Abstract
Background
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR TKIs) are established first-line treatments among patients with metastatic non-small cell lung cancer harboring EGFR-sensitizing mutations. Upon EGFR TKI resistance, there are scant data supporting a standard of care in subsequent lines of therapy.
Objective
We aimed to characterize real-world treatment patterns and adverse events associated with hospitalization in later lines of therapy.
Methods
This retrospective analysis of administrative claims included adults with metastatic non-small cell lung cancer who initiated a next line of therapy (index line of therapy) following EGFR TKI and platinum-based chemotherapy discontinuation on/after 1 November, 2015. Treatment regimens and adverse event rates during the index line of therapy were described.
Results
Among 195 eligible patients (median age: 59 years; female: 60%), the five most common index line of therapy regimens were immune checkpoint inhibitor monotherapy (29%), EGFR TKI monotherapy (21%), platinum-based chemotherapy (19%), non-platinum-chemotherapy (13%), and EGFR TKI combinations (9%). The overall median (95% confidence interval) time to discontinuation of the index line of therapy was 2.8 (2.1–3.2) months. Common adverse events associated with hospitalizations included infection/sepsis, pneumonia/pneumonitis, and anemia (2.9, 2.8, and 2.0 per 100 person-months, respectively).
Conclusions
Among EGFR TKI-resistant patients who discontinued platinum-based chemotherapy, the duration of the next line of therapy was short, treatment was highly variable, and re-treatment with EGFR TKIs and platinum-based regimens was common, suggesting a lack of standard of care in later lines. Adverse event rates associated with hospitalization were high, especially among platinum-treated patients. These results underscore the unmet need for new therapies in a later line of treatment to reduce the clinical burden among patients in this population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40801-023-00383-1.
Key Points
| Epidermal growth factor receptor-tyrosine kinase inhibitors are established first-line treatments for patients with metastatic non-small cell lung cancer harboring an epidermal growth factor receptor-sensitizing mutation. Upon epidermal growth factor receptor-tyrosine kinase inhibitor resistance, there are scant data to support the standard of care in subsequent lines of therapy. |
| This study found that after discontinuation of an epidermal growth factor receptor-tyrosine kinase inhibitor and platinum-based chemotherapy, the most common subsequent treatment was immune checkpoint inhibitor monotherapy (29%), and re-use of epidermal growth factor receptor-tyrosine kinase inhibitor monotherapy (21%) and platinum-based chemotherapy (19%) was common. The most frequent adverse events associated with hospitalizations included infection/sepsis, pneumonia/pneumonitis, and anemia (2.9, 2.8, and 2.0 per 100 person-months, respectively). |
| In the absence of standard of care, treatment in the subsequent line of therapy was highly variable, and the time to treatment discontinuation was short [median (95% confidence interval): 2.8 (2.1–3.2) months]. These results underscore the unmet need for new therapies to reduce the clinical burden among patients in this population. |
Introduction
Despite advances in therapy over the past decade, lung cancer remains the leading cause of cancer mortality in the USA [1] and is associated with a substantial clinical burden. The number of new lung cancer cases in the USA was estimated to be 236,740 in 2022 [2], with direct costs for lung cancer estimated at approximately $15 billion annually [3]. Non-small-cell lung cancer (NSCLC) is the most common form of lung cancer and comprises 80–85% of all cases [4]. Most NSCLC cases are diagnosed at an advanced or metastatic stage (i.e., 53–72% at Stages III or IV) [5–7], and NSCLC with distant metastases at diagnosis is associated with a 5-year relative survival rate of just 6% [8]. Even for those patients initially diagnosed with localized disease, nearly half develop recurrent and/or metastatic disease after a complete tumor resection [9].
Approximately 15% of US patients with metastatic NSCLC (mNSCLC) and adenocarcinoma histology harbor an epidermal growth factor receptor (EGFR)-sensitizing mutation (exon 19 deletions or exon 21 L858R mutations) [10], with a higher prevalence among never-smokers or former light smokers, and female or Asian patients. [11, 12] For patients with EGFR-sensitizing mutations, EGFR tyrosine kinase inhibitors (EGFR TKIs) have demonstrated superiority to chemotherapy in clinical trials [13], and for most EGFR mutations they are the first-line treatment in the USA per NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®; NCCN 2022) [14]. However, most patients treated with EGFR TKIs eventually develop disease progression [15] and a platinum-based chemotherapy regimen is a recommended subsequent treatment option for those with symptomatic disease [14]. The standard of care following disease progression on EGFR TKIs and platinum-based chemotherapy is less clear.
Non-platinum chemotherapies (e.g., docetaxel with or without ramucirumab, pemetrexed [for adenocarcinoma], albumin-bound paclitaxel, or gemcitabine if not previously given) are often recommended despite limited evidence on clinical efficacy and treatment benefit [14, 16–19]. An understanding of the real-world treatment patterns and outcomes of later lines of therapy (LOTs) will be informative to both healthcare payers and providers and will help evaluate how well existing treatments are meeting the needs of patients with mNSCLC. To this end, this retrospective administrative claim analysis aimed to characterize the treatment patterns and adverse events (AEs) associated with the LOT that followed discontinuation of EGFR TKI and platinum-based chemotherapy regimens in US patients with EGFR-mutated mNSCLC.
Methods
Data Source
This was a retrospective analysis of de-identified secondary medical and pharmacy claims of largely commercially insured patients in the IQVIA PharMetrics® Plus Database (January 2010–September 2019), a longitudinal health plan database of adjudicated medical and pharmacy claims data for individuals enrolled with national and regional US health plans and self-insured employer groups. The database contains de-identified data of more than 210 million enrollees since 2006, including demographics, pharmacy, procedure, and diagnostic codes associated with medical claims [20]. This study was conducted in accordance with ethical guidelines outlined in the Declaration of Helsinki. The research protocol followed in this retrospective study used de-identified secondary administrative claims data that were collected for routine medical care and billing purposes. These data were compliant with the Health Insurance Portability and Accountability Act Privacy rule, and thus did not require Institutional Review Board review [per Code of Federal Regulations Title 45, Subtitle A, Subchapter A, Part 46, Subpart A, §46.104(d)(4)(iii)]. It was not possible to contact patients for informed consent because the data were de-identified.
Study Design
Sample Selection
Patients were eligible for inclusion if they fulfilled the following criteria: (1) had one or more medical claims with a lung cancer diagnosis code (International Classification of Diseases, Ninth Edition [ICD-9]: 162.2–162.9; International Classification of Diseases, 10th Edition [ICD-10]: C34.x) between January 2010 and September 2019; (2) had one or more medical claims with a secondary malignancy diagnosis code, suggesting the presence of metastatic disease (ICD-9: 196.x-198.x; ICD-10: C77.x-C79.x) after the lung cancer diagnosis date (the first medical claim for a secondary malignancy was defined as the metastatic lung cancer diagnosis date); (3) were aged ≥ 18 years at the metastatic lung cancer diagnosis date; (4) had ≥ 6 months of continuous enrollment prior to the metastatic lung cancer diagnosis date; (5) had one or more prescription drug claims for an EGFR TKI (proxy identifier for EGFR-mutated NSCLC; including afatinib, dacomitinib, erlotinib, gefitinib, and osimertinib); (6) had no medical claims for other primary cancers (ICD-9: 140.xx-162.0, 163.xx-172.xx, 174.xx-195.xx, 200.xx-209.36; ICD-10: C00.xx-C33.xx, C35.xx-C43.xx, C45.xx-C76.xx, C81.xx-C96.xx) during the 6 months prior to the metastatic lung cancer diagnosis date, excluding the month immediately preceding this date when a cancer diagnosis could have reflected a rule-out diagnosis; and (7) had one or more medical claims for a platinum-based chemotherapy. The study sample included all patients who initiated a subsequent LOT on or after 13 November, 2015 after discontinuing the first EGFR TKI (or, if more than one EGFR TKI was used and included osimertinib, then the first discontinuation of osimertinib) and platinum-based chemotherapy regimens (in the same LOT or in sequential LOTs). Patients were also required to have a ≥ 1 month continuous enrollment after the subsequent LOT initiation.
LOTs
A treatment-based claims algorithm was applied to identify antineoplastic LOTs after the metastatic lung cancer diagnosis date. Combination regimens were defined as any new drug that was filled/administered within 14 days of a previous drug fill or administration. Termination of a LOT was defined as the discontinuation of all agents in a regimen for ≥ 60 days, or when a new agent was added (triggering advancement to a new LOT). A discontinued agent within a combination regimen did not advance the LOT. The LOT immediately after EGFR TKI and platinum-based chemotherapy discontinuation was defined as the index LOT.
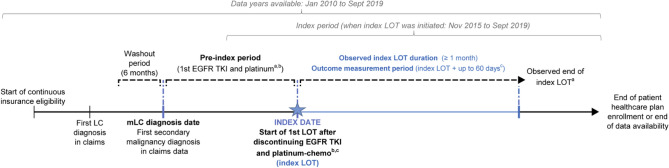
The identification period for the index LOT was between November 2015 and September 2019. As osimertinib was initially approved by the US Food and Drug Administration (13 November, 2015) for the treatment of EGFR T790M mutation-positive NSCLC after progression on prior EGFR TKI therapy [21], patients who initiated osimertinib after receiving a first-generation or second-generation EGFR TKI were included after they discontinued both osimertinib and platinum-based chemotherapy. The follow-up period began at the index date (i.e., initiation date of the index LOT) and ended at the first of the end of continuous insurance eligibility or data availability (Fig. 1).
Fig. 1.
Study design. aPatients who initiated osimertinib after another epidermal growth factor receptor tyrosine kinase inhibitor (EGFR TKI) were included in the study after osimertinib discontinuation; bbaseline use of EGFR TKI and platinum-based chemotherapy can be sequential or concurrent; cobserved duration of a line of therapy (LOT) was defined as the time from the initiation of the LOT until the LOT discontinuation (for patients who discontinued) or the end of the follow-up (for patients still on treatment at the end of the follow-up). The measurement period for outcomes was the observed duration of the index LOT plus 60 days, or prior to the beginning of the next LOT if <60 days, to account for any treatment-related AEs that may have resulted in index treatment discontinuation. chemo chemotherapy, LC lung cancer, mLC metastatic lung cancer
Treatment Groups
Index LOTs were classified into seven mutually exclusive treatment groups: (1) EGFR TKI monotherapy; (2) EGFR TKI combination (all other EGFR TKI-containing regimens); (3) platinum-based chemotherapy (± immune checkpoint inhibitor); (4) non-platinum-based chemotherapy (± immune checkpoint inhibitor; no platinum-based chemotherapy); (5) vascular endothelial growth factor receptor (VEGF/R) inhibitors plus any chemotherapy (± immune checkpoint inhibitor); (6) immune checkpoint inhibitor monotherapy; and (7) other regimens.
Measurements and Statistical Analyses
Patient Characteristics
Patient characteristics (i.e., demographics, payer type, year of the index date, selected comorbidities observed 6 months prior to the index date, and National Cancer Institute Comorbidity Index score [22]) were summarized overall and by the index LOT regimen. The National Cancer Institute Comorbidity Index differs from the Charlson Comorbidity Index in that it was designed for use with administrative data, excluding cancer diagnoses as comorbid conditions, and does not attribute points based on patient age [22].
Time to Discontinuation
The median time to discontinuation of the index LOT was measured from treatment initiation until discontinuation (event) or the end of the follow-up (censoring) using a Kaplan–Meier analysis.
AEs Associated with Hospitalization
The observation period for AE outcomes started from the initiation of the first drug in the index LOT regimen and extended to 60 days after discontinuation of the index LOT regimen (for patients who discontinued) or the end of the follow-up (for patients still receiving treatment at the end of the follow-up), or the day prior to the beginning of the next LOT if the patient had less than 60 days between the index LOT discontinuation and the next LOT start. The 60-day observation period was added after index LOT discontinuation to provide sufficient time to account for any treatment-related AEs that might have triggered the discontinuation of the index LOT.
Pre-specified AEs commonly associated with mNSCLC treatment in the literature [23–25] (e.g., infection/sepsis, pneumonia/pneumonitis, anemia, nausea and/or vomiting, constipation, fever, hypothyroidism, fatigue/asthenia, diarrhea, thrombocytopenia) were identified based on diagnosis codes (Table 1 of the Electronic Supplementary Material [ESM]) observed in any position during an inpatient admission and were reported per 100 person-months. The most common AEs associated with hospitalizations were reported.
Results
Characteristics of Patients in the Study Sample
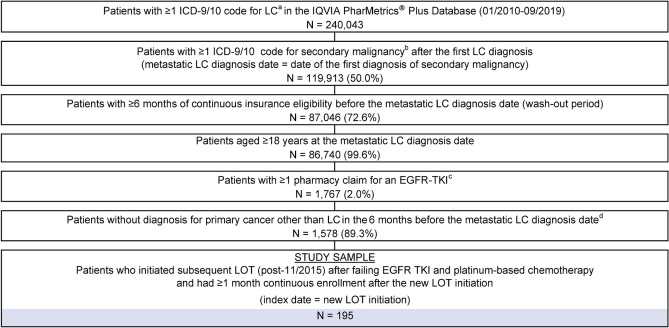
A total of 195 eligible patients were included in the study (Fig. 2). The median age of the study sample at index LOT initiation was 59 years; 60.0% of patients were female, and 39.5% of patients resided in the US South (Table 1). Overall, 59.0% of patients had commercial insurance, 38.5% were self-insured (through employer groups), and 1.5% had either Medicare or Medicaid. The mean National Cancer Institute Comorbidity Index score was 1.4 (standard deviation [SD]: 1.7), and the most common comorbid conditions were hypertension (37.4%) and chronic obstructive pulmonary disease (26.7%). The index date occurred between 2015 and 2017 for 59% and between September 2018 and 2019 for 41% of patients.
Fig. 2.
Selection of study sample. aLung cancer codes: ICD-9/10 International Classification of Diseases, Ninth Edition (ICD-9) 162.2–162.9; ICD-9/10 International Classification of Diseases, Tenth Edition (ICD-10) C34.x; bsecondary malignancy codes: ICD-9 196.xx–198.xx; ICD-10: C77.x–C79.x; cused as a proxy for epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC); included afatinib, dacomitinib, erlotinib, gefitinib, and osimertinib; dexcluding the month immediately preceding the metastatic lung cancer (LC) diagnosis date when a cancer diagnosis could have reflected a rule-out diagnosis. LOT line of therapy, TKI tyrosine kinase inhibitor
Table 1.
Baseline characteristics
| Study sample N = 195 |
Index LOT regimena | ||||||
|---|---|---|---|---|---|---|---|
| EGFR TKI based | Chemotherapy based | ICI monotherapy N = 57 |
|||||
| EGFR TKI monotherapy N = 41 |
EGFR TKI combination N = 18 |
Platinum-based chemotherapy N = 37 |
Non-platinum-based chemotherapy N = 25 |
Chemotherapy+ VEGF/R N = 14 |
|||
| Demographic characteristics | |||||||
| Age, years, median (Q1, Q3) | 59 (54, 64) | 57 (50, 63) | 59 (51, 61) | 58 (52, 63) | 62 (58, 68) | 61 (51, 65) | 60 (56, 64) |
| Female, % | 60.0 | 65.9 | 61.1 | 56.8 | 64.0 | 35.7 | 61.4 |
| US region, % | |||||||
| South | 39.5 | 48.8 | 22.2 | 37.8 | 48.0 | 28.6 | 40.4 |
| Midwest | 25.6 | 19.5 | 50.0 | 27.0 | 12.0 | 42.9 | 24.6 |
| Northeast | 17.4 | 24.4 | 22.2 | 13.5 | 20.0 | 7.1 | 15.8 |
| West | 17.4 | 7.3 | 5.6 | 21.6 | 20.0 | 21.4 | 19.3 |
| Payer type,b % | |||||||
| Commercial | 59.0 | 58.5 | 72.2 | 67.6 | 48.0 | 50.0 | 56.1 |
| Self-insured (through employer groups) | 38.5 | 36.6 | 27.8 | 32.4 | 52.0 | 50.0 | 38.6 |
| Medicaid or Medicare | 1.5 | 2.4 | 0.0 | 0.0 | 0.0 | 0.0 | 3.5 |
| Year of index date, % | |||||||
| 2015–17 | 59.0 | 56.1 | 44.4 | 48.6 | 56.0 | 50.0 | 75.4 |
| 2018/2019 | 41.0 | 43.9 | 55.6 | 51.4 | 44.0 | 50.0 | 24.6 |
| Clinical characteristics | |||||||
| NCICI,c mean ± SD | 1.4 ± 1.7 | 1.5 ± 1.9 | 1.0 ± 1.6 | 1.6 ± 1.6 | 1.2 ± 1.4 | 1.0 ± 1.4 | 1.7 ± 1.7 |
| Selected comorbiditiesd, % | |||||||
| Hypertension | 37.4 | 24.4 | 33.3 | 37.8 | 36.0 | 50.0 | 45.6 |
| COPD | 26.7 | 22.0 | 22.2 | 24.3 | 28.0 | 14.3 | 36.8 |
| Arrhythmias | 18.5 | 14.6 | 5.6 | 21.6 | 28.0 | 28.6 | 17.5 |
| Depression | 17.9 | 14.6 | 5.6 | 16.2 | 28.0 | 14.3 | 21.1 |
| Mild liver disease | 16.4 | 19.5 | 11.1 | 21.6 | 20.0 | 7.1 | 14.0 |
| Pulmonary circulation disorders | 15.9 | 12.2 | 27.8 | 13.5 | 24.0 | 14.3 | 12.3 |
| Coagulopathy | 13.8 | 9.8 | 11.1 | 16.2 | 28.0 | 14.3 | 10.5 |
| Alcohol/drug use disorders | 12.8 | 12.2 | 11.1 | 10.8 | 20.0 | 0.0 | 14.0 |
| Hypothyroidism | 12.3 | 17.1 | 0.0 | 13.5 | 16.0 | 21.4 | 7.0 |
| Peripheral vascular disease | 10.8 | 7.3 | 16.7 | 16.2 | 4.0 | 7.1 | 12.3 |
COPD chronic obstructive pulmonary disease, EGFR TKI epidermal growth factor receptor tyrosine kinase inhibitor, ICI immune checkpoint inhibitor, LOT line of therapy, NCICI National Cancer Institute Combined Index, Q1 quartile 1, Q3 quartile 3, SD standard deviation, VEGF/R vascular endothelial growth factor/receptor
aPatients treated with other index LOT regimens (i.e., those otherwise not listed) are not shown because of the small sample (n = 3)
bUnknown category (1%) not shown
cMeasured in the 6 months pre-index date
dPer Elixhauser et al. [50] and Klabunde et al. [51] with a prevalence > 10%
Treatment Patterns
Pre-Index LOT Regimens
The mean time from mNSCLC diagnosis to initiation of the index LOT was 21.4 (SD: 13.9) months and patients received an average of 2.8 (SD: 1.0) LOTs during this time period (Table 2). More than half (56.4%) of patients were treated with a platinum-based chemotherapy regimen before their first EGFR TKI therapy; 2.6% of patients received both therapies concurrently. Patients received on average 1.5 (SD: 0.7) pre-index LOTs with an EGFR TKI; erlotinib (n = 125; 64.1%) and osimertinib (n = 88; 45.1%) were most frequently prescribed. Immune checkpoint inhibitor prescribing was also noted among the pre-index regimens. Overall, 22.6% (n = 44) of patients had exposure to a pre-index immune checkpoint inhibitor either as monotherapy (10.3% [n = 20]), in combination with platinum-based chemotherapy (11.3% [n = 22]), or in combination with other agents (1.0% [n = 2]).
Table 2.
Pre-index LOTs in the metastatic NSCLC settinga
| Study sample N = 195 |
|
|---|---|
| Pre-index period duration,b months, mean ± SD | 21.4 ± 13.9 |
| Number of pre-index LOTs, mean ± SD | 2.8 ± 1.0 |
| 1 LOT, % | 1.5 |
| 2 LOTs, % | 46.2 |
| 3+ LOTs, % | 52.3 |
| EGFR TKI and platinum chemotherapy patterns | |
| Pre-index EGFR TKI/platinum-based chemotherapy sequence, % | |
| First EGFR TKI before first platinum-based chemotherapy | 41.0 |
| First platinum-based chemotherapy before first EGFR TKI | 56.4 |
| Concurrently | 2.6 |
| Number of pre-index EGFR TKI LOTsc, mean ± SD, % | 1.5 ± 0.7 |
| 1 LOT | 65.1 |
| 2 LOTs | 27.2 |
| 3+ LOTs | 7.7 |
| Patients with ≥ 1 pre-index LOT containing, % | |
| Afatinib | 21.5 |
| Dacomitinib | 0 |
| Erlotinib | 64.1 |
| Gefitinib | 2.6 |
| Osimertinib | 45.1 |
EGFR TKI epidermal growth factor receptor tyrosine kinase inhibitor, LOT line of therapy, NSCLC, non-small cell lung cancer, SD standard deviation
aBy design, all included patients have used EGFR TKIs and platinum-based chemotherapy pre-index (in same LOT or different LOTs)
bFrom the metastatic NSCLC diagnosis to the index date
cIncludes both monotherapy and combination therapy with EGFR TKIs
Index LOT Regimens
Following discontinuation of EGFR TKI and platinum-based chemotherapy, 36.9% (n = 72) of patients received an immune checkpoint inhibitor in the overall cohort, and retreatment with an EGFR TKI (n = 59; 30.3%) and/or platinum-based chemotherapy (n = 54; 27.7%) was common. Most patients (n = 153; 78.5%) started a new LOT at the index (i.e., no common drugs with the immediately prior LOT), 19.0% (n = 37) continued the immediately prior LOT with some modification at the index (i.e., adding, switching, or discontinuing at least one medication), and 2.6% (n = 5) used the same regimen as the immediately prior LOT at the index but had a treatment gap of more than 60 days (not shown).
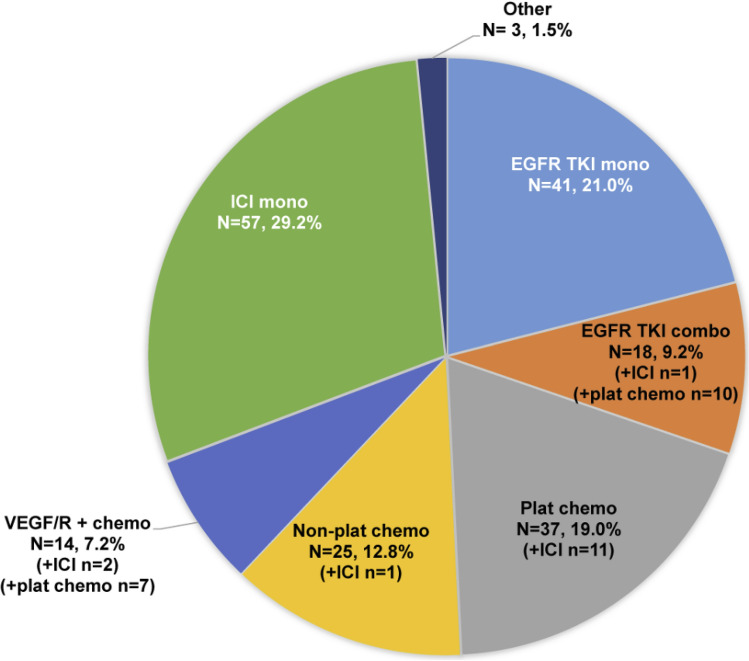
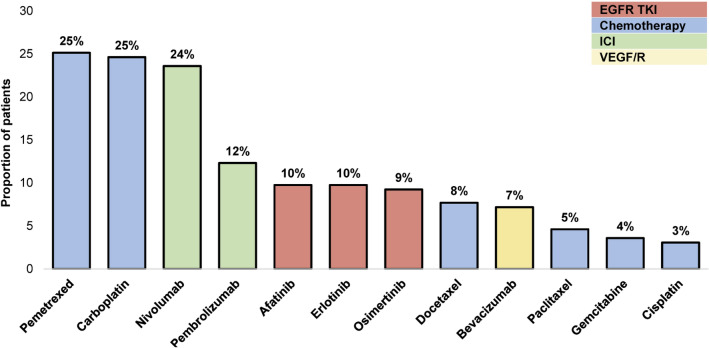
The most common index LOT regimen was immune checkpoint inhibitor monotherapy (n = 57; 29.2%), followed by EGFR TKI monotherapy (n = 41; 21.0%), platinum-based chemotherapy (n = 37; 19.0%), non-platinum-based chemotherapy (n = 25; 12.8%), EGFR TKI combination (n = 18; 9.2%), VEGF/R plus chemotherapy (n = 14; 7.2%), and other regimens (n = 3; 1.5%) with or without an immune checkpoint inhibitor (Fig. 3). Overall, the most frequently prescribed agents in the index LOT were pemetrexed (25.1%), carboplatin (24.6%), nivolumab (23.6%), and pembrolizumab (12.3%). Afatinib, erlotinib, and osimertinib were prescribed for 10%, 10%, and 9% of patients, respectively (Fig. 4).
Fig. 3.
Index line of therapy regimens. chemo chemotherapy, combo combination, EGFR TKI epidermal growth factor receptor tyrosine kinase inhibitor, ICI immune checkpoint inhibitor, LOT line of therapy, mono monotherapy, plat platinum-based, VEGF/R vascular endothelial growth factor/receptor
Fig. 4.
Common agents used in the index line of therapy (LOT) regimens (either as monotherapy or in combination with other agents). Agents with a > 3% frequency are shown. EGFR TKI epidermal growth factor receptor tyrosine kinase inhibitor, ICI immune checkpoint inhibitor, VEGF/R vascular endothelial growth factor receptor
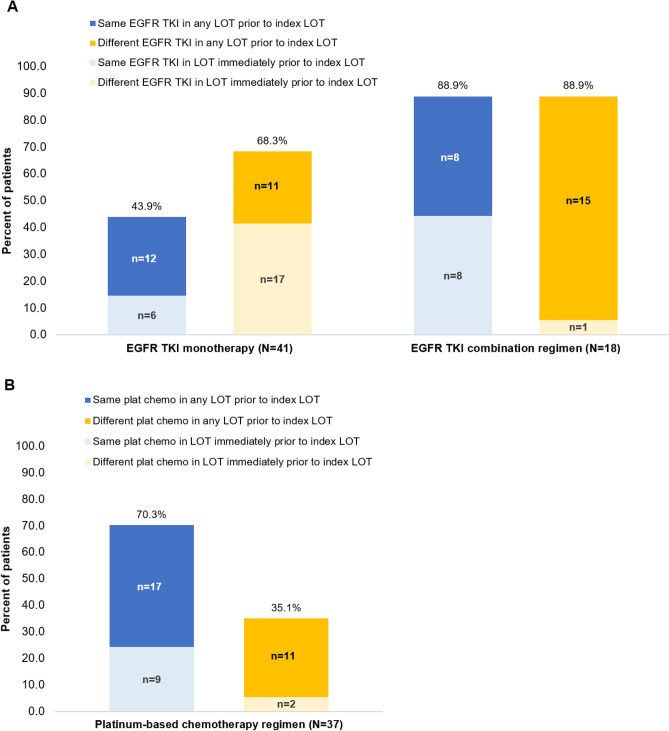
Among patients who received EGFR TKI monotherapy as the index LOT (n = 41), 43.9% (n = 18) had been treated with the same EGFR TKI and 68.3% (n = 28) had received a different EGFR TKI agent (vs the index agent) in a prior LOT (Fig. 5). Of patients who received an EGFR TKI as combination therapy at the index (n = 18), most were re-treated with an EGFR TKI that had been previously prescribed (n = 16; 88.9%), and most had also been previously treated with a different EGFR TKI agent (n = 16; 88.9%). For patients treated with a platinum-based chemotherapy regimen in the index LOT (n = 37), 70.3% (n = 26) had received the same platinum agent in a prior LOT. Only 35.1% (n = 13) of patients received a different platinum agent in a prior LOT.
Fig. 5.
Reutilization of epidermal growth factor receptor tyrosine kinase inhibitor [EGFR TKI] (A) and platinum-based chemotherapy (B) in the index line of therapy (LOT). The EGFR TKI agents included afatinib, erlotinib, gefitinib, and osimertinib. Platinum-based chemotherapy (Plat-chemo) agents included carboplatin and cisplatin
Index LOT Duration
The median study follow-up time after index LOT initiation was 6.9 (interquartile range 3.5–11.8) months, and 26.2% of patients were still receiving their index LOT at the end of the study follow-up. The overall median (95% CI) time to discontinuation of the index LOT was 2.8 (2.1–3.2) months (Table 3). At the regimen level, EGFR TKI combinations had the longest median time to discontinuation at 6.5 (3.1–not estimable) months, and EGFR TKI monotherapy was 3.1 (2.2–5.5) months. However, the median times to discontinuation for platinum-based chemotherapy, non-platinum-based chemotherapy, and immune checkpoint inhibitor monotherapy were all relatively short [1.6 (0.7–3.7), 1.4 (0.3–3.9), and 2.1 (1.4–3.2) months, respectively].
Table 3.
Duration of follow-up and time to treatment discontinuation for the index LOT
| Index LOTa | Patients, n (%) | Median [IQR] Duration of follow-upb, months | Patients on the index LOT at end of the follow-up, % | Median [95% CI] time to treatment discontinuationc, months |
|---|---|---|---|---|
| Study sample | 195 (100%) | 6.9 [3.5–11.8] | 26.2 | 2.8 [2.1–3.2] |
| Regimens | ||||
| EGFR TKI based | ||||
| EGFR TKI monotherapy | 41 (21.0%) | 8.3 [4.1–16.6] | 39.0 | 3.1 [2.2–5.5] |
| EGFR TKI combination | 18 (9.2%) | 5.4 [4.0–8.4] | 55.6 | 6.5 [3.1–NE] |
| Chemotherapy based | ||||
| Platinum-based chemotherapy | 37 (19.0%) | 6.5 [4.4–12.0] | 13.5 | 1.6 [0.7–3.7] |
| Non-platinum-based chemotherapy | 25 (12.8%) | 5.6 [2.8–9.0] | 24.0 | 1.4 [0.3–3.9] |
| VEGF/R + chemotherapy | 14 (7.2%) | 8.9 [4.7–14.4] | 14.3 | 2.3 [0.03–3.5] |
| ICI based | ||||
| ICI monotherapy | 57 (29.2%) | 6.1 [2.8–11.9] | 17.5 | 2.1 [1.4–3.2] |
CI confidence interval, EGFR TKI epidermal growth factor receptor tyrosine kinase inhibitor, ICI immune checkpoint inhibitor, IQR interquartile range, LOT line of therapy, NE not estimable, VEGF/R vascular endothelial growth factor/receptor
aIndex LOT regimens represent the first regimens initiated after the EGFR TKI and platinum-based chemotherapy discontinuation in a metastatic NSCLC setting; other treatment regimen (n = 3) not shown
bMeasured from index LOT initiation to the end of the follow-up
cMeasured from the initiation of the index LOT until discontinuation (event) or the end of the follow-up (censoring), and obtained from a Kaplan–Meier analysis
AEs Associated with Hospitalizations
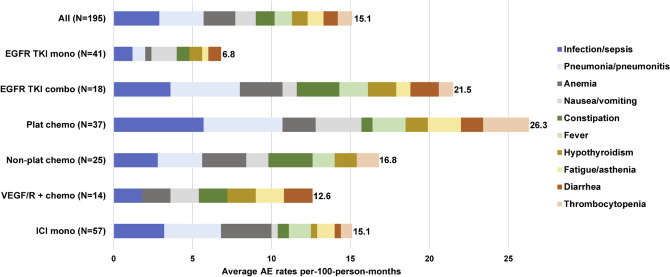
Overall, the most common AEs associated with inpatient admissions during the index LOT were infection/sepsis (2.9 events per-100-person-months on average), pneumonia/pneumonitis (2.8), and anemia (2.0) [Fig. 6 and Table 2 of the ESM]. Patients in the platinum-based chemotherapy group experienced the highest total number of AEs associated with hospitalization (26.3).
Fig. 6.
Common adverse events (AEs) associated with hospitalization during the index line of therapy (LOT). Patients treated with “other” index LOT regimens (n = 3) are not shown because of the small sample. Only the first occurrence of the AE was counted (i.e., based on the first inpatient admission at any diagnosis position because diagnoses observed on distinct days could be part of the same AE episode) for each of the ten most frequent AEs. The number at the end of each bar represents the total rate of AEs for the ten most frequent AEs. Adverse events were measured over the observed duration of the index LOT plus 60 days (or prior to the beginning of the next LOT if < 60 days) to account for any treatment-related AEs that may have resulted in index treatment discontinuation. chemo chemotherapy, combo combination, EGFR TKI epidermal growth factor receptor tyrosine kinas inhibitor, ICI immune checkpoint inhibitor, mono monotherapy, plat platinum, VEGF/R vascular endothelial growth factor/receptor
During the index LOT, infection/sepsis and pneumonia/pneumonitis associated with hospitalizations occurred frequently in the platinum-based chemotherapy (5.7 and 5.0 events per 100 person-months, respectively) and immune checkpoint inhibitor monotherapy (3.2 and 3.6) groups. Anemia was common among patients treated with immune checkpoint inhibitor monotherapy (3.2) and both types of chemotherapies (non-platinum based 2.8; platinum based 2.1); thrombocytopenia (2.9) and fatigue (2.1) were most frequent in the platinum-based chemotherapy group. Among patients treated with EGFR TKI monotherapy at the index, nausea and/or vomiting was the most frequent AE associated with hospitalization (1.6); hepatic toxicity (1.2) and diarrhea (0.8) were also present; however, there were no skin toxicity events recorded.
Discussion
To our knowledge, this retrospective claim database analysis is the first to characterize the real-world treatment patterns and AEs associated with the LOT following discontinuation of EGFR TKI and platinum-based chemotherapy regimens among patients with EGFR-mutated mNSCLC in the USA. Our study showed that a broad range of treatments were used in the index LOT, reflecting the lack of an established standard of care following discontinuation of EGFR TKI and platinum-based chemotherapy. Current NCCN Guidelines® recommended subsequent systemic therapies including docetaxel with or without ramucirumab, pemetrexed (for adenocarcinoma), albumin-bound paclitaxel (for squamous), or gemcitabine if the agent was not previously given and the patient maintains a good performance status [14]. However, only 13 and 7.2% of patients in this study were treated with a non-platinum-based chemotherapy or VEGF/R plus chemotherapy, respectively, post-EGFR TKI and platinum-based chemotherapy discontinuation, possibly reflecting the limited benefit of these therapies for patients with mNSCLC. A trial of docetaxel versus best supportive care in advanced NSCLC previously treated with platinum-based chemotherapy reported that median overall survival was extended by just over 2 months with docetaxel (7.0 vs 4.6 months) [26]. Other trials of docetaxel plus ramucirumab, pemetrexed, or gemcitabine have similarly noted limited improvements in median progression-free survival or overall survival in patients receiving these treatments versus placebo. [27–30]
Approximately half of the patients in this study were retreated with either an EGFR TKI or platinum-based chemotherapy regimen after prior discontinuation of these agents. Continuation of EGFR TKIs beyond disease progression is recommended by the current NCCN Guidelines for patients who experience disease progression upon TKI discontinuation (flare phenomenon) in which case the EGFR TKI might be restarted [14]. Rechallenge with EGFR TKIs has also been discussed in the literature [16–19, 31, 32]. In their review of EGFR resistance mechanisms in lung cancer, Tumbrink et al. noted that several on-target EGFR mutations that promote resistance to osimertinib remain sensitive to first-generation and second-generation EGFR inhibitors and suggest that rechallenging patients with these agents could be an effective strategy [16]. Rechallenging with EGFR TKIs in selected patients may improve tolerability relative to immune checkpoint inhibitor or cytotoxic therapies [24, 33]. Additional literature has discussed a rechallenge with EGFR TKIs, often as case studies or in small cohorts, noting that this approach is occasionally effective [17–19, 31, 32]. While a rechallenge may be effective in some cases, it highlights the lack of effective alternative treatment options in later LOTs as well as our limited knowledge on the optimal sequence of EGFR TKIs among patients with EGFR-mutated mNSCLC [34].
In this study, the use of an immune checkpoint inhibitor in the index LOT was high; approximately one third of patients (29.2%) received monotherapy, and an additional 7.7% received an immune checkpoint inhibitor in combination with other agent(s). Moreover, 75% of patients in the immune checkpoint inhibitor monotherapy treatment group had an index year prior to 2018. During the first half of the study observation period, pembrolizumab had a 2017 NCCN recommendation for patients who progressed on EGFR-TKIs, had multiple lesions, tested T790M negative, and were programmed death-ligand 1 (PD-L1) [≥ 50%] positive [35]. However, beginning in early 2018, the NCCN Guidelines (version 2.2018) noted that data in the second-line setting suggest that programmed cell death protein 1/PD-L1 inhibitors may be less effective in EGFR-mutated (i.e., exon 19, L858R exon 21) NSCLC, irrespective of PD-L1 expression [36, 37].
In our study, the overall median time to index LOT discontinuation was only 2.8 months, underscoring the limited efficacy and tolerability of existing treatment options in the later line setting. This finding is consistent with median treatment durations observed for ramucirumab plus docetaxel (12 weeks) [27] and nivolumab (2.6 months) [38] in clinical trials of treatment for mNSCLC after platinum-based chemotherapy. In addition, this finding is consistent with a retrospective cohort study by Arunachalam et al. of older patients (aged ≥65 years) with stage IIIB/IV disease who received second-line therapy after first-line platinum-based chemotherapy during 2007–13; the median time to second-line discontinuation was 2.7 months [39]. However, in the current study, time to treatment discontinuation was well above the median for patients who received EGFR TKI combinations (6.5 months). Additional studies in a larger sample population are needed to determine the validity of this result.
Inpatient admissions associated with diagnoses of known AEs of antineoplastic therapies were consistent with previous clinical trial data and were observed at exposure-adjusted incidence rates that ranged from 6.7 to 26.4 events per 100 person-months across treatment groups. Patients who received platinum-based chemotherapy experienced the highest total number of AEs associated with hospitalization, possibly because platinum-based chemotherapy was frequently used in combination in this study. A meta-analysis of 22 trials by Magee et al. compared immune checkpoint inhibitors (pembrolizumab, nivolumab, and atezolizumab) with chemotherapy for solid organ tumors (half of which were in lung cancer) and found that 41.1% of patients receiving chemotherapy experienced grade 3 or higher AEs versus 16.5% of those treated with immune checkpoint inhibitors [40]. Similarly, EGFR TKIs have been shown to have fewer associated grade 3 or higher AEs and lower toxicity compared with chemotherapy in the clinical trials of gefitinib [41], erlotinib [42], and osimertinib [43] monotherapy. Although skin toxicities have been previously reported to be common with EGFR TKIs [44, 45], there were no such events associated with hospitalization in this study. However, some patients who received EGFR TKI monotherapy experienced infection or sepsis, albeit at a lower rate than in the platinum-based chemotherapy treatment group as noted in prior real-world studies. [24] Finally, a high AE rate was noted for the EGFR TKI combination group. This may be because of the additive effect of treatment with multiple agents, but the sample size of this treatment group was small (n = 18) and further research is needed to confirm this finding. Findings from this study demonstrate that the AE burden is considerable among patients in later lines of therapy. Future treatments that provide improved tolerability profiles may help to improve patient quality of life and reduce the economic burden to the healthcare system.
The results of this study are subject to several limitations; some are common among claims database analyses such as coding errors, which may lead to misclassification of treatment groups and study outcomes. First, biomarker and histology information were unavailable in claims data, so treatment with EGFR TKI therapy was used as a proxy to identify EGFR-mutated NSCLC. Thus, patients with EGFR-mutated mNSCLC not treated with an EGFR TKI were excluded. Second, the reasons for discontinuation of prior EGFR TKI and platinum-based chemotherapy regimens were unavailable in the database, and their influence on the subsequent treatment is unknown. Third, this study examined the next LOT after EGFR TKI and platinum-based chemotherapy discontinuation starting in November 2015 (i.e., after osimertinib became US Food and Drug Administration approved for second-line treatment, with an expansion in 2018 to first-line treatment for adults with mNSCLC whose tumors have exon 19 deletions or exon L858R mutations). As a result, the treatment sequence of EGFR TKIs observed in this study may not reflect current clinical practice. Fourth, a claims-based algorithm was used to infer LOTs based on the start and end of therapies observed in the data, which may have led to misclassification of the LOT regimens for some patients. Fifth, only AEs that were identified during an inpatient hospital admission and not those managed outside of the hospital setting were presented; as a result, AE rates (e.g., fatigue) may be underestimated. Moreover, some AEs may have been pre-existing conditions, or associated with a previous LOT. Sixth, the sample sizes were small for several treatment groups; therefore, the results stratified by treatment group should be interpreted with caution. Seventh, patient performance status was not available in the administrative claims data and therefore it is unclear how the patient mix may have influenced treatment outcomes. Finally, this is a descriptive study that examined trends and numerical differences without statistical testing as no a priori hypothesis was considered.
Several new therapies are currently in development (e.g., patritumab deruxtecan [ClinicalTrials.gov identifier: NCT03260491] [46], RRx-001 [NCT02489903], and lazertinib plus amivantamab [NCT04077463] [47]) [48, 49] that may help to address the unmet need for patients with EGFR TKI-resistant mNSCLC. Moreover, further research is needed to assess the efficacy and sequencing of EGFR TKI reutilization in later lines of therapy, as well as the efficacy of immune checkpoint inhibitors in patients with EGFR mutations.
Conclusions
Among EGFR TKI-resistant patients with mNSCLC who discontinued platinum-based chemotherapy, treatment in the subsequent LOT was highly variable, suggesting the absence of a standard of care, and the median time to discontinuation was short, indicating that the benefit to patients was limited. Treatment with immune checkpoint inhibitor monotherapy and retreatment with an EGFR TKI or platinum-based chemotherapy agent was common. The occurrence of AEs associated with hospitalization was high, especially among patients receiving platinum-containing regimens compared to EGFR TKI or immune checkpoint inhibitor monotherapies. These results highlight the unmet need for new therapies to reduce the clinical burden among these patients. The present study may serve as a benchmark to assess the effectiveness of future therapies in this underserved patient population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing assistance was provided by Shelley Batts, PhD, an employee of Analysis Group, Inc. Support for this assistance was provided by Daiichi Sankyo, Inc.
Declarations
Funding
This study was funded by Daiichi Sankyo, Inc. The study sponsor was involved in all aspects of the research, including the study design, interpretation of data, and manuscript development.
Conflict of interest
Elizabeth Marrett and Winghan Jacqueline Kwong are employees of Daiichi Sankyo. Jipan Xie, Ameur M. Manceur, Selvam R. Sendhil, Eric Wu, and Raluca Ionescu-Ittu are employees of Analysis Group Inc., a consulting company that received funding from Daiichi Sankyo, Inc. for the conduct of this study. Janakiraman Subramanian has served in an advisory role and/or speakers bureau for Astra Zeneca, Axcess, BeiGene, Blueprint Medicines, Boehringer Ingelheim, Cardinal Health, Daiichi Sankyo, Eli Lilly, G1 Therapeutics, Janssen Oncology, Jazz Pharmaceuticals, Novartis, OncoCyte, Pfizer, and Takeda, and has received research funding from Canstem, G1 Therapeutics, Genentech, Helsinn Therapeutics, Incyte, Merck, Novartis, Novocure, and Tesaro/GSK.
Ethics approval
This retrospective database analysis used de-identifed claims data, which is compliant with the Health Insurance Portability and Accountability Act to protect patient privacy. No institutional board review was required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The database used to conduct this study is available from IQVIA with a licensing agreement (https://www.iqvia.com/solutions/real-world-evidence/real-world-data-and-insights). Sharing of data supporting the results reported in this article may be considered upon request by contacting emarrett@dsi.com.
Code availability
Not applicable.
Author contributions
EM, WJK, JX, AMM, SRS, EW, RII, and JS developed/approved the protocol. AMM, SRS, and RII analyzed the data. All authors contributed to the interpretation of data, revised the manuscript for intellectual content, and read and approved the final version.
Previous presentations
Portions of these results were presented in a poster format at the National Comprehensive Cancer Network 2021 Virtual Annual Conference held on 18–20 March, 2021 and the Academy of Managed Care Pharmacy 2021 Virtual Meeting held on 12–16 April, 2021.
References
- 1.World Health Organization. Global Cancer Observatory: cancer today. Lyon, France: International Agency for Research on Cancer. 2018. Available from: https://gco.iarc.fr/today. Accessed 27 Jan 2021.
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Cancer Society. Non-small cell lung cancer. 2022. Available from: https://www.cancer.org/cancer/lung-cancer/about/what-is.html. Accessed 20 May 2022.
- 5.Lin J, Kamamia C, Brown D, et al. Survival among lung cancer patients in the US military health system: a comparison with the SEER population. Cancer Epidemiol Biomark Prev. 2018;27(6):673–679. doi: 10.1158/1055-9965.EPI-17-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nations JA. Brown DW, Shao S, Shriver CD, Zhu K. Comparative trends in the distribution of lung cancer stage at diagnosis in the Department of Defense Cancer Registry and the Surveillance, Epidemiology, and End Results data, 1989–2012. Mil Med. 2020;185(11–12):e2044–e2048. doi: 10.1093/milmed/usaa218. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Ai Z, Xu G. Marital status and survival in patients with non-small cell lung cancer: an analysis of 70006 patients in the SEER database. Oncotarget. 2017;8(61):103518–103534. doi: 10.18632/oncotarget.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Cancer Society. Cancer facts and figures 2021. 2021. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html. Accessed 14 June 2021.
- 9.Sugimura H, Nichols FC, Yang P, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83(2):409–418. doi: 10.1016/j.athoracsur.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch FR, Suda K, Wiens J, Bunn PA., Jr New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016;388(10048):1012–1024. doi: 10.1016/S0140-6736(16)31473-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y-L, Yuan J-Q, Wang K-F, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48):78985–78993. doi: 10.18632/oncotarget.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Au JS-K, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9(2):154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oronsky B, Ma P, Reid TR, et al. Navigating the “No Man's Land” of TKI-failed EGFR-mutated non-small cell lung cancer (NSCLC): a review. Neoplasia. 2018;20(1):92–98. doi: 10.1016/j.neo.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.4.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed September 7, 2022. Available from: NCCN.org. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- 15.Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28(2):357–360. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tumbrink HL, Heimsoeth A, Sos ML. The next tier of EGFR resistance mutations in lung cancer. Oncogene. 2021;40(1):1–11. doi: 10.1038/s41388-020-01510-w. [DOI] [PubMed] [Google Scholar]
- 17.Tomizawa Y, Fujita Y, Tamura A, et al. Effect of gefitinib re-challenge to initial gefitinib responder with non-small cell lung cancer followed by chemotherapy. Lung Cancer. 2010;68(2):269–272. doi: 10.1016/j.lungcan.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Becker A, Crombag L, Heideman DAM, et al. Retreatment with erlotinib: regain of TKI sensitivity following a drug holiday for patients with NSCLC who initially responded to EGFR-TKI treatment. Eur J Cancer. 2011;47(17):2603–2606. doi: 10.1016/j.ejca.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 19.Kaira K, Kobayashi K, Shiono A, et al. Effectiveness of EGFR-TKI rechallenge immediately after PD-1 blockade failure. Thorac Cancer. 2021;12(6):864–873. doi: 10.1111/1759-7714.13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IQVIA. IQVIA PharMetrics® Plus. Available from: https://www.iqvia.com/library/fact-sheets/iqvia-pharmetrics-plus. Accessed 14 June 2021.
- 21.US Food and Drug Administration. Highlights of prescribing information: TAGRISSO (osimertinib) 2015. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf. Accessed 14 Sep 2021.
- 22.National Cancer Institute. NCI Comorbidity Index overview. Available from: https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html. Accessed 23 Jan 2020.
- 23.Chouaid C, Loirat D, Clay E, et al. Cost analysis of adverse events associated with non-small cell lung cancer management in France. Clinicoecon Outcomes Res. 2017;9:443–449. doi: 10.2147/CEOR.S138963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engel-Nitz NM, Johnson MP, Bunner SH, Ryan KJ. Real-world costs of adverse events in first-line treatment of metastatic non-small cell lung cancer. J Manag Care Spec Pharm. 2020;26(6):729–740. doi: 10.18553/jmcp.2020.26.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian J, Fernandes AW, Laliberté F, Pavilack M, DerSarkissian M, Duh MS. The rate of occurrence, healthcare resource use and costs of adverse events among metastatic non-small cell lung cancer patients treated with first- and second-generation epidermal growth factor receptor tyrosine kinase inhibitors. Lung Cancer. 2019;138:131–138. doi: 10.1016/j.lungcan.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18(10):2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 27.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 28.Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13(3):247–255. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 29.Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(23):2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 30.Pérol M, Chouaid C, Pérol D, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2012;30(28):3516–3524. doi: 10.1200/JCO.2011.39.9782. [DOI] [PubMed] [Google Scholar]
- 31.Otsuka K, Hata A, Takeshita J, et al. EGFR-TKI rechallenge with bevacizumab in EGFR-mutant non-small cell lung cancer. Cancer Chemother Pharmacol. 2015;76(4):835–841. doi: 10.1007/s00280-015-2867-8. [DOI] [PubMed] [Google Scholar]
- 32.Chic N, Mayo-de-Las-Casas C, Reguart N. Successful treatment with gefitinib in advanced non-small cell lung cancer after acquired resistance to osimertinib. J Thorac Oncol. 2017;12(6):e78–80. doi: 10.1016/j.jtho.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Hirsh V. Managing treatment-related adverse events associated with EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol. 2011;18(3):126–138. doi: 10.3747/co.v18i3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirsh V, Singh J. Optimal sequencing strategies in the treatment of EGFR mutation-positive non-small cell lung cancer: clinical benefits and cost-effectiveness. Am J Health Syst Pharm. 2020;77(18):1466–1476. doi: 10.1093/ajhp/zxaa197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.3.2017. © National Comprehensive Cancer Network, Inc. 2016. All rights reserved. Accessed September 7, 2022. Available from: NCCN.org. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- 36.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.2.2018. © National Comprehensive Cancer Network, Inc. 2018. All rights reserved. Accessed September 7, 2022. Available from: NCCN.org. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- 37.Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer: a meta-analysis. J Thoracic Oncol. 2017;12(2):403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arunachalam A, Li H, Bittoni MA, et al. Real-world treatment patterns, overall survival, and occurrence and costs of adverse events associated with second-line therapies for medicare patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2018;19(5):e783–e799. doi: 10.1016/j.cllc.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Magee DE, Hird AE, Klaassen Z, et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: a systematic review and meta-analysis of randomized clinical trials. Ann Oncol. 2020;31(1):50–60. doi: 10.1016/j.annonc.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 42.Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13(3):300–308. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 43.Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2016;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng L-C, Chen K-H, Wang C-L, Weng L-C. Effects of tyrosine kinase inhibitor therapy on skin toxicity and skin-related quality of life in patients with lung cancer: An observational study. Medicine (Baltimore) 2020;99(23):e20510. doi: 10.1097/MD.0000000000020510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 46.Janne PA, Baik CS, Su W-C, et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated (EGFRm) non-small cell lung cancer (NSCLC) J Clin Oncol. 2021;39(15_Suppl):9007. doi: 10.1200/JCO.2021.39.15_suppl.9007. [DOI] [Google Scholar]
- 47.Shu CA, Goto K, Ohe Y, et al. 1193MO: amivantamab plus lazertinib in post-osimertinib, post-platinum chemotherapy EGFR-mutant non-small cell lung cancer (NSCLC): preliminary results from CHRYSALIS-2. Ann Oncol. 2021;32:S949–1039. doi: 10.1016/j.annonc.2021.08.1798. [DOI] [Google Scholar]
- 48.Yuan M, Huang LL, Chen JH, Wu J, Xu Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct Target Ther. 2019;4:61. doi: 10.1038/s41392-019-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen R, Manochakian R, James L, et al. Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol. 2020;13(1):58. doi: 10.1186/s13045-020-00881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/S0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.