the expiratory flow pattern analysis, was selected arbitrarily within a time range of 0.4–0.8 s by 57 % of respondents. Miller et al., also reported that 39 % selected arbitrary numbers [11]. Given the patients variability and different disease condition and severity, the use of arbitrary numbers to set T-low without paying attention to the expiratory flow pattern, may compromise alveolar recruitment and oxygenation. This, in turn, will likely reduce the effectiveness of APRV mode, affecting patients’ outcomes.
The second variation was seen in the initial P-low, as 41 % of the respondents selected either a specific arbitrary number or matching PEEP from conventional mechanical ventilation, despite the guideline recommendations to set this at 0 cm H2O [10,13]. Our findings differ from those of Miller et al. [11] where only 16 % used similar strategies. Of note, 78 % of our respondents followed the guidelines and selected P low at 0 cm H2O. Setting P-low >0 cmH2O could result in the delay of peak expiratory flow rate, decreasing the percentage of the cycle time at T-high, which may affect alveolar recruitment [13]. Moreover, it has been demonstrated that using P-low >0 cm H20 result in CO2 retention and negative impact on secretion clearance [15]. This, in turn, may reduce the benefits of utilizing APRV, may negatively impact patient outcomes and potentially prolonging the duration of mechanical ventilation.
In the current study, the third variation occurred in the initial T-high setting. Surprisingly, 28 % of the respondents selected 2–3 s, despite the guideline recommendations that to optimize alveolar recruitment in APRV, T-high should be set at 80–95 % of the cycle time (to be set using a range of 4–6 s) [10,13]. The use of T-high for less than 4 s will result in decreased mean airway pressure, possibly leading to airway closure [13]. This, in turn, will affect alveolar recruitment by reducing alveolar surface area, likely resulting in less gas exchange compared to T-high >4 s. These variations of the initial setting highlight that there is a lack of knowledge and adherence to published guidelines, and by implementing a hospital-based standardized protocol, physicians can be guided not only to consider the APRV mode but also to use it safely and confidently.
For lung-protective strategies, it is recommended that plateau pressure (P-plat) should not exceed 30 cm H2O [16]; however, limiting P-plat is not included in the guidelines. Rather, the guidelines recommend that P-high should not exceed 30–35 cm H2O [10,13]. In our study, we found that 34 % of the respondents selected >35 as the maximum allowed setting for P-high. A similar finding was presented by Miller et al., in which 36 % of respondents exceeded the P-high limit [11]. P-plat equal to or greater than 30 cm H2O may result in ventilator-induced lung injury (VILI), such as barotrauma [17]. Given that P-plat is measured when there is no gas flow, the harm from exceeding P-high may be greater than the harm from exceeding P-plat. Hence, we recommend that P-plat be included in future guidelines.
In the current study, the maximum allowed tidal volume was assessed, and the majority of respondents (76 %) selected the safe range of 4 – 6 ml/kg. Our study findings differ from the Miller et al., who reported that 44 % of their respondents selected a tidal volume that is associated with a high chance of VILI in ARDS [11]. The guidelines do not provide any specific recommendations about tidal volume [10,13]. Given that our study showed 10 % selecting a less safe tidal volume (>8 ml/kg) and the findings from Miller et al. [11], guidelines should consider including tidal volume in their recommendations, helping to reduce the chances of VILI.
Managing ventilation and oxygenation in APRV mode may be challenging, especially without solid understanding of the underlying physiological concept. Guidelines recommend specific adjustments to the four APRV parameters to address hypoxemia and respiratory acidosis. In regards to managing hypoxemia, guidelines recommend increasing P-high by 2–5 cm H2O and/or increasing T-high by 0.5–1 s, and decreasing T-low (i.e., release time) [10,13]. Most respondents in the study followed these guidelines by adjusting P-high, T-high, or T-low accordingly. For managing respiratory acidosis in patients, published guidelines recommend increasing T-low by 0.5–1 s, increasing alveolar ventilation (increasing P-high and/or T-high second), or increasing minute ventilation (increasing P-high and decreasing T-high second) [10,13]. While there was variation in the parameters adjustment order, the surveyed physicians appeared to understand which parameters to consider for ventilation or oxygenation. Future guidelines should consider providing a detailed algorithm for ventilation and oxygenation management to assist healthcare providers in their decision-making process.
Guidelines recommend staring the weaning process in APRV mode by concurrently decreasing P-high and increasing T-high [10,13]. This will lead to a gradual decrease in the mean airway pressure and an increase in the contribution of spontaneous to total minute ventilation [13]. Before switching the patient to CPAP, it is recommended to set P-high at 16 and have T-high exceed 12–15 s, allowing for all minute volumes to be accounted for by spontaneous breathing. In the study, most respondents agreed on gradually decreasing P-high to 10–15 cm H2O and gradually increasing T-high until it reached >10–15 s. Moreover, most of the respondents (66 %) were aware of the criteria (FiO2 ≤ 40 %, P-high ≤10 cm H2O and T-high ≥10 s) before switching to CPAP [13]. While most physicians demonstrated knowledge of weaning from APRV, some responses deviated from the guidelines, suggesting potential barriers that need to be addressed.
While the APRV mode is an effective mode of mechanical ventilation in ARDS patients, certain barriers that may hinder its use in clinical practice. In the present study, inadequate training, lack of scientific evidence, and absence of protocol were reported by the physician as common barriers. These barriers may explain the variations in the initial settings, ventilation and oxygenation management, weaning, and discontinuation of the mode. To overcome these barriers, recommendations include providing healthcare professionals with adequate training, conducting large and better-designed RCTs, promoting knowledge sharing through workshops and conferences, and implementing hospital-based protocols. These recommendations, in turn, will increase healthcare providers confidence of using the mode.
The variations observed in the setting of P-high, P-low, T-high and T-low in our study have important clinical implications. These deviations from the current guidelines highlight the need for further standardization and consensus in clinical practice. Specifically, the discrepancies in the selection of P-high raise concerns about potential lung injury, as values exceeding the recommended setting were chosen by a considerable percentage of respondents. Similarly, the arbitrary selection of P-low, T-high, and T-low suggests a lack of adherence to guideline recommendations. It is crucial to address these variations and promote evidence-based practice to optimize patient care and outcomes while using APRV mode. Further research, education, and the development of clear guidelines are essential to improving the consistency and effectiveness of APRV mode in clinical practice.
The present cross-sectional study is the first to provide a comprehensive assessment of the APRV mode in the current clinical practice among physicians, evaluating several aspects, such as an indication of its use, initiation of the mode, adjustment of the four APRV parameters, weaning, and discontinuation of the mode, and the common barriers encountered. Another strength is that the study was multi-center and national, including physicians across Saudi Arabia. However, there are limitations to consider. First, the study utilized convenience sampling which may introduce bias; therefore, caution should be taken when interpret our results as it cannot be generalized. Second, the study design was cross-sectional and used surveys, that are dependent on self-reported data, perhaps leading to recall bias. Third, there may be a response bias, as only physicians interested in the APRV mode may have participated in the study. Fourth, the survey method limits a deeper understanding of the reasons behind the lack of knowledge and understanding of the APRV mode in physicians, although one question of the survey assessed the barriers to not using APRV mode. Mixed-method approach is needed for future study to capture respondents’ insight. Lastly, the study was conducted in Saudi Arabia and therefore, the findings may not reflect the practices or perspective of physician in other countries.
5. Conclusion
In conclusion, there is a lack of consensus on the use of APRV among physicians in Saudi Arabia, probably due to several barriers, including lack of training, encountered in its use. While there was some agreement on the management of ventilation and oxygenation, there were variations in the selection of the initial setting of APRV. This is mainly due to the lack of knowledge and understanding, as the mode requires a deep understanding of respiratory physiology. Education and training in the physiology, initiation, and manipulation of the APRV mode for physicians will help in overcoming this. Moreover, standardized protocols based on published guidelines may help to provide better management and use of APRV.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Abdulelah M. Aldhahir: Writing – review & editing, Project administration, Conceptualization. Abdullah A. Alqarni: Supervision, Methodology, Conceptualization. Mohammed A. Almeshari: Writing – review & editing, Writing – original draft, Formal analysis. Nowaf Y. Alobaidi: Writing – review & editing, Writing – original draft, Validation, Supervision, Formal analysis, Conceptualization. Omar A. Alqarni: Writing – review & editing, Writing – original draft, Resources. Saeed M. Alghamdi: Writing – original draft, Investigation. Foton S. Alkhonain: Project administration, Data curation. Esraa A. Qulisy: Project administration, Data curation. Rayan A. Siraj: Writing – review & editing, Writing – original draft, Visualization, Validation. Mansour S. Majrshi: Writing – original draft, Supervision, Resources, Project administration. Ahmed H. Alasimi: Writing – review & editing, Writing – original draft, Methodology. Mohammed M. Alyami: Supervision, Project administration, Conceptualization. Jaber S. Alqahtani: Investigation, Conceptualization. Hassan Alwafi: Writing – original draft, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22725.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Roth A. Elsevier; 2020. Mechanical Ventilation: Airway Pressure Release Ventilation (Respiratory Therapy) [Google Scholar]
- 2.Daoud E.G., Farag H.L., Chatburn R.L. Airway pressure release ventilation: what do we know? Respir. Care. 2012;57(2):282–292. doi: 10.4187/respcare.01238. [DOI] [PubMed] [Google Scholar]
- 3.Daoud E.G. Airway pressure release ventilation. Ann. Thorac. Med. 2007;2(4):176. doi: 10.4103/1817-1737.36556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demirkol D., Karabocuoglu M., Citak A. Airway pressure release ventilation: an alternative ventilation mode for pediatric acute hypoxemic respiratory failure. Indian J. Pediatr. 2010;77:1322–1325. doi: 10.1007/s12098-010-0214-y. [DOI] [PubMed] [Google Scholar]
- 5.Putensen C., et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am. J. Respir. Crit. Care Med. 2001;164(1):43–49. doi: 10.1164/ajrccm.164.1.2001078. [DOI] [PubMed] [Google Scholar]
- 6.Varpula T., et al. Combined effects of prone positioning and airway pressure release ventilation on gas exchange in patients with acute lung injury. Acta Anaesthesiol. Scand. 2003;47(5):516–524. doi: 10.1034/j.1399-6576.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 7.Sydow M., et al. Long-term effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am. J. Respir. Crit. Care Med. 1994;149(6):1550–1556. doi: 10.1164/ajrccm.149.6.8004312. [DOI] [PubMed] [Google Scholar]
- 8.Joseph D.A.K., et al. A Pilot Study of patients with COVID–19-related respiratory failure utilizing airway pressure release ventilation (APRV) Innovations in Surgery and Interventional Medicine. 2021;1(1):3–8. [Google Scholar]
- 9.González M., et al. Airway pressure release ventilation versus assist-control ventilation: a comparative propensity score and international cohort study. Intensive Care Med. 2010;36:817–827. doi: 10.1007/s00134-010-1837-1. [DOI] [PubMed] [Google Scholar]
- 10.Modrykamien A., Chatburn R.L., Ashton R.W. Airway pressure release ventilation: an alternative mode of mechanical ventilation in acute respiratory distress syndrome. Cleve. Clin. J. Med. 2011;78(2):101–110. doi: 10.3949/ccjm.78a.10032. [DOI] [PubMed] [Google Scholar]
- 11.Miller A.G., et al. Clinical management strategies for airway pressure release ventilation: a survey of clinical practice. Respir. Care. 2017;62(10):1264–1268. doi: 10.4187/respcare.05494. [DOI] [PubMed] [Google Scholar]
- 12.Dushianthan A., et al. Intensive care physicians' perceptions of the diagnosis & management of patients with acute hypoxic respiratory failure associated with COVID-19: a UK based survey. J. Intensive Care Soc. 2022;23(3):285–292. doi: 10.1177/17511437211002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habashi N.M. Other approaches to open-lung ventilation: airway pressure release ventilation. Crit. Care Med. 2005;33(3 Suppl):S228–S240. doi: 10.1097/01.ccm.0000155920.11893.37. [DOI] [PubMed] [Google Scholar]
- 14.Cherian S.V., et al. Salvage therapies for refractory hypoxemia in ARDS. Respir. Med. 2018;141:150–158. doi: 10.1016/j.rmed.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahajan M., et al. Time-controlled adaptive ventilation (TCAV) accelerates simulated mucus clearance via increased expiratory flow rate. Intensive Care Med Exp. 2019;7(1):27. doi: 10.1186/s40635-019-0250-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neto A.S., et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: a systematic review and individual patient data analysis. Crit. Care Med. 2015;43(10):2155–2163. doi: 10.1097/CCM.0000000000001189. [DOI] [PubMed] [Google Scholar]
- 17.Steinhorn R., Fitzsimons M.G. In: Evidence-Based Practice of Anesthesiology. fourth ed. Fleisher L.A., editor. Elsevier; Philadelphia: 2023. 55 - what works in a patient with acute respiratory distress syndrome? pp. 484–495. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.