Abstract
Six novel ciprofloxacin-1,2,3-triazole hybrids (6a-f) were synthesized via click reaction, by reacting of methyl 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-oxobutanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (5) with various aryl azides (9a-f). The new compounds were characterized using High-Resolution Mass Spectrometry (HRMS), 1H NMR, 13C NMR, and elemental analysis. Compounds (6a-f) screened for their in vitro anticancer activity against three cell lines, namely, non-small cell lung cancer (A549), glioblastoma (U-87 MG), and breast cancer (MCF7). Hybrids 6a and 6b exhibited remarkable anti-proliferative activity against all three cell-lines. IC50 values of 6b for all cancer cell lines were significantly lower comparing to the standard reference compound IC50. The IC50 of 6b for the normal cell (HDF) line was significantly higher than the reported for cisplatin [IC50 = 170.7 ± 8.1 μM/ml (HDF), (p ≤ 0.001)], indicating less toxicity towards normal cells and thereby has a better therapeutic index, with a selectivity index of 142.3 for U87 cell line. Compounds 6e, 6d, and 6f displayed significant cytotoxic activity against only U-87 and MCF-7 cancer cell lines, compared to normal cells (HDF). Compound 6f [IC50 = 7.9 ± 2.3 μM/ml (U-87) and 10.6 ± 3 μM/ml (MCF-7)] was more potent than cisplatin [IC50 = 28.3 ± 5.3 μM/ml (U-87) and 26.9 ± 4.7 μM/ml (MCF-7)] in displaying anti-proliferative effect against U-87 and MCF-7 cells, with less cytotoxic to normal cells [IC50 = 141.7 ± 4.1] than cisplatin [IC50 = 40.9 ± 5.4]. Moreover, they were tested for their antioxidant activity in DPPH and ABTS assays and antibacterial activity.
Keywords: Anti-tumor; Ciprofloxacin; 1,2,3-Triazoles; Huisgen 1,3-dipolar cycloaddition; Non-small cell lung cancer; Glioblastoma; Breast cancer
1. Introduction
Fluoroquinolones constitute a major class of antibacterial chemotherapeutic agents which have a broad spectrum against Gram-positive and Gram-negative bacteria. Examples include ciprofloxacin (1) and Norfloxacin (2), which were the first two fluoroquinolones marketed [1].
General fluoroquinolone structures contain β-keto carboxylic acid group at position 3 and 4 essential for binding to DNA-gyrase complex. N-1 position affects overall potency with cyclopropyl substituent display the best optimal activity. Fluorine atom at position 6 is essential for cell penetration and gyrase affinity. Piperazine at position 7 enhances the potency against Gram-negative bacteria [2]. Fluoroquinolones exhibit antimicrobial activity via the inhibition of DNA gyrase and topoisomerase II enzyme in bacteria [3]. Ciprofloxacin functions via expressing ternary complex with DNA-enzyme, thus restricting the repair of bacterial DNA and transcription of RNA [4,5]. Various fluoroquinolones continue to be the most effective and widely used common antibiotics for the treatment of urinary tract infections. Ciprofloxacin a second-generation fluoroquinolone displays broad-spectrum antibiotic activity, excessive use of ciprofloxacin in recent times led to the emergence of bacterial resistance, therefore; there is an urgent need to find an alternative drugs. A two-drug hybrid approach is a potential strategy in drug discovery that involves the combination of two drug pharmacophores into single molecule. The hybrid molecule acts through distinct modes of action on several targets at a given time with more efficacy and less susceptibility to resistance. 1,2,3-Triazoles form a powerful pharmacophore, since they have good and unique properties, such as strong dipole moment, high tendency to form hydrogen bond, π-stacking and good solubility, such features enabling 1,2,3-triazole moiety to interact with various enzymes, proteins, and receptors. 1,4-Disubsituted 1,2,3-triazole displays bio-isosteric effect due to their resemblance to amide bond in terms of bond length and planarity. Triazoles play a crucial role against fungal infections due to their broad spectrum and safety profile, but the widespread use of these agents has led to the development of resistance, the synthesis of new drugs has gained great importance. Thus, recently 1,2,3-triazoles core was found in several drugs that exhibit wide range of biological activities such as an antitumor [6,7], anti-HIV [8], and antibacterial [8]. Development of new antitumor agents has gained great focus in life-science research since cancer is the second leading cause of mortality globally after cardiovascular diseases. Several anticancer protocols are available, but due to their side effects such resistance, nondifferentiation between cancerous and noncancerous cells, and limitation of radiotherapy alternative treatments are required. 1,2,3-Triazoles have received great interest in drug discovery for the development of anticancer agents. Carboxyamidotriazole (3) and ceftrizine (4) are examples of drugs containing 1,2,3-triazole scaffold showing antitumor and antibacterial activities respectively, structures are shown below.
Herein, new hybrid compounds containing ciprofloxacin tethered to 1,2,3-triazole moiety via amide linkage were synthesized via enolate-azide click reaction and evaluated for their in vitro antitumor, and antibacterial activities, as well as their antioxidant activity.
2. Results with discussion
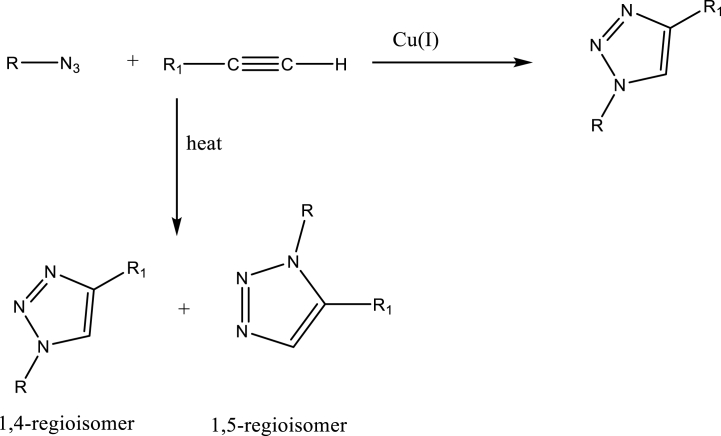
Chemistry: The most popular synthetic route to 1,2,3-triazoles is Cu (1)-catalyzed Huisgen 1,3-dipolar cycloaddtion [[9], a)], which involves the reaction between organic azides and alkynes to give only the 1,4-regioisomeric product, while the thermally induced reaction gives the 1,2,3-triazole adduct in poor regioselectivity and low chemical yield (Scheme 1). To avoid the toxicity of copper ions and other metals used as catalyst, great effort has been made to develop metal-free 1,3-dipolar cycloadditions. Chen [9b] developed a metal free reaction between nitroalkenes and organic azides for the synthesis of 1,2,3-triazoles. An excellent alternative for the synthesis of 1,2,3-triazoles which requires neither a metal catalyst nor alkyne substrates, is the reaction of enamine/enolate-mediated azide–carbonyl [3 + 2] cycloaddition [9c]. Also, cycloaddition reaction at room temperature between aryl azides and 1,3-dicarbonyl compounds in the presence of potassium carbonate in dimethyl sulfoxide yielded 1,2,3-triazoles [9d].
Scheme 1.
Huisgen 1,3-dipolar cycloaddition reaction.
Therefore, and to the best of our knowledge, ciprofloxacin-tethered to triazole nucleus via amide linkage have not been reported [6b]. Accordingly, we report herein the synthesis and in vitro evaluation of the antitumor, antibacterial, and antioxidant properties of a novel ciprofloxacin-1,2,3-triazole hybrid (6a-f), the structure is shown below.

New ciprofloxacin-1,2,3-triazole hybrid compounds (6a-f) were prepared in three simple steps starting from ciprofloxacin according to Scheme 3. The synthetic protocol is based on metal-free and alkyne-free synthesis using anhydrous K2CO3 as green catalyst. We have utilized β-ketoamide derivative (5) of ciprofloxacin methyl ester as the synthetic equivalent of alkyne to cycloadds to organic azides via enolate-click reaction.
Scheme 3.
Synthesis of ciprofloxacin-1,2,3-triazole hybrids(6a-f).
First, ciprofloxacin methyl ester (7) was prepared by refluxing ciprofloxacin with thionyl chloride (SOCl2) for 17 h, followed by addition of dry methanol after the removal of excess thionyl chloride, as shown below.
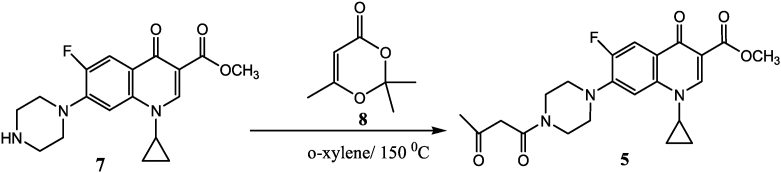
The acetoacetylation of nitrogen nucleophiles is an extremely important reaction to prepare β-ketoamides. Most of these acetoacetylation are affected by diketene, a four carbons synthon, highly reactive lachrymatory, and toxic reagent. So, the second step involves the reaction of 2,2,6-trimethy-4H-1,3-dioxoin-4-one (8) [10] with ciprofloxacin methyl ester (7) in preheated o-xylene at 1500C, to produce methyl 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-oxobutanoyl) piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (5) excellent yield, as shown below.
Carroll and Bader reported that diketene and acetone react to afford a 1:1 adduct namely, 2,2,6, trimethyl-4-H-1,3-dioxin-4-one (8) [[10], [11], [12]] the later compound is stable at room temperature and acetoacetylate alcohols and amines much better than ethyl acetoacetate. It has been suggested, that when pyrolyzed, 2,2,6-trimethyl-4-H-1,3-dioxin-4-one (8) decomposes into reactive acetyl ketene intermediate and acetone in retro Diels-Alder reaction as shown in (Scheme 2).
Scheme 2.
Acetoacetylation mechanism of ciprofloxacin methyl ester (7) with 2,2,6-trimethyl-4-H-1,3-dioxin-4-one.
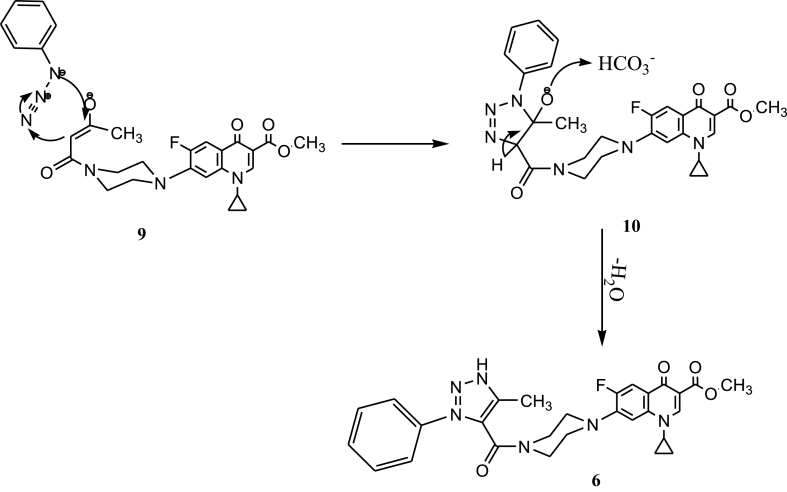
Finally, methyl 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-oxobutanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (5) was used as substrate to further generate ciprofloxacin 1,2,3-triazole hybrid (6a-f) by reacting with various organic azides via enolate-click reaction (Scheme 3). The new compounds were characterized by High-Resolution Mass Spectrometry (HRMS), 1H NMR, 13C NMR, and elemental analysis. The [3 + 2]-dipolar cycloaddition reaction of 5 with organic azides in dry dimethyl sulfoxide (DMSO) is catalyzed by anhydrous potassium carbonate(K2CO3). This strategy generates methyl 1,4-disubstituted 1,2,3-triazole caboxyamide of ciprofloxacin(6a-f) in high yield and high 1,4-regioselectivity at room temperature. A plausible mechanism has been proposed to explain (Scheme 4). First, the reaction of β-keto amides of ciprofloxacin methyl ester (5) with carbonate as base generates the enolate (9). Then, the enolate cycloadds to azide to afford five-membered intermediate (10), through [3 + 2] enolate-azide click cycloaddition. Finally, the rapid elimination of water induced by hydrogen carbonate transforms the intermediate into the final product (6). Work in progress to prepare 1,2,3-triazole derivatives for other fluoroquinolones.
Scheme 4.
Proposed mechanism for the formation of ciprofloxacin-1,2,3-triazole hybrid (6a-f) via enolate-click reaction.
3. Experimental
3.1. Material and Equipment
Ciprofloxacin were purchased form Thermo scientific, p-fluoroaniline, p-trifluoromethylaniline, m-fluorobenzyl bromide, p-flourobenzyl bromide were purchased form Aldrich, sodium azide, p-chloroaniline were purchased from Janssen chimica, p-nitroaniline was purchased from GPR, 2,2,6-trimethyl-4H-1,3-dioxine-4-one(diketene acetone adduct) was purchased from ACROS, silica gel for chromatography was purchased from Scharlau, Dimethylsulfoxide (DMSO) was purchased from Janseen chimica and was dried over anhydrous calcium sulfate, molecular sieves was purchased from Janssen chimica(5A, 8–12mesh) then distilled under vacuum, hexane, dimethyl formide (DMF) were purchased from Acilabscan and was dried overnight over barium oxide or 5A molecular sieves, followed by decantation of the drying agent and vacuum distilled (∼20 mmHg) then stored over 4A molecular sieves, chloroform, hexane were purchased from loba chemie, dichloromethane was purchased from Janssen chimica, o-xylene was purchased from Fluka. Potassium carbonate was purchased from pharmaccos LTD and was dried under vacuum with heating to 60 °C, and anhydrous sodium sulfate was purchased from AZ chem. Ultraviolet Fluorescence Analysis Cabinets were usedvisualizeuliz the colorless spots. Melting points(uncorrected) were determined on Electrothermal IA6304 Melting Point apparatus in open capillary tubes. H1-NMR was recorded on spinsolve 80 carbon (magritek/Germany) at Mutah University, for preliminary investigations and crude products before purification. 1D and 2D NMR spectra were recorded on a 500 MHz spectrometer (Bruker 500 MHz−Avance III) (University of Jordan) and 400 MHz spectrometer (Bruker Avance III 400 MHz) (Al-Yarmouk University) with TMS as the internal standard. High-resolution mass spectra (HRMS) were measured in positive ion mode using electrospray ion trap ESI technique by collision-induced dissociation on a Bruker APEX-IV (7 T) instrument (University of Jordan). The samples were dissolved in chloroform and acetonitrile and infused using a syringe pump with a flow rate of 2 μL/min. External calibration was conducted using an arginine cluster in a mass range of m/z 175–871. Mass error: 0.00–0.50 ppm. Elemental analysis was performed at the University of Jordan. Aryl azides(6c-f) were prepared from the reaction of corresponding aryldiazonium salts with sodium azide [13], while p-fluorbenzyl azide(9a) and m-fluorobenzyl azide(9b) were prepared from the reaction of their corresponding benzyl bromide with sodium azide [14]. Organic azides were used directly without further purification and stored in the freezer at −20 °C.
3.1.1. Preparation of methyl 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-quinoline-3-carboxylate (7)
Ciprofloxacin (1.91 g, 5.79 mmol) was dissolved in dry methanol (100 ml) and cooled in an ice bath. Thionyl chloride (8.38 ml, 114.88mmole) was added dropwise while stirring, resulting in a yellow solution. This solution was heated under reflux for 17 h. The solution was concentrated under reduced pressure giving a yellow oil. This oil was dissolved in saturated aqueous K2CO3(25 ml) and extracted with dichloromethane(4 × 40ml). The combined organic layer washed with water (40 ml), which was extracted with dichloromethane(3 × 40ml). The organic layer was combined and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the title compound as white crystalline solid with m.p = 220–222 °C, yield = 1.5 g, 75 %. 1H NMR (400 MHz, CDCl3) δ: 8.49 (s, 1H), 7.96(d, 3JH-F = 13.2 Hz, 1H), 7.24(d, 4JH-F = 6.9 Hz, IH), 3.90 (s, 3H), 3.5–2.8 (m, 9H), 1.8–1.22 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 172.9(s), 166.3(s), 153.3(d, JC-F = 248.3 Hz), 148.2(s), 144.9(d, 2JC-F = 10.5 Hz), 137.9(s), 122.7(d, 3JC-F = 7.7 Hz), 113.1(d, 2JC-F = 22.0 Hz), 109.8(s), 104.7(d, 3JC-F = 2.9 Hz), 52.0(s), 51.1(s), 45.9(s), 34.5(s), 8.0(s).
3.1.2. Preparation of methyl 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-oxobutanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (5)
A solution of 2,2,6-trimethyl-4H-1,3-dioxine-4-one (8) (0.5 g, 3mmole) and methyl 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-quinoline-3-carboxylate (7) (1.0 g, 3mmole) in o-xylene(20 ml) was placed in a 250 ml two-necked round-bottomed flask equipped with a condenser, the flask was immersed in an oil bath that had been preheated to 150 °C, and the solution was vigorously stirred. The evolution of acetone became apparent within several minutes as condensate on reflux condenser, heating was continued for a total of 30 min, the reaction was cooled. o-Xylene was evaporated in fume-hood overnight. The product was collected as faint-yellow solid with m.p = 210–212 °C, yield = 1.0 g, 78 %. Elemental analysis. Calcd: C, 61.53; H, 5.63; N, 9.78. Found: C, 62.23; H, 5.95; N, 10.36. 1H NMR (500 MHz, CDCl3) δ: 14.58 (s,1H), 8.54 (s,1H), 8.04 (d,3JH-F = 12.9 Hz, 1H), 7.27 (d,4JH-F = 6.1, 1H), 5.19 (s, 1H), 3.91 (s, 3H), 3.87 (m, 4H), 3.65 (s, 2H), 3.43 (m, 1H), 3.26 (m, 4H), 2.32 (s, 3H), 1.34 (m, 2H-cyclopropyl), 1.15 (m, 2H-cyclopropyl). 13C NMR (125 MHz, CDCl3) δ: 202.2(s), 171.9(d,4JC-F = 28 Hz), 166.4(s), 164.9(s), 153.3(d, 1JC-F = 247.5 Hz), 148.5(s), 144.0(d, 2JC-F = 11.25 Hz), 137.9(s), 123.2(d, 3JC-F = 121.2 Hz), 113.5(d, 2JC-F = 22.5 Hz), 110.0 (s), 106.5(d, 3JC-F = 382.5 Hz), 52.1(s), 50.3(s), 49.5(s), 49.5(s), 46.3(s), 41.6(s), 34.6(s), 30.5(s), 8.2(s).
3.1.3. General procedure for the synthesis of compounds (6a-f)
To a solution of methyl 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-oxobutanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (3) (0.1 g, 0.2 mmol), and organic azide (9) (0.24 mmol) in dry dimethyl sulfoxide (3 ml), dry potassium carbonate (0.1 g, 0.72 mmol)) was added, with stirring. Stirring was continued at room temperature overnight, at this point precipitation was formed, and the reaction was heated at 50 °C overnight. The reaction mixture was added to an ice-water bath, resulting in crude solid product, which was collected by suction filtration, washed with water, dried under vacuum, and recrystallized by CHCl3/hexane (1:1). (Scheme 2).
3.1.4. Synthesis of methyl 7-(4-(1-(4-fluorobenzyl)-4-methyl-1H-1,2,3-triazole-5-carbonyl) piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (6a)
Compound 6a was synthesized according to the general procedure from methyl 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-oxobutanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (3) (0.1 g, 0.2 mmol), and 1-azidomethyl-4-fluorobenzene (9a) (0.15 g, 0.1 mmol) to give a white solid, Yield: 86 %; m.p = 195–198 °C. 1H NMR: (400 MHz, CDCl3) δ = 8.56(s, 1H, H-2), 8.06(d, 3JF-H = 13.2 Hz, 1H, H-5), 7.31(d, 4JF-H = 7.2 Hz, 1H, H-8), 7.28–7.20 (m, 2H), 7.08–7.04(m, 2H), 5.49 (s, 2H, H-14″), 4.38 (m, 2H, H-3″-piperazinyl), 3.99(m, 2H, H-2″- piperazinyl), 3.91 (s, 3H, H-10), 3.45–3.36(m, 5H, H-1′-cyclopropyl, H-1″, H-4″- piperazinyl), 2.62 (s, 1H, H-7″), 2.46 (s, 1H, H-7″) indicating the presence of two regioisomers for 1,2,3-trizole, 1.37–1.32 (m, 2H, H-2′,H-3′- cyclopropyl), 1.71–1.55 (m, 2H, H-2′,H-3′- cyclopropyl). 13C NMR: (100 MHz, CDCl3) δ = 173.1, 166.4, 164.0, 161.5, 161.2, 153.4(d, 1JC-F = 247,10 Hz), 148.5, 144.3(d, 2JC-F = 10.50 Hz), 139.7, 138.0, 137.8, 129.8–129.3, 123.5(d, 3JC-F=7.10 Hz), 116.2(d, 2JC-F = 21.6 Hz), 113.5(d, 2JC-F = 22.8 Hz), 110.5(s), 105.2(d, 3JC-F = 2.60 Hz), 52.1, 51.2, 47.0, 42.3, 41.0, 34.6, 9.3, 8.2. HRMS (ESI) m/z: Calcd for C29H28F2N6NaO4 [M+Na] 585.20323; found 585.20352.
3.1.5. Synthesis of methyl 7-(4-(1-(3-fluorobenzyl)-4-methyl-1H-1,2,3-triazole-5-carbonyl) piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (6b)
Compound 6b was synthesized according to the general procedure from methyl 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-oxobutanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (5) (0.1 g, 0.2 mmol), and 1-azidomethyl-3-fluorobenzene (9b) (0.15 g, 0.1 mmol) to give a yellow solid. Yield: 88 %; m.p = 177–180 °C. 1H NMR: (400 MHz, CDCl3) δ = 8.56(s, 1H, H-2), 8.55(s, 1H, H-2), 8.06(d, 3JF-H = 13.2 Hz, 1H, H-5), 8.05(d, 3JF-H = 13.2 Hz, 1H, H-5), 7.38–7.34 (m, 2H), 7.32(d, 4JF-H = 6.8 Hz, 1H, H-8), 7.07–6.88 (m, 2H), 5.53 (s, 2H, H-14″), 4.40(t, 3JH-H = Hz, 2H, H-3″-piperazinyl), 3.99(m, 2H, H-2″-piperazinyl), 3.92 (s, 3H, H-10), 3.66 (s, 1H), 3.45–3.37(m, 5H, H-1′ cyclopropyl, H-1″, H-4″- piperazinyl), 2.46 (s, 3H, H-7″), 2.32 (s, 3H, H-7″) indicating the presence of two regioisomers for 1,2,3-trizole, 1.35(dd,3JH-H = 6.4 Hz, 2H, H-2′,H-3′-cyclopropyl), 1.15(dd, 3JH-H = 3.2 Hz, 2H, H-2′,H-3′- cyclopropyl). 13C NMR: (100 MHz, CDCl3) δ = 173.1, 173.1, 166.4, 164.3, 161.8, 161.2, 153.4(d, 1JC-F = 247.1 Hz), 148.5, 144.5(d, 2JC-F = 15.6 Hz), 139.8, 138.0, 137.8, 136.4(d, 3JC-F = 7.3 Hz), 130.8(d, 3JC-F = 8.2 Hz), 129.8–129.3, 123.6–123.5, 122.9(d, 3JC-F = 4 Hz), 115.9–115.1, 114.5–114.3, 113.5(d, 3JC-F = 2.8 Hz), 110.2, 105.2(d, 3JC-F = 2.6 Hz), 52.2, 52.1, 51.3, 50.5, 50.1 42.3, 34.6, 9.3, 8.2. Elemental analysis. Calcd: C, 61.91; H, 5.02; N, 14.64 Found: C, 60.92; H, 5.92; N, 13.75. HRMS (ESI) m/z: Calcd for C29H28F2N6NaO4 [M+Na] 585.20323; found 585.20352.
3.1.6. Synthesis of methyl 7-(4-(1-(4-chlorophenyl)-4-methyl-1H-1,2,3-triazole-5-carbonyl) piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (6c)
Compound 6c was synthesized according to the general procedure from methyl 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-oxobutanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate(5) (0.1 g, 0.2 mmol), and 1-azido-4-chlorobenzene (9c) (0.15 g, 0.1 mmol) to give yellow solid, Yield: 86 %; m.p = 225–229 °C. 1H NMR: (400 MHz, CDCl3) δ = 8.56(s, 1H, H-2), 8.07(d, 3JF-H = 13.2 Hz, 1H, H-5), 7.58(d, 3JH-H = 8.8 Hz 2H, H-10″, H-12″), 7.44(d, 4JF-H = 8.8 Hz, 1H, H-9″, H-13″), 7.33(d, 4JF-H = 6.8 Hz, 1H, H-8), 4.40(t, 3JH-H = 7 Hz, 2H, H-3″-piperazinyl), 4.03(t, 3JH-H = 7 Hz, 2H, H-2″- piperazinyl), 3.92 (s, 3H, H-10), 3.42(m, 5H, H-1′-cyclopropyl, H-1″, H-4″- piperazinyl), 2.57 (s, 3H, H-7″), 1.36(dd,3JH-H = 6.4 Hz, 2H, H-2′,H-3′- cyclopropyl), 1.15(dd, 3JH-H = 3.2 Hz, 2H, H-2′,H-3′- cyclopropyl). 13C NMR: (100 MHz, CDCl3) δ = 173.0, 166.4, 161.1, 153.4(d, 1JC-F = 247.1 Hz), 148.5, 144.3(d, 2JC-F = 11 Hz), 139.6, 138.4, 138.0, 136.2134.1, 130.0, 126.5, 123.63(d, 3JC-F = 6.8 Hz), 113.5(d, 3JC-F = 22.5 Hz), 110.2, 105.2(d, 3JC-F = 7.5 Hz), 52.1, 42.3, 41.0, 34.6, 10.2, 8.2. HRMS (ESI) m/z: Calcd for C28H27ClFN6O4 [M+H] 565.17609; found 565.17606.
3.1.7. Synthesis of methyl 7-(4-(1-(4-trifluoromethylphenyl)-4-methyl-1H-1,2,3-triazole-5-carbonyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (6d)
Compound 6d was synthesized according to the general procedure from methyl 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-oxobutanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (5) (0.1 g, 0.2 mmol), and 1-azido-4-trimethylbenzene (9d) (0.15 g, 0.1 mmol) to give a white solid, Yield: 96 %; m.p = 218–222 °C. 1H NMR: (400 MHz, CDCl3) δ = 8.57(s, 1H, H-2), 8.09(d, 3JF-H = 12.8 Hz, 1H, H-5), 7.89(d, 3JH-H = 8.4 Hz 2H, H-10″, H-12″), 7.66(d, 4JF-H = 8.4 Hz, 2H, H-9″, H-13″), 7.33(d, 4JF-H = 7.2 Hz, 1H, H-8), 4.40(t, 3JH-H = 7 Hz, 2H, H-3″-piperazinyl), 4.04(t, 3JH-H = 7 Hz, 2H, H-2″-piperazinyl), 3.92 (s, 3H, H-10), 3.44(m, 5H, H-1′-cyclopropyl, H-1″, H-4″- piperazinyl), 2.62 (s, 3H, H-7″), 1.36(dd,3JH-H = 6.4 Hz, 2H, H-2′,H-3' -cyclopropyl), 1.15(dd, 3JH-H = 3.2 Hz, 2H, H-2′,H-3′- cyclopropyl). 13C NMR: (100 MHz, CDCl3) δ = 173.1, 166.4, 161.0, 153.4(d, 1JC-F = 247.2 Hz), 148.5, 144.2(d, 2JC-F = 11 Hz), 144.2, 139.9, 138.4, 138.0, 132.1, 127.0, 127.0, 125.5, 123.6(d, 3JC-F = 6.8 Hz), 113.6(d, 3JC-F = 22.9 Hz), 110.2, 105.1(d, 3JC-F = 2.5 Hz), 52.2, 42.4, 34.6, 10.6, 8.2. HRMS (ESI) m/z: Calcd for C29H26F4N6O4 [M+Na] 621.18439; found 621.18380.
3.1.8. Synthesis of methyl 7-(4-(1-(4-nitrophenyl)-4-methyl-1H-1,2,3-triazole-5-carbonyl) piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (6e)
Compound 6e was synthesized according to the general procedure (mentioned previously in part 3.6) from methyl 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-oxobutanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (5) (0.1 g, 0.2 mmol), and 1-azido-4-nitrobenzene (9e) (0.15 g, 0.1 mmol) to give a white solid, Yield: 98 %; m.p = 217–220 °C. 1H NMR: (400 MHz, CDCl3) δ = 8.57(s, 1H, H-2), 8.49(d, 3JF-H = 8.8 Hz, 1H, H-10″, H-12″) 8.07(d, 3JF-H = 13.2 Hz, 1H, H-5), 7.75(d, 3JH-H = 8.8 Hz 2H, H-9″, H-13″), 7.33(d, 4JF-H = 6.8 Hz, 1H, H-8), 4.38(t, 3JH-H = 7.00 Hz, 2H, H-3″- piperazinyl), 4.05(t, 3JH-H = 7.0 Hz, 2H, H-2″- piperazinyl), 3.92 (s, 3H, H-10), 3.43(m, 5H, H-1′- cyclopropyl, H-1″, H-4″- piperazinyl), 2.66 (s, 3H, H-7″), 1.36(dd,3JH-H = 6.4 Hz, 2H, H-2′,H-3′-cyclopropyl), 1.17(dd, 3JH-H = 3.2 Hz, 2H, H-2′,H-3′- cyclopropyl). 13C NMR: (100 MHz, CDCl3) δ = 173.1, 166.4, 160.8, 153.4(d, 1JC-F = 247.3 Hz), 148.2, 144.2(d, 2JC-F = 10.7 Hz), 140.5, 140.2, 138.4, 138.0, 125.8, 125.2, 123.6(d, 3JC-F = 6.9 Hz), 113.6(d, 3JC-F = 22.8 Hz), 110.2, 105.2(d, 3JC-F = 7.0 Hz), 52.1, 50.6, 47.1, 42.4, 34.6, 10.4, 8.2. Elemental analysis. Calcd: C, 58.43; H, 4.55; N, 17.04. Found: C, 58.90; H, 5.77; N, 15.31. HRMS (ESI) m/z: Calcd for C28H26FN7O6 [M+Na] 598.18208; found 598.18102.
3.1.9. Synthesis of methyl 7-(4-(1-(4-fluorophenyl)-4-methyl-1H-1,2,3-triazole-5-carbonyl) piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (6f)
Compound 6f was synthesized according to the general procedure (mentioned previously in part 3.4) from methyl 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-oxobutanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate(5) (0.1 g, 0.2 mmol), and 1-azido-4-fluorobenzene (9f) (0.15 g, 0.1 mmol) to give a white solid, Yield: 87 %; m.p = 220–222 °C. 1H NMR: (400 MHz, CDCl3) δ = 8.58(s, 1H, H-2), 8.09(d, 3JF-H = 13.2 Hz, 1H, H-5), 7.48(d, 3JH-H = 89.2 Hz 2H, H-10″, H-12″), 7.46(d, 4JF-H = 9.2 Hz, 2H, H-9″, H-13″), 7.34(d, 4JF-H = 7.2 Hz, 1H, H-8), 4.41(t, 3JH-H = 7.0 Hz, 2H, H-3″-piperazinyl), 4.04(t, 3JH-H = 7.0 Hz, 2H, H-2″-piperazinyl), 3.93 (s, 3H, H-10), 3.42(m, 5H, H-1′-cyclopropyl, H-1″, H-4″-piperazinyl), 2.62 (s, 3H, H-7″), 2.55 (s, 3H, H-7″) indicating the presence of two regioisomers for 1,2,3-trizole, 1.36(dd,3JH-H = 6.8 Hz, 2H, H-2′,H-3′- cyclopropyl), 1.26(dd, 3JH-H = 3.2 Hz, 2H, H-2′,H-3′- cyclopropyl). 13C NMR: (100 MHz, CDCl3) δ = 173.1, 166.5, 162.0, 161.2, 153.5(d, 1JC-F = 247.30 Hz), 148.5, 144.3(d, 2JC-F = 10.7 Hz), 138.4, 139.5(s), 138.6, 138.0, 131.7(d, 3JC-F = 3.1 Hz), 127.3(d, 3JC-F = 9.0 Hz), 123.6(d, 3JC-F = 7.1 Hz), 117.0, 116.7, 113.6(d, 3JC-F = 22.9 Hz), 110.2, 105.2(d, 3JC-F = 2.50 Hz), 52.2, 50.2, 47.1, 47.0, 42.3, 41.0, 34.6, 10.1, 8.2. HRMS (ESI) m/z: Calcd for C28H26F2N6O4 [M+Na] 571.18758 found 571.18758.
4. Anticancer activity
4.1. Materials and methods
Cell culture: The non-small cell lung cancer (A549), glioblastoma (U-87 MG), human dermal fibroblast (HDF, human dermal cell line), and the breast cancer (MCF7), cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, United States). All culture mediums, l-glutamine, penicillin-streptomycin antibiotics (P-T) and heat-inactivated fetal Calf serum (FCS) were purchased from EuroClone, Milan, Italy. Roswell Park Memorial Institute Medium (RPMI) 1640 supplemented with 1 % P-T, 10 % (v/v) FCS and 2 mM l-glutamine was used to culture and maintain the A549 and MCF7 cell lines. Dulbecco's Modified Eagle Medium (DMEM) supplemented with 2 mM l-glutamine, 1 % P-T, and 10 % (v/v) FCS was used to culture and maintain other cell lines. All cells were kept and maintained in a tissue culture incubator (Memmert, Germany) at 37 °C in a 5 % CO2 humidified atmosphere.
Cytotoxicity assay: The Thiazolyl Blue Tetrazolium Bromide (MTT) (Bioworld, Dublin, USA) assay was carried out as described previously [15,16] to identify the IC50 of the synthesized compounds on the normal and tumor cell lines [17,18]. The assay is based on a mitochondrial reduction reaction that converts yellow compound (MTT) into formazan crystals (purple) within the cell. After cell harvesting, cells were inoculated at a density of 1 × 104 cells/1 ml (180 μL/well) in a round bottom 96-well plate (Corning, USA). Following overnight incubation and after cell adherence, a 20 μL of test compound was added to proper wells at concentration that is needed to make the required final concentration of test compound. By dissolving the test compound with proper growth medium, test compounds were serially diluted to make final concentrations of 1, 50, 100, 200, 400 and 600 μM/ml. Controls were wells filled with only growth medium (blank), wells containing cells at a concentration of 1 × 104 cells/1 ml as indicated above and finally wells containing cells treated with Dimethyl sulfoxide (DMSO, Sigma-Aldrich Chemie GmbH, Germany) at the same concentration found in each diluent solution. Cisplatin was used at different concentrations of 1, 10, 50 and 100 μM/mL as a standard positive control for all experiments (Sigma-Aldrich Chemie GmbH, Germany). After 96 h incubation, each well medium was aspirated and 0.2 ml of 5 mg/ml MTT solution was added to each well for 4 h. Following development of purple formazan crystals, the supernatant was pipetted carefully, and the crystals were solubilized in 150 L of DMSO. The absorbance at 540 nm of each of the formazan solutions was determined in the BioTek Synergy HTX multimode reader (Agilent Technologies, CA, USA). For each group, the assay was performed in triplicate. GraphPad Prism ver. 7 software California, U.S.A. was used to calculate the IC50 values of test compounds and cisplatin for each cell line used. Using Eq (1), cell growth inhibition (GI) was determined as follows:
| GI (%) = {100 – (At/Ac)}100 … … …. | (1) |
where At and Ac denote for the absorbance values of the test compound and control samples, respectively.
Selectivity index (SI): SI was utilized to assess the degree of test compounds selectivity towards cancer cells. This is the ratio of test compound IC50 values in human dermal fibroblast (HDF) to test compound IC50 values in each cancer cell line. Values > 3 indicate that the test compound has in vitro selective anti-tumor activity in cancer cell lines versus normal cells [19,20].
Morphological analysis: As previously mentioned, morphological analysis was carried out with some modifications [7,21] Cells of different cell lines were inoculated at a density of 1 × 104 cells/1 ml (180 μL/well) in a flat bottom 96-well plate (Corning, USA). Following overnight incubation to allow cell adherence, a 20 μL of test compound was added to proper wells at concentration that is needed to make the required final concentration of test compound. Serial dilutions of test compounds were used to make final concentrations of 1, 50, 100, 200, 400 and 600 μM/ml. Moreover, cells were also treated with test compounds at the respective IC50 for each cell line. Controls were wells containing cells at a concentration of 1 × 104 cells/1 ml as indicated above and wells containing cells treated with DMSO at the same concentration found in each diluent solution. Cisplatin was used at different concentrations of 1, 10, 50 and 100 μM/mL as a standard positive control for all experiments (Sigma-Aldrich Chemie GmbH, Germany). Following treatment, cells were stained with hematoxylin after fixation with ice-cold methanol for 30 min at 4 °C. Plates were viewed by inverted light microscopy using a Zeiss Axiovert 40 C microscope equipped with ToupCam digital camera.
Statistical analysis: All variables were analyzed using the Statistical Packages for Social Sciences (SPSS) software version 19. All experimental data were expressed in the form of mean ± standard deviation (SD) for each run. t-test was utilized to measure the statistical significance of differences between means of three experiments. Statistical difference was considered significant if the p values were less than 0.05.
5. Results with discussion
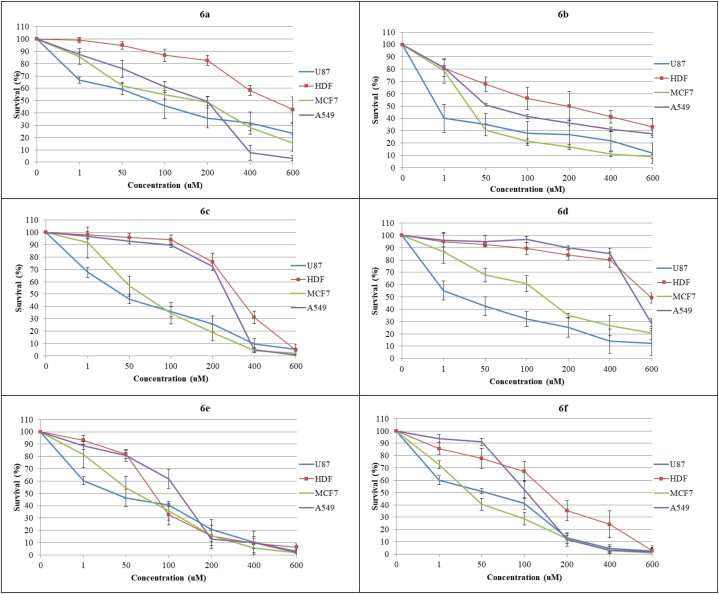
Cytotoxicity and Selectivity index: The 1,2,3-Triazole is a promising basic unit for the discovery of new anticancer agents, and some of the 1,2,3-triazole's derivatives are currently being used in clinics or in clinical trials to treat cancers [22]. They display their anticancer activity through a variety of mechanisms including inhibition of tubulin polymerization and inhibition of vital enzymes like kinases, aromatase and thymidylate synthase. In the last decade, a number of 1,2,3-triazole chalcone hybrids were developed as anticancer drugs with the goal of synergistic activity [22,23]. Some of these hybrids showed promising anti-proliferative activity against different cancer cell lines, with least cytotoxicity towards normal cells [24,25]. Inspired by the aforementioned perspectives, a new series of 1,2,3-triazole linked ciprofloxacin derivatives were developed and their anti-proliferative activity was assessed in different normal and cancer cell lines including the non-cancerous human dermal fibroblast (HDF, human dermal cell line), non-small cell lung cancer (A549), glioblastoma (U-87 MG), and the breast cancer (MCF7). Most of synthesized compounds showed a dose dependent cytotoxic activity against all cell lines used, as identified by MTT assay, albeit at varying degrees (Table 1, Fig. 1). Yet, two compounds namely 6b and 6a showed significant cytotoxic activity against all cancer cell lines, compared to normal cells (HDF) (p ≤ 0.001). However, 6b was more potent than 6a in exhibiting anti-proliferative effect against all cancer cell lines and the most potent among all compounds demonstrating cytotoxicity against U-87 and MCF-7 cancer cell lines, compared to normal cell line (HDF). Importantly, IC50 values of 6b for all cancer cell lines were significantly lower than that for the standard drug cisplatin [IC50 = 28.3 ± 5.3 μM/ml (U-87); 26.9 ± 4.7 μM/ml (MCF-7) and 30.4 ± 5.2 μM/ml (A549), (p ≤ 0.001)], thereby implying higher activity of 6b than the standard drug against cancer cells. In the other hand, 6b IC50 for normal cell line was significantly higher than the reported for cisplatin [IC50 = 170.7 ± 8.1 μM/ml (HDF), (p ≤ 0.001)], indicating less toxicity towards normal cells and thereby has better therapeutic index. Basically, these two compounds are fluorobenzyl derivatives of 1,2,3-triazole linked ciprofloxacin-chalcones, but they have different positions in aromatic ring. Fluorine atom substitution led to marked anti-proliferative effect against all cancer cell lines, with less cytotoxicity to normal cells. Such effect of Fluorine atom substitution in 1,2,3-triazole linked ciprofloxacin-chalcones has been reported previously [26,27]. However, the position of monosubstituted fluorine atom in aromatic ring seemed to play an important role in potency of derivative, which is not reported before. Here, the fluorine atom in position 12 of 6b compound is more optimal for anti-proliferative activity than the fluorine atom in position 11 of 6a compound. This position change led to higher potency of derivative (6b) in displaying anti-proliferative activity and to best selectivity towards all cancer cell lines (See SI, Table 1).
Table 1.
IC50 Values (μM) and Selectivity Index of Cancer Cell Lines Treated With Compounds (6a-f) for 96 h.
|
Drug/cell line |
IC50 |
SI |
|||||
|---|---|---|---|---|---|---|---|
| HDF | U87 | MCF-7 | A549 | U87 | MCF-7 | A549 | |
| 6c | 293.6 ± 11.4 | 12.29 ± 5.5 | 61.56 ± 11.1 | 238.8 ± 7.9 | 23.9 | 4.8 | 1.2 |
| 6d | 425.2 ± 18.5 | 3.817 ± 2.9 | 123.5 ± 10.6 | 307.6 ± 12.1 | 111.4 | 3.4 | 1.4 |
| 6b | 170.7 ± 8.1 | 1.2 ± 0.8 | 10.58 ± 2.3 | 29.4 ± 6.2 | 142.3 | 16.1 | 5.8 |
| 6a | 504.2 ± 19.9 | 34.57 ± 7.8 | 112 ± 10.1 | 138.6 ± 9.3 | 14.6 | 4.5 | 3.6 |
| 6e | 83 ± 5.8 | 45 ± 7.1 | 58.6 ± 5.9 | 79 ± 4.7 | 1.8 | 1.4 | 1.1 |
| 6f | 141.7 ± 4.1 | 7.9 ± 2.3 | 10.6 ± 3 | 103.7 ± 4.5 | 18 | 13.3 | 1.4 |
| Cisplatin | 40.9 ± 5.4 | 28.3 ± 5.3 | 26.9 ± 4.7 | 30.4 ± 5.2 | 1.5 | 1.5 | 1.3 |
Fig. 1.
Anti-proliferative activity of each CP derivatives non-cancerous cells (HDF) and cancerous cell lines (U87, MCF-7 and A549) upon 96 h treatment period. 6a and 6b showed significant cytotoxic activity against all cancer cell lines, compared to normal cells (HDF) (p ≤ 0.001). 6c, 6d, and 6f displayed significant cytotoxic activity against only U-87 and MCF-7 cancer cell lines, compared to normal cells (HDF). 6e derivative displayed no significant selective anti-proliferative activity against all cell lines used.
Other compounds namely 6c, 6d, and 6f displayed significant cytotoxic activity against only U-87 and MCF-7 cancer cell lines, compared to normal cells (HDF) (Table 1, Fig. 1). None of these compounds showed significant cytotoxic activity against A549 cell line (p ≤ 0.001). From these compounds, only 6f [IC50 = 7.9 ± 2.3 μM/ml (U-87) and 10.6 ± 3 μM/ml (MCF-7)] was more potent than cisplatin [IC50 = 28.3 ± 5.3 μM/ml (U-87) and 26.9 ± 4.7 μM/ml (MCF-7)] in displaying anti-proliferative effect against U-87 and MCF-7 cells, with less cytotoxic to normal cells [IC50 = 141.7 ± 4.1] than cisplatin [IC50 = 40.9 ± 5.4]. 6c and 6d showed higher anti-proliferative effect than cisplatin only against U-87 cells, while less cytotoxicity than cisplatin was seen in normal cells. Simple structure-activity relationship (SAR) showed that the presence of fluorine atom at positions in 4-fluorophenyl moiety of 6f derivative and 4-trifluoromethylphenyl moiety of 6d derivative is favorable for anti-proliferative activity and selectivity to cancer cells. This is consistent with our results above and previous reports regarding the importance of fluorine atom linked with 1,2,3-triazole moiety of Ciprofloxacin [26,27]. Similarly, presence of halogens particularly chlorine at same position of fluorine atom as in the case of 6c have anti-proliferative activity but at lower potency. Such finding was reported where the anti-proliferative activity is increased as the halogen size decreased [26,27]. Also the increase of the activity of fluoro derivatives may be attributed to electronegativity in addition to the small size of the atom. It is worth mentioning that all derivatives having phenyl moiety of 1,2,3-triazole linked Ciprofloxacin exhibited significant anti-proliferative activity and selectivity only towards U-87 and MCF-7 cancer cell lines (See SI, Table 1). In contrast, 6e derivative displayed anti-proliferative activity against all cancerous and non-cancerous cell lines, but the difference was not statistically significant. This further confirms the importance of halogens substituent as replacing fluorine and chlorine atoms with nitro group in corresponding NG-9 derivative led to least selective derivative among the series. Similar fashion of activity was reported previously where the halogen substituents at 1,2,3-triazole moiety particularly chlorine enhanced the anti-proliferative activity and led to better selectivity than nitro group substituent [27].
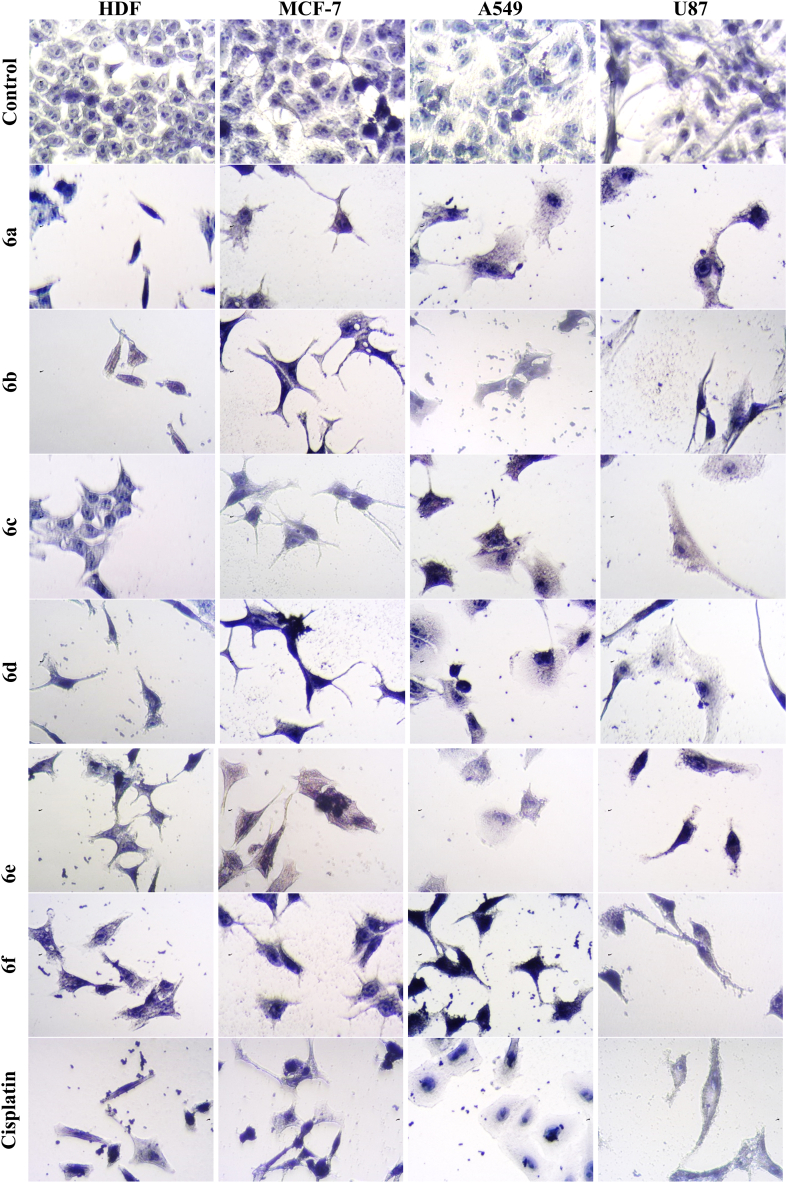
Morphological analysis: Several morphological changes such as cell rounding, and shrinkage were observed upon treatment with synthesized compounds and reference drug cisplatin (Fig. 2). These changes are indicative of cell cytoskeleton disruption [28]. Control cancer cells (untreated) had an atypical spindle shape, with the nucleus uniformly and heavily stained than cytoplasm, and the nucleolus remained clearly apparent. In contrast, cancer cells treated with synthesized compounds and reference drug cisplatin became rounded and separated from each other, shrank, and underwent nuclear condensation. This is particularly true for cisplatin where induces these morphological alterations leading to cell apoptosis [28,29]. Our active compounds showed a similar fashion to cisplatin connoting their ability to induce cytotoxicity via apoptosis.
Fig. 2.
Cytotoxic effects of CP derivatives and cisplatin against non-cancerous cells (HDF) and cancerous cell lines (U87, MCF-7 and A549) upon 96 h' treatment period. Treatment was carried out at the respective IC50 of each derivative for each cell line as indicated in Table 1. Control cancer cells (untreated) had an atypical spindle shape, with the nucleus uniformly and heavily stained than cytoplasm, and the nucleolus remained clearly apparent. Cancer cells treated with 6a-f derivatives and cisplatin became rounded and separated from each other, shrank, and underwent nuclear condensation. (40 × objective lens, total magnification = 400 × ).
5.1. Antioxidant activity
The antioxidant activities of ciprofloxacin-1,2,3-triazole hybrid (6a-f) were measured using 2,20-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) and 1,1-Diphenyl-2-picrylhydrazyl (DPPH) assays as mentioned in Al-Zereini [30,31](Table 2). Briefly, different concentrations of the tested compounds (50 μg/ml – 300 μg/ml) were placed in 1 ml of 7 mM ABTS in 2.45 mM potassium persulfate (A734nm = 0.700 ± 0.005), left at room temperature for 6 min, and the percentage of inhibition of absorbance at 734 nm was calculated. However, in the DPPH assay 50 μg/ml – 300 μg/ml of tested compounds were added to 1 ml of 0.1 mM DPPH in methanolic solution, mixed vigorously, and left to stand at room temperature for 30 min, and the decrease in blue color intensity was measured at 517 nm by UV/Vis spectrophotometer. The scavenging effect of the tested samples on the generated radicals was calculated according to the formula.
| Scavenging effect (%) = [(Acontrol - Asample)/ Acontrol] × | 100 |
Table 2.
Antioxidant activities of compounds (6a-f) in DPPH and ABTS assay.
|
Sample |
% inhibition ±SD* μg/ml |
IC50 ± SD μg/ml (μM) |
||||
|---|---|---|---|---|---|---|
| 5 | 10 | 25 | 50 | 100 | ||
| DPPH | ||||||
| 6d | 13.8 ± 0.2 | 26.0 ± 0.9 | 42.1 ± 0.6 | 46.2 ± 0.3 | 68.7 ± 0.1 | 58.8 ± 1.7 (35.2 ± 1.0)a |
| 6b | – | 18.9 ± 0.3 | 40.6 ± 0.9 | 58.5 ± 0.6 | 84.1 ± 0.2 | 32.4 ± 1.1(18.3 ± 0.6)b |
| 6a | 27.04 ± 0.2 | 40.2 ± 0.4 | 47.2 ± 1.0 | 53.2 ± 0.9 | 63.3 ± 0.8 | 32.6 ± 2.9(18.3 ± 1.6)b |
| 6e | 17.1 ± 0.6 | 33.8 ± 0.5 | 54.5 ± 0.4 | 60.7 ± 0.8 | 67.9 ± 0.6 | 18.5 ± 1.6(10.6 ± 0.9)d |
| 6f | 10.6 ± 0.9 | 20.7 ± 0.8 | 39.5 ± 0.5 | 48.9 ± 0.4 | 66.9 ± 0.3 | 56.2 ± 2.1 (30.8 ± 1.2)a |
| 6c | – | 24.6 ± 1.8 | 31.7 ± 0.3 | 52.2 ± 0.2 | 71.4 ± 0.7 | 43.1 ± 1.8 (24.3 ± 1.0)c |
| Trolox® | 5.2 ± 0.3 (1.3 ± 0.1)** | |||||
| ABTS | ||||||
| 6d | 21.1 ± 1.0 | 33.7 ± 0.7 | 42.8 ± 0.3 | 61.6 ± 0.6 | 66.5 ± 0.6 | 31.7 ± 1.9 (18.9 ± 1.1)e |
| 6b | 11.8 ± 0.5 | 28.3 ± 0.4 | 43.4 ± 0.3 | 53.9 ± 0.8 | 72.9 ± 0.5 | 35.3 ± 1.6 (19.8 ± 0.9)e |
| 6a | 22.7 ± 0.2 | 48.7 ± 0.5 | 53.5 ± 0.3 | 71.1 ± 1.0 | 75.5 ± 0.5 | 17.8 ± 1.1 (10.0 ± 0.6)f |
| 6e | 14.9 ± 1.4 | 37.0 ± 0.2 | 50.3 ± 0.5 | 71.2 ± 0.2 | 74.3 ± 0.4 | 24.2 ± 1.0 (13.9 ± 0.6)f |
| 6f | 13.3 ± 0.6 | 29.7 ± 0.7 | 46.1 ± 0.1 | 54.1 ± 0.7 | 64.2 ± 0.4 | 39.2 ± 2.1 (21.5 ± 1.2)e |
| 6c | 14.8 ± 0.6 | 33.3 ± 0.8 | 42.7 ± 0.4 | 59.8 ± 0.3 | 65.7 ± 0.1 | 35.1 ± 2.0 (19.8 ± 1.1)e |
| Trolox® | 2.7 ± 0.2 (0.7 ± 0.1)** | |||||
* Data represents the means ± standard deviation (SD). ** Statistically significant IC50 values compared to Trolox® (p˂0.05). The mean % inhibitions with similar letters are not significantly different from each other based on post hoc Tukey HSD test at p˂0.01.
Trolox® standard solutions (concentrations from 0 to 10 μg/ml) in ethanol were prepared and assayed using the same conditions. As blanks the same volume of solvents was run in each assay.
6. Results and discussion
Evaluation of the antioxidant activity of synthesized derivatives from 1,2,3-triazole ciprofloxacin hybrids revealed a dose-dependent radical scavenging activity, as well as potency depending on the employed test assay. The tested derivatives exhibited ability to neutralize the generated DPPH and ABTS radicals at IC50 values ranged 10.6 ± 0.9–35.2 ± 1.0 μM and 10.0 ± 0.6–21.5 ± 1.2 μM, respectively (Table 2). In DPPH assay, derivative 6e was the most potent antioxidant agent with the highest activity, while both derivative 6a and 6b revealed a comparable scavenging potential. However, the ciprofloxacin hybrid derivatives 6c, 6d, and 6f were less efficient in neutralizing the generated DPPH radicals. Intriguingly, the most active derivative in the ABTS assay was 6a followed by 6e, the remaining derivative revealed a non-significant difference in their ability to deactivate the ABTS radicals.
Due to lipophilicity of the 1,2,3-triazole ciprofloxacin derivatives, it is most convenient that the DPPH assay was more sensitive than the ABTS assay in revealing the antioxidant activity of the tested compounds. DPPH predicts the antioxidant capacity for hydrophobic antioxidants while ABTS (prepared in an aqueous phase) detected the hydrophilic [32] compounds. Moreover, there are no studies on the antioxidant of the triazole-ciprofloxacin derivatives and the results herein indicated the role of aryl ring linked to the 1,2,3-triazole ciprofloxacin derivative, type of electron withdrawing substitution, and its position on the aryl ring in the detected antioxidant activities. The derivatives with nitro group (NO2) were more effective in scavenging the DPPH radicals than chlorine, fluorine, and trifluoromethyl hybrids in an order: NO2 > -Cl > –F > –CF3; replacing the phenyl ring with the benzyl enhanced the activity of the fluorinated derivatives with non-effective role in the antioxidant activity between presence of the fluorine at meta- or para-position. These finding indicated that the antioxidant potency is enhanced with increasing in the electronic effect of the electron withdrawing substituent and agreed with the observation that most common derivatives with tethered electron-withdrawing substituents in the triazole containing hybrids proved antioxidant [33] capability.
6.1. Antibacterial activity
The antibacterial activities of ciprofloxacin-1,2,3-triazole hybrid (6a-f) were evaluated by agar diffusion test and deducing the minimum inhibitory concentration (MIC) by micro-broth dilution assay according to the standard methods followed by the Clinical and Laboratory Standards Institute (CLSI, 2012) and as described in Al-Zereini [31](Table 3). The included test bacterial strains the Gram-positive bacteria [Staphylococcus aureus (ATCC 10145), Bacillus cereus (ATCC 11778), and B. subtilis (ATCC 6633)] and two Gram-negative bacteria [Pseudomonas aeruginosa (ATCC 13048) and Escherichia coli (ATCC 25922)]. In the agar diffusion test, different concentrations (10, 50, and 100 μg/disc) of tested compounds were applied on a 6-mm blank filter disk that was placed on the top of Muller Hinton Agar plates (Oxoid, UK) seeded with bacterial test strains at a cell density of 106 cell/ml. The measured inhibition zone diameters are the mean of triplicate assays with standard deviation.
Table 3.
Antibacterial activity of 6a-f compounds and ciprofloxacin against tested bacterial strains.
| Compounds μg/disc |
Zone of inhibition [mm±SD] |
||||
|---|---|---|---|---|---|
| BS | BC | SA | EC | PA | |
| 6d | |||||
| 100 | – | 9.6 ± 1.2 | 10.3 ± 0.6 | 8.3 ± 1.5 | 11.7 ± 0.6 |
| 300 | – | 11.0 ± 0.0 | 12.3 ± 0.6 | 10.3 ± 0.7 | 13.0 ± 0.3 |
| 500 | – | 13.3 ± 0.6 | 14.6 ± 1.2 | 12.6 ± 0.7 | 13.3 ± 0.6 |
| MIC (μg/ml) | >500 | >500 | 500s | >500 | 500s |
| 6b | |||||
| 100 | – | – | – | 10.6 ± 1.2 | 12.3 ± 0.7 |
| 300 | – | – | – | 13.3 ± 1.5 | 13.6 ± 0.6 |
| 500 | – | – | – | 14.7 ± 0.6 | 15.3 ± 0.3 |
| MIC (μg/ml) | >500 | >500 | >500 | 500s | 500s |
| 6a | |||||
| 100 | 11.3 ± 1.2 | 12.7 ± 0.6 | – | – | – |
| 300 | 13.1 ± 0.6 | 14.6 ± 0.3 | – | – | – |
| 500 | 14.6 ± 0.7 | 15.3 ± 0.3 | – | – | – |
| MIC (μg/ml) | 500s | 500s | >500 | >500 | >500 |
| 6e | |||||
| 100 | 9.6 ± 0.6 | 10.3 ± 0.6 | – | – | – |
| 300 | 13.1 ± 0.3 | 11.3 ± 0.6 | – | – | – |
| 500 | 14.3 ± 0.6 | 13.3 ± 0.6 | – | – | – |
| MIC (μg/ml) | >500 | >500 | >500 | >500 | >500 |
| 6f | |||||
| 100 | – | – | – | – | – |
| 300 | – | – | – | – | – |
| 500 | – | – | – | – | – |
| MIC (μg/ml) | >500 | >500 | >500 | >500 | >500 |
| 6c | |||||
| 100 | – | 12.7 ± 0.6 | 10.6 ± 1.2 | 11.3 ± 0.7 | 12.3 ± 0.6 |
| 300 | – | 13.7 ± 1.1 | 12.3 ± 0.6 | 13.6 ± 1.2 | 14.6 ± 0.6 |
| 500 | – | 15.3 ± 0.7 | 13.3 ± 0.3 | 14.6 ± 0.3 | 16.3 ± 0.6 |
| MIC (μg/ml) | >500 | 500s | >500 | >500 | 500s |
| Ciprofloxacin | |||||
| 10 | 13.33 ± 1.15 | 13.67 ± 0.58 | 9.67 ± 0.58 | 12.33 ± 1.15 | 13.33 ± 0.58 |
| 50 | 15.0 ± 0.0 | 16.0 ± 0.0 | 11.67 ± 0.58 | 16.0 ± 0.0 | 20.0 ± 1.0 |
| 100 | 18.0 ± 0.0 | 19.33 ± 0.58 | 17.33 ± 0.58 | 18.33 ± 0.58 | 23.0 ± 0.0 |
| MIC (μg/ml) | 31.25c | 31.25c | 15.6c | 15.6 | 7.8c |
BS: B. subtilis BC: B. cereus SA: S. aureus EC: E. coli PA: P. aeruginosa.
NA: not active s: bacteriostatic c: bactericidal.
However, in the micro-broth dilution assay, a serial dilution of ciprofloxacin derivatives and control (ciprofloxacin) were performed starting from 150 μg/ml and 50 μg/ml, respectively. Type of inhibition, bacteriostatic or bactericidal, was determined by platting the preparations, where there were no visible growths, on Muller Hinton agar plates with incubation at 37 °C for 24 h.
7. Results and discussion
Recently, several pathogens have evolved ways to resist antibiotic activity with a drastic increase in the number of antibiotic-resistant [34,35] bacterial strains; an increase that is becoming a main public health problem. Fluoroquinolones [36] (e.g., Ciprofloxacin) are a large group of broad-spectrum bactericide antibiotics that bind and inhibit topoisomerase II (DNA gyrase) in Gram-negative bacteria and topoisomerase IV in the Gram-positive ones, which are vital for the bacterial survival. However, the number of fluoroquinolones-resistant [37] bacteria has rapidly increased which prompts scientists to conjugate these antibiotics with pharmacophoric active residues aiming to enhance their potency in overwhelming and treating pathogenic infections.
Not worthy, several studies pointed out that modification at C-7 of the piperazinyl-moiety might increase the hybrid fluoroquinolone analogs' lipophilicity compared with the parent drug and thereby enhance their antibacterial potency and impact their interaction with the target enzymes [38].Remarkably, the 1,2,3-triazole scaffold gained the researchers’ interest in developing promising bioconjugates due to its pharmacological properties; it has a hydrogen bonding capability, moderate dipole character, rigidity, and notable metabolic stability [34]. Furthermore, linking the 1,2,3-triazole [38] residue with an aryl ring having an electron-withdrawing group revealed remarkable antibacterial activities compared to the standard drug. Therefore, in the current study, several 1,2,3-triazole-ciprofloxacin hybrids having either benzyl or phenyl moiety with an electron-withdrawing group (e.g., chlorine, fluorine, trifluoromethyl) were synthesized and tested for their antibacterial activity against both Gram-negative and Gram-positive bacterial strains.
The 1,2,3-triazole-ciprofloxacin hybrids exhibited weak to moderate antimicrobial activity. Furthermore, the activity of benzyl- or phenyl-substituted triazole-ciprofloxacin hybrids was not drastically different rather than selectivity to target enzymes in the tested bacteria (Table 3). The para-substituted phenyl-triazole derivative revealed a broad-spectrum antimicrobial activity that becomes selective to Gram-positive bacteria on replacing the trifluoromethyl- and chloro-substitution with a more electronic effect group (i.e., NO2), interestingly, presence of fluorine on the para-position of the phenyl moiety cause inactivation of the examined derivative against tested bacterial strains. Derivatives 6c and 6d were active against most tested Gram-positive bacteria and both included Gram-negative strains; 6c was more potent than 6d causing the formation of inhibition zones ranging from 13 to 16 mm compared to 12–14 mm, respectively. The presence of less an electron-withdrawing group might enhance the potency of the tested compound against pathogenic bacteria (i.e., Cl− < CF3). In similar studies, it was reported that the bromine para-substituted phenyl linked to 1,2,3-triazole ciprofloxacin derivative [39] was less effective in inhibiting S. aureus and E. coli than trifluoromethyl group (Hryhoriv et al., 2022), moreover, it was documented that the antimycobacterial activity of para-benzene substituted ciprofloxacin hybrids increased with the decrease in the electronic effect of the substituent group Colliers [40]; in contrast to colliers findings where the para-fluoro-benzyl triazole ciprofloxacin hybrids were the most potent antimycobacterial agent, the 6f was deprived of any antibacterial activity indicating that compounds containing benzyl group linked to triazole moiety inhibited the growth of bacteria more strongly than the phenyl derivatives. Intriguingly, substituting of fluorine para-phenyl conjugated 1,2,3-triazole ciprofloxacin derivative with a benzyl moiety endows the compound an antibacterial activity and the position of the fluorine substitution para- or meta-has a crucial role in the selectivity to target enzyme. Compound 6a revealed bioactivity against Gram-positive bacterial test strains while the meta-fluoro substituted benzyl conjugate in the ciprofloxacin hybrid 6b exhibited activity against Gram-negative ones without inhibiting the Gram-positive test strains. Such observation support that the antibacterial activity of the 6a compound against Gram-positive bacteria might be a result of better interaction with topoisomerase IV while 6b with DNA gyrase. This change in antibacterial profile may be due to the change of selectivity to the target enzyme [36], fluoroquinolone derivatives that are effective against Gram-negative bacteria targeted DNA gyrase, whereas topoisomerase IV is the fundamental target for Gram-positive bacteria [40]. 1,2,3-triazole ciprofloxacin derivative with a benzyl moiety enhances the compound antibacterial activity and the position of the fluorine substitution para- or meta-has a crucial role in the selectivity to target enzyme, at the same time, simple structure-activity relationship (SAR) showed that the presence of fluorine atom at position 4 in 4-fluorophenyl moiety of 6f derivative and 4-trifluoromethylphenyl moiety of 6d derivative is favorable for anti-proliferative activity and selectivity to cancer cells, indicating the same interactions at the molecular level for both 1,2,3-triazole and fluoroquinolone pharmacophores in the hybrid compounds (6a-f).
8. Conclusions
Library of 1,2,3-triazoles encompassing ciprofloxacin core was designed and synthesized through an enolate-azide click reaction of methyl 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-oxobutanoyl) piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (5) with various organic azides using K2CO3 as catalyst. The reaction proceeded in good to excellent yield. The anticancer screening was investigated against some cancer cell lines including the non-small cell lung cancer (A549), glioblastoma (U-87 MG), human dermal fibroblast (HDF, human dermal cell line), and breast cancer (MCF7). The results revealed that the compound 6b emerged as the most potent tested compound against the U87 cancer cell line with IC50 value of 1.2 ± 0.8 μM and selectivity Index (SI) equal to 142.3. Moreover, they were tested for their antioxidant activity in DPPH and ABTS assays and antibacterial activity.
Data availability statement
There is no data availability associated with this work.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Samir Al-Taweel: Writing - review & editing, Writing - original draft, Supervision, Project administration, Methodology, Investigation, Conceptualization. Yousef Al-Saraireh: Writing - review & editing, Writing - original draft, Software, Methodology, Investigation. Salah Al-Trawneh: Writing - review & editing, Writing - original draft, Project administration, Methodology, Investigation, Conceptualization. Solhe Alshahateet: Writing - original draft, Methodology, Investigation, Conceptualization. Rakan Al- Tarawneh: Writing - original draft, Methodology, Investigation. Nadaa Ayed: Methodology, Investigation. Mohammad Alkhojah: Methodology. Wisam AL-Khaboori: Methodology. Wael Zereini: Writing - review & editing, Writing - original draft, Methodology, Investigation, Conceptualization. Omar Al-Qaralleh: Methodology.
Declaration of competing interest
The author declares no conflict of interest. This manuscript is the author's own original work, which has not been previously published elsewhere.
Acknowledgements
The authors would like to thank the Deanship of Scientific Research/Mutah University for the financial support through research grant 568/2022, for providing the necessary technical support needed to complete this research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22592.
Contributor Information
Samir Al-Taweel, Email: s_altaweel@mutah.edu.jo.
Yousef Al-Saraireh, Email: Yousef.sar@mutah.edu.jo.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ag H., Sumitra Chitra. Consummated review on prostatitis. Asian J. Pharmaceut. Clin. Res. 2019:1–7. doi: 10.22159/ajpcr.2019.v12i3.29307. [DOI] [Google Scholar]
- 2.Chu D.T., Fernandes P.B. Structure-activity relationships of the fluoroquinolones. Antimicrob. Agents Chemother. 1989;33:131–135. doi: 10.1128/aac.33.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitscher L.A. Bacterial topoisomerase inhibitors: quinolone and pyridone antibacterial agents. Chem. Rev. 2005;105:559–592. doi: 10.1021/cr030101q. [DOI] [PubMed] [Google Scholar]
- 4.Drlica K., Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang Z., Yang Y., Ji R., Zhang S. Synthesis and antibacterial activity of novel fluoroquinolones containing substituted piperidines. Bioorg. Med. Chem. Lett. 2007;17:4523–4526. doi: 10.1016/j.bmcl.2007.05.093. [DOI] [PubMed] [Google Scholar]
- 6.a) Xu Z., Zhao S.-J., Liu Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019;183 doi: 10.1016/j.ejmech.2019.111700. [DOI] [PubMed] [Google Scholar]; b) Agarwal A., Singh P., Maurya A., Patel U.K., Singh A., Nath G. Ciprofloxacin-tethered 1,2,3-triazole conjugates: new quinolone Family compounds to upgrade our antiquated approach against bacterial infections. ACS Omega. 2022;7:2725–2736. doi: 10.1021/acsomega.1c05303. [DOI] [PMC free article] [PubMed] [Google Scholar]; C) Al Sheikh Ali A., Khan D., Naqvi A., Al-Blewi F.F., Rezki N., Aouad M.R., Hagar M. Design, synthesis, molecular modeling, anticancer studies, and density functional theory calculations of 4-(1,2,4-Triazol-3-ylsulfanylmethyl)-1,2,3-triazole derivatives. ACS Omega. 2021;6:301–316. doi: 10.1021/acsomega.0c04595. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Liang T., Sun X., Li W., Hou G., Gao F. 1,2,3-Triazole-Containing compounds as anti-lung cancer agents: current developments, mechanisms of action, and structure-activity relationship. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.661173. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Sachdeva H., Saquib M., Tanwar K. Design and development of triazole derivatives as prospective anticancer agents: a review. Anti Cancer Agents Med. Chem. 2022;22:3269–3279. doi: 10.2174/1871520622666220412133112. [DOI] [PubMed] [Google Scholar]
- 7.Alam M.M. 1,2,3-Triazole hybrids as anticancer agents: a review. Arch. Pharm. 2022;355 doi: 10.1002/ardp.202100158. [DOI] [PubMed] [Google Scholar]
- 8.a) Altıntop M.D., Kaplancıklı Z.A., Turan-Zitouni G., Özdemir A., Demirci F., İşcan G., Revial G. Synthesis of some novel triazole derivatives and investigation of their antimicrobial activities. Synth. Commun. 2011;41:2234–2250. doi: 10.1080/00397911.2010.501475. [DOI] [Google Scholar]; b) Maji H.S., Maji S., Bhattacharya M. An exploratory study on the antimicrobial activity of cetirizine dihydrochloride. Indian J Pharm Sci. 2017;5:751–757. doi: 10.4172/pharmaceutical-sciences.1000288. [DOI] [Google Scholar]; c) Gonzaga D.T.G., Souza T.M.L., Andrade V.M.M., Ferreira V.F., de C da Silva F. Identification of 1-aryl-1H-1,2,3-triazoles as potential new antiretroviral agents. Med. Chem. 2018;14:242–248. doi: 10.2174/1573406413666170906121318. [DOI] [PubMed] [Google Scholar]
- 9.a) Breugst M., Reissig H.-U. The huisgen reaction: milestones of the 1,3-dipolar cycloaddition. Angew. Chem. Int. Ed Engl. 2020;59:12293–12307. doi: 10.1002/anie.202003115. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hui R., Zhao M., Chen M., Ren Z., Guan Z. One-pot synthesis of 4-aryl-NH-1,2,3-triazoles through three-component reaction of aldehydes, nitroalkanes and NaN3. Chin. J. Chem. 2017;35:1808–1812. doi: 10.1002/cjoc.201700367. [DOI] [Google Scholar]; c) Ramasastry S.S.V. Enamine/enolate-mediated organocatalytic azide-carbonyl [3+2] cycloaddition reactions for the synthesis of densely functionalized 1,2,3-triazoles. Angew. Chem. Int. Ed Engl. 2014;53:14310–14312. doi: 10.1002/anie.201409410. Epub 2014 Nov 17. PMID: 25404559. [DOI] [PubMed] [Google Scholar]; d) Nelson R., Kesternich V., Pérez-Fehrmann M., Jaldin S., Marcourt L., Christen P. Regiospecific synthesis of 1,4,5-trisubstituted 1,2,3-triazoles via enolate–azide cycloaddition between 1,3-dicarbonyl compounds and aryl azides. J.Chem. Res. 2016;40:453–457. doi: 10.3184/174751916X146566622669. [DOI] [Google Scholar]; e) Manetsch R., Krasiński A., Radić Z., Raushel J., Taylor P., Sharpless K.B., Kolb H.C. In situ click chemistry: enzyme inhibitors made to their own specifications. J. Am. Chem. Soc. 2004;126:12809–12818. doi: 10.1021/ja046382g. [DOI] [PubMed] [Google Scholar]
- 10.Hyatt J.A., Feldman P.L., Clemens R.J. Ketenes. 20. Thermal decomposition of 2,2,6-trimethyl-4H-1,3-dioxin-4-one and 1-ethoxybutyn-3-one. Acetylketene, J. Org. Chem. 1984;49:5105–5108. doi: 10.1021/jo00200a018. [DOI] [Google Scholar]
- 11.Clemens R.J., Hyatt J.A. Acetoacetylation with 2,2,6-trimethyl-4H-1,3-dioxin-4-one: a convenient alternative to diketene. J. Org. Chem. 1985;50:2431–2435. doi: 10.1021/jo00214a006. [DOI] [Google Scholar]
- 12.Clemens R.J., Witzeman J.S. Kinetic and spectroscopic studies on the thermal decomposition of 2,2,6-trimethyl-4H-1,3-dioxin-4-one. Generation of acetylketene. J. Am. Chem. Soc. 1989;111:2186–2193. doi: 10.1021/ja00188a037. [DOI] [Google Scholar]
- 13.Kutonova K.V., Trusova M.E., Filimonov V.D., Parello J. A simple and effective synthesis of aryl azides via arenediazonium tosylates. Synthesis. 2013;45:2706–2710. doi: 10.1055/s-0033-1339648. [DOI] [Google Scholar]
- 14.Barsoum D.N., Brassard C.J., Deeb J.H.A., Okashah N., Sreenath K., Tyler Simmons J., Zhu L. Synthesis of 5-Iodo-1,2,3-triazoles from organic azides and terminal Alkynes¬: ligand acceleration effect, substrate scope, and mechanistic insights. Synthesis. 2013;45:2372–2386. doi: 10.1055/s-0033-1339312. [DOI] [Google Scholar]
- 15.Al-Saraireh Y.M., Youssef A.M.M., Alsarayreh A.Z., Al Hujran T.A., Al-Sarayreh S., Al-Shuneigat J.M., Alrawashdeh H.M. Phytochemical and anti-cancer properties of Euphorbia hierosolymitana Boiss. crude extracts. J. Pharm. Pharmacogn. Res. 2021;9(13–23) ISSN 0719-4250. [Google Scholar]
- 16.Youssef A.M.M., Maaty D.A.M., Al-Saraireh Y.M. Phytochemical analysis and profiling of antitumor compounds of leaves and stems of calystegia silvatica (kit. Griseb, Molecules. 2023;28 doi: 10.3390/molecules28020630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youssef A.M.M., El-Swaify Z., Al-Saraireh Y.M., Dalain S. Cytotoxic activity of methanol extract of Cynanchumacutum L. seeds on human cancer cell lines. Latin Am. J. Pharm. 2018;37:1997–2003. ISSN 2362-3853. [Google Scholar]
- 18.Mohamed Youssef A., Said El-Swaify Z., Al-Saraireh Y., Al-Dalain S. Anticancer effect of different extracts of Cynanchum acutum L. seeds on cancer cell lines. Pharmacogn. Mag. 2019;15:261. doi: 10.4103/pm.pm_676_18. [DOI] [Google Scholar]
- 19.Youssef A.M.M., Maaty D.A.M., Al-Saraireh Y.M. Phytochemistry and anticancer effects of mangrove (Rhizophora mucronata lam.) leaves and stems extract against different cancer cell lines. Pharmaceuticals. 2022;16 doi: 10.3390/ph16010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-saraireh Y.M., Youssef A.M.M., Alshammari F.O.F., Al-Sarayreh S.A., Al-Shuneigat J.M., Alrawashdeh H.M., Mahgoub S.S. Phytochemical characterization and anti-cancer properties of extract of Ephedra foeminea (Ephedraceae) aerial parts. Trop. J. Pharm Res. 2021;20:1675–1681. doi: 10.4314/tjpr.v20i8.18. [DOI] [Google Scholar]
- 21.Xu Z., Zhao S.-J., Liu Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019;183 doi: 10.1016/j.ejmech.2019.111700. [DOI] [PubMed] [Google Scholar]
- 22.Ashour H.F., Abou-Zeid L.A., El-Sayed M.A.-A., Selim K.B. 1,2,3-Triazole-Chalcone hybrids: synthesis, in vitro cytotoxic activity and mechanistic investigation of apoptosis induction in multiple myeloma RPMI-8226. Eur. J. Med. Chem. 2020;189 doi: 10.1016/j.ejmech.2020.112062. [DOI] [PubMed] [Google Scholar]
- 23.Gurrapu N., Praveen Kumar E., Kolluri P.K., Putta S., Sivan S.K., Subhashini N.J.P. Synthesis, biological evaluation and molecular docking studies of novel 1,2,3-triazole tethered chalcone hybrids as potential anticancer agents. J. Mol. Struct. 2020;1217 [Google Scholar]
- 24.Mohammed H.H.H., Abd El-Hafeez A.A., Ebeid K., Mekkawy A.I., Abourehab M.A.S., Wafa E.I., Alhaj-Suliman S.O., Salem A.K., Ghosh P., Abuo-Rahma G.E.-D.A., Hayallah A.M., Abbas S.H. New 1,2,3-triazole linked ciprofloxacin-chalcones induce DNA damage by inhibiting human topoisomerase I& II and tubulin polymerization. J. Enzyme Inhib. Med. Chem. 2022;37:1346–1363. doi: 10.1080/14756366.2022.2072308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashour H.F., Abou-Zeid L.A., El-Sayed M.A.-A., Selim K.B. 1,2,3-Triazole-Chalcone hybrids: synthesis, in vitro cytotoxic activity and mechanistic investigation of apoptosis induction in multiple myeloma RPMI-8226. Eur. J. Med. Chem. 2020;189 doi: 10.1016/j.ejmech.2020.112062. [DOI] [PubMed] [Google Scholar]
- 26.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez R.P. Cellular and molecular determinants of cisplatin resistance. Eur. J. Cancer. 1998;34:1535–1542. doi: 10.1016/s0959-8049(98)00227-5. [DOI] [PubMed] [Google Scholar]
- 28.Al-Zereini W.A. Bioactive crude extracts from four bacterial isolates of marine sediments from red sea , gulf of Aqaba , Jordan, Jordan. J. Biol. Sci. 2014;7:133–173. doi: 10.12816/0008227. [DOI] [Google Scholar]
- 29.Al-Zereini W.A., Al-Trawneh I.N., Al-Qudah M.A., TumAllah H.M., Abudayeh Z.H., Hijazin T. Antibacterial, antioxidant, and cytotoxic activities of Syzygium aromaticum (L.) Merr. & Perry essential oil with identification of its chemical constituents. Z. Naturforsch. C. 2023;78:105–112. doi: 10.1515/znc-2022-0056. [DOI] [PubMed] [Google Scholar]
- 30.Rivero-Pérez M.D., Muñiz P., Gonzalez-Sanjosé M.L. Antioxidant profile of red wines evaluated by total antioxidant capacity, scavenger activity, and biomarkers of oxidative stress methodologies. J. Agric. Food Chem. 2007;55:5476–5483. doi: 10.1021/jf070306q. [DOI] [PubMed] [Google Scholar]
- 31.Bozorov K., Zhao J., Aisa H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: a recent overview. Bioorg. Med. Chem. 2019;27:3511–3531. doi: 10.1016/j.bmc.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faidallah H.M., Girgis A.S., Tiwari A.D., Honkanadavar H.H., Thomas S.J., Samir A., Kalmouch A., Alamry K.A., Khan K.A., Ibrahim T.S., Al-Mahmoudy A.M.M., Asiri A.M., Panda S.S. Synthesis, antibacterial properties and 2D-QSAR studies of quinolone-triazole conjugates. Eur. J. Med. Chem. 2018;143:1524–1534. doi: 10.1016/j.ejmech.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 33.Xu J.-H., Fan Y.-L., Zhou J. Quinolone-triazole hybrids and their biological activities. J. Heterocycl. Chem. 2018;55:1854–1862. doi: 10.1002/jhet.3234. [DOI] [Google Scholar]
- 34.Foroumadi A., Emami S., Mehni M., Moshafi M.H., Shafiee A. Synthesis and antibacterial activity of N-[2-(5-bromothiophen-2-yl)-2-oxoethyl] and N-[(2-5-bromothiophen-2-yl)-2-oximinoethyl] derivatives of piperazinyl quinolones. Bioorg. Med. Chem. Lett. 2005;15:4536–4539. doi: 10.1016/j.bmcl.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Jia Y., Zhao L. The antibacterial activity of fluoroquinolone derivatives: an update (2018–2021) Eur. J. Med. Chem. 2021;224 doi: 10.1016/j.ejmech.2021.113741. [DOI] [PubMed] [Google Scholar]
- 36.Dileep K., Polepalli S., Jain N., Buddana S.K., Prakasham R.S., Murty M.S.R. Synthesis of novel tetrazole containing hybrid ciprofloxacin and pipemidic acid analogues and preliminary biological evaluation of their antibacterial and antiproliferative activity. Mol. Divers. 2018;22:83–93. doi: 10.1007/s11030-017-9795-y. [DOI] [PubMed] [Google Scholar]
- 37.Hryhoriv H., Mariutsa I., Kovalenko S.M., Georgiyants V., Perekhoda L., Filimonova N., Geyderikh O., Sidorenko L. The search for new antibacterial agents among 1,2,3-triazole functionalized ciprofloxacin and Norfloxacin hybrids: synthesis, docking studies, and biological activity evaluation. Sci. Pharm. 2021;90:2. doi: 10.3390/scipharm90010002. [DOI] [Google Scholar]
- 38.Cilliers P. North-West University (South-Africa). Potchefstroom Campus; 2019. Synthesis and Antimycobacterial Activity of Ciprofloxacin-Triazole Hybrids.https://repository.nwu.ac.za/handle/10394/32784 [Google Scholar]
- 39.Strzelecka M., Świątek P. 1,2,4-Triazoles as important antibacterial agents. Pharmaceuticals. 2021;14 doi: 10.3390/ph14030224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao F., Wang P., Yang H., Miao Q., Ma L., Lu G. Recent developments of quinolone-based derivatives and their activities against Escherichia coli. Eur. J. Med. Chem. 2018;157:1223–1248. doi: 10.1016/j.ejmech.2018.08.095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There is no data availability associated with this work.