ABSTRACT
Background
Fatigue and impaired health-related quality of life (HRQoL) are common among kidney transplant recipients (KTR). We hypothesized that both may partially be attributable to poor sleep.
Methods
Cross-sectional and longitudinal data of KTR enrolled in the TransplantLines Biobank and Cohort Study were used. Sleep quality was assessed using the Pittsburgh Sleep Quality Index questionnaire. Individual strength (i.e. a composite of fatigue, concentration, motivation and physical activity), societal participation and HRQoL were assessed using validated questionnaires.
Results
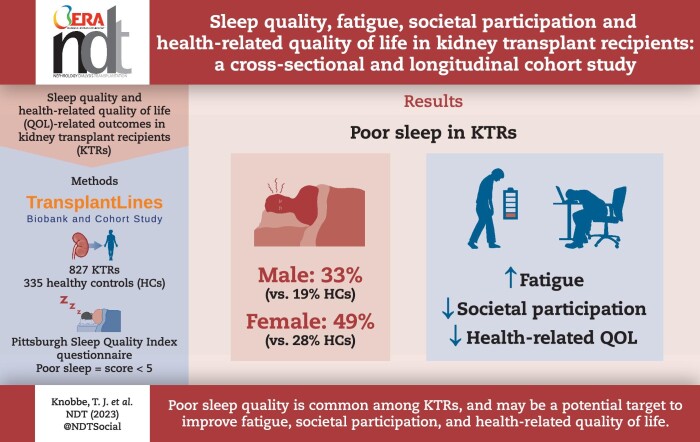
We included 872 KTR (39% female, age 56 ± 13 years) and 335 healthy controls. In total, 33% of male KTR and 49% of female KTR reported poor sleep quality, which was higher compared with male and female healthy controls (19% and 28%, respectively, P < .001 for both). In logistic regression analyses, female sex, anxiety, active smoking, low protein intake, physically inactive lifestyle, low plasma magnesium concentration, using calcineurin inhibitors, not using mTOR inhibitors and using benzodiazepine agonists were associated with poor sleep quality. In adjusted linear regression analyses, poor sleep was strongly and independently associated with lower individual strength [standardized β (st.β) = 0.59, 95% confidence interval (CI) 0.45 to 0.74, P < .001], poorer societal participation (frequency: st.β = −0.17, 95% CI −0.32 to −0.01, P = .04; restrictions: st.β = −0.36, 95% CI −0.51 to −0.21, P < .001; satisfaction: st.β = −0.44, 95% CI −0.59 to −0.28, P < .001) and lower HRQoL (physical: st.β = −0.53, 95% CI −0.68 to −0.38, P < .001; mental: st.β = −0.64, 95% CI −0.78 to −0.50, P < .001). The associations with poorer societal participation and lower HRQoL were strongly mediated by individual strength (P < .001 for all), yet the suggested direct effects of poor sleep quality on HRQoL remained significant (Pphysical = .03, Pmental = .002). Longitudinal data of 292 KTR showed that sleep quality improves after kidney transplantation in males (P < .001), but not in females (P = .9).
Conclusions
Poor sleep quality is common among KTR, and may be a potential target to improve fatigue, societal participation and HRQoL among KTR.
Keywords: insomnia, kidney transplantation, patient-reported outcome measurements, quality of life, tiredness
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Increasing numbers of patients are living with a kidney transplant, yet there is a lack of interventions to improve their limited health-related quality of life (HRQoL).
Poor sleep is common among patients with kidney disease, yet studies among kidney transplant recipients are sparse.
Poor sleep can contribute to a plethora of problems, including cognitive problems, psychosocial problems and fatigue, which are considered main causes of the impaired HRQoL in this population.
This study adds:
Approximately half of female kidney transplant recipients and one-third of male kidney transplant recipients report poor sleep quality. In addition, sleep quality improved after transplantation among males, but not among females.
Potential determinants of poor sleep are female sex, anxiety, active smoking, low protein intake, a physically inactive lifestyle, low plasma magnesium, using calcineurin inhibitor, not using mTOR inhibitors and using benzodiazepine agonist.
Poor sleep is associated with more fatigue, problems in concentration and motivation, and less physical activity. Moreover, poor sleep has detrimental effects on societal participation and lower HRQoL.
Potential impact:
The high prevalence of reported poor sleep, along with the suggested detrimental effects on poorer societal participation and lower HRQoL, highlight the magnitude of the problem among kidney transplant recipients.
We identified multiple potential targets for potential interventions to improve sleep quality among kidney transplant recipients, such as anxiety, calcineurin inhibitors, plasma magnesium, protein intake and physical activity
Sleep quality deserves attention in the (transplant) nephrologist's consultation rooms. Adequate recognition of sleep problems, and, where appropriate, referral to cognitive therapists may be useful in kidney transplant recipients suffering from poor sleep.
INTRODUCTION
Graft and patient survival among kidney transplant recipients (KTR) have improved drastically in the past decades [1]. As a result, increasing numbers of patients are living with a kidney transplant, for increasing periods of time. The subject of health-related quality of life (HRQoL) after transplantation thus keeps growing in importance, and rightfully receives increasing scientific attention. Unfortunately, there is a lack of interventions to improve HRQoL among KTR, and HRQoL thus remains limited compared with the general population [2, 3]. We hypothesized that poor sleep quality may be an important—yet rather overlooked—factor underlying the impaired HRQoL among KTR.
Poor sleep is common in patients with kidney disease [4]. This is partly explained by classic risk factors such as age, sex and obesity, but also by (the consequences of) kidney function decline [4]. However, there is limited evidence available regarding interventions to effectively improve sleep quality in adults with chronic kidney disease [5]. Although kidney transplantation restores kidney function, poor sleep is still frequently observed in KTR [6]. Currently, there is insufficient knowledge of potentially modifiable factors that may contribute to poor sleep [6]. In addition, there is limited evidence regarding trajectories of sleep quality before and after transplantation [7, 8], even though such evidence is needed for patient expectation management, to place the prevalence of poor sleep after transplantation into perspective, and to identify distinct pre- and post-transplantation factors that may contribute to poor sleep.
Importantly, poor sleep can contribute to a plethora of cognitive and psychosocial problems, as observed in multiple populations [9–16]. Notably, these suggested detrimental consequences of poor sleep include problems in concentration, motivation and physical activity [9–16], which are all frequently faced by KTR [17–19]. Moreover, poor sleep is considered to contribute to fatigue [20]—a complaint that is reported by 39%–59% of KTR, and is considered a main cause of the impaired HRQoL in this population [21, 22]. We therefore hypothesized that poor sleep may lower individual strength (i.e. a composite of fatigue, concentration, motivation and physical activity), which in turn may impair societal participation and HRQoL in KTR.
In this study, we therefore first examined patient-reported sleep quality among prevalent KTR, and aimed to identify potential clinical, biochemical and psychosocial factors associated with poor sleep. Second, we investigated potential consequences of poor sleep quality, by assessing cross-sectional associations with patient-reported fatigue, concentration, motivation, physical activity, societal participation and HRQoL. Third, we aimed to assess the potential causal path of poor sleep, by assessing whether the associations of poor sleep with poor societal participation and impaired HRQoL are mediated by lower individual strength. Finally, in longitudinal analyses, we studied trajectories of sleep quality before, and at 6 and 12 months after transplantation.
MATERIALS AND METHODS
Study design and population
Data from the ongoing, prospective, TransplantLines Biobank and Cohort study were used (ClinicalTrials.gov identifier: NCT03272841) [23], including solid organ transplant recipients and living organ donors (≥18 years) of the University Medical Center Groningen (UMCG, The Netherlands). All participants gave written informed consent. The study protocol was approved by the local Institutional Review Board (METc 2014/077) and adheres to the UMCG Biobank Regulation, and the Declarations of Helsinki and Istanbul. The timeline of assessments and outcomes are presented in Supplementary data, Fig. S1. In cross-sectional analyses, we included KTR with available data on sleep quality ≥1 year after kidney transplantation. A subgroup had available serial data on sleep quality before, at 6 months and/or 12 months after kidney transplantation, which were included in longitudinal analyses. Potential kidney donors were used as a healthy control group. This study was described following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Supplementary data, Table S1) [24].
Assessment of sleep quality
The validated Pittsburgh Sleep Quality Index (PSQI) questionnaire was used to assess sleep quality [25]. This validated 19-item questionnaire assesses seven components of sleep quality, including duration of sleep, sleep disturbances, sleep latency, day dysfunction due to sleepiness, sleep efficiency, overall sleep quality and medication needed to sleep, which are combined into one total score ranging from 0 to 21, with higher scores indicating worse sleep quality [25]. Poor sleep was defined as a PSQI score >5, a meaningful cut-off used previously in KTR [6, 25].
Assessments of outcomes and covariables
Assessments of outcomes and covariables are described in detail in Supplementary data, Table S2. In brief, fatigue severity, concentration, motivation and physical activity were assessed using the Checklist Individual Strength 20 Revised questionnaire. Societal participation and HRQoL were assessed using the Utrecht Scale for Evaluation of Rehabilitation-Participation and Short Form-36 questionnaires. Medication non-adherence was assessed using the Basel Assessment of Adherence to Immunosuppressive Medication Scale questionnaire. Clinical and demographic data were retrieved from medical files. Lifestyle and psychometric data were collected using questionnaires.
Statistical analyses
The statistical methodology is described in detail in Supplementary data, Table S3. In brief, associations of clinical, biochemical and psychosocial parameters with poor sleep quality were assessed by logistic regression analyses with adjustment for sex. Associations of poor sleep with individual strength, societal participation and HRQoL were assessed using univariable and adjusted linear regression. Causal mediation analyses [26] were performed to assess whether individual strength mediated the associations of poor sleep with societal participation and HRQoL. In longitudinal analyses, within-person changes in sleep were assessed using McNemar's tests.
Sensitivity analyses
To assess robustness of our findings and for ease of comparison with other studies [27], the linear regression and causal mediation analyses were repeated using an alternative PSQI cut-off score >7.
RESULTS
Population characteristics
In total, 872 KTR and 335 healthy controls (HC) with cross-sectional data regarding sleep quality were included (Supplementary data, Fig. S2). KTR were less frequently female compared with HC (39% vs 56%, P < .001), the mean age did not differ between the two groups (56 ± 13 vs 57 ± 11 years, P = .1). KTR were included at a median of 3 (1 to 10) years after transplantation, 37% was pre-emptively transplanted and estimated glomerular filtration rate (eGFR) at time of inclusion was 52 ± 18 mL/min/1.73 m2 (Table 1). Included patients were generally comparable to excluded patients, yet were longer after transplantation, more frequently received kidney from a living donor and transplantation was more often performed pre-emptively (Supplementary data, Table S4).
Table 1:
Characteristics and sleep of KTR and HC.
| KTR, N = 872 | HC, N = 335 | P | |
|---|---|---|---|
| Characteristics | |||
| Female sex, n (%) | 339 (39) | 187 (56) | <.001 |
| Age, years | 56 ± 13 | 57 ± 11 | .1 |
| Time after transplantation, years | 3 (1 to 10) | n/a | n/a |
| Pre-emptive transplantation | 323 (37) | n/a | n/a |
| eGFR, mL/min/1.73 m2 | 52 ± 18 | 85 ± 13 | <.001 |
| PSQI | |||
| Poor sleep quality, n (%) | 236 (20) | 42 (13) | <.001 |
| Female, n (%) | 165 (49) | 53 (28) | <.001 |
| Male, n (%) | 178 (33) | 28 (19) | <.001 |
Normally distributed data were presented as mean ± standard deviation, non-normally distributed data as median (interquartile range) and categorical data were presented as number (valid %). Significance of differences between groups were assessed using independent T-tests, Mann–Whitney U tests and Chi Square test depending on data distribution. n/a, not applicable
Sleep quality
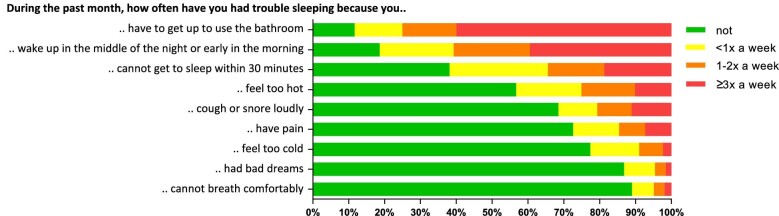
Poor sleep quality was reported by 33% of male KTR and 49% of female KTR, which was higher compared with male (19%, P < .001) and female (28%, P < .001) HC, respectively. Of note, 11% of KTR reported to take sleep medication three or more times per week, which was also higher compared with HC (5%, P = .008). KTR reported that their sleep was disturbed three or more times a week by the need to use the bathroom (60%), by waking up in the middle of the night or early morning (39%), or by not getting to sleep within 30 min (19%). Prevalence of sleep disturbances of KTR are presented in Fig. 1 and Supplementary data, Table S5.
Figure 1:
Sleep disturbances of KTR.
Factors associated with poor sleep quality among KTR
Univariable logistic regression analyses showed that female KTR had a higher risk of reporting poor sleep quality compared with male KTR [odds ratio (OR) 1.89, 95% confidence interval (CI) 1.43 to 2.50, P < .001, Table 2]. In analyses with adjustment for sex, symptoms of anxiety were associated with a higher risk of reported poor sleep quality (OR per SD = 1.93, 95% CI 1.65 to 2.26, P < .001), as was active smoking (OR 1.59, 95% CI 1.04 to 2.43, P = .03), lower protein intake (OR per SD = 0.85, 95% CI 0.73 to 0.99, P = .04), a physically inactive lifestyle (OR = 1.46, 95% CI 1.09 to 1.97, P = .01), lower plasma magnesium (OR per SD = 0.82, 95% CI 0.71 to 0.94, P = .006), using calcineurin inhibitors (OR = 1.74, 95% CI 1.18 to 2.56, P = .005), not using mammalian target of rapamycin (mTOR) inhibitors (OR 0.43, 95% CI 0.19 to 0.97, P = .04) and using benzodiazepine agonists (OR = 5.84, 95% CI 3.07 to 11.03, P < .001). Age, time after transplantation and eGFR were not associated with reported poor sleep quality.
Table 2:
Characteristics of KTR and cross-sectional associations with poor sleep quality (PSQI score >5).
| Logistic regression analyses adjusted for sex | |||
|---|---|---|---|
| KTR, N = 872 | OR (95% CI) | P | |
| Demographics | |||
| Female sex, n (%)a | 339 (39) | 1.89 (1.43 to 2.50) | <.001 |
| Age, years | 56 ± 13 | 1.00 (0.87 to 1.15) | .9 |
| Body mass index, kg/m2 | 27 ± 5 | 1.08 (0.94 to 1.23) | .3 |
| Diabetes, n (%) | 241 (28) | 1.32 (0.97 to 1.79) | .07 |
| Anemia, n (%) | 272 (31) | 1.07 (0.80 to 1.44) | .7 |
| Sleep apnea syndrome, n (%) | 37 (4) | 0.98 (0.49 to 1.98) | .9 |
| Congestive heart failure, n (%) | 51 (6) | 1.23 (0.69 to 2.20) | .5 |
| Partnered, n (%) | 692 (79) | 0.83 (0.59 to 1.17) | .3 |
| Anxiety total scoreb | 30 (23 to 37) | 1.93 (1.65 to 2.26) | <.001 |
| Educational level, n (%) | |||
| Low | 131 (19) | 1 (reference) | |
| Medium | 308 (46) | 1.03 (0.74 to 1.42) | .9 |
| High | 236 (35) | 0.89 (0.63 to 1.27) | .5 |
| Lifestyle factors | |||
| Smoking status, n (%) | |||
| Never | 414 (47) | 1 (reference) | |
| Past smoker | 348 (30) | 1.33 (0.99 to 1.78) | .06 |
| Active smoker | 110 (13) | 1.59 (1.04 to 2.43) | .03 |
| Alcohol intake, n (%) | |||
| None | 335 (38) | 1 (reference) | |
| <7 units/week | 349 (40) | 1.02 (0.75 to 1.39) | .9 |
| ≥7 units/week | 188 (22) | 0.98 (0.67 to 1.43) | .9 |
| Coffee consumption, n per day (%)c | 3 (2 to 4) | 0.96 (0.83 to 1.11) | .6 |
| Protein intake, g/day | 84 ± 22 | 0.85 (0.73 to 0.99) | .04 |
| Physically inactive lifestyle, n (%) | 344 (45) | 1.46 (1.09 to 1.97) | .01 |
| Transplant-specific characteristics | |||
| Time after transplantation, yearsb | 3 (1 to 10) | 0.87 (0.76 to 1.00) | .06 |
| Pre-emptive transplantation, n (%) | 323 (37) | 1.12 (0.84 to 1.49) | .4 |
| Living donor, n (%) | 500 (57) | 1.02 (0.77 to 1.35) | .9 |
| Spirometry assessment | |||
| Airflow limitation, n (%) | 195 (26) | 1.15 (0.82 to 1.61) | .4 |
| Laboratory measurements | |||
| Hemoglobin, mmol/L | 8.6 ± 1.1 | 0.97 (0.84 to 1.12) | .7 |
| Leukocyte count, 109/L | 7.6 ± 2.3 | 1.03 (0.89 to 1.18) | .7 |
| C-reactive protein, mg/Lb | 1.9 (0.7 to 4.4) | 1.12 (0.98 to 1.29) | .1 |
| Sodium, mmol/L | 140 ± 3 | 0.88 (0.76 to 1.01) | .07 |
| Potassium, mmol/L | 4.0 ± 0.4 | 1.09 (0.94 to 1.25) | .3 |
| Creatinine, µmol/L | 123 (102 to 152) | 0.99 (0.86 to 1.15) | .9 |
| eGFR, mL/min/1.73 m2 | 52 ± 18 | 1.04 (0.91 to 1.20) | .6 |
| Urea, mmol/L | 9.4 ± 4.9 | 0.99 (0.86 to 1.13) | .8 |
| Calcium, mmol/L | 2.4 ± 0.1 | 1.00 (0.87 to 1.15) | 1.0 |
| Phosphate, mmol/L | 0.92 ± 0.20 | 1.11 (0.96 to 1.28) | .2 |
| Magnesium, mmol/L | 0.74 ± 0.09 | 0.82 (0.71 to 0.94) | .006 |
| Albumin, g/L | 43 ± 3 | 1.01 (0.88 to 1.16) | .9 |
| NT-proBNP, ng/Lb | 207 (92 to 511) | 0.92 (0.80 to 1.06) | .3 |
| Medication use | |||
| Prednisolone, n (%) | 850 (98) | 1.19 (0.49 to 2.91) | .7 |
| Calcineurin inhibitor, n (%) | 722 (83) | 1.74 (1.18 to 2.56) | .005 |
| Proliferation inhibitor, n (%) | 748 (86) | 1.17 (0.78 to 1.74) | .4 |
| mTOR inhibitor, n (%) | 35 (4) | 0.43 (0.19 to 0.97) | .04 |
| Antidepressants, n (%) | 51 (6) | 1.07 (0.60 to 1.91) | .8 |
| Benzodiazepine agonists, n (%) | 59 (7) | 5.84 (3.07 to 11.03) | <.001 |
| Melatonin agonists, n (%) | 2 (0.2) | 1.45 (0.09 to 24.12) | .8 |
aResults for univariable analysis.
bVariables are log2 transformed in logistic regression analyses.
cVariable is square root–transformed in logistic regression analyses.
Normally distributed data were presented as mean ± standard deviation, non-normally distributed data as median (interquartile range) and categorical data were presented as number (valid %). ORs for continuous variables are presented per 1 SD increase. Data regarding coffee consumption, protein intake, physically inactive lifestyle and airflow limitation were missing in 40 (5%), 87 (10%), 104 (12%) and 119 (14%) KTR, respectively. Other variables were missing in <3% of the KTR.
NT-proBNP, N-terminal pro b-type natriuretic peptide.
Associations of poor sleep quality with individual strength among KTR
Reported poor sleep quality was associated with higher fatigue severity [standardized β (st.β) = 0.69, 95% CI 0.56 to 0.82, P < .001], lower ability to concentrate (st.β = 0.54, 95% CI 0.41 to 0.68, P < .001), less motivation (st.β = 0.48, 95% CI 0.35 to 0.62, P < .001) and less physical activity (st.β = 0.45, 95% CI 0.32 to 0.58, P < .001) among KTR. Finally, reported poor sleep was associated with a higher total score on these domains (st.β = 0.70, 95% CI 0.57 to 0.83, P < .001). These associations remained independent of adjustment for sex, age, time after transplantation, protein intake, physically inactive lifestyle, eGFR, plasma magnesium concentration, calcineurin inhibitor use, benzodiazepine use and symptoms of anxiety (Table 3).
Table 3:
Cross-sectional associations of poor sleep quality (PSQI score >5) with (domains of) individual strength.
| Linear regression analyses with poor sleep quality as independent variable | |||
|---|---|---|---|
| Dependent variable | N | St.β (95% CI) | P |
| Individual strength | |||
| Fatigue severity | |||
| Crude | 863 | 0.69 (0.56 to 0.82) | <.001 |
| Model 1 | 688 | 0.58 (0.43 to 0.72) | <.001 |
| Model 2 | 680 | 0.37 (0.24 to 0.51) | <.001 |
| Concentration | |||
| Crude | 863 | 0.54 (0.41 to 0.68) | <.001 |
| Model 1 | 688 | 0.54 (0.38 to 0.69) | <.001 |
| Model 2 | 680 | 0.29 (0.14 to 0.43) | <.001 |
| Motivation | |||
| Crude | 863 | 0.48 (0.35 to 0.62) | <.001 |
| Model 1 | 688 | 0.38 (0.24 to 0.53) | <.001 |
| Model 2 | 680 | 0.16 (0.02 to 0.30) | .03 |
| Physical activity | |||
| Crude | 863 | 0.45 (0.32 to 0.58) | <.001 |
| Model 1 | 688 | 0.33 (0.19 to 0.48) | <.001 |
| Model 2 | 680 | 0.17 (0.03 to 0.32) | .02 |
| Total score | |||
| Crude | 863 | 0.70 (0.57 to 0.83) | <.001 |
| Model 1 | 688 | 0.59 (0.45 to 0.74) | <.001 |
| Model 2 | 680 | 0.34 (0.21 to 0.47) | <.001 |
Model 1: adjusted for sex, age, log2 time after transplantation, protein intake, physically inactive lifestyle, eGFR, magnesium, calcineurin inhibitor use and benzodiazepine use. Model 2: Model 1 additionally adjusted for anxiety total score.
Associations of poor sleep quality with societal participation and HRQoL among KTR
In total, 22% of KTR had difficulties in keeping up enough enthusiasm to get things done, and 4% reported difficulties in staying awake while driving, eating meals or engaging in social activities. Reported poor sleep quality was associated with a lower frequency (st.β = −0.17, 95% CI −0.31 to −0.03, P = .02), more restrictions (st.β = −0.47, 95% CI −0.61 to −0.33, P < .001), and less satisfaction (st.β = −0.53, 95% CI −0.67 to −0.39, P < .001) regarding societal participation (Table 4).
Table 4:
Cross-sectional associations of poor sleep quality (PSQI score >5) with societal participation and HRQoL.
| Linear regression analyses with poor sleep quality as independent variable | |||
|---|---|---|---|
| Dependent variable | N | St.β (95% CI) | P |
| Societal participation | |||
| Frequency | |||
| Crude | 783 | −0.17 (−0.31 to −0.03) | .02 |
| Model 1 | 633 | −0.17 (−0.32 to −0.01) | .04 |
| Model 2 | 630 | −0.08 (−0.24 to 0.08) | .3 |
| Restriction | |||
| Crude | 777 | −0.47 (−0.61 to −0.33) | <.001 |
| Model 1 | 660 | −0.36 (−0.51 to −0.21) | <.001 |
| Model 2 | 625 | −0.24 (−0.39 to −0.09) | .002 |
| Satisfaction | |||
| Crude | 785 | −0.53 (−0.67 to −0.39) | <.001 |
| Model 1 | 637 | −0.44 (−0.59 to −0.28) | <.001 |
| Model 2 | 632 | −0.25 (−0.40 to −0.10) | .001 |
| HRQoL | |||
| Physical component score | |||
| Crude | 853 | −0.64 (−0.77 to −0.51) | <.001 |
| Model 1 | 683 | −0.53 (−0.68 to −0.38) | <.001 |
| Model 2 | 675 | −0.37 (−0.51 to −0.22) | <.001 |
| Mental component score | |||
| Crude | 853 | −0.77 (−0.89 to −0.64) | <.001 |
| Model 1 | 683 | −0.64 (−0.78 to −0.50) | <.001 |
| Model 2 | 675 | −0.34 (−0.47 to −0.22) | <.001 |
Model 1: adjusted for sex, age, log2 time after transplantation, protein intake, physically inactive lifestyle, eGFR, magnesium, calcineurin inhibitor use and benzodiazepine use. Model 2: Model 1 additionally adjusted for anxiety total score.
In addition, KTR reporting poor sleep quality had lower scores on all subdomains of HRQoL, indicating worse HRQoL, compared with KTR without poor sleep quality (P < .001 for all, Fig. 2). Reported poor sleep quality was strongly associated with lower physical and mental HRQoL (physical: st.β = −0.64, 95% CI −0.77 to −0.51, P < .001; mental: st.β = −0.77, 95% CI −0.89 to −0.64, P < .001).
Figure 2:
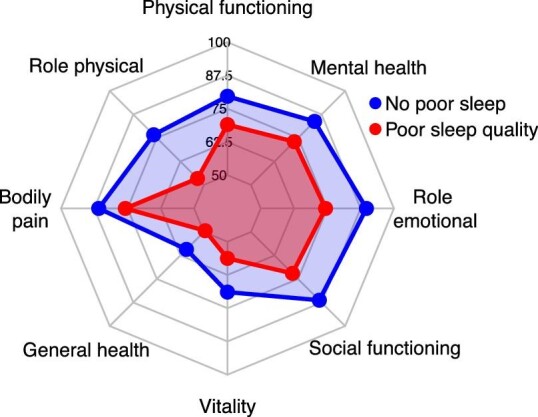
Radar plot of subdomains of HRQoL of KTR. Recipients with poor sleep quality (PSQI score >5, red) had lower scores on all subdomains of HRQoL compared with recipients with no poor sleep quality (blue, P < .001 for all).
All associations remained present in Model 1. After additional adjustment for symptoms of anxiety in Model 2, the association of poor sleep quality with frequency of societal participation lost statistical significance. Point estimates of all other associations were slightly lower, but associations remained present.
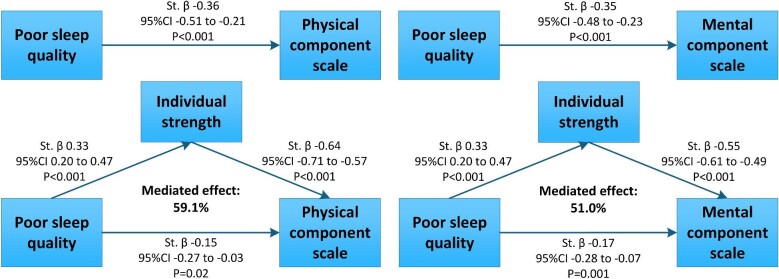
Mediation by individual strength
Mediation analyses with adjustment for potential confounders showed that individual strength (i.e. a composite of fatigue, concentration, motivation and physical activity) mediated the associations with restrictions and satisfaction of societal participation for 55.9% and 52.7%, respectively, and the associations with physical and mental HRQoL for 59.1% and 51.0%, respectively (Supplementary data, Table S6). The direct effect of poor sleep on restriction and satisfaction of societal participation was not statistically significant (P = .1 for both), indicating that the suggested effects of poor sleep quality on poor societal participation are mainly indirect, via individual strength (P < .001 for both). In contrast, poor sleep quality was both directly (physical: P = .03; mental: P = .002) and indirectly (P < .001 for both) associated with physical and mental HRQoL, suggesting that poor sleep quality has direct and indirect effects on HRQoL. The suggested causal path of these associations is presented in Fig. 3.
Figure 3:
The causal pathway suggested by mediation analyses for the association between poor sleep quality (PSQI score >5) and HRQoL. Presented st.β coefficients and 95% CI were adjusted for sex, age, log2 time after transplantation, protein intake, physically inactive lifestyle, eGFR, magnesium, calcineurin inhibitor use, benzodiazepine use and anxiety total score. Mediation analyses were performed using 1000 bootstrapped samples from 673 KTR.
Trajectory of sleep quality before and after transplantation
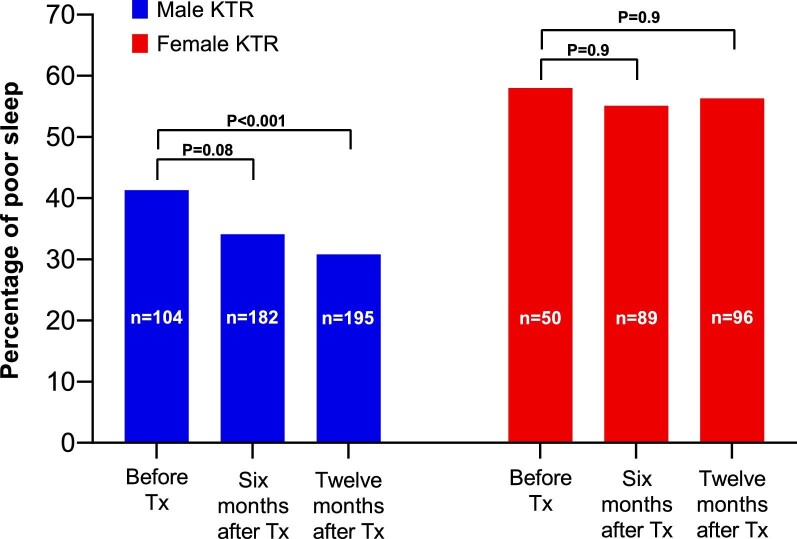
In a subgroup of 292 KTR (age 55 ± 13 years, 33% female and 46% pre-emptively transplanted) repeated measurements of sleep quality were performed before, 6 months after and/or 12 months after transplantation. Among male KTR, poor sleep quality was more frequently reported before transplantation compared with 1 year after transplantation (41% vs 31%, Pwithin patient < .001, respectively). In contrast, among female KTR, the prevalence of poor sleep was similar before versus 1 year after transplantation (58% vs 56%, Pwithin patient = .9, Fig. 4). This difference in the trajectory of sleep quality before and after transplantation was also observed among KTR with data on sleep quality at all time points, with a decrease in prevalence of poor sleep quality over time among males and no difference in prevalence of poor sleep quality over time among females (Supplementary data, Table S7). Among KTR who reported poor sleep quality before transplantation, those whose sleep quality improved after transplantation were more frequently male (77% vs 46%) and had a lower anxiety score before transplantation [33 (27 to 42) vs 40 (33 to 49)], compared with KTR who reported poor sleep quality before transplantation and did not experience improvement in sleep quality (Supplementary data, Table S8).
Figure 4:
Proportion of 292 KTR with a poor sleep quality (PSQI score >5) before, 6 months after and 12 months after Tx per sex. Among male KTR (blue), poor sleep quality was more prevalent before Tx compared with 12 months after Tx, while prevalence of poor sleep quality at these time points were comparable for female KTR (red). Within-participant differences were assessed using McNemar's tests. Tx, transplantation.
Exploratory analyses
Among 862 KTR with data regarding medication non-adherence, 41% of the KTR was regarded as non-adherent. Poor sleep was not associated with medication non-adherence in logistic regression analyses adjusted for potential confounders (OR = 1.36, 95% CI 0.96 to 1.91, P = .082). Furthermore, poor sleep was associated with neither rejection nor log2 24-h urinary protein excretion.
Sensitivity analyses
Using an alternative PSQI cut-off of >7 to define poor sleep, prevalence of reported poor sleep was 17% among male KTR and 31% among female KTR, which was again higher compared with HC (male HC 9%, P = .02; female HC: 16%, P < .001). Points estimates of the associations of poor sleep quality with individual strength, societal participation and HRQoL were higher compared with primary analyses. The causal mediation analyses showed comparable results (Supplementary data, Tables S9–S11).
DISCUSSION
This study shows that approximately half of the female KTR and one-third of the male KTR report poor sleep quality, which is much higher compared with HC. Female sex, anxiety, active smoking, low protein intake, a physically inactive lifestyle, low plasma magnesium, using calcineurin inhibitor, not using mTOR inhibitors and using benzodiazepine agonist appeared as potential determinants of poor sleep. Poor sleep was strongly associated with more fatigue, less motivation, less concentration and less physical activity, poorer societal participation and lower HRQoL. The associations between poor sleep and lower HRQoL were partly direct, and partly mediated through individual strength (including fatigue). Longitudinal analyses showed that sleep quality improved after transplantation in males, but not in females.
This study is one of the largest studies extensively assessing sleep quality among KTR, and is the first longitudinal study in which sleep quality is assessed from prior to transplantation to 12 months after transplantation in a large population of KTR [7, 8]. Our study confirms the high prevalence of poor sleep among KTR. Notably, this study shows that sleep problems are more prevalent and persist more after transplantation among female KTR, while most previous studies did not report sex differences in (trajectories of) sleep quality [6].
Interestingly, we observed no associations of partner status, education level, age, obesity, body mass index, alcohol or coffee intake, diagnosis of obstructive sleep apnea or heart failure with poor sleep, although these are generally considered risk factors for poor sleep [6, 28–30]. This suggests that, in the KTR population, primarily other factors underlie the observed sleep problems. Anxiety was strongly associated with poor sleep among KTR. Moreover, among KTR with poor sleep prior to transplantation, KTR with improving sleep quality after transplantation had a lower pre-transplantation anxiety score compared with KTR who did not experience improvement in sleep quality. This further suggests an important role of anxiety in sleep quality, which is in line with previous findings among KTR [6] and with the notion that sleep and cognitive-emotional reactivity have a bidirectional relationship [31, 32]. Such cognitive-emotional factors including anxiety and poor sleep may be difficult to target as treating transplant professionals. However, clinicians must realize that insomnia and anxiety can be treated, preferably by cognitive behavioral therapy rather than pharmaceutical treatment, because of its efficacy, safety and sustainability of benefit [33], also in KTR [34]. This notion, combined with our findings, highlights that is essential to recognize cognitive-emotional factors in the clinic to adequately refer patients for psychological treatment where appropriate. A recent publication by Chaput and Shiau has provided an excellent practical framework for busy clinicians to quickly and effectively get an impression of a patient's sleep health [35]. First, clinicians may get a snapshot of whether sleep quality is an issue by asking “How is your sleep in general.” To get a better impression of a patient's sleep health, five important characteristics of sleep should be assessed, including sleep duration, sleep quality, sleep timing, daytime alertness and the absence of a sleep disorder. Examples of questions that clinicians may use to assess a patient's sleep health in under two minutes are provided [35]. If poor sleep quality is present, clinicians are advised to discuss the option of referral to a sleep specialist with the patient.
In addition to anxiety, calcineurin inhibitor use was associated with poor sleep. This association is in line with the well-recognized adverse effects of calcineurin inhibitors on the nervous system [36]. Although the use of calcineurin inhibitors is generally recommended in clinical guidelines to prevent graft rejection, there are options to tailor immunosuppressive treatment by switching to alternative immunosuppressive drugs or by use of slow-release tacrolimus regimens which were infrequently prescribed in the current study population. Future (interventional) trials are needed to confirm potential effects of such immunosuppressive treatment alterations on sleep quality.
In addition, lower plasma magnesium was associated with poor sleep. Interestingly, magnesium status has previously been linked to sleep quality [37]. Magnesium is a NMDA antagonist and GABA agonist, and it has been hypothesized that magnesium deficiency can disrupt the sleep wake cycle through this pathway [38]. Moreover, it was suggested that magnesium deficiency can also cause neuroendocrine dysregulation by alterations in levels of melatonin and other hormones [38]. Importantly, hypomagnesemia is prevalent among KTR, partly due to calcineurin inhibitor use. Magnesium is affordable, safe and generally advisable in magnesium-deficient KTR [39]. Therefore, assessment (and potentially correction) of magnesium status may be considered in KTR reporting poor sleep.
Low protein intake was also associated with poor sleep, further underlining the importance of sufficient protein intake among KTR [40, 41]. This finding coincides with the association of a physically inactive lifestyle with poor sleep. This observation is in line with randomized controlled trials among KTR and other populations, which showed that increasing physical activity can improve sleep quality [42, 43]. These findings, together with a recent study on airflow limitation [44], underline the promising potential of exercise therapy to improve sleep quality, fatigue and HRQoL in KTR.
Generally, the association of sleep quality with HRQoL is well-established in the general population [13–15]. The current study underlines that sleep quality may be particularly relevant in KTR, as reflected by the strong and independent associations of poor sleep with fatigue, poorer concentration and motivation, and poorer societal participation.
Strengths of this study are the large study population, with the unique availability of extensive clinical, biochemical and psychosocial data, allowing for a comprehensive exploration of potential determinants of poor sleep quality, as well as potential repercussions of poor sleep quality with regard to societal participation and HRQoL. In addition, this study is the largest study in which sleep among KTR is assessed longitudinally. However, several limitations of this study must be acknowledged. First, due to its observational design, no conclusive interpretations regarding causality can be drawn. For example, future interventional studies are needed to identify the actual clinical benefit of improving sleep on HRQoL and other outcomes. Second, the data on sleep quality were strictly based upon self-reporting, rather than invasive and costly objective sleep monitoring methods. Third, we had no data regarding de novo donor-specific antibodies. In addition, the study population included patients from a single center in the Netherlands, with a predominantly Caucasian population, which may limit the extrapolation to KTR from other countries. Finally, the current study could not assess potential prospective somatic effects of poor sleep among KTR. However, the high prevalence of poor sleep is a cause for concern, particularly because, in other populations, poor sleep is consistently linked to cardiovascular disease, cancer and infections/septicemia [45–47], which are the leading causes of death among KTR [48].
CONCLUSION
We conclude that sleep quality deserves attention in the (transplant) nephrologist's consultation rooms. How this can best be done was not part of the current study, but the high prevalence of reported poor sleep makes clear that it is a topic worthy of further investigation. The suggested detrimental indirect effects (via individual strength) and direct effects on poorer societal participation and lower HRQoL highlight the magnitude of the problem among KTR, and the potential need of referral to cognitive therapists. In addition, we observed sex differences in prevalence of poor sleep, and in the trajectory of sleep quality after transplantation, showing that sleep quality improved among males, and not among females.
Supplementary Material
ACKNOWLEDGEMENTS
TransplantLines Investigators: Hans Blokzijl, Frank A.J.A. Bodewes, Marieke T. de Boer, Kevin Damman, Martin H. de Borst, Arjan Diepstra, Gerard Dijkstra, Caecilia S.E. Doorenbos, Michiel E. Erasmus, C. Tji Gan, Eelko Hak, Bouke G. Hepkema, Henri G.D. Leuvenink, Willem S. Lexmond, Vincent E. de Meijer, Hubert G.M. Niesters, L. Joost van Pelt, Robert A. Pol, Robert J. Porte, Adelta V. Ranchor, Jan Stephan F. Sanders, Marion J. Siebelink, Riemer J.H.J.A. Slart, Daan J. Touw, Marius C. van den Heuvel, Coretta van Leer-Buter, Marco van Londen, Erik A.M. Verschuuren, Michel J. Vos, Rinse K. Weersma.
Authors T.J.K., D.K., M.F.E., C.A., S.P.B. and S.J.L.B. are TransplantLines Investigators.
Contributor Information
Tim J Knobbe, Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Daan Kremer, Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Michele F Eisenga, Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Marco van Londen, Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Coby Annema, Department of Health Sciences, Section of Nursing Science, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Ute Bültmann, Department of Health Sciences, Community and Occupational Medicine, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Ido P Kema, Department of Laboratory Medicine Research, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Gerjan J Navis, Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Stefan P Berger, Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Stephan J L Bakker, Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
the TransplantLines Investigators:
Hans Blokzijl, Frank A J A Bodewes, Marieke T de Boer, Kevin Damman, Martin H de Borst, Arjan Diepstra, Gerard Dijkstra, Caecilia S E Doorenbos, Michiel E Erasmus, C Tji Gan, Eelko Hak, Bouke G Hepkema, Henri G D Leuvenink, Willem S Lexmond, Vincent E de Meijer, Hubert G M Niesters, L Joost van Pelt, Robert A Pol, Robert J Porte, Adelta V Ranchor, Jan Stephan F Sanders, Marion J Siebelink, Riemer J H J A Slart, Daan J Touw, Marius C van den Heuvel, Coretta van Leer-Buter, Marco van Londen, Erik A M Verschuuren, Michel J Vos, and Rinse K Weersma
CONFLICTS OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
T.J.K., D.K., M.F.E., M.v.L., C.A., U.B., I.P.K., G.J.N., S.P.B. and S.J.L.B. were responsible for conceptualization. T.J.K. and D.K. were responsible for original draft preparation, visualization and methodology. M.F.E., M.v.L., C.A., U.B., I.P.K., G.J.N., S.P.B. and S.J.L.B. contributed to writing, reviewing and editing. All authors approved the final version of the manuscript.
FUNDING
The TransplantLines Biobank and Cohort study was supported by a grant from Astellas BV and Chiesi Pharmaceuticals BV, and co-financed by the Dutch Ministry of Economic Affairs and Climate Policy by means of the PPP-allowance made available by the Top Sector Life Sciences & Health to stimulate public-private partnerships. The funders had no role in the study design, data collection, analysis, reporting, or the decision to submit for publication.
DATA AVAILABILITY STATEMENT
All data presented in this study can be made available upon reasonable request by the data manager of the TransplantLines study, by mailing to datarequest.transplantlines@umcg.nl.
REFERENCES
- 1. Hariharan S, Israni AK, Danovitch G. Long-term survival after kidney transplantation. N Engl J Med 2021;385:729–43. 10.1056/NEJMra2014530 [DOI] [PubMed] [Google Scholar]
- 2. van Sandwijk MS, Arashi DA, van de Hare FMet al. Fatigue, anxiety, depression and quality of life in kidney transplant recipients, haemodialysis patients, patients with a haematological malignancy and healthy controls. Nephrol Dial Transplant 2019;34:833–8. 10.1093/ndt/gfy103 [DOI] [PubMed] [Google Scholar]
- 3. Wang Y, Hemmelder MH, Bos WJWet al. Mapping health-related quality of life after kidney transplantation by group comparisons: a systematic review. Nephrol Dial Transplant 2021;36:2327–39. 10.1093/NDT/GFAB232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogna A, Ogna VF, Rubio JHet al. Sleep characteristics in early stages of chronic kidney disease in the HypnoLaus cohort. Sleep 2016;39:945–53. 10.5665/sleep.5660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Natale P, Ruospo M, Saglimbene VMet al. Interventions for improving sleep quality in people with chronic kidney disease. Cochrane Database Syst Rev 2019;5:CD012625. 10.1002/14651858.CD012625.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cordoza M, Koons B, Perlis MLet al. Self-reported poor quality of sleep in solid organ transplant: a systematic review. Transplant Rev 2021;35:1–33. 10.1016/j.trre.2021.100650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brekke FB, Waldum-Grevbo B, von der Lippe Net al. The effect of renal transplantation on quality of sleep in former dialysis patients. Transpl Int 2017;30:49–56. 10.1111/tri.12866 [DOI] [PubMed] [Google Scholar]
- 8. Hasanzamani B, Pourranjbar E, Ardani AR. Comparing sleep quality in patients before and after kidney transplantation. Iran J Kidney Dis 2020;14:133–8. [PubMed] [Google Scholar]
- 9. Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res 2010;185:105–29. 10.1016/B978-0-444-53702-7.00007-5 [DOI] [PubMed] [Google Scholar]
- 10. Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull 2010;136:375–89. 10.1037/A0018883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kyle SD, Morgan K, Espie CA. Insomnia and health-related quality of life. Sleep Med Rev 2010;14:69–82. 10.1016/J.SMRV.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 12. LeBlanc M, Beaulieu-Bonneau S, Mérette Cet al. Psychological and health-related quality of life factors associated with insomnia in a population-based sample. J Psychosom Res 2007;63:157–66. 10.1016/J.JPSYCHORES.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 13. Lee M, Choh AC, Demerath EWet al. Sleep disturbance in relation to health-related quality of life in adults: the Fels Longitudinal Study. J Nutr Heal Aging 2009;13:576–83. 10.1007/S12603-009-0110-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strine TW, Chapman DP. Associations of frequent sleep insufficiency with health-related quality of life and health behaviors. Sleep Med 2005;6:23–7. 10.1016/J.SLEEP.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 15. Darchia N, Oniani N, Sakhelashvili Iet al. Relationship between sleep disorders and health related quality of life—results from the Georgia SOMNUS study. Int J Environ Res Public Health 2018;15:1588. 10.3390/IJERPH15081588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reimer MA, Flemons WW. Quality of life in sleep disorders. Sleep Med Rev 2003;7:335–49. 10.1053/SMRV.2001.0220 [DOI] [PubMed] [Google Scholar]
- 17. Gordon E, Prohaska T, Gallant Met al. Self care strategies and barriers among kidney transplant recipients. Chronic Illn 2018;5:75–91. 10.1177/1742395309103558.Self-care [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zelle DM, Corpeleijn E, Klaassen Get al. Fear of movement and low self-efficacy are important barriers in physical activity after renal transplantation. PLoS One 2016;11:1–15. 10.1371/journal.pone.0147609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takahashi A, Hu SL, Bostom A. Physical activity in kidney transplant recipients: a review. Am J Kidney Dis 2018;72:433–43. 10.1053/j.ajkd.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 20. Ohayon MM. Prevalence and correlates of nonrestorative sleep complaints. Arch Intern Med 2005;165:35–41. 10.1001/ARCHINTE.165.1.35 [DOI] [PubMed] [Google Scholar]
- 21. Chan W, Bosch JA, Jones Det al. Predictors and consequences of fatigue in prevalent kidney transplant recipients. Transplantation 2013;96:987–94. 10.1097/TP.0B013E3182A2E88B [DOI] [PubMed] [Google Scholar]
- 22. Goedendorp MM, Hoitsma AJ, Bloot Let al. Severe fatigue after kidney transplantation: a highly prevalent, disabling and multifactorial symptom. Transpl Int 2013;26:1007–15. 10.1111/tri.12166 [DOI] [PubMed] [Google Scholar]
- 23. Eisenga MF, Gomes-Neto AW, Van Londen Met al. Rationale and design of TransplantLines: a prospective cohort study and biobank of solid organ transplant recipients. BMJ Open 2018;8:1–13. 10.1136/bmjopen-2018-024502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Von Elm E, Altman DG, Egger Met al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:1623–7. 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buysse DJ, Reynolds CF, Monk THet al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 26. Tingley D, Yamamoto T, Hirose Ket al. Mediation: R package for causal mediation analysis. J Stat Softw 2014;59:1–38. 10.18637/jss.v059.i0526917999 [DOI] [Google Scholar]
- 27. Liu HX, Lin J, Lin XHet al. Quality of sleep and health-related quality of life in renal transplant recipients. Int J Clin Exp Med 2015;8:16191–8. [PMC free article] [PubMed] [Google Scholar]
- 28. Dong X, Wang Y, Chen Yet al. Poor sleep quality and influencing factors among rural adults in Deqing, China. Sleep Breath 2018;22:1213–20. 10.1007/S11325-018-1685-8 [DOI] [PubMed] [Google Scholar]
- 29. Romero-Corral A, Caples SM, Lopez-Jimenez Fet al. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest 2010;137:711. 10.1378/CHEST.09-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parati G, Lombardi C, Castagna Fet al. Heart failure and sleep disorders. Nat Rev Cardiol 2016;13:389–403. 10.1038/nrcardio.2016.71 [DOI] [PubMed] [Google Scholar]
- 31. Kalmbach DA, Cuamatzi-Castelan AS, Tonnu CVet al. Hyperarousal and sleep reactivity in insomnia: current insights. Nat Sci Sleep 2018;10:193–201. 10.2147/NSS.S138823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fernández-Mendoza J, Vela-Bueno A, Vgontzas ANet al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med 2010;72:397–403. 10.1097/PSY.0b013e3181d75319 [DOI] [PubMed] [Google Scholar]
- 33. Sutton EL, Insomnia. Ann Intern Med 2021;174:ITC33–48. 10.7326/AITC202103160 [DOI] [PubMed] [Google Scholar]
- 34. Han Y, Kong Y, Peng Set al. Effect of attribution training on early postoperative depression of kidney transplant recipients. Curr Psychol 2022;41:5383–98. 10.1007/S12144-020-00954-3 [DOI] [Google Scholar]
- 35. Chaput JP, Shiau J. Routinely assessing patients’ sleep health is time well spent. Prev Med Rep 2019;14:100851. 10.1016/j.pmedr.2019.100851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Farouk SS, Rein JL. The many faces of calcineurin inhibitor toxicity—What the FK? Adv Chronic Kidney Dis 2020;27:56–66. 10.1053/j.ackd.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mah J, Pitre T. Oral magnesium supplementation for insomnia in older adults: a systematic review & meta-analysis. BMC Complement Med Ther 2021;21:1–11. 10.1186/S12906-021-03297-Z/FIGURES/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allen RP. Should we use oral magnesium supplementation to improve sleep in the elderly? Sleep Med 2003;4:263–4. 10.1016/S1389-9457(03)00067-4 [DOI] [Google Scholar]
- 39. Van De Cauter J, Sennesael J, Haentjens P. Long-term evolution of the mineral metabolism after renal transplantation: a prospective, single-center cohort study. Transplant Proc 2011;43:3470–5. 10.1016/J.TRANSPROCEED.2011.09.030 [DOI] [PubMed] [Google Scholar]
- 40.Gomes Neto AW, Boslooper-meulenbelt K, Geelink Met al. Protein intake, fatigue and quality of life in stable outpatient kidney transplant recipients. Nutrients 2020;12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deetman PE, Said MY, Kromhout Det al. Urinary urea excretion and long-term outcome after renal transplantation. Transplantation 2015;99:1009–15. 10.1097/TP.0000000000000464 [DOI] [PubMed] [Google Scholar]
- 42. Hartescu I, Morgan K, Stevinson CD. Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: a randomized controlled trial. J Sleep Res 2015;24:526–34. 10.1111/JSR.12297 [DOI] [PubMed] [Google Scholar]
- 43. Reid KJ, Baron KG, Lu Bet al. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med 2010;11:934–40. 10.1016/j.sleep.2010.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knobbe TJ, Kremer D, Eisenga MFet al. Airflow limitation, fatigue, and health-related quality of life in kidney transplant recipients. Clin J Am Soc Nephrol 2021;16:1686–94. 10.2215/CJN.06600521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Daghlas I, Dashti HS, Lane Jet al. Sleep duration and myocardial infarction. J Am Coll Cardiol 2019;74:1304–14. 10.1016/J.JACC.2019.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deng HB, Tam T, Zee BCet al. Short sleep duration increases metabolic impact in healthy adults: a population-based cohort study. Sleep 2017;40:1–11. 10.1093/SLEEP/ZSX130. [DOI] [PubMed] [Google Scholar]
- 47. Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep 2017;9:151–61. 10.2147/NSS.S134864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ying T, Shi B, Kelly PJet al. Death after kidney transplantation: an analysis by era and time post-transplant. J Am Soc Nephrol 2020;31:2887–99. 10.1681/ASN.2020050566 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this study can be made available upon reasonable request by the data manager of the TransplantLines study, by mailing to datarequest.transplantlines@umcg.nl.