Abstract
In recent decades, the development of non-destructive measurement methods for agricultural commodities has gained a lot of attention among scientists, but these techniques have different levels of accuracy for each instrument used. Therefore, this study aimed to compare the prediction accuracy of natural pigments, such as Total Carotenoid Content (TCC) and Total Flavonoid Content (TFC) using a color spectrophotometer and Visible/Near-Infrared (Vis/NIR) spectroscopy (381–1065 nm). The effect of ethephon concentration on the spectral characteristics and the accuracy of predicting pigments was studied. The samples used include cucumber fruit, which consisted of the 'Mars', 'Vanesa', and 'Roberto’ varieties. During the planting of the fruit, ethephon was applied at different concentrations of 0 ppm, 150 ppm, and 300 ppm. The results showed that the best accuracy for predicting TCC was obtained through a color spectrophotometer (Rcal = 0.89, Rpred = 0.90, RPD = 2.44), while the best prediction for TFC was the Vis/NIR spectroscopy (Rcal = 0.86, Rpred = 0.83, RPD = 1.78). Furthermore, the ethephon affects the spectral characteristics of cucumber fruit. Ethephon concentration of 150 ppm produced the highest accuracy value compared to others. This study proved that the use of non-destructive measurement methods with a color spectrophotometer and Vis/NIR spectroscopy has good performance in predicting TCC and TFC. The techniques are also easy to use, do not require chemicals, and have high accuracy.
Keywords: Food scanning, Fruit quality, Non-destructive, Prediction
1. Introduction
Cucumber (Cucumis sativus) is a horticultural commodity that is widely grown in many countries, and various varieties are often cultivated commercially. Furthermore, each of them has different qualities including size, shape, and color. In Indonesia, the 'Mars', 'Vanesa', and 'Roberto' varieties are often cultivated. Several studies revealed that cucumber is a source of natural antioxidants, and they help to counteract free radicals. Natural pigments, such as carotenoids and flavonoids are antioxidant compounds, which serve as secondary metabolites. They have several functions throughout the plant life cycle and strong antioxidant activity [1]. Cucumber contains 26.23 mg/100g–236 mg/100g carotenoid and 86.50 mg/100g–218.15 mg/100g flavonoid compounds that can nourish the body [2,3]. These compounds have strong antioxidant activity, hence, they can reduce various risks of chronic and degenerative diseases caused by oxidative stress [4].
Previous reports revealed that there has been a consistent increase in the production and quality of cucumber by farmers and studies. Plant growth regulators (PGRs), such as ethrel can improve the quality compound of agricultural products. The active ingredient in ethrel is ethephon, and its administration increased the number of female flowers [5]. This increase is expected to be directly proportional to the production of the plant. Gao et al. [6] also stated that the number of female flowers has a positive correlation with the number of fruits per plant, while Bajaj et al. [7] revealed that an increment led to higher fruit yield. The administration of ethephon can help increase the amount of natural pigments in fruits. Furthermore, Uchanski and Blalock et al. [8] stated that its application improved pigmentation. This finding is consistent with Puech et al. [9] that pigment changes were related to the application of ethephon. Shafiq et al. [10] stated that the initial stages of biosynthesis for the formation of flavonoids require the presence of ethylene.
Analysis of natural pigment content is often carried out on a laboratory/destructive analysis. Based on previous findings, the majority of quantitative analysis of measurements for testing carotenoids and flavonoids are performed using spectrophotometer evaluation, but it caused damage during the extraction process. Measurements of carotenoids and flavonoids are often carried out using wet/dry sample extraction [[11], [12], [13], [14], [15]]. However, these methods are time-consuming and require sample preparation as well as other chemicals. To avoid pigment oxidation during extraction, it is necessary to develop an analytical method that is affordable, and reliable, [16]. Spectroscopy is a method that uses non-destructive technology and can be used to overcome these problems. There are various types including Fourier-Transform Infrared (FTIR), Near-Infrared (NIR), Ultraviolet–Visible (UV/Vis), Visible/Near-Infrared (Vis/NIR), Nuclear Magnetic Resonance (NMR), Raman, and X-ray spectroscopies. These technologies have been used to quantitatively detect various ingredients in agricultural products [[17], [18], [19], [20], [21], [22], [23], [24], [25], [26]]. The differences between the various types of spectroscopy usually depend on the wavelength, the type of sample, and the analytical parameters required. Vis/NIR spectroscopy has a wavelength that is closely related to color, which can be measured with a spectrophotometer.
A color spectrophotometer was used to determine the quantitative value of fruit skin coloration. In vegetables or fruit, skin color is an important component because it can serve as an indicator of freshness/maturity. The values displayed from the color spectrophotometer include L*, a*, and b*, while Vis/NIR spectroscopy produced absorbance values [27,28]. It is interesting to be able to compare the accuracy of natural pigment prediction models from color spectrophotometer and Vis/NIR spectroscopy, which have similar wavelength. Color spectrophotometer can make prediction based on color variables (L*, a*, and b*), while Vis/NIR spectroscopy is based on spectral (absorbance) variables. Vis/NIR spectroscopy has been used to make prediction model for natural pigments in agricultural products. Several studies also revealed that spectroscopy technology has succeeded in predicting the content of flavonoids [29,30] and carotenoids [31,32]. Furthermore, previous studies have compared predictions based on color and spectra together. Tilahun et al. [16] and Goisser et al. [33] carried out a comparison between the use of color variables and Vis/NIR spectra on the parameters of natural pigments (lycopene and β-carotene) in tomato, which is a climacteric fruit. The color spectrophotometer yielded sufficient accuracy (lycopene, Rcv2 = 0.94 and β-carotene Rcv2 = 0.74), whereas Vis/NIR spectroscopy resulted comparable accuracy (lycopene, Rcv2 = 0.74 dan β-carotene R cv2 = 0.94). Hence, our study was done on cucumber. To the best of our knowledge, no study has predicted natural pigments such as total carotenoids and flavonoids using color variables (color spectrophotometer) and Vis/NIRS spectra (Vis/NIR spectroscopy) of various cucumber varieties. The Vis/NIRS spectra of cucumber subjected to various ethephon concentrations and as well as the effect of the ethephon concentration on the accuracy of prediction models have never been studied.
Therefore, this study aimed to compare the prediction accuracy of the Total Carotenoids Content (TCC) and Total Flavonoids Content (TFC) in multi-variety cucumbers using a color spectrophotometer and Vis/NIR spectroscopy. The effect of the cultivation techniques in the field on the accuracy of the multi-variety cucumber model was also assessed. It is important to evaluate the influence of various ethephon concentrations on the spectral characteristics and accuracy values for TCC and TFC. Each variety of cucumber at each ethephon concentration was also classified to determine the similarities or differences in the characteristics of each treatment.
2. Materials and methods
2.1. Sample material
Planting was carried out in a screen house with a height of 829 m above sea level. The cucumbers used include varieties of 'Mars', 'Vanesa', and 'Roberto'. A total of 10 mL of ethephon with different concentrations of 0, 150, and 300 ppm was applied to all parts of the samples at 21 Days After Planting (DAP) on all parts. The same cultivation techniques were applied to the varieties, except for the concentration of ethephon. A total of 100 cucumbers were used in this study, and they were divided into calibration and prediction sets with a total of 70 and 30 samples, respectively. The samples were harvested at the same maturity stage. Laboratory analysis was then carried out at the Laboratory of Horticulture, Faculty of Agriculture, Universitas Padjadjaran, Indonesia.
2.2. Color measurement
Samples of fruit that have been harvested were cleaned and cut off their stalks. Measurement of color of the whole cucumber was carried out on different positions, namely the top, middle, and bottom, and the average was then obtained. Color was described with L*, a*, and b* values using a CM-600d color spectrophotometer (Konica Minolta Inc., Japan). L* refers to brightness, while a* and b* represent green-red and blue-yellow gradations, respectively [17]. Furthermore, the color value in this study was used as a predictor. The calibration and prediction sets were used to assess the performance of TCC and TFC regression models.
2.3. Vis/NIR spectra measurement
The portable NirVana AG410 spectrometer (Integrated Spectronics Pty, Ltd, Australia) was used to collect Vis-NIR spectra with spectral sample intervals of 3 nm and wavelengths of 381–1065 nm. The Vis-NIR spectra were measured at 6 different points, namely, at the top, middle, and bottom on 2 different sides, and the values obtained were averaged for each sample. Further, each spectral measurement was carried out for 6–8 s. The Vis/NIR spectra were measured at the same spots, where color evaluation was performed. After the collection of spectra data, the data were transferred to a laptop. Then, the data were interpreted using the Integrated Software for Imagers and Spectrometers (Integrated spectronics Pty, Ltd, Australia). The software automatically processed the Vis/NIR spectra into absorbance data [[34], [35], [36]].
2.4. Reference measurement: TCC and TFC
TCC and TFC were measured using a UV/Vis spectrophotometer (Shimadzu UV mini-1240, Tokyo, Japan), and the extract used was obtained from dry samples. The sample was weighed as much as 0.05g and placed in a 10 mL vial, followed by the addition of 10 mL acetone. It was then placed in the sonication apparatus (Ultrasonic Cleaner BK-2000 Guangzhou, China) for 15 min at 33 °C. The extraction results were transferred into a 10 mL Eppendorf tube using a micropipette, followed by centrifugation (Corona 80-2 Centrifuge) at 4000 rpm for 10 min. The samplewas then measured using a UV–Vis spectrophotometer (Shimadzu UV mini-1240, Tokyo, Japan) with a wavelength of 449 nm [37].
TFC analysis was carried out on the results of dry sample extraction. The same extraction method was used to determine TCC, but the chemical solvent was ethanol. A total of 1 mL of the extracted sample was placed into a 5 mL volumetric flask, followed by the addition of 0.5 mL AlCl3, 0.5 mL acetic acid, and 3 mL ethanol. The solution was shaken until it was mixed, and then incubated for 30 min at room temperature. Absorbance was measured using a UV–Vis spectrophotometer (Shimadzu UV mini-1240, Tokyo, Japan) with a wavelength of 415 nm [38]. TCC and TFC were obtained as mg/100g dry weight by comparing the sample values with the β-Carotene and quercetin standard curve for TCC and TFC, respectively.
2.5. Data analysis
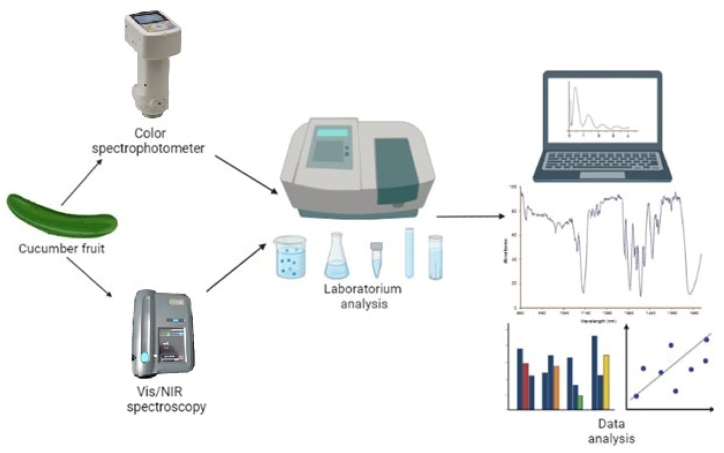
Calibration model including three varieties and three different ethephon concentrations were constructed using the Partial Least Square Regression (PLSR). Calibration and prediction sets were then randomly selected. Data analysis was carried out using The Unscrambler X 10.4 (Camo Software USA, Oslo, Norway) for further evaluation. In developing the model, an analysis was carried out to assess the linear relationship between reference measurements using color measurements and reference measurements with Vis/NIR spectra. The statistical indicators used to evaluate the performance of the developed PLSR model include the correlation coefficient on the calibration set (Rcal), Root Mean Square Error of the Calibration set (RMSEC), Correlation coefficient on the prediction set (Rpred), Root Mean Square Error of the Prediction set (RMSEP) and Ratio of Prediction to Deviation (RPD) [39]. The laboratory analysis processes conducted during the study are described in Fig. 1.
Fig. 1.
Illustration of experimental procedures using a color spectrophotometer and Vis/NIR spectroscopy for TCC and TFC in cucumbers fruit. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Results and discussion
3.1. Spectra of various varieties in different ethephon concentrations
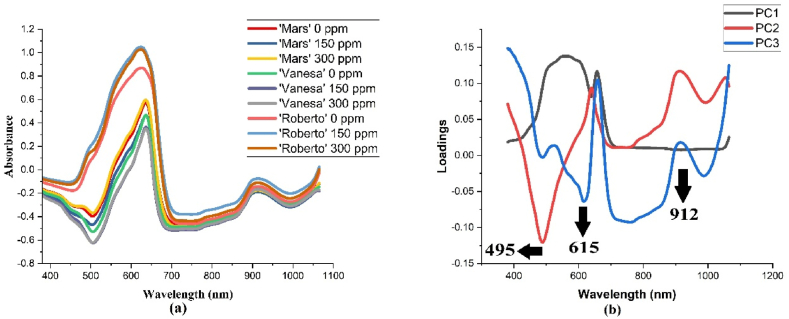
Fig. 2 displays the absorbance spectra of cucumber samples in the Vis.NIR range. Fig. 2a depicts a spectrum representation for each variety, while Fig. 2b depicts the first, second, and third PCs of the loading plot. Fig. 2a shows the average absorbance value of each variety with different ethephon concentration. The absorbance spectra of cucumber varieties ‘Mars', 'Vanesa', and 'Roberto' are illustrated in Fig. 2b, which shows the presence of peaks at 495 nm 615 nm 912 nm. The wavelengths of 495 nm and 615 are closely related to pigment, especially the content of beta-carotene and chlorophyll. The 475 nm region is a strong absorption area for beta-carotene, which has a yellow to orange-red color, while 680 nm is for chlorophyll [40]. Meanwhile, the wavelength of 912 nm is closely related to the absorption of water and starch. The results also showed that the water property caused by the O–H bonds and starch can be detected at 970 nm [41,42,43] The recorded spectrum contributes to the presence of organic substances, as inferred from the spectral bands that arise from the interaction between molecular bonds such as O–H, C–H, C–O, and N–H with the incoming radiation. The vibrational energy changes visible in these interactions encompass two distinct vibration patterns: stretch vibration and bend vibration. The Vis/NIR spectra of cucumber exhibit distinct absorbance bands according to the presence of prominent pigments, water, and starch. These spectral differences were caused by variations in variety and ethephon concentration. The study observed that certain wavelengths, specifically the peaks and valleys of the spectrum, were identified as significant or influential in contributing to the models. The spectrum patterns are also similar, but the level of absorbance was different. The similarities or differences of each spectrum group can be visualized with the Principal Component Analysis (PCA) method. The principle of PCA is to combine previously associated data into new variants to find variations across the samples [44].
Fig. 2.
Average spectra from each variety and ethephon concentrations using Vis/NIR spectroscopy and loading plot of first two three PCs.
3.2. Development of calibration model of various varieties in different ethephon concentrations
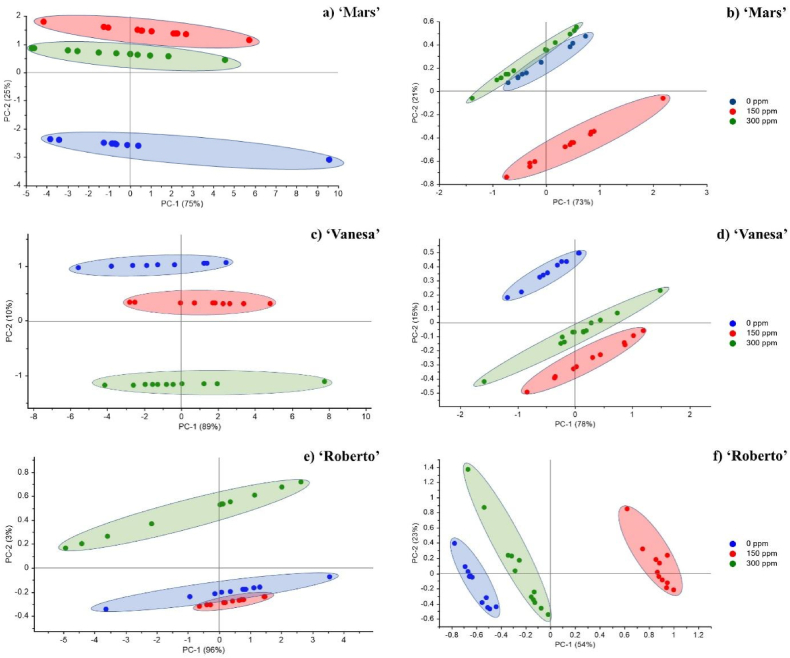
Classification was carried out using PCA to visualize products based on their characteristics. PCA is widely employed as the primary technique for qualitative analysis of Vis/NIR spectral data. This method is specifically useful for product separation and grouping purposes. PCA is widely utilized in data synthesis because of its ability to identify the principal components (PCs) that capture the highest variance in Vis/NIR spectroscopic data. These PCs can potentially replace wavelength as an input in data analysis. The utilization of a PCA score plot was applied to identify potential outliers by means of Hotelling's T2 statistics. Additionally, it was used to conduct an unsupervised classification of cucumber samples that varied in cultivars and ethephon concentrations. Furthermore, Fig. 3 shows the results of PCA and Multiplicative Scatter Correction (MSC) visualization. The clustering process was visualized based on color reference data (Fig. 3 a, c, and e) and Vis/NIR spectra (Fig. 3 b, d, and f). In the 'Mars' variety grouping, various concentrations of ethephon yielded 75 % PC1 and 25 % PC2 in Fig. 3a. The process also successfully classified 73 % in PC1 and 21 % in PC2, as shown in Fig. 3b. The yields for the 'Vanesa' variety with yielded 89 % for PC1 and 10 % for PC2 in Fig. 3c as well as 78 % for PC1 and 15 % for PC2 in Fig. 3d. 'Roberto' was successfully classified with a PC1 value of 96 % and 3 % PC2 in Fig. 3e as well as 54 % PC1 and 23 % PC2 in Fig. 3f. Based on these findings, each ethephon concentration for the 3 varieties can be separated properly. The results showed that each sample in this study did not overlap and was perfectly separated. Furthermore, the combination of PC1 and PC2 showed good results because the value obtained was >70 %. A two-dimensional score scatters plot can be used to visualize PCA findings because PC1 and PC2 combination explained more than 70 % of the total variance [45].
Fig. 3.
Classification PCA + MSC from each cultivar with different ethephon concentrations using color spectrophotometer (a, c, and e), and Vis/NIR spectroscopy (b, d, and f). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In this study, the dynamics of the accuracy of each model for ethephon concentrations were also studied. Furthermore, Table 1 showed the models, which were obtained from a combination of various varieties. The best result was recorded at 150 ppm ethephon for the 2 parameters predicted using a color spectrophotometer or Vis/NIR spectroscopy. In the prediction results using a color spectrophotometer for TCC and TFC, models with sufficient accuracy were obtained. In TCC, the results were Rcal (0.92), Rcv (0.92), RMSEC (48.99), RMSECV (52.44), and Dcv (3.45), while in TFC Rcal (0.84), Rcv (0.84), RMSEC (45.29), RMSECV (49.32), and Dcv (4.03) were recorded. Accuracy values using Vis/NIR spectroscopy obtained the model with the highest accuracy value. In TCC, the accuracy values for Rcal were 0.93, RMSEC 44.30, Rcv 0.91, RMSECV 51.27, and Dcv 6.97, while TFC gave Rcal 0.92, RMSEC 33.39, Rcv 0.87, RMSECV 41.67 and Dcv 8.28. These results showed that the specified model was not overfitted or under fitted based on the values of Rcal, Rpred, and Rcv, which were close [46].
Table 1.
Statistics for Model Predictions of TCC and TFC from each Ethephon Concentration using Color Spectrophotometer and Vis/NIR Spectroscopy.
| Instruments | Quality | Ethephon | Rcal | RMSEC | Rcv | RMSECV | Dcv |
|---|---|---|---|---|---|---|---|
| Color spectrophotometer | TCC | 0 ppm | 0.85 | 60.56 | 0.86 | 63.45 | 2.89 |
| 150 ppm | 0.92 | 48.99 | 0.92 | 52.44 | 3.45 | ||
| 300 ppm | 0.93 | 44.30 | 0.91 | 48.52 | 4.22 | ||
| TFC | 0 ppm | 0.86 | 34.36 | 0.81 | 39.07 | 4.71 | |
| 150 ppm | 0.84 | 45.29 | 0.84 | 49.32 | 4.03 | ||
| 300 ppm | 0.86 | 39.37 | 0.83 | 45.30 | 5.93 | ||
| Vis/NIR spectroscopy | TCC | 0 ppm | 0.89 | 51.57 | 0.87 | 61.05 | 9.48 |
| 150 ppm | 0.93 | 44.30 | 0.91 | 51.27 | 6.97 | ||
| 300 ppm | 0.91 | 48.12 | 0.87 | 57.44 | 9.32 | ||
| TFC | 0 ppm | 0.84 | 36.47 | 0.78 | 44.85 | 8.38 | |
| 150 ppm | 0.92 | 33.39 | 0.87 | 41.67 | 8.28 | ||
| 300 ppm | 0.83 | 43.48 | 0.65 | 56.68 | 13.2 |
Dcv: the difference between RMSECV and RMSEC.
The application of ethephon at various concentrations produced a dynamic trend, as shown in Table 1. The accuracy of the predicted Rcal and Rcv values using the color spectrophotometer and Vis/NIR spectroscopy was partly dominated by an increasing trend at 150 ppm. The value of the difference between RMSECV and RMSEC (Dcv) was also evaluated in this study. In the color spectrophotometer, the higher the ethephon concentration, the higher the Dcv value. Meanwhile, in the Vis/NIR spectroscopy, the lowest Dcv value was recorded at 150 ppm. Good models are characterized by low RMSEC, RMSEP, and RMSECV, as well as high Rcal, with little difference between RMSEC and RMSECV/RMSEP (Dcv) [47,48].
3.3. Measured data and model comparison between two instruments
Carotenoids and flavonoids are important qualities of cucumber fruit. These two parameters are part of natural pigments. Generally, fruit and vegetables are rich in carotenoids. On the other hand, flavonoids, characterized by their polyphenolic structure, are an essential secondary metabolite. Flavonoids are recognized for their beneficial benefits on health and are an essential element in a range of nutraceutical, pharmacological, medical, and cosmetic uses. The reference data used in this study are show in Table 2. In the calibration set, the smallest TCC value was 3.86 mg/100g, while the highest was 410.68 mg/100g, with an average of 112.75 mg/100g as well as an SD of 120.83 mg/100g. Meanwhile, in the prediction set, the smallest and highest TCC values were 13.78 mg/100g and 390.32 mg/100g, respectively with an average of 132.40 mg/100g as well as an SD of 126.54 mg/100g. In TFC, the calibration ranged from 26.12 to 349.84 mg/100g with an average of 120.45 mg/100g and an SD of 81.01 mg/100g. In the prediction set, it ranged from 52.66 to 330.73 mg/100g with a mean of 133.26 as well as an SD of 78.68 mg/100g.
Table 2.
Reference data of TCC and TFC in calibration and prediction set.
| Quality |
Calibration |
Prediction |
||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | |
| TCC (mg/100g) | 3.86 | 410.68 | 112.75 | 120.83 | 13.78 | 390.32 | 132.40 | 126.54 |
| TFC (mg/100g) | 26.12 | 349.84 | 120.45 | 81.01 | 52.66 | 330.73 | 133.26 | 78.68 |
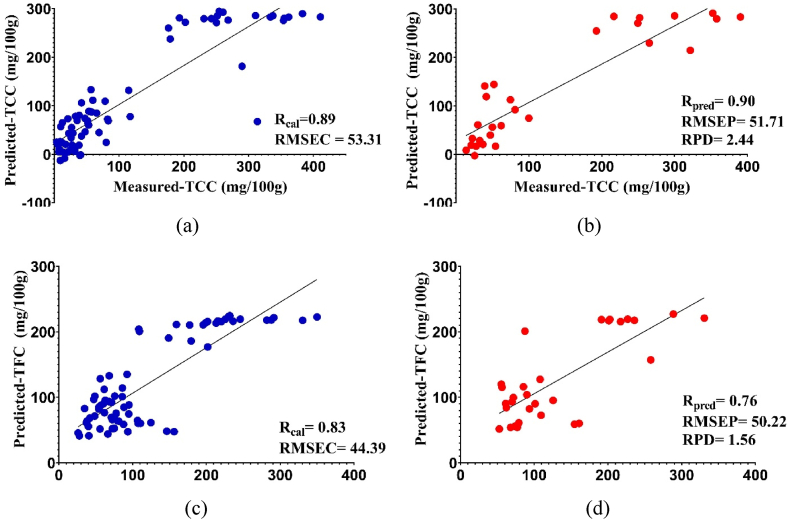
Cucumber samples were subjected to PLSR to create prediction models for TCC and TFC. The PLSR approach is commonly used for the calibration of Vis/NIR spectroscopy. The PLSR algorithm is relevant due to its unique characteristic of incorporating reference and predicted variables, distinguishing it from other linear regression algorithms. During the calibration process, PLSR was optimized using a cross-validation technique. The optimization was based on identifying the lowest minimum root mean square error cross-validation value for the corresponding latent variable. The prediction set applied a methodology of analyzing independent samples to make predictions for other cases. PLSR typically has the benefit of effectively addressing the challenges posed by irrelevant and noisy variables. Based on the statistical indicators from the prediction of TCC and TFC values using a color spectrophotometer and Vis/NIR spectroscopy, there was only a slight difference in accuracy, as shown in Table 3. The models developed from both methods have sufficient accuracy (Rcal and Rpred ≥ 0.76 and RPD ≥1.56). The poor prediction and discrepancy were indicated by RPD <1.5, while values between 1.5 and 2 indicate the ability of the model to distinguish between low and high response variables. RPD values of 2 and 2.5 show the ability to make rough quantitative predictions, while values 2.5 and 3 or more indicate good and excellent prediction accuracy [49,50]. Based on this classification, the results obtained from these two methods for the prediction of TCC and TFC were considered good. Prediction using color and spectra variables allows quantitative detection of TCC and TFC from cucumber fruit. This shows that non-destructive measurements can be used to detect fruit quality accurately, quickly, and easily. The use of a color spectrophotometer and Vis/NIR spectroscopy makes it easy to detect the quality of agricultural products. These methods do not require chemicals as well as a multi stages process. Non-destructive measurement allows for on-site analysis, has a low maintenance cost, requires only small sample volumes, and does not cause destruction of the sample [51,52].
Table 3.
Comparison of accuracy between color spectrophotometer and Vis/NIR spectroscopy.
| Quality | Instruments | Rcal | RMSEC | Rpred | RMSEP | RPD |
|---|---|---|---|---|---|---|
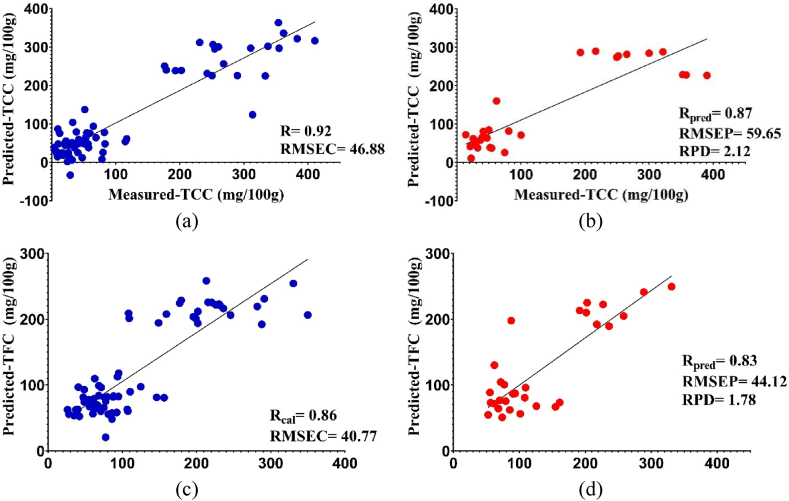
| TCC | Color spectrophotometer | 0.89 | 53.31 | 0.90 | 51.71 | 2.44 |
| Vis/NIR spectroscopy | 0.92 | 46.88 | 0.87 | 59.65 | 2.12 | |
| TFC | Color spectrophotometer | 0.83 | 44.39 | 0.76 | 50.22 | 1.56 |
| Vis/NIR spectroscopy | 0.86 | 40.77 | 0.83 | 44.12 | 1.78 |
According to Table 4, showing the correlation coefficient for concentrations with color (L*,a*, and b*) and pigments (TCC and TFC). The correlation coefficient is a statistical measure that quantifies the strength and direction of the linear relationship between two variables. When the correlation coefficient is close to 0, it suggests a weak or no correlation, whereas values close to 1 or -1 indicate a strong positive or negative linear correlation, respectively. The study produced both negative and positive correlation coefficient values. Color, TCC, and TFC have a strong correlation with each other. This can be observed from the correlation coefficients. TCC and TFC are natural pigments known for their important role in the appearance of a wide range of colors displayed by different fruits. However, these chemicals are mostly correlated with biochemical activities in plants.
Table 4.
Correlation of ethephon concentration, color (L*, a*, and b*) and pigments (TCC and TFC).
| Ethephon Concentrations | Color (L*, a*, and b*) | TCC | TFC | |
|---|---|---|---|---|
| Ethephon concentrations | 1 | |||
| Color (L*, a*, and b*) | 0.034 | 1 | ||
| TCC | −0.008 | −0.901** | 1 | |
| TFC | −0.053 | −0.783** | 0.858** | 1 |
The scatter plots generated from the most optimal model (Fig. 4, Fig. 5) demonstrate that the slope of the curve closely approximates the ideal 45°, suggesting that the projected TCC and TFC exhibit minimal bias in relation to the measured TCC and TFC. The study observed an array of actual TCC and TFC, which perhaps had a favorable impact on the model's performance. A broader sample range could potentially lead to improved model performance. The most optimal correlation observed between the reference data and the Vis/NIR spectra data is often achieved by the application of PLSR throughout the regression procedure. The main goal of the PLSR is to identify the optimal correlation between the reference data and the Vis/NIR spectra data in the regression analysis. The closeness of the data distribution to the regression line indicates the similarity of the values between the predictions of the color spectrophotometer or Vis/NIR spectroscopy and laboratory reference. Fig. 4 (a – d) shows the scatter plot using a color spectrophotometer, while Fig. 5 (a – d) presents of Vis/NIR spectroscopy. The prediction approach by comparing the use of both methods on cucumbers has never been carried out and the results showed their feasibility. The results also showed that the application of ethephon at the time of planting cucumbers can affect the accuracy of the model. Furthermore, based on these findings, color spectrophotometer and Vis/NIR spectroscopy showed the potential to predict TCC and TFC as a whole fruit. Detection using these methods makes field measurements easier, but one of the advantages of prediction with Vis/NIR spectroscopy is that it can perform non-destructive quantitative evaluation.
Fig. 4.
Correlation of measured and predicted TCC (a,b) and TFC (c,d) in calibration set (blue) and prediction set (red) using a color spectrophotometer. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
Correlation of measured and predicted TCC (a, b) and TFC (c,d) in calibration set (blue) and prediction set (red) using Vis/NIR spectroscopy. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Conclusions
Non-destructive technology has several advantages, namely it does not damage the sample, easy to use, time efficient, and does not use chemicals. Moreover, measurement of natural pigments, such as total carotenoids and flavonoids has the potential to be detected non-destructively using a color spectrophotometer and Vis/NIR spectroscopy. Prediction using these two methods is categorized as sufficient to detect TCC and TFC in cucumbers. The highest accuracy model resulted Rcal and Rpred ≥ 0.83 and RPD ≥1.78. In summary, the findings were: 1) color spectrophotometer produced better accuracy in predicting TCC, 2) TFC prediction had better accuracy using a Vis/NIR spectroscopy, 3) each variety and ethephon concentration could be classified properly, 4) ethephon of 150 ppm had the highest level of accuracy compared to the concentrations of 0 and 300 ppm. This has implications for quality control processes within the agricultural industry, enabling improved monitoring and assessment of product quality. Furthermore, the classification of cucumber varieties and the impact of ethephon concentrations were successfully demonstrated using the prediction model. To expand upon these findings, future studies can consider incorporating a wider range of cucumber varieties and explore additional plant cultivation treatments that may influence sample quality. This is expected to affect the quality of the sample to increase the robustness of the prediction model. Such investigations would enhance the robustness and applicability of the prediction model, further contributing to advancements in the field of non-destructive quality assessment in the agricultural sector.
Data availability statement
Data included in article/supp. material/referenced in article.
CRediT authorship contribution statement
Kusumiyati Kusumiyati: Writing - original draft, Methodology, Funding acquisition, Conceptualization. Ine Elisa Putri: Writing - review & editing, Validation, Methodology, Formal analysis.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kusumiyati Kusumiyati reports article publishing charges was provided by Padjadjaran University.
Acknowledgments
The authors are grateful to Yuda Hadiwijaya, Sarah Nurul Fajr, and Yusuf Eka Maulana for their assistance, technical support, and chemical acquisition in this study.
References
- 1.Lu W., Shi Y., Wang R., Su D., Tang M., Liu Y., Li Z. Antioxidant activity and healthy benefits of natural pigments in fruits: a review. Int. J. Mol. Sci. 2021;22:1–18. doi: 10.3390/ijms22094945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C., Zhou G., Chen J., Liu X., Lu X., Chen H., Tian Y. Integrated metabolome and transcriptome analysis unveils novel pathway involved in the formation of yellow peel in cucumber. Int. J. Mol. Sci. 2021;22:1–19. doi: 10.3390/ijms22031494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusumiyati, Fajri S.N., Sutari W., Hamdani J.S. Growth, yield, and fruit quality responses of three cucumber (Cucumis sativus L.) varieties to different ethephon concentrations. Emir. J. Food Agric. 2022;34:1–8. doi: 10.9755/ejfa.2022.v34.i1.2813. [DOI] [Google Scholar]
- 4.Andarwulan N., Puspita N.C., Saraswati, Średnicka-Tober D. Antioxidants such as flavonoids and carotenoids in the diet of Bogor, Indonesia residents. Antioxidants. 2021;10:1–20. doi: 10.3390/antiox10040587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Özgür M., Skirvin R.M., Al-Juboory K.H., Kushad M. Effects of ethylene on the production of female flowers by “burpless hybrid” cucumber (Cucumis sativus L.) in vitro. Biotechnol. Biotechnol. Equip. 2004;18:35–38. doi: 10.1080/13102818.2004.10819227. [DOI] [Google Scholar]
- 6.Gao L., Yu G., Hu F., Li Z., Li W., Peng C. The patterns of male and female flowers in flowering stage may not be optimal resource allocation for fruit and seed growth. Plants. 2021;10 doi: 10.3390/plants10122819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajaj S., Kumar D., Singh N., Gangwar V., Wamiq M., Pal O., Dishri M. Effect of different plant growth regulators on fruit yield and quality parameters of cucumber (Cucumis sativus L.) cv. Punjab Naveen. Int. J. Environ. Clim. Chang. 2022;10:2310–2315. doi: 10.9734/ijecc/2022/v12i1131225. [DOI] [Google Scholar]
- 8.Uchanski M.E., Blalock A. Ethephon improved pigmentation but had no effect on cayenne pepper fruit yield in Southern New Mexico. Hortscience. 2013;48:738–741. doi: 10.21273/hortsci.48.6.738. [DOI] [Google Scholar]
- 9.Puech A.A., Rebeiz C.A., Crane J.C. Pigment changes associated with application of ethephon ((2-Chloroethyl) phosphonic Acid) to Fig (Ficus carica L.) fruits. Plant Physiol. 1976;57:504–509. doi: 10.1104/pp.57.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shafiq M., Singh Z., Khan A.S. Pre-harvest ethephon application and training systems affect colour development, accumulation of flavonoids and fruit quality of “Cripps Pink” apple. Aust. J. Crop. Sci. 2014;8:1579–1589. [Google Scholar]
- 11.Szabo K., Teleky B.E., Ranga F., Roman I., Khaoula H., Boudaya E., Ben Ltaief A., Aouani W., Thiamrat M., Vodnar D.C. Carotenoid recovery from tomato processing by-products through green chemistry. Molecules. 2022;27 doi: 10.3390/molecules27123771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zulueta A., Esteve M.J., Frígola A. Carotenoids and color of fruit juice and milk beverage mixtures. J. Food Sci. 2007;72:C457–C463. doi: 10.1111/j.1750-3841.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 13.Abid M., Jabbar S., Wu T., Hashim M.M., Hu B., Lei S., Zeng X. Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrason. Sonochem. 2014;21:93–97. doi: 10.1016/j.ultsonch.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Sharma V., Janmeda P. Extraction, isolation and identification of flavonoid from Euphorbia neriifolia leaves. Arab. J. Chem. 2017;10:509–514. doi: 10.1016/j.arabjc.2014.08.019. [DOI] [Google Scholar]
- 15.Chaves J.O., de Souza M.C., da Silva L.C., Lachos-Perez D., Torres-Mayanga P.C., Machado A.P. da F., Forster-Carneiro T., Vázquez-Espinosa M., González-de-Peredo A.V., Barbero G.F., Rostagno M.A. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020;8 doi: 10.3389/fchem.2020.507887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tilahun S., Park D.S., Seo M.H., Hwang I.G., Kim S.H., Choi H.R., Jeong C.S. Prediction of lycopene and β-carotene in tomatoes by portable chroma-meter and VIS/NIR spectra. Postharvest Biol. Technol. 2018;136:50–56. doi: 10.1016/j.postharvbio.2017.10.007. [DOI] [Google Scholar]
- 17.Kusumiyati K., Putri I.E., Munawar A.A., Suhandy D. A data fusion model to merge the spectra data of intact and powdered cayenne pepper for the fast inspection of antioxidant properties. Sustainability. 2022;14:1–11. doi: 10.3390/su14010201. [DOI] [Google Scholar]
- 18.Kusumiyati K., Hadiwijaya Y., Putri I.E., Munawar A.A. Enhanced visible/near-infrared spectroscopic data for prediction of quality attributes in Cucurbitaceae commodities. Data Brief. 2021;39 doi: 10.1016/j.dib.2021.107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunghez I.R., Raduly M., Doncea S., Aksahin I., Ion R.M. Lycopene determination in tomatoes by different spectral techniques (UV-VIS, FTIR and HPLC) Dig. J. Nanomater. Biostruct. 2011;6:1349–1356. doi: 10.1002/14651858.CD006489.pub4. [DOI] [Google Scholar]
- 20.Fan S., Wang Q., Tian X., Yang G., Xia Y., Li J., Huang W. Non-destructive evaluation of soluble solids content of apples using a developed portable Vis/NIR device. Biosyst. Eng. 2020;193:138–148. doi: 10.1016/j.biosystemseng.2020.02.017. [DOI] [Google Scholar]
- 21.Genis H.E., Durna S., Boyaci I.H. Determination of green pea and spinach adulteration in pistachio nuts using NIR spectroscopy. LWT--Food Sci. Technol. 2021;136 doi: 10.1016/j.lwt.2020.110008. [DOI] [Google Scholar]
- 22.Haughey S.A., Galvin-King P., Ho Y.C., Bell S.E.J., Elliott C.T. The feasibility of using near infrared and Raman spectroscopic techniques to detect fraudulent adulteration of chili powders with Sudan dye. Food Control. 2015;48:75–83. doi: 10.1016/j.foodcont.2014.03.047. [DOI] [Google Scholar]
- 23.Mathanker S.K., Weckler P.R., Bowser T.J. X-ray applications in food and agriculture: a review. Trans. ASABE (Am. Soc. Agric. Biol. Eng.) 2013;56:1227–1239. doi: 10.13031/trans.56.9785. [DOI] [Google Scholar]
- 24.Kamal T., Cheng S., Khan I.A., Nawab K., Zhang T., Song Y., Wang S., Nadeem M., Riaz M., Khan M.A.U., Zhu B.W., Tan M. Potential uses of LF-NMR and MRI in the study of water dynamics and quality measurement of fruits and vegetables. J. Food Process. Preserv. 2019;43 doi: 10.1111/jfpp.14202. [DOI] [Google Scholar]
- 25.Martin D., Gonzalvez A.G., Medina R.M., González Ureña A. Modeling tomato ripening based on carotenoid Raman spectroscopy: experimental versus kinetic model. Appl. Spectrosc. 2017;71:1310–1320. doi: 10.1177/0003702816681012. [DOI] [PubMed] [Google Scholar]
- 26.Saad A., Jha S.N., Jaiswal P., Srivastava N., Helyes L. Non-destructive quality monitoring of stored tomatoes using VIS-NIR spectroscopy. Eng. Agric. Environ. Food. 2016;9:158–164. doi: 10.1016/j.eaef.2015.10.004. [DOI] [Google Scholar]
- 27.Munawar A.A., Zulfahrizal, Meilina H., Pawelzik E. Near infrared spectroscopy as a fast and non-destructive technique for total acidity prediction of intact mango: comparison among regression approaches. Comput. Electron. Agric. 2022;193 doi: 10.1016/J.COMPAG.2021.106657. [DOI] [Google Scholar]
- 28.Kusumiyati K., Hadiwijaya Y., Suhandy D., Munawar A.A. Prediction of water content and soluble solids content of ‘manalagi’ apples using near infrared spectroscopy. IOP Conf. Ser. Earth Environ. Sci. 2021;922 doi: 10.1088/1755-1315/922/1/012062. [DOI] [Google Scholar]
- 29.Tian Z., Tan Z., Li Y., Yang Z. Rapid monitoring of flavonoid content in sweet tea (Lithocarpus litseifolius (Hance) Chun) leaves using NIR spectroscopy. Plant Methods. 2022;18:1–9. doi: 10.1186/s13007-022-00878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassan H., Fan M., Zhang T., Yang K. Prediction of total phenolics and flavonoids contents in Chinese wild rice (Zizania latifolia) using FT-NIR spectroscopy. Am. J. Food Technol. 2015;10:109–117. doi: 10.3923/ajft.2015.109.117. [DOI] [Google Scholar]
- 31.Kusumiyati K., Putri I.E., Hamdani J.S., Suhandy D. Real-time detection of the nutritional compounds in green ‘Ratuni UNPAD’ cayenne pepper. Horticulturae. 2022;8:554. doi: 10.3390/horticulturae8060554. [DOI] [Google Scholar]
- 32.Davey M., Saeys W., Hof E., Ramon H., Swennen R., Keulemans J. Application of visible and near-infrared reflectance spectroscopy (vis/nirs) to determine carotenoid contents in banana (Musa spp.) fruit pulp. J. Agric. Food Chem. 2009;57:1742–1751. doi: 10.1021/jf803137d. [DOI] [PubMed] [Google Scholar]
- 33.Goisser S., Wittmann S., Fernandes M., Mempel H., Ulrichs C. Comparison of colorimeter and different portable food-scanners for non-destructive prediction of lycopene content in tomato fruit. Postharvest Biol. Technol. 2020;167 doi: 10.1016/j.postharvbio.2020.111232. [DOI] [Google Scholar]
- 34.Kusumiyati, Mubarok S., Sutari W., Farida, Hamdani J.S., Hadiwijaya Y., Putri I.E. Non-destructive method for predicting sapodilla fruit quality using near infrared spectroscopy. IOP Conf. Ser. Earth Environ. Sci. 2019;334:1–8. doi: 10.1088/1755-1315/334/1/012045. [DOI] [Google Scholar]
- 35.Kusumiyati, Hadiwijaya Y., Putri I.E., Mubarok S. Water content prediction of “crystal” guava using visible-near infrared spectroscopy and chemometrics approach. IOP Conf. Ser. Earth Environ. Sci. 2019;393:1–5. doi: 10.1088/1755-1315/393/1/012099. [DOI] [Google Scholar]
- 36.Kusumiyati, Mubarok S., Sutari W., Hadiwijaya Y. Application of spectra pre-treatments on firmness assessment of intact sapodilla using vis-nir spectroscopy. IOP Conf. Ser. Earth Environ. Sci. 2021;644:1–8. doi: 10.1088/1755-1315/644/1/012001. [DOI] [Google Scholar]
- 37.Biswas A.K., Sahoo J., Chatli M.K. A simple UV-Vis spectrophotometric method for determination of β-carotene content in raw carrot, sweet potato and supplemented chicken meat nuggets. LWT--Food Sci. Technol. 2011;44:1809–1813. doi: 10.1016/j.lwt.2011.03.017. [DOI] [Google Scholar]
- 38.Sytar O., Hemmerich I., Zivcak M., Rauh C., Brestic M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018;25:631–641. doi: 10.1016/j.sjbs.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kusumiyati Y. Hadiwijaya, Putri I. Elisa. Determination of water content of intact sapodilla using near infrared spectroscopy. IOP Conf. Ser. Earth Environ. Sci. 2018;207:1–7. doi: 10.1088/1755-1315/207/1/012047. [DOI] [Google Scholar]
- 40.Walsh K.B., Blasco J., Zude-Sasse M., Sun X. Visible-NIR ‘point’ spectroscopy in postharvest fruit and vegetable assessment: the science behind three decades of commercial use. Postharvest Biol. Technol. 2020;168 doi: 10.1016/j.postharvbio.2020.111246. [DOI] [Google Scholar]
- 41.Kusumiyati K., Hadiwijaya Y., Sutari W., Munawar A.A. Global model for in-field monitoring of sugar content and color of melon pulp with comparative regression approach. AIMS Agric. Food. 2022;7:312–325. doi: 10.3934/agrfood.2022020. [DOI] [Google Scholar]
- 42.Xue J., Zhang S., Sun H., Zhou J. Study of Malus Asiatica Nakai's firmness during different shelf lives based on visible/near-infrared spectroscopy. Math. Comput. Model. 2013;58:1829–1836. doi: 10.1016/j.mcm.2012.12.021. [DOI] [Google Scholar]
- 43.Ibrahim A., Daood H.G., Égei M., Takács S., Helyes L. A comparative study between vis/NIR spectroradiometer and NIR spectroscopy for the non-destructive quality assay of different watermelon cultivars. Horticulturae. 2022;8:1–17. doi: 10.3390/horticulturae8060509. [DOI] [Google Scholar]
- 44.Siregar S.D., Rindang A., Ayu P.C. Principle component analysis (PCA) - classification of arabica green bean coffee of north sumatera using FT-NIRS. IOP Conf. Ser. Earth Environ. Sci. 2020;454:1–7. doi: 10.1088/1755-1315/454/1/012046. [DOI] [Google Scholar]
- 45.Saganowska P., Wesolowski M. Principal component and cluster analyses as supporting tools for co-crystals detection. J. Therm. Anal. Calorim. 2017;130:45–55. doi: 10.1007/s10973-017-6436-8. [DOI] [Google Scholar]
- 46.Pandiselvam R., Mahanti N.K., Manikantan M.R., Kothakota A., Chakraborty S.K., Ramesh S.V., Beegum P.P.S. Rapid detection of adulteration in desiccated coconut powder: vis-NIR spectroscopy and chemometric approach. Food Control. 2022;133 doi: 10.1016/J.FOODCONT.2021.108588. [DOI] [Google Scholar]
- 47.Xie L., Ye X., Liu D., Ying Y. Prediction of titratable acidity, malic acid, and citric acid in bayberry fruit by near-infrared spectroscopy. Food Res. Int. 2011;44:2198–2204. doi: 10.1016/j.foodres.2010.11.024. [DOI] [Google Scholar]
- 48.Wang A., Hu D., Xie L. Comparison of detection modes in terms of the necessity of visible region (VIS) and influence of the peel on soluble solids content (SSC) determination of navel orange using VIS-SWNIR spectroscopy. J. Food Eng. 2014;126:126–132. doi: 10.1016/j.jfoodeng.2013.11.011. [DOI] [Google Scholar]
- 49.Saeys W., Mouazen A.M., Ramon H. Potential for onsite and online analysis of pig manure using visible and near infrared reflectance spectroscopy. Biosyst. Eng. 2005;91:393–402. [Google Scholar]
- 50.Nicolaï B.M., Beullens K., Bobelyn E., Peirs A., Saeys W., Theron K.I., Lammertyn J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: a review. Postharvest Biol. Technol. 2007;46:99–118. doi: 10.1016/j.postharvbio.2007.06.024. [DOI] [Google Scholar]
- 51.Folli G.S., Santos L.P., Santos F.D., Cunha P.H.P., Schaffel I.F., Borghi F.T., Barros I.H.A.S., Pires A.A., Ribeiro A.V.F.N., Romão W., Filgueiras P.R. Food analysis by portable NIR spectrometer. Food Chem. Adv. 2022;1 doi: 10.1016/J.FOCHA.2022.100074. [DOI] [Google Scholar]
- 52.Kusumiyati, Hadiwijaya Y., Putri I.E., Mubarok S., Hamdani J.S. Rapid and non-destructive prediction of total soluble solids of guava fruits at various storage periods using handheld near-infrared instrument. IOP Conf. Ser. Earth Environ. Sci. 2020;458:1–7. doi: 10.1088/1755-1315/458/1/012022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.